Abstract
Suboptimal adherence to guidelines for hepatocellular carcinoma (HCC) surveillance among high‐risk patients is a persistent problem with substantial detriment to patient outcomes. While patients cite cost as a barrier to surveillance receipt, the financial burden they experience due to surveillance has not been examined. We conducted a retrospective administrative claims study to assess HCC surveillance use and associated costs in a US cohort of insured patients without cirrhosis but with hepatitis B virus (HBV) infection, monitored in routine clinical practice. Of 6831 patients (1122 on antiviral treatment, 5709 untreated), only 39.3% and 51.3% had received any abdominal imaging after 6 and 12 months, respectively, and patients were up to date with HCC surveillance guidelines for only 28% of the follow‐up time. Completion of surveillance was substantially higher at 6 and 12 months among treated patients (51.7% and 69.6%, respectively) compared with untreated patients (36.9% and 47.6%, respectively) (p < 0.001). In adjusted models, treated patients were more likely than untreated patients to receive surveillance (hazard ratio [HR] 1.75, 95% confidence interval [CI] 1.53–2.01, p < 0.001), and the proportion of those up to date with surveillance was 9.7% higher (95% CI 6.26–13.07, p < 0.001). Mean total and patient‐paid daily surveillance‐related costs ranged from $99 (ultrasound) to $334 (magnetic resonance imaging), and mean annual patient costs due to lost productivity for surveillance‐related outpatient visits ranged from $93 (using the federal minimum wage) to $321 (using the Bureau of Labor Statistics wage). Conclusion: Use of current HCC surveillance strategies was low across patients with HBV infection, and surveillance was associated with substantial patient financial burden. These data highlight an urgent need for accessible and easy‐to‐implement surveillance strategies with sufficient sensitivity and specificity for early HCC detection.
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for approximately 75% of primary liver cancer, and is the third leading cause of cancer deaths worldwide.[ 1 , 2 ] The most common risk factor for HCC globally is chronic hepatitis B virus (HBV) infection,[ 2 ] which can increase HCC risk even in the absence of cirrhosis.[ 2 , 3 ] A large randomized controlled trial among patients with HBV demonstrated that HCC surveillance significantly increased early HCC detection and reduced HCC‐related mortality.[ 4 ] These data have informed practice guidelines such as those issued by the American Association for the Study of Liver Diseases (AASLD), which recommends HCC surveillance using abdominal ultrasound with or without alpha‐fetoprotein (AFP) every 6 months for patients with HBV infection with cirrhosis as well as those without cirrhosis at higher risk for HCC—including Asian or Black men over 40 years of age, Asian women over 50 years of age, and patients with hepatitis delta virus coinfection or a first‐degree family history of HCC.[ 5 , 6 ]
Although HCC has an overall 5‐year survival rate of only 19.6%,[ 7 ] patients who are diagnosed at an early stage may be eligible for curative treatment such as resection, ablation, or liver transplantation, which increases 5‐year survival to 50%–80%.[ 8 ] Unfortunately, HCC surveillance is widely underused,[ 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 ] despite evidence that it can promote early detection and potentially improve survival among patients with chronic HBV.[ 4 , 17 , 18 , 19 , 20 ] In a recent systematic review and meta‐analysis of 22 studies including 19,511 patients with cirrhosis or chronic viral hepatitis, the adherence rate to AASLD surveillance guidelines was only 52% overall and was 39% when limited to retrospective analyses, which may be a better reflection of real‐world practice.[ 21 ] Due in part to such low surveillance rates, most individuals with HCC are diagnosed at an intermediate or advanced stage, when the prognosis is much poorer.[ 22 ]
Given that suboptimal adherence to HCC surveillance guidelines is a persistent problem with substantial detriment to patient outcomes, the elucidation of potential barriers to HCC surveillance in routine practice is an important research goal. Previous assessments conducted among US patients with HBV infection have indicated that those who were not under specialist care were less likely to receive guideline‐based HCC surveillance.[ 13 , 15 , 23 , 24 ] However, the patient‐side financial burden of surveillance—which many patients cite as a significant barrier to surveillance receipt[ 25 , 26 ]—has not been previously examined. The present study was conducted to assess HCC surveillance use and associated costs in a US cohort of insured patients without cirrhosis but with HBV infection, monitored in routine clinical practice.
METHODS
Study design and data source
This was a retrospective observational study conducted using administrative claims data from the Optum Research Database (ORD) from January 1, 2013, through December 31, 2018 (study period; Figure 1). The ORD is geographically diverse across the United States and contains deidentified medical and pharmacy claims data and linked enrollment information for individuals enrolled in US health plans. Medical claims include diagnosis and procedure codes from the International Classification of Diseases, 9th and 10th Revisions, Clinical Modification; Current Procedural Terminology or Healthcare Common Procedure Coding System codes; site of service codes; paid amounts; and other information. Pharmacy claims include drug name, national drug code, dosage form, drug strength, fill date, and financial information for health plan–provided outpatient pharmacy services. Because no identifiable protected health information was accessed in the conduct of this study, institutional review board approval or waiver of approval was not required.
FIGURE 1.
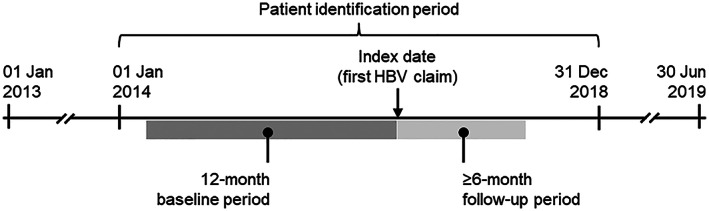
Study design schematic. The 12‐month baseline period was designed to capture previous hepatocellular carcinoma (HCC) screening. A minimum 6‐month follow‐up period was chosen to allow sufficient opportunity for guideline‐recommended HCC screening to occur. HBV, hepatitis B virus
Patient selection
The study included commercial insurance enrollees and Medicare Advantage with Part D (MAPD) beneficiaries with two or more claims for HBV and no claims for cirrhosis (Table S1) from January 1, 2014, through December 31, 2018 (patient identification period; Figure 1). The date of the first qualifying claim for HBV was designated as the index date. The 12 months before the index date were designated as the baseline period. Included patients were also required to be 40 years old or older for men, or 50 years or older for women, on the index date; and to have continuous health plan enrollment with medical and pharmacy benefits during the baseline and follow‐up periods. Those with claims evidence of liver cancer (two or more nondiagnostic claims with diagnosis codes for liver cancer ≥ 30 days apart within a 365‐day period) or liver transplantation during the baseline period or on the index date were excluded from the study (Table S1).
Patients were observed for at least 6 months, beginning on the index date and ending at the earlier of disenrollment from the health plan or the end of the study period. Patients with medical or pharmacy claims for HBV treatments (adefovir dipivoxil, entecavir, interferon alfa‐2b, lamivudine, peginterferon alfa‐2a, telbivudine, tenofovir disoproxil, or tenofovir alafenamide fumarate) any time between the start of the baseline period and the end of the follow‐up period were categorized as treated, whereas others were categorized as untreated. Follow‐up for study outcomes was truncated at liver cancer diagnosis or liver transplantation.
Study variables
Patient demographic and clinical characteristics measured during the baseline period included age, sex, US census region, insurance type, baseline Quan‐Charlson comorbidity score,[ 27 ] baseline comorbidities identified using Clinical Classifications Software from the Agency for Healthcare Research and Quality,[ 28 ] and prior HCC surveillance. Health care provider specialty was captured from claims with diagnosis codes for HBV during the follow‐up period.
HCC surveillance events
HCC surveillance events (abdominal ultrasounds, magnetic resonance imaging [MRI] scans, computed tomography [CT] scans, and AFP tests) were identified from claims data. AFP tests occurring within 14 days of an ultrasound were considered to accompany the ultrasound. As a sensitivity analysis, AFP tests occurring within 60 days of an ultrasound were also captured. Surveillance events that included any abdominal imaging were considered to be complete, while events that included only AFP were considered incomplete.
Proportion of days covered
The proportion of follow‐up time during which patients were up to date with recommended HCC surveillance was assessed using proportion of days covered (PDC), which was calculated as (days covered)/(days of follow‐up). Any abdominal imaging was considered to provide 6 months of days covered. PDC was analyzed separately for all patients and for patients with evidence of any surveillance during the follow‐up period.
Cost outcomes
Cost outcomes were analyzed during the first surveillance episode among patients with no inpatient admission or emergency room visit during the follow‐up period. The first surveillance episode was defined as the first outpatient surveillance event during follow‐up plus outpatient surveillance events within the following 60 days. For each surveillance mechanism, the mean and median daily costs during the first surveillance episode were calculated as health plan–paid and patient‐paid amounts. For patients with ultrasound plus AFP testing, costs on the day of the AFP test were added to the costs on the day of the ultrasound if the tests occurred on different days. All combinations of surveillance types that occurred on the same day were assessed; however, data are shown only for ultrasound plus AFP, as very few surveillance days included any other combinations (nine other combinations totaling only 1.1% of surveillance days, with no single combination exceeding 0.3%).
Yearly patient productivity costs due to surveillance‐related outpatient health care encounters were estimated by assuming 4 working hours lost per outpatient visit, multiplied by the patient's estimated average wage derived from the US Bureau of Labor Statistics (BLS) data[ 29 ] and the federal minimum wage.[ 30 ] Costs were adjusted to 2018 USD using the annual medical care component of the Consumer Price Index.[ 31 ]
Statistical analysis
Study variables were analyzed descriptively. Numbers and percentages were provided for categorical variables; means, medians, and SDs were provided for continuous variables. Time to follow‐up surveillance events and the censoring‐adjusted proportion of patients receiving surveillance during the follow‐up period were evaluated using Kaplan–Meier analysis. Proportional hazards models were used to evaluate the effect of baseline provider specialty on receipt of surveillance. An ordinary least squares model was used to evaluate the effect of baseline provider specialty on PDC among patients with at least one follow‐up surveillance event. All multivariable models were adjusted for treatment status, age group, sex, geographic region, presence of high‐deductible health plan, baseline Charlson comorbidity score category, and select comorbidities; the ordinary least squares model was also adjusted for follow‐up length. To examine follow‐up surveillance from a similar starting point, Kaplan–Meier and multivariable analyses were performed among patients without surveillance during the baseline period. All results were stratified by treated versus untreated patients. Statistical analyses were performed using SAS software version 9.4 (SAS Institute). Statistical significance was defined as p ≤ 0.05.
RESULTS
Study population
Of 16,091 potential patients with HBV infection, 6831 met the study inclusion criteria (Figure 2). There were 1122 patients in the treated cohort (16.4%) and 5709 in the untreated cohort (83.6%) (Table 1). In the total patient population, mean (SD) age was 60.1 (11.7) years, 64.1% were male, and 43.1% had MAPD insurance. A higher proportion of treated versus untreated patients was from the Northeast (30.2% vs. 25.0%; p < 0.001), whereas a higher proportion of untreated patients was seen in the South (40.7% of untreated patients vs. 36.9% of treated patients; p = 0.016). Although mean (SD) baseline Charlson comorbidity scores were higher for treated patients (2.4 [1.9] compared with 1.9 [2.1] for untreated; p < 0.001), the prevalence of several common comorbidities was significantly higher among untreated patients, including hypertension (51.6% vs. 46.4%; p = 0.002), connective tissue disease (34.5% vs. 27.1%; p < 0.001), and back disorders (30.7% vs. 24.9%; p < 0.001).
FIGURE 2.
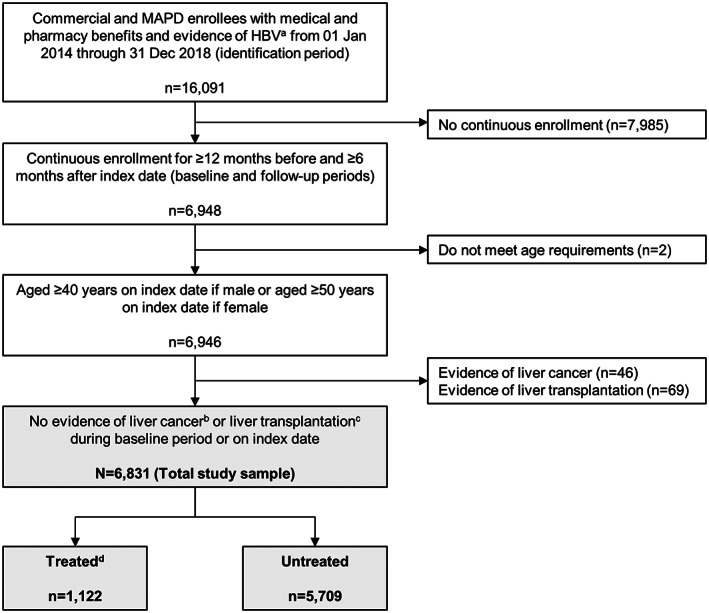
Patient identification and attrition. aAt least two nondiagnostic claims for HBV in any position on different dates during the identification period and age ≥ 40 years on the claim if male or age ≥ 50 years on the claim if female (only required on the second of the two claims; the first claim that meets the age criteria is the index date). bAt least two nondiagnostic claims ≥ 30 days apart in positions 1 or 2 on the claim. cAt least one claim in any position. dMedical or pharmacy claims for HBV treatments (adefovir dipivoxil, entecavir, interferon alfa‐2b, lamivudine, peginterferon alfa‐2a, telbivudine, tenofovir disoproxil, or alafenamide fumarate) any time between the start of the baseline period and the end of the follow‐up period. MAPD, Medicare Advantage with Part D
TABLE 1.
Patient demographic and clinical characteristics
| Characteristics | Total (n = 6831; 100.0%) | Treated (n = 1122; 16.4%) | Untreated (n = 5709; 83.6%) | Treated vs. untreated p value |
|---|---|---|---|---|
| Age, years (mean [SD]) | 60.1 (11.7) | 58.8 (11.9) | 60.3 (11.7) | <0.001 |
| Male sex (n [%]) | 4375 (64.1) | 762 (67.9) | 3613 (63.3) | 0.003 |
| Geographic region (n [%]) | ||||
| Northeast | 1767 (25.9) | 339 (30.2) | 1428 (25.0) | <0.001 |
| Midwest | 947 (13.9) | 125 (11.1) | 822 (14.4) | 0.004 |
| South | 2740 (40.1) | 414 (36.9) | 2326 (40.7) | 0.016 |
| West | 1375 (20.1) | 244 (21.8) | 1131 (19.8) | 0.139 |
| Other | 2 (0.0) | 0 (0.0) | 2 (0.0) | 0.531 |
| MAPD insurance (n [%]) a | 2944 (43.1) | 401 (35.7) | 2543 (44.5) | <0.001 |
| Baseline Quan‐Charlson comorbidity score (mean [SD]) | 2.0 (2.1) | 2.4 (1.9) | 1.9 (2.1) | <0.001 |
| Top 10 baseline AHRQ comorbidities (n [%]) b | ||||
| Hypertension | 3464 (50.7) | 521 (46.4) | 2943 (51.6) | 0.002 |
| Dyslipidemia | 3272 (47.9) | 510 (45.5) | 2762 (48.4) | 0.073 |
| Diseases of the heart c | 2424 (35.5) | 367 (32.7) | 2057 (36.0) | 0.457 |
| Diseases of the urinary system d | 2453 (35.9) | 399 (35.6) | 2054 (36.0) | 0.034 |
| Nontraumatic joint disorders | 2393 (35.0) | 313 (27.9) | 2080 (36.4) | 0.790 |
| Diabetes mellitus | 2313 (33.9) | 361 (32.2) | 1952 (34.2) | <0.001 |
| Eye disorders | 2294 (33.6) | 378 (33.7) | 1916 (33.6) | 0.933 |
| Connective tissue disease other than SLE | 2272 (33.3) | 304 (27.1) | 1968 (34.5) | <0.001 |
| Lower respiratory disease e | 2154 (31.5) | 330 (29.4) | 1824 (32.0) | 0.094 |
| Spondylosis, intervertebral disc disorders, and other back problems | 2029 (29.7) | 279 (24.9) | 1750 (30.7) | <0.001 |
| Baseline surveillance (n [%]) f | ||||
| Any | 2957 (43.3) | 755 (67.3) | 2202 (38.6) | <0.001 |
| Ultrasound | 2270 (33.2) | 585 (52.1) | 1685 (29.5) | <0.001 |
| AFP | 2010 (29.4) | 600 (53.5) | 1410 (24.7) | <0.001 |
| MRI | 189 (2.8) | 55 (4.9) | 134 (2.4) | <0.001 |
| CT | 122 (1.8) | 28 (2.5) | 94 (1.7) | 0.050 |
| Gastroenterologist visit (n [%]) g | ||||
| Baseline | 2284 (33.4) | 627 (55.9) | 1657 (29.0) | <0.001 |
| Follow‐up | 2454 (35.9) | 706 (62.9) | 1748 (30.6) | <0.001 |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; MAPD, Medicare Advantage with Part D, NAFLD, nonalcoholic fatty liver disease; SLE, systemic lupus erythematosus.
Patients without MAPD insurance were covered by commercial plans.
Top 10 most prevalent AHRQ comorbidities in the total population are shown, excluding liver disease and viral infection.
Includes valve disorders, cardiomyopathy, myocarditis, angina, myocardial infarction, coronary atherosclerosis, pulmonary heart disease, conduction disorders, dysrhythmias, ventricular fibrillation, and heart failure.
Includes renal failure; chronic kidney disease; urinary tract infections and kidney infections; and other diseases of the kidneys, bladder, and urethra.
Does not include lung disease due to external agents (e.g., environmental lung disease or lung conditions due to fumes or chemicals).
Not mutually exclusive; patients may have received more than one type of baseline surveillance.
Provider specialty was captured from claims with diagnosis codes for cirrhosis during the baseline and follow‐up periods. Patients could have received baseline or follow‐up care from more than one type of provider.
Only 43.3% of patients had evidence of HCC surveillance during the baseline period, with ultrasound being the most common modality (33.2%) followed by AFP (29.4%). The proportion of patients with prior surveillance was almost twice as high among treated versus untreated patients (67.3% vs. 38.6%; p < 0.001). Overall, 33.4% of patients received health care from a gastroenterologist (GI) during the baseline period, and 35.9% received GI care during follow‐up. The proportion of patients with a GI visit was also almost twice as high for treated versus untreated patients (55.9% vs. 29.0% during baseline; 62.9% vs. 30.6% during follow‐up; p < 0.001 for both).
Study outcomes
HCC surveillance events
The proportions of patients who received any abdominal imaging (ultrasound, CT, or MRI regardless of AFP) during follow‐up were 39.3% and 51.3% at 6 and 12 months, respectively (Figure 3), with completion of abdominal imaging being substantially higher at 6 and 12 months among treated patients (51.7% and 69.6%, respectively) compared with untreated patients (36.9% and 47.6%, respectively) (p < 0.001). Results were similar when considering only ultrasound ± AFP, with 36.0% and 48.1% of patients overall completing any abdominal ultrasound at 6 and 12 months, respectively, and higher receipt among treated patients (45.7% and 65.2%, respectively) compared with untreated patients (34.1% and 44.7%, respectively) (p < 0.001) (Figure 4A). The proportions of patients who received ultrasound with AFP were even lower overall (13.9% and 19.6% at 6 and 12 months, respectively), although receipt was still higher among treated versus untreated patients at both time points (p < 0.001) (Figure 4B).
FIGURE 3.
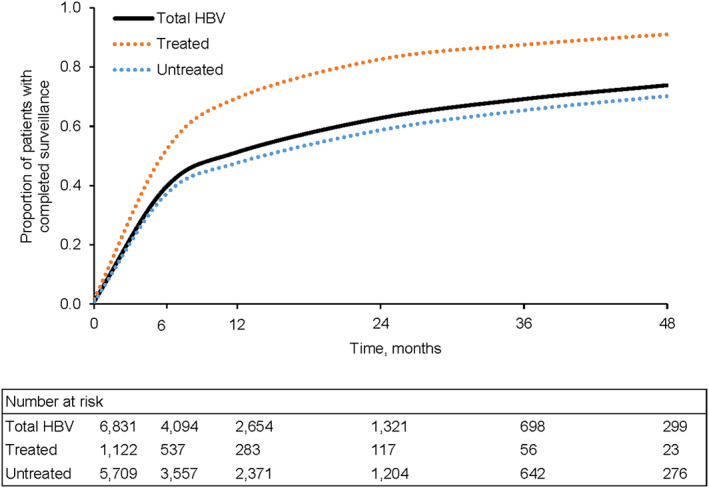
Completed follow‐up surveillance events. Surveillance events that included any abdominal imaging were considered to be complete. p < 0.001 for difference among survival curves.
FIGURE 4.
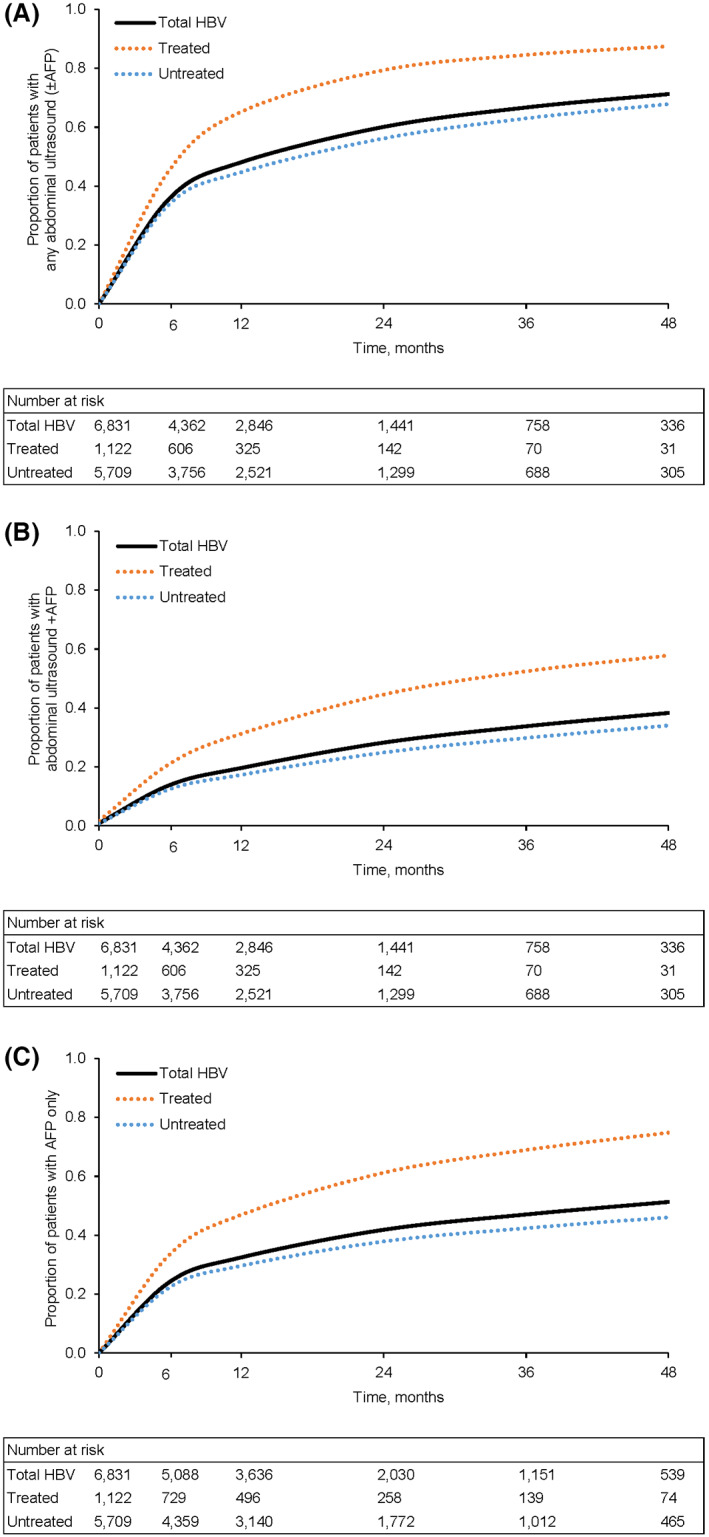
Follow‐up ultrasound and AFP testing surveillance events. (A) Any abdominal ultrasound (±alpha‐fetoprotein [AFP]). (B) Abdominal ultrasound + AFP. (C) AFP only. In each panel, p < 0.001 for difference among survival curves.
Notably, a relatively large proportion of patients received AFP alone: 24.2% at 6 months and 32.5% at 12 months (Figure 4C). In a sensitivity analysis that increased the time permitted between ultrasounds and AFP tests from 14 days to 60 days, the overall proportion of patients receiving AFP alone remained substantial: 22.0% and 29.3% at 6 and 12 months, respectively.
Proportion of days covered
Overall, patients' PDC with imaging‐based HCC surveillance was only 0.28 (SD 0.30) during the follow‐up period (Figure 5A). PDC was higher for treated versus untreated patients (0.43 vs. 0.25; p < 0.001). In the subset of individuals with at least one surveillance event during follow‐up (n = 4250), PDC was 0.45 (SD 0.26) and was higher for treated versus untreated patients (PDC 0.53 vs. 0.43; p < 0.001) (Figure 5B).
FIGURE 5.
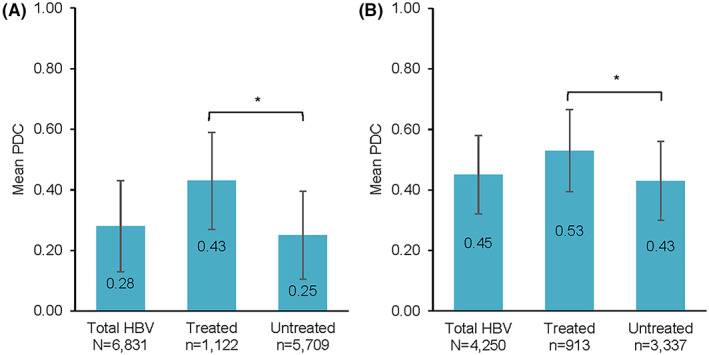
Follow‐up proportion of days covered (PDC). (A) PDC among all patients (n = 6831). (B) PDC among patients with follow‐up surveillance (n = 4250). Error bars represent 1 SD. *p < 0.001
Factors associated with HCC surveillance
In a proportional hazards model adjusted for treatment status, patient demographics, presence of high‐deductible health plan, baseline Charlson comorbidity score category, and select comorbidities, patients with treated HBV were more likely to receive HCC surveillance during follow‐up compared with untreated patients (hazard ratio [HR] 1.75, 95% confidence interval [CI] 1.53–2.01, p < 0.001) (Table 2). Younger age and Northeast or West/Other geographic region (vs. South) were associated with increased follow‐up surveillance, whereas higher baseline comorbidity burden was associated with lower surveillance receipt. The effect of baseline gastroenterology care was not significant (95% CI 0.99–1.24; p = 0.068) (Table 2).
TABLE 2.
Proportional hazards model of surveillance receipt
| Independent variable | Univariable unadjusted model | Multivariable adjusted model | ||
|---|---|---|---|---|
| HR of surveillance receipt (95% CI) | p value | HR of surveillance receipt (95% CI) | p value | |
| Baseline gastroenterologist a | 1.05 (0.94–1.17) | 0.376 | 1.11 (0.99–1.24) | 0.068 |
| HBV | ||||
| Untreated | Reference | Reference | ||
| Treated | 1.76 (1.54–2.00) | <0.001 | 1.75 (1.53–2.01) | <0.001 |
| Age group, years | ||||
| 65+ | Reference | Reference | ||
| 51–64 | 1.33 (1.2–1.48) | <0.001 | 1.34 (1.2–1.49) | <0.001 |
| 40–50 | 1.79 (1.59–2.01) | <0.001 | 1.65 (1.45–1.89) | <0.001 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 1.13 (1.03–1.24) | 0.009 | 1.03 (0.93–1.14) | 0.550 |
| Geographic region | ||||
| South | Reference | Reference | ||
| Northeast | 1.2 (1.07–1.35) | 0.002 | 1.27 (1.13–1.43) | <0.001 |
| Midwest | 0.94 (0.82–1.07) | 0.36 | 0.96 (0.84–1.09) | 0.498 |
| West or Other | 1.31 (1.16–1.48) | <0.001 | 1.17 (1.03–1.32) | 0.013 |
| High‐deductible health plan | ||||
| No or missing info | Reference | Reference | ||
| Yes | 1.27 (1.14–1.43) | <0.001 | 1.02 (0.9–1.15) | 0.799 |
| Baseline Charlson comorbidity score category | ||||
| 0 | Reference | Reference | ||
| 1–2 | 0.74 (0.66–0.82) | <0.001 | 0.78 (0.69–0.87) | <0.001 |
| 3+ | 0.52 (0.46–0.58) | <0.001 | 0.55 (0.48–0.63) | <0.001 |
| Baseline viral infection b | 0.76 (0.69–0.84) | <0.001 | 0.87 (0.78–0.97) | 0.014 |
| Baseline liver disease b | 1.24 (1.1–1.41) | 0.001 | 1.46 (1.28–1.66) | <0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Gastroenterologist visit during the baseline period.
Identified using Clinical Classifications Software from the Agency for Healthcare Research and Quality.[ 27 ]
In an ordinary least squares model adjusted for treatment status, patient demographics, presence of high‐deductible health plan, baseline Charlson comorbidity score category, select comorbidities, and follow‐up length, PDC during follow‐up was 9.7% higher for treated versus untreated patients (95% CI 6.26–13.07; p < 0.001) and was 4.2% higher for patients aged 40–50 years versus those aged ≥ 65 (95% CI 1.25–7.21; p = 0.005) (Table 3). In contrast, PDC was 3.2% lower for patients with baseline Charlson comorbidity scores ≥ 3 compared with scores of 0 (95% CI −6.26 to −0.07; p = 0.045). Longer follow‐up length was also associated with lower PDC, but this decrease was likely too small to be clinically relevant (−0.03%, 95% CI −0.03 to −0.03; p < 0.001). The effect of baseline gastroenterology care on PDC was not significant (1.08% increase, 95% CI −1.36 to 3.53; p = 0.386) (Table 3).
TABLE 3.
Ordinary least squares model of PDC
| Independent variable | PDC a for follow‐up imaging (95% CI) | p value |
|---|---|---|
| Baseline gastroenterologist b | 1.08 (−1.36 to 3.53) | 0.386 |
| HBV | ||
| Untreated | Reference | – |
| Treated | 9.66 (6.26–13.07) | <0.001 |
| Age group, years | ||
| 65+ | Reference | – |
| 51–64 | 1.96 (−0.52 to 4.45) | 0.005 |
| 40–50 | 4.23 (1.25–7.21) | 0.005 |
| Sex | ||
| Female | Reference | – |
| Male | 1.51 (−0.66 to 3.68) | 0.172 |
| Geographic region | ||
| South | Reference | – |
| Northeast | 1.29 (−1.26 to 3.84) | 0.321 |
| Midwest | −0.25 (−3.31 to 2.80) | 0.871 |
| West or Other | 2.25 (−0.55 to 5.04) | 0.116 |
| High‐deductible health plan | ||
| No or missing info | Reference | – |
| Yes | −2.39 (−5.06 to 0.29) | 0.081 |
| Baseline Charlson comorbidity score category | ||
| 0 | Reference | – |
| 1–2 | 1.11 (−1.43 to 3.64) | 0.393 |
| 3+ | −3.17 (−6.26 to −0.07) | 0.045 |
| Baseline viral infection c | −1.40 (−3.89 to 1.10) | 0.272 |
| Follow‐up length | −0.03 (−0.03 to −0.03) | <0.001 |
PDC is presented on a 0–100 scale and calculated among patients with at least one follow‐up surveillance event.
Gastroenterologist visit during the baseline period.
Identified using Clinical Classifications Software from the Agency for Healthcare Research and Quality.[ 27 ] Includes alcohol‐related liver disease, liver cirrhosis without mention of alcohol, liver abscess and sequelae of chronic liver disease, ascites, and other/unspecified liver disorders.
Cost outcomes
Total and patient‐paid mean daily costs of outpatient surveillance were highest for MRI only ($1717 and $334, respectively) and lowest for ultrasound only ($415 and $99, respectively) (Figure 6A). Total median daily costs were lower than mean daily costs due to a skewed distribution, but remained highest for MRI only ($1261) and lowest for US only ($234) (Figure 6A). Daily surveillance costs were not appreciably different between treated and untreated patients (Table S2).
FIGURE 6.
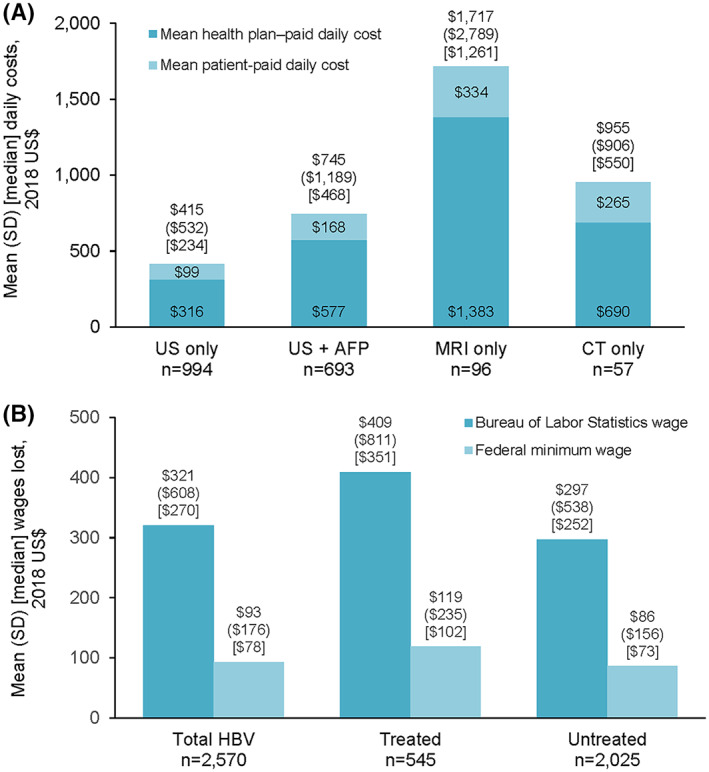
HCC surveillance costs. Cost outcomes were calculated among patients with no inpatient stay or emergency room visit during the follow‐up period (n = 2570). (A) Daily costs for completed outpatient surveillance. (B) Yearly patient productivity costs for completed outpatient surveillance. For both wage calculations, p = 0.002 for difference between treated and untreated patients. CT, computed tomography; MRI, magnetic resonance imaging; US, ultrasound
Overall, the estimated mean (SD) yearly patient productivity costs of outpatient surveillance using BLS wage data and the federal minimum wage were $321 ($609) and $93 ($176), respectively (Figure 6B). Productivity costs were higher for treated versus untreated patients: $409 ($811) versus $297 ($538) using BLS wage data, and $119 ($235) versus $86 ($156) using the federal minimum wage (p = 0.002 for both) (Figure 6B).
DISCUSSION
Routine surveillance is essential for patients with chronic HBV infection—including those without cirrhosis, who generally have well‐preserved hepatic function and are therefore more likely to be eligible for curative treatments if diagnosed with HCC at an early stage.[ 5 , 6 , 32 ] However, in this study we found that after 6 months of follow‐up, only 36% of individuals without cirrhosis but with HBV infection had received an abdominal ultrasound (the primary recommended HCC surveillance modality), and close to half of patients had received no abdominal imaging at all. Although surveillance was significantly higher among those with evidence of HBV treatment versus untreated individuals (45.7% vs. 34.1% at 6 months and 65.2% vs. 44.7% at 12 months), it was still notably underused even in the former group, which would presumably include the highest‐risk patients. Moreover, patients who underwent surveillance experienced a substantial financial burden, with mean out‐of‐pocket costs ranging from $99 to $334 on the day of surveillance, depending on modality, and sizeable annual productivity costs.
Survey data indicate that many patients perceive cost as a significant barrier to HCC surveillance receipt.[ 25 , 26 ] The present study quantitatively assesses the patient financial burden associated with HCC surveillance among individuals with HBV without cirrhosis in the United States.[ 33 ] As in our previous analysis conducted among patients with cirrhosis,[ 34 ] health plans paid the majority of costs for surveillance‐related visits but patients' out‐of‐pocket expenses remained high, particularly for MRI and CT surveillance. The estimated yearly patient productivity costs of $321 (using BLS wage data) were markedly lower than the $1471 we observed previously for patients with cirrhosis and HBV infection[ 34 ]—likely attributable to patients with versus without cirrhosis being sicker and requiring more testing, higher‐intensity care, and more frequent outpatient visits[ 35 ]—but would nevertheless constitute a substantial burden for many Americans.
We also found that a substantial proportion of patients with HBV infection received only AFP testing during follow‐up. AFP testing in the absence of abdominal imaging is not a guideline‐recommended mechanism for HCC surveillance; however, its frequent use may suggest broad acceptance of blood‐based screening tests on the part of providers and patients alike. Taken together with our cost findings, these results point to the development of blood‐based biomarkers as a potential avenue for improving HCC surveillance underuse and increasing test effectiveness in this population.[ 36 ] Compared with imaging, blood tests are generally more accessible and require minimal time commitment.[ 37 ] Furthermore, as they are a familiar feature of routine primary care visits for many patients, inclusion of another test on the panel would require no additional effort or productivity loss, potentially decreasing barriers to surveillance. This may be particularly relevant for patients with HBV infection, who typically undergo regular blood‐based assessments to monitor HBV status. The development of novel biomarkers may also represent a cost‐effective way to expand HCC screening to other groups that are not included in current HCC surveillance recommendations but have been found to have increased risk, such as men under age 40 or women under age 50 with chronic HBV infection but not cirrhosis.[ 38 ]
The findings of this study also augment a large body of existing evidence that surveillance is underused among multiple subgroups of patients at high risk for HCC.[ 9 , 12 , 14 , 21 , 33 ] Interestingly, adherence to surveillance guidelines in the present study was similar to that observed in our previous analysis conducted among patients with cirrhosis, in which 34% had received an abdominal ultrasound at 6 months.[ 33 , 34 ] This outcome was somewhat surprising, as patients with HBV infection without cirrhosis have previously been reported to have lower adherence to HCC surveillance guidelines, despite having high HCC risk.[ 12 , 14 ] As these earlier studies were conducted in 2009 and 2014, respectively, this could suggest that some progress has been made in reducing surveillance underuse among patients with HBV in the past decade.
We found that receipt of GI care during the baseline period did not have a significant effect on adherence to recommended surveillance in the present analysis. This was in contrast to our previous study and others, which have found specialist care to be associated with improved adherence.[ 12 , 13 , 15 , 34 , 39 ] Given that HBV is often managed by GIs and we found that treated patients had significantly higher follow‐up surveillance than untreated patients, we hypothesize that HBV treatment status may essentially have functioned as a surrogate for guideline‐concordant provider behavior due to collinearity between HBV treatment status and provider specialty. Our findings may also reflect providers' assessment of patient risk, as providers may have been more likely to recommend surveillance for patients perceived to be at high risk for liver‐related outcomes (such as those whose HBV was sufficiently progressed to warrant antiviral treatment). However, it should be noted that substantial underuse of surveillance was observed even among HBV‐treated patients, who are presumably at high risk for HCC.
Geographic region also had a significant effect on HCC surveillance adherence in the present study, with patients located in the Northeast or West being more likely to have surveillance during follow‐up than those in the South. We speculate that these findings are due to regional differences in distribution of both patients and providers. Although information on patient race and ethnicity was not available in this analysis, the burden of HBV in the United States is known to fall disproportionately on foreign‐born individuals, who constitute an estimated 60%–70% of those living with HBV and are primarily of Asian or African origin.[ 40 , 41 , 42 , 43 , 44 ] As these high‐risk populations are concentrated in the northeastern and western United States,[ 45 ] it is plausible that HBV awareness and/or availability of care providers with knowledge of HBV management and HCC surveillance guidelines would be higher in these regions than in the South. In addition, localities with sizeable foreign‐born populations have been targeted for community‐based HBV outreach programs that have been shown to increase awareness of HBV and facilitate linkage to care for infected individuals.[ 46 , 47 , 48 , 49 ]
Notably, older age and higher baseline comorbidity burden were significantly associated with lower HCC surveillance. These findings suggest that the challenges involved in managing multiple conditions for patients who are in poorer health may increase the likelihood that surveillance recommendations will be overlooked—a concerning possibility, given that increased age is a risk factor for HCC.[ 5 ] Conversely, this finding may reflect appropriate provider decisions regarding the lower value of HCC surveillance in patients with a high competing risk of mortality.[ 50 ]
Study limitations
The results of this study should be considered in light of several limitations. First, surveillance estimates were modeled in a population that was screening‐naïve during the baseline period; however, surveillance receipt may be higher among patients with prior surveillance before the index date. Second, the presence of a diagnosis code on a claim is not proof of disease, as codes may have been entered incorrectly or included as rule‐out diagnoses. Patient misidentification was minimized by requiring at least two nondiagnostic claims for HBV during the identification period; however, this may have caused surveillance to be overestimated, as patients with only one HBV code were excluded. Third, information on factors that contribute to HCC risk and may affect screening recommendations for patients with HBV infection (e.g., patient race/ethnicity, hepatitis delta virus infection) were not available for this study, and while diagnosis codes for family history of HCC exist, they were not included in this analysis as it is unclear to what extent they would have been captured in the 12‐month baseline period. Without these data, it is possible that some patients who did not meet HCC surveillance criteria were inadvertently included in the study population. This may be particularly true of untreated patients, which may have contributed to the lower estimates of surveillance receipt observed for this group. Fourth, while blood tests may generally require less time than imaging‐based surveillance, a standard estimate of 4 work hours lost per encounter was used for all surveillance methods in the patient productivity cost calculations to help account for factors such as travel time and work hours lost by individuals providing transportation assistance; this may underestimate the cost differential between imaging‐based and blood test–based surveillance. In addition, it was not possible for this study to distinguish the ancillary costs of services that occurred on the same day as the AFP laboratory test, including phlebotomy and other charges related to testing or office visits. Together, these factors may have led to overestimation of costs for AFP testing. Finally, because this analysis was conducted in a US population with commercial or MAPD insurance, study results may not be generalizable to populations such as patients who are uninsured, enrolled in Medicaid, or outside the United States. However, uninsured or underinsured populations may have more barriers to medical care overall, potentially resulting in even poorer adherence to HCC surveillance.
CONCLUSIONS
Patients with HBV infection experienced substantial economic burden due to health care encounters related to HCC surveillance. Furthermore, HCC surveillance was low in this patient population, potentially mitigating surveillance effectiveness in clinical practice. The development of accessible and easy‐to‐implement biomarkers with sufficient accuracy for effective early‐stage HCC detection could help reduce barriers to patient adherence and thereby improve implementation of surveillance programs.
FUNDING INFORMATION
Supported by Exact Sciences Corp.
CONFLICTS OF INTEREST
MN consults and advises for Intercept, GSK, Eli Lily, and Laboratory of Advanced Medicine. She receives grants from Pfizer, Enanta, Vir Biotech, Glycotest, CurveBio, and Helio Health. She consults, advises, and receives grants from Exact Sciences and Gilead. LR consults for and received grants from Bayer and Exact Sciences. He consults for QED, Grail, and Norvatis Venture Fund. He advises Astra Zeneca Clinical Care Options Envision, Genetech, the Lynx Group, MJH Life Sciences, and Pontifax Venture. He has received royalties from Five Prime Therapeutics. AS consults for Exact Sciences, Bayer, Glycotest, Grail, and Roche Fuji Film Medical Sciences. TB owns stock in UnitedHealth Group. AO owns stock in Exact Sciences.
DISCLOSURES
This work was funded by Exact Sciences Corp. Burak Ozbay is an employee of and owns stock in Exact Sciences Corporation. Nicole Engel‐Nitz and Tim Bancroft are employees of Optum, which was contracted by Exact Sciences Corp. to conduct this study. Mindie Nguyen has received grants from B.K. Kee Foundation, Enanta Pharmaceuticals Inc., Gilead Sciences Inc., Glycotest, National Cancer Institute, Pfizer and Vir Biotechnology, and has served as a consultant for Bayer, Eisai Co. Ltd., Eli Lilly and Co., Exact Sciences Corp., Gilead Sciences, Intercept Pharmaceuticals, Janssen, Laboratory of Advanced Medicine, Novartis, and Spring Bank Pharmaceuticals. Lewis Roberts has received grants from ARIAD Pharmaceuticals, Bayer, BTG International Inc., Exact Sciences Corp., Gilead Sciences Inc., and Glycotest; has received royalties from Five Prime Therapeutics; has received payment for presentations or educational events from AstraZeneca Pharmaceuticals LP, Clinical Care Options, Envision Communications, Genentech Inc., The Lynx Group LLC, MJH Life Sciences, and Pontifax Venture Capital; and has served as a consultant or on advisory boards for AstraZeneca Pharmaceuticals LP, Bayer, Clinical Care Options, Exact Sciences Corp., Genentech Inc., Gilead Sciences Inc., GRAIL, Tavec Inc., MJH Life Sciences, Pontifax Venture Capital, and QED Therapeutics. Amit Singal has served as a consultant or on advisory boards for Bayer, Exact Sciences Corp., Glycotest, GRAIL, Roche, and Wako Diagnostics. Medical writing assistance was provided by Yvette Edmonds, Ph.D. (Optum) and contracted by Exact Sciences Corp.
Supporting information
Table S1 Codes used in patient selection
Table S2 Total and patient‐paid daily hepatocellular carcinoma (HCC) surveillance costs for completed outpatient surveillance by hepatitis B virus (HBV) treatment status. Costs were calculated among patients with no inpatient stay or emergency room visit during the follow‐up period (n = 2570).
Nguyen MH, Roberts LR, Engel‐Nitz NM, Bancroft T, Ozbay AB, Singal AG. Gaps in hepatocellular carcinoma surveillance among insured patients with hepatitis B infection without cirrhosis in the United States. Hepatol Commun. 2022;6:3443–3456. 10.1002/hep4.2087
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [DOI] [PubMed] [Google Scholar]
- 3. Global Burden of Disease Liver Cancer Collaboration , Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–50. [DOI] [PubMed] [Google Scholar]
- 7. Noone A, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. Seer cancer statistics review, 1975–2017. Bethesda, MD, USA: National Cancer Institute;2020. April 9. [cited 2020 Jul]. Available from: https://seer.cancer.gov/csr/1975_2017/ [Google Scholar]
- 8. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–80. [DOI] [PubMed] [Google Scholar]
- 9. Singal AG, Yopp A, Skinner CS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27:861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang Y, Wallace MC, Adams LA, MacQuillan G, Garas G, Ferguson J, et al. Rate of nonsurveillance and advanced hepatocellular carcinoma at diagnosis in chronic liver disease. J Clin Gastroenterol. 2018;52:551–6. [DOI] [PubMed] [Google Scholar]
- 11. Mittal S, Kanwal F, Ying J, Chung R, Sada YH, Temple S, et al. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: a United States cohort. J Hepatol. 2016;65:1148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang C, Chen V, Vu V, le A, Nguyen L, Zhao C, et al. Poor adherence and low persistency rates for hepatocellular carcinoma surveillance in patients with chronic hepatitis B. Medicine (Baltimore). 2016;95:e4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu Y, Johnson KB, Roccaro G, Lopez J, Zheng H, Muiru A, et al. Poor adherence to AASLD guidelines for chronic hepatitis B Management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong CR, Garcia RT, Trinh HN, Lam KD, Ha NB, Nguyen HA, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54:2712–21. [DOI] [PubMed] [Google Scholar]
- 15. Sarkar M, Shvachko VA, Ready JB, Pauly MP, Terrault NA, Peters MG, et al. Characteristics and management of patients with chronic hepatitis B in an integrated care setting. Dig Dis Sci. 2014;59:2100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldberg DS, Valderrama A, Kamalakar R, Sansgiry SS, Babajanyan S, Lewis JD. Hepatocellular carcinoma surveillance rates in commercially insured patients with noncirrhotic chronic hepatitis B. J Viral Hepat. 2015;22:727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204–9. [DOI] [PubMed] [Google Scholar]
- 18. Yang B, Zhang B, Xu Y, Wang W, Shen Y, Zhang A, et al. Prospective study of early detection for primary liver cancer. J Cancer Res Clin Oncol. 1997;123:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bae H, Lee SA, Choi JW, Hwang SH, Park S, Park MS. Effectiveness of hepatocellular carcinoma surveillance and an optimal surveillance interval: nationwide cohort of Korea. Yonsei Med J. 2021;62:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su F, Weiss NS, Beste LA, Moon AM, Jin GY, Green P, et al. Screening is associated with a lower risk of hepatocellular carcinoma‐related mortality in patients with chronic hepatitis B. J Hepatol. 2021;74:850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao C, Jin M, Le RH, Le MH, Chen VL, Jin M, et al. Poor adherence to hepatocellular carcinoma surveillance: a systematic review and meta‐analysis of a complex issue. Liver Int. 2018;38:503–14. [DOI] [PubMed] [Google Scholar]
- 22. Zhao C, Xing F, Yeo YH, Jin M, Le R, Le M, et al. Only one‐third of hepatocellular carcinoma cases are diagnosed via screening or surveillance: a systematic review and meta‐analysis. Eur J Gastroenterol Hepatol. 2020;32:406–19. [DOI] [PubMed] [Google Scholar]
- 23. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US veterans with hepatitis B: a national cohort study. Hepatology. 2016;63:1774–82. [DOI] [PubMed] [Google Scholar]
- 24. Tran S, Jeong D, Henry L, Cheung RC, Nguyen MH. Initial evaluation, long‐term monitoring, and hepatocellular carcinoma surveillance of chronic hepatitis B in routine practice: a nationwide US study. Am J Gastroenterol. 2021;116:1885–95. [DOI] [PubMed] [Google Scholar]
- 25. Singal AG, Tiro JA, Murphy CC, Blackwell JM, Kramer JR, Khan A, et al. Patient‐reported barriers are associated with receipt of hepatocellular carcinoma surveillance in a multicenter cohort of patients with cirrhosis. Clin Gastroenterol Hepatol. 2021;19:987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farvardin S, Patel J, Khambaty M, Yerokun OA, Mok H, Tiro JA, et al. Patient‐reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology. 2017;65:875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–82. [DOI] [PubMed] [Google Scholar]
- 28. Clinical classifications software (CCS) for ICD‐9‐CM/ICD‐10‐CM. Rockville, MD: Agency for Healthcare Research and Quality;2018. [cited 2017 Apr]. Available from: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp [Google Scholar]
- 29. US Department of Labor, Bureau of Labor Statistics . National occupational employment and wage estimates. Washington, DC, USA: United States Department of Labor. [cited 2017 Apr]. Available from: https://www.bls.gov/oes/current/oes_nat.htm#00‐0000 [Google Scholar]
- 30. US Department of Labor . Minimum wage. [cited 2017 Apr]. Available from: https://www.dol.gov/agencies/whd/minimum‐wage
- 31. US Department of Labor, Bureau of Labor Statistics . Consumer Price Index. Medical Care. Series ID: CUUR0000SAM. 2018. [cited 2019]. Available from: http://data.bls.gov/cgi‐bin/surveymost?cu
- 32. Bruix J, Sherman M, Practice Guidelines Committee AAftSoLD . Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. [DOI] [PubMed] [Google Scholar]
- 33. Wolf E, Rich NE, Marrero JA, Parikh N, Singal AG. Utilization of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta‐analysis. Hepatology. 2021;73:713–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen MH, Engel‐Nitz NM, Bancroft T, Ozbay AB, Singal AG. Gaps in surveillance for hepatocellular carcinoma among high‐risk patients in an insured United States population. APHA Virtual Annual Meeting and Expo. Washington, DC, USA: American Public Health Association;2020. [Google Scholar]
- 35. Nguyen MH, Burak Ozbay A, Liou I, Meyer N, Gordon SC, Dusheiko G, et al. Healthcare resource utilization and costs by disease severity in an insured national sample of US patients with chronic hepatitis B. J Hepatol. 2019;70:24–32. [DOI] [PubMed] [Google Scholar]
- 36. Onyirioha K, Mittal S, Singal AG. Is hepatocellular carcinoma surveillance in high‐risk populations effective? Hepat Oncol. 2020;7:HEP25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Woolen SA, Singal AG, Davenport MS, Troost JP, Khalatbari S, Mittal S, et al. Patient preferences for hepatocellular carcinoma surveillance parameters. Clin Gastroenterol Hepatol. 2022;20:204–15.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu M, Tseng TC, Jun DW, Yeh ML, Trinh H, Wong GLH, et al. Transition rates to cirrhosis and liver cancer by age, gender, disease and treatment status in Asian chronic hepatitis B patients. Hepatol Int. 2021;15:71–81. [DOI] [PubMed] [Google Scholar]
- 39. Goldberg DS, Taddei TH, Serper M, Mehta R, Dieperink E, Aytaman A, et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology. 2017;65:864–74. [DOI] [PubMed] [Google Scholar]
- 40. Le MH, Yeo YH, Cheung R, Henry L, Lok AS, Nguyen MH. Chronic hepatitis B prevalence among foreign‐born and U.S.‐born adults in the United States, 1999–2016. Hepatology. 2020;71:431–43. [DOI] [PubMed] [Google Scholar]
- 41. Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR‐8):1–20. [PubMed] [Google Scholar]
- 42. Lim JK, Nguyen MH, Kim WR, Gish R, Perumalswami P, Jacobson IM. Prevalence of chronic hepatitis B virus infection in the United States. Am J Gastroenterol. 2020;115:1429–38. [DOI] [PubMed] [Google Scholar]
- 43. Wong RJ, Brosgart CL, Welch S, Block T, Chen M, Cohen C, et al. An updated assessment of chronic hepatitis B prevalence among foreign‐born persons living in the United States. Hepatology. 2021;74:607–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. National Center for Health Statistics . Prevalence and trends in hepatitis B virus infection in the United States, 2015–2018. Atlanta, GA, USA: Centers for Disease Control and Prevention;2020. [cited 2021 Dec 2]. Available from: https://www.cdc.gov/nchs/products/databriefs/db361.htm [Google Scholar]
- 45. Migration Policy Institute . Frequently requested statistics on immigrants and immigration in the United States. Washington, DC, USA: Migration Policy Institute;2021. [cited 2021 Dec 1]. Available from: https://www.migrationpolicy.org/article/frequently‐requested‐statistics‐immigrants‐and‐immigration‐united‐states [Google Scholar]
- 46. Shankar H, Blanas D, Bichoupan K, Ndiaye D, Carmody E, Martel‐Laferriere V, et al. A novel collaborative community‐based hepatitis B screening and linkage to care program for African immigrants. Clin Infect Dis. 2016;62((Suppl 4)):S289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zacharias T, Wang W, Dao D, Wojciechowski H, Lee WM, do S, et al. HBV outreach programs significantly increase knowledge and vaccination rates among Asian pacific Islanders. J Community Health. 2015;40:619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dang JH, Chen MS Jr. Increasing hepatitis B testing and linkage to care of foreign‐born Asians, Sacramento, California, 2012–2013. Public Health Rep. 2016;131(Suppl 2):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harris AM, Link‐Gelles R, Kim K, Chandrasekar E, Wang S, Bannister N, et al. Community‐based services to improve testing and linkage to care among non‐U.S.‐born persons with chronic hepatitis B virus infection—three U.S. Programs, October 2014‐September 2017. MMWR Morb Mortal Wkly Rep. 2018;67:541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rich NE, Singal AG. Overdiagnosis of hepatocellular carcinoma: prevented by guidelines? Hepatology. 2022;75:740–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Codes used in patient selection
Table S2 Total and patient‐paid daily hepatocellular carcinoma (HCC) surveillance costs for completed outpatient surveillance by hepatitis B virus (HBV) treatment status. Costs were calculated among patients with no inpatient stay or emergency room visit during the follow‐up period (n = 2570).


