Abstract
Alcohol‐associated liver fibrosis accumulates over decades, driven by hepatic inflammation and cell death. We investigated the diagnostic accuracy of keratin‐18 degradation, measured using serum M30 and M65 levels, and the ActiTest for hepatic inflammatory activity in patients with compensated alcohol‐associated liver disease (ALD). Furthermore, we evaluated the prognostic accuracy of markers for liver‐related events and all‐cause mortality. All findings were compared with routine liver function tests: Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma‐glutamyltransferase. Our prospective, biopsy‐controlled, single‐center study included 265 patients with ongoing or prior excessive alcohol intake, representing the full spectrum of compensated ALD. We defined hepatic inflammatory activity as a combined score of lobular inflammation and ballooning. For severe hepatic inflammatory activity (n = 40), we found excellent diagnostic accuracy for M30 (area under the receiver operating characteristics curve [AUROC] = 0.90), M65 (AUROC = 0.86), and AST (AUROC = 0.86). Elevated M30 (M30 > 240 U/L) had the highest positive predictive value (PPV) and specificity, significantly higher than M65, ActiTest and ALT, but not AST (M30: sensitivity = 83%, specificity = 82%, positive predictive value = 45%, negative predictive value = 95%). Patients were followed up for 1445 patient‐years. All markers, except for ALT, significantly predicted liver‐related events and all‐cause mortality. After adjusting for advanced fibrosis, drinking behavior and body mass index, M30 and M65 remained significant predictors of liver‐related events, whereas M30 and AST were significant predictors of all‐cause mortality. Conclusion: M30 and AST accurately detect severe hepatic inflammatory activity in patients with compensated ALD. M30 was the only significant predictor of both liver‐related events and all‐cause mortality after adjusting for advanced fibrosis, body mass index, and drinking behavior at inclusion.
In this biopsy‐controlled study in 265 patients with compensated, alcohol‐associated liver disease and long‐term follow‐up, we investigated the diagnostic and prognostic potential of serum M30, M65 and ActiTest for hepatic inflammation, liver‐related events and all‐cause mortality, compared to routine liver function tests (AST, ALT and GGT). We found M30 to accurately detect severe hepatic inflammatory activity (AUROC: 0.90), and as the only marker significantly predict liver‐related events and all‐cause mortality when stratifying for advanced fibrosis, abstinence and BMI.

INTRODUCTORY STATEMENT
Globally, approximately 300 million people have an alcohol use disorder.[ 1 ] Excessive alcohol intake can cause alcohol‐associated liver disease (ALD). The end stage of ALD is liver cirrhosis, which is the result from years of accumulating liver fibrosis and is driven by immune activation, hepatic inflammation, and hepatocyte degeneration.[ 2 , 3 , 4 , 5 , 6 , 7 ] In the precirrhotic stages of chronic ALD, routine liver function tests often fail to detect and monitor liver damage. Thus, reliable noninvasive methods for measuring the hepatic inflammation that drives disease progression are needed.[ 7 , 8 ]
Full‐length keratin‐18 (K18) and caspase‐cleaved K18 (cK18) are released from the hepatocyte cytoskeleton during degeneration and can be detected by the antibody‐based serum markers M30 and M65. Whereas M30 detects cK18, generated during apoptosis, M65 detects both K18 and cK18 and therefore reflects overall cell death.[ 5 , 9 ] Previous studies have found that cK18‐based and K18‐based serum markers correlate with hepatic inflammation and perform well as diagnostic and prognostic serum markers of alcoholic hepatitis (AH) in hospitalized patients.[ 10 , 11 , 12 ] Another potential serum marker for detecting hepatic inflammation is the algorithm‐based ActiTest, which combines age, sex, alpha‐2‐macroglobulin, haptoglobin, apolipoprotein A1, bilirubin, gamma‐glutamyltransferase (GGT), and alanine aminotransferase (ALT). The ActiTest was shown to correlate with the degree of ballooning and lobular inflammation in patients with nonalcoholic fatty liver disease (NAFLD).[ 13 , 14 ]
Our primary aim was to evaluate the diagnostic accuracy of M30, M65, and ActiTest for biopsy‐verified hepatic inflammatory activity, evidenced by ballooning and lobular inflammation in patients with compensated ALD. Our secondary aim was to investigate the prognostic value of M30, M65, and ActiTest for liver‐related events that indicate disease progression, as well as all‐cause mortality.
METHODS
We conducted a prospective, biopsy‐controlled, single‐center study in patients with ALD to investigate the diagnostic and prognostic performance of serum markers, with liver biopsies for reference.[ 12 ] This manuscript follows the Standards for Reporting of Diagnostic Accuracy checklist for reporting study results (Supporting Table S8).[ 15 ] The study protocol was approved by the Region of Southern Denmark's ethical committee (ethical ID S‐20120071 and S‐20160021), and all patients provided written consent to participate before inclusion, in accordance with the Declaration of Helsinki. All data were securely collected and stored using REDCap electronic data capture tools and Sharepoint software, hosted by the Open Patient data Explorative Network, the Region of Southern Denmark, Denmark.
Patients
We recruited patients with no evidence of decompensated liver cirrhosis from two alcohol rehabilitation centers and three outpatient hospital clinics in the Region of Southern Denmark between April 2013 and October 2016, as previously published.[ 16 , 17 , 18 ] Inclusion criteria were as follows: age 18–75 years, a self‐reported history of previous or ongoing excessive alcohol intake for minimum 1 year (>14 units/week for women, >21 units/week for men), and the ability to comply with the study protocol. All participants were screened for competing etiologies of liver disease and excluded if they were diagnosed with hepatitis B or C, autoimmune hepatitis, biliary diseases, or overload diseases. Moreover, patients were excluded if they had clinical signs of severe AH, malignancy, or if a liver biopsy was contraindicated due to an increased risk of bleeding or ultrasonic evidence of decompensated liver cirrhosis. We did not exclude patients with coexisting metabolic risk factors (i.e., patients who were overweight, had diabetes, or metabolic syndrome) because these are frequently present in patients with ALD and constitute an increased risk of severe liver disease.[ 19 , 20 , 21 ] In January 2016, we revised the inclusion criteria to a minimum age of 30 years, and liver biopsies were considered redundant if transient elastography values were below 6 kilopascals, due to the particularly low a priori risk of advanced liver fibrosis.[ 16 ]
Investigations
After 6 h of fasting, all included patients underwent same‐day investigations performed by experienced personnel, in accordance with standard operating procedures. We performed abdominal ultrasonography, transient elastography (FibroScan; Echosens), and percutaneous liver biopsies using a 17‐gauge Menghini‐type needle. Tissue was immediately stored in 10% neutral‐buffered formalin and embedded in paraffin. Blood was collected for routine liver function tests and for storage in our project biobank at −80°C. Finally, patients underwent physical examinations (height, weight, body mass index [BMI], and blood pressure measurements) and survey evaluation of comorbidities, lifestyle factors, and drinking behavior including alcohol use disorder screening (the AUDIT and CAGE questionnaires).[ 22 , 23 ]
Liver biopsy evaluation
A single, experienced pathologist (SD), who was blinded to patient characteristics and serum marker results, scored all liver biopsies according to the Clinical Research Network NAFLD Activity Score: steatosis (S0–S3), lobular inflammation (0–3), and ballooning (B0–B2).[ 24 ] Fibrosis stages (F0–F4) were also scored according to the Pathology Committee of the NASH Clinical Research Network.[ 24 ] Biopsies were deemed adequate if they contained at least six portal tracts, were at least 10 mm in length, or in case of present regeneration nodules. Liver tissue was stained with sirius red and hematoxylin and eosin for scoring and was also stained immunohistochemically with antibodies raised against M30 (IHC‐M30) to detect apoptotic cells.[ 25 ] For IHC‐M30 staining, we used the monoclonal mouse antibody clone M30 (1:4000; Roche) after epitope retrieval with protease. The staining procedure was automated using the BenchMark Ultra immunostainer (Ventana Medical Systems) with the OptiView‐DAB detection and OptiView Amplification kits (Ventana Medical Systems). The apoptotic index was calculated as apoptotic cells detected by IHC‐M30 per millimeter of liver biopsy sample and multiplied by 100.
We combined the lobular inflammation and ballooning scores and constructed a semi‐quantitative scale of hepatic inflammatory activity (grade 0–5), which was grouped into mild (grade 0–1), moderate (grade 2–3), and severe (grade 4–5) hepatic inflammatory activity.[ 17 ] We grouped fibrosis stages into minimal fibrosis (F0–F1), significant fibrosis (F ≥ 2), and advanced fibrosis (F3–F4). Finally, we defined steatohepatitis as the presence of steatosis (S ≥ 1) combined with both lobular inflammation (≥1) and ballooning (≥1).[ 26 ]
Serum markers
At the day of inclusion, the following routine liver function tests were analyzed: aspartate aminotransferase (AST), ALT, and GGT. We used serum from the project biobank to analyze M30, M65, and ActiTest using the enzyme‐linked immunosorbent assay (ELISA) kit M30 Apoptosense and M65 (both from PEVIVA VLV bio) and ActiTest (Biopredictive) in accordance with the manufacturer's instructions. Laboratory personnel who analyzed the serum markers (M30, M65, and ActiTest) were blinded to clinical information and biopsy results.
Assessment of clinical end points
Trained clinicians collected clinical endpoints data by systematically examining electronic patient records and noting liver‐related events and all‐cause mortality on the entire cohort, as previously published.[ 18 ] The patient records consisted of all hospital contacts in Denmark, and patients were followed until October 1, 2020. However, patients included before April 4, 2016, who had moved outside the region before the end of the follow‐up period, were censored at the day of moving due to protocol restrictions. Liver‐related events included liver failure–induced jaundice, AH, varices that required treatment, ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, hepatocellular carcinoma, and hepatorenal syndrome. Detailed definitions are found in the Supporting Information.
Statistical analysis
We report descriptive data as means and SDs or medians and interquartile ranges (IQRs), depending on the distribution of data. We performed between‐group comparisons using Student's t test, the Wilcoxon rank sum test, or the Kruskal‐Wallis test if there were more than two groups. We performed correlation analyses using the Spearman Rho test.
We evaluated diagnostic accuracy for the serum markers (i.e., M30, M65, and ActiTest) and routine liver function tests (i.e., AST, ALT, and GGT) by calculating the area under the receiver operating characteristic curve (AUROC) for severe hepatic inflammatory activity, significant fibrosis (F ≥ 2), and the presence of steatohepatitis. We compared the performances using the DeLong test. Furthermore, we assessed the pretest and posttest probabilities of dichotomized serum markers using cutoffs determined by the Youden index.
To investigate the prognostic potential of the serum markers, we used Harrell's C‐statistic and we performed univariate and multivariable regression analyses based on serum markers and variables available in an outpatient setting (e.g., age, sex, BMI, and alcohol abstinence).
To determine the prognostic value of serum markers to predict liver‐related events, we performed competing‐risk univariate and multivariable regression analyses using the Fine and Gray method. We also constructed cumulative incidence curves and tested for significance using the Pepe‐Mori test. To investigate the prognostic value of the serum markers for all‐cause mortality, we performed univariate and multivariate Cox regression analyses. Additionally, we constructed Kaplan–Meier survival curves and tested for significance using the log‐rank test. All analyses were also performed for routine liver function tests (AST, ALT, and GGT). We used Stata software (ver. 15; StataCorp) for all analyses, and we considered a p‐value < 0.05 to be statistically significant.
RESULTS
Patient characteristics
From April 2013 to October 2016, we recruited 297 patients, of whom 278 were eligible for liver biopsy. After inclusion, we excluded 2 patients due to insufficient liver biopsies and 11 patients due to lack of serum measurements of both M30 and M65. The final cohort consisted of 265 liver‐biopsied patients (Figure 1). M65 serum levels were missing for 2 patients, and the ActiTest failed to generate results for 5 patients (2%) due to unusual deviations below the first or above the 99th percentile for individual test components. Our pathologist (SD) successfully evaluated IHC‐M30 tissue expression in 260 patients. For the remaining five liver biopsies, there was insufficient tissue in the paraffin blocks for IHC‐M30 staining.
FIGURE 1.
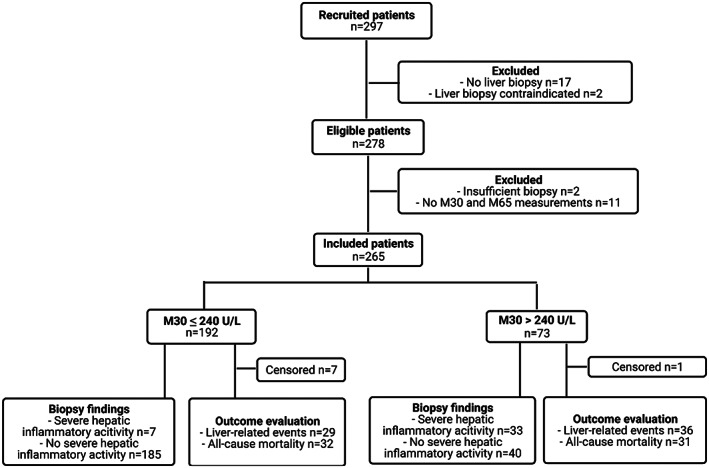
Standards for Reporting of Diagnostic Accuracy (STARD) flowchart. The flowchart illustrates inclusion of patients and evaluation of M30 levels for biopsy‐verified severe hepatic inflammatory activity and outcomes: liver‐related events and all‐cause mortality. We defined hepatic inflammatory activity (0–5) as the sum of biopsy‐verified lobular inflammation (0–3) and ballooning (0–2). Severe hepatic inflammatory activity was defined as a sum total of ≥4
The median age was 56 ± 14 years, and most patients were male (74%). Approximately half of the patients were active drinking at inclusion (48%), of whom 51% drank above their sex‐specific high‐risk limit the week before inclusion (female > 14 units/week, male > 21 units/week). In total, 76% of abstinent patients had been abstinent for less than 1 year; for these patients, the median time of abstinence before the investigations was 12 weeks (IQR: 12–20). When drinking alcohol, the maximum level of daily alcohol intake was significantly higher among abstinent patients than active drinking patients (p < 0.01; Table 1). Although there was no significant difference in BMI, obesity, or diabetes between the two groups, we observed a higher prevalence of metabolic syndrome in active drinking patients (p = 0.03; Table 1).[ 27 ]
TABLE 1.
Baseline patient characteristics
| Full cohort | Abstinent patients | Nonabstinent patients | p‐Value | |
|---|---|---|---|---|
| Patients (n, %) | 265 | 138 (52%) | 127 (48%) | N/A |
| Male sex (n, %) | 195 (74%) | 99 (72%) | 96 (76%) | 0.48 |
| Age (years) | 56 (48–62) | 55 (46–61) | 57 (50–64) | 0.01 |
| BMI (kg/m2) | 26.7 ± 5 | 26.4 ± 5 | 27 ± 5 | 0.22 |
| HbA1c (mmol/mol) | 37 (33–40) | 37 (33–41) | 36 (31–39) | 0.07 |
| Bilirubin (μmol/L) | 10 (7–14) | 9 (7–14) | 11.5 (7–17) | 0.01 |
| Albumin (g/L) | 41 (39–44) | 42 (39–44) | 41 (38–43) | 0.50 |
| INR | 1 (0.9–1.1) | 1 (0.9–1.1) | 1 (0.9–1.1) | 0.43 |
| Creatinine (μmol/L) | 73 ± 15 | 76 ± 14 | 70 ± 16 | <0.01 |
| Ferritin (μg/L) | 131 (72–290) | 93 (52–176) | 208 (95–400) | <0.00 |
| Alkaline phosphatase (U/L) | 88 (71–118) | 89 (72–115) | 87 (69–122) | 0.71 |
| AST (U/L) | 34 (25–54) | 29 (22–40) | 43 (29–73) | <0.01 |
| ALT (U/L) | 32 (20–48) | 26 (18–38) | 38 (24–56) | <0.01 |
| GGT (U/L) | 74 (38–215) | 48 (25–103) | 131 (57–297) | <0.01 |
| Platelet count (109/L) | 234 (186–292) | 241 (188–296) | 225 (175–289) | 0.27 |
| M30 (U/L) | 154 (95–260) | 118 (85–208) | 187 (113–390) | <0.01 |
| M65 (U/L) | 432 (267–833) | 358 (229–671) | 512 (330–940) | <0.01 |
| ActiTest (U/L) | 0.17 (0.07–0.31) | 0.12 (0.06–0.24) | 0.23 (0.11–0.37) | <0.01 |
| Median alcohol consumption the week until inclusion (units) | 0 ± 20 | 21 ± 28 | N/A | |
| Maximum daily alcohol intake when active drinking (units daily) | 15 (9–25) | 18 (10–30) | 12 (7–20) | <0.01 |
| Time of abstinence in years (%) <1 year/1–5/6–10/11–20/> 30 years | N/A | 76/13/5/4/1 | N/A | N/A |
| Years with alcohol‐overuse (%) <5/6–10/11–20/21–30/> 30 years | 15/18/24/20/16 | 14/20/25/17/13 | 17/17/22/23/20 | 0.26 |
| Overweight, BMI > 30 (n, %) | 66 (25%) | 28 (20%) | 38 (30%) | 0.09 |
| Diabetes (n, %) | 38 (14%) | 20 (14%) | 18 (14%) | 0.94 |
| Metabolic syndrome (n, %) a | 58 (22%) | 23 (17%) | 35 (28%) | 0.03 |
| Liver biopsy scores | ||||
| Fibrosis stage (%) 0/1/2/3/4 | 12/35/29/7/17 | 17/37/22/6/18 | 7/33/37/7/16 | 0.07 |
| Steatosis (%) 0/1/2/3 | 52/22/19/7 | 75/17/7/1 | 28/26/33/13 | <0.01 |
| Ballooning (%) 0/1/2 | 52/30/18 | 56/34/10 | 48/25/27 | 0.03 |
| Lobular inflammation (%) 0/1/2/3 | 28/42/23/8 | 38/43/14/4 | 16/40/33/11 | <0.01 |
| NAS score (%) 0/1/2/3/4/5/6/7/8 | 25/11/16/14/15/8/8/2/1 | 35/14/19/14/12/4/2 | 14/6/13/14/20/13/13/5/2 | <0.01 |
| Hepatic inflammatory activity (%) 0/1/2/3/4/5 | 25/23/23/14/9/6 | 35/22/24/13/3/4 | 15/24/22/15/17/8 | <0.01 |
Note: The table sums up the patient characteristics for included patients and subcohort analyses of abstinent and active drinking patients. Continuous variables are listed as median (IQR) or mean ± SD, depending on distribution, and categorical variables are listed as counts (n, %). Ferritin levels are missing for 32 patients and HbA1c for 19 patients. BMI was missing for 1. M65 serum levels is missing for 2 patients, while ActiTest serum levels are missing for 5 patients. Maximum alcohol intake was not reported by 5 patients, and time of abstinence was not reported by 1 patient. Years of alcohol overuse was defined as >21 units/week for men and >14 units/week for women and not reported by 17 patients.
Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; HDL‐P, high‐density lipoprotein particle concentration; INR, international normalized ratio; IQR, interquartile range; N/A, not applicable; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score.
Defined according to the criteria by the International Diabetes Federation using BMI > 30 instead of abdominal obesity and adding HbA1c ≥ 48 mmol/mol as diagnostic for diabetes type 2. For 27 patients, either BMI, p‐triglyceride, HDL‐P, or p‐glucose was missing.
The cohort represented the full histopathological spectrum of ALD covering all stages of steatosis, inflammation, and fibrosis. In total, 15% of patients in the cohort presented with severe hepatic inflammatory activity, and 23% had advanced fibrosis (Table 1 and Supporting Figure S1). Less than half of the patients presented with steatosis (48%) and 29% with steatohepatitis. In the subgroup of patients with normal weight (BMI < 25 kg/m2, n = 96), of whom 57% were abstinent (n = 55), 13% presented with severe hepatic inflammatory activity (n = 12), 19% had advanced fibrosis (n = 18), and 25% had steatohepatitis (n = 24).
Serum marker levels, stages of fibrosis, and outcome evaluations are described in the Supporting Information (Tables S6 and S7).
M30 levels diagnose severe hepatic inflammatory activity with a high accuracy
We found that M30, M65, and ActiTest levels correlated significantly with lobular inflammation, ballooning, and hepatic inflammatory activity (correlation coefficients: 0.37–0.55, p < 0.05; Supporting Table S1). M30, M65, and ActiTest levels significantly increased from mild to moderate and to severe hepatic inflammatory activity (Figure 2). However, only M30 and AST levels showed a subgroup‐dependent increase in a step‐wise manner after adjusting for abstinence (Supporting Figure S2).
FIGURE 2.
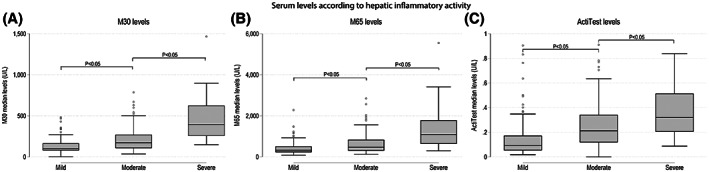
Box plots for serum markers levels according to mild, moderate, and severe hepatic inflammatory activity. The box plots illustrate the serum maker levels of M30 (A), M65 (B), and ActiTest (C) for biopsy‐verified hepatic inflammatory activity (0–5), which we defined as the sum of lobular inflammation (0–3) and ballooning (0–2). We separated hepatic inflammatory activity into three subgroups: mild (≤1), moderate (2–3), and severe (≥4). The levels of all three serum markers increased significantly in between each subgroup of hepatic inflammatory activity (p < 0.05). One patient was excluded from the analyses of M30 and M65 due to outlier values (M30 = 3817 U/L and M65 = 10,016 U/L)
Overall, M30 showed the greatest diagnostic accuracy for severe hepatic inflammatory activity and performed significantly better than ActiTest and ALT (AUROCM30 = 0.90 [0.86–0.95]; Figure 3A). Interestingly, in patients without steatosis (n = 139), M30 performed an excellent diagnostic accuracy for severe hepatic inflammatory activity (AUROCM30 = 0.94 [0.87–1.00]), greater than M65, AST, and GGT (AUROCM65 = 0.87 [0.79–0.96]; AUROCAST = 0.89 [0.79–0.98]; AUROCGGT = 0.84 [0.73–0.95]), and significantly higher than ActiTest and ALT (AUROCActiTest = 0.79 [0.68–0.91]; AUROCALT = 0.57 [0.38–0.76]). However, the overall diagnostic accuracy for significant fibrosis in patients without steatosis remained stable (not reported).
FIGURE 3.
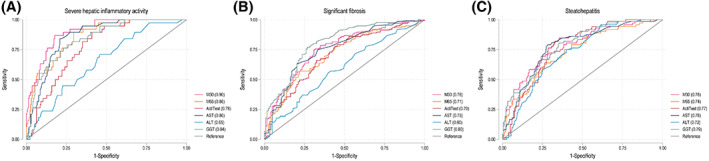
Receiver operating characteristic (ROC) curves for serum markers according to severe hepatic inflammatory activity, significant fibrosis, and steatohepatitis. The ROC illustrates the diagnostic performance of serum markers (M30, M65, and Actitest) and routine liver function tests (aspartate aminotransferase [AST], alanine aminotransferase [ALT], and gamma‐glutamyltransferase [GGT]) for relevant biopsy‐verified histopathological findings. We defined hepatic inflammatory activity (0–5) as the sum of biopsy‐verified lobular inflammation (0–3) and ballooning (0–2). Severe hepatic inflammatory activity was defined as a sum total of ≥4. Steatohepatitis was defined as the presence of steatosis (S ≥ 1) in combination with both lobular inflammation (≥1) and ballooning (≥1). Significant fibrosis was defined as fibrosis grade F ≥ 2. (A) Severe hepatic inflammatory activity: AUROCM30 = 0.90 (0.86–0.95), AUROCM65 = 0.86 (0.80–0.91), AUROCActiTest = 0.78 (0.71–0.84), AUROCAST = 0.86 (0.81–0.91), AUROCALT = 0.65 (0.56–0.74), and AUROCGGT = 0.84 (0.78–0.90). (B) Significant fibrosis (Kleiner ≥ F2): AUROCM30 = 0.76 (0.70–0.82), AUROCM65 = 0.71 (0.65–0.77), AUROCActiTest = 0.70 (0.64–0.77), AUROCAST = 0.75 (0.69–0.81), AUROCALT = 0.59 (0.52–0.66), and AUROCGGT = 0.80 (0.75–0.86). (C) Steatohepatitis: AUROCM30 = 0.78 (0.72–0.84), AUROCM65 = 0.74 (0.67–0.80), AUROCActiTest = 0.77 (0.71–0.83), AUROCAST = 0.78 (0.71–0.84), AUROCALT = 0.72 (0.66–0.78), and AUROCGGT = 0.78 (0.73–0.85). Abbreviation: AUROC, area under the receiver operating characteristic curve
We then dichotomized the serum markers using the optimal cutoff determined by the Youden index, and found that M30 was the only serum marker that exhibited a sensitivity and specificity that were both above 80% (Table 2). Furthermore, M30 had a high negative predictive value (NPVM30 = 96%), and the greatest positive predictive value (PPVM30 = 45%) was significantly greater than M65, ActiTest, and ALT (Table 2).
TABLE 2.
Diagnostic accuracy of serum markers for severe hepatic inflammatory activity
| Cutoff | Sensitivity (%, 95 CI) | Specificity (%, 95 CI) | Positive predictive value (%, 95 CI) | Negative predictive value (%, 95 CI) | |
|---|---|---|---|---|---|
| M30 (U/L) | 240 | 83 (67–93) | 82 (77–87) | 45 (34–57) | 96 (93–99) |
| M65 (U/L) | 545 | 88 (73–96) | 70 (63–76) | 34 (25–44) | 97 (93–99) |
| ActiTest (U/L) | 0.180 | 84 (69–94) | 60 (53–66) | 26 (19–35) | 96 (91–98) |
| AST (U/L) | 45 | 85 (70–94) | 78 (72–83) | 41 (30–52) | 97 (93–99) |
| ALT (U/L) | 35 | 58 (41–73) | 58 (52–65) | 20 (13–28) | 89 (82–93) |
| GGT (U/L) | 150 | 78 (62–89) | 77 (71–82) | 36 (26–47) | 95 (91–98) |
Note: The table lists the sensitivity, specificity, positive predictive values, and negative predictive values with 95% CI in parentheses. The analyses were based on 2 × 2 tables of the listed serum markers at cutoffs set by the Youden index. We defined hepatic inflammatory activity (0–5) as the sum of biopsy‐verified lobular inflammation (0–3) and ballooning (0–2). Severe hepatic inflammatory activity was defined as a sum total of ≥4.
We found similar results in patients with normal weight (BMI < 25 kg/m2). M30 showed the greatest diagnostic accuracy (AUROCM30 = 0.88 [0.79–0.98]), but only performed significantly better than ALT (Supporting Figure S3A). After we re‐dichotomized the serum markers for severe hepatic inflammatory activity, M30 was still the only serum marker that exhibited a sensitivity and specificity above 80%. M30 also had the highest PPV, significantly better than ALT (PPVM30 = 37%; Supporting Table S2).
Correlation with tissue expression of M30 and other histopathological findings
When correlating the serum markers to the IHC‐M30‐based apoptotic index in tissue, serum M30 showed the greatest correlation coefficient compared with M65, ActiTest, AST, ALT, and GGT (rM30 = 0.53; Supporting Table S1). For fibrosis stage and grade of steatosis, AST and GGT were superior to M30, M65, and ActiTest (Supporting Table S1).
Overall, we observed moderate diagnostic accuracy for significant fibrosis (AUROCM30 = 0.76, AUROCM65 = 0.71, AUROCActiTest = 0.70; Figure 3B) and steatohepatitis (AUROCM30 = 0.78, AUROCM65 = 0.74, AUROCActiTest = 0.77; Figure 3C). Similar results were observed in patients with normal weight (BMI < 25 kg/m2) for both significant fibrosis (AUROCM30 = 0.76, AUROCM65 = 0.71, AUROCActiTest = 0.69) and steatohepatitis (AUROCM30 = 0.81, AUROCM65 = 0.76, AUROCActiTest = 0.81). In this subgroup, GGT showed an excellent diagnostic accuracy for both outcomes (AUROCfibrosis = 0.86 [0.79–0.94], AUROCsteatohepatitis = 0.86 [0.78–0.95]; Supporting Figure S3B,C).
We observed that M30, M65, and ActiTest were significantly elevated in patients with steatohepatitis (n = 76; Supporting Figure S4). We re‐dichotomized the serum markers for steatohepatitis and observed moderate sensitivity overall (range: 16–69; Supporting Table S3). Consequently, M30 showed a relatively low NPV, but a relatively high PPV (NPVM30 = 82%, PPVM30 = 58%; Supporting Table S3), compared with the findings for severe hepatic inflammatory activity (Table 2).
M30 levels at inclusion predict liver‐related events and all‐cause mortality
Follow‐up ended on October 1, 2020, and consisted of 1445 person‐years, with a median follow‐up time of 5.9 years (IQR: 4.9–6.7). Eight patients were censored due to relocation outside the Region of Southern Denmark during the follow‐up period (Figure 1). A total of 65 patients (25%) experienced at least one liver‐related event during the follow‐up period, with a median time to the first event of 24.8 months (IQR: 7.2–37.2). Furthermore, a total of 63 patients (24%) died (all‐cause mortality), with a median time until death of 45.9 months (IQR: 19.3–60.8).[ 18 ]
Initially, we performed Harrell's C‐statistic and found that M30, M65, and AST were moderate predictors of liver‐related events and all‐cause mortality (range: 0.62–0.69; Table 3).
TABLE 3.
Harrell's C‐statistics for the prognostic accuracy of the serum markers
| Liver‐related event | All‐cause mortality | |
|---|---|---|
| M30 > 240 U/L | 0.69 | 0.64 |
| M65 > 545 U/L | 0.69 | 0.62 |
| ActiTest > 0.18 U/L | 0.61 | 0.58 |
| AST > 45 U/L | 0.68 | 0.66 |
| ALT > 35 U/L | 0.52 | 0.54 |
| GGT > 150 U/L | 0.66 | 0.63 |
Note: The table sums up Harrell's C‐statistics for each dichotomized serum marker as predictors of liver‐related events and all‐cause mortality. The cutoffs for the serum markers were set by the Youden index.
We then performed univariate and multivariate competing‐risk regression analyses for liver‐related events of elevated serum markers (i.e., M30, M65, ActiTest, AST, ALT, and GGT) and variables available in an outpatient setting (i.e., age, sex, BMI, and abstinence). We found that all serum markers, except for ALT, were significant predictors of liver‐related events in both univariate and multivariate analyses (p < 0.01; Supporting Tables S4 and S5). Overall, M30 showed the greatest subdistribution hazard ratios (SHRs) among all serum markers tested in both univariate (SHRM30 = 4.36) and multivariate analyses (SHRM30 = 4.42), significantly greater than ActiTest and ALT (p < 0.05; Supporting Tables S4 and S5). We also constructed cumulative incidence curves and found that elevated serum levels of all markers, except for ALT, were significantly correlated with an increased incidence of liver‐related events (Figure 4). After adjusting for grouped fibrosis stage at inclusion (F0–F1, minimal fibrosis; F2, significant fibrosis; F3–F4, advanced fibrosis), we found that among all serum markers, elevated M30 and M65 were the only serum markers that significantly correlated with an increased cumulative incidence of liver‐related events in patients with significant and advanced fibrosis (p < 0.01; Supporting Figure S5). None of the serum markers showed significant correlations in patients with minimal fibrosis, while elevated AST and GGT were significantly correlated with an increased cumulative incidence of liver‐related events in patients with significant fibrosis, but not in patients with advanced fibrosis (Supporting Figure S5). In both active drinking and abstinent patients at inclusion, we found that elevated M30, M65, AST, and GGT significantly predicted an increased rate of liver‐related events, while elevated ActiTest was only significant in active drinking patients (SHRActiTest = 1.99 [1.20–3.29]). The SHR was the highest for M30 (SHRM30 = 4.35 [2.64–7.18]), but not significant from M65, AST, or GGT (SHR range: 3.76–3.94; Supporting Figure S7). We also grouped patients dependent on BMI at inclusion (either BMI ≥ 25 kg/m2 or BMI < 25 kg/m2) and found all serum markers, except for ALT (SHRALT = 0.81 [0.48–1.37]), to be significant predictors of liver‐related events in both groups (Supporting Figure S8).
FIGURE 4.
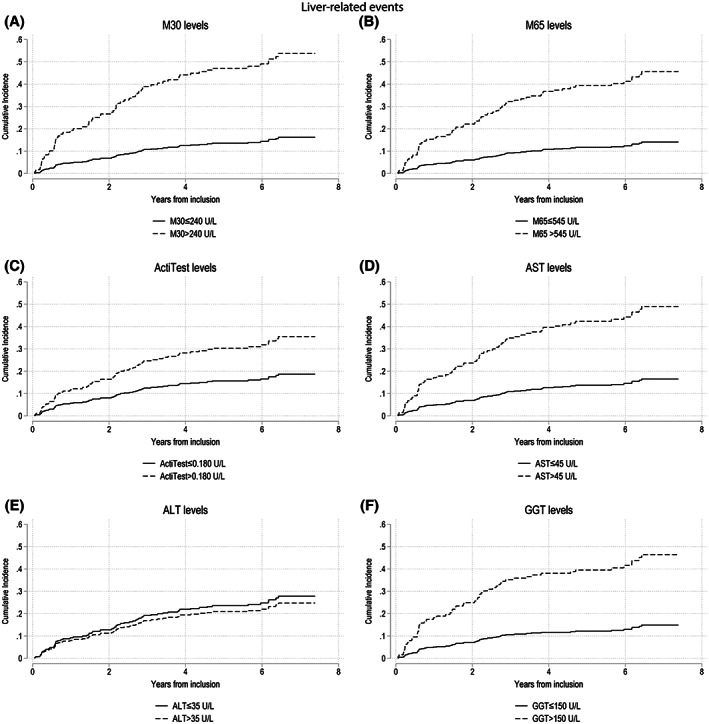
Competing‐risk regression analyses for developing a liver‐related event based on serum marker levels. The graphs illustrate competing‐risk regression analyses of serum markers M30 (A), M65 (B), ActiTest (C), AST (D), ALT (E), and GGT (F) for developing a liver‐related event, with death before an event as a competing risk. The cutoffs for the serum markers were set using the Youden index, and levels above the cutoff values were considered elevated. We used the Fine and Gray method to construct the cumulative incidence curves and compared elevated and nonelevated serum markers levels for significant difference using the Pepe‐Mori test. With the exception of ALT (p > 0.05), elevated serum marker levels were significantly related to an increased cumulative incidence of liver‐related events (p < 0.01). (A) Subdistribution hazard ratio (SHR) (M30 > 240 U/L) = 4.36 (2.67–7.12); p < 0.01. (B) SHR (M65 > 545 U/L) = 4.02 (2.40–6.71); p < 0.01. (C) SHR (ActiTest > 0.180 U/L) = 2.12 (1.29–3.51); p < 0.01. (D) SHR (AST > 45 U/L) = 3.73 (2.29–6.07); p < 0.01. (E) SHR (ALT > 35 U/L) = 0.87 (0.53–1.43); p = 0.64. (F) SHR (GGT > 150 U/L) = 3.88 (2.31–6.51); p < 0.01
For all‐cause mortality, we performed univariate and multivariate Cox regression analyses and found that all serum markers, except for ALT, were significant predictors (Supporting Tables S4 and S5). These findings were supported by Kaplan–Meier survival curves (Figure 5). After adjusting for grouped fibrosis stage, we found that elevated AST levels significantly predicted an increased mortality rate in patients with minimal, significant, and advanced fibrosis (Supporting Figure S6). In addition, elevated M30 predicted an increased mortality in patients with advanced fibrosis (p < 0.05) and trended toward predicting mortality in patients with significant fibrosis (p = 0.07). Elevated GGT significantly predicted an increased mortality in patients with minimal fibrosis (p = 0.01). Elevated levels of M65, ActiTest, and ALT were not significant predictors of an increased rate of all‐cause mortality in any of the three fibrosis groups (p > 0.05; Supporting Figure S6). After adjusting for drinking behavior at inclusion (active drinking or abstinent), we found that elevated M30 and AST levels were predictors of increased all‐cause mortality in both groups (p < 0.01; Supporting Figure S9). When patients were grouped according to their BMI at inclusion (i.e., BMI ≥ 25 kg/m2 or BMI < 25 kg/m2), all serum markers, except for ALT, were significant predictors of all‐cause mortality (Supporting Figure S10).
FIGURE 5.
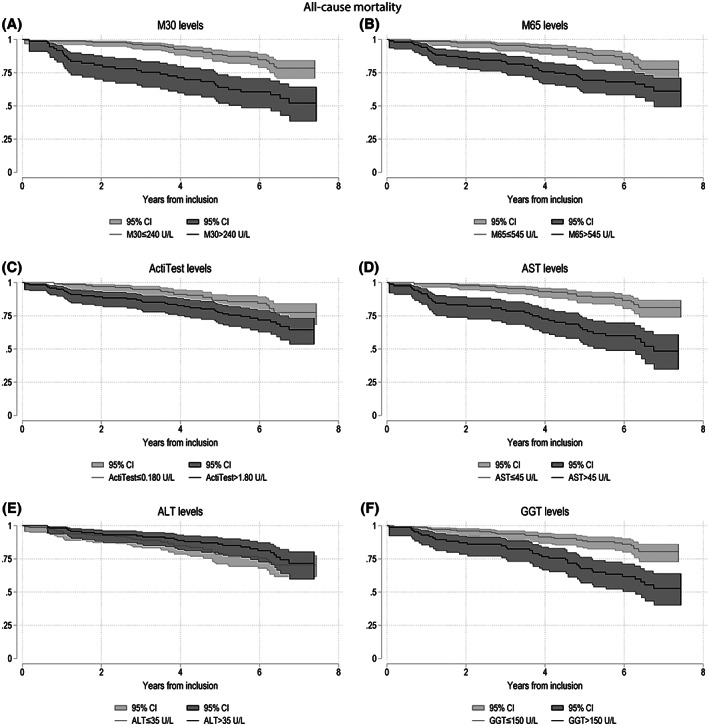
Kaplain‐Meier survival curves for all‐cause mortality based on serum marker levels. The Kaplain‐Meier survival curves show the serum markers M30 (A), M65 (B), ActiTest (C), AST (D), ALT (E), and GGT (F) as predictors of all‐cause mortality with 95% confidence intervals (CIs). All cutoffs were based on the Youden index, and serum markers levels above the cufoffs were considered elevated and illustrated with a darker color. Elevated and nonelevated serum markers were compared using log‐rank tests. With the exception of ALT (p = 0.33), elevated levels of all serum markers were significantly related to an increased all‐cause mortality (p < 0.05)
DISCUSSION
In our biopsy‐controlled study of 265 patients with compensated ALD, we found that M30 and AST exhibited excellent diagnostic accuracy for severe hepatic inflammatory activity, and were superior to M65, ActiTest, ALT, and GGT. Furthermore, we found that M30 had the highest specificity and PPV of all evaluated serum markers. Finally, based on 1445 patient‐years of follow‐up, we found that elevated levels of M30 at inclusion significantly predicted an increased risk of liver‐related events and all‐cause mortality, even after adjusting for advanced fibrosis, drinking behavior, and BMI at inclusion.
Notably, our cohort also included patients with coexisting metabolic risk factors as well as abstinent patients, of whom most had been abstinent for less than 1 year (76%). We included these patients to test the utility of the serum markers on a population of real‐life diverse patients with ALD with both high and low a priori risk of progressive liver disease.[ 19 , 21 ] Furthermore, we recruited patients in close collaboration with alcohol rehabilitation centers, and most abstinent patients therefore showed a tendency of more hazardous drinking pattern when active, compared with nonabstinent patients (Table 1). However, we cannot rule out the possibility that the hepatic inflammatory activity exhibited in these patients is driven by metabolic dysfunction.
As with studies in patients with AH, we observed increased serum levels of M30 and M65 according to the degree of hepatic inflammation.[ 10 , 11 , 28 ] Two of these studies found M65 to be superior to M30, in contrast to our findings. This is likely due to acute AH being driven primarily by necrotic liver damage rather than by apoptotic nonacute inflammation, which was predominant in our cohort.[ 9 ] Furthermore, we also found AST to be effective in detecting hepatic inflammatory activity. This challenges the clinical utility of the K18 markers for hepatic inflammation in patients with nonacute ALD, as these analyses require expensive and time‐consuming ELISA kits, whereas AST is already accessible and often routinely analyzed.
Our results demonstrate that K18‐based serum marker levels correlate more strongly with inflammation than with fibrosis and steatosis. This observation is consistent with the results from a study of patients hospitalized for alcohol rehabilitation, but contrasts with the results of a French study in heavy drinkers.[ 11 , 29 ] Notably, the French study used a composite AH score to assess hepatic inflammation, based on lobular inflammation, necrosis, and Mallory Denk bodies.[ 30 ] The different results may be due to apoptosis and inflammation triggering fibrogenesis, rather than K18 fragments being direct markers of fibrosis.[ 5 ] This interpretation is supported by a recent meta‐analysis in patients with NAFLD, which found that M30 and M65 levels showed moderate diagnostic accuracy for fibrosis staging and detecting advanced fibrosis.[ 31 ] Similar to this meta‐analysis, we also found that M30 and M65 showed moderate diagnostic accuracy for steatohepatitis.[ 31 ] For our outcome evaluation, we found that both elevated M30 and M65 could predict long‐term liver‐related events, even after adjusting for significant and advanced fibrosis. This observation is consistent with results from a cohort of patients with cirrhosis, which showed increased levels of M30 and M65 according to decompensation and short‐term disease progression.[ 32 ] While the patient cohort in the study by MacDonald et al. consisted of patients with clinical signs of severe liver disease, our results demonstrate the potential of K18‐based serum markers for monitoring disease progression in patients with subclinical, compensated ALD. We also observed that elevated levels of M30, M65, AST, and GGT were significant predictors of liver‐related event, regardless of drinking behavior and BMI.
We also found that M30 and M65 could predict long‐term all‐cause mortality, similar to a study in active drinking patients with liver cirrhosis.[ 11 ] Our cutoff for both M30 and M65 levels were slightly lower than those reported by Mueller et al., which may be explained by our cohort representing more stable patients with a remarkably lower cell death rate. After adjusting for advanced fibrosis, we found that elevated M30 and AST levels, but not M65 levels, were predictors of a significant increase in all‐cause mortality. This suggests a more prominent role of apoptosis, than necrosis, in nonacute ALD disease progression. Furthermore, we observed that M30 and AST were the only significant predictors of all‐cause mortality, after adjusting for drinking behavior. However, both M30, M65, ActiTest, AST, and GGT were all significant predictors when adjusting for BMI. Notably, elevated ALT levels did not predict liver‐related events or mortality after adjusting for fibrosis, drinking behavior or BMI, whereas elevated AST was the only predictor of all‐cause mortality independently on the fibrosis group, which questions the current clinical favorability of ALT.[ 18 , 33 ]
Our results validate using M30 as an accurate serum marker for hepatic inflammation and demonstrate the prognostic potential of M30 for liver‐related events and all‐cause mortality in patients with compensated ALD. To our knowledge, this is the first biopsy‐controlled study that evaluate the clinical potential of K18‐based serum markers in compensated patients representing the full disease spectrum of nonacute ALD, including both patients with no fibrosis and those with compensated cirrhosis. However, one limitation of our study is the cross‐sectional analyses of the serum markers. The clinical potential of M30 as a tool for disease monitoring and prognostication requires further longitudinal analyses. Moreover, M30 levels can fluctuate depending on a few days of abstinence from alcohol, which our study could not address in detail, due to questionnaires focusing primarily on general alcohol habits.[ 11 ] However, all participants were recruited based on a concern of at‐risk behavior for ALD from alcohol rehabilitations centers and hospital departments.
Moreover, our suggested cutoffs were lower than cutoffs reported by previous studies, and therefore need external validation. However, this difference may be due to our selected population of patients without acute liver disease. Additionally, cost benefit analyses that compare AST and M30 are needed to evaluate the clinical utility of M30 for detecting hepatic inflammatory activity in patients with nonacute ALD. Finally, the rate of outcomes (i.e., liver‐related events and all‐cause mortality) in this study was relatively low, questioning the power of the outcome evaluation. Therefore, the prognostic potential of the serum markers needs external validation.
In conclusion, M30 and AST are accurate noninvasive markers for severe hepatic inflammatory activity in patients with compensated ALD. M30 significantly predict long‐term liver‐related events and all‐cause mortality, even in patients with advanced fibrosis and regardless of drinking behavior and BMI. Our results hereby suggest M30 as a potential noninvasive tool for clinical disease monitoring and prognostication in patients with compensated ALD.
AUTHOR CONTRIBUTIONS
Study design: Maja Thiele and Aleksander Krag. Study administration: Katrine Holtz Thorhauge, Maja Thiele, Ditlev Nytoft Rasmussen, Stine Johansen, Steen Antonsen, Lars Melholt Rasmussen, and Sönke Detlefsen. Data analysis: Katrine Holtz Thorhauge, Maja Thiele, and Ditlev Nytoft Rasmussen. Manuscript draft: Katrine Holtz Thorhauge, Katrine Prier Lindvig, and Maja Thiele. Manuscript revisions: Aleksander Krag. Data interpretation and approval of the final version of the manuscript: all authors.
FUNDING INFORMATION
Supported by the European Union's Horizon 2020 Research and Innovation Program (668031); the Innovation Fund Denmark; Odense University Hospital's Foundation for Independent Research and PhD stipends; the University of Southern Denmark's PhD stipends; OPEN (Odense Patient data Exploratory Network's biobank) research grants; and the Region of Southern Denmark's postdoc stipends. The assays used for the M30 Apoptosense and M65 ELISA tests were provided without restrictions by VLVbio AB (Nacka, Sweden). The ActiTest calculations were provided without restrictions at a 20% discount from Biopredictive (Paris, France). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST
MT received a speaker's fee from Echosens, Norgine and Siemens Healthcare, and an advisory fee from GE Healthcare.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENT
The authors thank the Center Manager Louise Skovborg Just, Data Manager Peter Andersen, Research Support Worker Vibeke Nielsen, Department Manager Karsten Lauridsen and Louise Vestring, the Odense Patient data Exploratory Network, the staff at the Center for Liver Research, and our patient advisory board.
Thorhauge KH, Thiele M, Detlefsen S, Rasmussen DN, Johansen S, Madsen BS, et al. Serum keratin‐18 detects hepatic inflammation and predicts progression in compensated alcohol‐associated liver disease. Hepatol Commun. 2022;6:3421–3432. 10.1002/hep4.2075
REFERENCES
- 1. World Health Organization . WHO Global Status Report on Alcohol and Health. WHO; 2018. https://www.who.int/publications/i/item/9789241565639 [Google Scholar]
- 2. Ziol M, Tepper M, Lohez M, Arcangeli G, Ganne N, Christidis C, et al. Clinical and biological relevance of hepatocyte apoptosis in alcoholic hepatitis. J Hepatol. 2001;34:254–60. [DOI] [PubMed] [Google Scholar]
- 3. Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lackner C, Gogg‐Kamerer M, Zatloukal K, Stumptner C, Brunt EM, Denk H. Ballooned hepatocytes in steatohepatitis: the value of keratin immunohistochemistry for diagnosis. J Hepatol. 2008;48:821–8. [DOI] [PubMed] [Google Scholar]
- 5. Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. French SW, Nash J, Shitabata P, Kachi K, Hara C, Chedid A, et al. Pathology of alcoholic liver disease. VA Cooperative Study Group 119. Semin Liver Dis. 1993;13:154–69. [DOI] [PubMed] [Google Scholar]
- 7. Thursz M, Kamath PS, Mathurin P, Szabo G, Shah VH. Alcohol‐related liver disease: areas of consensus, unmet needs and opportunities for further study. J Hepatol. 2019;70:521–30. [DOI] [PubMed] [Google Scholar]
- 8. EASL Clinical Practice Guidelines: management of alcohol‐related liver disease. J Hepatol. 2018;69:154–81. [DOI] [PubMed] [Google Scholar]
- 9. Ku NO, Strnad P, Bantel H, Omary MB. Keratins: biomarkers and modulators of apoptotic and necrotic cell death in the liver. Hepatology. 2016;64:966–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bissonnette J, Altamirano J, Devue C, Roux O, Payance A, Lebrec D, et al. A prospective study of the utility of plasma biomarkers to diagnose alcoholic hepatitis. Hepatology. 2017;66:555–63. [DOI] [PubMed] [Google Scholar]
- 11. Mueller S, Nahon P, Rausch V, Peccerella T, Silva I, Yagmur E, et al. Caspase‐cleaved keratin‐18 fragments increase during alcohol withdrawal and predict liver‐related death in patients with alcoholic liver disease. Hepatology. 2017;66:96–107. [DOI] [PubMed] [Google Scholar]
- 12. Moreno C, Mueller S, Szabo G. Non‐invasive diagnosis and biomarkers in alcohol‐related liver disease. J Hepatol. 2019;70:273–83. [DOI] [PubMed] [Google Scholar]
- 13. Imbert‐Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–75. [DOI] [PubMed] [Google Scholar]
- 14. Munteanu M, Tiniakos D, Anstee Q, Charlotte F, Marchesini G, Bugianesi E, et al. Diagnostic performance of FibroTest, SteatoTest and ActiTest in patients with NAFLD using the SAF score as histological reference. Aliment Pharmacol Ther. 2016;44:877–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thiele M, Detlefsen S, Sevelsted Moller L, Madsen BS, Fuglsang Hansen J, Fialla AD, et al. Transient and 2‐dimensional shear‐wave elastography provide comparable assessment of alcoholic liver fibrosis and cirrhosis. Gastroenterology. 2016;150:123–33. [DOI] [PubMed] [Google Scholar]
- 17. Thiele M, Madsen BS, Hansen JF, Detlefsen S, Antonsen S, Krag A. Accuracy of the enhanced liver fibrosis test vs fibrotest, elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology. 2018;154:1369–79. [DOI] [PubMed] [Google Scholar]
- 18. Rasmussen DN, Thiele M, Johansen S, Kjærgaard M, Lindvig KP, Israelsen M, et al. Prognostic performance of seven biomarkers compared to liver biopsy in early alcohol‐related liver disease. J Hepatol. 2021;75:1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Israelsen M, Juel HB, Detlefsen S, Madsen BS, Rasmussen DN, Larsen TR, et al. Metabolic and genetic risk factors are the strongest predictors of severity of alcohol‐related liver fibrosis. Clin Gastroenterol Hepatol. 2022;20:1784–94. [DOI] [PubMed] [Google Scholar]
- 20. Åberg F, Helenius‐Hietala J, Puukka P, Färkkilä M, Jula A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology. 2018;67:2141–9. [DOI] [PubMed] [Google Scholar]
- 21. Boyle M, Masson S, Anstee QM. The bidirectional impacts of alcohol consumption and the metabolic syndrome: cofactors for progressive fatty liver disease. J Hepatol. 2018;68:251–67. [DOI] [PubMed] [Google Scholar]
- 22. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—part II. Addiction. 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 23. Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry. 1974;131:1121–3. [DOI] [PubMed] [Google Scholar]
- 24. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 25. McPartland JL, Guzail MA, Kendall CH, Pringle JH. Apoptosis in chronic viral hepatitis parallels histological activity: an immunohistochemical investigation using anti‐activated caspase‐3 and M30 cytodeath antibody. Int J Exp Pathol. 2005;86:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seitz HK, Bataller R, Cortez‐Pinto H, Gao B, Gual A, Lackner C, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16. [DOI] [PubMed] [Google Scholar]
- 27. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world‐wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. [DOI] [PubMed] [Google Scholar]
- 28. Vatsalya V, Cave MC, Kong M, Gobejishvili L, Falkner KC, Craycroft J, et al. Keratin 18 is a diagnostic and prognostic factor for acute alcoholic hepatitis. Clin Gastroenterol Hepatol. 2020;18:2046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lavallard VJ, Bonnafous S, Patouraux S, Saint‐Paul MC, Rousseau D, Anty R, et al. Serum markers of hepatocyte death and apoptosis are non invasive biomarkers of severe fibrosis in patients with alcoholic liver disease. PLoS One. 2011;6:e17599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Orrego H, Blake JE, Blendis LM, Medline A. Prognosis of alcoholic cirrhosis in the presence and absence of alcoholic hepatitis. Gastroenterology. 1987;92:208–14. [DOI] [PubMed] [Google Scholar]
- 31. Lee J, Vali Y, Boursier J, Duffin K, Verheij J, Brosnan MJ, et al. Accuracy of cytokeratin 18 (M30 and M65) in detecting non‐alcoholic steatohepatitis and fibrosis: a systematic review and meta‐analysis. PLoS One. 2020;15:e0238717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Macdonald S, Andreola F, Bachtiger P, Amoros A, Pavesi M, Mookerjee R, et al. Cell death markers in cirrhotic patients with acute decompensation. Hepatology. 2017;67:989–1002. [DOI] [PubMed] [Google Scholar]
- 33. Xu Q, Higgins T, Cembrowski GS. Limiting the testing of AST: a diagnostically nonspecific enzyme. Am J Clin Pathol. 2015;144:423–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information


