Abstract
Background and Aims:
Postoperative pain after laparoscopic cholecystectomy is very common complication hindering the early return of routine activity. Since agonist opioids are not easily available, the most common drug used for intraoperative analgesia is intravenous butorphanol in our institute. The purpose of our study is to compare the analgesic effect of intraperitoneal butorphanol and nalbuphine as additives with ropivacaine in laparoscopic cholecystectomy for postoperative pain.
Setting and Design:
Randomized, double-blind prospective study undertaken after approval from the Institutional Ethics Committee.
Materials and Methods:
In this study, 90 patients undergoing laparoscopic cholecystectomy were randomly divided into three groups: group A received intraperitoneal ropivacaine 0.2% of 20 mL with butorphanol 2 mg; Group B received intraperitoneal ropivacaine 0.2% 20 mL with nalbuphine 10 mg; and Group C received intraperitoneal ropivacaine 0.2% 20 mL with 0.9% normal saline. The primary outcome was to compare the analgesic efficacy of butorphanol with nalbuphine and the duration of postoperative pain relief. The secondary outcomes included the comparison of hemodynamic parameters, frequency of rescue analgesia, and complications among the three groups.
Statistical Analysis:
The data analysis was carried out with ANOVA and Chi-square test using the SPSS software version 26.0.
Results:
The mean of the Numeric Rating Scale pain score was insignificant in Group A versus B at all-time intervals indicating similar efficacy of butorphanol and nalbuphine in terms of pain relief postoperatively. However, the time to first rescue analgesia was significantly higher in Group A (5.70 ± 3.57 h), followed by Group B (3.95 ± 2.06 h) and Group C (2.50 ± 1.24 h).
Conclusion:
Butorphanol is better analgesic than nalbuphine as postoperative pain-free period was relatively more with lesser complications.
Keywords: Analgesia, butorphanol, intraperitoneal, laparoscopic cholecystectomy, nalbuphine, ropivacaine
INTRODUCTION
The first laparoscopic cholecystectomy was performed in 1985.[1] It has emerged as the procedure of choice with advancements in technology and awareness among patients. Pain remains the most common complaint in the early postoperative period.[2] This pain is classified into three components: somatic (abdominal wall incision), visceral (surgical manipulation), and shoulder tip pain (residual CO2 in the peritoneal cavity).[2,3]
Various drugs have been studied using intraperitoneal instillation technique for postoperative pain relief.[4,5,6,7,8,9,10,11,12,13,14,15,16] In the present study, we chose butorphanol and nalbuphine using their equianalgesic doses as they belong to the same class of drug.[17] This study aimed to provide an impetus for further research, minimal pharmacological expenses, and better patient outcome.
MATERIALS AND METHODS
The present study was a randomized, parallel double-blind study, undertaken after approval from the Institutional Ethical Committee and CTRI registration number CTRI/2020/08/027342 dated August 24, 2020 in our institute. The study was conducted from September 2020 to December 2021. Our study follows the guidelines laid down in the Declaration in Helsinki. A total of 90 patients scheduled for elective laparoscopic cholecystectomy under general anesthesia of American Society of Anesthesiologists (ASA) physical Status I and II between the age group of 18 and 60 years were included in the study. Patients with acute cholecystitis, carcinoma gall bladder, hypersensitivity to any of the study drugs, pregnant females, bleeding disorders, opioid addiction, and refusal to participate in the study were excluded from the study. The sample size was determined by considering alpha error of 0.05 and power of study of 80%, the number needed to study was calculated to be 26 in each group and a total sample size of 78. Considering 8%–10% of dropout rate, the sample size was increased to 90, with 30 patients in each group. Computed-generated random numbers were obtained and sealed in an envelope. The slip was taken out by consultant on duty not involved in the study and drug was prepared according to the coded slip. The assessor and the patient both were blinded. Group A (n = 30): received 20 mL of ropivacaine 0.2% plus 1 mL of butorphanol 2 mg intraperitoneally, Group B (n = 30): received 20 mL of ropivacaine 0.2% plus 1 mL of nalbuphine 10 mg intraperitoneally, and Group C (n = 30): received 20 mL ropivacaine 0.2% plus 1 mL of 0.9% normal saline (NS) intraperitoneally.
An informed written consent was taken from all patients after explaining the details of the study being conducted. A detailed preanesthetic checkup and were explained the Numeric Rating Scale (NRS) using a 100-mm scale (0: no pain and 100: worst possible pain). Details pertaining to the patient's clinical history, general physical and systemic examination, and routine investigations such as complete hemogram, coagulation profile, random blood sugar, renal function tests, liver function tests, and viral markers were recorded.
After detailed preanesthetic checkup, a common conduct of anesthesia was followed in all patients. They were kept fasting for 6 h before surgery. Tablet alprazolam 0.5 mg was administered the night before and on the morning of surgery. The patient was shifted to operation theater, a peripheral intravascular access was obtained; baseline heart rate (HR), peripheral arterial oxygen saturation (SpO2), noninvasive blood pressure, systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP), respiratory rate (RR) and five-lead electrocardiogram were recorded. Glycopyrrolate 0.2 mg, midazolam 1 mg, and fentany l 2.0 μg.kg−1 were administered intravenously. Preoxygenation with 100% O2 for 3–5 min was done, followed by induction with intravenous (i.v.) propofol 2 mg.kg−1. After confirming that the patient is being ventilated on bag and mask, i.v., succinylcholine 2.0 mg.kg−1 was used to facilitate endotracheal intubation. Then, the patient was intubated with a cuffed endotracheal tube of appropriate size. Muscle relaxant, atracurium 0.5 mg.kg−1 was administered intravenously. HR, SpO2, SBP, DBP, MAP, RR, and end-tidal carbon dioxide (EtCO2) were recorded at 5 min interval for the first 20 min and thereafter at 10 min intervals throughout the surgery. Maintenance of anesthesia was done with isoflurane (1%–2%) along with O2 and N2O and volume control ventilation which was adjusted to maintain an EtCO2 between 30 and 40 mmHg. Muscle relaxation was maintained with one-fourth the loading dose of i.v. atracurium earlier administered. The residual effects of atracurium were reversed with injection of neostigmine 50 μg.kg−1 and glycopyrrolate 10 μg.kg−1 intravenously and with adequate respiratory efforts, patients were extubated and shifted to postanesthetic care unit (PACU), which was considered 0 h for postoperative pain.
Instillation technique
Intraperitoneal instillation of study drugs was done by the operating surgeon through laparoscopic ports guided by surgical camera after the removal of the gall bladder, onto the gall bladder fossa, under the diaphragm, on the liver bed and patient position was changed to 15°-20° Trendelenburg position for 10 min. Drain, if placed by the surgeon, was clamped for 30 min in the PACU.
Postoperative pain intensity was measured using NRS from 0 h to 24 h. The time duration of the first demand for analgesia, total analgesic consumption and side effects if any, were observed and recorded for 24 h postoperatively, i.v., diclofenac 75 mg in 100 mL of 0.9% NS was given as rescue analgesia if NRS ≥40. Side effects such as cardiovascular, respiratory, nausea, vomiting, and any other were recorded and managed accordingly.
Statistics
At the end of the study, the data were statistically analyzed using Statistical Package IBM SPSS (Statistical Package for the Social Sciences) Statistics for Windows, version 26.0. Armonk, NY, USA: IBM Corp. The quantitative data were expressed as mean and standard deviation. The qualitative data were presented in frequency and percentage. To find the analgesic efficacy between the groups, post hoc Tukey test was applied. To find the significance between the groups, Chi-square test and ANOVA were applied on demographic and hemodynamic variables. The level of significance was determined as its “P” value with P < 0.05 as statistically significant and P < 0.001 as highly significant at a 95% confidence interval.
RESULTS
Thirty patients in each group completed the study as shown in Figure 1. The three groups were comparable in terms of age, gender, weight, height, body mass index, and ASA physical status. Furthermore, no statistically significant difference (P > 0.05) was observed between the groups with respect to the duration of surgery [Table 1].
Figure 1.
Consort Diagram showing flow of the participants
Table 1.
Demographic profile of study participants
| Group A (n=30) | Group B (n=30) | Group C (n=30) | P | |
|---|---|---|---|---|
| Age | 40.13±11.66 | 38.47±11.05 | 38.8±12.63 | 0.846 |
| Weight | 64.67±7.37 | 62.9±10.1 | 59.57±7.05 | 0.059 |
| Height | 163.72±7.22 | 163.44±8.21 | 161.66±4.44 | 0.450 |
| BMI | 24.08±1.69 | 23.43±2.56 | 22.75±2.13 | 0.064 |
| Gender (male/female) | 8/22 | 6/24 | 3/27 | 0.252 |
| ASA grade (I/II) | 19/11 | 22/8 | 20/10 | 0.700 |
| Duration of surgery | 65.67±10.4 | 71±9.23 | 66.33±8.5 | 0.063 |
BMI=Body mass index, ASA=American Society of Anesthesiology
All three groups were comparable in terms of preoperative vitals. There was no significant difference (P > 0.05) observed in the vitals both intraoperatively and postoperatively.
NRS was analyzed for a period of 24 h postoperatively; every half an hour for the first 2 h, every two hourly for the next 4 h, and every four hourly till 14 h and thereafter at 24th h. The NRS was observed to be highest in Group C at all times as compared to Group A and Group B and the difference was statistically significant (P < 0.05) at 0.5, 1, and 1.5 h and statistically highly significant (P < 0.001) at 2 h [Figure 2]. The intergroup comparison of Group A versus B showed no significant difference (P > 0.05). However, on comparing the study groups with the placebo group, a statistically significant difference was observed. On comparing Group A versus C, statistically significant difference was observed at 1, 1.5, and 2 h, P = 0.019, P = 0.032, and P = 0.001, respectively. On comparing Group B versus C, statistically significant difference was observed at 1.5, 2, 6, and 24 h, which were P 0.002, 0.019, 0.045, and 0.046, respectively [Table 2]. This intergroup comparison indicates that both butorphanol and nalbuphine when added to ropivacaine provide superior analgesia as compared to when ropivacaine used alone intraperitoneally.
Figure 2.
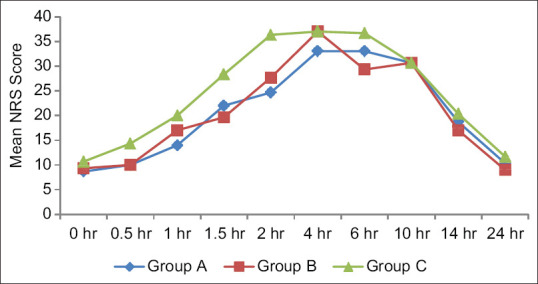
Postoperative NRS of the study participants among three groups. NRS = Numeric Rating Scale
Table 2.
Intergroup comparison of postoperative Numeric Rating Scale of the study participants among three groups
| Intergroup comparison | ||||||
|---|---|---|---|---|---|---|
| Postoperative NRS (h) | A/B |
A/C |
B/C |
|||
| P | 95% CI | P | 95% CI | P | 95% CI | |
| 0 | 0.847 | −3.56-2.23 | 0.231 | −0.89-4.89 | 0.517 | −4.23-1.56 |
| 0.5 | 1.000 | −3.19-3.19 | 0.005* | 1.14-7.52 | 0.005* | −7.52-−1.14 |
| 1 | 0.355 | −8.18-2.18 | 0.019* | 0.82-11.18 | 0.355 | −8.18-2.18 |
| 1.5 | 0.615 | −3.57-8.23 | 0.032* | 0.43-12.23 | 0.002* | −14.57-−2.77 |
| 2 | 0.605 | −10.46-4.46 | 0.001* | 4.2-19.13 | 0.019* | −16.13-−1.2 |
| 4 | 0.550 | −13.12-5.12 | 0.550 | −5.12-13.12 | 0.999 | −9.12-9.12 |
| 6 | 0.448 | −3.53-10.86 | 0.448 | −3.53-10.86 | 0.045* | −14.53-−0.14 |
| 10 | 1.000 | −7.46-7.46 | 1.000 | −7.46-7.46 | 0.998 | −7.46-7.46 |
| 14 | 0.686 | −3.13-6.46 | 0.686 | −3.13-6.46 | 0.227 | −8.13-1.46 |
| 24 | 0.450 | −1.29-3.96 | 0.450 | −1.29-3.96 | 0.046* | −5.29-−0.04 |
*P<0.05=Significant. CI=Confidence interval, NRS=Numeric Rating Scale
The mean time to demand first rescue analgesia was longer in Group A (5.70 ± 3.57 h) compared to Group B (3.95 ± 2.06 h) and C (2.50 ± 1.24 h), which was statistically highly significant (P < 0.001), as shown in Table 3. On intergroup comparison, statistically significant difference was observed P = 0.023 in Group A versus B, whereas statistically highly significant difference was observed P < 0.001 in Group A versus C, but there was no statistically significant difference in Group B versus C [Table 4] indicating that patients were pain-free for a longer time period in Group A followed by Group B and C.
Table 3.
Analgesia requirement 24 h postoperatively among three groups
| Group A (n=30) | Group B (n=30) | Group C (n=30) | P | |
|---|---|---|---|---|
| Time to first rescue analgesia | 5.7±3.57 | 3.95±2.06 | 2.5±1.24 | <0.001† |
| Total dose of rescue analgesia | 105±54.3 | 125±41 | 172.5±40.12 | <0.001† |
†P<0.001=Highly significant. n=Number of patients
Table 4.
Intergroup comparison of analgesia requirement 24 h postoperatively among three groups
| Intergroup comparison | ||||||
|---|---|---|---|---|---|---|
| A/B |
A/C |
B/C |
||||
| P | 95% CI | P | 95% CI | P | 95% CI | |
| Time to first rescue analgesia | 0.023* | 0.20-3.31 | <0.001† | −4.75-−1.66 | 0.065 | −0.07-2.97 |
| Total dose of rescue analgesia | 0.212 | −48.08-8.08 | <0.001† | −95.58-−39.42 | <0.001† | −75.58-−19.42 |
*P<0.05=Significant, †P<0.001=Highly significant. CI=Confidence interval
The frequency of rescue analgesia in 24 h postoperatively in Figure 3 shows that rescue analgesia was consumed three times (that is the maximum number) by Group C (10 patients), two times by Group B (21 patients), and 1 time by Group A (12 patients).
Figure 3.
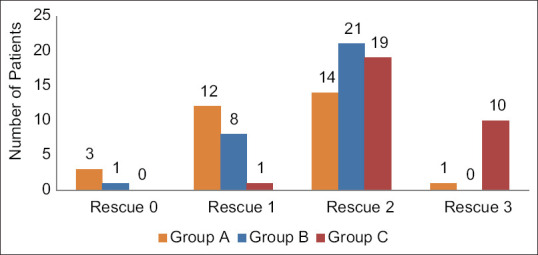
Number of rescue analgesia given 24 h postoperatively among three groups
Similarly, this corresponded to higher total dose rescue analgesic consumption in Group C (172.50 ± 40.12 mg), followed by Group B (125.00 ± 41.00 mg) and Group A (105.00 ± 54.30 mg). The difference was statistically highly significant (P < 0.001) among the three groups [Table 3].
While comparing complications, nausea was the most frequent one, seen in 40% of patients in Group B and 3% of patients in Group A and C each. This finding was statistically highly significant (P < 0.001). Furthermore, hypertension was seen in 10% of patients in Group A and B each and 7% of patients in Group C, which was statistically insignificant (P > 0.05). Tachycardia was seen in 7%, 3%, and 7% of patients in Groups A, B, and C, respectively, which was also statistically insignificant (P > 0.05). Bradycardia and vomiting were observed in 7% of patients in Group B and none in Groups A and C, which was statistically insignificant (P > 0.05). Postoperative sedation was observed in 13% of patients in Group B and none in Groups A and C, which was statistically insignificant (P > 0.05). No episode of hypotension and respiratory depression was observed in any of the three groups [Figure 4].
Figure 4.
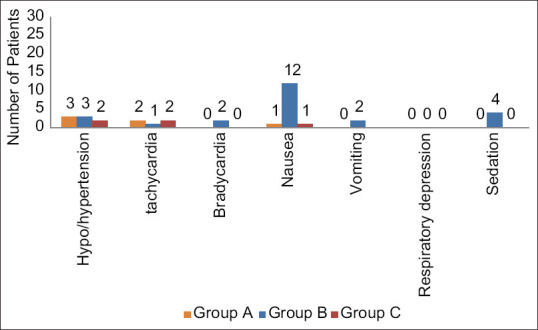
Adverse effects 24 h postoperatively among three groups
DISCUSSION
Optimal control of pain is essential for mobilization and early discharge of patients from the hospital. Adequate pain relief helps in reducing perioperative morbidity and better patient outcome.
We compared the analgesic effect of intraperitoneal instillation of butorphanol with ropivacaine versus nalbuphine with ropivacaine in patients undergoing laparoscopic cholecystectomy in a placebo-controlled study.
Our results show that the addition of both, butorphanol and nalbuphine to ropivacaine decreases postoperative pain. The postoperative mean NRS at different time intervals was significantly higher in Group C as compared to Groups A and B. Furthermore, it was observed that NRS started increasing at 4 h in Group A and Group B and was 33.00 ± 14.89 and 37.00 ± 15.79, respectively, whereas the NRS started increasing after 2 h in the placebo group and was 36.33 ± 13.26, as shown in Figure 2 indicating superior analgesia when butorphanol and nalbuphine are used as an adjuvant with ropivacaine intraperitoneally. Butorphanol, as an adjuvant to ropivacaine, provides a longer pain-free period and lesser number of analgesic consumption than nalbuphine. In the present study, the earliest dose was demanded at 6th h in Group A, when compared to Group B, where they received rescue analgesia at 4th h and in Group C, earliest rescue analgesia had been after 2 h indicating a longer pain-free period with butorphanol. Furthermore, maximum number of rescue analgesia was used in the placebo group (three times), followed by nalbuphine group (2 times), and minimum in butorphanol group (1 time). Similarly, the total dose requirement of rescue analgesia was maximum in the placebo group, followed by ropivacaine with nalbuphine group and minimum in ropivacaine with butorphanol Group. A study that compared intraperitoneal instillation of ropivacaine alone and in addition to nalbuphine in a dose 2 mg in laparoscopic cholecystectomy, concluded that patients receiving ropivacaine with nalbuphine after the removal of gall bladder had better pain relief compared with patients who were administered only ropivacaine.[18] These findings were in accordance with the findings of the present study. Another study by Vidhya et al. comparing the efficacy of i.v. butorphanol and nalbuphine in laparoscopic cholecystectomy concluded lower Visual Analog Scale scores with butorphanol compared to nalbuphine.[19]
Literature shows no use of butorphanol intraperitoneally till date. Hence, the above findings of i.v. route were taken as a bare minimum reference for the intraperitoneal route.
Postoperatively, hypertension was observed in 3 (10%) patients in Group A and 3 (10%) patients in Group B and two patients in Group C (7%), which was statistically insignificant. Hypotension was not observed in any of the patients in any of the three groups. The findings of the present study are different from Singh et al. who observed hypotension in 3 (10%) of the patients among a group of 30 patients who were administered 2 mg nalbuphine with 0.2% of 20 mL ropivacaine intraperitoneally, which was statistically insignificant.[18] The finding of hypertension observed in the present study and hypotension observed in Singh et al.'s study are clinically seen in both studies; however, the findings are statistically insignificant.[18] The finding of hypertension can be attributed to pain in the postoperative period.
In the present study, bradycardia in the postoperative period was observed in two patients (7%) in the nalbuphine with ropivacaine groups and none in the butorphanol with ropivacaine and ropivacaine alone group, which was statistically insignificant. Singh et al. observed that 9 (30%) patients out of 30 who received ropivacaine with nalbuphine had bradycardia as compared to 1 (3%) in 30 patients who received ropivacaine alone and NS.[18] This observation was also statistically insignificant. Although bradycardia was observed in the present study, it was lesser in incidence as compared to the study by Singh et al.[18]
Nausea in the postoperative period was a significant finding in the present study, whereas vomiting was present in only 2 (7%) patients in Group B and no episode of vomiting was observed in Group A and C. Injection ondansetron 4 mg i.v. was administered to relieve nausea and vomiting. In a study conducted by Ali et al., a higher incidence of nausea and vomiting was found in the control group as compared to bupivacaine alone and bupivacaine with the nalbuphine group, which was statistically significant.[20] In yet another study, a higher incidence of emetic symptoms in the control group as compared to ropivacaine alone and ropivacaine with the nalbuphine group was observed, which was statistically insignificant.[18] This finding of nausea and vomiting seen in our study can be attributed to the pharmacodynamic effect of nalbuphine on the chemoreceptor trigger zone, and due to its direct effect on the gastro-intestinal tract.[21] Sedation was seen in 4 (13%) patients in Group B and no sedation was seen in Group A and Group C. The level of sedation of the patients was assessed based on the Modified Ramsay Sedation Scale, which was observed to be 3. This observation was in concordance with the study conducted by Singh et al. in 2017.[18]
Limitation
The pain perception is dependent on the threshold, emotional, and psychological well-being of the person and varies from person to person. The tools used to quantify pain were subjective. For a better assessment, more large-scale trials and researches need to be conducted in future.
CONCLUSION
Ropivacaine, when combined with butorphanol or nalbuphine, provides excellent quality of postoperative analgesia for laparoscopic cholecystectomy. Both the groups were effective in providing adequate postoperative pain relief with hemodynamic stability. The butorphanol group appears to be superior to the nalbuphine group in terms of prolonged postoperative analgesia, lesser number of rescue analgesia consumed, the total dose of rescue analgesia consumed, and lesser complications such as bradycardia, nausea, vomiting, and sedation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Reynolds W., Jr The first laparoscopic cholecystectomy. JSLS. 2001;5:89–94. [PMC free article] [PubMed] [Google Scholar]
- 2.Kaushal-Deep SM, Lodhi M, Anees A, Khan S, Khan MA. Randomised prospective study of using intraoperative, intraincisional and intraperitoneal ropivacaine for the early discharge of postlaparoscopic cholecystectomy patients as a day case in a cost-effective way in government setup of low income and middle-income countries: Opening new horizons. Postgrad Med J. 2019;95:78–84. doi: 10.1136/postgradmedj-2018-135662. [DOI] [PubMed] [Google Scholar]
- 3.Nyerges A. Pain mechanisms in laparoscopic surgery. Semin Laparosc Surg. 1994;1:215–8. doi: 10.1053/SLAS00100215. [DOI] [PubMed] [Google Scholar]
- 4.Ram D, Sistla SC, Karthikeyan VS, Ali SM, Badhe AS, Mahalakshmy T. Comparison of intravenous and intraperitoneal lignocaine for pain relief following laparoscopic cholecystectomy: A double-blind, randomized, clinical trial. Surg Endosc. 2014;28:1291–7. doi: 10.1007/s00464-013-3325-5. [DOI] [PubMed] [Google Scholar]
- 5.Sharan R, Singh M, Kataria AP, Jyoti K, Jarewal V, Kadian R. Intraperitoneal instillation of bupivacaine and ropivacaine for postoperative analgesia in laparoscopic cholecystectomy. Anesth Essays Res. 2018;12:377–80. doi: 10.4103/aer.AER_6_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernández-Palazón J, Tortosa JA, Nuño de la Rosa V, Giménez-Viudes J, Ramírez G, Robles R. Intraperitoneal application of bupivacaine plus morphine for pain relief after laparoscopic cholecystectomy. Eur J Anaesthesiol. 2003;20:891–6. doi: 10.1017/s0265021503001431. [DOI] [PubMed] [Google Scholar]
- 7.Jairath A, Gupta S, Singh K, Katyal S. Can intraperitoneal tramadol decrease pain in patients undergoing laparoscopic cholecystectomy in postoperative period? A randomized controlled trial. World J Laparosc Surg. 2017;10:26–9. [Google Scholar]
- 8.Khurana S, Garg K, Grewal A, Kaul TK, Bose A. A comparative study on postoperative pain relief in laparoscopic cholecystectomy: Intraperitoneal bupivacaine versus combination of bupivacaine and buprenorphine. Anesth Essays Res. 2016;10:23–8. doi: 10.4103/0259-1162.164731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morsy KM, Mohamad Abdalla EE. Postoperative pain relief after laparoscopic cholecystectomy: Intraperitoneal lidocaine versus nalbuphine. Ain Shams J Anesthesiol. 2014;7:40–4. [Google Scholar]
- 10.Gupta B, Verma V, Chaudhary UK, Sidhu R, Chandel A. Effect of intraperitoneal instillation of dexmedetomidine or fentanyl as adjuvants to bupivacaine on fast tracking discharge criteria in patients undergoing ambulatory laparoscopic cholecystectomy: A randomised double-blind control trial. Ain Shams J Anesthesiol. 2021;13:60–6. [Google Scholar]
- 11.Abd El-Hamid AM, El-Moutaz H, Abdel Moneim AT. Evaluation of intraperitoneal levobupivacaine with and without sufentanil for postoperative analgesia after laparoscopic cholecystectomy. Ain Shams J Anesthesiol. 2016;9:371–6. [Google Scholar]
- 12.Kumhar G, Mayank A. comparative study of intraperitoneal instillation of levobupivacaine (0.25%) plus dexmedetomidine versus ropivacaine (0.25%) plus dexmedetomidine for postoperative analgesia in patients undergoing laparoscopic cholecystectomy. Eur J Mole Clin Med. 2020;7:3666–72. [Google Scholar]
- 13.Kaarthika T, Radhapuram SD, Samantaray A, Pasupuleti H, Hanumantha Rao M, Bharatram R. Comparison of effect of intraperitoneal instillation of additional dexmedetomidine or clonidine along with bupivacaine for post-operative analgesia following laparoscopic cholecystectomy. Indian J Anaesth. 2021;65:533–8. doi: 10.4103/ija.IJA_231_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saadati K, Razavi MR, Nazemi Salman D, Izadi S. Postoperative pain relief after laparoscopic cholecystectomy: Intraperitoneal sodium bicarbonate versus normal saline. Gastroenterol Hepatol Bed Bench. 2016;9:189–96. [PMC free article] [PubMed] [Google Scholar]
- 15.Elfiky M, Stohy M, Ibrahim W, Ibrahim A. Comparative study between intravenous and intraperitoneal magnesium sulphate as adjuvant to general anesthesia for pain management in laparoscopic cholecystectomy. Egypt J Hosp Med. 2018;72:5695–704. [Google Scholar]
- 16.Ng A, Swami A, Smith G, Robertson G, Lloyd DM. Is intraperitoneal levobupivacaine with epinephrine useful for analgesia following laparoscopic cholecystectomy? A randomized controlled trial. Eur J Anaesthesiol. 2004;21:653–7. doi: 10.1017/s0265021504008117. [DOI] [PubMed] [Google Scholar]
- 17.Miller RD, Cohen NH, Eriksson LI, Wiener-Kronish JP, Young WL. Miller's Anesthesia International Edition. 8th. Philadelphia: Elsevier, Saunders; 2014. Opioid analgesics; p. 903. Ch. 31. [Google Scholar]
- 18.Singh S, Giri MK, Singh M, Giri NK. A clinical comparative study of intraperitoneal instillation of ropivacaine alone or ropivacaine with nalbuphine for postoperative analgesia in laparoscopic cholecystectomy. Anaesth Pain Intensive Care. 2017;21:335–9. [Google Scholar]
- 19.Vidhya N, Prakash V, Irshad B, Kumar VS. Comparative efficacy of butorphanol versus nalbuphine for balanced anaesthesia and post-operative analgesia in patients undergoing laparoscopic surgery. Indian J Clin Anaesth. 2019;6:143–7. [Google Scholar]
- 20.Ali WA, Ali NS, Sewefy AM, Ahmed AH. Comparative study between intraperitoneal bupivacaine and bupivacaine-nalbuphine for postoperative pain relief after laparoscopic cholecystectomy. Res Opin Anesth Intensive Care. 2020;7:57–64. [Google Scholar]
- 21.Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Stock MC, Ortega R, et al. South Asian Edition of Clinical Anesthesia. 8th. New Delhi: Wolters Kluwer; 2017. Opioids; pp. 505–26. Ch. 20. [Google Scholar]


