Abstract
Fenofibrate (FF) has shown potential benefits in patients with primary biliary cholangitis (PBC) who have an incomplete response to ursodeoxycholic acid (UDCA). However, the efficacy and safety of FF in patients with cirrhosis remain unclear. To evaluate the efficacy and safety of additional FF therapy in patients with PBC‐related cirrhosis with an incomplete response to UDCA, we conducted a retrospective analysis comparing the clinical results of additional FF therapy and continued UDCA monotherapy. A total of 59 patients were included; 27 cases underwent UDCA monotherapy and 32 cases underwent UDCA combined with FF therapy. A significant difference in alkaline phosphatase (ALP) normalization was achieved in the FF group compared to the UDCA group (37% vs. 11%, respectively; p = 0.020). Additional FF therapy was an independent risk factor for ALP normalization (hazard ratio, 7.679; 95% confidence interval, 2.059–28.633; p = 0.003). Hepatic deterioration was experienced by 40% versus 48% (p = 0.562) while 11% vs. 37% (p = 0.111) experienced liver‐related mortality or liver transplantation in the FF and UDCA groups, respectively. Compared to UDCA monotherapy, additional FF therapy was associated with lower United Kingdom (UK)‐PBC risk score and surrogate serum indices of liver fibrosis. After 12 months of add‐on FF therapy, median ALP level and UK‐PBC risk score decreased 35% and 52% from baseline (p = 0.001 and 0.210, respectively). Serum aminotransferase, triglyceride, and cholesterol decreased progressively, while total bilirubin, serum creatinine, blood urea, estimated glomerular filtration rate, aspartate aminotransferase‐to‐platelet ratio index, and fibrosis‐4 index remained stable in FF‐treated cirrhotic cases during follow‐up. No significant adverse effects associated with additional FF therapy were observed in our cohort. Conclusion: Additional FF therapy was associated with higher ALP normalization rates and lower UK‐PBC risk scores in patients with cirrhotic PBC with an incomplete response to UDCA. In addition, FF therapy seemed safe and well tolerated with a low frequency of adverse effects in patients with cirrhosis.
INTRODUCTION
Primary biliary cholangitis (PBC) is a progressive cholestasis featuring chronic nonsuppurative cholangitis that occurs mainly in the background of genetic susceptibilities and environmental inducers.[ 1 ] Currently, the only first‐line drug licensed for disease‐modifying therapy is ursodeoxycholic acid (UDCA).[ 2 ] Long‐term UDCA treatment is effective in ameliorating cholestasis‐related indicators, delaying histologic progression, and extending liver transplantation (LT)‐free survival.[ 3 ] However, approximately 40% of patients with PBC fail to achieve biochemical responses to UDCA in clinical practice and have limited long‐term survival, indicating a clear need for additional treatment.[ 4 ]
Fenofibrate (FF), a peroxisome proliferator‐activated alpha receptor (PPARα) agonist, has been unexpectedly found to improve indicators of cholestasis in the treatment of hyperlipidemia in patients with PBC.[ 5 , 6 ] Recently, FF has been recognized as a potential anticholestatic agent to reduce parameters of cholestasis and abnormal liver function and has had good safety features in patients with an incomplete response to UDCA in multiple studies.[ 7 , 8 , 9 , 10 , 11 ] Guidance from the American Association for the Study of Liver Diseases recommends that off‐label therapy be recognized as an alternative in patients with PBC who are inadequate responders to UDCA, notably with the PPAR agonist fibrates.[ 12 ] However, the safety profile of FF remedial treatment on clinical outcomes in patients with cirrhosis raises concern. Cheung et al.[ 13 ] showed that serum bilirubin increased more rapidly in advanced PBC treated with FF for a mean duration of 11 months. A 24‐month follow‐up study observed that two of the three patients with elevated serum bilirubin suffered from cirrhosis.[ 14 ] Notably, it also demonstrated that the estimated glomerular filtration rate (eGFR) and serum creatinine (SCr) deteriorated over time, possibly due to the high proportion of patients with advanced liver disease in the cohort, suggesting caution and avoidance of this drug in patients with cirrhosis. In addition, the efficacy of FF in patients with cirrhosis is limited, with lower rates of alkaline phosphatase (ALP) normalization and increased mortality or liver‐related outcomes.[ 13 , 14 ] Nevertheless, because the majority of the current studies include small numbers of patients and have short follow‐up periods, the efficacy and safety of FF in patients with cirrhosis cannot be well addressed.
To further explore these issues, we conducted a study including 59 patients with cirrhotic PBC who had an incomplete response to UDCA therapy. The aim of this study was to evaluate the efficacy profile of add‐on FF, which was assessed by ALP normalization and the decline in the United Kingdom (UK)‐PBC risk score. Our secondary objective was to assess the safety of additional fenofibrate treatment.
MATERIALS AND METHODS
Study population
We analyzed 59 consecutive subjects with PBC who had an incomplete response to prior UDCA monotherapy and who were diagnosed and treated in Xijing Hospital of Digestive Diseases (Xi'an, Shanxi, China) from February 2010 to November 2021. Patients with evidence of concomitant liver disease (autoimmune hepatitis, alcoholic hepatitis, drug‐induced liver injury, viral hepatitis, hemochromatosis, hepatocellular carcinoma) were excluded from our design. A diagnosis of PBC is made if it meets two of the three following standards: (1) evidence of cholestasis characterized by elevated ALP levels; (2) detection of anti‐mitochondrial antibody (AMA) or other disease‐specific autoantibodies; (3) histologic evidence suggesting typical PBC.[ 15 ] The definition of incomplete response to UDCA was failure to meet the ALP cut‐off value (serum ALP > 1.67 × ULN) used in Toronto criteria after 6 months of prior UDCA monotherapy.[ 16 ] A diagnosis of cirrhosis was made if it met one of the three following criteria: (1) liver stiffness measurement >16.9 KPa; (2) liver biopsy showing stage F4 fibrosis; and (3) magnetic resonance imaging or computed tomography suggestive of cirrhosis.[ 17 , 18 ] UDCA and FF were administered orally at doses of 13–15 mg/kg/day and 200 mg/day, respectively.
Study design
A retrospective cohort study was conducted. Patients were divided into a UDCA group and an FF group depending on treatment with either UDCA monotherapy or UDCA along with FF, respectively. We explored the efficacy and safety of FF by comparing the clinical characteristics of the two groups. Biochemical response was determined by achieving normal serum ALP levels during follow‐up. To evaluate the efficacy of FF, the primary outcome was the percentage of cases that obtained biochemical responses. The secondary outcomes included the development of liver deterioration and LT‐free survival. The safety of FF was assessed primarily in terms of FF‐related symptoms, hepatotoxicity, and nephrotoxicity. Hepatic deterioration was determined by the presence of a decompensatory event (such as hepatic encephalopathy, ascites, or variceal bleeding) and/or progression of the Child‐Pugh grade by at least one level.[ 19 ] To estimate the risk of transplantation or liver‐related mortality, we introduced the UK‐PBC risk score.[ 20 ] Two surrogate serum indices of liver fibrosis were also assessed, the aspartate aminotransferase‐to‐platelet ratio index (APRI) score and the fibrosis‐4 (FIB‐4) index.[ 21 , 22 ] The Chronic Kidney Disease Epidemiology Collaboration equation was used to calculate eGFR values.[ 23 ] The study design was approved by the ethics committee of the Xijing Hospital of the Air Force Military Medical University.
Data collection and analysis
We systematically collected clinical information at presentation and each follow‐up. Data included general characteristics, clinical symptoms, serology results, and histologic findings. Liver biopsies were analyzed based on the METAVIR and Ludwig Symposium scoring systems for chronic hepatitis and PBC, respectively. Biopsy specimens were assessed by two qualified and experienced pathologists who were blinded to the results of serologic tests.
Statistical analysis
SPSS, version 26.0 (IBM), was applied to all statistical analyses. Quantitative variables were described as median or mean and interquartile range or SD and were analyzed with the Mann‐Whitney U test or the paired t test. Qualitative variables were assessed using the chi‐squared test. Cox regression analyses were used to assess the relation between FF and time to events, which was assessed by ALP normalization, hepatic deterioration, LT, or liver‐related death. Univariate analysis was used to obtain crude hazard ratios (HRs). All variables that were statistically significant (p < 0.05) at univariate analysis were adjusted for in a multivariate model. The Kendall‐Tau‐b correlation model was used for consistency analysis. Statistical significant was identified as a two‐sided p < 0.05.
RESULTS
Study population
The study flowchart is shown in Figure 1. Of the 59 patients with cirrhosis with PBC and incomplete response to UDCA, 32 (54%) were treated with UDCA along with FF (FF group) and 27 (46%) continued with UDCA monotherapy (UDCA group). The mean patient age was 55 ± 8 years. The majority of patients were women (89%), Child‐Pugh A (84%), and AMA positive (86%). Notably, no patients with Child‐Pugh C were included in our study cohort at baseline (Table S1). FF was administrated at a median of 12 months (range, 6–36 months) after UDCA. The median time of exposure to FF was 36 months (range, 12–108 months). The median Model for End‐Stage Liver Disease (MELD) score and APRI score for the study cohort at baseline were 8.0 and 1.6, respectively. No significant differences apart from serum gamma‐glutamyl transpeptidase (GGT), immunoglobulin G, and triglyceride (TG) levels were identified for both groups at baseline (p = 0.001, p = 0.001, and p < 0.001, respectively; Table 1).
FIGURE 1.
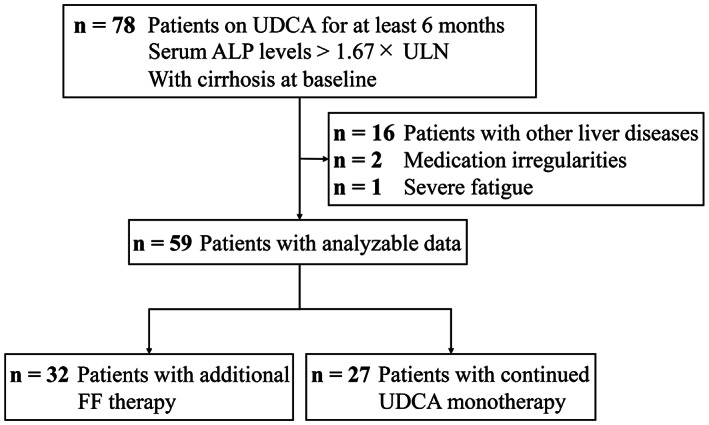
Study flowchart. ALP, alkaline phosphatase; FF, fenofibrate; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
TABLE 1.
Baseline characteristics of patients with UDCA‐refractory cirrhotic primary biliary cholangitis
| Characteristic | FF group | UDCA group | p value |
|---|---|---|---|
| n = 32 | n = 27 | ||
| Age, years (mean ± SD) | 54 ± 10 | 55 ± 7 | p = 0.671 |
| Female, n (%) | 28 (88) | 25 (93) | p = 0.678 |
| Follow‐up time, months | 36 (24–48) | 36 (24–60) | p = 0.895 |
| AMA positive, n (%) | 28 (87) | 23 (85) | p = 1.000 |
| Child‐Pugh A/B, n (%) | 27 (85)/5 (15) | 23 (86)/4 (14) | p = 1.000 |
| MELD score | 8.0 (7.0–10.0) | 8.0 (7.0–9.0) | p = 0.658 |
| APRI index | 1.6 (1.0–2.6) | 1.8 (1.3–2.8) | p = 0.263 |
| ALP × ULN | 2.6 (2.0–3.0) | 2.2 (1.9–3.0) | p = 0.479 |
| GGT × ULN | 6.7 (4.1–12.2) | 3.6 (2.5–5.6) | p = 0.001 |
| ALT × ULN | 1.6 (1.1–2.0) | 1.2 (0.9–1.9) | p = 0.105 |
| AST × ULN | 1.7 (1.4–2.4) | 1.9 (1.3–2.6) | p = 0.939 |
| ALB × LLN | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | p = 0.558 |
| Tbil × ULN | 1.1 (0.8–1.6) | 1.0 (0.8–1.5) | p = 0.744 |
| PLT (×109/L) | 118 (78–161) | 91 (76–100) | p = 0.115 |
| IgG × ULN | 0.8 (0.7–0.9) | 1.0 (0.9–1.1) | p = 0.001 |
| IgM × ULN | 1.3 (0.8–1.7) | 1.0 (0.8–1.6) | p = 0.760 |
| TG × LLN | 0.9 (0.7–1.5) | 0.6 (0.5–0.7) | p < 0.001 |
| BU × ULN | 0.6 (0.5–0.7) | 0.7 (0.5–0.8) | p = 0.188 |
| Scr × ULN | 1.0 (0.8–1.1) | 0.9 (0.8–1.0) | p = 0.891 |
| eGFR (ml/minute/1.73 m2) | 96 (91–101) | 95 (87–103) | p = 0.692 |
Note: Shown are the median values and interquartile ranges.
Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMA, anti‐mitochondrial antibody; APRI, aspartate aminotransferase‐to‐platelet ratio index; AST, aspartate aminotransferase; BU, blood urea; eGFR, estimated glomerular filtration rate; FF, fenofibrate; GGT, gamma‐glutamyl transpeptidase; IgG, immunoglobulin G; IgM, immunoglobulin M; LLN, lower limit of normal; MELD, Model for End‐Stage Liver Disease; PLT, platelet; Scr, serum creatinine; Tbil, total bilirubin; TG, triglyceride; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Primary and secondary outcomes
The primary outcome was obtained in 37% of additional FF‐treated cases versus only 11% of UDCA‐treated cases during follow‐up (p = 0.020; Figure 2A). Eight patients in the FF group and no patient in the UDCA group achieved ALP normalization at 12 months (25% vs. 0%, p = 0.006); 10 patients in the FF group and no patient in the UDCA group achieved ALP normalization at 24 months (40% vs. 0%, p = 0.001). Other dichotomic response criteria are shown in Table 2. Univariate analysis of the ALP normalization and non‐normalization cohorts demonstrated that additional FF therapy and younger age were associated with increased ALP normalization (Table S2). A multivariate Cox analysis was performed to evaluate the independent contribution of ALP normalization, adjusting for age which was significant in the univariate analysis. Additional FF therapy was an independent risk factor for ALP normalization (HR, 7.679; 95% confidence interval [CI], 2.059–28.633; p = 0.003; Table S3). The other variable that remained significant in the multivariate cox analysis was age (HR, 1.095 per 1 year; 95% CI, 1.021–1.174; p = 0.011).
FIGURE 2.
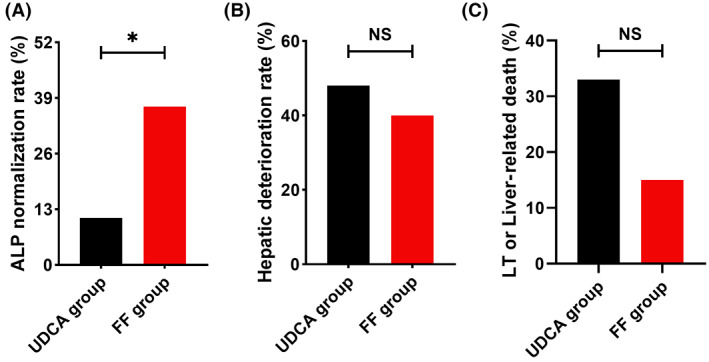
Incidence of primary and secondary outcomes in patients with UDCA‐refractory PBC treated with additional FF (the FF group) or UDCA monotherapy (the UDCA group). (A) ALP normalization rate. (B) Hepatic deterioration rate. (C) LT or liver‐related death. Data were analyzed by the chi‐squared test. ALP, alkaline phosphatase; FF, fenofibrate; LT, liver transplant; UDCA, ursodeoxycholic acid.
TABLE 2.
Patients who met dichotomic response criteria between the FF group and the UDCA group
| Response criteria, n (%) | FF group | UDCA group | p value |
|---|---|---|---|
| ALP normalization (at 12 months) | 8 (24) | 0 (0) | 0.006 |
| ALP normalization (at 24 months) | 10 (40) | 0 (0) | 0.001 |
| Toronto criteria | 18 (56) | 10 (37) | 0.141 |
| Paris‐I criteria | 13 (40) | 9 (33) | 0.564 |
| Paris‐II criteria | 6 (18) | 3 (11) | 0.488 |
Abbreviations: ALP, alkaline phosphatase; FF, fenofibrate; UDCA, ursodeoxycholic acid.
Thirteen FF‐treated cases and 13 UDCA‐treated cases experienced hepatic deterioration, and no statistical difference was found between the groups by the study end (40% vs. 48%, p = 0.562; Figure 2B). The deterioration events of these patients with cirrhosis are shown in Table 3. In our study cohort, the major constituent event of hepatic deterioration in the FF group was mild ascites (53%), whereas the major constituent event of hepatic deterioration in the UDCA group was variceal bleed (46%) and moderate to severe ascites (30%). A multivariate Cox analysis demonstrated that additional FF therapy was not an independent risk factor for hepatic deterioration (crude HR, 0.879; 95% CI, 0.406–1.903; p = 0.743; Table S4).
TABLE 3.
Patients who experienced hepatic decompensation, death, or liver transplantation when treated with additional FF therapy or UDCA monotherapy
| Outcome | FF group | UDCA group |
|---|---|---|
| Hepatic deterioration, n (%) | 13 | 13 |
| Variceal bleed | 1 (8) | 6 (46) |
| Ascites | ||
| Mild | 7 (53) | 1 (8) |
| Moderate–severe | 1 (8) | 4 (30) |
| Encephalopathy | 1 (8) | 1 (8) |
| Child‐Pugh progression | 3 (23) | 1 (8) |
| Transplantation or died, n (%) | 5 | 9 |
| Transplantation | 1 (20) | 1 (11) |
| Died | 4 (80) | 8 (89) |
Abbreviations: FF, fenofibrate; UDCA, ursodeoxycholic acid.
Liver‐related death and LT occurred in eight and one patient, respectively, in the UDCA group and four and one patient, respectively, in the FF group. Compared to UDCA‐treated cases, FF‐treated cases reported a lower rate of liver‐related mortality or LT by study end despite the absence of statistical differences (15% vs. 33%, p = 0.111; Figure 2C). A multivariate Cox analysis demonstrated that additional FF therapy was not an independent risk factor for liver‐related death or LT (crude HR, 0.567; 95% CI, 0.189–1.702; p = 0.311; Table S5). However, we did not find a relationship between ALP normalization (at 12 or 24 months) and clinical outcomes in our study cohort. The p values of Kendall‐Tau‐b correlation analysis are shown in Table S6.
Biochemical measures
The dynamic changes in ALP, total bilirubin (Tbil), UK‐PBC risk score, alanine aminotransferase (ALT), aspartate aminotransferase (AST), TG, cholesterol (CHO), APRI score, FIB‐4 index, SCr, blood urea (BU), and eGFR during treatment are shown in Figure 3. At 12 months, the levels of ALP decreased 35% from baseline in FF‐treated cases and 20% in UDCA‐treated cases (p = 0.001 and p = 0.003, respectively). Similar reductions were found in ALT in both groups (p < 0.05, both). The median AST, TG, and CHO in the FF group and eGFR in the UDCA group decreased progressively, with no statistical differences between baseline and 60 months, while Tbil, UK‐PBC risk score, APRI score, FIB‐4 index, BU, and Scr remained stable in both groups. Lower median APRI score and FIB‐4 index and higher median eGFR were observed in the FF group during follow‐up. Compared to UDCA‐treated cases, patients with FF add‐on therapy had significantly lower ALP level, UK‐PBC risk score, APRI score, and FIB‐4 index during follow‐up, while no statistical differences were observed in Tbil, ALT, AST, TG, CHO, BU, Scr, and eGFR. Elevated ALP levels were observed in three patients after stopping FF but not UDCA for 0.25–3 months, and two patients reached normal values after resuming FF therapy.
FIGURE 3.
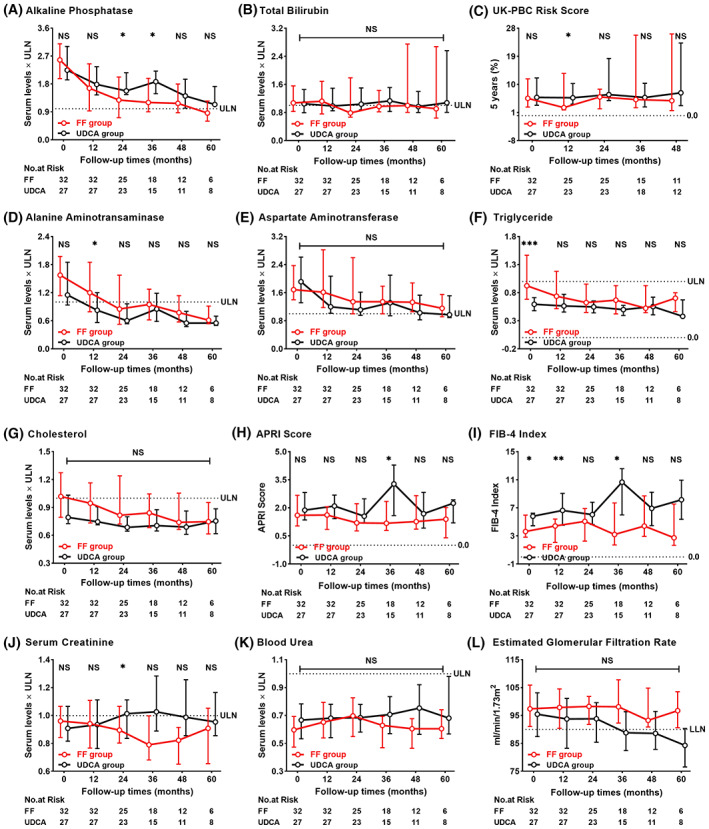
Dynamic changes between the FF group and the UDCA group at each follow‐up visit. (A) Alkaline phosphatase, (B) total bilirubin, (C) UK‐PBC risk score, (D) alanine aminotransferase, (E) aspartate aminotransferase, (F) triglyceride, (G), cholesterol, (H) APRI score, (I) FIB‐4 index, (J) serum creatinine, (K) blood urea, and (L) estimated glomerular filtration rate. Shown are the median values and interquartile ranges. Data were compared by the Mann‐Whitney U test. APRI, aspartate aminotransferase‐to‐platelet ratio index; FF, fenofibrate; FIB‐4, fibrosis‐4; LLN, lower limit of normal; NS, not significant; UDCA, ursodeoxycholic acid; UK‐PBC, United Kingdom primary biliary cholangitis; ULN, upper limit of normal.
Safety of additional FF
Adverse events are listed in Table 4. One patient discontinued using FF due to increased fatigue at 3 months that resolved after stopping FF. Three patients experienced self‐limiting nausea, cramps and myalgia, and bloating in the first 3 months. One patient was found to have elevated ALT levels (5–7 × ULN) at 12 months of treatment, but ALT levels gradually decreased with continued FF therapy under monthly monitoring. Two patients experienced first severe progression of Tbil levels (>100 μmol/L) at 73 and 96 months of additional FF therapy, respectively, one of whom had fluctuating Tbil levels during follow‐up without discontinuing FF; the other patient also experienced renal deterioration, with normal renal function in Child‐Pugh B cirrhosis at baseline, progressed to Child‐Pugh C cirrhosis with eGFR of 28 mL/minute/1.73 m2 at 96 months, and subsequently died. No serious adverse events were identified in other patients treated with FF for more than 12 months.
TABLE 4.
Adverse events of additional FF therapy in patients with cirrhosis
| Adverse event | Number/severity | Relationship to FF |
|---|---|---|
| Fatigue (severe) | 1 persistent | Probably related |
| Gastrointestinal disorders | 2 transient | Possibly related |
| Cramps and myalgia | 1 transient | Possibly related |
| Elevated ALT and AST | Probably related | |
| Severe (5–7 × ULN) | 1 transient | |
| Moderate (2–5 × ULN) | 3 transient | |
| Elevated Tbil (≥100 μmol/L) | Probably not related | |
| First occurrence after enrollment | 1 died; 1 fluctuate | |
| Decreased eGFR | Probably not related | |
| Severe (<30 ml/minute/1.73 m2) | 1 persistent, died |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; FF, fenofibrate; Tbil, total bilirubin; ULN, upper limit of normal.
DISCUSSION
The results of our study suggest that additional FF therapy is associated with higher ALP normalization rates and lower UK‐PBC risk scores in patients with cirrhotic PBC with an incomplete response to UDCA. Parallel changes in surrogate serum indices of liver fibrosis were consistent with this effect. No statistically significant associations between FF and clinical outcome parameters were observed. Median serologic measures of liver or kidney function did not worsen significantly in FF‐treated cases. During follow‐up, we did not observe severe FF‐related adverse events. In this light, FF‐addition therapy seemed to be an alternative option for patients with cirrhosis with an incomplete response to UDCA.
Reduced biochemical responses with increased mortality or liver‐related outcomes have been observed for FF in patients with cirrhosis with an incomplete response to UDCA.[ 13 , 14 ] In this way, the benefit of adding FF to this population appears to be limited. To better illustrate the efficacy of FF, we compared patients with cirrhosis between FF add‐on therapy and UDCA monotherapy.
In the current study, FF was related to a prompt and persistent decrease in ALP levels in patients with cirrhosis with an incomplete response to UDCA. The primary outcome obtained in patients treated with FF was significantly higher than the UDCA‐treated during follow‐up. In addition, the salutary effect of adding FF with decreasing ALP levels persisted even after 60 months. The efficacy of FF can also be supported by observing the relapse of ALP levels after discontinuing the drug. Three patients with cirrhosis who discontinued FF but not UDCA were found to have elevated ALP levels while two patients reached normal values after resuming FF therapy. This beneficial effect was also reached in the serum levels of TC and CHO.
Our study demonstrated that the rate of liver‐related death or LT was lower in FF‐treated cirrhotic cases than the UDCA‐treated cases by study end. However, our sample size was too limited to determine whether these changes were related to an effective increased survival rate. Importantly, we found these changes in patients treated with FF were accompanied by persistently lower APRI score and FIB‐4 index, which were significantly lower at 36 months of follow‐up, compared to the patients treated with UDCA. Together with a lower UK‐PBC risk score, our study revealed that FF add‐on therapy appears to show prolonged LT‐free survival in patients with cirrhosis. However, there was no significant difference in the incidence of liver deterioration between the two groups. This suggests that FF did not delay the onset of liver deterioration in patients with cirrhosis. The compositional difference between the FF and UDCA groups may to a certain extent explain this lack of reduced decompensating events despite FF therapy. Meanwhile, in the current study, FF has failed to show any significant reduction in the predicted 5‐, 10‐, and 15‐year risk rate even after 60 months of treatment in patients with and without cirrhosis, although a disease‐stabilizing effect was seen. Overall, FF addition therapy is effective in patients with cirrhosis with an incomplete response to UDCA.
FFs are generally well tolerated, with symptoms of gastrointestinal and musculoskeletal problems; elevations of aminotransferase and Scr are the most common adverse events.[ 7 , 8 , 9 , 10 , 13 , 14 , 24 ] Several studies have found that FF may trigger hepatotoxicity and nephrotoxicity in patients with cirrhosis and have advised caution and avoidance in the use of the drug.[ 13 , 14 ] Multiple studies have found that abnormal serum Tbil levels were factors in the poor prognosis of PBC, with Tbil >30 μmol/L increasing the incidence of LT or death by 6‐fold.[ 25 , 26 ] In contrast to a previous study that reported FF was associated with an accelerated rise in serum Tbil levels in patients with cirrhosis,[ 13 ] our cohort demonstrated that the median serum Tbil levels remained stable in patients treated with FF during 60 months of follow‐up. For patients with cirrhotic PBC, the evidence evaluating FF use is still limited to a small cohort of patients with limited follow‐up. Together with the different backgrounds of the included population, the conclusions drawn may be different. In addition, a previous study of a Chinese cohort[ 14 ] observed that the Tbil level was stable during follow‐up in patients with cirrhosis who were treated with FF, which is consistent with our findings. Two patients experienced their first severe progression of serum Tbil levels after relatively long periods of FF therapy, suggesting the progression seemed to be related to disease progression rather than FF therapy.
Notably, deterioration in serum Scr levels and eGFR has been reported in patients with cirrhosis after 24 months of FF therapy.[ 14 ] Nevertheless, it has been shown that the increase in serum SCr levels resulting from FF therapy was temporary and reversible, even if treatment was not discontinued.[ 27 ] In our cohort, the median Scr, BU, and eGFR was stable in patients with cirrhosis treated with FF during 60 months of follow‐up. The development of renal deterioration in one patient may have been due to worsening liver function rather than FF therapy. One patient suffered from potential FF‐related hepatotoxicity, which spontaneously resolved within a few months, presumably due to the activation of transaminase gene expression by PPAR‐a.[ 6 , 28 ] Considering that only one patient discontinued FF due to adverse effects and four patients had self‐limiting symptoms, our results clearly indicate that FF add‐on therapy appears to be clinically safe in patients with cirrhosis.
Our study has some notable limitations, such as its single‐center retrospective design and relatively small sample size, which limit the ability to generalize the results. Unlike randomized controlled studies, there is the potential for selection bias, and there were some significant differences in baseline characteristics between the two treatment groups; however, important factors, such as baseline ALP level or MELD score, were not significantly different. We also acknowledge that only a minority of patients completed 60 months of treatment. Furthermore, because our patients did not undergo paired liver biopsy at baseline and after FF therapy, the effect of FF on liver histology remains unanswered. The findings of our study cohort might have been caused by the differences between groups or simply by sampling error; therefore, particular caution is required in the interpretation. Nevertheless, we believe our results are novel and provide further insights into the utility of additional FF therapy in PBC.
In conclusion, the higher ALP normalization rates and lower UK‐PBC risk scores compared to UDCA monotherapy suggest that additional FF therapy would lead to more salutary clinical effects in patients with cirrhosis. In addition, this combined therapy appears to be safe and well tolerated with a low frequency of adverse effects during treatment. In this light, it seems to be an alternative option for patients with cirrhosis with an incomplete response to UDCA to receive additional FF therapy. Further, larger long‐term cohort studies should be performed to confirm these results.
AUTHOR CONTRIBUTIONS
Guarantor of the article: Ying Han. Conceived and designed the study: Ying Han, Yulong Shang, Changcun Guo. Data collection: Dawei Ding, Juan Deng, Gui Jia. Analyzed the data: Guanya Guo, Xiufang Wang, Ruiqing Sun. Drafted the paper: Yansheng Liu, Linhua Zheng. All authors critically reviewed the manuscript. All authors approved the final version of the manuscript and authorship list.
FUNDING INFORMATION
National Natural Science Foundation of China; Grant Numbers: 81820108005, 81900502, and 82173241; National Key Research and Development Program of China; Grant Number: 2017YFA0105704; Key Research and Development Program of Shaanxi; Grant Number: 2021ZDLSF02‐07.
CONFLICT OF INTEREST
Nothing to report.
Supporting information
Table S1‐S6
Ding D, Guo G, Liu Y, Zheng L, Jia G, Deng J, et al. Efficacy and safety of fenofibrate addition therapy in patients with cirrhotic primary biliary cholangitis with incomplete response to ursodeoxycholic acid. Hepatol Commun. 2022;6:3487–3495. 10.1002/hep4.2103
Dawei Ding, Guanya Guo, and Yansheng Liu contributed equally to this work as first authors.
Contributor Information
Changcun Guo, Email: guochc@sina.com.
Yulong Shang, Email: shangyul870222@163.com.
Ying Han, Email: hanying1@fmmu.edu.cn.
REFERENCES
- 1. Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386(10003):1565–75. Erratum in: Lancet. 2015;386(10003):1536. [DOI] [PubMed] [Google Scholar]
- 2. Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ, et al. Primary biliary cirrhosis. Hepatology. 2009;50(1):291–308. [DOI] [PubMed] [Google Scholar]
- 3. Poupon RE, Poupon R, Balkau B. Ursodiol for the long‐term treatment of primary biliary cirrhosis. The UDCA‐PBC Study Group. N Engl J Med. 1994;330(19):1342–7. [DOI] [PubMed] [Google Scholar]
- 4. Parés A, Caballería L, Rodés J. Excellent long‐term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130(3):715–20. [DOI] [PubMed] [Google Scholar]
- 5. Zumoff B. Effect of clofibrate on plasma levels of alkaline phosphatase. N Engl J Med. 1977;297(12):669. [DOI] [PubMed] [Google Scholar]
- 6. Edgar AD, Tomkiewicz C, Costet P, Legendre C, Aggerbeck M, Bouguet J, et al. Fenofibrate modifies transaminase gene expression via a peroxisome proliferator activated receptor alpha‐dependent pathway. Toxicol Lett. 1998;98(1–2):13–23. [DOI] [PubMed] [Google Scholar]
- 7. Han XF, Wang QX, Liu Y, You ZR, Bian ZL, Qiu DK, et al. Efficacy of fenofibrate in Chinese patients with primary biliary cirrhosis partially responding to ursodeoxycholic acid therapy. J Dig Dis. 2012;13(4):219–24. [DOI] [PubMed] [Google Scholar]
- 8. Liberopoulos EN, Florentin M, Elisaf MS, Mikhailidis DP, Tsianos E. Fenofibrate in primary biliary cirrhosis: a pilot study. Open Cardiovasc Med J. 2010;4:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grigorian AY, Mardini HE, Corpechot C, Poupon R, Levy C. Fenofibrate is effective adjunctive therapy in the treatment of primary biliary cirrhosis: a meta‐analysis. Clin Res Hepatol Gastroenterol. 2015;39(3):296–306. [DOI] [PubMed] [Google Scholar]
- 10. Levy C, Peter JA, Nelson DR, Keach J, Petz J, Cabrera R, et al. Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment Pharmacol Ther. 2011;33(2):235–42. [DOI] [PubMed] [Google Scholar]
- 11. Carrion AF, Lindor KD, Levy C. Safety of fibrates in cholestatic liver diseases. Liver Int. 2021;41(6):1335–43. [DOI] [PubMed] [Google Scholar]
- 12. Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2021 practice guidance update from the American Association for the Study of Liver Diseases. Hepatology. 2022;75(4):1012–3. [DOI] [PubMed] [Google Scholar]
- 13. Cheung AC, Lapointe‐Shaw L, Kowgier M, Meza‐Cardona J, Hirschfield GM, Janssen HLA, et al. Combined ursodeoxycholic acid (UDCA) and fenofibrate in primary biliary cholangitis patients with incomplete UDCA response may improve outcomes. Aliment Pharmacol Ther. 2016;43(2):283–93. [DOI] [PubMed] [Google Scholar]
- 14. Duan W, Ou X, Wang X, Wang Y, Zhao X, Wang Q, et al. Efficacy and safety of fenofibrate add‐on therapy for patients with primary biliary cholangitis and a suboptimal response to UDCA. Rev Esp Enferm Dig. 2018;110:557–63. [DOI] [PubMed] [Google Scholar]
- 15. Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69(1):394–419. [DOI] [PubMed] [Google Scholar]
- 16. Kumagi T, Guindi M, Fischer SE, Arenovich T, Abdalian R, Coltescu C, et al. Baseline ductopenia and treatment response predict long‐term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 2010;105(10):2186–94. [DOI] [PubMed] [Google Scholar]
- 17. Corpechot C, Carrat F, Poujol‐Robert A, Gaouar F, Wendum D, Chazouillères O, et al. Noninvasive elastography‐based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56(1):198–208. [DOI] [PubMed] [Google Scholar]
- 18. Aubé C, Oberti F, Korali N, Namour MA, Loisel D, Tanguy JY, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol. 1999;30(3):472–8. [DOI] [PubMed] [Google Scholar]
- 19. Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23(1):164–76. [DOI] [PubMed] [Google Scholar]
- 20. Carbone M, Sharp SJ, Flack S, Paximadas D, Spiess K, Adgey C, et al.; UK‐PBC Consortium. The UK‐PBC risk scores: derivation and validation of a scoring system for long‐term prediction of end‐stage liver disease in primary biliary cholangitis. Hepatology. 2016;63(3):930–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wai C, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26. [DOI] [PubMed] [Google Scholar]
- 22. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al.; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–25. [DOI] [PubMed] [Google Scholar]
- 23. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. Erratum in: Ann Intern Med. 2011;155(6):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hegade VS, Khanna A, Walker LJ, Wong L, Dyson JK, Jones DEJ. Long‐term fenofibrate treatment in primary biliary cholangitis improves biochemistry but not the UK‐PBC risk score. Dig Dis Sci. 2016;61(10):3037–44. [DOI] [PubMed] [Google Scholar]
- 25. Bonnand AM, Heathcote EJ, Lindor KD, Poupon RE. Clinical significance of serum bilirubin levels under ursodeoxycholic acid therapy in patients with primary biliary cirrhosis. Hepatology. 1999;29(1):39–43. [DOI] [PubMed] [Google Scholar]
- 26. Ohira H, Sato Y, Ueno T, Sata M. Fenofibrate treatment in patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97(8):2147–9. [DOI] [PubMed] [Google Scholar]
- 27. Ansquer J, Dalton RN, Caussé E, Crimet D, Le Malicot K, Foucher C. Effect of fenofibrate on kidney function: a 6‐week randomized crossover trial in healthy people. Am J Kidney Dis. 2008;51(6):904–13. [DOI] [PubMed] [Google Scholar]
- 28. Ahmad J, Odin JA, Hayashi PH, Chalasani N, Fontana RJ, Barnhart H, et al. Identification and characterization of fenofibrate‐induced liver injury. Dig Dis Sci. 2017;62(12):3596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S6


