Abstract
Nasopharyngeal Carcinoma (NPC) is one of the leading cancers in India’s north-eastern (NE) region affecting a section of the population each year. A proportion of the NPC cases are observed to recur even after therapy, indicating the involvement of other factors. We aimed to explore the NPC and Epstein-Barr virus (EBV) burden in the NE region and investigate the prognostic factors for the NPC patients’ poor survival and recurrence. NPC patients’ information was obtained from different state hospitals between 2014 and 2019. PCR and Sanger sequencing were performed to detect EBV types. Statistical analysis, including forest plot analysis, Kaplan-Mayer survival plot, Log-rank test, cox hazard regression, and Aalen’s additive regression model, were performed to determine prognostic factors for the NPC patients’ lower survival and recurrence. We observed an increased incidence of NPC and EBV infection in the past five years. Step-wise statistical analyses pointed out that variable such as non-professionals (B = 1.02, HR = 2.8, 95%CI = 1.5,4.9) workers (B = 0.92, HR = 2.5, 95%CI = 1.4,4.4), kitchen cum bedroom (B = 0.61, HR = 1.8, 95%CI = 1.2,2.8), mosquito repellent (B = 0.60, HR = 1.7, 95%CI = 1.1,2.7), nasal congestion (B = 0.60, HR = 1.8, 95%CI = 1.2,2.8), lower haemoglobin level (B = 0.92, HR = 2.5, 95%CI = 1.3,4.9), tumor stage IV (B = 2.8, HR = 1.8, 95%CI = 1.6,14.3), N2 (B = 1.4, HR = 4.0, 95%CI = 1.8,9.1), N3 (B = 1.9, HR = 6.4, 95%CI = 2.8,15.3), and M+ (B = 2.02, HR = 7.5, 95%CI = 4.1,13.7) revealed significant correlation with NPC patients’ poor prognosis (p < 0.05). The presence of viral factors also showed a significant association with NPC patients’ decreased survival. We concluded that factors related to day-to-day life with EBV infection could be the individual predictor for NPC incidence, lower survival, and disease recurrence.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13337-022-00789-5.
Keywords: Nasopharyngeal carcinoma, EBV, Risk factors, Survival, Public health, Survivorship
Introduction
70% of the global cancer deaths occur in lower and middle-income countries [1]. In 2008, 8% of overall cancer deaths were reported in India [2]. According to the population-based cancer registry report published by Cancer Registry Programme (NCRP) in 2012–2014, NE has the highest cancer incidence [3]. Survey of the Ministry of health and family welfare, Govt. of India, in 2014-15 showed additional factors that contributed to the increased incidence of cancer as well as other diseases [4]. Nasopharyngeal Carcinoma (NPC) is a growing public health issue in the Asian sub-continent, particularly in India. According to the report of GLOBOCAN in 2020, the highest number of NPC incidence (1,13,659) with five years prevalence (3,28,036) was observed in Asia. Among which, China (62,444 new cases with a cumulative risk of 0.32 and 5-year prevalence at all ages 1,86,908) ranked first, and India (5,697 new cases with a cumulative risk of 0.05 and 5-year prevalence at all ages 14, 196) was at the third position [5]. Reports of the national cancer registry program (2012–2016) published by NCDIR and ICMR in 2020 indicated that NPC is uniquely distributed in India’s north-eastern part than any other region, where males are more affected. Age-Adjusted Rates (AAR) in males were reported highest in East Khasi Hills district (78.5/100,000), followed by Kamrup urban (62.4/100,000), Meghalaya (58.4/100,000), Nagaland (46.3/100,000), and Aizawl district (45.6/100,000). The maximum AAR in females was cited in Papumpare district (21.7/100,000), followed by Kamrup urban (19.2/100,000), East Khasi Hills district (18.7/100,000), Meghalaya (16.6/100,000), and Cachar district (14.8/100,000) [6]. Thus, the morbidity and mortality rates linked with NPC are a significant concern in the north-eastern region.
NPC’s distribution depends on ethnic variation. Unique geography, racial, cultural, socio-economical variation, and different dietary habits in different regions are thought to be discrete NPC occurrences [7]. The North-East region is the homeland of a large number of tribes with various distinct religions. As a result, there exist considerable dissimilarity in culture and daily lifestyle practices [8]. This diversity is thought to increase NPC susceptibility to this region. The Ministry of Health and Family Welfare, Government of India, identified factors responsible for inadequate disease control and prevention in north-eastern India. These include lack of access to healthcare facilities due to remote, far-flung areas, poorly populated, a high level of tobacco consumption, absence of screening facilities and shortage of skilled human resources, and lack of public health experts in health care administration [9, 10]. Moreover, the significant association of Epstein-Barr virus (EBV) with NPC was investigated [11].
Among the anti-cancerous therapies, chemotherapy and radiation are the routine treatments for NPC in this region. However, about 20–30% of the NPC patients with the same or similar stages receiving similar treatment regimens showed local relapse or distal metastasis [12–14], indicating the involvement of factors such as genetic, epigenetic, day to day life styles and others, which could induce treatment failure. Everyday lifestyle-related factors accountable for NPC progression, survival, and recurrence in NE are not yet completely understood. This may be due to the incorporation of small study size, lack of evidence, improper screening, and inappropriate investigation strategies. Day-to-day lifestyle, viral infection, or clinicopathological factors could significantly impact NPC patients’ poor survival and disease recurrence. Other than a few reports, no publication is available for the last five years; thus, necessitating detailed evaluation of factors responsible for NPC patients’ poor survival is required to facilitate better cancer management and improved treatment regimens.
The present study aimed to explore NPC incidence and EBV burden in the last five years in India’s north-eastern region. Then we evaluated possible poor prognostic factors correlated with NPC patients’ lower survival and recurrence.
Materials and methods
Recruitment of the study population
Medical reports of 399 NPC patients were obtained from the cancer registry of different state hospitals in North-Eastern India between May 2014 and April 2019 on a half-yearly basis (Table S1). Clinical examination and NPC types were determined according to WHO guidelines as keratinizing squamous cell carcinoma (KSCC), non-keratinizing undifferentiated carcinoma (NKUC), and non-keratinizing differentiated carcinoma (NKDC). Patient information relating to demographic, dietary, addictions, cooking mode, use of mosquito repellent, and clinoco-pathological characteristics were screened. Forty-five cases containing incomplete information were removed, and 354 patients’ reports were included in the study (Table S2).
Clinical signs were recorded, including neck mass, nasal bleeding with headache, and congestion. Patients were examined before and post-treatment during the follow-up period. Examinations included detailed medical history, physical check-up, Nasopharyngioscopy, serum biochemistry, blood count, chest X-ray, CT or MRI examination of the nasopharynges, skull base, and any suspect metastatic sites (paranasal sinuses) [15, 16]. Treatment efficacy was evaluated according to WHO criteria. Generally, radical concurrent chemoradiation (CTRT) with neo-adjuvant/adjuvant chemotherapy was administered. The chemotherapy regimen was based on stage, age, nutritional status, and biochemical parameters. Post-treatment, each patient was monitored once every three months for up to two years. Six monthly reviews were done after two years until relapse or death. The follow-up period started from diagnosis until the last follow-up time (April 2019) or death. Patients were considered deceased when they failed to reach the endpoint; maybe they withdrew from the study or died during follow-up. 13% of the total NPC cases were found to have recurred. Informed and written consent was obtained from each patient for the study. The statement of STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) was followed in the current observational study [17].
Detection of EBV types
As the correlation of EBV is a well-known risk factor for the susceptibility of NPC [18], its role as an individual prognostic factor of NPC patients’ survival is needed to be investigated. Therefore, 130 NPC patients’ blood samples were obtained separately among 399 to detect EBV. Age, sex, and ethnicity matched disease-free healthy controls (n = 130) were selected (Table S3). Ethical approval was obtained from the participating institutes. Genomic DNA was isolated from all samples using a genomic DNA isolation kit as per the manufacturer’s instruction (Invitrogen, CA, US). The EBV and determination of EBV type were detected using the two-step PCR. After the initial PCR amplification of the EBV-associated nuclear antigen 2 gene (EBNA2), two separate PCR reactions were performed to amplify two distinctive regions of type1 and type 2 as described earlier [19]. Sanger sequencing was performed for confirmation of the variant(s). The online open-access tool ‘CLUSTALW’ (https://www.genome.jp/tools-bin/clustalw) was used to confirm the sequence similarity with the reference (Accession number V01555 for type1 and K03332 for type2) [19]. Odds Ratio (OR), 95% Confidence Interval (CI), and P-value were calculated using GraphPad Prism (version 6.0) to determine the association of EBV types with the mortality of NPC patients. The P-value of < 0.05 was considered significant.
Statistical analysis
Statistical analysis was carried out using RStudio (version 1.2.1335) and Graphpad Prism (version 6). The odds ratio (OR), 95% confidence interval (CI), and the p-value were calculated using an unconditional logistic regression model to identify risk factors associated with NPC patients’ lower survival. A nonparametric Kaplan–Meier survival analysis was conducted to estimate survival probability (S) from observed survival times (t), using the following formula:
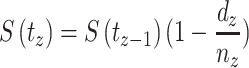 |
Where, 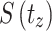 signified survival probability at the time
signified survival probability at the time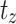 ,
, 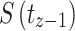 indicated the probability of being alive at
indicated the probability of being alive at 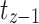 ,
,  designated the number of patients alive just before
designated the number of patients alive just before 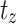 , and
, and 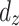 indicated the number of events at
indicated the number of events at  . At time 0, S (
. At time 0, S ( ) = 1 [20, 21]. A survival plot was generated measuring the median survival time. Further, survival curves of two or more groups were compared by calculating the chi-square statistic (χ2) on degrees of freedom for the test of equality and p-value using the log-rank (Mantel-Cox) test [22]. Finally, a semi-parametric Cox proportional model was conducted to assess the poor prognostic factors of NPC patients’ lower survival of the Log-rank test’s statistically significant covariates. This can be calculated as:
) = 1 [20, 21]. A survival plot was generated measuring the median survival time. Further, survival curves of two or more groups were compared by calculating the chi-square statistic (χ2) on degrees of freedom for the test of equality and p-value using the log-rank (Mantel-Cox) test [22]. Finally, a semi-parametric Cox proportional model was conducted to assess the poor prognostic factors of NPC patients’ lower survival of the Log-rank test’s statistically significant covariates. This can be calculated as:
 |
Where 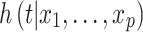 denoted the hazard function, determined by sets of p covariates (
denoted the hazard function, determined by sets of p covariates ( ),
), 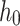 signified the baseline hazard, bi measure the effect size of i covariates (
signified the baseline hazard, bi measure the effect size of i covariates (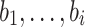 ), and
), and ) is called hazard ratio (HR) [23]. The regression coefficient (B) was calculated to identify the risk of survival; a positive value indicated the increased hazard (poor prognosis). HR and 95% CI was calculated to test the effect of covariates on the disease survival; a value of 1, > 1, and 0 indicated no effect, increased hazard, and reduced hazard. The wald statistics (z) and p-value determined the significance level. The proportional hazard assumption was tested using Schoen’s method [24]. The result was further justified by Aalen’s additive regression model, an alternative to the Cox regression model. It is a fully nonparametric linear model where the covariates are modeled as the additive risk to a baseline hazard and allowed to vary freely over time [25]. The slope was calculated to determine whether a specific covariate has a constant or a time-dependent effect. A positive slope results when increasing the covariate increases the hazard [26]. Finally, a risk prediction model in survival analysis was built using a random forest model by combining many survival trees [27].
) is called hazard ratio (HR) [23]. The regression coefficient (B) was calculated to identify the risk of survival; a positive value indicated the increased hazard (poor prognosis). HR and 95% CI was calculated to test the effect of covariates on the disease survival; a value of 1, > 1, and 0 indicated no effect, increased hazard, and reduced hazard. The wald statistics (z) and p-value determined the significance level. The proportional hazard assumption was tested using Schoen’s method [24]. The result was further justified by Aalen’s additive regression model, an alternative to the Cox regression model. It is a fully nonparametric linear model where the covariates are modeled as the additive risk to a baseline hazard and allowed to vary freely over time [25]. The slope was calculated to determine whether a specific covariate has a constant or a time-dependent effect. A positive slope results when increasing the covariate increases the hazard [26]. Finally, a risk prediction model in survival analysis was built using a random forest model by combining many survival trees [27].
Results
Incidence and distribution of NPC and EBV
Crude incidence rate (CIR) /100,000 population was measured. Results indicated a higher frequency of NPC occurrence from the middle of 2017 (11%, CIR = 0.24). NKUC frequency was highest, followed by KSCC and NKDC, respectively (NKUC: 57.6%, KSCC: 21.6%, and NKDC: 20.8%) (Fig. 1a and Table S1). The distribution of NPC types indicated that the Nagaland state displayed the highest NPC occurrence frequency (Nagaland: 29.3%, Manipur: 24.8%, Assam: 24.6%, and Mizoram: 21.3%). Nagaland also showed the highest prevalence of NKUC and NKDC (NKUC: 19.3%, NKDC: 29.3%); however, Manipur showed an increased incidence of KSCC (7.3%) (Fig. 1b and Table S4).
Fig. 1.
Incidence and distribution of NPC and EBV infection in NPC patients in NE of India. The overall scenario of NPC occurrence and their types between 2014 and 2019 is depicted in Fig. 1a. The state-wise distribution of NPC is represented in Fig. 1b. The detection of EBV in NPC samples is shown in Fig. 1c, where lane 2 and 3 indicated the presence of EBNA2; lane 4 to 6 stated type1 and lane 7 marked type 2 EBV; PCR product was run on 2% of agarose gel with 1 kb plus DNA ladder (Thermo Fisher, MA, US) as the marker (M) living lane 1 as the negative control. The distribution of EBV and their serotypes in different states is denoted in Fig. 1d
The detection and distributions of EBV types are presented in Fig. 1c, d, and Table 1. The aligned sequence of EBV types 1 and 2 with their reference is illustrated in Figure S1. Among the NPC cases and Healthy control, 60.8% and 10.8% were EBV positive. Among EBV types, type 1 variant was found higher than type 2 (Type1: NPC = 40.8%, control = 8.4%; Type 2: NPC = 0.8). No type 2 variant was detected in the control sample. Type1 and type2 dual positive variant with an increased frequency (NPC = 19.2%, control = 2.3%) was also observed. The higher incidence of EBV was observed in Nagaland (21.5%), followed by Assam (16.2%), Manipur (13.8%), and Mizoram states (9.2%). Nagaland also showed an increased incidence of type 1 (13.1%) and dual variant (7.7%) than other states.
Table 1.
Occurrence and distribution of EBV and their types in different states of NE, India
| Study (Case/ Control) (130/130) |
EBV (EBNA2 positive) | EBV Types | ||||||
|---|---|---|---|---|---|---|---|---|
| Type 1 | Type 2 | Type 1 & 2 dual positive | ||||||
| Case (%) | Control (%) | Case (%) | Control (%) | Case (%) | Control (%) | Case (%) | Control (%) | |
| Overall | 79 (60.8) | 14 (10.8) |
53 (40.8) |
11 (8.5) | 1 (0.8) | 0 | 25 (19.2) | 3 (2.3) |
| Assam | 21 (16.2) | 4 (3.1) | 14 (10.8) | 3 (2.3) | 00 | 00 | 7 (5.4) | 1(0.8) |
| Manipur | 18 (13.9) | 2 (1.5) | 13 (10.0) | 2 (1.5) | 00 | 00 | 5 (3.4) | 00 |
| Mizoram | 12.(9.2) | 1 (0.8) | 9 (6.9) | 1 (0.8) | 00 | 00 | 3 (2.3) | 00 |
| Nagaland | 28 (21.5) | 7 (5.4) | 17 (13.1) | 5 (3.9) | 1 (0.8) | 00 | 10 (7.7) | 2 (1.5) |
The frequency is represented by %
Factors associated with NPC patients’ lower survival
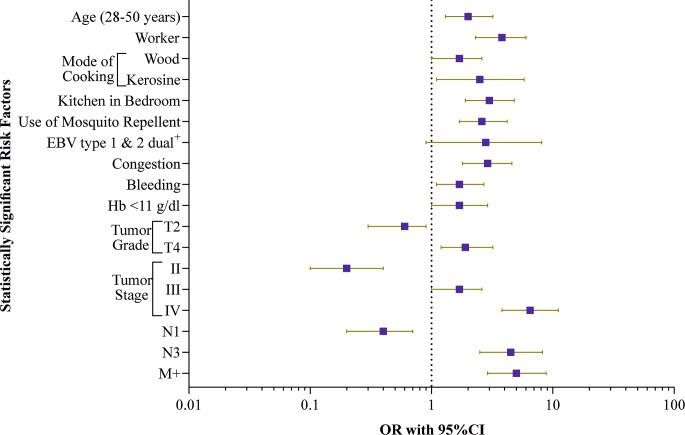
Factors associated with lower survival in patients are represented in Table S5. Demographic characteristics indicated that patients between the ages of 28 and 50 were associated with a risk of lower survival than other age groups (OR = 2.0, 95% CI = 1.3, 3.2, P = 0.002). Patients who belonged to the worker category, used wood or kerosene for cooking, had kitchen in the bedroom, and users of mosquito repellents were more likely to have lower survival risk [(worker: OR = 3.8, 95%CI = 2.3, 6.0, P = 0.0001), (wood: OR = 0.41, 95%CI = 0.25, 0.68, P = 0.0006), (kerosene: OR = 2.5, 95%CI = 1.1, 5.8, P = 0.03), (kitchen in the bedroom: OR = 3.0, 95%CI = 1.9, 4.8, P = 0.0001), (mosquito repellent: OR = 2.6, 95%CI = 1.7, 4.2, P = 0.0001)]. In clinicopathological category, patients with nasal bleeding and congestion showed a significant correlation with the risk of decreased survival [(Nasal bleeding: OR = 1.7, 95%CI = 1.1, 2.7, P = 0.02), (congestion: OR = 2.9, 95%CI = 1.8, 4.6, P = 0.0001)]. Patients who had lower haemoglobin level revealed a significant correlation with lower survival [OR = 1.7, 95%CI = 1.0, 2.9, P = 0.03]. In TNM staging, patients with tumor gradeT4, stage III, stage IV, N3, and M + indicated significantly lower survival [(gradeT4: OR = 1.9, 95% CI = 1.2, 3.2, P = 0.006), (stage III: OR = 1.7, 95% CI = 1.0, 2.6, P = 0.02), (stage IV: OR = 6.5, 95% CI = 3.8, 11.1, P = 0.0001), (N3: OR = 4.5, 95% CI = 2.5, 8.2, P = 0.0001), and M+: OR = 5.0, 95% CI = 2.9, 8.8, P = 0.0001)]. Among the EBV variants, type 1and 2 dual positives were associated with the risk of decreased survival of NPC patients (OR = 2.8, 95% CI = 0.9, 8.1, P = 0.05) (Table S6). Figure 2 illustrates statistically significant risk factors associated with decreased survival.
Fig. 2.
Forest plot analysis of the significant factors associated with the lower survival in NPC patients. The odds ratios (OR) are represented by the square, and the 95% CIs are denoted by horizontal lines
Factors responsible for NPC patients’ poor prognosis and recurrence
The result of the Kaplan–Meier survival curve is highlighted in Fig. 3. Results demonstrated that multiple factors like occupation, mode of cooking, kitchen in the bedroom, mosquito repellent uses, Nasal congestion, Nasal bleeding, low haemoglobin, tumor grade, tumor stage, N, M, and EBV were shown to be statistically significant for lower survival (as p < 0.05). The difference of survival between two groups were noticed when comparing two or more survival curve using the Log-Rank test (Table S7), which further rationalized the result of Kaplan–Meier analysis [(occupation: χ2 = 37·7, p < 0.001), (mode of cooking: χ2 = 12.9, p = 0.005), (kitchen in bedroom: χ2 = 26.9, p < 0.001), (mosquito repellent: χ2 = 14.7, p < 0.001), (Nasal congestion: χ2 = 22.1, p < 0.001), (Nasal bleeding: χ2 = 4.1, p = 0.04), (low haemoglobin: χ2 = 42·4, p = 0.04), (tumor grade: χ2 = 29·3, p < 0.001), (tumor stage: χ2 = 104·2, p < 0.001), (N: χ2 = 91·9, p < 0.001), (M: χ2 = 226·9, p < 0.001), (EBV: χ2 = 11.2, p < 0.001)]. The proportion surviving at five years were also estimated for each group which showed that NPC patient who belonged to the worker category (45.95%), used kerosene for cooking (45.83%), had the kitchen in the bedroom (49.07%), used mosquito repellent (56.76%), suffered from nasal congestion (51.52%), nasal bleeding (58.26%), had lower haemoglobin level (52.3%), under tumor grade T4 (55.32%), stage IV (33.73%), N3 (19.30%) and M+ (7.25%) revealed proportionally lower survival at five years. Patients infected with both type 1 and 2 EBV (45.45%) also depicted the same.
Fig. 3.
Kaplan-Mayer survival plot of statistically significant risk factors associated with NPC. Plot (a) represents occupation; (b) Mode of cooking; (c) Kitchen in the bedroom; (d) Use of Mosquito Repellent; (e) Congestion; (f) Bleeding; (g) Haemoglobin; (h) Tumor Grade; (i) Tumor stage; (j) The number of nearby lymph nodes that have cancer; (k). Metastasized NPC; and (l). EBV
Cox regression backward stepwise analysis for the predictors of mortality is demonstrated in table S8 and Fig. 4. Result indicated that factors like non-professional, worker, kitchen in bedroom, mosquito repellents, nasal congestion, lower haemoglobin are individual predictors of poor survival [(non-professional: B = 1.02, HR = 2.8, 95% CI = 1.5, 4.9, p < 0.001), (worker: B = 0.92, HR = 2.5, 95% CI = 1.4, 4.4, p = 0.002), (kitchen in bedroom: B = 0.61, HR = 1.8, 95% CI = 1.2,2.8 p = 0.004), (mosquito repellents: B = 0.60, HR = 1.7, 95% CI = 1.1, 2.7, p = 0.01), (nasal congestion: B = 0.60, HR = 1.8, 95% CI = 1.2, 2.8, p = 0.008), and (lower haemoglobin: B = 0.92, HR = 2.5, 95% CI = 1.3, 4.9, p = 0.007)]. In TNM staging, tumor stage II-IV, N2-4, and M + are shown to be the poor prognostic factors for NPC patients survival [(stage II: B = 2.2, HR = 9.1, 95% CI = 1.1, 45.7, p = 0.04), (stage III: B = 2.83, HR = 1.9, 95% CI = 0.7, 14.8, p = 0.01), (stage IV: B = 2.8, HR = 1.8, 95% CI = 1.6, 14.3, p = 0.01), (N2: B = 1.4, HR = 4.0, 95% CI = 1.8, 9.1, p = 0.001), (N3: B = 1.9, HR = 6.4, 95% CI = 2.8, 15.3, p < 0.001), (N4: B = 1.9, HR = 6.7, 95% CI = 1.9, 22.9, p = 0.003), and (M+: B = 2.02, HR = 7.5, 95% CI = 4.1, 13.7, p < 0.001)]. The global p value was found to be significant (p < 0.05) for the concordance (0.91), likelihood ratio test (305 on 23 degrees of freedom), wald test (183.9 on 23 degrees of freedom), and score log-rank statistics (349.1 on 23 degrees of freedom).
Fig. 4.
Cox regression analysis of the risk factor associated with the poor survival of NPC patients. The Hazard Ratios (HR) are represented by the square, and the 95% CIs are denoted by horizontal lines
The result of Aalen’s additive regression model is depicted in table S9 and Fig. 5. Result demonstrated that patients who belonged to the non-professional and worker category, had kitchen in bedroom, used mosquito repellents, suffered from nasal congestion and lower haemoglobin showed positive slope with statistically significant p-value [(non-professional: slope = 0.004, p = 0.01), (worker: slope = 0.004, p = 0.03), (kitchen in bedroom: slope = 0.003, p = 0.01), (mosquito repellents: slope = 0.004, p = 0.03), (nasal congestion: slope = 0.001, p = 0.03)]. The similar result was observed in NPC patients under tumor stage IV, N2-3, and M+ [(stage IV: slope = 0.003, p = 0.02), (N2: slope = 0.005, p = 0.05), (N2: slope = 0.01, p = 0.01), and (M+: slope = 0.052, p < 0.001)]. Finally, a risk prediction model was developed by calculating the global Harrell’s c-index of 0.87, which is similar to the concordance index, indicating a shorter time to disease. The overall prediction error was also measured to be 0.13, showing that the predictor correctly ranks two random individuals in terms of survival. The result of the random forest model is illustrated in figure S2.
Fig. 5.
Aalen’s additive regression model
Discussion
Despite the current treatment regimens, many NPC cases have recurred. The recurrence rate, including local and regional, can vary from 10 to 60% depending on the ethnic variation with high incidence area, where more local relapsed cases are noted than regional [13, 14, 28]. Early evidence reported that even after complete treatment, some patients manifested regional recurrence of NPC with an eight-year disease-free survival [29], which ascends concern about cancer detection, management, choice of therapy, and global public health. Moreover, the overall scenario has raised questions about the role of external factors linked to the day-to-day life for NPC’s poor prognosis and disease recurrence. Risk factors associated with increased NPC and other cancers have been examined [30]. Several reports showed that compromise factors for NPC prognosis were also investigated in India [30–36]. However, failure to identify NPC risk may be due to the small sample size, shortfalls in the appropriate screening of patients, or absence of precise statistical evaluation made the previous findings vague to elucidate the accurate picture of cancer incidence, survival, risk, etc. comprehensively. In view of these, efforts were made to carry out a retrospective study with large sample size and accurate statistical analysis to investigate NPC incidence and identify factors directly associated with the poor NPC prognosis.
The present research has indicated a different occurrence and distribution pattern of NPC throughout NE states, with an increased mortality rate among middle-aged patients (28–50 years). Nagaland state showed the most significant NPC proportion in the last five years than any other state in the region. NPC types were also distributed differently; mostly non-keratinizing types were observed in Nagaland and keratinizing types in Assam. The National Family Health survey declared Mizoram as the highest tobacco-consuming state. A global adult tobacco survey in 2017 showed increasing tobacco use in Assam, Tripura, and Manipur states [9, 37]. Thus, the increased frequency of NPC in Nagaland pointed out other risk factors additional to tobacco use.
Nagaland state also ranked higher in the occurrence of type 1 EBV and the co-infection of both types (type 1 and 2). This observation is consistent with the previous reports, which suggested that the non-keratinizing undifferentiated type is the most common in the Asian continent, and communities are prone to EBV infections [33, 38, 39]. The first report on EBV variant detection in India’s NPC patients was published in 1999 [40], which showed a higher occurrence of type 1 EBV than type 2 in 40 NPC patients. A similar pattern was followed in our previous report [41]. As these involved a small study population, it did not provide the accuracy to the population mean. However, it laid the foundation for further exploration to detect type-specific linkages in India. According to another report, the transformation potential of the Type 1 variant was more excellent than type 2. They further showed the enhanced transformation potential of type 1 in the presence of recombinant type 2 [42]. In contrast to the early report, we have noted an increased type 2 infection that was elevated further in the presence of type 1, suggesting that EBV viral co-infection and the disease evolution could be linked.
The assessment of prognostic factors by Kaplan Meier and Lag- rank test revealed that occupation (including non-professionals, workers), kitchen cum bedroom, mode of cooking, mosquito repellent, nasal congestion, nasal bleeding, and low haemoglobin level and TNM staging were significantly linked to the NPC patients’ poor survival. Additionally, EBV Co-infection was found to be a crucial parameter that lowered NPC patients’ survival probabilities. Cox regression analysis disclosed that non-professionals and workers are the individual predictors for NPC patients’ mortality in occupation. This report is in sync with earlier reports which suggested that occupation is firmly related to severe cancer prognosis due to exposure to risk factors like chemicals or other carcinogens, especially for workers [43]. The kitchen in the bedroom and mosquito repellent was the harmful prognostic factor for NPC patients’ decreased survival. Ethnicity-based research in China indicated that indoor kitchen usage increased lung cancer risk [44]. Inadequate ventilation in the kitchen and the burning of household inhalants could enhance the risk. Another report from the same ethnicity showed that mosquito repellent use did not affect NPC prognosis [45]. As the distribution of NPC is ethnicity-specific, it is likely that the intake of the fumes of the burning chemical compounds in mosquito repellent makes it carcinogenic and could promote the risk of NPCs. We found that nasal congestion is a poor prognostic factor of NPC. Infection, inadequate inhalation of tobacco smoke, burning fuels, or smoke of mosquito repellent may induce inflammation and blockage in the nasal passage, thus causing NPC development [7, 44–46]. Earlier NPC and other cancer reports proposed a correlation between low haemoglobin and cancer susceptibility [47, 48]. We noticed that low haemoglobin was a bad prognostic factor for NPC patients’ mortality. Tumor stage II-IV; tumor spread to the nearby lymph nodes (N1-N4), and metastasized NPC (M+) was detected to be the NPC’s poor prognostic factors. Aalen’s additive regression model further eliminated the mode of cooking, nasal bleeding, tumor stage II-III, N1, and N4 from the poor prognostic factors list. We built a theoretical model from the overall study explaining the effect of environmental inducers and other factors on the NPC’s patients’ poor survival and recurrence (Fig. 6).
Fig. 6.
Theoretical model of multiple risk factor’s effect on NPC patients’ survival and recurrence
The current study presented a lucid picture of the NPC in India, variants of EBV infection, and factors correlated with NPC patients’ poor survival, which could set the goal and direction for further studies to develop a fair distribution of available human resources and efficient control programs. More importantly, it could improve and individualize therapy through better prescriptions in selecting and delivering treatment regimens. Though our current study is stable and more comprehensive than previous reports, some limitations exist. Firstly, information from all patients could not be obtained directly; thus, certain patients’ data were excluded. This exclusion might affect risk factor evaluation and statistical analyses. Secondly, ‘EBV infection’ could not be included in the multivariate analysis due to the low number of samples, which could lead to wider confidence intervals.
Conclusion
In the past five years, an increased incidence of NPC and EBV infection was noticed in India’s northeastern states. Day-to-day lifestyle and other factors were found to be associated with the NPC patients’ poor survival. Thus, it can be said that these factors are the individual predictors affecting NPC survivorship and influence disease recurrence. Furthermore, it could be used as risk factors/markers to predict outcomes/ disease prognosis and patients’ survival.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by Visva-Bharati University, West Bengal, India. We express sincere gratitude to the doctors and workers of Eden Hospital Dimapur, Nagaland, Dr. Bhubaneswar Borooah Cancer Institute, Guwahati, Assam; Cachar Cancer Hospital & Research Center, Silchar, Assam; Guwahati Medical College & Hospital, Guwahati, Assam; Civil Hospital, Aizawl, Mizoram and Regional Institute of Medical Sciences, Imphal. No funding was received for this project.
Authors’ contributions
Koustav Chatterjee: Conceptualization, Study design, Data Collection, analysis, literature search, data interpretation, figure and manuscript writing; Koushik Chakraborty, Asmaul Haque and Syamantak Mukherjee: Helped on Statistical analysis, literature search and figure drawing; Sudipta Chakrabarti and Sushil Kumar Sahu: Helped on study design; Sankar Deb Roy, Moatoshi Aier, Ashok Kumar Das, Nizara Baishya, R Ravi Kannan, Zoreng puii, Eric Zomawia, Y Indibar Singh, Sam Tserin, and Komri Riba: provided the NPC samples, patient’s information and helped on data collection; Nabanita Roy Chattopadhyay, Piyanki Das, Sudipa Mal, Nilanjana Das: assisted on literature search and data interpretation; Amol Suryawanshi and R Rajendra Reddy: Assisted on compiling the parent’s report; Arindom Chakraborty: Assisted on the statistical analysis; Bhabani Sankar Das, Sandeep Ghatak and Shanmugam Rajasubramaniamn: Helped on the data interpretation and manuscript writing; Tathagata Choudhuri: Supervised the work, assisted on the data interpretation and reviewed the manuscript.
Funding
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Conflicts of interest/Competing interests
We do not have any competing interests to disclose.
Ethics approval
Taken from Visva-Bharati and other participating institutes.
Consent to participate
Informed consent was taken from each patient.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA: a cancer journal for clinicians 68(6) (2018) 394–424.10.3322/caac.21492. [DOI] [PubMed]
- 2.Sharma JD, Kalit M, Nirmolia T, Saikia SP, Sharma A, Barman D. Cancer: scenario and relationship of different geographical areas of the globe with special reference to North East-India. Asian Pac J Cancer Prev. 2014;15(8):3721–9. doi: 10.7314/apjcp20141583721. [DOI] [PubMed] [Google Scholar]
- 3.Medical ICo. Three-Year Report ofPopulation Based Cancer Registries 2012–2014. NATIONAL CENTRE FOR DISEASE INFORMATICS AND RESEARCHNATIONAL CANCER REGISTRY PROGRAMME; 2016.
- 4.Ministry of H, M.o.W. Family Welfare Statistics Division, D. Child, Rural Health Statistics, Ministry of Women & Child Development (2014), https://wcd.nic.in/acts/rural-health-statistics-2014-15-ministry-health-and-family-welfare-statistics-division.
- 5.Cancer today. 2021. http://gco.iarc.fr/today/home.
- 6.Report of National Cancer Registry Programme. 2020, 2021. https://www.ncdirindia.org/All_Reports/Report_2020/.
- 7.Roy Chattopadhyay N, Das P, Chatterjee K, Choudhuri T. Higher incidence of nasopharyngeal carcinoma in some regions in the world confers for interplay between genetic factors and external stimuli. Drug Discov Ther. 2017;11(4):170–80. doi: 10.5582/ddt.2017.01030. [DOI] [PubMed] [Google Scholar]
- 8.Ali ANM, Das I. Tribal Situation in North East India, Stud. Tribes and Tribals. 2003;1:141–8. doi: 10.1080/0972639X.2003.11886492. [DOI] [Google Scholar]
- 9.Prakash A, Saxena A. Health in north-east region of India – The new focus of attention. Indian J Med Specialities. 2016;7:93–4. doi: 10.1016/j.injms.2016.09.013. [DOI] [Google Scholar]
- 10.Ngaihte P, Zomawia E, Kaushik I. Cancer in the NorthEast India: Where we are and what needs to be done?, 63(3) (2019) 251–253.10.4103/ijph.IJPH_323_18. [DOI] [PubMed]
- 11.El-Sharkawy A, Al Zaidan L, Malki A. Epstein–Barr Virus-Associated Malignancies: Roles of Viral Oncoproteins in Carcinogenesis, 8(265) (2018).10.3389/fonc.2018.00265. [DOI] [PMC free article] [PubMed]
- 12.Jiang W, Liu N, Chen XZ, Sun Y, Li B, Ren XY, Qin WF, Jiang N, Xu YF, Li YQ, Ren J, Cho WC, Yun JP, Zeng J, Liu LZ, Li L, Guo Y, Mai HQ, Zeng MS, Kang TB, Jia WH, Shao JY, Ma J. Genome-Wide Identification of a Methylation Gene Panel as a Prognostic Biomarker in Nasopharyngeal Carcinoma. Mol Cancer Ther. 2015;14(12):2864–73. doi: 10.1158/1535-7163.MCT-15-0260. [DOI] [PubMed] [Google Scholar]
- 13.Xu T, Tang J, Gu M, Liu L, Wei W, Yang H. Recurrent nasopharyngeal carcinoma: a clinical dilemma and challenge. Curr Oncol. 2013;20(5):e406. doi: 10.3747/co.20.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J-X, Lu T-X, Huang Y, Han F. Clinical Characteristics of Recurrent Nasopharyngeal Carcinoma in High-Incidence Area. Sci World J. 2012;2012:719754. doi: 10.1100/2012/719754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.P.D.Q.A.T E, Board, Nasopharyngeal Cancer Treatment (Adult) (PDQ®): Health Professional Version, PDQ Cancer Information Summaries, National Cancer Institute (US), Bethesda (MD), 2002.
- 16.Pastor M, Lopez Pousa A, Del Barco E, Perez Segura P, Astorga BG, Castelo B, Bonfill T, Martinez Trufero J, Grau JJ, Mesia R. SEOM clinical guideline in nasopharynx cancer (2017), Clinical & translational oncology: official. publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2018;20(1):84–8. doi: 10.1007/s12094-017-1777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int J Surg. 2014;12(12):1500–24. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Tsao SW, Tsang CM, Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. 2017;372(1732):20160270. doi: 10.1098/rstb.2016.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan R, White LR, Stefanoff CG, de Oliveira DE, Felisbino FE, Klumb CE, Bacchi CE, Seuanez HN, Zalcberg IR. Epstein-Barr virus (EBV) detection and typing by PCR: a contribution to diagnostic screening of EBV-positive Burkitt’s lymphoma. Diagn Pathol. 2006;1:17. doi: 10.1186/1746-1596-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goel MK, Khanna P, Kishore J. Understanding survival analysis: Kaplan-Meier estimate. Int J Ayurveda Res. 2010;1(4):274–8. doi: 10.4103/0974-7788.76794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P.J.J.o.t.A.S.A. Nonparametric Estimation from Incomplete Observations. 1958;53:457–81. [Google Scholar]
- 22.Riffenburgh RH, Gillen DL. 19 - Analysis of censored time-to-event data, in: R.H. Riffenburgh, D.L. Gillen, editors, Statistics in Medicine (Fourth Edition), Academic Press2020, pp. 477–490.
- 23.Goerdten J, Carrière I, Muniz-Terrera G. Comparison of Cox proportional hazards regression and generalized Cox regression models applied in dementia risk prediction. Alzheimers Dement (N Y) 2020;6(1):e12041–1. doi: 10.1002/trc2.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abeysekera WWM, Sooriyarachchi R. Use of Schoenfeld’s global test to test the proportional hazards assumption in the Cox proportional hazards model: An application to a clinical study, J Natl Sci Foundation Sri Lanka - J NATL SCI FOUND SRI LANKA 37 (2009).10.4038/jnsfsr.v37i1.456.
- 25.Comparison of Aalen’s Additive and Cox Proportional Hazards Models for Breast Cancer Survival: Analysis of Population-Based Data from British Columbia, Can %J Asian Pac J Cancer Prev, 12(11) (2011) 3113–6, http://journal.waocp.org/article_26022_cf9f540d3d028e18a4ddffe3561ac1be.pdf. [PubMed]
- 26.Bb_ar EJSM. Aalen’s Additive, Cox Proportional Hazards and the Cox-Aalen Model: Application to Kidney Transplant Data, 46 (2017) 469–476.
- 27.Mogensen UB, Ishwaran H, Gerds TA. Evaluating Random Forests for Survival Analysis using Prediction Error Curves. J Stat Softw. 2012;50(11):1–23. doi: 10.18637/jss.v050.i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perri F, Della Vittoria Scarpati G, Caponigro F, Ionna F, Longo F, Buonopane S, Muto P, Di Marzo M, Pisconti S, Solla R. Management of recurrent nasopharyngeal carcinoma: current perspectives. Onco Targets Ther. 2019;12:1583–91. doi: 10.2147/OTT.S188148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nozaki M, Matsumoto H, Takahashi M, Yoshida K, Inagaki M, Mitsuhashi N, Niibe H. Nasopharyngeal Cancer with Neck Recurrence at 8 Years and a Lung Metastasis at 15 Years After the First Definitive Radiotherapy: A Case Report. Jpn J Clin Oncol. 1998;28(11):702–4. doi: 10.1093/jjco/28.11.702. [DOI] [PubMed] [Google Scholar]
- 30.Kunheri B, Agarwal G, Sunil PS, Nair AR, Pushpaja KU. Nasopharyngeal carcinoma: Experience and treatment outcome with radical conformal radiotherapy from a tertiary care center in India. Indian J Cancer. 2017;54(3):502–7. doi: 10.4103/ijc.IJC_287_17. [DOI] [PubMed] [Google Scholar]
- 31.Radhakrishnan V, Kumar P, Totadri S, Ganesan P, Selvaluxmy G, Ganesan T, Dhanushkodi M, Sagar T. Pediatric nasopharyngeal carcinoma: Experience from a tertiary cancer center in India. Indian J Cancer. 2016;53(3):377–80. doi: 10.4103/0019-509X.200663. [DOI] [PubMed] [Google Scholar]
- 32.Haleshappa RA, Thanky AH, Kuntegowdanahalli L, Kanakasetty GB, Dasappa L, Jacob L. Epidemiology and outcomes of nasopharyngeal carcinoma: Experience from a regional cancer center in Southern India. South Asian J Cancer. 2017;6(3):122–4. doi: 10.4103/2278-330X.214578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma TD, Singh TT, Laishram RS, Sharma LD, Sunita AK, Imchen LT. Nasopharyngeal carcinoma–a clinico-pathological study in a regional cancer centre of north-eastern India. Asian Pac J Cancer Prev. 2011;12(6):1583–7. [PubMed] [Google Scholar]
- 34.Chelleng PK, Narain K, Das HK, Chetia M, Mahanta J. Risk factors for cancer nasopharynx: a case-control study from Nagaland, India. Natl Med J India. 2000;13(1):6–8. [PubMed] [Google Scholar]
- 35.Lourembam D, Singh A, Sharma T, Singh T, Singh T, Singh L. Evaluation of Risk Factors for Nasopharyngeal Carcinoma in a High-risk Area of India, the Northeastern Region. Asian Pac J cancer prevention: APJCP. 2015;16:4927–35. doi: 10.7314/APJCP.2015.16.12.4927. [DOI] [PubMed] [Google Scholar]
- 36.Wani S, Khan T, Wani S, Mir L, Lone M, Rasool M, Najmi A, Afroz F, Teli M, Khan N. Nasopharyngeal Carcinoma: A 15 Year Study with Respect to Clinicodemography and Survival Analysis, Indian J Otolaryngol Head Neck Surg 68 (2016).10.1007/s12070-016-1018-9. [DOI] [PMC free article] [PubMed]
- 37.Asthana S, Patil RS, Labani S. Tobacco-related cancers in India: A review of incidence reported from population-based cancer registries. Indian J Med Paediatr Oncol. 2016;37(3):152–7. doi: 10.4103/0971-5851.190357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei K, Xu Y, Liu J, Zhang W, Liang Z. No incidence trends and no change in pathological proportions of nasopharyngeal carcinoma in Zhongshan in 1970–2007. Asian Pac J Cancer Prev. 2010;11(6):1595–9. [PubMed] [Google Scholar]
- 39.McDermott AL, Dutt SN, Watkinson JC. The aetiology of nasopharyngeal carcinoma. Clin Otolaryngol Allied Sci. 2001;26(2):82–92. doi: 10.1046/j.1365-2273.2001.00449.x. [DOI] [PubMed] [Google Scholar]
- 40.Rathaur RG, Chitale AR, Banerjee K. Epstein-Barr virus in nasopharyngeal carcinoma in Indian patients. Indian J Cancer. 1999;36(2–4):80–90. [PubMed] [Google Scholar]
- 41.Roy Chattopadhyay N, Chakrabarti S, Chatterjee K, Deb Roy S, Kumar Sahu S, Reddy RR, Das P, Bijay Kanrar B, Kumar Das A, Tsering S, Puii Z, Zomawia E, Singh YI, Suryawanshi A, Choudhuri T. Histocompatibility locus antigens regions contribute to the ethnicity bias of Epstein-Barr virus-associated nasopharyngeal carcinoma in higher-incidence populations, 90(4) (2019) e12796.10.1111/sji.12796. [DOI] [PubMed]
- 42.Rickinson AB, Young LS, Rowe M. Influence of the Epstein-Barr virus nuclear antigen EBNA 2 on the growth phenotype of virus-transformed B cells. J Virol. 1987;61(5):1310–7. doi: 10.1128/jvi6151310-13171987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinar T, Occupation, Cancer Int J Hematol Oncol. 2012;22(3):202–10. doi: 10.4999/uhod.11069. [DOI] [Google Scholar]
- 44.Mu L, Liu L, Niu R, Zhao B, Shi J, Li Y, Swanson M, Scheider W, Su J, Chang SC, Yu S, Zhang ZF. Indoor air pollution and risk of lung cancer among Chinese female non-smokers. Cancer Causes Control. 2013;24(3):439–50. doi: 10.1007/s10552-012-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He YQ, Xue WQ, Shen GP, Tang LL, Zeng YX, Jia WH. Household inhalants exposure and nasopharyngeal carcinoma risk: a large-scale case-control study in Guangdong, China. BMC Cancer. 2015;15:1022. doi: 10.1186/s12885-015-2035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okekpa SI, RB SMNM, Mangantig E, Azmi NSA, Zahari SNS, Kaur G, Musa Y. Nasopharyngeal Carcinoma (NPC) Risk Factors: A Systematic Review and Meta-Analysis of the Association with Lifestyle, Diets, Socioeconomic and Sociodemographic in Asian Region. Asian Pac J Cancer Prev. 2019;20(11):3505–14. doi: 10.31557/APJCP.2019.20.11.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo S-S, Tang L-Q, Chen Q-Y, Zhang L, Liu L-T, Huang P-Y, Cao K-J, Guo L, Mo H-Y, Guo X, Hong M-H, Zeng M-S, Qian C-N, Mai H-Q. Is Hemoglobin Level in Patients with Nasopharyngeal Carcinoma Still a Significant Prognostic Factor in the Era of Intensity-Modulated Radiotherapy Technology? PLoS ONE. 2015;10(8):e0136033–3. doi: 10.1371/journal.pone.0136033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Topkan E, Ekici NY, Ozdemir Y, Besen AA, Yildirim BA, Mertsoylu H, Sezen D, Selek U. Baseline hemoglobin < 11.0 g/dL has stronger prognostic value than anemia status in nasopharynx cancers treated with chemoradiotherapy, 34(2) (2019) 139–147.10.1177/1724600818821688. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.