Abstract
We analyzed homotypic and heterotypic antibody responses to a type-specific antigen (Tsa), a 56-kDa protein of Orientia tsutsugamushi, by using sera from mice immunized with strains Gilliam, Karp, Kato, and Boryong. We generated a series of deletion constructs of the tsa gene and expressed them as MalE fusion proteins. Variable domain I (VD I) showed strong responses to homotypic antibodies. Antigenic domain II (AD II) from Boryong and Karp showed cross-reactivities to each other. VD III showed no responses to any of the antibodies. Sera from Kato-immunized mice showed only homotypic responses to AD III. On the other hand, sera of the mice immunized with Gilliam, Karp, or Boryong showed homotypic as well as heterotypic responses to this region. VD IV showed the strongest heterotypic antibody responses among the fragments tested. These data suggest that VD I is important in homotypic antibody responses and that AD II, AD III, and VD IV are important in heterotypic antibody responses of the mice to Tsa.
Scrub typhus is characterized by fever, rash, eschar, pneumonitis, meningitis, and disseminated intravascular coagulation in some cases leading to circulatory collapse (10). It is caused by infection with Orientia tsutsugamushi, which belongs to the family Rickettsiaceae (21).
The mechanisms responsible for protective immunity of O. tsutsugamushi-infected humans may involve both humoral and cell-mediated immunity (1, 2, 7–9, 13, 15–17, 20). Type-specific antigen (Tsa), a 56-kDa protein of O. tsutsugamushi, is a surface-exposed (22), major integral membrane protein (19). The immune responses to Tsa are important in preventing infection (16, 17). Animals immunized with Tsa develop both humoral and cellular immune responses to O. tsutsugamushi markedly (16, 17, 19). Mice immunized with recombinant Bor56, one of the Tsa, were protected from challenge with the homotype of O. tsutsugamushi (16). Recent study has shown that antibody to Bor56 neutralizes oriental infection in vitro (17). The strong immune response of humans to this surface protein shows its potent immunogenicity (4, 6, 12, 14). As a result, Tsa has become the primary candidate for a genetically engineered scrub typhus vaccine. Since distinct determinants on this molecule could form the basis of a recombinant vaccine, determination of antigenicity and immunoaccessibility of epitopes should permit the rational selection of candidate domains. In an effort to identify strain-specific and cross-reactive epitopes of Tsa from strains Gilliam, Karp, Kato, and Boryong, we have generated a group of deletion fragments of the tsa gene encoding various regions of the protein. By using these constructs, we have identified domains which react with homotypic and heterotypic antibodies from the hyperimmunized mice.
Sera from hyperimmunized mice.
Ten female BALB/c mice were immunized subcutaneously with O. tsutsugamushi as described previously (16). Three weeks after the third immunization, mice were bled and sera were prepared (3). Titers of antibody to O. tsutsugamushi and to MalE were examined (11, 12). Sera that showed a titer of antibody to a homotypic strain of more than 1:320 were used after heat inactivation by incubation at 56°C for 30 min.
Generation of ΔTsa mutants.
To obtain the desired Tsa deletion (ΔTsa) mutants, parts of tsa were amplified by PCR, creating a series of fusion proteins that contain NH2-terminal MalE fused with various lengths of coding sequences, as indicated in Fig. 1. tsa open reading frames of O. tsutsugamushi Gilliam, Karp, Kato, and Boryong were retrieved from the oriental genomic DNAs by PCR (12). Prokaryotic expression plasmids encoding truncated forms of Tsa were expressed in Escherichia coli XL1-Blue (Stratagene, La Jolla, Calif.). The nucleotide sequences of the 5′ ends of the deletion constructs were determined by using malE primer (New England Biolabs, Beverly, Mass.). The first amino acids inferred from the 5′ end of each deletion clone are shown in Fig. 1. Each of these expression clones was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma, St. Louis, Mo.). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting were performed as described previously (11, 12). The constructs encoded a fusion product that was clearly distinguishable on a Coomassie-stained gel (data not shown). MalE from the lysate of E. coli transformed by expression vector pIH821 was also analyzed. Figure 2A shows an immunoblot analysis of the constructs illustrated in Fig. 1 following the induced overexpression (19).
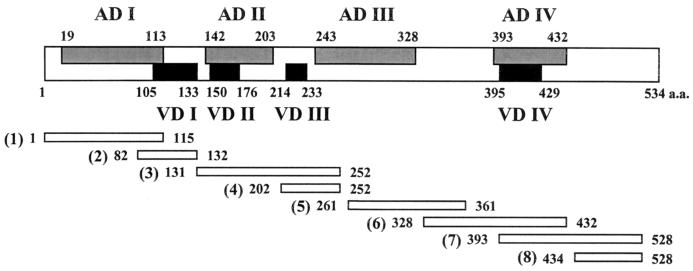
FIG. 1.
Schematic representation of the fragments (1 to 8) of cloned tsa genes based on the bor56 nucleotide sequences and inferred amino acid sequences (GenBank accession no. L04956). The malE sequences of expression vector pIH821 are fused to portions of the fragments corresponding to the amino terminus and are not depicted. Numbers beside the fragments refer to amino acid residues of the translated Δtsa gene.
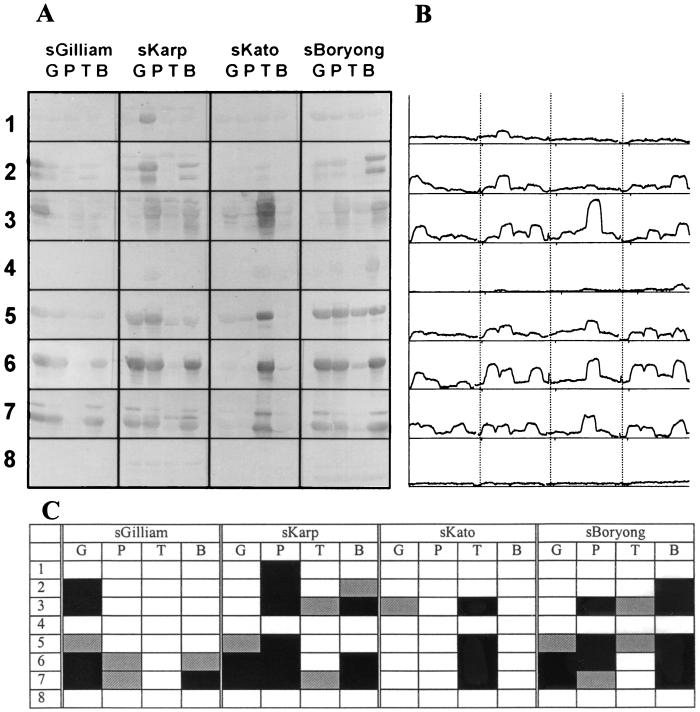
FIG. 2.
(A) Immunoblot of ΔTsa fusion proteins with sera from hyperimmunized mice. Induced fusion constructs were lysed, electrophoresed, transferred to nitrocellulose papers, and reacted with the indicated polyclonal sera (see below). Numbers indicate ΔTsa fragments shown in Fig. 1. (B) Densitometry analysis of the immunoblot. (C) Summary of immunoblotting analysis of sera from hyperimmunized mice with ΔTsa fragments. Black squares and gray squares indicate strongly positive and positive reactions (see the text), respectively. sGilliam, sKarp, sKato, and sBoryong, sera from mice immunized with Gilliam, Karp, Kato, and Boryong, respectively. G, P, T, and B, amino acid fragments derived from Gilliam, Karp, Kato, and Boryong, respectively.
Antibody responses to Tsa.
The reactivities of the ΔTsa constructs with sera from hyperimmunized mice were analyzed after the immunostained bands were digitized (Fig. 2B). The images on the immunostained nitrocellulose membranes were digitized with a scanner (ScanJet 4100C; Hewlett-Packard, Boise, Idaho). The images were converted to gray scale. The densities of the bands were measured by using ScionImage (version beta 2; Scion Corporation, Frederick, Md.). The density values were assigned arbitrarily by ScionImage (Fig. 2B). The minimum value among the background gray scales was subtracted from the density values of the bands. Sera from the mice immunized with O. tsutsugamushi were analyzed for reactivity to MalE after proteins from pIH821-transformed E. coli were separated. Average values (AV) and standard deviations (SD) of the band densities derived from the antibody responses to MalE were calculated as described above. The AV of the sera from the mice immunized with Gilliam, Karp, Kato, and Boryong were 6.6, 6.3, 11.5, and 9.9, respectively. The SD were 2.2, 3.3, 2.3, and 2.3, respectively. The density values of the bands over the sum of the AV and the number obtained by multiplying the SD by 2 were considered positive reactions. The density values larger than the sum of the AV and the number obtained by multiplying the SD by 5 were regarded as strongly positive reactions.
The reactivities of the hyperimmunized sera with ΔTsa mutants are shown in Fig. 2C. The 115 amino-terminal amino acids from Gilliam, Kato, and Boryong were not reactive with homotypic antibodies or heterotypic antibodies, although amino acids (aa) 1 to 115 from Karp was reactive with homotypic antibodies. Sera from the mice immunized with Gilliam, Karp, and Boryong were reactive with homotypic aa 82 to 132. Sera from Kato-immunized mice were not reactive with homotypic or heterotypic aa 82 to 132. Sera from Karp-immunized mice were cross-reactive with aa 82 to 132 from Boryong. Although the 113 amino-terminal amino acids were highly reactive with patient immunoglobulin M antibodies, this region was not immunogenic in mice (19). This region contains the variable domain I (VD I) sequence, which has been suggested to be important in strain-specific antibody responses.
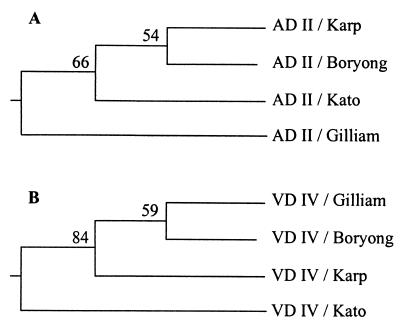
aa 131 to 252 from Karp, Kato, and Boryong were reactive with both homotypic and heterotypic antibodies. However, sera from Gilliam-immunized mice were not reactive with heterotypic aa 131 to 252. They were reactive only with the homotypic region. Considering the fact that aa 202 to 252 were not reactive with any of the antibodies, antibody responses to aa 131 to 252 could be thought of as responses directed to aa 131 to 201. aa 131 to 201 contain antigenic domain II (AD II) and VD II. Sera from the mice immunized with Karp or Boryong showed cross-reactivity to aa 131 to 201 derived from Karp, Kato, and Boryong. Sera from the Kato-immunized mice showed only strain-specific responses to ΔTsa mutants derived from homotypic strains, except to aa 131 to 201. These sera showed only one heterotypic response to aa 131 to 201 from Gilliam. The similarity of the sequences of aa 131 to 252 from Karp and Boryong was 70%, and this value was the highest among the pairs of aa sequences of this region. When we clustered the O. tsutsugamushi strains based on the AD II sequences, Karp and Boryong were grouped into same cluster (Fig. 3A). Gilliam was located in a cluster different from that of Karp, Kato, and Boryong. Considering the cross-reactivities of this region together with the clustering patterns based on the primary amino acid sequences, the antigenicity of the AD II region might be largely due to the linear epitopes. With only this observation, however, we could not completely exclude the possibility of the existence of conformational epitopes in this region.
FIG. 3.
UPGMA dendrogram, derived from similarity coefficients, showing the relationships among O. tsutsugamushi strains based on the amino acid sequences of AD II and VD IV. Amino acid sequences were aligned and analyzed as described previously (18). The statistical validity of the trees was investigated by the bootstrap method of numerical resampling, using the SEQBOOT, PRODIST, NEIGHBOR, and CONSENS programs of the PHYLIP package (5). One hundred bootstrapped data sets of amino acid sequences for AD II and VD IV were generated. The distance matrices from each of the replicate data sets were calculated, and the tree was generated by successive clustering by using an average linkage method. The numbers at the forks indicate the number of times the group consisting of the species which are to the right of that fork occurred among the trees.
aa 202 to 252 were not reactive with sera from hyperimmunized mice. This region contains VD III. In a previous study (19), it was reported that VD III was weakly reactive with patient sera and induced weak humoral immune responses in mice. Hence, it is thought that although the VD III region is not responsible for the binding of serotype-specific antibodies, it might contribute to conformational changes of the Tsa which lead to the alterations of the conformational strain-specific epitopes.
aa 261 to 433 showed homotypic and heterotypic antibody responses. However, fragments from Kato spanning this region showed reactions only to homotypic antibodies. aa 261 to 361 did not show any unique patterns of reactivity. Sera from Boryong-immunized mice were reactive with aa 261 to 361 from all of the strains. However, sera from the mice immunized with Kato or Gilliam were reactive only with homotypic aa 261 to 361. This region contains part of AD III. Considering the similar reactivities of aa 328 to 432 and aa 393 to 528, the reactivities of aa 328 to 361 were thought to contribute little to the antibody responses to aa 261 to 361. For this reason, antibody responses to aa 261 to 361 could be thought of as responses directed to aa 261 to 327. Considering the absence of reactions of aa 202 to 252, reactivities to AD III could be confined to aa 261 to 327.
aa 328 to 432 showed extensive cross-reactions among strains Gilliam, Karp, and Boryong. aa 393 to 528 showed patterns of reactivities similar to those of aa 328 to 432. However, the reactivities of aa 393 to 528 were abrogated by deleting amino acids up to the 433rd residue, as was shown in reactivities of aa 434 to 528. Considering the absence of reactivity to aa 434 to 528, responses to aa 393 to 528 could be thought of as responses to aa 393 to 433. For these reasons, antibody responses to aa 328 to 528 could be analyzed in the same manner as reactions to the region of aa 393 to 433, containing VD IV. This region showed cross-reactivities with heterotypic antibodies. However, that from Kato was not reactive with heterotypic antibodies. VD IV is thought to be a cross-reactive antigenic determinant among Gilliam, Karp, and Boryong. When we clustered strains on the basis of the primary amino acid sequences of VD IV, we found that patterns of heterotypic antibody responses of this region were largely dependent on primary amino acid sequence homology (Fig. 3B). Gilliam, Karp, and Boryong were grouped into the same cluster. Kato was segregated from the cluster. It was observed in a previous study that recombinant ΔBor56 protein expressing this region was not immunogenic in C3H mice and was not reactive with patient sera (19). This lack of immunogenicity might have been caused by variable major histocompatibility complexes of the host and by variable forms of immunizing antigens. Recombinant antigens were used for the immunization in the previous study (19), and we used live O. tsutsugamushi organisms in the present study. While we could not elucidate exactly why the variable responses for VD IV were obtained in this study, this region could be thought of as one of the ADs, namely, AD IV, in another H-2 haplotype.
In summary, although AD I is reactive with patient sera, it was not reactive with sera derived from hyperimmunized BALB/c mice. AD II overlapping with VD II has immunogenic potential both in humans and in mice with the H-2k as well as the H-2d haplotype. These residues determined the homotypic antibody responses of Gilliam and Kato as well as the heterotypic antibody responses of Karp and Boryong. AD III could be confined to aa 261 to 327 and showed various responses according to the strains. AD IV contributed to the cross-reactivities of Tsa to various heterotypic antibodies. The further elucidation of heterotypic protective immunity induced by these domains of Tsa could provide a rationale for the development of a more efficacious vaccine.
Acknowledgments
This work was supported by the Ministry of Science and Technology of the Republic of Korea (grant 97-N1-02-01-A-06).
REFERENCES
- 1.Bourgeois A L, Olson J G, Fang R C, Huang J, Wang C L, Chow L, Bechthold D, Dennis D T, Coolbaugh J C, Weiss E. Humoral and cellular responses in scrub typhus patients reflecting primary infection and reinfection with Rickettsia tsutsugamushi. Am J Trop Med Hyg. 1982;31:532–540. doi: 10.4269/ajtmh.1982.31.532. [DOI] [PubMed] [Google Scholar]
- 2.Catanzaro P J, Shirai A, Agniel L D, Jr, Osterman J V. Host defenses in experimental scrub typhus: role of spleen and peritoneal exudate lymphocytes in cellular immunity. Infect Immun. 1977;18:118–123. doi: 10.1128/iai.18.1.118-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang W H, Kang J S, Lee W K, Choi M S, Lee J H. Serological classification by monoclonal antibodies of Rickettsia tsutsugamushi isolated in Korea. J Clin Microbiol. 1990;28:685–688. doi: 10.1128/jcm.28.4.685-688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisemann C S, Osterman J V. Antigens of scrub typhus rickettsiae: separation by polyacrylamide gel electrophoresis and identification by enzyme-linked immunosorbent assay. Infect Immun. 1981;32:525–533. doi: 10.1128/iai.32.2.525-533.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felsenstein J. PHYLIP (phylogenetic inference package), version 3.5c. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 6.Hanson B. Identification and partial characterization of Rickettsia tsutsugamushi major protein immunogens. Infect Immun. 1985;50:603–609. doi: 10.1128/iai.50.3.603-609.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanson B A. Effect of immune serum on infectivity of Rickettsia tsutsugamushi. Infect Immun. 1983;42:341–349. doi: 10.1128/iai.42.1.341-349.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hickman C J, Stover C K, Joseph S W, Oaks E V. Murine T-cell response to native and recombinant protein antigens of Rickettsia tsutsugamushi. Infect Immun. 1993;61:1674–1681. doi: 10.1128/iai.61.5.1674-1681.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jerrells T R, Osterman J V. Development of specific and cross-reactive lymphocyte proliferative responses during chronic immunizing infections with Rickettsia tsutsugamushi. Infect Immun. 1983;40:147–156. doi: 10.1128/iai.40.1.147-156.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawamura A, Tanaka H, Tamura A. Tsutsugamushi disease. Tokyo, Japan: University of Tokyo Press; 1995. [Google Scholar]
- 11.Kim I-S, Seong S-Y, Woo S-G, Choi M-S, Chang W-H. High-level expression of a 56-kilodalton protein gene (bor56) of Rickettsia tsutsugamushi Boryong and its application to enzyme-linked immunosorbent assays. J Clin Microbiol. 1993;31:598–605. doi: 10.1128/jcm.31.3.598-605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim I-S, Seong S-Y, Woo S-G, Choi M-S, Kang J-S, Chang W-H. Rapid diagnosis of scrub typhus by a passive hemagglutination assay using recombinant 56-kilodalton polypeptides. J Clin Microbiol. 1993;31:2057–2060. doi: 10.1128/jcm.31.8.2057-2060.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodama K, Kawamura S, Yasukawa M, Kobayashi Y. Establishment and characterization of a T-cell line specific for Rickettsia tsutsugamushi. Infect Immun. 1987;55:2490–2495. doi: 10.1128/iai.55.10.2490-2495.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohashi N, Tamura A, Suto T. Immunoblotting analysis of anti-rickettsial antibodies produced in patients of tsutsugamushi disease. Microbiol Immunol. 1988;32:1085–1092. doi: 10.1111/j.1348-0421.1988.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 15.Rikihisa Y, Ito S. Effect of antibody on entry of Rickettsia tsutsugamushi into polymorphonuclear leukocyte cytoplasm. Infect Immun. 1983;39:928–938. doi: 10.1128/iai.39.2.928-938.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seong S-Y, Huh M-S, Jang W-J, Park S-G, Kim J-G, Woo S-G, Choi M-S, Kim I-S, Chang W-H. Induction of homologous immune response to Rickettsia tsutsugamushi Boryong with a partial 56-kilodalton recombinant antigen fused with the maltose-binding protein MBP-Bor56. Infect Immun. 1997;65:1541–1545. doi: 10.1128/iai.65.4.1541-1545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seong S Y, Kim H R, Huh M S, Park S G, Kang J S, Han T H, Choi M S, Chang W H, Kim I S. Induction of neutralizing antibody in mice by immunization with recombinant 56 kDa protein of Orientia tsutsugamushi. Vaccine. 1997;15:1741–1747. doi: 10.1016/s0264-410x(97)00112-6. [DOI] [PubMed] [Google Scholar]
- 18.Seong S Y, Park S G, Huh M S, Jang W J, Choi M S, Chang W H, Kim I S. T-track PCR fingerprinting for the rapid detection of genetic polymorphism. FEMS Microbiol Lett. 1997;152:37–44. doi: 10.1111/j.1574-6968.1997.tb10406.x. [DOI] [PubMed] [Google Scholar]
- 19.Seong S-Y, Park S-G, Huh M-S, Jang W-J, Kim H-R, Han T-H, Choi M-S, Chang W-H, Kim I-S. Mapping of antigenic determinant regions of the Bor56 protein of Orientia tsutsugamushi. Infect Immun. 1997;65:5250–5256. doi: 10.1128/iai.65.12.5250-5256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirai A, Catanzaro P J, Phillips S M, Osterman J V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976;14:39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura A, Ohashi N, Urakami H, Miyamura S. Classification of Rickettsia tsutsugamushi in a new genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int J Syst Bacteriol. 1995;45:589–591. doi: 10.1099/00207713-45-3-589. [DOI] [PubMed] [Google Scholar]
- 22.Tamura A, Ohashi N, Urakami H, Takahashi K, Oyanagi M. Analysis of polypeptide composition and antigenic components of Rickettsia tsutsugamushi by polyacrylamide gel electrophoresis and immunoblotting. Infect Immun. 1985;48:671–675. doi: 10.1128/iai.48.3.671-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]