Abstract
Liver cancer, comprised primarily of hepatocellular carcinoma (HCC), is the third leading cause of cancer deaths worldwide and increasing in Western countries. We previously identified the transcription factor zinc fingers and homeoboxes 2 (Zhx2) as a regulator of hepatic gene expression, and many Zhx2 target genes are dysregulated in HCC. Here, we investigate HCC in Zhx2‐deficient mice using the diethylnitrosamine (DEN)–induced liver tumor model. Our study using whole‐body Zhx2 knockout (Zhx2 KO ) mice revealed the complete absence of liver tumors 9 and 10 months after DEN exposure. Analysis soon after DEN treatment showed no differences in expression of the DEN bioactivating enzyme cytochrome P450 2E1 (CYP2E1) and DNA polymerase delta 2, or in the numbers of phosphorylated histone variant H2AX foci between Zhx2 KO and wild‐type (Zhx2 wt ) mice. The absence of Zhx2, therefore, did not alter DEN bioactivation or DNA damage. Zhx2 KO livers showed fewer positive foci for Ki67 staining and reduced interleukin‐6 and AKT serine/threonine kinase 2 expression compared with Zhx2 wt livers, suggesting that Zhx2 loss reduces liver cell proliferation and may account for reduced tumor formation. Tumors were reduced but not absent in DEN‐treated liver‐specific Zhx2 knockout mice, suggesting that Zhx2 acts in both hepatocytes and nonparenchymal cells to inhibit tumor formation. Analysis of data from the Cancer Genome Atlas and Clinical Proteomic Tumor Consortium indicated that ZHX2 messenger RNA and protein levels were significantly higher in patients with HCC and associated with clinical pathological parameters. Conclusion: In contrast to previous studies in human hepatoma cell lines and other HCC mouse models showing that Zhx2 acts as a tumor suppressor, our data indicate that Zhx2 acts as an oncogene in the DEN‐induced HCC model and is consistent with the higher ZHX2 expression in patients with HCC.
INTRODUCTION
Liver cancer is the sixth most common cancer and the third leading cause of cancer deaths worldwide.[ 1 ] Hepatocellular carcinoma (HCC), which accounts for 85%–90% of primary liver cancer, is increasing dramatically in Western countries due, in large part, to the continued rise of obesity‐associated nonalcoholic fatty liver disease (NAFLD).[ 2 ] NAFLD, with a globally estimated frequency of 25%,[ 3 ] can progress to nonalcoholic steatohepatitis (NASH), which includes the development of inflammation and fibrosis,[ 4 ] which, if left untreated, can worsen to cirrhosis and HCC.[ 4 , 5 ] With the alarming rise in obesity in the United States, the incidence of both NAFLD and HCC is also expected to grow in coming years[ 5 , 6 ]; by 2030, liver cancer is projected to be the third leading cause of cancer deaths in the United States.[ 7 ] HCC is also among the most lethal cancers, with a 5‐year survival rate of 5%–8%, which is attributed to both late‐stage diagnosis and few treatment options.[ 8 ] Liver transplantation is one option, but the need far exceeds the number of donors.[ 9 ] Sorafenib and Regorafenib, the only approved drugs for HCC, have limited efficacy and extend survival by an average of fewer than 3 months,[ 10 ] and numerous other pharmaceutical compounds have failed to meet clinical end points in Phase 3 trials.[ 11 ] Thus, an improved understanding of HCC development and additional HCC biomarkers are needed to monitor and combat this increasingly prevalent cancer.
A hallmark of many cancers, including HCC, is the reactivation of genes that are normally expressed during fetal developed and silenced at birth. Alpha‐fetoprotein (AFP), which is abundantly expressed in the fetal liver, was the first HCC‐associated “oncofetal” gene identified.[ 12 ] This has led to considerable interest in AFP regulation, including a study showing that postnatal AFP serum levels were higher in BALB/cJ mice than any other strain.[ 13 ] The persistent AFP expression in BALB/cJ was transmitted as a monogenic trait.[ 13 ] By positional cloning, we showed that this trait was due to a hypomorphic mutation in the BALB/cJ zinc fingers and homeoboxes 2 (Zhx2) gene that dramatically reduced Zhx2 levels.[ 14 , 15 ] Zhx2 is a member of a small family that also contains Zhx1 and Zhx3, all of which contain two C2‐H2 zinc fingers motifs and four or five homeodomains.[ 16 ] Several studies indicate that Zhx2 is a transcriptional regulator, although the mechanisms by which Zhx2 controls target genes are not known, and a consensus Zhx2 binding site has not been identified.[ 16 ]
In addition to AFP, several additional HCC‐associated oncofetal genes, including Glypican 3 (Gpc3), lipoprotein lipase (Lpl) and the long noncoding RNA H19, continue to be expressed in the adult BALB/cJ liver. More recently, we developed C57BL/6 mice in which a floxed Zhx2 gene has been deleted in hepatocytes. AFP, Gpc3, Lpl, and H19 continue to be expressed in Zhx2‐deficient adult livers similarly to BALB/cJ mice, confirming that this trait is due to the absence of Zhx2.[ 14 , 17 , 18 ]
Dysregulation of oncofetal genes in the absence of Zhx2 in the postnatal liver has led us to propose that Zhx2 could function as a tumor suppressor to control liver tumorigenesis. Human clinical data in support of this notion are conflicting. Several studies suggest that Zhx2 levels are increased in HCC, whereas others indicate reduced Zhx2 levels.[ 19 , 20 ] Tissue culture and xenograft studies using human hepatoma cell lines indicated that Zhx2 inhibits cell proliferation and reduces levels of cyclins A and E,[ 21 ] supporting the possibility that Zhx2 functions as a tumor suppressor. Liver‐specific Zhx2 knockout mice had increased tumors compared with wild‐type controls after hydrodynamic tail‐vein injection (HTVI) of the oncogenes AKT and Myc,[ 22 ] consistent with tumor suppressor activity of Zhx2.
To further investigate the mechanisms by which Zhx2 affects HCC in mice, we used the well‐established diethylnitrosamine (DEN) model of HCC.[ 23 ] Here, 14‐day‐old wild‐type (Zhx2 wt ) and whole‐body Zhx2 knockout (Zhx2 KO ) mice were given a single injection of DEN. Following bioactivation, toxic DEN metabolites can damage cell components, including DNA, resulting in the appearance of HCC tumors after 8–9 months. Both male and female Zhx2 wt mice treated with DEN exhibited numerous tumors, with the tumor burden being greater in males than in females. Although we expected greater tumor incidence in Zhx2 KO mice based on the predicted Zhx2 function as a tumor suppressor, our data surprisingly showed that DEN‐treated Zhx2 KO mice had a complete absence of tumors. Analysis of livers within a week after DEN treatment indicates that this absence could be due to decreased cell proliferation in Zhx2 KO livers. Furthermore, tumors were reduced, but not absent, when the DEN treatment was performed in liver‐specific Zhx2 knockout mice, suggesting that Zhx2 acts in both hepatocytes and nonparenchymal cells (NPCs) to inhibit tumor formation after DEN treatment. UALCAN analysis of data from the Cancer Genome Atlas (TCGA) and Clinical Proteomic Tumor Consortium (CPTAC) indicated that ZHX2 mRNA and protein levels were significantly higher in patients with HCC, suggesting that our results may provide insight into the role of ZHX2 in HCC progression in humans.
EXPERIMENTAL PROCEDURES
Experimental animals
All mice used in this study were on the C57BL/6 background and were housed in the University of Kentucky Division of Laboratory Animal Research facility in accordance with Institutional Animal Care and Use Committee–approved protocols. To generate Zhx2 knockout mice, exon 3, which encodes the entire Zhx2 coding region, was flanked by loxP sites to generate a Zhx2 floxed allele (Zhx2 fl ).[ 24 ] The Zhx2 fl mice were crossed with Alb‐Cre mice (cat.# 003574; Jackson) or E2a‐Cre mice (cat.# 003724; Jackson) to generate liver‐specific Zhx2 knockout mice (Zhx2 ∆liv ) or whole‐body Zhx2 knockout mice (Zhx2 KO ), respectively.[ 25 ]
Tumor induction and analysis
For the DEN‐induced HCC model, Zhx2 KO and Zhx2 wt littermates were given a single intraperitoneal injection of DEN (10 mg/kg; Sigma‐Aldrich), diluted in phosphate‐buffered saline (PBS), or vehicle (PBS) at 14 days of age, weaned and maintained on a regular chow diet. Tumor loads were examined at 9 or 10 months. Externally visible tumors (≥0.5 mm) were counted and measured. Partial large lobes were fixed in formalin, and paraffin‐embedded sections were processed for hematoxylin and eosin (H&E) staining. Remaining lobes were microdissected into tumors and nontumor tissues and stored at −80°C. For short‐term studies, Zhx2 KO and Zhx2 wt littermates were given DEN (10 mg/kg) or vehicle (PBS) at 14 days of age and killed at designated timepoints within a week after DEN exposure. H&E‐stained liver sections were used for histopathological evaluation of HCC by Eun Lee in a blinded manner.
Immunohistochemistry and quantitative image analysis
Immunohistochemical (IHC) staining was performed on paraffin‐embedded tissue sections. Detailed methods and antibodies used for IHC can be found in the Supporting Materials.
The Aperio ScanScope XT high‐throughput digital slides scanner system was used to image entire stained slides at ×20 magnification to create a single high‐resolution digital image. Quantification of nuclear staining for Ki67 was done using HALO imaging software (Indica Labs).
Real‐time quantitative polymerase chain reaction
Total RNAs were extracted from samples with RNAzol RT reagent (Molecular Research Center) according to the manufacturer's instructions. One microgram of RNA was reverse‐transcribed to complementary DNA using the High‐Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Real‐time quantitative polymerase chain reactions (PCRs) were prepared with SsoAdvanced Universal SYBR Green Supermix (Bio‐Rad) and amplified in a Bio‐Rad CFX96 real‐time PCR system. Oligonucleotides (Table S1) were obtained from Integrated DNA Technologies. The quantitative PCR Ct values were normalized to ribosomal protein L30 or glyceraldehyde 3‐phosphate dehydrogenase levels and reported as the normalized expression of the indicated gene using the ∆Ct method.[ 26 ]
Western blot analysis
For western blotting, liver or tumor protein lysates were prepared and quantified. Each sample was resolved by electrophoresis using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes or Immobilon‐FL membranes. After incubation with corresponding primary antibodies overnight at 4°C and the corresponding secondary antibodies, protein expression was visualized. Detailed methods and antibodies used for western blotting can be found in the Supporting Materials.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9.0.2 software. All values within a group were averaged and plotted as mean ± SEM. One‐way or two‐way analysis of variance or Student's t test were used to assess statistical significance. A p value less than or equal to 0.05 was considered significant.
UALCAN analysis
The UALCAN database (http://ualcan.path.uab.edu) was used to analyze RNA‐sequencing (RNA‐seq) data from the TCGA[ 27 ] and protein expression data from the CPTAC.[ 28 ] ZHX2 protein expression values from the CPTAC data portal were log2‐normalized in each sample. Then a Z‐value for each sample was calculated as SDs from the median across samples.[ 28 ] ZHX2 messenger RNA (mRNA) expression in TCGA Liver Hepatocellular Carcinoma (LIHC) samples was compared with normal samples. LIHC samples were then categorized using clinical patient data from the TCGA‐LIHC project as follows: (1) tumor histology; (2) individual cancer stages (based on American Joint Committee on Cancer pathologic tumor stage information; samples were divided into stages I, II, III, and IV); (3) and tumor grade (where tumor grade information is available, samples were categorized into grades 1, 2, 3, and 4).[ 27 ]
RESULTS
Zhx2 expression is effectively eliminated in the livers of Zhx2 knockout mice
We previously described C57BL/6J mice with a floxed Zhx2 allele (Zhx2 fl[ 24 ]; Figure 1A); Zhx2 levels are the same in Zhx2 fl mice as in mice with the wild‐type Zhx2 gene (Zhx2 wt ; data not shown). We also showed that breeding Zhx2 fl mice to albumin‐Cre to delete Zhx2 in hepatocytes (Zhx2 ∆liv ) or βactin‐Cre to delete Zhx2 in all cells (Zhx2 KO ) dramatically reduced or completely eliminated liver Zhx2 mRNA levels, respectively.[ 25 ] To confirm changes in Zhx2 protein levels in knockout mice, immunohistochemical staining was performed using adult liver from Zhx2 wt , Zhx2 KO , and Zhx2 ∆liv mice. Zhx2 was present in the nuclei of both hepatocytes and NPCs in Zhx2 wt mice (Figure 1B). Hepatocytes from Zhx2 ∆liv mice no longer expressed Zhx2, although protein was still detected in NPCs, whereas Zhx2 was not detected in any cells of Zhx2 KO liver (Figure 1B). These data confirm the complete absence of Zhx2 in Zhx2 ∆liv hepatocytes and Zhx2 expression in NPCs.
FIGURE 1.
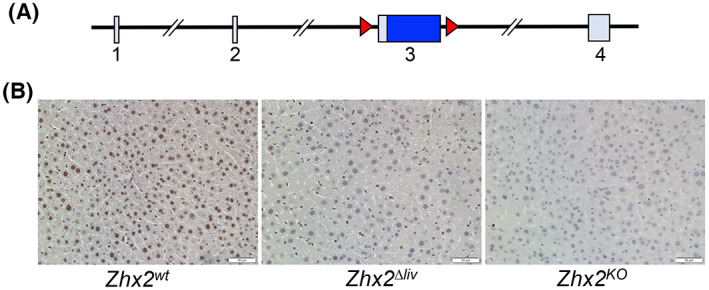
The zinc fingers and homeoboxes 2 (Zhx2) gene is expressed in hepatocytes and nonparenchymal cells and efficiently deleted in knockout mouse livers. (A) The mouse Zhx2 gene spans 145 kb and consists of 4 exons, including a 2.7‐kb exon 3 that encodes the entire 836 amino acid of Zhx2 protein; loxP sites (red triangles) are used for deletion of flanked exon 3. (B) Immunohistochemical (IHC) staining of adult livers with anti‐Zhx2. Adult mouse liver sections from homozygous Zhx2 wt mice, Zhx2 Δliv mice (Zhx2 fl/fl , Alb‐Cre + ), and Zhx2 KO mice (Zhx2 fl/fl , βactin‐Cre + ) were stained with Zhx2 antibodies and counterstained with hematoxylin. Magnification, ×20.
Zhx2 is essential for HCC formation after DEN treatment in male and female mice
Previous studies using hepatoma cell lines indicated that increased Zhx2 expression resulted in reduced cell proliferation in vitro and reduced xenograft growth in nude mice[ 21 ] and that HTVI of oncogenes AKT/Myc led to more tumors in Zhx2 liver‐knockout mice,[ 22 ] consistent with Zhx2 functioning as a tumor suppressor. To explore the role of Zhx2 in the initiation and progression of tumor development in vivo, we used the well‐established DEN model in Zhx2 wt and Zhx2 KO mice. The whole‐body Zhx2 KO mice were used, as any Zhx2‐expressing cells could potentially contribute to a liver tumor phenotype. Mice were injected with DEN or PBS on postnatal day 14 (p14) and killed at 9 months after DEN exposure, at which time livers were removed and analyzed. No tumors were observed in cohorts that received PBS injections (Table 1, Figure 2A,B). Tumors were present in 100% (14 of 14) of the Zhx2 wt male mice and 25% (3 of 12) of Zhx2 wt female mice. The tumor incidence and tumor number were less in female mice, consistent with previous studies.[ 29 ] In stark contrast to Zhx2 wt mice, visible liver tumors were completely absent in all male (0 of 11) and female (0 of 10) Zhx2 KO mice. To test whether there was a delay in tumor formation, DEN‐treated Zhx2 wt and Zhx2 KO male and female mice were killed at 10 months after p14 DEN treatment (for ethical reasons, DEN‐treated Zhx2 wt male mice could not be maintained longer than this time). While maximal tumor size and tumor numbers increased in Zhx2 wt male mice (Figure 2B) and tumor incidence increased to 36% (4 of 11) in Zhx2 wt female mice (Table 1), no visible tumors were present in Zhx2 KO male and female livers. These data demonstrate that Zhx2 is required for HCC progression after perinatal DEN treatment and indicate that Zhx2 promotes tumor growth in this model.
TABLE 1.
Tumor incidence in Zhx2 KO mice and Zhx2 wt littermates after DEN exposure
| Treatment | Tumor incidence | ||||
|---|---|---|---|---|---|
| Male | Female | ||||
| Zhx2 wt | Zhx2 KO | Zhx2 wt | Zhx2 KO | ||
| 9 months | PBS | 0% (0 of 15) | 0% (0 of 9) | 0% (0 of 14) | 0% (0 of 10) |
| DEN | 100% (14 of 14) | 0% (0 of 11) | 25% (3 of 12) | 0% (0 of 10) | |
| 10 months | PBS | 0% (0 of 10) | 0% (0 of 11) | 0% (0 of 9) | 0% (0 of 10) |
| DEN | 100% (10 of 10) | 0% (0 of 11) | 36% (4 of 11) | 0% (0 of 11) | |
Note: Zhx2 KO mice and Zhx2 wt littermates were given a single intraperitoneal injection of DEN (10 mg/kg; Sigma‐Aldrich) or vehicle (PBS) at 14 days of age. Livers were removed and tumor loads were examined 9 or 10 months after DEN exposure. Number of mice with tumor/cohort in parentheses.
FIGURE 2.
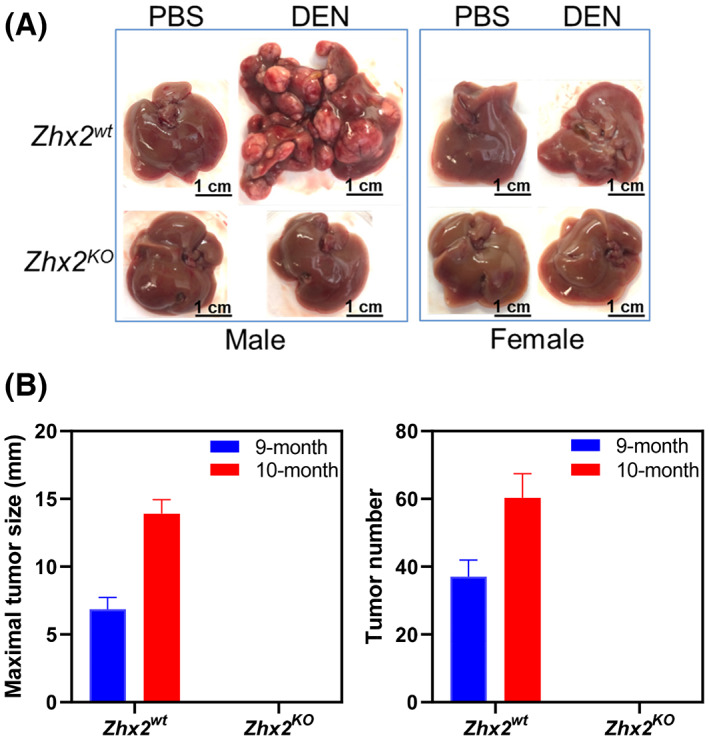
The absence of Zhx2 in Zhx2 KO mice completely blocks DEN‐induced HCC. (A) Male and female Zhx2 wt and Zhx2 KO mice (9–15 mice/cohort) were given a single intraperitoneal injection of diethylnitrosamine (DEN; 10 mg/kg; Sigma‐Aldrich) or vehicle (phosphate buffered saline [PBS]) at p14. After 9 months, livers were removed, and tumors were quantitated. Representative livers are shown; tumor incidence data are given in Table 1. (B) The maximal tumor size (left panel) and the average number of visible tumors (right panel) in Zhx2 wt male mice at 9 months (n = 14) and 10 months (n = 10) and Zhx2 KO male mice at 9 months (n = 11) and 10 months (n = 11) after DEN treatment.
H&E‐stained liver sections from DEN‐treated Zhx2 wt male mice confirmed that tumors were HCC (Figure 3A) and confirmed the absence of tumors or any small foci in livers of DEN‐treated Zhx2 KO male mice or any of the PBS‐treated control mice. Previous studies indicated that DEN‐initiated tumors are glutamine synthetase (GS)–negative, which was confirmed in tumors in Zhx2 wt mice; the absence of Zhx2 did not alter GS staining after PBS or DEN treatment (Figure 3B).
FIGURE 3.
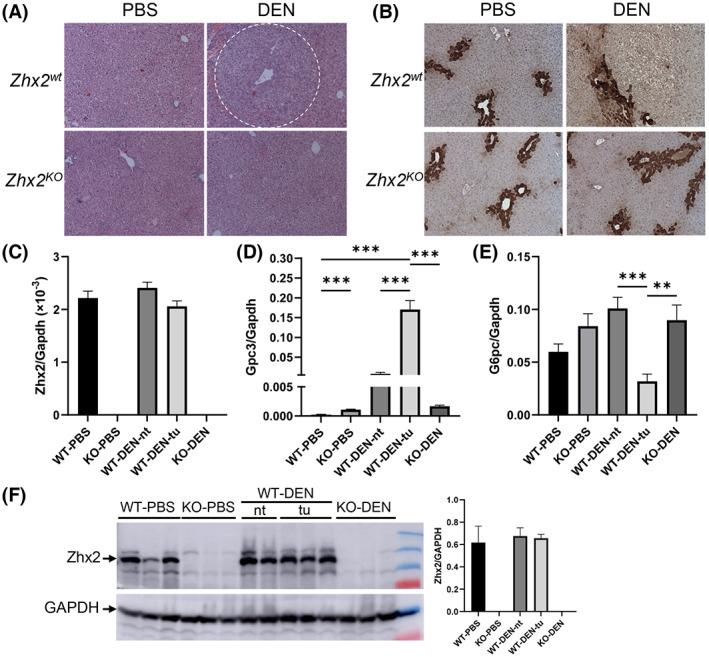
Histological and messenger RNA (mRNA) analysis of livers 9 months after PBS or DEN treatment confirm the presence of HCC in Zhx2 wt mice and the absence of tumors in Zhx2 KO mice. (A,B) Hematoxylin and eosin staining (A) and immunohistochemical staining for glutamine synthetase (B) in Zhx2 wt and Zhx2 KO male mice after PBS or DEN treatment. (C–E) Quantitation of hepatic Zhx2 (C), Gpc3 (D), and G6PC (E) mRNA levels by real‐time quantitative polymerase chain reaction (PCR) in PBS‐treated or DEN‐treated Zhx2 KO mice (n = 8 and 10, respectively) or livers from PBS‐treated Zhx2 wt mice (n = 9) or tumors or nontumor tissues from DEN‐treated Zhx2 wt mice (n = 10). mRNA levels were normalized to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH). **p < 0.01 and ***p < 0.001. (F) Zhx2 protein levels were analyzed by western blot of liver lysate from PBS‐treated or DEN‐treated Zhx2 KO mice or livers from PBS‐treated Zhx2 wt mice or tumors or nontumor tissues from DEN‐treated Zhx2 wt mice. Blots were reprobed with antibodies against GAPDH. The Zhx2 protein levels were quantified using ImageQuant TL Image Analysis Software (GE Healthcare) and normalized to GAPDH. Abbreviations: KO, knockout; nt, nontumor tissues; tu, tumors; WT, wild‐type.
Gpc3, one of many oncofetal genes controlled by Zhx2, has emerged as an important HCC biomarker in humans.[ 18 , 30 ] We therefore analyzed Zhx2 and Gpc3 levels in the 9‐month liver samples. As expected, Zhx2 mRNA and protein were not detected in PBS‐treated or DEN‐treated Zhx2 KO livers (Figure 3C,F). In contrast, Zhx2 was expressed at similar levels in PBS‐treated Zhx2 wt livers and in both nontumor regions and tumors present in DEN‐treated Zhx2 wt livers, indicating that Zhx2 mRNA and protein levels are not different in HCC (Figure 3C,F). Gpc3 mRNA levels were barely detectable in PBS‐treated Zhx2 wt livers and approximately 5‐fold higher in PBS‐treated Zhx2 KO livers, consistent with previous studies showing the incomplete silencing of Gpc3 after birth in the absence of Zhx2. Hepatic Gpc3 levels were dramatically higher in both nontumor and tumor tissue of DEN‐treated Zhx2 wt mice compared with PBS‐treated Zhx2 wt livers; however, this increase was significantly greater in HCC tumors. In contrast, Gpc3 mRNA levels in DEN‐treated Zhx2 KO livers were similar to those of the PBS‐treated Zhx2 KO controls; this similarity is consistent with the absence of tumor in DEN‐treated Zhx2 KO livers. It has been reported that glucose‐6‐phosphatase (G6PC) is down‐regulated in DEN‐induced premalignant lesions.[ 31 ] When compared with PBS‐treated controls, the G6PC mRNA level was significantly decreased in tumors of DEN‐treated Zhx2 wt mice as seen previously but remained unchanged in Zhx2 KO livers (Figure 3E).
Hepatic CYP2E1 levels and DNA damage are the same in Zhx2 wt and Zhx2 KO mice soon after DEN treatment
The striking absence of liver tumors in DEN‐treated Zhx2 KO mice led us to focus on events associated with the initiation of tumorigenesis soon after DEN administration. DEN must be hydroxylated to a bioactive form that is toxic to cells and can damage DNA, both of which can lead to cellular transformation that will ultimately manifest in tumors.[ 23 ] Because CYP2E1 is the primary enzyme for DEN bioactivation, we analyzed CYP2E1 levels in Zhx2 wt and Zhx2 KO livers before and up to 24 h after DEN treatment. No difference in hepatic CYP2E1 mRNA levels was observed in mice with or without Zhx2 at 0, 4, 8, or 24 h after DEN treatment (Figure 4A), although levels in both groups of mice increased slightly at 24 h. Western blot analysis indicated that CYP2E1 protein levels also were the same in Zhx2 wt and Zhx2 KO livers at all timepoints tested (Figure 4C, Figure S1A). We also determined whether Zhx2 levels changed in response to DEN treatment. Western analysis confirmed the absence of Zhx2 in Zhx2 KO livers but indicated that Zhx2 protein levels did not change 24 h after DEN treatment in Zhx2 wt mice (Figure 4D, Figure S1B).
FIGURE 4.
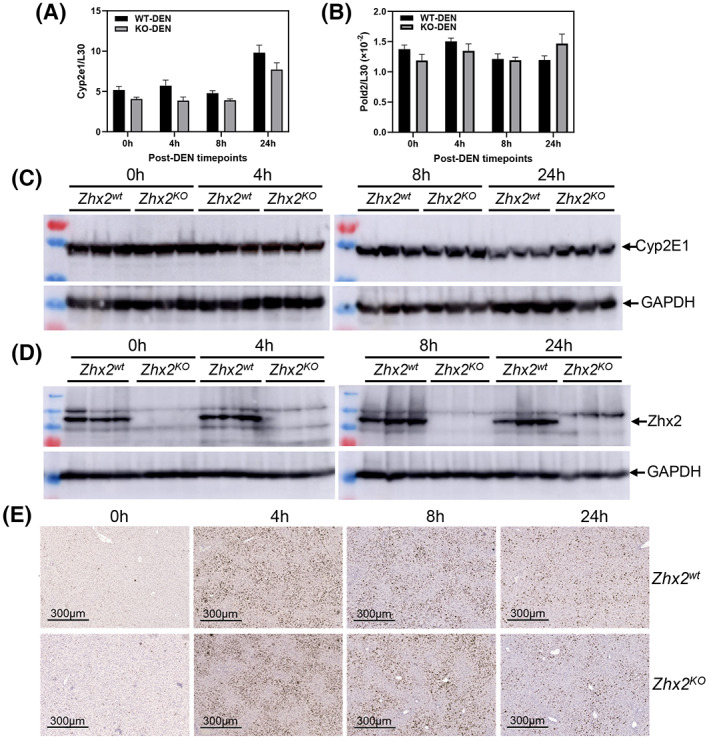
Cytochrome P450 2E1 (CYP2E1), Pold2 expression, and phosphorylated histone variant H2AX (γH2AX) staining are the same in Zhx2 wt and Zhx2 KO mice 24 hours after DEN treatment. Data were obtained from p14 Zhx2 wt and Zhx2 KO mice before (0 h) and 4, 8, or 24 h after DEN treatment. (A,B) Hepatic Cyp2e1 (A) and Pold2 (B) mRNA levels were quantitated by real‐time quantitative PCR and normalized to ribosomal protein L30. (C,D) Hepatic CYP2E1 (C) and Zhx2 (D) protein levels were analyzed by western blot; each lane represents an individual mouse. Blots were reprobed with antibodies against GAPDH. (E) Representative images from liver sections after IHC staining for γH2AX and counterstained with hematoxylin.
Bioactive DEN damages DNA, causing a rapid increase in double‐strand breaks that can be detected by phosphorylated histone variant H2AX (γH2AX) staining.[ 32 ] Therefore, we analyzed γH2AX staining in Zhx2 wt and Zhx2 KO livers. Before DEN treatment, very little staining was observed in either Zhx2 wt or Zhx2 KO livers. Staining increased dramatically 4 h after treatment, indicating a large number of double‐strand breaks, with a gradual reduction by 24 h. However, there was no difference in γH2AX staining between the two cohorts (Figure 4E). Expression of the four members of the DNA polymerase delta (Pold) family, which are involved in DNA repair after damage, were also measured after DEN treatment. No difference in mRNA levels of Pold2 (Figure 4B) or other Pold family members (data not shown) was observed between Zhx2 wt and Zhx2 KO between 0 h and 24 h after DEN treatment. Taken together, these data indicate that differences in DNA damage, or DNA repair soon after DEN treatment, are unlikely to explain the lack of tumors in Zhx2 KO livers.
Hepatic cell proliferation is decreased in Zhx2 KO mice compared with Zhx2 wt soon after DEN treatment
Although DEN metabolites can act as genotoxins, DEN is also hepatotoxic and triggers an inflammatory response that results in activation of NPCs and elevated expression of interleukin‐6 (IL‐6), a mitogen that promotes compensatory proliferation of surviving hepatocytes; this proliferation plays a critical role in DEN‐induced hepatocarcinogenesis.[ 29 , 33 ] This led us to test whether proliferation was affected by the absence of Zhx2 after DEN exposure. Livers from p14 Zhx2 wt and Zhx2 KO mice were removed before DEN treatment or at several timepoints up to 1 week after treatment, and sections were stained for Ki67. Similar Ki67 staining was seen in untreated livers from both cohorts (Figure 5A,B). The number of Ki67‐positive nuclei was slightly lower in DEN‐treated Zhx2 KO livers than in Zhx2 wt livers at 8 h and 1 day after DEN treatment. However, at 2 days after DEN treatment, there are dramatically fewer Ki67‐positive nuclei in Zhx2 KO livers compared with the Zhx2 wt livers (Figure 5A,B). After 7 days, numerous Ki67‐positive nuclei were still present in Zhx2 wt liver sections, whereas very few positive cells were seen in Zhx2 KO liver sections. Real‐time quantitative PCR data showed that hepatic IL‐6 mRNA levels were significantly decreased in Zhx2 KO livers compared with Zhx2 wt livers at 2 days and 7 days following DEN treatment, whereas hepatic tumor necrosis factor α mRNA levels remain unchanged between Zhx2 KO livers and Zhx2 wt livers at all time points 7 days following DEN treatment (Figure 5C). These results suggest that decreased cell proliferation may be a consequence of reduced IL‐6 expression in NPCs of Zhx2 KO livers. This, in turn, could account for the lack of tumors after DEN treatment in Zhx2 KO mice. The reduced proliferation rates are analogous to the lower phosphorylation of AKT (pAKT) observed in the Zhx2 KO mice compared to the Zhx2 wt (Figure 5D). We further analyzed the AKT expression in DEN‐treated livers. We found that AKT serine/threonine kinase 2 (AKT2) expression was significantly lower in the DEN‐treated Zhx2 KO mice compared with Zhx2 wt littermates, but there was no change in AKT1 levels (Figure 5E,F). Nuclear factor kappa B (NF‐κB), a downstream effector of AKT, was lower, although this difference did not reach statistical significance (Figure 5E,F). These results suggest that reduced AKT2 expression and lower pAKT are associated with reduced proliferation rates in the Zhx2 KO mice compared to Zhx2 wt littermates after DEN treatment.
FIGURE 5.
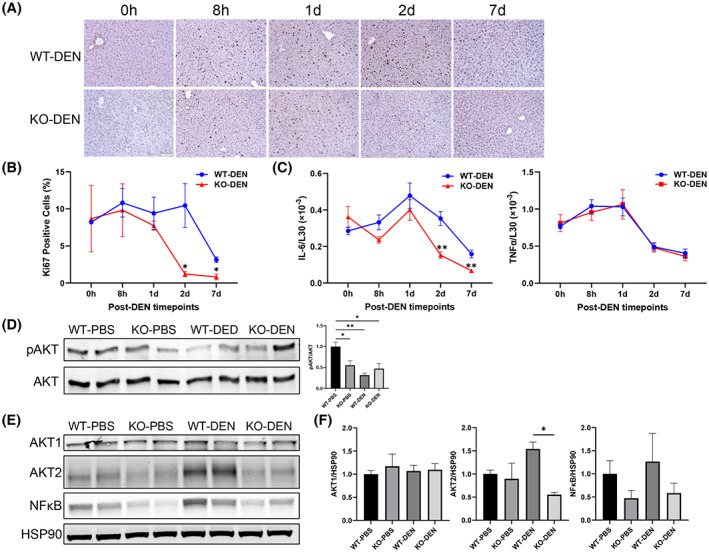
Cell proliferation is reduced in the livers of Zhx2 KO mice compared with Zhx2 wt mice during the 7 days after DEN treatment. (A) Liver sections were obtained from p14 mice before (0 h) and 8 h, 1 day, 2 days, and 7 days after DEN treatment and stained for the proliferation marker Ki67. Representative images from liver sections at designated timepoints are shown. (B) Quantitation of Ki67 IHC staining. Aperio ScanScope XT digital slides scanner was used to scan the entire stained slide at ×20 magnification to create a single high‐resolution digital image. Quantification of nuclear staining for Ki67 was done using HALO imaging software (Indica Labs). Data were from three mice in each group. *p < 0.05. (C) Hepatic interleukin‐6 (IL‐6) and tumor necrosis factor α (TNFα) mRNA levels were quantitated by real‐time quantitative PCR and normalized to ribosomal protein L30. **p < 0.01. (D–F) Immunoblotting and densitometry of phosphorylation of AKT (pAKT; D), AKT serine/threonine kinase 1 (AKT1), AKT2, nuclear factor kappa B (NF‐κB) p65, and heat shock protein 90 (HSP90) (E,F) from the 7‐day livers of PBS‐treated or DEN‐treated Zhx2 KO mice or Zhx2 wt mice. *p < 0.05 and **p < 0.01 (n = 4).
Zhx2 expression in both hepatocytes and NPCs contributes to reduced tumor formation after DEN treatment
As noted previously, Zhx2 is expressed in both hepatocytes and NPCs. Although DEN is metabolized in hepatocytes, it is possible that the absence of Zhx2 in other liver cell types could explain the lack of tumors after DEN treatment. To address this, DEN was injected into male p14 Zhx2 ∆liv mice and control littermates. As shown previously (Figure 1), the loss of Zhx2 in the Zhx2 ∆liv mice is restricted to hepatocytes. After 9 months, mice were killed, and livers were analyzed. In contrast to the complete absence of tumors in Zhx2 KO livers, a small number of tumors were present in Zhx2 ∆liv livers (Figure 6), although tumor numbers were considerably less than in Zhx2 fl livers. Furthermore, the maximal tumor size and the liver/body weight ratios were lower in Zhx2 ∆liv mice than in Zhx2 fl mice. These data indicate that the absence of Zhx2 in both hepatocytes and NPCs contributes to the reduced tumors after DEN treatment.
FIGURE 6.
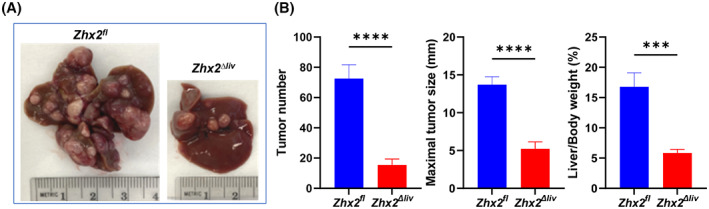
DEN‐induced tumors are significantly reduced in Zhx2 Δliv mice compared with Zhx2 fl littermate controls. (A) Representative livers from Zhx2 fl and Zhx2 Δliv mice 9 months after DEN treatment. (B) Liver tumor number (left panel), maximal tumor size (middle panel), and liver/body weight ratio (right panel) of Zhx2 fl (n = 7) and Zhx2 Δliv (n = 9) mice 9 months after DEN treatment. ***p < 0.001 and ****p < 0.0001.
ZHX2 mRNA and protein levels are significantly higher in patients with HCC and associated with clinical pathological parameters
UALCAN can be used to compare relative gene‐expression and protein levels between normal and tumor samples, as well as between tumor subgroups stratified by pathological grade, clinical stage, age, sex, and other clinical features.[ 27 , 28 ] We used UALCAN to analyze the ZHX2 mRNA levels in the TCGA database and protein levels in the CPTAC database. ZHX2 protein levels were significantly higher in HCC samples compared with normal samples (Figure 7A). From TCGA‐LIHC RNA‐seq data, ZHX2 mRNA levels were significantly higher in LIHC samples than normal samples (Figure 7B). Based on histology subtype, ZHX2 mRNA levels were significantly higher in HCC (361 cases, most of the 371 LIHC cases), fibrolamellar carcinoma (FLC, 3 cases), and hepatocholangiocarcinoma (HCC‐CC, 7 cases) (Figure 7C). Also, ZHX2 mRNA expression significantly increased with the individual cancer stage 1 to 3 and the tumor grade 1 to 4 (Figure 7D,E). Expression in cancer stage 4 did not differ, possibly due to the low number of cases. Overall, these results show that ZHX2 expression was significantly correlated with HCC tumor stage and grade.
FIGURE 7.
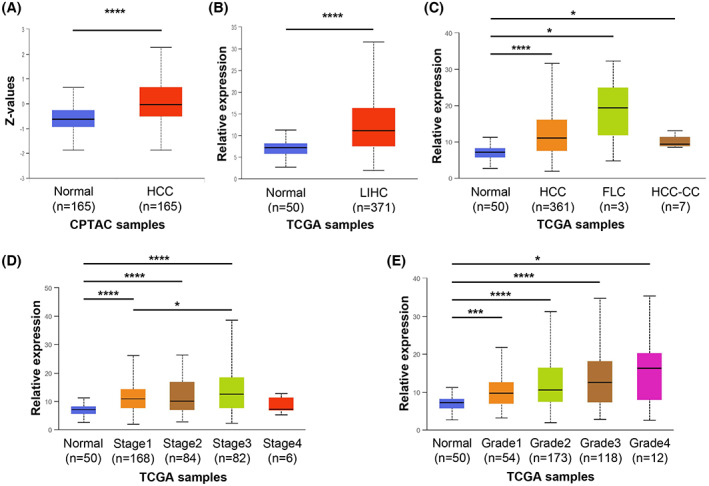
ZHX2 mRNA and protein levels are significantly higher in patients with hepatocellular carcinoma (HCC) and associated with clinical pathological parameters. UALCAN was used to analyze data from the Cancer Genome Atlas (TCGA) and the Clinical Proteomic Tumor Consortium (CPTAC). (A) ZHX2 protein expression in CPTAC samples. (B) ZHX2 mRNA expression in TCGA Liver Hepatocellular Carcinoma (LIHC) samples. (C–E) ZHX2 mRNA expression in TCGA‐LIHC samples based on histological subtypes (C), individual cancer stages (D), and tumor grade (E). *p < 0.05, ***p < 0.001, and ****p < 0.0001. Abbreviations: FLC, fibrolamellar carcinoma; HCC‐CC, hepatocholangiocarcinoma.
DISCUSSION
Data presented here show that DEN‐initiated hepatocarcinogenesis is completely blocked in the absence of Zhx2, with a complete lack of tumors at 9 months and 10 months of age in both male and female mice. H&E‐stained sections and molecular analyses both support the absence of tumors in these mice. Most tellingly, Gpc3 levels dramatically increased in tumors (~700‐fold) of DEN‐treated Zhx2 wt livers compared with PBS‐treated Zhx2 wt livers. “Nontumor” tissues from DEN‐treated Zhx2 wt livers also had higher level of Gpc3 (33‐fold), likely due to the presence of multiple smaller tumor nodules present in these tissues. However, Gpc3 levels were not increased in Zhx2 KO livers after DEN treatment compared with PBS‐treated Zhx2 KO livers. In addition, when compared with PBS‐treated controls, G6PC mRNA levels significantly decreased in tumors of DEN‐treated Zhx2 wt mice, as seen previously,[ 31 ] but remained unchanged in DEN‐treated Zhx2 KO livers.
Because differences between Zhx2 wt and Zhx2 KO mice soon after DEN treatment could explain the disparity in tumor formation, we focused on the first week after treatment (Figure 8). CYP2E1, the primary bioactivator of DEN in the liver to generate reactive radicals that can damage cellular components, including DNA, and lead to an inflammatory response, is present at similar levels in Zhx2 wt and Zhx2 KO livers. γH2AX staining and Pold mRNA levels were also similar between Zhx2 wt and Zhx2 KO livers during the early timepoints, suggesting that there was no difference in cellular or DNA damage and repair in these cohorts after DEN treatment. However, cell proliferation, as measured by Ki67 staining, was higher and persisted longer in Zhx2 wt mice than in Zhx2 KO mice during the week after DEN treatment. This increased proliferation could increase the likelihood of DNA damage and/or other tumor initiation events in Zhx2 wt mice that resulted in tumors 9 months later. The reduced proliferation rates align with the lower pAKT and AKT2 levels observed in the Zhx2 KO mice compared with the Zhx2 wt mice. AKT2 expression has been correlated with HCC prognosis in humans,[ 34 ] suggesting that targeting Zhx2 may have the potential to regulate this pathway for HCC therapy. How Zhx2 may regulate the AKT pathway, by gene regulation or protein–protein interaction, needs further investigation. Targeting the AKT‐pathway in HCC has been proposed.[ 35 ] Possible concerns of antagonizing this pathway alone for HCC therapy may cause other deleterious effects such as inducing insulin‐resistant diabetes. However, these effects have not been observed in the Zhx2 KO mice and implies that this could be a better and safer target. Knockout studies of the AKT isozymes in mice have shown positive results, as Akt1 ablation impaired carcinogenesis,[ 36 ] and Akt2 deletion reduced the occurrence of HCC in c‐Met‐transfected mice.[ 37 ] Future studies that dissect the AKT isozyme function in HCC and how each might impact growth or metabolic pathways are needed.
FIGURE 8.
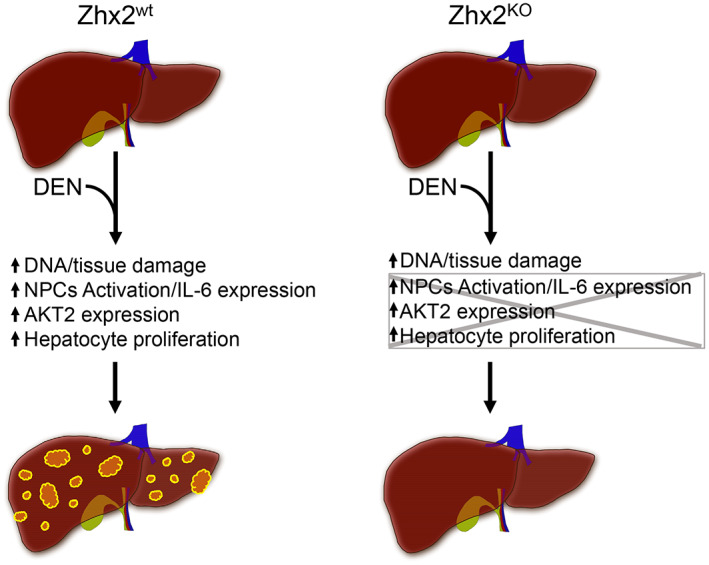
Graphic summary of action of Zhx2 in DEN‐induced HCC. The absence of Zhx2 completely blocks liver tumor formation 9 months after DEN treatment. Although DEN‐mediated DNA damage is the same in both Zhx2 KO and Zhx2 wt mice, hepatocyte proliferation, AKT2 activation, and IL‐6 expression are reduced in Zhx2 KO livers soon after DEN exposure. These data suggest that Zhx2 functions in both hepatocytes and nonparenchymal cells (NPCs)—a possibility that is supported by the fact that tumors are reduced, but not absent, in DEN‐treated Zhx2 Δliv mice.
Our data that Zhx2 acts as a tumor promoter after DEN treatment are consistent with higher ZHX2 expression in human HCC from TCGA and CPTAC data, and suggest that this model may provide insight into Zhx2 and HCC progression in humans. However, our mouse data appear paradoxical with other liver tumor models indicating that Zhx2 functions as a tumor suppressor. This suggests that the action of Zhx2 in liver cancer is context‐dependent—a notion that is consistent with a growing number of studies of other tumor promoters and/or suppressors.[ 38 ] For example, the tyrosine phosphatase Shp2,[ 38 ] Stat3,[ 38 ] beta‐catenin,[ 38 ] the microRNA miR‐21,[ 39 ] and proteins in the NF‐kB pathway[ 29 ] can all promote or inhibit tumor formation in different tumor models. Interestingly, the Zhx2 target H19 has also been shown to have both tumor‐promoting and tumor‐suppressing activities in different liver tumor models.[ 40 ] In vitro growth and xenograft tumor studies suggested that Zhx2 represses the proliferation of liver tumor cell lines, which may be due to Zhx2‐mediated repression of cyclin A and cyclin E expression.[ 21 ] In contrast, we found that cell proliferation, as measured by Ki67 staining, was greater in the presence of Zhx2. Thus, differences in the control of cell proliferation by Zhx2 in transformed liver cell lines and perinatal hepatocytes could at least partially explain the seemingly contradictory results in these distinct model systems and help explain the conflicting data that have been obtained in human studies.[ 19 , 20 ]
A recent study by Zhao et al. showed that Zhx2 alleviates NASH through phosphatase and tensin homolog activation.[ 41 ] This study used a high‐fat high‐cholesterol diet to induce NASH and used both liver‐specific Zhx2 knockout and liver‐specific Zhx2 transgenic mice. Mice in this study did not develop tumors, so we cannot compare this model with our DEN model regarding HCC formation. However, it should be noted that Zhao et al.[ 41 ] and Yue et al[ 21 ] focused on hepatocytes, although it is increasing clear that NPCs also play critical roles in NAFLD progression from simple steatosis to NASH, cirrhosis, and HCC.[ 42 ] DEN‐induced HCC depends on an inflammatory response and IL‐6 production in activated Kupffer cells.[ 29 , 33 ] Our data showed that hepatic IL‐6 levels were significantly decreased in whole‐body Zhx2 knockout mice, which might due to the absence of Zhx2 in NPCs. Thus, understanding Zhx2 function in both NPCs and hepatocytes will be necessary to fully understand the role of Zhx2 in HCC initiation and progression, and could help elucidate the basis for the contradictory data with Zhx2 in different liver disease models and may provide valuable insight into the complexity of HCC progression.
Many Zhx2 target genes are dysregulated in HCC, and it is possible that one or more of these target genes is responsible for the dramatic difference in HCC progression between Zhx2 wt and Zhx2 KO mice after DEN treatment. Several known Zhx2 target genes, including H19, Gpc3 and Lpl, have been associated with liver cancer. H19, which acts as a tumor suppressor specifically in the DEN model,[ 43 ] could help explain the lack of tumors in Zhx2 KO livers, as it is expressed at higher levels in the absence of Zhx2. Most reports indicate that Gpc3 is pro‐oncogenic[ 30 ] and Lpl levels were higher in human and mouse HCC.[ 44 ] However, hepatic Gpc3 and Lpl levels are higher in Zhx2 KO than in Zhx2 wt mice, which indicates that these proteins are not involved in the absence of tumors in DEN‐treated Zhx2 KO mice. Altered lipid metabolism is found in HCC, including DEN‐induced tumors.[ 45 ] Many enzymes regulating lipid homeostasis are controlled by Zhx2,[ 46 , 47 ] so changes in hepatic lipids could also contribute to tumor differences between Zhx2 wt and Zhx2 KO livers.
Zhx2 is expressed in hepatocytes and NPCs in the adult liver (Figure 2).[ 25 ] The fact that tumors are reduced in DEN‐treated Zhx2 Δliv mice, compared with the absence of tumors in DEN‐treated Zhx2 KO mice, indicates that NPCs contribute to tumor formation. BALB/cJ mice, which have a natural Zhx2 mutation, have reduced atherosclerotic lesions compared to mice with a wild‐type Zhx2 gene when placed on a high‐fat diet. These BALB/cJ mice have greater numbers of anti‐inflammatory M2 macrophages, which could account for reduced damage compared to Zhx2‐positive mice with higher pro‐inflammatory M1 macrophages.[ 46 ] Based on these data, Zhx2 expression in Kupffer cells and/or infiltrating macrophages in Zhx2 Δliv mice compared with Zhx2 KO mice could lead to different inflammatory environments after DEN treatment, which would impact tumorigenesis. Future studies will need to investigate the role of Zhx2 in various liver cell populations in relation to liver disease, including HCC.
Although early Zhx2 studies focused on Zhx2 function in HCC due to its control of AFP and other hepatic genes, a growing number of reports have associated Zhx2 with other cancers. Zhx2 levels are low in Hodgkin's lymphoma and multiple myeloma, suggesting that Zhx2 may function as a tumor suppressor in these B‐cell malignancies, although functional studies were not performed.[ 48 ] Zhx2 inhibits the metastatic potential of several thyroid cancer cell lines, possibly by inhibition of S100A14, suggesting a tumor suppressor role in this cancer.[ 49 ] In contrast to these tumors, Zhx2 is oncogenic in clear‐cell renal cell carcinoma, the most common kidney tumor, potentially through co‐regulation of target genes with NF‐κB,[ 50 ] and in triple‐negative breast cancer, where it co‐regulates target genes with hypoxia inducible factor 1 alpha subunit.[ 51 ] Future studies will require different liver tumor model systems to fully understand Zhx2 control of tumor initiation and promotion. Because Zhx2 is known to regulate many genes that control lipid homeostasis, and that obesity and NAFLD are currently major drivers of the increased incidence of HCC, it will be particularly interesting to investigate the role of Zhx2 in high‐fat diet–induced liver tumor models.
SYNOPSIS
We found that the absence of Zhx2 completely blocks liver tumor formation in mice after DEN treatment. Our data indicate that reduced cell proliferation and AKT2 expression could result in lower tumors. The reduced IL‐6 expression in Zhx2 KO mice suggests that NPCs also contribute to the lack of tumors, possibly by reducing compensatory hepatocyte proliferation after DEN‐mediated damage (Figure 8). These data indicate that Zhx2 is pro‐oncogenic in the DEN‐induced mouse HCC model and is consistent with the higher expression of ZHX2 in patients with HCC.
AUTHOR CONTRIBUTIONS
Study concept and design: Jieyun Jiang and Brett T. Spear. Experiments: Jieyun Jiang, Courtney Turpin, and Guofang (Shirley) Qiu. Immunoblotting and calculations for Figure 5D–F: Mei Xu. Evaluation of histopathology of liver H&E sections: Eun Lee. Data analysis and manuscript draft and edit: Jieyun Jiang and Brett T. Spear. Discussions of experimental design, data interpretation, and manuscript editing: Martha L. Peterson and Terry D. Hinds. All authors have read and approved the final version of the manuscript.
FUNDING INFORMATION
Supported by the American Cancer Society (IRG 16‐182‐28); National Institutes of Health (DK‐074816); the University of Kentucky Markey Cancer Center Support Grant and the Biospecimen Procurement and Translational Pathology Shared Resource Facility of the University of Kentucky Markey Cancer Center (P30CA177558).
CONFLICT OF INTEREST
Nothing to report.
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGMENT
Floxed Zhx2 mice were obtained from the trans‐NIH Knock‐Out Mouse Project.
Jiang J, Turpin C, Qiu G, Xu M, Lee E, Hinds TD, et al. Zinc fingers and homeoboxes 2 is required for diethylnitrosamine‐induced liver tumor formation in C57BL/6 mice. Hepatol Commun. 2022;6:3550–3562. 10.1002/hep4.2106
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. Karagozian R, Derdak Z, Baffy G. Obesity‐associated mechanisms of hepatocarcinogenesis. Metabolism. 2014;63:607–17. [DOI] [PubMed] [Google Scholar]
- 3. Eslam M, Sanyal AJ, George J, International Consensus Panel . MAFLD: a consensus‐driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. [DOI] [PubMed] [Google Scholar]
- 4. Creeden JF, Kipp ZA, Xu M, Flight RM, Moseley HNB, Martinez GJ, et al. Hepatic kinome atlas: an in‐depth identification of kinase pathways in liver fibrosis of humans and rodents. Hepatology. 2022. Mar 21. 10.1002/hep.32467. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weaver L, Hamoud AR, Stec DE, Hinds TD Jr. Biliverdin reductase and bilirubin in hepatic disease. Am J Physiol Gastrointest Liver Physiol. 2018;314:G668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang DQ, El‐Serag HB, Loomba R. Global epidemiology of NAFLD‐related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
- 8. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lozanovski VJ, Unterrainer C, Dohler B, Susal C, Mehrabi A. Outcome of extended right lobe liver transplantations. Liver Transpl. 2022;28:807–18. [DOI] [PubMed] [Google Scholar]
- 10. Desai JR, Ochoa S, Prins PA, He AR. Systemic therapy for advanced hepatocellular carcinoma: an update. J Gastrointest Oncol. 2017;8:243–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Llovet JM, Hernandez‐Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072–9. [DOI] [PubMed] [Google Scholar]
- 12. Abelev GI. Alpha‐fetoprotein in ontogenesis and its association with malignant tumors. Adv Cancer Res. 1971;14:295–358. [DOI] [PubMed] [Google Scholar]
- 13. Olsson M, Lindahl G, Ruoslahti E. Genetic control of alpha‐fetoprotein synthesis in the mouse. J Exp Med. 1977;145:819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perincheri S, Dingle RW, Peterson ML, Spear BT. Hereditary persistence of alpha‐fetoprotein and H19 expression in liver of BALB/cJ mice is due to a retrovirus insertion in the Zhx2 gene. Proc Natl Acad Sci U S A. 2005;102:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perincheri S, Peyton DK, Glenn M, Peterson ML, Spear BT. Characterization of the ETnII‐α endogenous retroviral element in the BALB/cJ Zhx2 Afr1 allele. Mamm Genome. 2008;19:26–31. [DOI] [PubMed] [Google Scholar]
- 16. Spear BT, Jin L, Ramasamy S, Dobierzewska A. Transcriptional control in the mammalian liver: liver development, perinatal repression, and zonal gene regulation. Cell Mol Life Sci. 2006;63:2922–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peterson ML, Ma C, Spear BT. Zhx2 and Zbtb20: novel regulators of postnatal alpha‐fetoprotein repression and their potential role in gene reactivation during liver cancer. Semin Cancer Biol. 2011;21:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morford LA, Davis C, Jin L, Dobierzewska A, Peterson ML, Spear BT. The oncofetal gene glypican 3 is regulated in the postnatal liver by zinc fingers and homeoboxes 2 and in the regenerating liver by alpha‐fetoprotein regulator 2. Hepatology. 2007;46:1541–7. [DOI] [PubMed] [Google Scholar]
- 19. Hu S, Zhang M, Lv Z, Bi J, Dong Y, Wen J. Expression of zinc‐fingers and homeoboxes 2 in hepatocellular carcinogenesis: a tissue microarray and clinicopathological analysis. Neoplasma. 2007;54:207–11. [PubMed] [Google Scholar]
- 20. Lv Z, Zhang M, Bi J, Xu F, Hu S, Wen J. Promoter hypermethylation of a novel gene, ZHX2, in hepatocellular carcinoma. Am J Clin Pathol. 2006;125:740–6. [DOI] [PubMed] [Google Scholar]
- 21. Yue X, Zhang Z, Liang X, Gao L, Zhang X, Zhao D, et al. Zinc fingers and homeoboxes 2 inhibits hepatocellular carcinoma cell proliferation and represses expression of Cyclins A and E. Gastroenterology. 2012;142:1559–70.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu X, Lin Q, Wu Z, Zhang Y, Wang T, Zhao S, et al. ZHX2 inhibits SREBP1c‐mediated de novo lipogenesis in hepatocellular carcinoma via miR‐24‐3p. J Pathol. 2020;252:358–70. [DOI] [PubMed] [Google Scholar]
- 23. Verna L, Whysner J, Williams GM. N‐nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA‐adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther. 1996;71:57–81. [DOI] [PubMed] [Google Scholar]
- 24. Creasy KT, Jiang J, Ren H, Peterson ML, Spear BT. Zinc Fingers and homeoboxes 2 (Zhx2) regulates sexually dimorphic cyp gene expression in the adult mouse liver. Gene Expr. 2016;17:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang J, Creasy KT, Purnell J, Peterson ML, Spear BT. Zhx2 (zinc fingers and homeoboxes 2) regulates major urinary protein gene expression in the mouse liver. J Biol Chem. 2017;292:6765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C[T]) Method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 27. Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce‐Rodriguez I, Chakravarthi B, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, et al. UALCAN: an update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine‐driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–90. [DOI] [PubMed] [Google Scholar]
- 30. Shih TC, Wang L, Wang HC, Wan YY. Glypican‐3: a molecular marker for the detection and treatment of hepatocellular carcinoma(). Liver Res. 2020;4:168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Q, Li J, Zhang W, Xiao C, Zhang S, Nian C, et al. Glycogen accumulation and phase separation drives liver tumor initiation. Cell. 2021;184:5559–76.e19. [DOI] [PubMed] [Google Scholar]
- 32. Connor F, Rayner TF, Aitken SJ, Feig C, Lukk M, Santoyo‐Lopez J, et al. Mutational landscape of a chemically‐induced mouse model of liver cancer. J Hepatol. 2018;69:840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88‐dependent IL‐6 production. Science. 2007;317:121–4. [DOI] [PubMed] [Google Scholar]
- 34. Xu X, Sakon M, Nagano H, Hiraoka N, Yamamoto H, Hayashi N, et al. Akt2 expression correlates with prognosis of human hepatocellular carcinoma. Oncol Rep. 2004;11:25–32. [PubMed] [Google Scholar]
- 35. Mroweh M, Roth G, Decaens T, Marche PN, Lerat H, Jilkova ZM. Targeting Akt in hepatocellular carcinoma and its tumor microenvironment. Int J Mol Sci. 2021;22:1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)‐ErbB2/neu and MMTV‐polyoma middle T transgenic mice. Cancer Res. 2007;67:167–77. [DOI] [PubMed] [Google Scholar]
- 37. Wang C, Che L, Hu J, Zhang S, Jiang L, Latte G, et al. Activated mutant forms of PIK3CA cooperate with RasV12 or c‐Met to induce liver tumour formation in mice via AKT2/mTORC1 cascade. Liver Int. 2016;36:1176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Feng GS. Conflicting roles of molecules in hepatocarcinogenesis: paradigm or paradox. Cancer Cell. 2012;21:150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang T, Yang Z, Kusumanchi P, Han S, Liangpunsakul S. Critical role of microRNA‐21 in the pathogenesis of liver diseases. Front Med (Lausanne). 2020;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tietze L, Kessler SM. The good, the bad, the question‐H19 in hepatocellular carcinoma. Cancers (Basel). 2020;12:1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao Y, Gao L, Jiang C, Chen J, Qin Z, Zhong F, et al. The transcription factor zinc fingers and homeoboxes 2 alleviates NASH by transcriptional activation of phosphatase and tensin homolog. Hepatology. 2022;75:939–54. [DOI] [PubMed] [Google Scholar]
- 42. Hernandez‐Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schultheiss CS, Laggai S, Czepukojc B, Hussein UK, List M, Barghash A, et al. The long non‐coding RNA H19 suppresses carcinogenesis and chemoresistance in hepatocellular carcinoma. Cell Stress. 2017;1:37–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cao D, Song X, Che L, Li X, Pilo MG, Vidili G, et al. Both de novo synthetized and exogenous fatty acids support the growth of hepatocellular carcinoma cells. Liver Int. 2017;37:80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haberl EM, Pohl R, Rein‐Fischboeck L, Horing M, Krautbauer S, Liebisch G, et al. Accumulation of cholesterol, triglycerides and ceramides in hepatocellular carcinomas of diethylnitrosamine injected mice. Lipids Health Dis. 2021;20:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Erbilgin A, Seldin MM, Wu X, Mehrabian M, Zhou Z, Qi H, et al. Transcription factor Zhx2 deficiency reduces atherosclerosis and promotes macrophage apoptosis in mice. Arterioscler Thromb Vasc Biol. 2018;38:2016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu Z, Ma H, Wang L, Song X, Zhang J, Liu W, et al. Tumor suppressor ZHX2 inhibits NAFLD‐HCC progression via blocking LPL‐mediated lipid uptake. Cell Death Differ. 2020;27:1693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nagel S, Schneider B, Meyer C, Kaufmann M, Drexler HG, Macleod RA. Transcriptional deregulation of homeobox gene ZHX2 in Hodgkin lymphoma. Leuk Res. 2012;36:646–55. [DOI] [PubMed] [Google Scholar]
- 49. Zhang Y, Sun M, Gao L, Liang X, Ma C, Lu J, et al. ZHX2 inhibits thyroid cancer metastasis through transcriptional inhibition of S100A14. Cancer Cell Int. 2022;22:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang J, Wu T, Simon J, Takada M, Saito R, Fan C, et al. VHL substrate transcription factor ZHX2 as an oncogenic driver in clear cell renal cell carcinoma. Science. 2018;361:290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fang W, Liao C, Shi R, Simon JM, Ptacek TS, Zurlo G, et al. ZHX2 promotes HIF1alpha oncogenic signaling in triple‐negative breast cancer. Elife. 2021;10:e70412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


