Abstract
Aim:
This study evaluates the relationship of tumour and anatomical features with operative difficulty in robotic low anterior resection performed by four experienced surgeons in a high-volume colorectal cancer practice.
Methods:
Data from 382 patients who underwent robotic low anterior resection by four expert surgeons between January 2016 and June 2019 were included in the analysis. Operating time was used as a measure of operative difficulty. Univariate and multivariate mixed models were used to identify associations between baseline characteristics and operating time, with surgeon as a random effect, thereby controlling for variability in surgeon speed and proficiency. In an exploratory analysis, operative difficulty was defined as conversion to laparotomy, a positive margin or an incomplete mesorectum.
Results:
Median operating time was 4.28 hours (range, 1.95–11.33 hours) but varied by surgeon from 3.45 (1.95–6.10) hours to 5.93 (3.33–11.33) hours (p<0.001). Predictors of longer operating time in multivariate analysis were male sex, higher body mass index, neoadjuvant radiotherapy, low tumour height, greater sacral height, and larger mesorectal area at the S5 vertebral level. Conversion occurred in 2 cases (0.5%), and incomplete mesorectum and positive margins were found in 9 (2.4%) and 19 (5.0%) patients, respectively. Neoadjuvant radiotherapy and larger pelvic outlet were the only characteristics associated with the exploratory measure of difficulty.
Conclusion:
Predicting operative difficulty based on easy to identify, preoperative radiological and clinical variables is feasible in robotic anterior resection.
Introduction:
Minimally invasive total mesorectal excision (TME) is a technically challenging procedure. Robotic surgery offers potential advantages over standard laparoscopy for rectal cancer surgery, including better visualization (secondary to stable camera control and three-dimensional optics) and finer tissue handling (secondary to articulating instruments, motion scaling and removal of tremor). Nonetheless, tumour-specific and anatomical characteristics still contribute significantly to operative complexity. Predictors of operative difficulty in laparoscopic surgery for rectal cancer have been well described [1, 2], but the impact of preoperative clinical and anatomical parameters on difficulty of robotic surgery for rectal cancer has not been sufficiently elucidated.
Understanding which parameters are associated with increased difficulty may have many practical implications, including optimal surgical preparation, selection of appropriate teaching cases, administration of hospital resources and management of patient expectations. Previous studies investigating operative difficulty in robotic low anterior resection had small samples [3–6], grouped robotic surgeries with other operative approaches [6], did not account for variability in surgeons’ baseline speed and proficiency [4, 5], and/or were based on operative data from a single surgeon [3]. Moreover, studies have used widely different outcome measures, including a variety of intraoperative and postoperative events as surrogates for operative difficulty, which may be multifactorial in nature (e.g. morbidity, length of stay, anastomotic leak).
In this study, we evaluated the impact of tumour-specific and anatomical parameters with operative difficulty in robotic low anterior resection. Surgeries were performed by four expert colorectal surgeons in a high-volume colorectal oncology center, and variability in operating time was used as a surrogate for operative difficulty. The analysis also controlled for baseline surgeon speed and proficiency using a mixed methods model. Finally, we conducted an exploratory analysis with a composite outcome of intraoperative and pathological outcomes. Our hypothesis was that factors influencing difficulty would be similar to those described in open and laparoscopic surgery.
Materials and Methods:
Patient selection
We searched prospectively maintained institutional databases to identify patients with rectal cancer (i.e., a tumour within 15 cm of the anal verge) who had undergone robotic low anterior resection between January 2016 and June 2019. All procedures had been performed by an expert surgeon (JGG, MRW, PBP, or JGA) who had more than 20 years of experience and had performed more than 40 robotic rectal resections each for rectal cancer. Patients were excluded if they had undergone abdominoperineal resection, lateral pelvic lymph node dissection, multivisceral resection or Hartmann’s procedure. Clinical and tumour characteristics, including those related to preoperative imaging and endoscopy, were identified by chart review. Clinical stage was assessed on preoperative MRI by specialized GI radiologists. Clinical T stage (cT) was classified as cT1/2, cT3, or cT4 when a clinical stage was given on preoperative magnetic resonance imaging (MRI), and as cTx when this data was missing or the primary tumour was not visualized. The study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center, and a waiver of informed consent was obtained.
Surgical technique
All surgeries were performed in the presence of colorectal or surgical oncology fellows. The majority of each procedure was performed by experienced attending colorectal surgical oncologists. Patient and robot set up was performed in a standardized manner by experienced physician assistants (PAs) trained in a dedicated robotic PA program. Our colorectal service’s technique for robotic low anterior resection for rectal cancer has been previously published [7]. In brief, resections were performed in medial-to-lateral fashion using the da Vinci Surgical System Xi (Intuitive Surgical, Sunnyvale, CA). Entry into the peritoneum was accomplished with a Veress needle or, less commonly, the Hasson technique. A supraumbilical port was used for the 0° robotic camera. Right-sided abdominal ports, and one or two 5-mm assistant ports, were utilized for identification and ligation of the inferior mesenteric artery and inferior mesenteric vein, and for mobilization of the splenic flexure, descending colon, and sigmoid. The robot was then undocked and rotated towards the pelvis for pelvic dissection, rectal division, and anastomosis. The rectum was divided by a linear robotic stapler. Anastomoses were completed using a double-stapling technique. Diverting ileostomies were created at the discretion of the operating surgeon.
Outcome Measures
For the primary analysis, we used operating time to quantify operative difficulty. Operating time was defined as the time from incision to complete application of all dressings. Operating time is a widely used surrogate of difficulty for which data is reliably and easily gathered from the operative record [1–3, 6, 8–10]. Understanding that operating time may not encompass all facets of operative difficulty and that a difficult surgery may be performed quickly but sub-optimally, we also performed an exploratory analysis in which we defined operative difficulty as a categorical outcome measure consisting of conversion to laparotomy, a positive distal or circumferential margin (≤1mm), or an incomplete mesorectum. Presence of any of these was considered an event.
Covariates:
Candidate covariates were selected for data collection based on clinical relevance to rectal cancer surgery, as determined by surgeons in the colorectal division and literature review. These variables included sex, age, BMI, tumour height, clinical stage, anterior tumour location, threatened circumferential margins on MRI (≤2mm), neoadjuvant chemoradiation, and history of abdominal surgery or colorectal endoscopic stenting.
Pelvimetry measurements were assessed by two blinded surgeons (JBY and HMT) based on baseline high resolution rectal MRI on the midsagittal (midpoint of anterosuperior aspect of S1) and axial planes using the Centricity Universal Viewer (GE Healthcare, Chicago, IL). In line with previous research [[3, 6]], midsagittal plane measurements of the inlet length, pubic tubercle height, outlet length, sacral height, and sacral depth were recorded. Axial plane measurements included the interspinous distance at the level of the fovea of the femoral head, and the mesorectal area at the S5 vertebral level (Figure 1). When preoperative MRI was not available, all pelvimetry measurements (except for mesorectal area, which was omitted in these cases) were performed using preoperative computed tomography scans of the midsagittal and axial planes. These measurements of osseous landmarks should be consistent between MRI and computed tomography.
Figure 1:
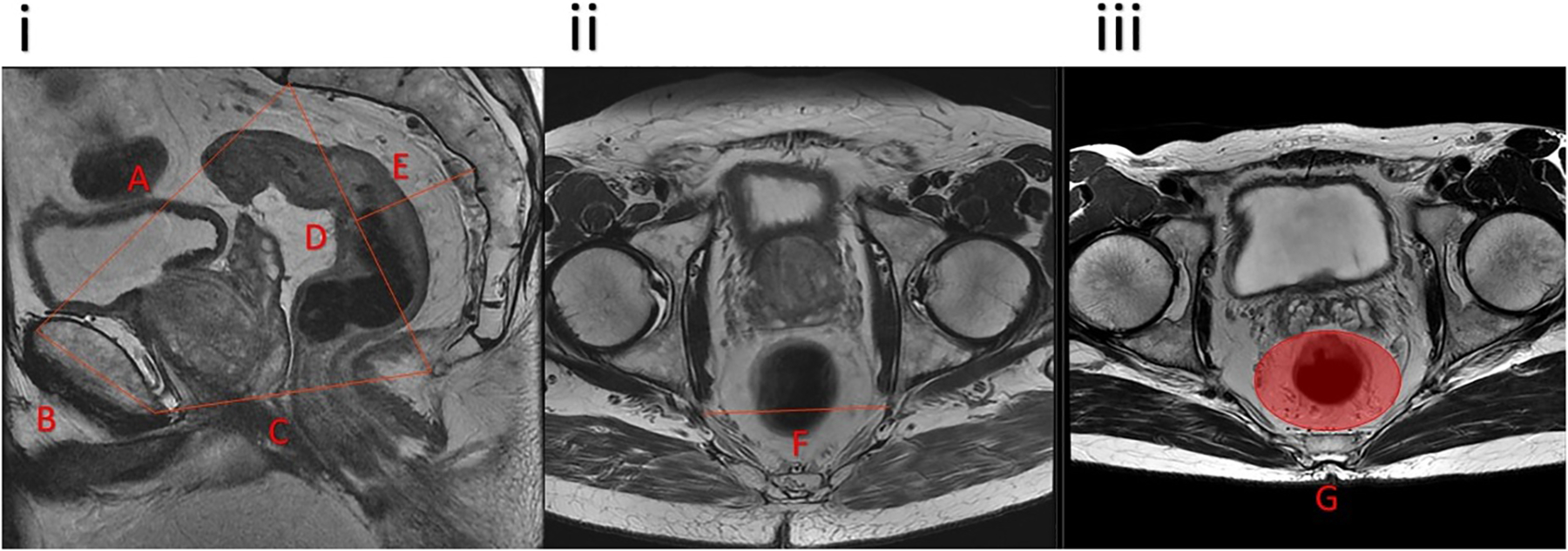
Pelvimetry measurements on midsagittal (i) and axial (ii and iii) planes. A, pelvic inlet; B, pubic tubercle height; C, pelvic outlet length; D, sacral height; E, sacral depth; F, interspinous distance; G, mesorectal area.
Statistical analysis
We first present descriptive data; categorical variables are presented as frequencies and percentages, and continuous variables as medians (with ranges). Univariate analysis was used to identify potential predictors of increased operating time. A multivariate mixed models approach was used to predict increased operating time controlling for important covariates identified in univariable analysis. In order to account for heterogeneity in baseline surgeon speed and proficiency, the surgeon was considered as a random effect.
In a separate and exploratory analysis, operative difficulty was defined as the occurrence of one of the following: conversion to laparotomy; positive distal or circumferential margins (≤1mm); or incomplete mesorectum. The baseline tumour and anatomical characteristics of patients experiencing any of the three aforementioned outcomes were compared with those of patients who experienced none of these outcomes.
Statistical significance was defined as p<0.05 and was assessed using chi-square tests and Fisher’s exact test for categorical variables, and Wilcoxon rank-sum tests for continuous variables. Interobserver agreement was evaluated using Spearman’s correlation test. All analyses were performed using R software version 3.6.2.
Results
Patient Characteristics:
During the study period, 382 patients met the inclusion criteria. Table 1 provides an overview of these patients’ demographic, preoperative and tumour characteristics. Pelvimetry was performed on CT and not MRI in 47 patients (12%). All pelvic distances and area measurements, except for sacral depth, differed by patient sex (Table 2).
Table 1:
Baseline characteristics of patients who underwent robotic low anterior resection for rectal cancer
| Characteristic | N | n (%) or median (range) |
|---|---|---|
| Sex | 382 | |
| Female | 159 (42%) | |
| Male | 223 (58%) | |
| BMI | 382 | 27.1 (17.3–59.7) |
| Age | 382 | 53 (21–83) |
| Previous neoadjuvant chemoradiation | 382 | 202 (53%) |
| Previous abdominal surgery | 382 | 141 (37%) |
| Previous stenting | 382 | 2 (0.5%) |
| Tumor height (cm) | 382 | |
| 0–5 | 65 (17%) | |
| 6–10 | 201 (53%) | |
| 11–15 | 116 (30%) | |
| Threatened CRM on MRI | 226 | 84 (37%) |
| cT stage | 382 | |
| cT1/cT2 | 76 (20%) | |
| cT3 | 247 (65%) | |
| cT4 | 28 (7.3%) | |
| cTx | 31 (8.1%) |
BMI, body mass index; CRM, circumferential resection margin; cT, clinical tumor.
Table 2:
Median values of pelvimetry measurements, by sex
| Characteristic | N | Females | Males | P value |
|---|---|---|---|---|
| Inlet length (cm) | 372 | 12.46 (10.03–14.78) | 11.62 (9.50–13.80) | <0.001 |
| Outlet length (cm) | 372 | 9.33 (6.84–12.39) | 8.99 (6.60–12.20) | 0.002 |
| Sacral height (cm) | 372 | 11.64 (7.69–15.22) | 12.54 (8.31–16.76) | <0.001 |
| Sacral depth (cm) | 372 | 3.91 (1.87–5.46) | 4.01 (1.53–8.26) | 0.2 |
| Pubic tubercle height (cm) | 372 | 3.88 (2.63–4.97) | 4.40 (3.19–5.86) | <0.001 |
| Interspinous distance (cm) | 378 | 10.88 (7.41–12.98) | 9.38 (7.27–11.74) | <0.001 |
| Mesorectal area (cm2) | 327 | 28 (10–58) | 32 (8–65) | <0.001 |
Values in parentheses are ranges. P values were calculated using the Wilcoxon rank sum test.
Diverting ileostomies were performed in 235 patients (62%) and the splenic flexure was mobilized in 209 patients (55%). Complete mesorectal excision was performed in 248 patients (65%), tumour specific TME was performed in 121 patients (32%) and extent of TME was missing for 13 patients (3%). Post-operative complications were reported in 40 (10.4%) patients, of these 9 (2.3%) patients experienced a grade 3 or higher complication. There was no post-operative mortality.
Primary Analysis:
The median operating time was 4.28 hours (range, 1.95–11.33), but varied significantly by individual surgeon (Figure 2), (3.45 (1.95–6.10), 3.83 (2.45–8.55), 5.40 (2.93–9.60) and 5.93 (3.33–11.33) hours, p<0.001).
Figure 2:
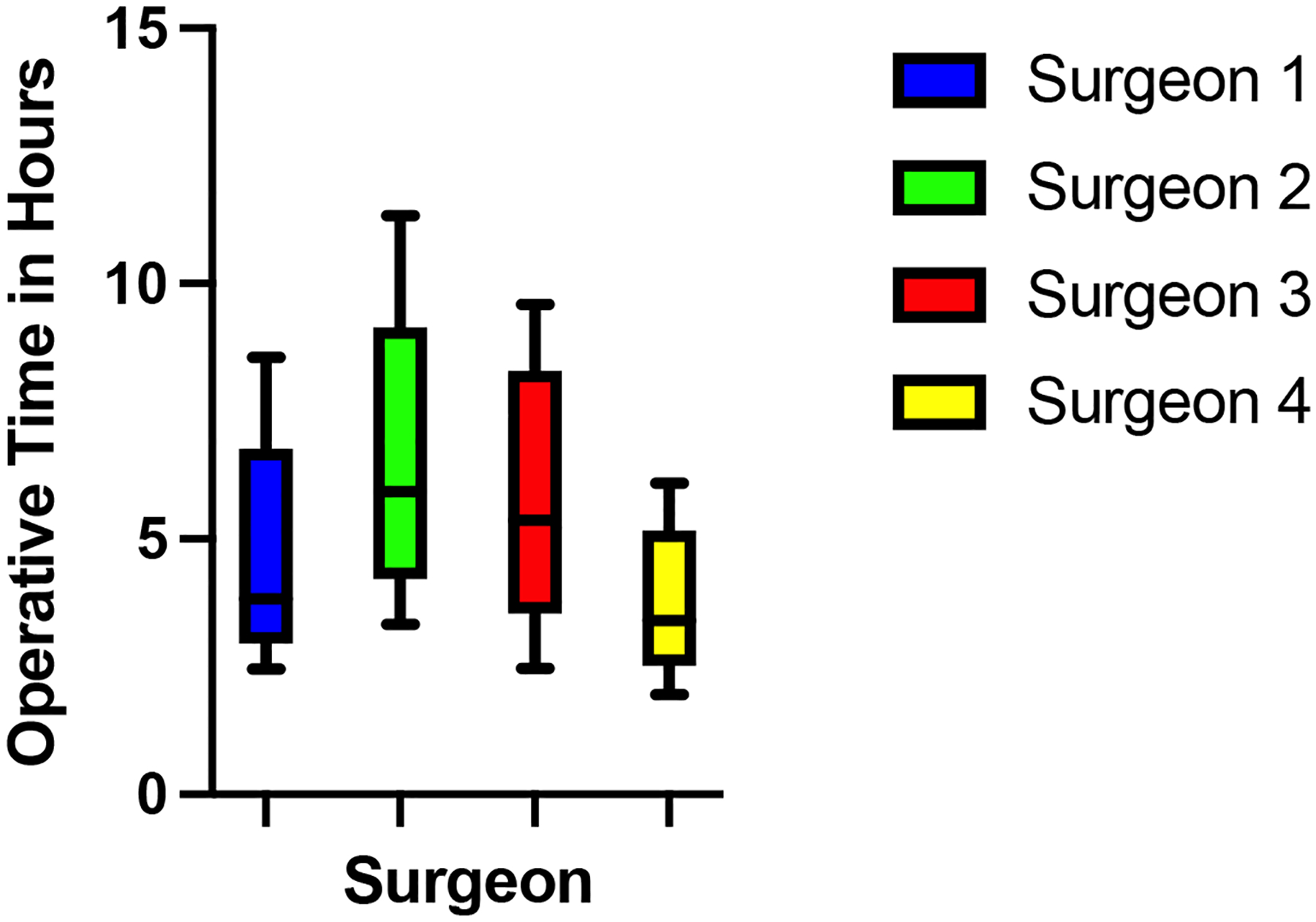
Box and whisker plot of the variability of each surgeon’s operating time. The whiskers represent minimum and maximum values. The box represents median, first and third quartile of operating times.
In the univariate analysis, longer operating time was associated with male sex, higher body mass index (BMI), previous neoadjuvant radiotherapy, lower tumour height, anterior tumour location, greater sacral height, greater pubic tubercle height, and larger mesorectal area at the S5 vertebral level (Supplementary Table 1). All of these characteristics, except anterior tumour extension and pubic tubercle height, remained significantly associated with increased operating time in the multivariate analysis (Table 3).
Table 3:
Coefficients (and 95% CIs) from mixed-model multivariate regression assessing associations between baseline characteristics and operative time, with surgeon as random effect
| Characteristic | Beta | 95% CI | P value |
|---|---|---|---|
| Sex | |||
| Female | Reference group | ||
| Male | 0.29 | 0.04, 0.55 | 0.024 |
| Body mass index | 0.06 | 0.04, 0.08 | <0.001 |
| Previous neoadjuvant chemoradiation | 0.51 | 0.24, 0.78 | <0.001 |
| Tumor height (cm) | |||
| 0–5 | 0.50 | 0.14, 0.86 | 0.007 |
| 6–10 | 0.15 | −0.13, 0.43 | 0.3 |
| 11–15 | Reference group | ||
| cT stage | |||
| cT1/cT2 | Reference group | ||
| cT3 | −0.29 | −0.62, 0.04 | 0.084 |
| cT4 | −0.19 | −0.68, 0.30 | 0.5 |
| cTx | 0.37 | −0.75, 1.5 | 0.5 |
| Anterior tumor location | −0.09 | −0.35, 0.16 | 0.5 |
| Sacral height (cm) | 0.09 | 0.01, 0.18 | 0.029 |
| Pubic tubercle height (cm) | −0.07 | −0.31, 0.17 | 0.6 |
| Mesorectal area (cm2) | 0.02 | 0.01, 0.03 | <0.001 |
CI, confidence interval; BMI, body mass index, cT, clinical tumor.
Interobserver Variation:
Interobserver variation in pelvimetry measurements are summarized in Table 4. The Spearman’s correlation coefficients ranged from 0.645 to 0.951 (p<0.001 for each), indicating that interobserver agreement was either strong (0.6–0.79) or very strong (0.8–1.0) for all measurements.
Table 4:
Interobserver variation in pelvimetry measurements
| Characteristic | Median (range) | r † | P value | |
|---|---|---|---|---|
| First observer | Second observer | |||
| Inlet length | 11.87 (9.51–14.55) | 12.00 (9.30–15.00) | 0.951 | <0.001 |
| Outlet length | 9.03 (6.50–12.11) | 9.20 (6.70–12.80) | 0.858 | <0.001 |
| Sacral height | 12.10 (7.57–16.83) | 12.10 (5.10–16.70) | 0.901 | <0.001 |
| Sacral depth | 3.97 (1.46–6.18) | 4.00 (1.60–11.70) | 0.873 | <0.001 |
| Pubic tubercle height | 4.23 (2.56–6.03) | 4.10 (2.70–7.80) | 0.675 | <0.001 |
| Interspinous distance | 9.99 (7.04–13.34) | 9.80 (7.10–13.20) | 0.905 | <0.001 |
| Mesorectal area | 34 (7–72) | 27 (2–62) | 0.645 | <0.001 |
Spearman’s correlation coefficient. N=372 for all measurements except interspinous distance (N=378) and mesorectal area (N=327).
Exploratory Analysis:
Twenty subjects were excluded from the exploratory analysis due to variability in pathological reporting, whereby the completeness of the mesorectal resection was not specifically reported. Among the 362 patients included the analysis, 29 (8.0%) of the 362 patients with complete data experienced a total of 30 events included in the composite outcome of operative difficulty: 2 conversions to laparoscopy (0.6%); 9 cases of incomplete mesorectum (2.5%); and 19 cases of positive (≤1mm) margins (5.2%), of which two were positive distal margins and 17 were positive circumferential margins. The rate of the composite outcome was 13% for surgeon 1, 8% for surgeons 2 and 3, and 4% for surgeon 4. The only patient characteristics associated with the composite outcome were preoperative chemoradiotherapy and pelvic outlet diameter (Supplementary Table 2).
The operating time for patients with the composite outcome was not significantly different than that for the rest the cohort. In analyses that compared surgeons, operating time for patients with the composite outcome was similar to that of other patients for three of the surgeons (Surgeons 1, 2, and 4). However, for Surgeon 3, median operating time was longer for the 5 patients with the composite outcome (8.40 hours; range, 6.95–9.12 hours) than for the remaining 61 patients (5.35 hours; range, 2.93–9.6 hours) (p=0.001).
Discussion and conclusions:
To our knowledge, this is the largest study evaluating operative difficulty in robotic anterior resection for rectal cancer, and the first to control for variability in multiple surgeons’ baseline speeds. Independent predictors of longer operating time were male sex, high BMI, neoadjuvant radiotherapy, low tumour height, large sacral height, and large mesorectal area. Defining operative difficulty based on operative time without adjusting for surgeons’ speed introduces clear confounding. Studies based on a single surgeon may avoid this confounding, but may be less generalizable.
In their series of 182 robotic rectal cancer resections, Baek et al. demonstrated that high BMI, low tumour height, and neoadjuvant chemoradiation were associated with longer operating time, but pelvimetry measurements were not [5]. Moreover, no baseline characteristics in their study were predictive of a positive circumferential resection margin (CRM). Because a previous study by the same group had found that pelvimetry measurements predicted longer operating time in laparoscopic rectal cancer resections [11], but not in robotic resections [5], the authors concluded that the robotic platform allows surgeons to overcome difficulties associated with pelvic anatomy in rectal cancer surgery. However, the authors did not control for variability in the operative speed of the 5 participating surgeons, and did not measure mesorectal area. Yamaoka et al. did measure baseline mesorectal area in their series of 98 patients undergoing robotic low anterior resections for rectal cancer by a single surgeon, and in their analysis this parameter was the only independent predictor of longer operating time [3].
In previous studies of laparoscopic and open rectal cancer surgery, the characteristics associated with greater difficulty have included male sex, high BMI, low tumour height, neoadjuvant radiation therapy, narrow outlet diameter, short interspinous distance, long sacral height and depth, and large mesorectal area [1, 2, 6, 12]. This list overlaps substantially with the predictors of longer operating time in robotic surgery that we identified in analyses that controlled for variability in surgeons’ operative speed, suggesting that despite its multiple advantages, robotic surgery cannot completely overcome the complexity posed by pelvic anatomy. In fact, the variables that make TME surgery difficult are somewhat consistent across all surgical approaches. Escal et al. investigated predictors of surgical difficulty in 164 rectal cancer patients undergoing either open, laparoscopic or robotic rectal resection according to surgeon’s preference. In line with our findings, predictors of difficulty in these three approaches included high BMI, coloanal anastomosis (colinear with tumour height), short intertuberous distance, and large mesorectal area [6].
Most pelvimetry measurements in our cohort were different between men and women. However, sex remained an independent predictor of longer operating time despite inclusion of pelvimetry measurements in the multivariate analysis, suggesting that operating time was influenced by additional anatomic differences between the sexes that were not accounted for by the measurements performed (e.g., prostate vs. uterus).
Of all studied baseline parameters, only pelvic outlet and preoperative chemoradiation were associated with the composite outcome of conversion, positive margins, and incomplete mesorectum. Perhaps surprisingly, patients who experienced one or more of these outcomes had, on average, a larger outlet diameter than those who did not (9.50 cm vs. 9.07 cm; p=0.023). In previous studies, larger outlet diameter in rectal cancer surgery was associated with both shorter [2] and longer [9] operating time, as well as with anastomotic leak [2]. The proportion of patients who received preoperative chemoradiation was higher among those who subsequently had the exploratory composite outcome than among those who did not (72% vs. 52%, p=0.032). The main driver of this difference was a higher rate of CRM positivity in irradiated patients, likely reflecting the fact that these more advanced tumours had been selected for preoperative radiation. This finding has been recapitulated in prior studies [13, 14]. Given the small number of other evaluable events, additional analyses are likely underpowered to find any meaningful associations.
The limitations of our study include the potential selection bias inherent to a retrospective design. Additionally, the study was conducted at a single specialized cancer center, which may limit generalizability. Pelvimetry was performed on CT and not on MRI in a small minority of patients and pelvimetry measurements were recorded by surgeons rather than by radiologists, both of which may have affected accuracy. We chose to have surgeons perform measurements because we wanted to identify parameters that could feasibly be identified by surgeons in radiological review as part of routine preoperative planning. In fact, we found that the measurements of our two surgeon observers were strongly correlated, which supports the feasibility of accurate pelvimetry measurement by surgeons. The strengths of the study include the relatively large number of patients, and the experience of the participating surgeons. Additionally, the uniform assistance of skilled robotic physician assistants at our medical center should have minimized differences in time related to robot set up and docking.
In summary, in this study we were able to delineate easily evaluable baseline anatomical, clinical, and tumour-related parameters which influence the operating times of robotic low anterior resections performed by expert surgeons with varying operative speeds. These parameters are predictive for surgeons with faster and slower baseline speed and include male sex, higher BMI, preoperative chemoradiation, low tumour height, high sacral height and greater mesorectal area. Routine assessment of the anatomic and tumour-related parameters described herein may have practical relevance for surgical preparation, selection of appropriate teaching cases, hospital resource planning and management of patient expectations.
In conclusion, we identified six anatomical features that can be easily assessed by surgeons in the preoperative setting, and that are associated with increased operative difficulty in robotic low anterior resection for rectal cancer. These features should be routinely examined by all surgeons in pre-operative planning for this challenging surgery.
Supplementary Material
What does this paper add to the literature?
To our knowledge, this is the largest study evaluating operative difficulty in robotic anterior resection for rectal cancer, and the first to control for variability in multiple surgeons’ speeds. Predictors of longer operating time were similar to those in prior studies focused on open and laparoscopic approaches for rectal cancer, and include many easy to identify anatomical and clinical variables. Routine pre-operative assessment of these variables by surgeons is feasible and can help in the management of rectal cancer patients.
Acknowledgments:
The authors thank Peter Doskoch for editing the manuscript. JBY acknowledges Yael Renert-Yuval for insightful conversations.
Disclosures:
Dr. Garcia-Aguilar has received fees from Medtronic, Johnson & Johnson and Intuitive Surgical. Dr. Smith has served as an advisor for Guardant Health, Inc. Drs. Yuval, Thompson, Patil, Wei, Pappou, Guillem, Nash, Paty, Weiser and Widmar have no conflicts of interest to disclose. Ms. Fiasconaro has no conflicts of interest to disclose.
Financial support:
Research at Memorial Sloan Kettering is funded in part by grant P30 CA008748 from the National Cancer Institute. J. B. Yuval’s research fellowship at Memorial Sloan Kettering was funded in part by grant T32 CA009501 from the National Cancer Institute.
Footnotes
This study was accepted as an oral presentation in the 2021 SAGES meeting
References:
- 1.Targarona EM, Balague C, Pernas JC, Martinez C, Berindoague R, Gich I, et al. Can we predict immediate outcome after laparoscopic rectal surgery? Multivariate analysis of clinical, anatomic, and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann Surg 2008; 247: 642–9. [DOI] [PubMed] [Google Scholar]
- 2.Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, et al. Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery 2009; 146: 483–9. [DOI] [PubMed] [Google Scholar]
- 3.Yamaoka Y, Yamaguchi T, Kinugasa Y, Shiomi A, Kagawa H, Yamakawa Y, et al. Mesorectal fat area as a useful predictor of the difficulty of robotic-assisted laparoscopic total mesorectal excision for rectal cancer. Surg Endosc 2019; 33: 557–66. [DOI] [PubMed] [Google Scholar]
- 4.Jimenez Rodriguez RM, De la Portilla De Juan F, Diaz Pavon JM, Rodriguez Rodriguez A, Prendes Sillero E, Cadet Dussort JM, et al. Analysis of conversion factors in robotic-assisted rectal cancer surgery. Int J Colorectal Dis 2014; 29: 701–8. [DOI] [PubMed] [Google Scholar]
- 5.Baek SJ, Kim CH, Cho MS, Bae SU, Hur H, Min BS, et al. Robotic surgery for rectal cancer can overcome difficulties associated with pelvic anatomy. Surg Endosc 2015; 29: 1419–24. [DOI] [PubMed] [Google Scholar]
- 6.Escal L, Nougaret S, Guiu B, Bertrand MM, de Forges H, Tetreau R, et al. MRI-based score to predict surgical difficulty in patients with rectal cancer. Br J Surg 2018; 105: 140–6. [DOI] [PubMed] [Google Scholar]
- 7.Guend H, Widmar M, Patel S, Nash GM, Paty PB, Guillem JG, et al. Developing a robotic colorectal cancer surgery program: understanding institutional and individual learning curves. Surg Endosc 2017; 31: 2820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veenhof AA, Engel AF, van der Peet DL, Sietses C, Meijerink WJ, de Lange-de Klerk ES, et al. Technical difficulty grade score for the laparoscopic approach of rectal cancer: a single institution pilot study. Int J Colorectal Dis 2008; 23: 469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Killeen T, Banerjee S, Vijay V, Al-Dabbagh Z, Francis D, Warren S. Magnetic resonance (MR) pelvimetry as a predictor of difficulty in laparoscopic operations for rectal cancer. Surg Endosc 2010; 24: 2974–9. [DOI] [PubMed] [Google Scholar]
- 10.Lee JM, Han YD, Cho MS, Hur H, Min BS, Lee KY, et al. Prediction of transabdominal total mesorectal excision difficulty according to the angle of pelvic floor muscle. Surg Endosc 2020; 34: 3043–50. [DOI] [PubMed] [Google Scholar]
- 11.Kim JY, Kim YW, Kim NK, Hur H, Lee K, Min BS, et al. Pelvic anatomy as a factor in laparoscopic rectal surgery: a prospective study. Surg Laparosc Endosc Percutan Tech 2011; 21: 334–9. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez Ananin S, Targarona EM, Martinez C, Pernas JC, Hernandez D, Gich I, et al. Predicting the pathological features of the mesorectum before the laparoscopic approach to rectal cancer. Surg Endosc 2014; 28: 3458–66. [DOI] [PubMed] [Google Scholar]
- 13.Klein MF, Vogelsang RP, Gogenur I. Circumferential Resection Margin After Laparoscopic and Open Rectal Resection: A Nationwide Propensity Score Matched Cohort Study. Dis Colon Rectum 2019; 62: 1177–85. [DOI] [PubMed] [Google Scholar]
- 14.Patel SH, Hu CY, Massarweh NN, You YN, McCabe R, Dietz D, et al. Circumferential Resection Margin as a Hospital Quality Assessment Tool for Rectal Cancer Surgery. J Am Coll Surg 2020; 230: 1008–18 e5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


