Abstract
Background
Poor sexual function is a widespread problem affecting about 40% of women and this may worsen their quality of life. Ashwagandha (Withania somnifera) an adaptogenic herb has been reported to improve sexual satisfaction, sleep, and quality of life in women.
Objective
The purpose of the study was to evaluate the efficacy and safety of standardized Ashwagandha root extract in improving sexual function in healthy females.
Methods
In this prospective, randomized, placebo-controlled study, 80 women between 18 and 50 years of age without any hormonal disturbances and having hypoactive sexual desire disorder (HSDD) with a Female Sexual Function Index (FSFI) score <26, or Female Sexual Distress Scale (FSDS) score >11 were randomized to receive either capsule containing standardized Ashwagandha root extract 300mg twice daily (n=40), or identical placebo (n=40) for eight weeks. Sexual function was assessed using FSFI, FSDS, and Satisfying Sexual Encounters (SSEs). Assessments were done at baseline, four weeks, and eight weeks. Quality of life (QoL) was assessed using the general health questionnaire (GHQ-28) scale, and safety was assessed using clinical signs and symptoms. Repeat measures analysis of variance (ANOVA) was used for the assessment of treatment effect at different time periods. Nominal data were analyzed for differences using Fischer’s Chi-square test.
Results
There was statistically significant improvement (p<0.0001) in FSFI scores with Ashwagandha [14.20 (0.98) at baseline to 22.62 (2.06) at week 8] as compared to placebo [14.17 (0.71) at baseline to 19.25 (2.23) at eight weeks], and this improvement was observed in all sub-scales (desire, arousal, lubrication, orgasm, sexual satisfaction, and pain) of the FSFI scale. There was a greater improvement (p<0.0001) in FSDS scores with AG as compared to placebo. Although not statistically significant (p, 0.078), there was a greater reduction (improvement) in GHQ-28 scores at eight weeks with Ashwagandha as compared to placebo, and this trend was observed for all domains of GHQ-28 (global, physical, psychological, and social function). More women with Ashwagandha had improvement in SSEs at week 4 (p, 0.017) and week 8 (p, 0.002) as compared to placebo. Adverse events were comparable in the two groups. Two women reported nausea and one reported drowsiness with AG, whereas two reported nausea, one reported drowsiness and one reported nausea with drowsiness in the placebo group.
Conclusions
Oral administration of Ashwagandha 300mg twice daily administered for eight weeks improves the female sexual health in otherwise healthy women who do not have any hormonal disturbances. Ashwagandha is a known adaptogen, maintains general well-being and improves vitality.
Keywords: ghq-28, satisfying sexual encounters, fsds, fsfi, female sexual function, ashwagandha
Introduction
Sexual function is an essential component of life and poor sexual function may worsen the quality of life in women. Female sexual dysfunction (FSD) is a prevalent problem, affecting about 40% of women [1]. Women with sexual dysfunction may have female sexual arousal disorder (FSAD), Female Orgasmic Disorder (FOD), or Hypoactive Sexual Desire Disorder (HSDD). Some women may have combined genital and subjective arousal disorders or isolated genital sexual arousal disorders [2]. Several factors including medical and physical conditions like heart disease, diabetes, thyroid disease, neurological, and even simple fatigue could be the plausible causes for FSD [3]. In addition, psychological and emotional issues like depression, stress, and anxiety states could contribute significantly to FSD.
Ashwagandha (Withania somnifera [WS] fam. Solanaceae) commonly known as “Indian Winter cherry” is one of the most important herbs in Ayurveda (the traditional system of medicine in India) used for millennia as a Rasayana for its wide-ranging health benefits [4]. Ashwagandha is found across India and south Asia and is cultivated in the dry western parts of India. The important phytochemicals in Ashwagandha which are the active constituents responsible for the therapeutic efficacies of the plant are the withanolides which are steroidal lactones. Ashwagandha is considered an “adaptogen” as it protects the body from stress and helps the body to recover from the effects of stress. It reduces serum cortisol levels in patients with chronic stress, restores adrenal functions and normalizes the sympathetic nervous system [5]. Ashwagandha improves memory and promotes a healthy sexual and reproductive balance. It helps in improving cell-mediated immunity with its potent antioxidant properties. It is an adaptogenic botanical grown in India that is revered for its ability to balance, energize, rejuvenate, and revitalize [6]. The chemical profile of several extracts and formulations of WS has been well documented in previous studies, and the extracts contain a variety of phytochemicals that makes it a strong therapeutic agent [7]. WS has numerous physiological actions including anti-inflammatory, neuroprotective, adaptogen, cognitive-enhancing effects and normalizing of the sleep cycle. Extract of Ashwagandha root helps manage stress by reducing the stress hormone levels in the blood. This stress-relieving effect of Ashwagandha may have a direct contribution towards better sexual health. Ashwagandha can improve energy, stamina, and endurance. Fatigue is very often cited as an inhibiting factor in reduced sexual activity, and stress is associated with an increase in serum cortisol which can be associated with gonadal and sexual dysfunction [8]. Consumption of extract of Ashwagandha root may reduce FSD in women with androgen deficiency syndrome, which is seen as contributing factor towards a lack of sexual desire [9]. Also, animal studies have confirmed Ashwagandha's influence on sex hormone production, as demonstrated by its effects on luteinizing hormone, follicle-stimulating hormone, testosterone, and progesterone [10,11]. Thus, regular consumption of Ashwagandha has the potential to improve sexual function and improve the quality of life in women.
Materials and methods
Study design
This 8-week prospective, randomized, double-blind, placebo-controlled clinical study was conducted to evaluate the efficacy and safety of oral administration of standardized Ashwagandha root extract in improving sexual health in healthy women who do not have any hormonal disturbances.
The study was conducted in accordance with the Helsinki Declaration (1989 amendment) and the study protocol was approved by the Institutional Ethics Committee of DY Patil Medical College and Hospital, Navi Mumbai (Ref. #: DYP/IEC/2019-08-158, dated 12 December 2019). The Consolidated Standards of Reporting Trials (CONSORT) guidelines for designing and reporting controlled trials were followed in the conduct and reporting of this study. Written informed consent was obtained from all participants prior to the enrolment. Each participant was explained in detail about the study objective and the expected outcome before taking the consent.
Sample size
The sample size was based on the results of previously reported data for reduction in FSDS with eight weeks of ashwagandha. [9] A sample size of seven in each group provides 90% power to detect a difference of -5.6 for change in FSDS score after eight weeks between the AG and PL with a significance level (alpha) of 0.050 using a one-sided two-sample t-test. However, we planned to include 100 women in the study with 50 in each group.
Study participants
Married or unmarried healthy women between 18 and 50 years of age attending the study site were screened for study eligibility based on the study inclusion and exclusion criteria. Women having regular menstrual periods were inquired about any signs and symptoms suggestive of HSDD. Those who had HSDD as per the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV Sexual Desire Screening Questionnaire were included in the study. HSDD is defined as “Persistently or recurrently deficient (or absent) sexual fantasies and desire for sexual activity” along with “marked distress or interpersonal difficulty.” Women who had a Female Sexual Function Index (FSFI) total score of <26 and/or a Female Sexual Distress Scale (FSDS) score of >11 at screening were enrolled. All women and their partners were willing to try to have regular sexual intercourse activity (four or more attempts in four weeks) and were able and willing to maintain a diary of sexual events. Women were in a stable, monogamous, heterosexual relationship that was secure and communicative, for at least one year prior to the screening. Participating women's partner was expected to be physically present at their place of stay for a minimum of 50% of the study period. All couples used a medically acceptable method of contraception for at least three months prior to screening and continue to use that during the study period.
Women having any acute illness which may hamper the study participation, and those with any clinically significant medical history, medical finding or an on-going medical or psychiatric condition were excluded. Baseline serum hormones were estimated as a screening procedure in all women. Women with any deviation from the normal serum levels for estrogen, progesterone and testosterone were excluded from participation. Women with any other situation which in the opinion of the investigator could jeopardize their safety, impact the validity of the study results or interfere with study completion were excluded. Those who participated in other studies, including macro/micro/any other forms of dietary supplements/multivitamins or disease-specific oral nutrition supplements (for improving sexual function) during the three months prior to screening were not included. Women with a known history of hypersensitivity reactions, or a history of alcohol or substance abuse within the past year were excluded. Women who indicated that their sexual partner has inadequately treated sexual problems that could interfere with the response to treatment were also excluded. Those within the menopausal transition or menopause or who have had a hysterectomy were excluded.
Randomization and blinding
Enrolled women were randomly assigned to Ashwagandha (AG) or Placebo (PL) in a 1:1 randomization ratio. Randomization was performed using a computer-generated pre-determined randomization list (Rando version 1.2, Windows). The AG and PL products were manufactured and packed in identical containers and labeled equivalently to ensure blinding. The investigator received the randomization codes in separate envelopes for each study participant and was instructed to open the envelope only after assigning the study number to the eligible participant. An independent investigator who was blinded collected the outcome measures at each scheduled visit.
Interventions
Women in the AG group received a capsule containing standardized Ashwagandha root extract (Ixoreal Biomed, LA, CA) 300mg, whereas women in the PL group received a capsule identical to AG containing 300mg starch. Both treatments were administered orally two times daily with water for a period of eight weeks. The sexual partners of the participating women were not provided with any study-specific intervention.
Efficacy assessments
All women underwent a detailed medical and surgical history and were subjected to detailed clinical examination for any physical signs/symptoms, and vital parameters at baseline and all follow-up visits. The primary efficacy outcomes were the mean improvement in FSFI scores and FSDS scores from baseline to the end of four and eight weeks. Secondary efficacy outcomes were: i) mean change in the quality of life assessed using the GHQ-28 questionnaire from baseline to end of eight weeks; ii) improvement in the total sexual encounters and satisfying sexual encounter (SSE) computed as mean change in SSEs from baseline to end of four and eight weeks, and iii) proportion of patients reporting improvement in SSEs at four and eight weeks.
FSFI scale is a 19-item self-reported (patient) survey instrument developed by Rosen et al. [12] assessed the extent of sexual dysfunction in participating women. The instrument has sub-scores for different domains for desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI total score is a weighted sum of these. FSDS is a self-report 12-item survey instrument [13] that measures sexually related personal distress in women. The items correspond to different dimensions of sexual distress and were rated based on the occurrence during the previous 30 days. A record of all sexual activity was maintained to collect data for “total sexual encounters” and “satisfying sexual encounters.” The FSFI, FSDS and sexual activity records were recorded at baseline, at four weeks, and the study termination (eight weeks). The 28-item General Health Questionnaire (GHQ-28) was used to assess the quality of life [14]. The GHQ-28 has four sub-scales which include: i) physical symptoms (1-7), ii) anxiety symptoms (8-14), iii) social function (15-21), and iv) depression symptoms (22-28). Each sub-scale had seven items with each question scored on a Likert scale (0-3). The total scores range from 0 to 84, with lower scores indicating a better quality of life. The 28-question form had the advantage of being useful for all the members of the society [15].
Safety assessments
The safety outcomes were clinical safety recorded as the number and proportion of Treatment Emergent Adverse Events (TEAEs) and Treatment Emergent Serious Adverse Events (TESAE) during the study period.
Compliance assessment
Compliance was assessed using the tablet count, and the patient was considered compliant if more than 80% of the medication was consumed as per the protocol.
Statistical methods
The study sample size was determined based on the improvement in total FSFI scores reported by Dongre et al. [9]. The mean (SD) reduction in FSFI total scores at the end of eight weeks of treatment with Ashwagandha and placebo were 10.29 (1.75) and 6.43 (1.89), respectively [9]. Group sample sizes of seven (AG) and seven (PL) achieve 90% power to detect a score difference of 3.9 for FSFI between AG and PL at a significance level (alpha) of 0.050 using a two-sided two-sample t-test. However, we planned to include 80 women in the study with 40 in each of the two treatment groups.
All statistical computations were done on the windows-based program MedCalc® Statistical Software version 20.015 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021). Efficacy analysis was performed on the per-protocol (PP) data (n = 72), whereas safety analysis was performed on the intent-to-treat (ITT) data (n = 80). Summary statistics for all the parameters were performed and the results were presented as means with a 95% confidence interval (C.I.) for continuous data and counts with percentages for categorical data. Categorical and nominal data were analyzed for differences between the two groups using the chi-square test (Fisher’s exact test). For measurement data, repeat measures analysis of variance (ANOVA) was used for the assessment of treatment effect at different time periods, with Dunnett’s test for between group comparisons. All analyses were done using two-sided tests at alpha 0.05 (95% confidence levels), and a p-value of <0.05 was the cut-off for rejecting the null hypothesis.
Results
Demography and baseline data
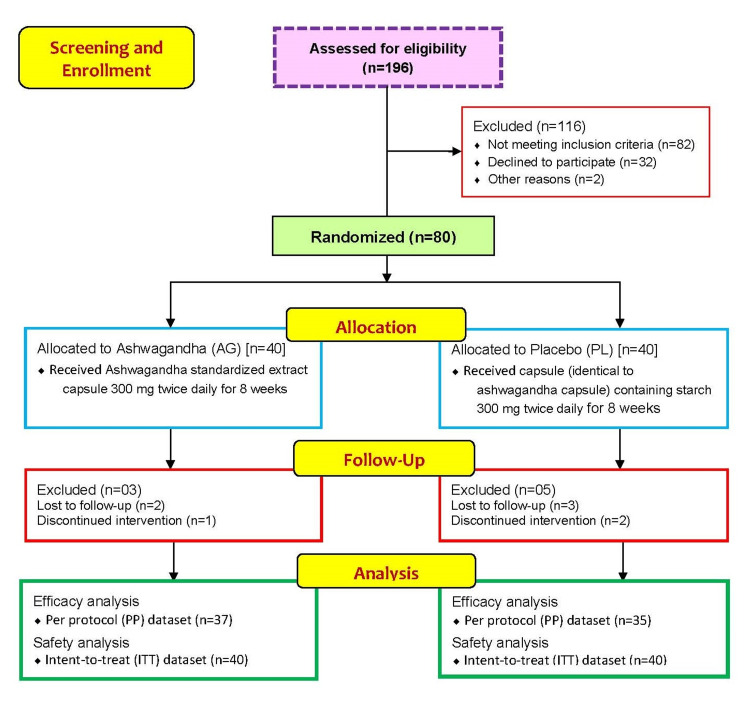
A total of 196 participants were screened and assessed for eligibility initially, of which, 116 failed the eligibility criteria (Figure 1). The remaining 80 participants underwent randomization and were allocated to receive either capsule containing Ashwagandha standardized root extract 300mg twice daily (n=40), or an identical placebo (n=40) in a 1:1 ratio. Three participants from the Ashwagandha group and five from the placebo group were excluded due to follow-up loss or failure of medication adherence. Compliance was poor in one patient from AG and two patients from the PL group, and data of these women was excluded from efficacy analyses. Poor compliance was not due to adverse events. The final efficacy analyses were done on the per-protocol (PP) dataset of seventy-two women (37 in AG and 35 in the PL group), whereas safety analyses were done on the intent-to-treat (ITT) dataset of all eighty women.
Figure 1. CONSORT flow representation of the patient enrollment, allocation, follow-up and analysis for this study.
CONSORT: Consolidated Standards Of Reporting Trials
Table 1 shows the demography and baseline data for patients enrolled and randomized (ITT dataset). The two groups were comparable at baseline with respect to the demography and baseline scores.
Table 1. Demography and baseline data for all parameters in the ITT dataset.
SBP: systolic blood pressure; DBP: diastolic blood pressure; FSFI: female sexual function index; FSDS: female sexual distress scale; GHQ-28: general health questionnaire-28; ITT: intent-to-treat; CI: confidence intervals
| ITT dataset | Ashwagandha (AG, n=40) | Placebo (PL, n=40) | t-test | Mean Diff. | 95% C.I. for diff. | |||
| Mean | SD | Mean | SD | ‘t’ | ‘p’ | |||
| Age (yrs.) | 29.10 | 5.69 | 29.85 | 6.15 | -0.566 | 0.573 | -0.75 | -3.39 to 1.89 |
| Height (cm) | 147.10 | 7.96 | 148.58 | 9.04 | -0.775 | 0.441 | -1.47 | -5.27 to 2.32 |
| Weight (Kg) | 69.83 | 9.25 | 68.85 | 11.04 | 0.428 | 0.670 | 0.98 | -3.56 to 5.51 |
| BMI (Kg/sq.m) | 32.33 | 4.22 | 31.26 | 4.77 | 1.059 | 0.293 | 1.07 | -0.94 to 3.07 |
| SBP (mm Hg) | 119.35 | 11.25 | 117.00 | 15.06 | 0.791 | 0.432 | 2.35 | -3.57 to 8.27 |
| DBP (mm Hg) | 77.30 | 4.83 | 76.50 | 8.34 | 0.525 | 0.601 | 0.80 | -2.23 to 3.83 |
| Pulse rate (/min.) | 72.05 | 2.29 | 70.70 | 2.24 | 2.665 | 0.009 | 1.35 | 0.34 to 2.36 |
| Temperature (0F) | 98.19 | 0.11 | 98.19 | 0.17 | -0.233 | 0.817 | -0.01 | -0.07 to 0.06 |
| Respiratory rate (per min.) | 17.05 | 1.50 | 17.35 | 1.59 | -0.866 | 0.389 | -0.30 | -0.99 to 0.39 |
| FSFI scale | ||||||||
| Desire | 2.04 | 0.57 | 2.08 | 0.54 | -0.282 | 0.779 | -0.04 | -0.28 to 0.21 |
| Arousal | 2.36 | 0.38 | 2.29 | 0.30 | 0.916 | 0.362 | 0.07 | -0.08 to 0.22 |
| Lubrication | 2.45 | 0.49 | 2.40 | 0.40 | 0.450 | 0.654 | 0.05 | -0.15 to 0.24 |
| Orgasm | 1.92 | 0.39 | 1.93 | 0.36 | -0.118 | 0.906 | -0.01 | -0.18 to 0.16 |
| Satisfaction | 2.44 | 0.45 | 2.40 | 0.38 | 0.453 | 0.652 | 0.04 | -0.14 to 0.23 |
| Pain | 3.05 | 0.49 | 3.06 | 0.53 | -0.066 | 0.948 | -0.01 | -0.23 to 0.22 |
| Total FSFI Score | 14.26 | 1.02 | 14.16 | 0.73 | 0.530 | 0.598 | 0.10 | -0.29 to 0.50 |
| FSDS | ||||||||
| FSDS score | 17.55 | 3.40 | 17.53 | 3.07 | 0.733 | 0.466 | 0.10 | -0.17 to 0.37 |
| Sexual encounters | ||||||||
| Total encounters | 4.40 | 1.71 | 4.33 | 2.08 | 0.176 | 0.861 | 0.08 | -0.77 to 0.92 |
| Successful encounters | 1.75 | 0.90 | 1.98 | 1.25 | -0.924 | 0.358 | -0.23 | -0.71 to 0.26 |
| GHQ-28 scale | ||||||||
| Total score | 52.95 | 11.96 | 52.03 | 10.80 | 0.363 | 0.718 | 0.93 | -4.15 to 6.00 |
| Physical score | 14.05 | 2.89 | 12.28 | 3.34 | 2.540 | 0.013 | 1.78 | 0.38 to 3.17 |
| Anxiety score | 12.25 | 4.11 | 11.58 | 3.85 | 0.758 | 0.451 | 0.68 | -1.10 to 2.45 |
| Social function score | 13.75 | 3.15 | 14.10 | 2.59 | -0.543 | 0.589 | -0.35 | -1.63 to 0.93 |
| Depression score | 12.90 | 3.40 | 14.08 | 2.63 | -1.727 | 0.088 | -1.18 | -2.53 to 0.18 |
Female sexual function index (FSFI)
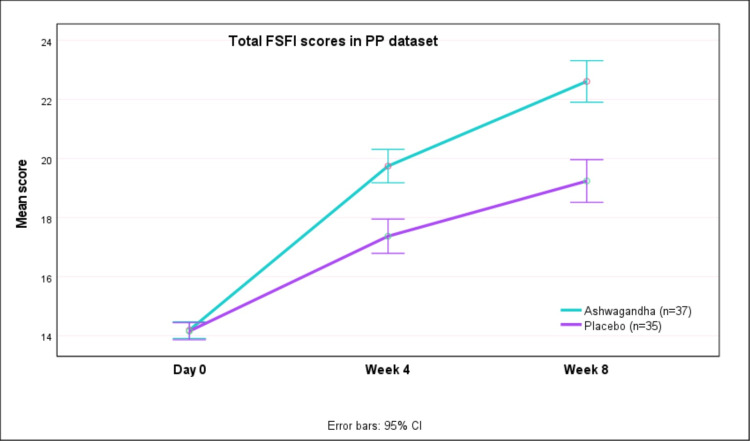
Table 2 presents the FSFI (total and sub-scale) scores at baseline and the change in scores at week 4 and week 8 after therapy in the PP dataset. Greater improvements in FSFI scores were seen with AG as compared to PL (p<0.0001). The mean (SD) FSFI total scores increased from 14.20 (0.98) at baseline to 22.62 (2.06) at week 8 with AG, whereas with PL the scores increased from 14.17 (0.71) at baseline to 19.25 (2.23) after eight weeks (Figure 2). Thus, there was greater improvement in FSFI scores with AG as compared to placebo (p<0.0001). This improvement was observed in all sub-scales (desire, arousal, lubrication, orgasm, sexual satisfaction, and pain) of the FSFI scale.
Table 2. FSFI scale scores at baseline and during study period in PP dataset.
FSFI: female sexual function index; PP: per-protocol; CI: confidence interval
| Ashwagandha (AG, n=37) | Placebo (PL, n=35) | t-test | Mean Difference | 95% C.I. for diff. | Effect size (Cohen’s) | |||||
| Mean | SD | Mean | SD | t' | p' | ‘d' | 95% C.I. for ‘d’ | |||
| Desire sub-scale | ||||||||||
| Day 0 | 2.01 | 0.56 | 2.07 | 0.54 | -0.445 | 0.657 | -0.06 | -0.32 to 0.20 | -0.11 | -0.57 to 0.36 |
| Change at Week 4 | 0.68 | 0.54 | 0.55 | 0.59 | 0.954 | 0.344 | 0.13 | -0.14 to 0.39 | 0.22 | -0.24 to 0.69 |
| Change at Week 8 | 1.44 | 0.59 | 1.25 | 0.79 | 1.204 | 0.233 | 0.20 | -0.13 to 0.52 | 0.28 | -0.18 to 0.75 |
| Arousal sub-scale | ||||||||||
| Day 0 | 2.35 | 0.36 | 2.30 | 0.30 | 0.660 | 0.511 | 0.05 | -0.10 to 0.21 | 0.16 | -0.31 to 0.62 |
| Change at Week 4 | 0.57 | 0.38 | 0.36 | 0.23 | 2.832 | 0.006 | 0.21 | 0.06 to 0.36 | 0.67 | 0.19 to 1.14 |
| Change at Week 8 | 0.94 | 0.48 | 0.34 | 0.51 | 5.236 | <0.0001 | 0.61 | 0.38 to 0.84 | 1.23 | 0.73 to 1.74 |
| Lubrication sub-scale | ||||||||||
| Day 0 | 2.46 | 0.50 | 2.43 | 0.42 | 0.206 | 0.838 | 0.02 | -0.20 to 0.24 | 0.05 | -0.41 to 0.51 |
| Change at Week 4 | 0.96 | 0.53 | 0.54 | 0.58 | 3.210 | 0.002 | 0.42 | 0.16 to 0.68 | 0.76 | 0.28 to 1.23 |
| Change at Week 8 | 1.20 | 0.61 | 0.69 | 0.50 | 3.892 | <0.0001 | 0.51 | 0.25 to 0.77 | 0.92 | 0.43 to 1.40 |
| Orgasm sub-scale | ||||||||||
| Day 0 | 1.90 | 0.36 | 1.95 | 0.36 | -0.668 | 0.507 | -0.06 | -0.23 to 0.11 | -0.16 | -0.62 to 0.31 |
| Change at Week 4 | 1.37 | 0.68 | 0.77 | 0.49 | 4.236 | <0.0001 | 0.60 | 0.32 to 0.88 | 1.00 | 0.50 to 1.49 |
| Change at Week 8 | 1.44 | 0.63 | 0.87 | 0.46 | 4.371 | <0.0001 | 0.57 | 0.31 to 0.84 | 1.03 | 0.53 to 1.52 |
| Satisfaction sub-scale | ||||||||||
| Day 0 | 2.44 | 0.47 | 2.39 | 0.38 | 0.462 | 0.645 | 0.05 | -0.15 to 0.25 | 0.11 | -0.35 to 0.57 |
| Change at Week 4 | 0.78 | 0.60 | 0.27 | 0.50 | 3.953 | <0.0001 | 0.52 | 0.26 to 0.78 | 0.93 | 0.44 to 1.42 |
| Change at Week 8 | 1.24 | 0.71 | 0.30 | 0.68 | 5.778 | <0.0001 | 0.95 | 0.62 to 1.27 | 1.36 | 0.84 to 1.87 |
| Pain sub-scale | ||||||||||
| Day 0 | 3.05 | 0.48 | 3.02 | 0.53 | 0.240 | 0.811 | 0.03 | -0.21 to 0.27 | 0.06 | -0.41 to 0.52 |
| Change at Week 4 | 1.19 | 0.83 | 0.73 | 0.80 | 2.448 | 0.017 | 0.47 | 0.09 to 0.85 | 0.58 | 0.10 to 1.05 |
| Change at Week 8 | 2.15 | 0.82 | 1.65 | 0.76 | 2.691 | 0.009 | 0.50 | 0.13 to 0.87 | 0.63 | 0.16 to 1.11 |
| Total FSFI Score | ||||||||||
| Day 0 | 14.20 | 0.98 | 14.17 | 0.71 | 0.169 | 0.866 | 0.03 | -0.37 to 0.44 | 0.04 | -0.42 to 0.50 |
| Change at Week 4 | 5.55 | 1.90 | 3.21 | 1.76 | 5.404 | <0.0001 | 2.34 | 1.48 to 3.20 | 1.27 | 0.76 to 1.78 |
| Change at Week 8 | 8.42 | 1.96 | 5.08 | 2.05 | 7.061 | <0.0001 | 3.34 | 2.39 to 4.28 | 1.67 | 1.12 to 2.20 |
Figure 2. Total FSFI scores on day 0, week 4 and week 8 in PP dataset.
FSFI: female sexual function index; PP: per-protocol; CI: confidence interval
FSDS and SSEs
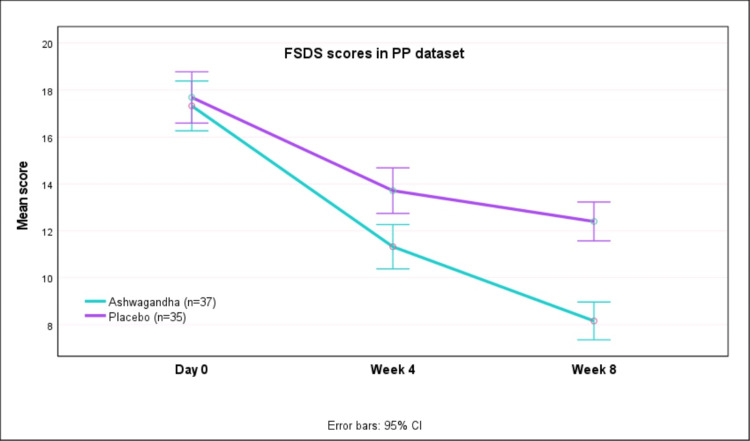
Table 3 presents the FSDS and sexual encounters at baseline and after week 4/week 8 of therapy in the PP dataset. Greater reductions in FSDS scores were seen with AG as compared to PL (p<0.0001). Also, the mean number of SSEs increased significantly with AG as compared to PL (p<0.0001). The mean (SD) total FSDS scores decreased from 17.32 (3.38) at baseline to 8.16 (2.36) at week 8 with AG, whereas with PL the scores decreased from 17.69 (3.08) at baseline to 12.40 (2.55) after eight weeks (Figure 3). Thus, there was greater improvement in FSDS scores with AG as compared to PL (p<0.0001).
Table 3. FSDS and number of sexual encounters at baseline and during study period in PP dataset.
FSDS: female sexual distress scale; PP: per-protocol; CI: confidence interval
| Ashwagandha (AG, n=37) | Placebo (PL, n=35) | t-test | Mean Difference | 95% C.I. for diff. | Effect size (Cohen’s) | |||||
| Mean | SD | Mean | SD | t' | p' | ‘d' | 95% C.I. for ‘d’ | |||
| FSDS scale | ||||||||||
| Day 0 | 17.32 | 3.38 | 17.69 | 3.08 | -0.473 | 0.638 | -0.36 | -1.89 to 1.16 | -0.11 | -0.57 to 0.35 |
| Change at Week 4 | -6.00 | 3.11 | -3.97 | 2.73 | -2.936 | 0.004 | -2.03 | -3.41 to -0.65 | -0.69 | -1.17 to -0.21 |
| Change at Week 8 | -9.16 | 2.91 | -5.29 | 3.21 | -5.370 | <0.0001 | -3.88 | -5.32 to -2.44 | -1.27 | -1.77 to -0.75 |
| Total sexual encounters | ||||||||||
| Day 0 | 4.41 | 1.77 | 4.17 | 2.04 | 0.521 | 0.604 | 0.23 | -0.66 to 1.13 | 0.12 | -0.34 to 0.59 |
| Change at Week 4 | 0.78 | 1.18 | 0.49 | 1.80 | 0.833 | 0.407 | 0.30 | -0.42 to 1.01 | 0.197 | -0.27 to 0.66 |
| Change at Week 8 | 0.81 | 1.78 | 0.06 | 2.11 | 1.641 | 0.105 | 0.75 | -0.16 to 1.67 | 0.387 | -0.08 to 0.85 |
| Successful encounters | ||||||||||
| Day 0 | 1.76 | 0.93 | 1.89 | 1.21 | -0.510 | 0.611 | -0.13 | -0.63 to 0.37 | -0.12 | -0.58 to 0.34 |
| Change at Week 4 | 1.70 | 0.97 | 1.03 | 1.27 | 2.540 | 0.013 | 0.67 | 0.14 to 1.20 | 0.599 | 0.12 to 1.07 |
| Change at Week 8 | 2.32 | 1.08 | 1.11 | 1.08 | 4.752 | <0.0001 | 1.21 | 0.70 to 1.72 | 1.120 | 0.62 to 1.61 |
Figure 3. FSDS scores on day 0, week 4 and week 8 in PP dataset.
FSDS: female sexual distress scale; PP: per-protocol; CI: confidence interval
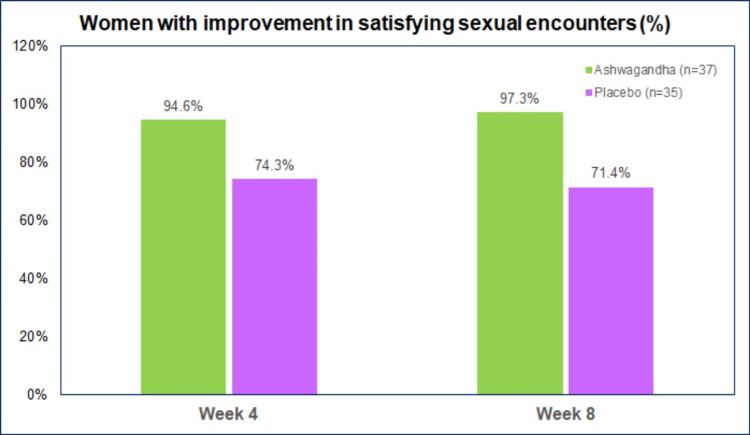
There was an improvement in the number of women reporting “satisfying sexual encounters” with both AG and PL (Figure 4). However, more women with Ashwagandha had improvement in SSEs than placebo both at week 4 (p, 0.017) and week 8 (p, 0.002). A decrease in SSEs was observed in two (5.4%) women on AG and seven (20.0%) on PL after four weeks, and three (8.6%) women on PL after eight weeks. There were no women with decreased SSEs after eight weeks.
Figure 4. Women with improvement in SSEs at week 4 and week 8.
SSE: satisfying sexual encounters
General health questionnaire (GHQ-28)
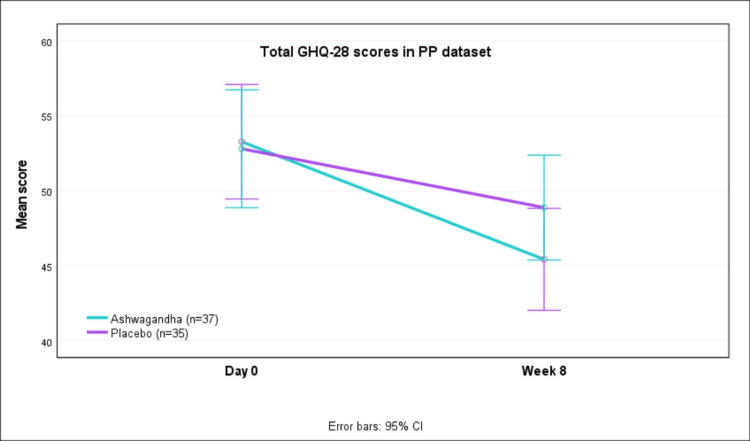
Table 4 presents the GHQ-28 scores at baseline and after week 8 in the PP datasA greaterater reduction in scores was seen with AG as compared to PL (p<0.05). The mean (SD) GHQ-28 scores decreased from 53.27 (12.25) at baseline to 45.43 (11.90) at week 8 with AG, whereas with PL the scores decreased from 52.80 (10.94) at baseline to 48.89 (8.43) after eight weeks (Figure 5). Although not statistically significant (p, 0.078), there was a greater reduction (improvement) in GHQ-28 scores with AG as compared to PL. This trend was seen for all domains of GHQ-28 (global, physical, psychological and social function).
Table 4. GHQ-28 scores at baseline and during study period in PP dataset.
GHQ-28: general health questionnaire-28; PP: per-protocol; CI: confidence interval
| Ashwagandha (AG, n=37) | Placebo (PL, n=35) | t-test | Mean Difference | Effect size (Cohen’s) | ||||||
| Mean | SD | Mean | SD | t' | p' | 95% C.I. for diff. | ‘d' | 95% C.I. for ‘d’ | ||
| Total GHQ-28 score | ||||||||||
| Day 0 | 53.27 | 12.25 | 52.80 | 10.94 | 0.171 | 0.864 | 0.47 | -5.00 to 5.94 | 0.04 | -0.42 to 0.50 |
| Change at Week 8 | -7.84 | 8.74 | -3.91 | 9.87 | -1.789 | 0.078 | -3.92 | -8.30 to 0.45 | -0.42 | -0.89 to 0.05 |
| Physical score | ||||||||||
| Day 0 | 14.14 | 2.93 | 12.60 | 3.37 | 2.067 | 0.042 | 1.54 | 0.05 to 3.02 | 0.49 | 0.02 to 0.95 |
| Change at Week 8 | -1.78 | 3.00 | -0.74 | 3.04 | -1.461 | 0.148 | -1.04 | -2.46 to 0.38 | -0.34 | -0.81 to 0.12 |
| Anxiety score | ||||||||||
| Day 0 | 12.35 | 4.18 | 11.77 | 3.93 | 0.606 | 0.546 | 0.58 | -1.33 to 2.49 | 0.14 | -0.32 to 0.61 |
| Change at Week 8 | -2.95 | 3.83 | -1.54 | 5.77 | -1.222 | 0.226 | -1.40 | -3.69 to 0.89 | -0.29 | -0.75 to 0.18 |
| Social function score | ||||||||||
| Day 0 | 13.92 | 3.17 | 14.29 | 2.61 | -0.535 | 0.594 | -0.37 | -1.73 to 1.00 | -0.13 | -0.59 to 0.34 |
| Change at Week 8 | -1.76 | 3.38 | -0.83 | 3.02 | -1.226 | 0.224 | -0.93 | -2.44 to 0.58 | -0.29 | -0.75 to 0.18 |
| Depression score | ||||||||||
| Day 0 | 12.86 | 3.54 | 14.14 | 2.59 | -1.741 | 0.086 | -1.28 | -2.74 to 0.19 | -0.41 | -0.88 to 0.06 |
| Change at Week 8 | -1.35 | 3.15 | -0.80 | 3.35 | -0.720 | 0.474 | -0.55 | -2.08 to 0.98 | -0.17 | -0.63 to 0.29 |
Figure 5. GHQ-28 total scores at day 0 and week 8 in PP dataset.
GHQ-28: general health questionnaire-28; PP: per=protocol; CI: confidence interval
Adverse events
Three women from each AG (7.5%) and PL (7.5%) group reported adverse events. Nausea was reported by two (5.0%) women from each AG and PL group, while drowsiness was reported by two (5.0%) women from the AG group and one (1.5%) from the PL group. One (1.5%) woman receiving AG reported both nausea and drowsiness. All events were of mild severity, were not associated with the study treatments, and were self-limiting.
Vital parameters
Table 5 presents the vital parameters at baseline (day 0) and after week 4 and week 8. No significant changes were observed in the vital parameters in the two groups (p>0.05).
Table 5. Vital parameters at baseline and during study period in PP dataset.
SBP: systolic blood pressure; DBP: diastolic blood pressure; PP: per-protocol; CI: confidence interval; 0F: degree fahrenheit
| Ashwagandha (AG, n=37) | Placebo (PL, n=35) | t-test | Mean Difference | 95% C.I. for diff. | Effect size (Cohen’s) | |||||
| Mean | SD | Mean | SD | t' | p' | ‘d' | 95% C.I. for ‘d’ | |||
| SBP (mm Hg) | ||||||||||
| Day 0 | 118.75 | 11.178 | 116.28 | 15.734 | 0.771 | 0.443 | 2.47 | -3.92 to 8.86 | 0.18 | -0.28 to 0.64 |
| Week 4 | 118.27 | 9.106 | 117.14 | 9.570 | 0.512 | 0.610 | 1.13 | -3.26 to 5.52 | 0.12 | -0.34 to 0.58 |
| Week 8 | 117.73 | 8.00 | 116.00 | 7.35 | 0.953 | 0.344 | 1.73 | -1.89 to 5.35 | 0.22 | -0.24 to 0.69 |
| DBP (mm Hg) | ||||||||||
| Day 0 | 77.29 | 4.90 | 76.00 | 7.746 | 0.854 | 0.396 | 1.30 | -1.73 to 4.33 | 0.20 | -0.26 to 0.66 |
| Week 4 | 76.81 | 7.46 | 75.42 | 7.413 | 0.788 | 0.433 | 1.38 | -2.12 to 4.88 | 0.19 | -0.28 to 0.65 |
| Week 8 | 76.16 | 4.76 | 74.57 | 6.10 | 1.235 | 0.221 | 1.59 | -0.98 to 4.16 | 0.29 | -0.17 to 0.75 |
| Pulse rate (per min.) | ||||||||||
| Day 0 | 72.05 | 2.37 | 70.45 | 2.17 | 2.968 | 0.004 | 1.60 | 0.52 to 2.67 | 0.70 | 0.22 to 1.17 |
| Week 4 | 72.70 | 1.83 | 72.08 | 1.77 | 1.448 | 0.152 | 0.62 | -0.23 to 1.47 | 0.34 | -0.13 to 0.81 |
| Week 8 | 72.54 | 2.60 | 72.51 | 1.83 | 0.049 | 0.961 | 0.03 | -1.04 to 1.09 | 0.01 | -0.45 to 0.47 |
| Temperature (0F) | ||||||||||
| Day 0 | 98.19 | 0.11 | 98.18 | 0.16 | 0.269 | 0.789 | 0.01 | -0.06 to 0.08 | 0.06 | -0.40 to 0.53 |
| Week 4 | 98.25 | 0.13 | 98.23 | 0.21 | 0.468 | 0.642 | 0.02 | -0.06 to 0.10 | 0.11 | -0.35 to 0.57 |
| Week 8 | 98.24 | 0.13 | 98.23 | 0.10 | 0.508 | 0.613 | 0.01 | -0.04 to 0.07 | 0.12 | -0.34 to 0.58 |
| Respiratory rate (per min.) | ||||||||||
| Day 0 | 17.13 | 1.53 | 17.25 | 1.46 | -0.345 | 0.731 | -0.12 | -0.83 to 0.58 | -0.08 | -0.54 to 0.38 |
| Week 4 | 18.54 | 1.46 | 18.00 | 1.18 | 1.714 | 0.091 | 0.54 | -0.09 to 1.17 | 0.40 | -0.06 to 0.87 |
| Week 8 | 18.54 | 1.86 | 17.88 | 1.60 | 1.593 | 0.116 | 0.65 | -0.16 to 1.47 | 0.38 | -0.09 to 0.84 |
Discussion
FSD is a multifaceted disorder, comprising anatomical, psychological, physiological, as well as social-interpersonal components. With several existing definitions for FSD, the most descriptive definition encompasses FSD as the persistent/recurring decrease in sexual desire or arousal, the difficulty/inability to achieve an orgasm, and/or the feeling of pain during sexual intercourse [16]. Currently, there are limited pharmacological options available for the treatment of FSD, which include psychoactive drugs (flibanserin and bremelanotide), transdermal testosterone, intravaginal dehydroepiandrosterone (DHEA), local estrogen therapy (LET) and ospemifene [17]. Ospemifene is a third-generation Selective Estrogen Receptor Modulator (SERM) reported to be useful in women with vulvovaginal atrophy, painful intercourse, vaginal dryness, and vulvar vestibular symptoms [18]. The newer agents aim at increasing blood flow to the genitals, improving androgen deficiencies and enhancing central nervous system stimulation. However, most of these pose safety issues [16]. Flibanserin, which is approved by the United States Food and Drug Administration (USFDA) for the treatment of low sexual desire in premenopausal women, causes potentially serious side effects like hypotension, sleepiness, nausea, fatigue, dizziness, and fainting, particularly if the drug is mixed with alcohol [16]. Another agent bremelanotide which is administered as an injection causes nausea, vomiting, flushing, headache, and local reaction at the injection site [16]. Estrogen therapy may lead to heart and blood vessel disease and cancer [19].
Sexual dysfunction is a frequent problem in women during pregnancy and counseling about healthy sexual function during pregnancy is required for most couples [20]. Women who underwent an operative vaginal delivery (n=132) had poor sexual function (lower orgasm scores, p<0.05) after six months compared to those with cesarean section delivery (n=92) and spontaneous vaginal delivery (n=45). Also, breastfeeding women were reported to have poor sexual function [21]. Poor sexual function is more common in women with rheumatoid arthritis (RA) and psoriatic arthritis (PsA), compared to healthy women [22], and female patients with RA and PsA should be screened for sexual dysfunction. Findings of a cross-sectional analysis of 3433 (2487 sexually active) women presenting for menopause or sexual health consult were reported from the Data Registry on Experiences of Aging, Menopause and Sexuality (DREAMS) by King et al. [23]. Of these, 75% had poor sleep quality, and 54% met the criteria for female sexual dysfunction. As reported by King et al., multivariable analysis shows that women with poor sleep quality were 1.48 times more likely to report female sexual dysfunction (95% C.I. 1.21 to 1.80, p < 0.001), and women with <5 hours sleep time had 63.3% prevalence of sexual dysfunction (p = 0.004). The authors also report that sexually active women were more likely to report good sleep quality compared with sexually inactive women (25.3% vs 20.5%, p = 0.003). The authors concluded that good sleep quality was linked to sexual activity. Hence, improving sleep quality can be helpful to improve sexual function.
Due to the complexity of FSD, a multifaceted approach, addressing neurobiological, vasoactive, hormonal as well as psychosocial/cultural aspects would be more comprehensive and would address the needs and concerns of the women that suffer from this disorder [24]. Ashwagandha has a member potential to be useful in improving female sexual function due to being an “adaptogen.” which can regulate body metabolic functions in individuals with physical or mental stress [25].
We compared the clinical efficacy and safety of Ashwagandha root extract with an identical placebo in women for improvement of female sexual function. The study product used by us was KSM-66® Ashwagandha capsule (300 mg) administered twice daily orally with water for a period of eight weeks. Ashwagandha has been reported to improve sexual function in women in a randomized, double-blind, placebo-controlled study, where an aqueous extract of Ashwagandha root was used [9]. In this pilot study of 50 patients randomized to AG (n=25) and PL (n=25), there was a greater improvement (reduction) in FSDS scores with AG. In our study, we observed similar findings as reported in this study. The study by Dongre et al. observed improvements in serum testosterone. We did not measure testosterone levels since none of the women participating in our study had any hormonal abnormalities at baseline. We estimated a sample size of 14 required to test a superiority hypothesis that AG is better than PL for improvement in FSFI. However, we included 80 women in our study to have a substantial sample. Our results indicate improvement in female sexual function with both placebo and Ashwagandha. However, there were significantly greater improvements with Ashwagandha over placebo. Thus, these findings suggest a potentially useful role of Ashwagandha in improving sexual health in otherwise healthy women. Ashwagandha roots extract is reported to be safe for human use with a daily dosage of up to 1,000 mg [26,27].
Strengths and limitations of the study
A robust double-blind, placebo-controlled design is the major strength of the study. A major limitation of our study is the well-defined cohort with a homogeneous subject group coming from a specific cross-section of society. Although this design provides greater statistical discrimination power and lower p values for any observed effect size, there is a concern regarding the generalization of these results across the entire population. Future research in a real-world scenario is needed with participants from a wider range of demographics, occupations, and socio-economic statuses. Also, a shorter study duration of eight weeks could prevent any derivations for the long-term benefits.
Conclusions
This study evaluated the efficacy and safety of Ashwagandha standardized root extract in improving sexual health in otherwise healthy women aged 18 to 50 years having HSDD. Significant improvements in the FSFI scores and FSDS scores were observed with Ashwagandha. Oral administration of standardized Ashwagandha root extract for eight weeks improves female sexual health in otherwise healthy women who do not have any hormonal disturbances.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Institutional Ethics Committee, D.Y. Patil Medical College & Hospital, Navi Mumbai issued approval DYP/IEC/2019-08-158
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Female sexual dysfunction: therapeutic options and experimental challenges. Allahdadi KJ, Tostes RC, Webb RC. Cardiovasc Hematol Agents Med Chem. 2009;7:260–269. doi: 10.2174/187152509789541882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Female sexual dysfunction: anatomy, physiology, evaluation and treatment options. Berman JR, Berman LA, Werbin TJ, Goldstein I. Curr Opin Urol. 1999;9:563–568. doi: 10.1097/00042307-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Fordyce K. Sexual Problems in Women. [ May; 2022 ]. 2022. https://www.webmd.com/sexual-conditions/sexual-dysfunction-women https://www.webmd.com/sexual-conditions/sexual-dysfunction-women
- 4.An overview on ashwagandha: a rasayana (rejuvenator) of ayurveda. Singh N, Bhalla M, de Jager P, Gilca M. Afr J Tradit Complement Altern Med. 2011;8:208–213. doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Chandrasekhar K, Kapoor J, Anishetty S. Indian J Psychol Med. 2012;34:255–262. doi: 10.4103/0253-7176.106022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Immune enhancing effects of WB365, a novel combination of Ashwagandha (Withania somnifera) and Maitake (Grifola frondosa) extracts. Vetvicka V, Vetvickova J. N Am J Med Sci. 2011;3:320–324. doi: 10.4297/najms.2011.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.High catechin concentrations detected in Withania somnifera (ashwagandha) by high performance liquid chromatography analysis. Alam N, Hossain M, Khalil MI, Moniruzzaman M, Sulaiman SA, Gan SH. BMC Complement Altern Med. 2011;11:65. doi: 10.1186/1472-6882-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Withania somnifera improves semen quality in stress-related male fertility. Mahdi AA, Shukla KK, Ahmad MK, Rajender S, Shankhwar SN, Singh V, Dalela D. Evid Based Complement Alternat Med. 2011;2011:1–9. doi: 10.1093/ecam/nep138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efficacy and safety of ashwagandha (Withania somnifera) root extract in improving sexual function in women: a pilot study. Dongre S, Langade D, Bhattacharyya S. Biomed Res Int. 2015;2015:284154. doi: 10.1155/2015/284154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Effect of Withania somnifera (L.) Dunal on sex hormone and gonadotropin levels in addicted male rats. Rahmati B, Ghosian Moghaddam MH, Khalili M, Enayati E, Maleki M, Rezaeei S. Int J Fertil Steril. 2016;10:239–244. doi: 10.22074/ijfs.2016.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belal N. World J Dairy Food Sci. Vol. 7. 7; 2012. Effect of dietary intake ashwagandha roots powder on the levels of sex hormones in the diabetic and non-diabetic male rats; pp. 160–166. [Google Scholar]
- 12.The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. Rosen R, Brown C, Heiman J, et al. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 13.The Female Sexual Distress Scale (FSDS): initial validation of a standardized scale for assessment of sexually related personal distress in women. Derogatis LR, Rosen R, Leiblum S, Burnett A, Heiman J. J Sex Marital Ther. 2002;28:317–330. doi: 10.1080/00926230290001448. [DOI] [PubMed] [Google Scholar]
- 14.General Health Questionnaire-28 (GHQ-28) Sterling M. J Physiother. 2011;57:259. doi: 10.1016/S1836-9553(11)70060-1. [DOI] [PubMed] [Google Scholar]
- 15.Comparison between the GHQ-28 and SF-36 (MH 1-5) for the assessment of the mental health in patients with ischaemic heart disease. Failde I, Ramos I, Fernandez-Palacín F. Eur J Epidemiol. 2000;16:311–316. doi: 10.1023/a:1007688525023. [DOI] [PubMed] [Google Scholar]
- 16.Women's sexual dysfunction: a pathophysiological review. Salonia A, Munarriz RM, Naspro R, et al. BJU Int. 2004;93:1156–1164. doi: 10.1111/j.1464-410X.2004.04796.x. [DOI] [PubMed] [Google Scholar]
- 17.Pharmacotherapy for female sexual dysfunctions (FSDs): What is on the market and where is this field heading? [PREPRINT] Nappi RE, Tiranini L, Cucinella L, et al. Expert Opin Pharmacother. 2022 doi: 10.1080/14656566.2022.2066997. [DOI] [PubMed] [Google Scholar]
- 18.Ospemifene efficacy and safety data in women with vulvovaginal atrophy. Pup LD, Sánchez-Borrego R. Gynecol Endocrinol. 2020;36:569–577. doi: 10.1080/09513590.2020.1757058. [DOI] [PubMed] [Google Scholar]
- 19.Female sexual dysfunction - diagnosis and treatment. [ May; 2022 ];https://www.mayoclinic.org/diseases-conditions/female-sexual-dysfunction/diagnosis-treatment/drc-20372556#:~:text=Flibanserin%20(Addyi).&text=A%20daily%20pill%2C%20Addyi%20may 2022 20372556:20. [Google Scholar]
- 20.Effect of pregnancy on sexual function of couples. Khalesi ZB, Bokaie M, Attari SM. Afr Health Sci. 2018;18:227–234. doi: 10.4314/ahs.v18i2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Impact of Mode of Delivery on Female Postpartum Sexual Functioning: Spontaneous Vaginal Delivery and Operative Vaginal Delivery vs. Cesarean Section. Barbara G, Pifarotti P, Facchin F, et al. J Sex Med. 2016;13:393–401. doi: 10.1016/j.jsxm.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Comparison of Female Sexual Function Index in patients with psoriatic and rheumatoid arthritis and healthy controls. Boone D, Ronson A, Karsh J. Musculoskeletal Care. 2019;17:226–230. doi: 10.1002/msc.1405. [DOI] [PubMed] [Google Scholar]
- 23.Associations of sleep and female sexual function: good sleep quality matters. Kling JM, Kapoor E, Mara K, Faubion SS. Menopause. 2021;28:619–625. doi: 10.1097/GME.0000000000001744. [DOI] [PubMed] [Google Scholar]
- 24.Clinical application of CNS-acting agents in FSD. Perelman MA. J Sex Med. 2007;4 Suppl 4:280–290. doi: 10.1111/j.1743-6109.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- 25.Increasing vitality with adaptogens: multifaceted herbs for treating physical and mental stress. Abascal K, Yarnell E. Altern Complement Ther. 2003;9:54–60. [Google Scholar]
- 26.Efficacy and tolerability of ashwagandha root extract in the elderly for improvement of general well-being and sleep: a prospective, randomized, double-blind, placebo-controlled study. Kelgane SB, Salve J, Sampara P, Debnath K. Cureus. 2020;12:0. doi: 10.7759/cureus.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical evaluation of the pharmacological impact of ashwagandha root extract on sleep in healthy volunteers and insomnia patients: a double-blind, randomized, parallel-group, placebo-controlled study. Langade D, Thakare V, Kanchi S, Kelgane S. J Ethnopharmacol. 2021;264:113276. doi: 10.1016/j.jep.2020.113276. [DOI] [PubMed] [Google Scholar]