Abstract
BACKGROUND:
Intraoperative molecular imaging (IMI) has emerged as a potential tool in addressing challenges faced during lung cancer surgery by localizing small lesions, ensuring negative margins, and identifying synchronous cancers. CEACAM5 glycoprotein has emerged as a potential target in fluorescent labeling of NSCLC given the high antigen density in tumor cells and absence expression in normal parenchyma. The goal of our study was to determine if anti-CEACAM5 targeted NIR -fluorochrome could be a suitable target in NSCLC.
METHODS:
CEACAM5 expression was evaluated in AB-12 (known negative control), HT29 (known positive control),and H460 (NSCLC) cell-lines via PCR. SGM-101, a CEACAM5 Ab coupled with a BM-104 near-infrared fluorescent tracer was evaluated with dose escalation, in-vitro cellular localization, and immunofluorescence microscopy. Subsequently, in-vivo validation was performed in 52 athymic nude xenografts.
RESULTS:
PCR analysis demonstrated 3000x relative expression of CEACAM5 in HT-29 cells compared with AB-12. H460 cells showed 1000x relative expression compared to AB12 (p<0.05). Both HT29 and H460 cells showed tracer internalization with SBR of 4.5 (SD 0.34) while there was minimal uptake by AB12 cells (SBR 1.1 (SD 0.1))(p<0.05). There was linear fluorescence increase with increasing tracer dosing in receptor expressing cell-lines. In pre-clinical models, HT-29 and H460 cells lines produced NIR fluorescence with average TBR of 3.89 (SD 0.25) irrespective of tumor size compared to no fluorescence by AB12 tumors (p<0.05). CEACAM5 expressing tumors had excellent dye uptake compared to AB12 tumors.
CONCLUSIONS:
CEACAM5 serves as a possible receptor for targeted IMI resections in lung cancer. This study sets a path for evaluation of CEACAM5 target in future clinical trials.
Keywords: CEACAM5, CEA, Molecular Imaging, SGM-101, Fluorescence Guided Surgery
Non-Small Cell Lung Cancer (NSCLC) is a leading cause of oncologic morbidity and mortality worldwide[1].. Stage by stage, complete disease clearance offers the best oncologic outcomes in non-disseminated disease[2]. Early nodule detection coupled with widespread adaptation of minimally invasive techniques such as video-assisted thoracoscopic surgery (VATS) and robotics have further improved surgical outcomes and recovery for the patients. However, increased use minimally invasive techniques have challenged the thoracic surgeon’s ability to localize lesions using their visual and tactile feedback[3].
One of the technologies that has the potential to address this conundrum is Intraoperative Molecular Imaging (IMI). IMI employs the use of targeted fluorochromes that localize to cancer cells which then emit fluorescence in the far-red or near infrared (NIR) spectrum that can be detected using specialized cameras[4]. The technology has been increasingly studied in advanced clinical thoracic oncology clinical trials. One of the key tracers that has been extensively evaluated in this realm is pafolacianine, which is a folate receptor targeted NIR fluorochrome[5]. Multiple clinical trials have demonstrated increased benefit in detecting visually occult primary lesions, synchronous lesions not detected by screening pre-operative imaging, and confirm intra-operative margin assessment[4–7]. Despite the observed benefits, not all patients harbor folate receptor positive tumors. Therefore, new tracers which would capture large patient pool should be explored.
One of these cell surface receptors of interests is the Carcinoembryonic Antigen Cell Adhesion Molecule 5 (CEACAM5), also known as CEA or CD66e. CEACAM5 is expressed in various solid organ adenocarcinomas and serum detection of the glycoprotein are prognostic and predictive marker of lung cancer (adenocarcinoma)[8,9]. Assessment of the protein levels can be used for cancer diagnosis, prognosis, and assessment of therapy[10]. Additionally, CEACAM5 has been found to be overexpressed in 60–80% of lung adenocarcinomas, making it an optimal receptor target to be exploited in IMI guided lung cancer resections[11]. Various literature reports have demonstrated the potential association of CEACAM5 presence in NSCLC with tumor aggressiveness, lymph node metastases, and worse survival outcomes[9,12,13] Therefore, the glycoprotein has the potential of being further explored in NSCLC both in terms of fluorescent labeling and targeted therapy delivery. Furthermore, CEACAM5 has the additional benefit of being detected in the serum, which would identify optimal patients pre-operatively with blood tests and not be subjected to core biopsies to assess receptor expression via immunohistochemistry[10]. SGM-101, a CEACAM5 antibody targeted NIR tracer has been studied in advanced CEA expressing gastrointestinal adenocarcinomas with good success but no studies to date have been performed in lung cancer despite the known receptor expression[7,14].
Our goal in this proof of principle study was to show that SGM-101 can specifically identify CEACAM5 expressing NSCLC in-vitro. We hypothesize that the CEACAM5 antibody conjugated fluorochrome will produce NIR emissions both microscopically and macroscopically in only CEA positive cell lines. We first performed cancer genome database to identify lung adenocarcinoma cell lines that express CEA and selected appropriate controls to test our hypothesis. We then analyzed dye binding analysis using immunofluorescence microscopy and fluorescence activated single cell sorting flow cytometric analysis. Our results demonstrate that SGM-101 is highly specific NIR tracer for CEA positive lung cancer cell line.
MATERIAL AND METHODS
Additional description of technical details, reagents, PCR sequences, in-vivo protocols used in the study are described in the supplementary file.
Near-Infrared Fluorochrome and Imaging System
The tumor-specific imaging agent, SGM-101 (molecular weight, 148.6 kDa), consists of an anti-CEACAM5 chimeric monoclonal antibody covalently bound to NIR fluorophore BM-104 (excitation, 686 nm; emission, 704 nm)[15]. SGM-101 was supplied by Surgimab (Montpellier, France) as a sterile solution.
Cell Lines
Cell line selection rationale was based on review of literature studies and the Cancer Cell Line Encyclopedia analysis. Mesotheliomas are widely known not to express CEACAM5 glycoprotein and therefore selected as a negative control[16]. >90% of colorectal adenocarcinomas express CEACAM5 and HT29 have been extensively studied as in-vitro model for CEACAM5 intracellular effects, therefore the cell line was selected as a positive control[17]. H460 (NSCLC) was selected as cell line of interest given the potential expression of the glycoprotein as depicted by CCLE
Live Cell Imaging, SGM-101 Cell Uptake
Cell lines were cultured on poly-L-lysine-coated glass coverslips in 6-well plates with DMEM media supplemented with 10% FBS, L-glutamine, and penicillin/streptomycin for 24 hours. For internalization time course studies, cells were treated with differing concentrations of SGM-101 for 2 hours.. For internalization time course studies, cells were treated with 200 nM LysoTracker (Invitrogen, Waltham, MA) for 2 hours prior to incubation with SGM-101. Cells were mounted on glass slides with ProLong Gold antifade reagent with DAPI (Fisher Scientific, Waltham, MA) and imaged on a Leica DM6 B fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
Murine Studies
Animal studies were performed according to protocols approved by the University of Pennsylvania Institutional Animal Care and Use. 6–8 weeks old athymic nude mice were purchased from Charles River. Mice were housed in a sterile environment on a standard 12-hour light and dark cycle for the duration of the study (Supplementary Methods).
Statistical Analysis:
Data are presented as mean ± SEM or ±SD as indicated. Statistical differences between 2 groups were assessed using t test with GraphPad Prism software and SPSS version 27 (IBM Technologies). A one-way ANOVA followed by post hoc Tukey test was used for analyzing differences between more than two groups. P<0.05 was considered a statistically significant difference.
RESULTS
CEACAM5 Expression Lung Cancer
In order to confirm the feasibility of targeting CEACAM5 in pre-clinical lung cancer models, we explored frequency of glycoprotein expression and mutation using The Cancer Genome Atlas (TCGA) database. Analysis of mRNA expression of CEACAM5 expression in various cancer demonstrated highest median detection in colorectal adenocarcinomas (188k) followed by NSCLC (98k), esophagogastric adenocarcinoma (89k), and pancreatic adenocarcinoma (91k). Glycoprotein expression was significantly higher expressed in adenocarcinomas than squamous cell malignancies (102k vs 11k, p<0.05). Additionally, in adenocarcinomas with elevated CEACAM5 expression, the number and type mutations were significantly elevated with shallow deletion and amplification the most common protein mutation noted (Figure 1).
Figure 1:

TCGA analysis of cancer type and CEACAM5 mRNA expression with associated mutation frequency for each cancer type.
Analysis of CCLE similarly demonstrated increased CEACAM5 expression in colorectal adenocarcinoma cell lines with log2 (TPM+1) of 6.22. NSCLC CEACAM5 mRNA log2(TPM+1) was found to be 3.44. Highest glycoprotein expression was noted in HT29 colorectal cell line and H460 (NSCLC). Mesothelioma cell line AB12 and A549 cell line did not have any CEACAM5 expression noted in the databases. Therefore, these cell lines were selected for in-vitro validation.
To further analyze the feasibility of CEACAM5 for NSCLC, particularly lung adenocarcinomas, we performed immunohistochemical analysis of glycoprotein expression from core biopsies in 33 consecutive lung adenocarcinoma patients. 23/33 (69.6%) patients had positive CEACAM5 staining noted. No CEACAM5 expression was noted in normal adjacent lung parenchyma.
Cell Line CEACAM5 Expression Validation
qPCR analysis(Supplementary methods)for CEACAM5 demonstrated highest expression in HT29 cell line (2388 relative mRNA expression). H460 cell line mRNA expression was statistically lower at 898 (p<0.05). HT29 and H460 had statistically higher significant expression than AB12 or A549 cells, which did not demonstrate any detectable mRNA levels (p<0.01) (Figure 2). qPCR results were corroborated by RT-PCR analysis of the cell lysates which demonstrated highest relative chemiluminescence in HT29 cells (411) vs H460 (130) vs AB12 (0) vs A549 (0) (p<0.01)(Figure 2). CEACAM5 glycoprotein expression with flow cytometry confirmed mRNA expression results with primary antibody staining demonstrating no detection in AB12 cell line and statistically significant binding in H460 and HT29 cell lines (Figure 3). HT29 cells similarly had higher CEACAM5 expression compared to H460 cell line (1.45-fold difference)(p<0.05).
Figure 2:

(A) qPCR analysis of CEACAM5 relative mRNA expression in HT29, H460, A549, and AB12 cell lines. (B) RT-PCR analysis of AB12, HT29, H460, and A549 cell lines showing detection of CEACAM5 in HT29 and H460 cells with relative chemiluminescence levels displayed below. Analyses were performed in triplicates.
Figure 3:
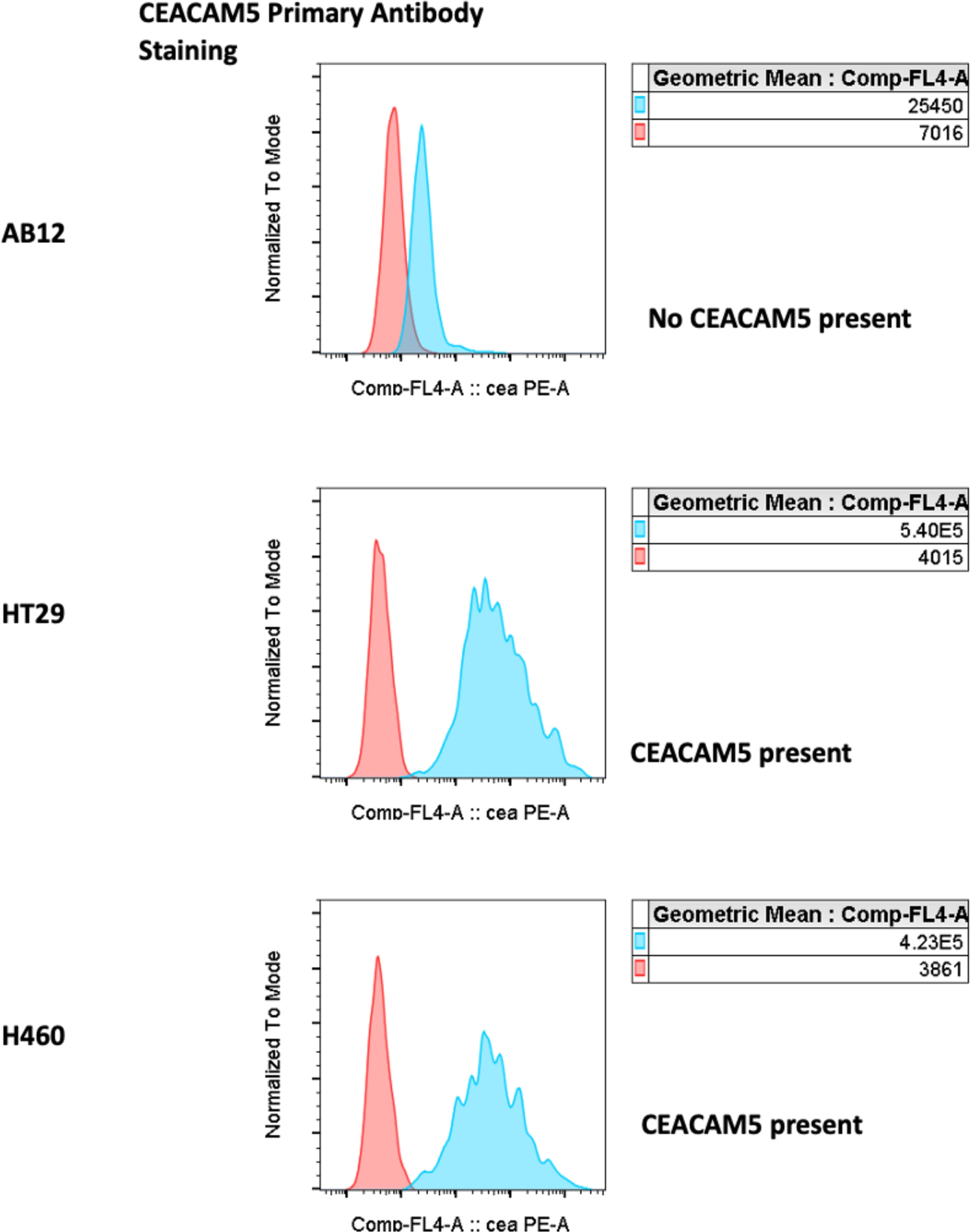
Flow cytometry analysis for primary CEACAM5 antibody binding to AB12, H460, and HT29 cells with no binding observed in AB12 cells. Anti-CEACAM5 to irrelevant Ab GMean ratios were 3.6, 134.5 and 109.6 for AB12, HT29 and H460 cells, respectively.
CEACAM5 Antibody Conjugated Dye Binding Analysis
Fluorochrome titration showed concentration dependent dye binding and fluorescence emission in CECAM5+ cell lines. HT29 had 1.48x higher fluorescence at all concentration levels compared to H460 cell lines. AB12 cell lines did not have any fluorescence detected by immunofluorescence microscopy (IF) (p<0.05)(Figure 4,5). Mean Signal to Background Ratio (SBR) was 4.11 (Absorbance Units (A.U) for HT29, 3.23 A.U for H460, and 1.0 A.U for AB12 (p<0.05). SGM-101 accumulates in the cells in a predictable and concentration dependent manner only in CECAM5+ cells (Figure 4-A). Concentration kinetic analysis demonstrated that SGM-101 had high affinity towards CEACAM5 expressing cells with a Kd of (12 pM)(Figure 4-B). When compared the fluorescence properties of SMG-101 to ICG which is an established lung adenocarcinoma tracer, at concentrations 0.5 uM and above, there is similar SBRs (3.45(SGM-101) vs (3.67(ICG)(p=0.11). (Figure 5-C)(Figure 6).
Figure 4:

Immunofluorescence microscopy of SGM-101 in HT29, AB12, H460 cells showing dye uptake and internalization in CEACAM5+ cells.
Figure 5:

(A): Immunofluorescence NIR microscopy shows concentration dependent increase in cellular uptake and fluorescence in HT29 and H460 cell lines with absence of fluorescence detected in AB12 cell lines at 24 hours.
(B): Concentration kinetics quantifying observed fluorescence intensity in the cell lines with Kd of 12 pM demonstrating high specificity of the tracer.
(C): Near Infrared emission comparison of SGM-101 to ICG at concentrations of 1 uM to 100nM.
Figure 6:

Time course and site-specific fluorescence of SGM-101 in HT29 positive control cells.
SGM-101 Localizes to CEACAM5 Expressing Xenografts
1,000,000 cells from HT29, H460, and AB12 cell-lines were implanted into flanks of athymic nude mice. After xenografts developed, SGM-101 was systemically injected at a dose of 200 uL and imaged 72 hours post injection, given the highest TBR observed at 72 hours as compared to other time points. Nevertheless, SGM-101 based fluorescence of the xenografts was observed up to 14 days post infusion in-vivo. Similar to in-vitro analysis, only the CEACAM5 expressing tumors produced fluorescence with SGM-101 (Figures 7-A,B). HT29 tumors had a TBR of 4.11 vs 3.04 in H460 vs 1.0 AB12 (p<0.05). CEACAM5 presence in the xenografts correlated with SGM-101 localization (p<0.05). AB12 tumors did not have any dye uptake. Analysis of organ biodistribution of SGM-101 revealed expected uptake in the gastrointestinal organs (stomach, intestines, liver, and CEACAM5 tumors) but no uptake was noted in the lungs of both AB12 and HT-29 xenograft bearing mice. The fluorescence of the intestinal tract was not visualized prior to organ harvest by laparotomy indicating a depth of penetration of around 1 cm, which is similar to other NIR-I range (700–1000nm) wavelength tracers. No SGM-101 fluorescence was visualized in the normal lung parenchyma, coupled with strong CEACAM5+ tumor fluorescence, points to potential high tumor to background ratio of fluorescence and visual contrast noted by the operator.
Figure 7:

(A): Mouse xenografts of HT29, H460, and AB12 cell lines demonstrates SGM-101 localization to CEACAM5 expressing cells which is concordant with in-vitro findings. (B) SGM-101 fluorescence emission in positive and negative controls as well as organ biodistribution in both the control groups demonstrating expected uptake of the tracer in the GI tract and CEACAM5+ xenograft while no uptake or fluorescence was detected in the lung parenchyma of both controls. (C): Histopathologic assessment of CEACAM5 targeted SGM-101 demonstrating tumor specific localization.
Observations in flank tumors correlated with histopathologic examination of the tissues. CEACAM5+ tumors (HT29, H460) demonstrated SGM-101 uptake in tumor cells (Figure 7-C). Individual CEACAM5+ cells were able to be detected using immunofluorescence microscopic analysis. No tumor associated dye localization with SGM-101 was noted in AB12 cells concordant with absence of CEACAM5 expression. ELISA serum CEACAM5 (CEA) analysis demonstrated high concordance with SGM-101 fluorescence and presence of the glycoprotein in the murine plasma. Receiver operator curve (ROC) of normalized plasma serum with SGM-101 fluorescence presence (TBR >1.5) generated an AUC of 0.895 (Supplementary Figure 1).
SGM-101 Margin Assessment
18 athymic nude mice bearing H460 tumors were randomly assigned to tumor resection using standard techniques or SGM-101 NIR guided tumor resections. Tumors operated using conventional techniques (white light) had 3/9 positive margins as detected by NIR imaging systems vs 0/9 in the SGM-101 arm (p<0.05). Positive margins were confirmed using histologic assessment. SGM-101 was successful in localizing margins as small as 2 mm. Negative NIR fluorescence detection correlated with negative tumor presence in both arms (Figure 8) (Supplemental Figure-2).
Figure 8:

Representative mice images of H460 tumor resection with positive and negative margin detection.
COMMENT
Non-Small Cell Lung Cancer is a major cause of cancer related morbidity and mortality globally. Surgical intervention coupled with improved resection techniques have favorable outcomes[2]. IMI has been shown to address some of the pressing surgical challenges such as identifying sub-centimeter lesions, identifying positive margins, and detecting occult synchronous lesions[5]. In this study we show that SGM-101 which is a CEACAM5 antibody conjugated NIR fluorochrome can identify CEACAM5 cells in pre-clinical models. Our findings are one of the few in literature exploring CEACAM5 as a potential molecular imaging target in lung cancer.
CEACAM5 has been widely studied and adopted in screening, monitoring, and prognostic evaluation in colorectal and pancreatic adenocarcinomas[19]. Nevertheless, the glycoprotein expression is present in various other adenocarcinomas including esophagogastric and lung[20]. While there is considerable debate, there remains subset of patients with lung adenocarcinoma in whom CEACAM5 expression have been found to be prognostic [9,10]. These observations in conjunction with successful implementation of SGM-101 in early-stage colorectal cancer trials, makes CEACAM5 expressing lung cancers as an optimal malignancy to be explored for SGM-101 guided resections (Figure 1). Validation of CEACAM5 targeted fluorophores for lung adenocarcinomas have previously not been explored.
Our analysis of the TCGA and CCLE showed increased CEACAM5 expression in colorectal and lung adenocarcinomas further reinforcing the cell-surface glycoprotein as an attractive target for SGM-101[21]. Glycoprotein was highly expressed in lung adenocarcinomas as compared to squamous cell cancers which were consistent with clinical literature. Therefore, in conjunction with the database analysis and in literature, we explored colorectal cell lines(HT29) as a positive control, lung adenocarcinoma(H460) for cell line of interest, and mesothelioma(AB12) cell lines as a negative control.
Prior to examining SGM-101 effectiveness, we wanted to confirm CEACAM5 presence in the cell lines. HT29 as described in literature is a widely studied CEACAM5+ cell line and in our PCR analyses the cell line had the highest expression of the glycoprotein (Figure 2)[17]. H460 cell line while scantly described in literature reports, is known to harbor CEACAM5 expression. AB12 which is used as a negative control, did not demonstrate any expression in any of the analysis[16]. Similarly, our SGM-101 dye binding analysis showed strong concordance with the PCR analysis and confirmed in-vitro validation of our controls as well as our cell-line of interest(Figures 2,3,4).
Furthermore on fluorescence microscopy, the degree of dye binding was concentration and time dependent with 1uM concentration producing the highest fluorescence (p<0.05). Only CEACAM5+ cells had dye internalization with HT29 cells having higher SBR (4.11) as compared to H460 (3.23). The affinity of the tracer to the cells were also strong with Kd of ~12 pM. Although, they produce NIR emission at different wavelength (700 nm vs 800 nm(ICG)), the fluorescence intensity comparison at concentrations 1 uM and above were comparable potentially pointing to clinical viability of SGM-101(Figure 5).
Validation of in-vitro results naturally yields to developing a pre-clinical animal model prior to clinical investigation. SGM-101 was previously studied in advanced colorectal and pancreatic adenocarcinomas with variable success[7,22]. However, results from these trials cannot be generalized to lung cancer patients as anatomic consideration, tumor biology, and disease spread patterns are vastly different. The xenografts after infusion of SGM-101 demonstrated CEACAM5+ correlated fluorescence. HT29 and H460 mice had a median TBR of 4.11 vs 3.04 respectively (p<0.05) whereas AB12 cells did not demonstrate any fluorescence at all. The targeting of SGM-101 to CEACAM5 harboring tumors were clearly evident on histopathologic examination (p<0.05) (Figure 7). Furthermore, CEACAM5 shedding into the plasma appears to be a predictor of SGM-101 fluorescence with serum CEA ELISA analysis demonstrating high concordance with success of antibody-fluorochrome conjugate labeling. This demonstrates that SGM-101 is highly specific and sensitive in targeting CEACAM5+ tumors.
A large part of morbidity and mortality associated with lung adenocarcinomas are local recurrences[5,23]. Therefore, we explored if SGM-101 can identify positive margins compared to visual assessment alone. The NIR imaging guided xenograft resection group did not demonstrate any positive margins post resection whereas there was almost 33% margin positivity in the white light group. Of note, the investigators performing resections in the control group were not familiar with the study or NIR imaging technology. IMI guided resections identified positive margins as small as 2mm which was confirmed by histopathologic assessment (Figure 8, Supplemental Figure-2). The goal of this experiment was not to suggest that there are positive margins in one-third of specimen resections, which is significantly different than rates observed in clinical practice for anatomic resections. However, it was to assess the ability of SGM-101 to detect sub-centimeter areas of margin positivity. Clinically, anatomic resections have excellent margin safety but in cases of metastasectomy and wedge resections where the margins can be very close, the tracer appears to have the ability to inform the surgeon of potentially close margins that would otherwise be discovered after frozen section analysis, which can take >30 minutes at high-volume institutions [5]. Based on the in-vitro and pre-clinical data, SGM-101 could be a suitable NIR agent that can be studied in IMI guided resections of lung nodules. Optimal patient benefit is dependent on selecting the ideal candidates based on pre-operative data given the variable CEACAM5 expression. We envision two groups of patients in whom SGM-101 could be evaluated. One group of patients are those with advanced stage colorectal malignancies who develop pulmonary metastases in the setting of adequate primary disease control. CEACAM5 expression is noted to be >90% in colorectal malignancies and the presence of the glycoprotein is routinely evaluated after index operation[7]. Secondly this group is routinely followed up with serum CEA/CEACAM5 levels and serum positivity of the glycoprotein in our preclinical models is strongly associated with SGM-101 fluorescence (Supplementary Figure 1). The surgeon in this setting would have prior knowledge on the glycoprotein expression in primary tumor and patterns of serum CEA levels to select patients that would benefit from SGM-101 guided resections. Future clinical studies should further assess this potential correlation of SGM-101 labeling and serum CEA/CEACAM5 levels. Fortunately, in patients with pulmonary metastases of colorectal adenocarcinoma, metastasectomy offers survival advantage. SGM-101 can potentially help assess localization, detection of occult disease, and margin positivity for wedge resections[4].
Second group of patients that would potentially benefit from SGM-101 resections are those with CEACAM5 expressing primary lung adenocarcinomas. CEACAM5 levels can be readily assessed via IHC on pre-operative biopsy samples and patients with high expression of the glycoprotein can be selected for IMI guided resections. While these hypotheses been explored in gastrointestinal malignancies both in pre-clinical and human trials, it has yet to be done for NSCLC. Additionally, while the literature reports vary greatly, preliminary analyses have found glycoprotein expression associated with inferior survival and oncologic outcomes, therefore, there remains a theoretical potential that SGM-101 fluorescence in these patients suggest an aggressive disease that should be managed with aggressive systemic therapy by medical oncologists[9,13,24,25].
The use of SGM-101 guided lung cancer resections would complement current surgical practice both in minimally invasive resections and open thoracotomies. There are various thoracoscopes such as Quest Spectrum (Quest, Olympus, Japan) which can excite and detect fluorescence emission at the range of SGM-101. These thoracoscopic instruments are inserted through standard chest wall port sites and integrate easily into standard operating room video equipment. The surgeon can then use the fluorescence detected on the screen to amend or complement their surgical decisions. Additionally, the surgeon has the ability to assess the nodule ex-vivo on back table using the exoscope attachment of the camera in order to assess margin distances prior to sending for pathologic evaluation. This would be a further area of exploration in early clinical trials.
There are multiple factors and limitations that need to be considered in our study. Although CEACAM5 targeting NIR tracer has shown considerable promise in-vitro and in small animal models, one must keep in mind that murine models and cell-culture analysis only crudely simulate lung cancer evolution in humans. Secondly, the degree of CEACAM5 expression in lung adenocarcinomas have not been fully explored as compared to gastrointestinal malignancies. The retrospective studies in Asia have demonstrated anywhere between 30–80% expression rates in lung adenocarcinomas but results in western population is confounding[10]. However, our study supports the notion that if the glycoprotein is expressed in the tumor, SGM-101 will likely produce NIR emission that can be visualized in real time and immunofluorescence microscopy. Additionally, highly specific tumor labeling by SGM-101 paves a potential pathway of assessing CEACAM5 as a target for treatment of glycoprotein positive lung cancers. Given that CEACAM5 is not expressed in normal lung parenchyma, it could be explored as an option in theranostics or antibody targeted intra-tumoral chemotherapeutic delivery. The presence of highly targetable antibody conjugates makes this hypothesis as an actionable area of study. Randomized clinical trials can potentially assess the viability of SGM-101 in lung cancer resections and assess its efficacy compared to current standards.
Conclusion
In summary, our study demonstrated that CEACAM5 surface glycoprotein targeting NIR tracer, SGM-101 binds to CEACAM5 expressing adenocarcinoma cells both in-vitro and xenograft models in a CEACAM5 dependent manner. Additionally, SGM-101 can identify macroscopic and microscopic margins which is a significant concern in thoracic oncology. Our study is one of the first studies in literature exploring CEACAM5 targeting in lung adenocarcinomas and paves a path for its validation in early phase clinical studies.
Supplementary Material
Acknowledgments:
Figures formatted and edited with BioRender
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
AP and FC are co-founders of SurgiMab. AP and FC are employees of SurgiMab.
REFERENCES
- [1].Lung Cancer Statistics | How Common is Lung Cancer n.d. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html (accessed August 15, 2020).
- [2].Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Translational Lung Cancer Research 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Azari F, Kennedy G, Bernstein E, Hadjipanayis CG, Vahrmeijer AL, Smith BL, et al. Intraoperative molecular imaging clinical trials: a review of 2020 conference proceedings. JBO 2021;26:050901. 10.1117/1.JBO.26.5.050901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Azari F, Kennedy G, Bernstein E, Hadjipanayis CG, Vahrmeijer AL, Smith BL, et al. Intraoperative molecular imaging clinical trials: a review of 2020 conference proceedings. Journal of Biomedical Optics 2021;26:050901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kennedy GT, Azari FS, Bernstein E, Marfatia I, Din A, Kucharczuk JC, et al. Targeted Intraoperative Molecular Imaging for Localizing Nonpalpable Tumors and Quantifying Resection Margin Distances. JAMA Surgery 2021. 10.1001/jamasurg.2021.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].De Jesus E, Keating JJ, Kularatne SA, Jiang J, Judy R, Predina J, et al. Comparison of Folate Receptor Targeted Optical Contrast Agents for Intraoperative Molecular Imaging. Int J Mol Imaging 2015;2015. 10.1155/2015/469047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Boogerd LSF, Hoogstins CES, Schaap DP, Kusters M, Handgraaf HJM, van der Valk MJM, et al. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: a dose-escalation pilot study. The Lancet Gastroenterology & Hepatology 2018;3:181–91. 10.1016/S2468-1253(17)30395-3. [DOI] [PubMed] [Google Scholar]
- [8].Chae YK, Choi WM, Bae WH, Anker J, Davis AA, Agte S, et al. Overexpression of adhesion molecules and barrier molecules is associated with differential infiltration of immune cells in non-small cell lung cancer. Scientific Reports 2018;8:1023. 10.1038/s41598-018-19454-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gao Y, Song P, Li H, Jia H, Zhang B. Elevated serum CEA levels are associated with the explosive progression of lung adenocarcinoma harboring EGFR mutations. BMC Cancer 2017;17:484. 10.1186/s12885-017-3474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012;76:138–43. 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- [11].Klaile E, Klassert TE, Scheffrahn I, Müller MM, Heinrich A, Heyl KA, et al. Carcinoembryonic antigen (CEA)-related cell adhesion molecules are co-expressed in the human lung and their expression can be modulated in bronchial epithelial cells by non-typable Haemophilus influenzae, Moraxella catarrhalis, TLR3, and type I and II interferons. Respir Res 2013;14:85. 10.1186/1465-9921-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang X, Han X, Zuo P, Zhang X, Xu H. CEACAM5 stimulates the progression of non-small-cell lung cancer by promoting cell proliferation and migration. J Int Med Res 2020;48:300060520959478. 10.1177/0300060520959478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maeda R, Suda T, Hachimaru A, Tochii D, Tochii S, Takagi Y. Clinical significance of preoperative carcinoembryonic antigen level in patients with clinical stage IA non-small cell lung cancer. Journal of Thoracic Disease 2017;9. 10.21037/jtd.2017.01.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de Valk KS, Deken MM, Schaap DP, Meijer RP, Boogerd LS, Hoogstins CE, et al. Dose-Finding Study of a CEA-Targeting Agent, SGM-101, for Intraoperative Fluorescence Imaging of Colorectal Cancer. Ann Surg Oncol 2020. 10.1245/s10434-020-09069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gutowski M, Framery B, Boonstra MC, Garambois V, Quenet F, Dumas K, et al. SGM-101: An innovative near-infrared dye-antibody conjugate that targets CEA for fluorescence-guided surgery. Surgical Oncology 2017;26:153–62. 10.1016/j.suronc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- [16].Muley T, Dienemann H, Herth FJF, Thomas M, Meister M, Schneider J. Combination of mesothelin and CEA significantly improves the differentiation between malignant pleural mesothelioma, benign asbestos disease, and lung cancer. J Thorac Oncol 2013;8:947–51. 10.1097/JTO.0b013e31828f696b. [DOI] [PubMed] [Google Scholar]
- [17].Soeth E, Wirth T, List HJ, Kumbhani S, Petersen A, Neumaier M, et al. Controlled ribozyme targeting demonstrates an antiapoptotic effect of carcinoembryonic antigen in HT29 colon cancer cells. Clin Cancer Res 2001;7:2022–30. [PubMed] [Google Scholar]
- [18].Cerami1 E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov 2012;2:401–4. 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shinkins B, Nicholson BD, Primrose J, Perera R, James T, Pugh S, et al. The diagnostic accuracy of a single CEA blood test in detecting colorectal cancer recurrence: Results from the FACS trial. PLoS One 2017;12:e0171810. 10.1371/journal.pone.0171810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Blumenthal RD, Leon E, Hansen HJ, Goldenberg DM. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer 2007;7:2. 10.1186/1471-2407-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhou X, Xie G, Wang S, Wang Y, Zhang K, Zheng S, et al. Potent and Specific Antitumor Effect for Colorectal Cancer by CEA and Rb Double Regulated Oncolytic Adenovirus Harboring ST13 Gene. PLOS ONE 2012;7:e47566. 10.1371/journal.pone.0047566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hoogstins CES, Boogerd LSF, Sibinga Mulder BG, Mieog JSD, Swijnenburg RJ, van de Velde CJH, et al. Image-Guided Surgery in Patients with Pancreatic Cancer: First Results of a Clinical Trial Using SGM-101, a Novel Carcinoembryonic Antigen-Targeting, Near-Infrared Fluorescent Agent. Ann Surg Oncol 2018;25:3350–7. 10.1245/s10434-018-6655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee GD, Kim DK, Jang SJ, Choi SH, Kim HR, Kim Y-H, et al. Significance of R1-resection at the bronchial margin after surgery for non-small-cell lung cancer. Eur J Cardiothorac Surg 2017;51:176–81. 10.1093/ejcts/ezw242. [DOI] [PubMed] [Google Scholar]
- [24].Shintani T, Matsuo Y, Iizuka Y, Mitsuyoshi T, Mizowaki T, Hiraoka M. Prognostic Significance of Serum CEA for Non-small Cell Lung Cancer Patients Receiving Stereotactic Body Radiotherapy. Anticancer Research 2017;37:5161–7. [DOI] [PubMed] [Google Scholar]
- [25].Azari F, Kennedy G, Zhang K, Bernstein E, Maki RG, Gaughan C, et al. Impact of Intraoperative Molecular Imaging after Fluorescent-Guided Pulmonary Metastasectomy for Sarcoma. Journal of the American College of Surgeons n.d.: 10.1097/XCS.0000000000000132. 10.1097/XCS.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


