Summary
Background:
We present a 2020 estimate of the global burden of HIV-associated cryptococcal infection (antigenemia), cryptococcal meningitis, and cryptococcal-associated deaths. The estimates of national, regional, and global burden of cryptococcal meningitis are critical to guide prevention strategies and determine needs for diagnostic tests and treatments.
Methods:
We used Joint UN Programme on HIV and AIDS (UNAIDS) estimates (2019 to 2020) and population-based HIV impact assessment (PHIA) surveys from 2016 to 2018 to estimate the number of adults with CD4 counts of <200 cells/μL at risk for cryptococcosis, by country and region. Secondly, we summarized cryptococcal antigenemia (CrAg) prevalence in CD4<200 cells/μL by reviewing published literature. Thereafter, we calculated the number of CrAg-positive people by country and region by multiplying the number with advanced HIV disease at risk for cryptococcal infection by the CrAg-positive prevalence of the respective country or region. We estimated progression from CrAg-positive to meningitis and/or death based on estimates from the published literature.
Findings:
We estimate 4.3 million adults with CD4 counts of <200 cells/μL globally. We calculated a mean global cryptococcal antigenemia prevalence of 4·4% (95% CI, 1·6% to 7·4%) among HIV-infected people with CD4 counts of <200 cells/μL, corresponding to 179,000 (IQR 133,000 to 219,000) cases of cryptococcal antigenemia (infection) globally in 2020. Annually, we estimate 152,000 (IQR 111,000 to 185,000) cases of cryptococcal meningitis, resulting in 112,000 cryptococcal-related deaths (IQR 79,000 to 134,000). Globally cryptococcal disease results in 19% (IQR 13% to 24%) of AIDS-related mortality.
Interpretation:
Despite a reduction in the estimated absolute global burden of HIV-associated cryptococcal meningitis compared to 2014, likely due to ART expansion, cryptococcal disease still accounts for 19% of AIDS-related deaths, similar to 2014 estimates. To end cryptococcal meningitis deaths by 2030 cryptococcal diagnostics, meningitis treatments, and implementation of preventive screening are critically needed.
Keywords: advanced HIV disease, disease burden, AIDS, AIDS-related opportunistic infections, cryptococcal antigen screening, cryptococcal meningitis, tuberculosis
Introduction
Cryptococcal meningitis remains the most common etiology of meningitis in adults living with HIV in sub-Saharan Africa.1 In 2014, an estimated 223,100 persons developed cryptococcal meningitis globally, resulting in 181,100 deaths, accounting for 15% of AIDS-related deaths.2 Most recently in 2020, UNAIDS estimated 580,000 (range 400,000 to 850,000) AIDS-related deaths occurred among adults globally.3
Cryptococcosis is unique among AIDS-related opportunistic infections in that cryptococcal antigen (CrAg) is detectable in the blood weeks to months before onset of meningitis symptoms. This cryptococcal antigenemia represents early disseminated infection which is initially subclinical or minimally symptomatic. CrAg screening and preemptive treatment with fluconazole for those with cryptococcal antigenemia, coupled with antiretroviral therapy (ART) reduces mortality in persons with advanced HIV disease.4,5 Despite clinical trial data confirming the survival benefit of CrAg screening and preemptive treatment,5 a limited number of nations have adopted a national CrAg screening program, with variable uptake. More countries are considering the cost-benefit of this screen and treat strategy.6,7
Estimates of the current incidence of cryptococcal disease are needed to quantify the testing, treatment commodities needed, and the potential lives saved with implementation of prevention and improved treatment strategies. Furthermore, new therapeutic strategies are being evaluated in order to reduce the high mortality burden associated with cryptococcal meningitis.8 Since the last estimate in 2014 of 223,100 cases of cryptococcal infection globally,2 global AIDS-related deaths among adults have decreased by an estimated 28% from 800,000 deaths in 2014 to 580,000 in 2020. By 2020, ART coverage had increased to 27.5 million adults, up from 15 million in 2014, and integrase inhibitors are now first-line therapy in many large HIV programs.
Here we present an updated estimate of the global burden of HIV-associated cryptococcal infection (antigenemia), cryptococcal meningitis, and cryptococcal-associated deaths using data through 2020.
Methods
Definition of Advanced HIV Disease
We defined advanced HIV disease as adults with CD4 cell count of <200 cells/μL, as this group is at highest risk for cryptococcosis. While the WHO defines advanced disease as CD4 <200 cells/μL or those with WHO clinical stage 3 or 4.9 We used only CD4 estimates, as WHO clinical staging is frequently inaccurate when assessed by non-medical healthcare workers, and therefore may not appropriately identify those severely immunosuppressed.10 While children under 5 years with HIV are considered to have advanced disease,9 we did not consider children in this analysis, as risk of opportunistic infections such as cryptococcosis, along with screening and treatment recommendations differ substantially from adults.11
Data Sources
For estimates of proportion with CD4<200 cells/μL, we used UNAIDS 2020 estimates2 and population-based HIV impact assessment (PHIA) surveys from 2016 to 2018, conducted by the International Center for AIDS Care and Treatment Program (ICAP)12 and Centers for Diseases Control and Prevention (CDC) in partnership with national HIV programs.
The Joint United Nations Programme on HIV/AIDS (UNAIDS) publishes annual reports of the HIV epidemic by country and region. The most recent 2021 report uses data gathered from 2020. Specifically, we used UNAIDS published estimates of the number of adults living with HIV, percent of people living with HIV (PLWH) who know their status, in order to estimate the number of PLWH who do not know their status. We used UNAIDS estimate of adults on antiretroviral therapy (ART), to calculate the number of adults not on ART. Finally, we used the UNAIDS point estimates and range of uncertainty of percent with suppressed viral loads to calculate the proportion on ART with virologic non-suppression (suppression defined as viral load <1000 copies/mL). If no country-level estimate was reported in the last three years for the percentage achieving viral suppression, we utilized the regional estimate. If no UNAIDS estimates were provided for the number of adults living with HIV in a country, this country was not included in our country-level analysis; however, such countries were included in regional estimates.
UNAIDS also publishes estimates of percentages of PLWH with a “Late HIV diagnosis” by country, defined as having an initial CD4 cell count <200 cells/μL (Appendix pages 4–5). Detailed methodology on how UNAIDS estimates were obtained have been published.13 Where country-specific data were available, we used the 2020 estimate for proportion with CD4<200 cells/μL at HIV diagnosis. If unavailable for 2020, we used estimates from 2019 or 2018. For countries not reporting proportion with CD4<200 cells/μL at time of HIV diagnosis between 2018 and 2020, we combined all available country-level estimates within the region (e.g. Southern & Eastern Africa, or Western & Central Africa) and used the median and interquartile range (IQR) for regional estimates. In summary, we used PHIA estimates to estimate the percentage of persons a) not previously diagnosed with HIV with CD4<200 cells/μL, b) on ART with CD4<200 cells/μL, and UNAIDS data to estimate the proportion c) newly entering HIV care (ART naïve) with CD4<200 cells/μL.
A. Estimate number with Advanced HIV disease
As cryptococcal infection occurs in persons with advanced immunosuppression, we first sought to generate estimates of the number of individuals with advanced HIV disease. For the purposes of this analysis, we assumed that the vast majority of PLWH with CD4<200 cells/μL fall into one of three categories: i) those who do not know their HIV status, ii) those newly diagnosed with HIV infection, entering HIV care but not yet on ART, and iii) those on ART without virologic suppression (Figure 1). See appendix (page 1) for additional methods.
Figure 1.

Model structure. Inputs summarized in supplemental tables.
B. Summarize CrAg prevalence by country and region
Thereafter, we summarized the prevalence of cryptococcal antigenemia among those with advanced HIV disease, whereby detectable cryptococcal antigen in peripheral blood reflects disseminated systemic infection.14 To summarize cryptococcal antigenemia prevalence, we identified all published studies and conference abstracts from 1989 to 2021. We included studies from both outpatient and inpatient settings. Further details of the search strategy are included in the Appendix (page 1).
CrAg prevalence among people with CD4<200 cells/μL was used by country, along with the associated 95% confidence interval (95% CI), calculated by Fisher’s exact test. We identified 53 CrAg prevalence studies from sub-Saharan Africa, 10 from Asia, 10 from Latin America, 2 from Europe, 2 from the Middle East, and 1 from North America (Figure 1). Outpatient and inpatient CrAg prevalence studies were standardized (see Supplemental Methods for details). For countries without cryptococcal antigenemia prevalence data, we used the pooled, weighted regional mean estimate.
C. Estimate number of CrAg-positive
Next, we calculated the number of people with cryptococcal antigenemia by country and region (Figure 1). Specifically, we calculated number of CrAg-positive among people with: i) advanced HIV disease with unknown HIV status, ii) advanced HIV disease not receiving ART, and, iii) advanced HIV disease without virologic suppression (>1000 copies/mL). We multiplied the number of persons within each of these groups by the CrAg prevalence for the respective country. The sum of these three groups was the total estimate for CrAg-positive persons by country. We also present country-specific absolute numbers and number per 100,000 HIV infections (Appendix, page 15).
D. Estimate progression from antigenemia to meningitis
Progression to cryptococcal meningitis was calculated considering the likelihood of a CrAg-positive person engaging in HIV care, receiving preemptive fluconazole through a CrAg screening program, and receiving effective ART. We identified 35 studies from the published literature to calculate the weighted mean of progression from asymptomatic CrAg-positive infection to cryptococcal meningitis with fluconazole preemptive therapy. We identified 17 studies that summarized prevalence of meningitis (subclinical or mildly symptomatic) at the time of CrAg screening. The weighted percentage of those with meningitis at time of CrAg screening was 35%, and once these people were excluded, we estimated that an additional 11% would progress to develop meningitis over a 6-month period, despite preemptive fluconazole, and ART (Appendix page 8).
Progression from asymptomatic CrAg-positive to cryptococcal meningitis without preemptive fluconazole is no longer ethical to study, though there is evidence that nearly 67% (95% CI 46% to 84%) of persons with CD4<100 cells/μL progress to meningitis or death without fluconazole (despite ART).4,15,16 Despite the recommendation for lumbar puncture and cerebrospinal fluid (CSF) testing among those with asymptomatic CrAg-positive by some national guidelines, frequently this is difficult to implement in resource-limited settings, due to lack of lumbar puncture capability, and patient refusal especially if asymptomatic. Without access to either preemptive fluconazole or ART, we presumed 95% of CrAg-positive persons would progress to meningitis or death.4,16
E. Estimate progression of cryptococcal meningitis to death
Mortality estimates include death from HIV-associated cryptococcal meningitis and other causes of death among CrAg-positive persons. One-year mortality after cryptococcal meningitis is summarized in the Appendix (page 11). Mortality among persons who have been hospitalized has been estimated from the published literature.17 However, these estimates do not include persons who do not present to the hospital, or present late to hospital.
We stratified those who present to hospital for meningitis care based on underlying HIV status (Appendix pages 10–11). We assumed that those who do not know their HIV status and have meningitis may be less likely to access medical care compared to a person on ART with virologic failure who has previously attended outpatient HIV clinics.
Finally, we estimated that approximately 10% of CrAg-positive persons without cryptococcal meningitis die of other concomitant conditions, not necessarily of cryptococcosis (Appendix page 8). Potential alternative causes of death include tuberculosis, histoplasmosis, malnutrition/wasting, sepsis, other opportunistic infections, or cancer.
See Supplemental Methods (Appendix pages 1–3) for estimates of cryptococcal deaths as a percentage of AIDS deaths, calculations related to benefit of CrAg screening program, estimates of uncertainty to generate interquartile range (IQR), ethics statement, and role of the funding source. We adhered to the STROBE guidelines for reporting observational studies.18 All modeling and calculations were performed using Microsoft Excel 2016.
F. Role of the funding body
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
In 2020, UNAIDS estimated that 36 million (range 28·9 to 43·2 million) adults were living with HIV globally, of which 23·8 million (range 19·5 million to 28·5 million) reside in sub-Saharan Africa. Globally, 27·5 million (range 16·2 million to 38·0 million) adults were receiving ART. We estimated 4·3 million (IQR 3·0 million to 4·8 million) adults globally had advanced HIV disease (12% of PLWH; range 10% to 15%), of whom 56% live in sub-Saharan Africa (2.5 million/4.3 million).
We calculated the mean global cryptococcal antigenemia prevalence of 4·4% (95% CI 1.6% to 7.4%) among people with CD4 counts of <200 cells/μL, corresponding to 179,000 (IQR 133,000 to 219,000) cases of cryptococcal antigenemia globally per year (Figure 2). Of these 179,000 cases, we estimated 56% (IQR 40% to 66%) were among people who are newly diagnosed with HIV infection and are not yet on ART, 21% (IQR 20% to 23%) were among persons with virologic failure, and 22% (IQR 21% to 23%) were unaware of their HIV infection. Annually, we estimated that there are 152,000 (IQR 111,000 to 185,000) cases of cryptococcal meningitis, resulting in 112,000 cryptococcal-related deaths (IQR CI 79,000 to 134,000) (Figure 3, Table 1). In sub-Saharan Africa where historically the burden of cryptococcal infection has been the greatest, an estimated 97,000 (IQR 73,000 to 120,000) adults had cryptococcal antigenemia, 82,000 (IQR 61,000 to 101,000) adults had cryptococcal meningitis, and 71,000 (IQR 52,000 to 88,000) cryptococcal-related deaths occurred. Sixty-three percent of deaths occur in sub-Saharan Africa (71,000/112,000). The region with the second highest incidence of cryptococcal meningitis is Asia & Pacific region with 44,000 (IQR 35,000 to 51,000) meningitis cases and 26,000 (IQR 21,000 to 30,000) cryptococcal deaths annually. Figure 4 and Supplemental Table 7 (Appendix page 11) highlight the countries with the highest annual incidence of cryptococcal antigenemia. Supplemental Figure 1 summarizes cryptococcal infection per 100,000 HIV infections, (Appendix page 13). Globally cryptococcal disease results in 19% (IQR 13% to 24%) of AIDS-related mortality.
Figure 2.

CrAg prevalence by Country among HIV-infected people with CD4<200 cells/μL.
Figure 3.
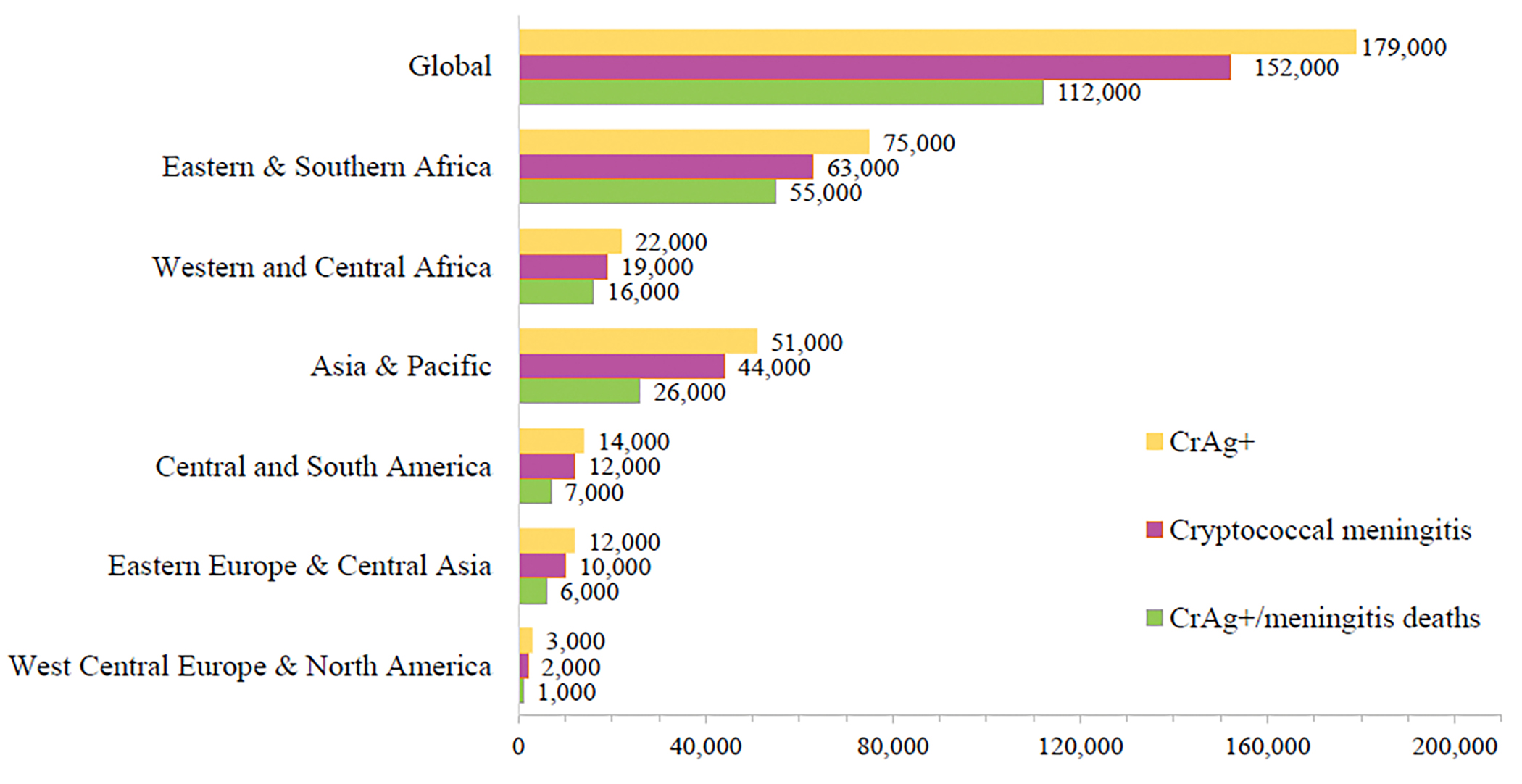
Global and regional estimates of the burden of cryptococcal antigenemia, cryptococcal meningitis, and deaths.
Table 1.
Regional estimates of Burden of Cryptococcosis
| Region | CrAg-positive | Cryptococcal meningitis | Cryptococcal deaths | % of AIDS deaths |
|---|---|---|---|---|
|
| ||||
| Global | 179,000 (IQR 133,000 to 219,000) | 152,000 (IQR 111,000 to 185,000) | 112,000 (IQR 79,000 to 134,000) | 19% (IQR 13% to 24%) |
| Eastern & Southern Africa | 75,000 (IQR 55,000 to 95,000) | 63,000 (IQR 45,000 to 80,000) | 55,000 (IQR 39,000 to 70,000) | 21% (IQR 15% to 28%) |
| Western & Central Africa | 22,000 (IQR 19,000 to 26,000) | 19,000 (IQR 16,000 to 22,000) | 16,000 (IQR 13,000 to 19,000) | 15% (IQR 12% to 18%) |
| Asia & Pacific | 51,000 (IQR 42,000 to 60,000) | 44,000 (IQR 35,000 to 51,000) | 25,000 (IQR 21,000 to 30,000) | 20% (IQR 13% to 22%) |
| Eastern Europe & Central Asia | 12,000 (IQR 11,000 to 13,000) | 10,000 (IQR 9,000 to 11,000) | 6,000 (IQR 5,000 to 7,000) | 19% (IQR 17% to 22%) |
| Latin America | 14,000 (IQR 10,000 to 17,000) | 12,000 (IQR 9,000 to 14,000) | 7,000 (IQR 5,000 to 9,000) | 23% (IQR 16% to 30%) |
| Western Central Europe & North America | 3,000 (IQR 2,000 to 4,000) | 2,000 (IQR 1,500 to 3,000) | 1,000 (IQR 700 to 1,400) | 8% (IQR 5% to 11%) |
| Caribbean | 2,000 (IQR 1,700 to 2,300) | 1,700 (IQR 1,400 to 1,900) | 1,000 (IQR 800 to 1,100) | 19% (IQR 15% to 23%) |
| Middle East & North Africa | 500 (IQR 100 to 600) | 400 (IQR 100 to 500) | 200 (IQR <100 to 300) | 3% (IQR 1% to 5%) |
Cryptococcal deaths include deaths among those with diagnosed meningitis as well as deaths in CrAg-positive persons without overt meningitis. IQR: Interquartile range. Sub-Saharan Africa consists of total estimates of Eastern and Southern Africa and Western and Central Africa.
Figure 4.

Annual incidence of cryptococcal antigenemia in sub-Saharan Africa. Top 10 countries include: South Africa, Mozambique, Kenya, Democratic Republic of Congo, Tanzania, Zambia, Nigeria, Malawi, Zimbabwe, and Ethiopia. Supplemental Table 7 provides country-level estimates.
If all regions implemented national CrAg screening programs, where at least 80% of persons entering/re-entering HIV care were screened and received fluconazole preemptive therapy, we estimate that 34,000 cases of cryptococcal meningitis could be avoided, that is 22% of the current meningitis cases annually. With implementation of CrAg screening programs globally to identify CrAg-positive people before onset of meningitis, 21,000 lives could be saved annually, which is 19% of current cryptococcal-related deaths. With wider implementation of lumbar punctures to identify early meningitis among CrAg-positive people, especially those who are symptomatic or with high CrAg titers, more deaths could be averted. Sub-Saharan African and the Asia-Pacific region have the highest proportion of cases of cryptococcal infection, and thus would experience the greatest reduction in mortality with implementation of CrAg screening programs.
Discussion
While there has been a reduction in the estimated absolute global burden of HIV-associated cryptococcal meningitis since 2014, likely due to ART expansion, and, to some degree, CrAg screening programs, cryptococcosis still results in 19% of AIDS-related deaths; the latter is largely unchanged from prior estimates in 2014. The persistent burden of infection suggests that death from cryptococcal infection remains a marker for failure in the HIV cascade of care. Persons at risk for cryptococcal infection have advanced HIV disease, either because they do not know their HIV status, have not engaged in outpatient HIV care and initiated ART, are on a failing ART regimen, or have had interruptions in treatment. Indeed, the majority of persons presenting with cryptococcal meningitis are now ART-experienced.19
UNAIDS estimates that globally 73% of PLWH are receiving ART, but only 61% of PLWH have suppressed viral loads. Contributing to the high continued prevalence of cryptococcosis are 3 key gaps in the HIV care cascade: 1) 27% of PLWH are not receiving ART, 2) 39% of PLWH do not have suppressed viral loads, and 3) 30% (IQR 24 to 41%) of PLWH present for the first time with an initial CD4<200 cells/μL. Earlier identification and engagement in HIV care of PLWH would reduce the proportion presenting late to care, and would therefore reduce the number at risk for opportunistic infections. In conjunction with rapid ART initiation, identification of advanced HIV disease, and appropriate screening for opportunistic infections, including cryptococcal infection, are essential. Enhanced active monitoring for virologic failure, and evidence-based interventions to improve adherence to care would increase the proportion with virologic suppression and reduce the population at risk for opportunistic infections. Novel strategies to reduce ART interruption and improve treatment adherence, such as long-acting injectable ART, would likely reduce the burden of opportunistic infections. Furthermore, more implementation science programs to address non-adherence to ART and optimize ART adherence are warranted.
Cryptococcal antigen detection using FDA approved lateral flow devices is perhaps the ultimate test in microbiology – highly accurate, quick, easy to read, and inexpensive.20 Diagnosis of antigenemia prior to the onset of meningitis is optimal. Initiation of ART without diagnosing cryptococcal infection carries a considerable risk of death.4,21 Greater access to CD4 testing to identify advanced HIV disease, followed by CrAg testing if advanced HIV disease is identified is required to assist with expeditious diagnosis, and access to effective pre-emptive therapy, together with health system strengthening interventions centered on improving ART adherence and early identification of ART failure. There are currently many limitations to early diagnosis of cryptococcal meningitis, primarily related to access to CD4 testing, timely receipt of results, and availability of CrAg lateral flow assays and fluconazole.
How can cryptococcal deaths be reduced? Global scale up and investment into CrAg screening programs along with adequate supply of preemptive treatment can ultimately prevent meningitis and thereby deaths, while potentially reducing costs. Thus, testing is a start but rapid linkage to preemptive therapy following laboratory testing is necessary.22 In South Africa, where reflexive laboratory CrAg screening of all CD4 samples <100 cells/μL is implemented on a national scale, only approximately 50% of CrAg-positive persons receive fluconazole treatment (unpublished data). Unfortunately, national-level interventions and implementation strategies to retain persons with advanced HIV disease in care, screen for opportunistic infections, and rapidly initiate ART are lacking in many countries and districts. Increased coverage of CrAg screening programs would reduce cryptococcal deaths.
Therapeutics for cryptococcal meningitis remain inaccessible in many resource-limited settings, where the burden is greatest, because therapeutics are not locally manufactured. The preferred induction regimen for adults with HIV-associated cryptococcal meningitis is amphotericin B and flucytosine for 7 days, followed by 7 days of high dose fluconazole.23 Most recently, a randomized controlled trial demonstrated that single dose liposomal amphotericin B with flucytosine and fluconazole is non-inferior to the above WHO-recommended regimen, with significantly reduced side effects.8 Unfortunately, flucytosine is generally unavailable in sub-Saharan Africa, Asia, and Latin America, though efforts are underway to improve access.24,25 Without flucytosine regimens there is increased morality with the typical course for cryptococcal meningitis treatment being 14 days of amphotericin B with fluconazole, and where amphotericin is unavailable or cannot be safely administered suboptimal fluconazole monotherapy prevails.23 Amphotericin B deoxycholate is notorious for medication-related side effects including rigors, fevers, chills, life threatening hypokalemia, and kidney injury. Manometers, which are considered standard of care to measure and control intracranial pressure in cryptococcal meningitis to improve outcomes,26 are likewise unavailable in most resource-limited settings. Finally, training of healthcare workers in meningitis care, and point-of-care diagnostic algorithms is needed to improve meningitis diagnosis in routine settings.27 Clinical trials are underway to investigate new treatments and new implementation strategies to improve meningitis care in resource-poor settings. An initiative entitled “Ending HIV-associated CM deaths by 2030” has been announced recently which lays out a comprehensive framework to address cryptococcal meningitis deaths, including targets and interventions for countries to minimize meningitis deaths.28
The primary limitation of our estimate is the quality of data inputs. We used UNAIDS published estimates on the current state of HIV care globally.13 These estimates sometimes had no confidence intervals, and may not reflect the variation within a country or region. Countries without UNAIDS estimates for the number of PLWH were excluded from country-level analyses but included in regional estimates. The COVID-19 pandemic likely affected 2020 HIV estimates, though there is likely a high degree of variability on the impact of COVID-19 on HIV infection. Our unpublished data suggest that lockdowns resulted in fewer visits to HIV clinics and increased late presentation to care with advanced HIV disease. As a result, the numbers derived from UNAIDS may have underreported the true number with HIV infection, not on ART and/or with virologic failure. Our assessment of the burden of cryptococcal infection, meningitis, and associated deaths may be underestimated given fewer PLWH presenting to care due to the COVID-19 pandemic, and a greater proportion at risk for cryptococcal infection.
Our estimates may underestimate the burden of cryptococcal infection, as UNAIDS estimates of HIV infection are considerably lower than estimates from 2019 Global Burden of Disease data.29 Specifically, UNAIDS estimates 680,000 AIDS-related deaths whereas the Global Burden of Diseases data estimates 864,000 HIV-related deaths. If the latter is more accurate, then the burden of cryptococcal infection may be 20% higher than we estimate here.
There are many unknowns regarding persons with advanced HIV disease. Specifically, it is unknown what proportion of PLWH with virological non-suppression have advanced HIV disease or asymptomatic cryptococcal antigenemia,30 especially outside of sub-Saharan Africa. It is unknown (and challenging to study) what proportion of PLWH who do not know their HIV status have advanced HIV disease. CrAg prevalence studies in high and middle-income regions, including North America, Europe, and the Middle East are needed to further understand the burden of meningitis, and value of CrAg screening. It is unknown among people with cryptococcal antigenemia, what proportion develop meningitis and/or die at home, without presenting to a hospital.31,32 Among those few countries with CrAg screening programs in place, detailed outcome data related to the proportion screened, proportion treated, and incidence of cryptococcal meningitis and death would be valuable metrics for stakeholders in other countries considering their own programs. Timely results are crucial for identifying meningitis as early as possible, or even before it occurs in those who are positive.
Finally, our estimates exclude children, where the burden of cryptococcal infection is lower, and persons with non-HIV-associated cryptococcal infection. In South Africa, 2% of cryptococcal meningitis occurs in those <18 years of age.11 Pregnant and breastfeeding women are frequently excluded from cryptococcal studies, and research in general, thus our estimates may slightly underestimate the total burden of HIV-associated cryptococcal infection. A recent study on minimally invasive autopsies identified cryptococcosis as a leading cause of maternal death.33
With the expansion of HIV therapy, the absolute number of cryptococcal infections, cryptococcal meningitis, and cryptococcal-related deaths has declined, yet we estimate that cryptococcosis still accounts for 19% of AIDS-related deaths. This is unchanged from the estimate made in 2014.2 Yet in the past decade since CrAg screening was first recommended by the WHO,34 there has been some progress in implementation of cryptococcal prevention, which is generally cost-effective across low, middle, and high income settings.6,35 Implementation of the new framework to end cryptococcal meningitis deaths by 2030 is critically needed.24 Specifically, to end cryptococcal meningitis deaths by 2030, the following steps are needed: 1) increased availability of diagnostics, 2) implementation of CrAg screening in persons with advanced HIV, and 3) access and implementation of WHO-recommended optimized treatment regimens for cryptococcal meningitis, along with strengthening the HIV cascade of care and retention in ART programs.
Supplementary Material
Research in Context.
Evidence before this study
Cryptococcus is the most common cause of meningitis among adults in sub-Saharan Africa, due to the burden of human immunodeficiency virus (HIV) infection. We searched PubMed using the terms “cryptococcal meningitis” and “burden” on May 31, 2022, with no restrictions on language or date. Two prior studies have estimated the global burden of cryptococcal infection. The first was published in 2008. The global annual incidence of cryptococcosis was estimated as 957,900 cases per year (range, 371,700–1,544,000 cases). This estimate was based on published cohorts primarily from the pre-ART era, and the wide ranges indicate the high level of uncertainty of these estimates. This 2008 study used just three incidence publications to derive sub-Saharan African estimates. A publication from 2017 estimated the global burden of cryptococcal meningitis at 223,100 cases. This estimate used data from 2014 Joint UN Programme on HIV and AIDS, and emerging country-specific data on cryptococcal antigen (CrAg) prevalence.
Since the last estimate, acquired immunodeficiency syndrome (AIDS)-related deaths have declined by 28%. Antiretroviral therapy coverage has increased to 27.5 million adults, up from 15 million in 2014, and integrase inhibitors are now first-line therapy in many large HIV programs.
Added value of this study
This is an updated global estimate of cryptococcal infection, using data from 2020. The landscape of HIV infection has changed dramatically since the last estimate of global burden in 2014. We provide an updated estimate of the global incidence of HIV-associated cryptococcal disease using published Joint United National Programme on HIV and AIDS (UNAIDS) data on HIV incidence, ART access, retention-in-care, and published CRAG prevalence data.
Implications of all the available evidence
The estimates of national, regional, and global burden of cryptococcal meningitis are critical to guide prevention strategies and determine needs for diagnostic tests, antifungal medicines, and medical supplies, such as diagnostics, lumbar puncture needles, and manometers.
Acknowledgements:
RR, DRB, and NPG are supported by the National Institute of Allergy and Infectious Diseases (K23AI138851, R01AI118511, U01AI125003). AL is in receipt of grants from EDCTP and ANRS outside the scope of this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention.
Footnotes
Declaration of Interest: We declare that we have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing:
Raw data tables and individual country information will be made available with publication. Persons should email the corresponding author at radha@umn.edu to make such a request.
References
- 1.Ellis J, Bangdiwala AS, Cresswell FV, et al. The Changing Epidemiology of HIV-Associated Adult Meningitis, Uganda 2015–2017. Open forum infectious diseases 2019; 6(10): ofz419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. The Lancet Infectious diseases 2017; 17(8): 873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. AIDSinfo. 2020. https://aidsinfo.unaids.org/ (accessed March 22 2021).
- 4.Meya DB, Manabe YC, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2010; 51(4): 448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mfinanga S, Chanda D, Kivuyo SL, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet (London, England) 2015; 385(9983): 2173–82. [DOI] [PubMed] [Google Scholar]
- 6.Rajasingham R, Meya DB, Greene GS, et al. Evaluation of a national cryptococcal antigen screening program for HIV-infected patients in Uganda: A cost-effectiveness modeling analysis. PloS one 2019; 14(1): e0210105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajasingham R, Meya DB, Boulware DR. Integrating cryptococcal antigen screening and pre-emptive treatment into routine HIV care. Journal of acquired immune deficiency syndromes (1999) 2012; 59(5): e85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarvis JN, Lawrence DS, Meya DB, et al. Single-Dose Liposomal Amphotericin B Treatment for Cryptococcal Meningitis. The New England journal of medicine 2022; 386(12): 1109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. 2017. p. 56. [PubMed]
- 10.Hakim J, Musiime V, Szubert AJ, et al. Enhanced Prophylaxis plus Antiretroviral Therapy for Advanced HIV Infection in Africa. The New England journal of medicine 2017; 377(3): 233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Communicable Diseases. GERMS-SA Annual Report 2019. In: Lebaka T, Quan V, editors. Johanesburg; 2019. [Google Scholar]
- 12.ICAP. Population-based HIV impact assessment, Guiding the Global HIV Response. . https://phia.icap.columbia.edu/resource_categories/final-reports/ (accessed June 4 2021). [Google Scholar]
- 13.2020 U. Global AIDS Monitoring 2021. 2020. https://www.unaids.org/sites/default/files/media_asset/global-aids-monitoring_en.pdf (accessed March 22, 2021.
- 14.Rajasingham R, Wake RM, Beyene T, Katende A, Letang E, Boulware DR. Cryptococcal Meningitis Diagnostics and Screening in the Era of Point-of-Care Laboratory Testing. Journal of clinical microbiology 2019; 57(1): e01238–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longley N, Jarvis JN, Meintjes G, et al. Cryptococcal Antigen Screening in Patients Initiating ART in South Africa: A Prospective Cohort Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2016; 62(5): 581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Letang E, Müller MC, Ntamatungiro AJ, et al. Cryptococcal Antigenemia in Immunocompromised Human Immunodeficiency Virus Patients in Rural Tanzania: A Preventable Cause of Early Mortality. Open forum infectious diseases 2015; 2(2): ofv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajasingham R, Rolfes MA, Birkenkamp KE, Meya DB, Boulware DR. Cryptococcal meningitis treatment strategies in resource-limited settings: a cost-effectiveness analysis. PLoS medicine 2012; 9(9): e1001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UK EQUATOR Centre. Enhancing the Quality and Transparency of Health Research: Equtor network. 2022. https://www.equator-network.org/reporting-guidelines/strobe/ (accessed June 7 2022).
- 19.Flynn AG, Meya DB, Hullsiek KH, et al. Evolving Failures in the Delivery of Human Immunodeficiency Virus Care: Lessons From a Ugandan Meningitis Cohort 2006–2016. Open Forum Infect Dis 2017; 4(2): ofx077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tadeo KK, Nimwesiga A, Kwizera R, et al. Evaluation of the Diagnostic Performance of a Semiquantitative Cryptococcal Antigen Point-of-Care Assay among HIV-Infected Persons with Cryptococcal Meningitis. Journal of clinical microbiology 2021; 59(8): e0086021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhein J, Hullsiek KH, Evans EE, et al. Detrimental Outcomes of Unmasking Cryptococcal Meningitis With Recent ART Initiation. Open forum infectious diseases 2018; 5(8): ofy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medina N, Alastruey-Izquierdo A, Bonilla O, et al. A Rapid Screening Program for Histoplasmosis, Tuberculosis, and Cryptococcosis Reduces Mortality in HIV Patients from Guatemala. Journal of fungi (Basel, Switzerland) 2021; 7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. 2018. http://www.who.int/hiv/pub/guidelines/cryptococcal-disease/en/ (accessed April 1, 2018. [PubMed]
- 24.Shroufi A, Chiller T, Jordan A, et al. Ending deaths from HIV-related cryptococcal meningitis by 2030. The Lancet Infectious diseases 2021; 21(1): 16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UNITAID. Nine quick facts about Unitaid’s investment in advanced HIV. 2019. https://unitaid.org/news-blog/targeting-opportunistic-infections-to-cut-hiv-related-deaths/#en (accessed August 26 2021).
- 26.Rolfes MA, Hullsiek KH, Rhein J, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis 2014; 59(11): 1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oladele RO, Jordan A, Akande P, et al. Tackling cryptococcal meningitis in Nigeria, one-step at a time; the impact of training. PloS one 2020; 15(7): e0235577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ending cryptoocccal meningitis deaths by 2030: strategic framework. . 2021. https://msfaccess.org/ending-cryptococcal-meningitis-deaths-2030-strategic-framework (accessed February 1 2022).
- 29.Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England) 2020; 396(10258): 1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mpoza E, Rajasingham R, Tugume L, et al. Cryptococcal Antigenemia in Human Immunodeficiency Virus Antiretroviral Therapy-Experienced Ugandans With Virologic Failure. Clin Infect Dis 2020; 71(7): 1726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.French N, Gray K, Watera C, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS (London, England) 2002; 16(7): 1031–8. [DOI] [PubMed] [Google Scholar]
- 32.Liechty CA, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Tropical medicine & international health : TM & IH 2007; 12(8): 929–35. [DOI] [PubMed] [Google Scholar]
- 33.Letang E, Rakislova N, Martinez MJ, et al. Minimally Invasive Tissue Sampling: A Tool to Guide Efforts to Reduce AIDS-Related Mortality in Resource-Limited Settings. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2021; 73(Supplement_5): S343–s50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Rapid advice: Diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. December 2011. www.who.int/hiv/pub/cryptococcal_disease2011 (accessed 1 Jan 2017). [PubMed]
- 35.Rajasingham R, Boulware DR. Reconsidering cryptococcal antigen screening in the U.S. among persons with CD4 <100 cells/mcL. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2012; 55(12): 1742–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data tables and individual country information will be made available with publication. Persons should email the corresponding author at radha@umn.edu to make such a request.


