Abstract
The permeability of the outer membrane (OM) to hydrophobic probes and its susceptibility to bactericidal cationic peptides were investigated for natural rough Brucella ovis and for mutant rough Brucella abortus strains. The OM of B. ovis displayed an abrupt and faster kinetic profile than rough B. abortus during the uptake of the hydrophobic probe N-phenyl-naphthylamine. B. ovis was more sensitive than rough B. abortus to the action of cationic peptides. Bactenecins 5 and 7 induced morphological alterations on the OMs of both rough Brucella strains. B. ovis lipopolysaccharide (LPS) captured considerably more polymyxin B than LPSs from both rough and smooth B. abortus strains. Polymyxin B, poly-l-lysine, and poly-l-ornithine produced a thick coating on the surfaces of both strains, which was more evident in B. ovis than in rough B. abortus. The distinct functional properties of the OMs of these two rough strains correlate with some structural differences of their OMs and with their different biological behaviors in animals and culture cells.
The genus Brucella is a gram-negative, facultative, intracellular pathogen that produces disease in a large number of mammals, including humans (8). The outer membrane (OM) of Brucella, which has been implicated in the virulence of the species, is unique in many respects. It has been demonstrated, for instance, that this layer is permeable to hydrophobic permeants and is considerably more resistant to bactericidal cationic peptides than most gram-negative bacteria (9, 15, 16). In smooth pathogenic Brucella species (Brucella abortus, Brucella melitensis, and Brucella suis), the polysaccharide O chain of the lipopolysaccharide (LPS) has been implicated as a virulence factor. This proposition is based on the observation that smooth Brucella survives and replicates in animals and in cultured macrophages more efficiently than rough mutants, and on the increased susceptibility of rough strains to microbicidal cationic peptides relative to that of their smooth counterparts (14, 16, 31).
Brucella ovis and Brucella canis, pathogenic for rams and dogs, respectively, do not possess O-chain polysaccharides; their LPSs react with antibodies against core epitopes specific to the genus and are lysed by the R/C phages specific for rough Brucella strains (3, 5, 8). Due to the fact that these properties are shared by mutants derived from smooth strains, these two Brucella species have been regarded as natural rough variants (8). Although B. ovis has been observed within trophoblasts of placentomes from infected sheep (17), this species attaches in lower numbers to epithelial HeLa cells than the rough mutants B. abortus 45/20 and B. abortus RB51 (22, 27). B. abortus, moreover, is able to proliferate inside nonprofessional phagocytes and induce loss of viability of the infected cells, while B. ovis is readily destroyed within lysosomes of these cells and does not induce cellular death (22). Since the differences observed between the natural and mutant rough Brucella strains cannot be due to their roughness, we have asked whether this distinct behavior may be correlated with different OM properties displayed by these strains. For this purpose, we have compared the permeabilities of the OM, the susceptibilities to bactericidal cationic peptides, and the levels of binding of the LPS to polymyxin B for mutant rough B. abortus strains and natural rough B. ovis.
The growth conditions of the bacterial strains used, as well as the purification and characterization of their LPS molecules, have been described elsewhere (6, 10, 15, 16, 18). Briefly, smooth B. abortus S19, rough B. abortus 45/20, and rough B. ovis REO 198 (CO2 independent) strains were originally obtained from Lois Jones (University of Wisconsin, Madison, Wis.). Attenuated rough B. abortus RB51 was obtained from Gerhardt Schurig (Virginia Polytechnic Institute and State University). Smooth Salmonella montevideo SH94 serogroup D1 is maintained as part of the collection of the Division of Clinical Bacteriology, Karolinska Institute, Huddinge, Sweden. All strains are maintained in lyophilized stocks at the Veterinary School (National University, Heredia, Costa Rica, and University of Navarra, Pamplona, Spain) and have been demonstrated to be stable strains throughout the years, without detectable phenotypic or biochemical changes (2, 9, 10, 15, 16, 18, 25). B. abortus and S. montevideo strains were propagated in tryptic soy broth, and B. ovis was propagated in the same medium with 0.5% yeast extract (Difco Laboratories, Detroit, Mich.). Bacteria were harvested (5,000 × g for 15 min at 4°C) in the exponential phase of growth. Attenuated B. abortus S19 is phenotypically a smooth strain with 90% smooth-type LPS, 10% rough-type LPS (10), and a considerable quantity of surface native hapten (NH) polysaccharide (2, 25). The biological, chemical, and physical characteristics of B. abortus S19 LPS are indistinguishable from those of preparations isolated from virulent strains (10). Attenuated B. abortus 45/20 strain contains more than 99% rough-type LPS and a small number of lipid-bound O-polysaccharide-containing molecules (4, 10, 26), which has been estimated by immunogold electron microscopy with monoclonal antibody against epitope C/Y of the O chain (9) to be from 0 to 12 molecules per cell (Fig. 1). This bacteria does not contain any detectable NH (18). B. ovis REO 198 does not possess O chain or NH as demonstrated by immunogold detection (Fig. 1) and by sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) analysis (18, 25). The rough-type LPSs of Brucella strains demonstrate extensive immunological cross-reactivity and similar chemical and physical properties (18, 25). Cationic protein 18A, bactenecin 5, and bactenecin 7 were provided by R. Gennaro and D. Romeo (Department of Biochemistry, Biophysics and Chemistry of Macromolecules, Trieste University, Trieste, Italy) or synthesized by Chiron Mimotopes Pty. Ltd. (Victoria, Australia). The polymyxin B sulfate, dansyl-polymyxin B, melittin, poly-l-lysine, and poly-l-ornithine were purchased from Sigma Chemical Co. (St. Louis, Mo.). Lactoferricin B was provided by W. Bellamy (Morinaga Dairy Company, Higashihara, Japan). The bactericidal sensitivity assays (results expressed as either the number of viable CFU or the diameters of bactericidal halos in agar plates) and the method for adsorption of the peptides to different bacteria (reported as the reduction of bactericidal halos in agar plates) were performed as described previously (9). Fluorimetric assays of peptide-treated bacteria with N-phenyl-naphthylamine (NPN) as the fluorescent probe were performed as reported by Martínez de Tejada and Moriyón (15). Binding of polymyxin B to LPSs isolated from rough and smooth Brucella strains was estimated by fluorometric analysis. Briefly, LPS suspensions (yielding concentrations of 60 to 63 nM lipid A in 2.5 mM HEPES, pH 7.2, and prepared by sonic dispersion) were incubated with different concentrations (5.5, 8.25, and 13.75 μM) of dansyl-polymyxin B. The fluorescence was estimated at room temperature under conditions of excitation at 340 nm, with an LS-50 fluorimeter (Perkin-Elmer Ltd., Beaconsfield, England) with a slit width (for both excitation and emission) of 2.5 nm and a range of 400 to 600 nm. Bacterial cell damage was evaluated by observation of the peptide-treated bacteria on a Hitachi 1100 transmission electron microscope (Hitachi Scientific Instruments, Mountain View, Calif.) operating at 100 kV as previously described (9). Experiments were performed in quadruplicate, and the results were expressed either as the percentage in the reduction of the bactericidal activity inhibition or the lethal concentration of the peptides in micrograms per milliliter (mean ± standard deviation) with respect to the control. Both the Student t test and variance analysis were performed for statistical examination.
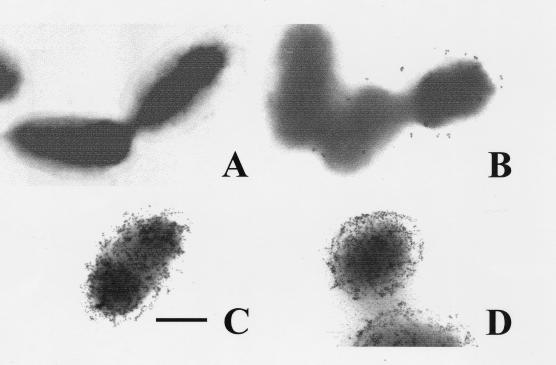
FIG. 1.
Immunogold detection of O chain (C/Y) and core polysaccharide epitopes (R) in the surface of rough Brucella. (A) B. ovis REO 198 stained with anti-O-chain (C/Y epitope) monoclonal antibody and anti-mouse immunoglobulin gold (5 nm) conjugate; (B) B. abortus 45/20 stained with anti-O-chain (C/Y epitope) monoclonal antibody and anti-mouse immunoglobulin gold (5 nm) conjugate; (C) B. ovis REO 198 stained with anti-R (R1 epitope) monoclonal antibody and anti-mouse immunoglobulin gold (5 nm) conjugate; (D) B. abortus 45/20 stained with anti-R (R1 epitope) monoclonal antibody and anti-mouse immunoglobulin gold (5 nm) conjugate. Bar, 0.125 μm.
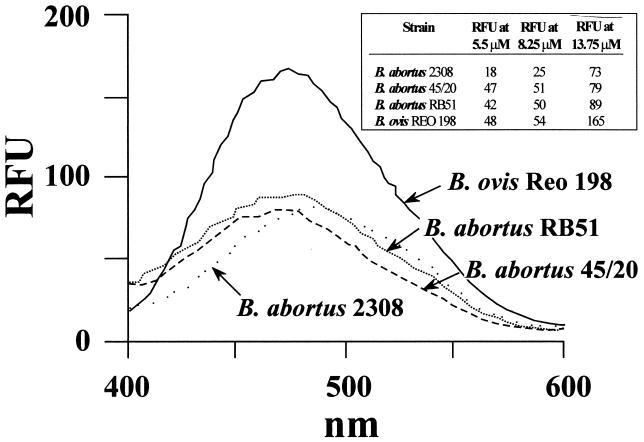
Just as the reduction of CFU showed the bactericidal action of cationic peptides in various Brucella strains (9, 11, 16), the MICs showed that the rough B. abortus 45/20 and RB51 strains were more susceptible than their smooth counterparts, although B. abortus displayed more resistance than B. ovis (Table 1). The results of the adsorption experiments were consistent with those of the bactericidal assays in that B. ovis cells adsorbed more peptide than did the rough B. abortus cells (Table 2). Moreover, B. ovis LPS bound considerably more polymyxin B than did LPSs from both rough and smooth B. abortus strains (Fig. 2). As with the binding of cationic peptides by Brucella cells, LPS molecules from rough strains captured more polymyxin B than LPS from smooth B. abortus, as demonstrated by the increased fluorescence at low concentrations of dansyl-polymyxin B (Fig. 2). At higher concentrations, quenching due to turbidity of the dansyl-polymyxin B-LPS suspensions was observed for B. abortus 45/20 LPS (optical density [OD] at 400 nm = 0.723), and to a lesser extent for B. abortus RB51 (OD at 400 nm = 0.357). Little quenching was observed with B. ovis (OD at 400 nm = 0.288) and smooth B. abortus (OD at 400 nm = 0.157) LPSs. These results are consistent with micelle size and solubility of rough B. abortus LPS with respect to those of LPS from smooth strains (18).
TABLE 1.
Minimal lethal concentrations of cationic peptides needed to inhibit the growth of various Brucella strains in agar plates
| Peptide | Minimum lethal concn (μg/ml)
|
||
|---|---|---|---|
| B. abortus S19 | B. abortus 45/20 | B. ovis REO 198 | |
| Bactenecin 7 | 4.25 ± 1.08 | 0.72 ± 0.02 | 0.45 ± 0.06a |
| Bactenecin 5 | 24.37 ± 2.57 | 4.05 ± 0.48 | 0.07 ± 0.00a |
| Polymyxin B | 0.36 ± 0.02 | 0.13 ± 0.01 | 0.12 ± 0.01 |
| Lactoferricin B | >95 | 43.72 ± 2.14 | 1.63 ± 0.18a |
| Cationic protein 18A | 2.85 ± 0.07 | 1.40 ± 0.06 | 1.18 ± 0.03b |
| Melittin | 1.67 ± 0.12 | 1.56 ± 0.19 | 1.05 ± 0.11b |
| Poly-l-lysine | >95 | 23.67 ± 0.23 | 12.69 ± 2.45a |
| Poly-l-ornithine | >95 | 90.03 ± 0.82 | 66.17 ± 14.83c |
Significantly different from the results obtained with B. abortus 45/20 (P < 0.001).
Significantly different from the results obtained with B. abortus 45/20 (P < 0.02).
Significantly different from the results obtained with B. abortus 45/20 (P < 0.01).
TABLE 2.
Reduction of the bactericidal activity against Escherichia coli ATCC 29648 after adsorption of the peptides with live Brucella or Salmonella strainsa
| Peptide | % Reduction in bactericidal activity
|
|||
|---|---|---|---|---|
| B. abortus S19 | B. abortus 45/20 | B. ovis REO 198 | S. montevideo | |
| Bactenecin 5 | 3 ± 0.25 | 34 ± 4.00 | 34 ± 1.00 | 16 ± 1.10 |
| Bactenecin 7 | 5 ± 0.25 | 20 ± 2.50 | 40 ± 5.00 | 55 ± 2.00 |
| Poly-l-lysine | 3 ± 0.25 | 20 ± 2.50 | 20 ± 2.50 | 30 ± 2.50 |
| Poly-l-ornithine | 12 ± 1.00 | 20 ± 2.50 | 30 ± 2.00 | 40 ± 3.00 |
| Melittin | 17 ± 1.40 | 41 ± 4.00 | 53 ± 3.10 | 41 ± 2.15 |
| Lactoferricin B | 5 ± 1.25 | 25 ± 2.00 | 50 ± 2.50 | 60 ± 3.00 |
| Polymyxin B | 27 ± 1.5 | 47 ± 1.50 | 60 ± 3.50 | 71 ± 3.50 |
Ten micrograms of the peptide to be tested was mixed with 1010 bacterial cells in a 0.1-ml volume. The mixture was centrifuged and 15 μl of the supernatant was dispensed into wells punched in peptone-glucose-agar plates previously inoculated with E. coli ATCC 29648 as target cells, as described previously (9).
FIG. 2.
Binding of polymyxin B by LPSs from different Brucella strains. LPS suspensions adjusted to 60 to 63 nM concentrations of lipid A were incubated with a 13.75 μM concentration of dansyl-polymyxin B, and the fluorescence was estimated at a range of 400 to 600 nm. The inserted table shows the binding of different concentrations of dansyl-polymyxin B of the different LPSs at 480 nm. Dansyl-polymyxin B alone did not produce detectable fluorescence under these conditions. RFU, relative fluorescence units.
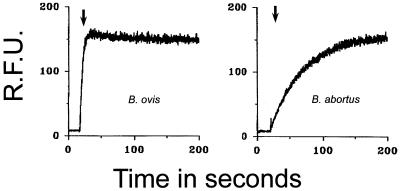
In previous investigations it was observed that, in contrast to enterobacterial OM, the Brucella spp. OMs were not barriers to hydrophobic permeants and that bactericidal cationic peptides did not alter the kinetics during the entrance of the hydrophobic probe NPN (9, 15). Figure 3 shows that the NPN partition kinetics in the OM of B. ovis differs considerably from that in the OM of B. abortus. None of the peptides tested altered the partition of NPN in the OM of Brucella strains, although the abrupt partition of NPN in the B. ovis OM makes it difficult to evaluate the action of the peptides in this bacterium.
FIG. 3.
Fluorimetry assays of bacteria (B. ovis and B. abortus 45/20 or RB51) treated with bactenecins 5 and 7, lactoferricin B, or polymyxin B. The NPN uptake profiles of B. abortus 45/20 and B. abortus RB51 are practically the same. The arrow indicates the time of addition of NPN or NPN and the peptide. No effect was observed when NPN or NPN plus peptide was added. RFU, relative fluorescence units.
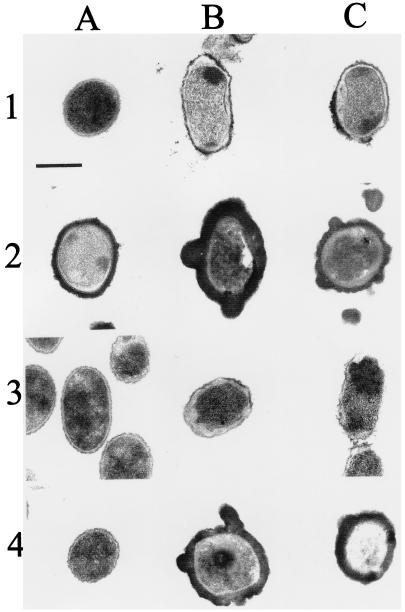
Transmission electron microscopy demonstrated that the effects induced by bactenecins 5 and 7 were more evident in S. montevideo than they were in rough Brucella (Fig. 4 and 5). The effects of bactenecins 5 and 7 on rough B. abortus and on B. ovis appeared to be similar (Fig. 4). The morphological changes observed in peptide-treated Brucella were mild and evident only at high concentrations of the bactericidal agents. These changes were characterized by detachment of the internal membrane, vacuolization, and the appearance of electron-dense bodies within the cytoplasm. Most of the time, the OM maintained its appearance. Poly-l amino acids produced a thick coating on rough B. abortus and B. ovis, being more conspicuous in the latter than in the former bacteria (Fig. 4). This phenomenon hampered the infiltration of the resin, making observation difficult. In spite of this problem, minimal morphological effects were observed in the Brucella OM treated with poly-l amino acids. Some spotted precipitation and vacuolization of the cytoplasm was evident; however, the significance of these was unclear. In contrast, poly-l amino acids produced severe damage on S. montevideo cells, characterized by severe vacuolization, formation of cytoplasmic electron-dense bodies, and distortion of the cell shape (Fig. 5). These effects were more evident in the bacterial poles. Treatment with a dose of polymyxin B lethal for S. montevideo (Fig. 5) did not affect the OM structure of the rough Brucella strains. The use of large quantities of polymyxin B resulted in the deposition of an electron-dense layer on the surface of B. ovis with little detectable cell damage (Fig. 4), an effect that may be related to the augmented binding of polymyxin B by the LPS of this rough Brucella (Fig. 2). Control experiments showed that insoluble polymyxin B in agarose was stained with uranyl and osmium salts in a pattern similar to that of the electron-dense coat observed around polymyxin B-treated B. ovis (data not shown). No morphological differences were observed between smooth and rough B. abortus control strains.
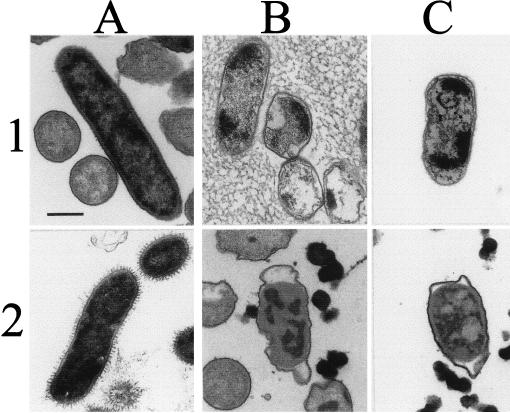
FIG. 4.
Transmission electron microscopy of peptide-treated rough Brucella. Rows 1 and 2 show B. ovis REO 198, and rows 3 and 4 show rough B. abortus 45/20. (A) Untreated controls (rows 1 and 3) and bacteria treated with 20 μg of polymyxin B (rows 2 and 4); (B) bacteria treated with 20 μg of bactenecin 5 or 20 μg of poly-l-lysine (rows 2 and 4); (C) bacteria treated with 20 μg of bactenecin 7 (rows 1 and 3) or 20 μg of poly-l-ornithine (rows 2 and 4). Bar, 0.25 μm.
FIG. 5.
Transmission electron microscopy of peptide-treated smooth Salmonella. Row 1 shows the following: untreated S. montevideo SH94 (A) and Salmonella treated with 10 μg of bactenecin 5 (B) or 10 μg of bactenecin 7 (C). Row 2 shows results of treatment with 10 μg of polymyxin B (A), 10 μg of poly-l-lysine (B), or 10 μg of poly-l-ornithine (C). Bar, 0.25 μm.
The OM structure of Brucella organisms differ from that of other pathogenic groups (6, 15, 16, 19), including several facultative intracellular bacteria such as Salmonella or Shigella spp. However, within the genus Brucella, which is a close monophyletic bacterial group (28), most of the OM components have been found to be very similar (19). Some of the structural features of the Brucella OM correlate well with the observed permeability of the Brucella OM to hydrophobic compounds (9, 15), and with the resistance of these bacteria to EDTA, Tris, some detergents (20), and oxygen-dependent and -independent killing mechanisms (13, 16, 24). Features which have been implicated in this resistance are the O chain (linked to the core lipid A) and the NH, both being characteristic of smooth Brucella strains (2, 9). Since the resistance of B. ovis and rough B. abortus strains to cationic peptides has been demonstrated to be lower than that displayed by the smooth Brucella, we concluded that the lack of any O chain in the LPS and the absence of NH are factors which contribute to the sensitivity of the OM. It is unlikely that the small amounts of O chains present on the surface of Brucella 45/20 account for this difference. Findings supporting this proposition include the almost identical NPN kinetics displayed by the rough B. abortus RB51 (which does not possess any O chain) and the B. abortus 45/20 cells, and the similar levels of binding of polymixin B of their LPSs (Fig. 2 and 3). Other OM characteristics may also participate in this susceptibility, however, since the pathogenic B. ovis is more affected by the peptides than the rough B. abortus. In this respect, it has been demonstrated that naturally occurring rough Brucella strains expose a uniform set of anionic sites on their surfaces, which are easily visualized with positively charged probes (30). Although the peptides did not have any clear effect on the uptake of NPN in the Brucella strains, the partition of this hydrophobic probe on the OM of B. ovis was strikingly different in smooth and rough B. abortus mutant strains (Fig. 3 and reference 15). The sudden uptake of NPN by the OM of B. ovis marked a definitive difference between this species and the other Brucella spp. In conclusion, the higher susceptibility of B. ovis to cationic peptides, the increased uptake of hydrophobic probes, the augmented polymyxin binding of its LPS, and the distinct structural properties of its OM correlate with the different biological behavior of this rough strain in animal hosts and culture cells (Table 3). We must be cautious, however, since other factors not necessarily related to the structure of the OM may also participate in the host preferences of B. ovis, the most differentiated species among the genus Brucella (19).
TABLE 3.
Invasiveness and OM properties of rough B. abortus and B. ovis
| Characteristic | B. abortus S19a | B. abortus 45/20 | B. abortus RB51 | B. ovis REO 198 | Reference |
|---|---|---|---|---|---|
| Invasiveness to host and cellsb | |||||
| Ovine infection | Moderate | Moderate | Moderate | Severe | 8, 12, 17 |
| Bovine infection | Moderate | Moderate | Moderate | None | 1, 8, 21 |
| No. (%) of bacterium-associated cells after 2 h | 75 | 98 | 100 | 12 | 22, 23, 27 |
| No. of adherent bacteria per cell after 2 h | 4 | 40 | 38 | 4 | 22, 23, 27 |
| No. of intracellular bacteria per cell after 2 h | 1–2 | 20 | 18 | 0–1 | 22, 23, 27 |
| No. of CFU within cells at 48 h | 5 × 105 | 6.2 × 106 | NDc | 0 | 22, 23, 27 |
| No. (%) of infected cells dead at 48 h | 0 | 73 | ND | 0 | 22, 23 |
| Bacteria outside lysosomes | + | ++ | ND | − | 22, 23, 27 |
| Skin reaction of B. melitensis-infected animals | Positive | Positive | ND | Negative | 13, 26 |
| OM properties | |||||
| Reactivity with polyclonal anti-B. canis R-LPS | Total | Total | Total | Partial | 18, unpublished data |
| Access to surface R epitopes | + | ++++ | ++++ | ++++ | 4 |
| Estimated no. of anti-O chain gold conjugate particles per bacterial cell | 3,000–5,000 | 0–12 | 0 | 0 | 4, 10, 26, this work |
| Heterogeneity of R-LPS in SDS-PAGE (no. of bands) | 1 | 4 | 3 | 1 | 10, 26 |
| OM protein 25 | Complete | Complete | Complete | Truncated | 4, 7 |
| OM protein 31 | Absent | Absent | Absent | Present | 4, 29 |
| Sensitivity to hydrophobic antibiotics | + | ++ | ND | ++++ | 15 |
| Maximal uptake of NPN (s) | 200 | 200 | 200 | 10 | This work |
| Binding of polymyxin B by LPS | + | ++ | ++ | ++++ | 16, this work |
| Sensitivity to cationic peptides | + | ++ | ++ | ++++ | 16, this work |
This is an attenuated vaccine strain. Virulent B. abortus 2308 induces severe infections, penetrates cells at an efficiency of close to 100%, evades lysosomes, and replicates within cells (23). LPS preparations from smooth Brucella contain close to 10% of rough-type LPS displayed in a single band in SDS-PAGE (10). −, absent; +, present in <25%; ++, present in >25% to <50%; ++++, present in >75%.
Tested in human epithelial HeLa cells.
ND, not done with RB51.
Acknowledgments
This work was supported in part by grants from the Karolinska International Research Training (KIRT) Program of the Swedish Agency for Research Co-operation with Developing Countries (SAREC/SIDA), the Centre National de la Recherche Scientifique (PICS), and the Institut National de la Santé et de la Recherche Médicale, Nord-Sud, France; the Dirección General de Investigación Científica y Tecnológica, Madrid, Spain (BIO96-1398-C02-01) and Fundación CR-USA, San José, Costa Rica (R-215-99).
We are grateful to Reynaldo Pereira for his expert assistance in electron microscopy and Daphnne Garita for her unfaltering technical assistance.
REFERENCES
- 1.Alton G. Experiences with Brucella vaccines. In: Crawford R P, Hidalgo R, editors. Bovine brucellosis. College Station, Tex: Texas A & M University Press; 1977. pp. 198–212. [Google Scholar]
- 2.Aragón V R, Díaz R, Moreno E, Moriyón I. Characterization of Brucella abortus and Brucella melitensis native haptens outer membrane O-type polysaccharides independent from the smooth lipopolysaccharide. J Bacteriol. 1996;178:1070–1079. doi: 10.1128/jb.178.4.1070-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blasco J M. Brucella ovis. In: Nielsen K, Duncan B, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 351–378. [Google Scholar]
- 4.Bowden R A, Cloeckaert A, Zygmunt M, Bernard S, Dubray G. Surface exposure of outer membrane protein and lipopolysaccharide epitopes in Brucella species studied by enzyme-linked immunosorbent assay and flow cytometry. Infect Immun. 1995;63:3945–3952. doi: 10.1128/iai.63.10.3945-3952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmichael L E, Kenny R M. Canine abortion caused by Brucella canis. J Am Vet Med Assoc. 1968;152:605–616. [PubMed] [Google Scholar]
- 6.Cherwonogrodzky J W, Dubray G, Moreno E, Mayer H. Antigens of Brucella. In: Nielsen K, Duncan B, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 19–64. [Google Scholar]
- 7.Cloeckaert A, Verger J M, Grayon M, Zygmunt M S, Grepient O. Nucleotide sequence and expression of the gene encoding the major 25-kilodalton outer membrane protein of Brucella ovis: evidence for antigenic shift, compared with other Brucella species, due to a deletion in the gene. Infect Immun. 1996;64:2047–2055. doi: 10.1128/iai.64.6.2047-2055.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbel M J, Brinley-Morgan W J. Genus Brucella Meyer and Shaw 1920, 173AL. In: Krieg N R, Holt J C, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams and Wilkins Co.; 1984. pp. 377–388. [Google Scholar]
- 9.Freer E, Moreno E, Moriyon I, Pizarro-Cerdá J, Weintraub A, Gorvel J-P. Brucella-Salmonella lipopolysaccharide chimeras are less permeable to hydrophobic probes and more sensitive to cationic peptides and EDTA than are their native Brucella sp. counterparts. J Bacteriol. 1996;178:5867–5876. doi: 10.1128/jb.178.20.5867-5876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freer E, Rojas N, Weintraub A, Lindberg A, Moreno E. Heterogeneity of Brucella abortus lipopolysaccharides. Res Microbiol. 1995;146:569–578. doi: 10.1016/0923-2508(96)80563-8. [DOI] [PubMed] [Google Scholar]
- 11.Halling S M. The effects of magainin 2, cecropin, mastoparan and melittin on Brucella abortus. Vet Microbiol. 1996;51:187–192. doi: 10.1016/0378-1135(96)00027-2. [DOI] [PubMed] [Google Scholar]
- 12.Jiménez-de-Bagües M P, Barberán M, Marín C M, Blasco J M. The Brucella abortus RB51 vaccine does not confer protection against Brucella ovis in rams. Vaccine. 1995;13:301–304. doi: 10.1016/0264-410x(95)93317-3. [DOI] [PubMed] [Google Scholar]
- 13.Jones L M, Diaz R, Taylor A G. Characterization of allergens prepared from smooth and rough strains of Brucella melitensis. Br J Exp Pathol. 1973;54:492–508. [PMC free article] [PubMed] [Google Scholar]
- 14.Kreutzer D L, Robertson D C. Surface macromolecules and virulence in intracellular parasitism: comparison of cell envelope components of smooth and rough strains of Brucella abortus. Infect Immun. 1979;23:819–828. doi: 10.1128/iai.23.3.819-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez de Tejada G, Moriyón I. The outer membranes of Brucella spp. are not barriers to hydrophobic permeants. J Bacteriol. 1993;175:5273–5275. doi: 10.1128/jb.175.16.5273-5275.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez de Tejada G, Pizarro-Cerdá J, Moreno E, Moriyón I. The outer membranes of Brucella spp. are resistant to bactericidal cationic peptides. Infect Immun. 1995;63:3054–3061. doi: 10.1128/iai.63.8.3054-3061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mollelo J A, Jensen R, Flint J C, Collier J R. Placental pathology. I. Placental lesions of sheep experimentally infected with Brucella ovis. Am J Vet Res. 1963;102:897–904. [PubMed] [Google Scholar]
- 18.Moreno E, Jones L M, Berman D T. Immunochemical characterization of rough Brucella lipopolysaccharides. Infect Immun. 1984;43:779–782. doi: 10.1128/iai.43.3.779-782.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno E. Evolution of Brucella. In: Plommet M, editor. Prevention of brucellosis in the Mediterranean countries. International Center for Advanced Mediterranean Agronomic Studies. Wageningen, The Netherlands: Pudoc Scientific Publishers; 1992. pp. 198–218. [Google Scholar]
- 20.Moriyon I, Berman D T. Effects of non-ionic, ionic, and dipolar ionic detergents and EDTA on Brucella cell envelope. J Bacteriol. 1982;152:822–828. doi: 10.1128/jb.152.2.822-828.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer M V, Olsen S C, Gilsdorf M J, Philo L M, Clarke P R, Cheville N F. Abortion and placentitis in pregnant bison (Bison bison) induced by vaccine candidate, Brucella abortus RB51. Am J Vet Res. 1996;57:1604–1607. [PubMed] [Google Scholar]
- 22.Pizarro-Cerdá J. Traffic intracellulaire et survie de Brucella abortus dans les phagocytes professionnels et non professionnels. Doctoral thesis. Marseille-Luminy, France: University de la Mediterranee Aix-Marseille II; 1998. [Google Scholar]
- 23.Pizarro-Cerdá J, Moreno E, Sanguedolce V, Mege J L, Gorvel J P. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect Immun. 1998;66:2387–2392. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasool O, Freer E, Moreno E, Jarstrand C. Effect of Brucella abortus lipopolysaccharide on oxidative metabolism and lysozyme release by human neutrophils. Infect Immun. 1992;60:1699–1702. doi: 10.1128/iai.60.4.1699-1702.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rojas N, Freer E, Weintraub A, Ramírez M, Lind S, Moreno E. Immunochemical identification of Brucella abortus lipopolysaccharide epitopes. Clin Diagn Lab Immunol. 1994;1:206–213. doi: 10.1128/cdli.1.2.206-213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schurig G G, Roop II R M, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51; a stable rough strain of B. abortus. Vet Microbiol. 1991;28:171–188. doi: 10.1016/0378-1135(91)90091-s. [DOI] [PubMed] [Google Scholar]
- 27.Sola-Landa A, Pizarro-Cerdá J, Grilló M, Moreno E, Moriyón I, Blasco J, Gorvel J, López-Goñi I. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol Microbiol. 1998;29:125–138. doi: 10.1046/j.1365-2958.1998.00913.x. [DOI] [PubMed] [Google Scholar]
- 28.Verger J, Grimont F, Grimont P A D, Grayon M. Brucella, a monospecific genus as shown by deoxyribonucleic acid hybridization. Int J Syst Bacteriol. 1985;35:292–295. [Google Scholar]
- 29.Vizcaíno N, Verger J-M, Grayon M, Zygmunt M S, Cloeckaert A. DNA polymorphism at the omp-31 locus of Brucella spp.: evidence for a large deletion in Brucella abortus, and other species specific markers. Microbiology. 1997;143:2913–2921. doi: 10.1099/00221287-143-9-2913. [DOI] [PubMed] [Google Scholar]
- 30.Weber, A., H.-D. Schiefer, and H. Krauss. 1977. Ultrastructural investigations on anionic surface sites of Brucella canis. 239:365–374. [PubMed]
- 31.Young E J, Borchert M, Kretzertf L, Musher D M. Phagocytosis and killing of Brucella by human polymorphonuclear leukocyte. J Infect Dis. 1985;151:682–688. doi: 10.1093/infdis/151.4.682. [DOI] [PubMed] [Google Scholar]