Abstract
Bipolar disorder is a chronic mental illness associated with early mortality, elevated risk of comorbid cardiovascular disease, enormous burden of disability, and large societal costs. Patients often seek treatment for symptoms of bipolar disorder in the primary care setting but are frequently misdiagnosed. This article provides primary care providers with an evidence-based approach to the screening, diagnosis, and pharmacological management of bipolar disorder. Guidance is also provided for helping patients connect with higher levels of specialty psychiatric care when clinically indicated.
Keywords: bipolar disorder, mood disorders, integrated behavioral care, primary care mental health
Introduction
Bipolar disorder, encompassing bipolar 1 and bipolar 2 disorders, is a chronic, functionally impairing mental illness with a lifetime prevalence of 1% and with onset prior to age 25 in the majority of patients.1 The burdens faced by individuals with bipolar disorder are enormous: early mortality of 10 or more years,2–4 substantially elevated rates of suicide and suicide attempt,3 a large burden of disability despite a low prevalence relative to other psychiatric diagnoses (bipolar disorder accounts for approximately 7% of all mental illness-related disability worldwide),5 and a large economic burden - total societal costs associated with bipolar 1 disorder in the US are approximately 80,000 US dollars per individual per year.6
Bipolar disorder is characterized by periods of depression and mania/hypomania. Although mania/hypomania are the hallmarks of bipolar disorder, these individuals experience depressive symptoms far more frequently. A 20 year prospective study revealed individuals with bipolar 1 experienced depressive symptoms approximately 30% of the year and manic or hypomanic symptoms during 10%; individuals with bipolar 2 experienced depressive symptoms for greater than half of the year and only experienced hypomanic symptoms 1.4% of the year.7 In addition, individuals with bipolar disorder may experience mood episodes with mixed features (or mixed states). In mixed states, manic or hypomanic symptoms co-occur with depressive symptoms. A substantial portion of bipolar mood episodes are mixed states, with nearly 30% of manic/hypomanic episodes having mixed features,8 and mixed states have been associated with greater clinical severity, including a higher risk of suicidal ideation and suicidal behavior.9
Patients with bipolar disorder are often initially misdiagnosed and experience a resulting delay in appropriate treatment; approximately 3 in 20 patients with depressive symptoms in primary care settings have unrecognized bipolar depression.10–13 A longitudinal study in Quebec (1998–2010) demonstrated a consistently low rate of concordance between general practitioner suspicion for bipolar disorder and psychiatric specialist diagnosis of bipolar disorder, with no improvement in concordance over time.14 Proper identification of bipolar disorder is crucial in the primary care setting given the greater prevalence of common medical conditions among individuals with bipolar disorder, including hypertension, obesity, diabetes, dyslipidemia, and metabolic syndrome.4,15 Cardiovascular disease (CVD) is the leading contributor to early mortality in bipolar disorder, more than accidents, suicides, and homicide combined; and individuals with bipolar disorder have an approximately two-fold greater risk of death due to CVD than the general population.2
Given the enormous morbidity and mortality associated with bipolar disorder and its suboptimal detection and treatment, bipolar disorder remains an important diagnosis for primary care providers. This article reviews the diagnosis and management of bipolar disorder in the primary care setting, with a focus on psychopharmacology and medical management.
Presentation, Diagnosis, and Differential Diagnosis
Patients with bipolar disorder most often present to primary care with active depressive symptoms. Bipolar disorder presents with a major depressive episode 54% of the time, compared to 22% presenting with mania/hypomania and 24% with a mixed episode.16 Bipolar disorder may also be characterized by anxious distress, in which individuals feel tense, restless, have difficulty concentrating, are afraid something awful may happen, or experience a sense of having lost control.17
Bipolar depression can differ from unipolar depression.18,19 When compared to unipolar depression, bipolar depression has higher heritability rates, earlier age of onset, and more frequent recurrence. It is more likely to be associated with hypersomnia, motor retardation, mood lability, delusions, and hallucinations. All patients with bipolar depression should be asked about suicidal ideation (including urges to act on those thoughts, having a plan to act on those thoughts, or rehearsing an attempt) and risk factors associated with suicide attempts (eg, psychotic or delusional symptoms, substance use or abuse, mixed mania/hypomania, prior attempts).
Patients with undiagnosed bipolar depression may initially present with treatment-resistant depression (up to 40% of treatment-resistant unipolar depression diagnoses were unrecognized bipolar disorder).19 When these patients are treated with antidepressants, their depression may actually worsen.20 Therefore, all patients with apparent treatment-resistant depression should be re-evaluated for prior hypomanic or manic episodes.
In primary care, patients may present with bipolar symptoms as the chief complaint, or symptoms may be elicited as part of routine screening. The US Preventive Services Task Force recommends screening for depression among adults in primary care.21 Whether in response to a patient’s presenting problem or as part of a routine screening, we recommend use of the Patient Health Questionnaire-9 (PHQ-9) as a method of measuring current and recent depressive symptoms. A cut-off score of 10 or greater is associated with a sensitivity of 88% and specificity of 88% for major depressive disorder.22 As the PHQ-9 assesses symptoms within the past 2 weeks, it can be used on an ongoing basis for monitoring clinical progress.
To screen for bipolar disorder, we recommend the use of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI).23 The CIDI screens for a history of any manic or hypomanic symptoms in the patient’s history and begins with two screening “stem” questions. If both stem questions are negative, the screen is considered negative. Routine use of the CIDI for universal screening has demonstrated feasibility in the Collaborative Care setting.24 It is important to note that the CIDI needs to be administered by healthcare staff and cannot be completed by the patient on their own. When asking patients the CIDI questions, we recommend reminding them that we are asking for an episode that occurred independently of substance use or an unusually aggravating event. Additionally, we often clarify during the CIDI questioning whether or not the symptoms are occurring only during that episode (this would count towards CIDI score), daily (this would not count towards CIDI score), or arising only during aggravating circumstances (eg, arguing with a partner or parent) and then subsiding once the argument is over (this would not count towards CIDI score).
In addition to screening, we recommend several other standard components of assessing an individual with a prior diagnosis of bipolar disorder or suspected bipolar disorder. Given the enormous risk of suicidal ideation and behavior, we recommend routine assessment of suicidality, preferably with a structured tool such as the Columbia-Suicide Severity Rating Scale.25 Family psychiatric history is important to assess given the high heritability of bipolar disorder (monozygotic twin concordance of 40–70%) and shared genetic susceptibility between bipolar disorder and schizophrenia as well as between bipolar disorder and autism spectrum disorder.26 Psychiatric comorbidity is common, and we recommend a general review of psychiatric symptoms. Specific psychiatric comorbidities are discussed below.
The formal diagnosis of bipolar illness and the specific type is based upon the diagnosis and characterization of the mood episodes. Mania/hypomania are periods characterized by the so-called “DIG FAST” symptoms: distractibility (D), high-risk behavior (I, for “impulsivity” or “indiscretion”), grandiosity (G), racing thoughts (F, for “flight of ideas”), increase in goal-directed activity (A), decreased need for sleep (S), and talkativeness (T). Per the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, mania lasts for at least 7 consecutive days whereas hypomania lasts for at least 4; both require at least 3 or 4 of these symptoms to be present throughout the episode.17,27 In addition, mania is characterized by severe impairment in social or occupational functioning, associated with psychotic symptoms, or necessitates psychiatric hospitalization; these features are not present in hypomania. Hypomania distinguishes itself from mania by not causing marked disruptions in daily functioning.17 One episode of mania is consistent with a diagnosis of bipolar 1 disorder; one episode of hypomania and one major depressive episode are consistent with a diagnosis of bipolar 2 disorder; cyclothymic disorder is characterized by depressive and hypomania symptoms for at least 2 years without meeting criteria for a major depressive or hypomanic episode.
The psychiatric differential for bipolar disorder should include other psychiatric diagnoses that may include symptoms of affective instability, depression, anxiety, impulsivity, altered cognitive processes, or psychosis. Evaluating the psychiatric differential diagnosis for bipolar disorder can be challenging as it is often comorbid with other psychiatric diagnoses. Around 75% of individuals with bipolar 1 or 2 have a comorbid anxiety disorder. Upwards of 25% of adults with bipolar 1 or 2 meet criteria for ADHD.28
Substance use, including states of intoxication and withdrawal, may lead to symptoms consistent with depression, mania/hypomania, or mixed episodes. Approximately half of all individuals with bipolar 1 disorder and one-third of individuals with bipolar 2 disorder have a comorbid substance use disorder (SUD).28 Cannabis and alcohol are most commonly used, with fewer patients using cocaine and opioids.29 Comorbid SUD is associated with more severe affective symptoms, greater number of suicidal gestures, poorer treatment adherence, and overall worse quality of life.29 We recommend routine and regular screening for substance use in the clinical interview, as well as through urine and serum testing when clinically indicated.
Borderline personality disorder (BPD) and post-traumatic stress disorder (PTSD) can be difficult to distinguish from bipolar disorder. The impulsivity, reactivity, and anger in borderline personality disorder and the hyperarousal of PTSD often mimic mixed or hypomanic episodes. The comorbidity of bipolar disorder with BPD is approximately 15%, and it has been posited that these conditions have distinct clinical courses, even when comorbid.30 The critical difference between bipolar disorder and BPD or PTSD is that the mood episodes in bipolar disorder represent sustained changes from baseline occurring during discrete time periods (several days or longer). Even in “rapid cycling” bipolar disorder, mood episodes must last a minimum of several consecutive days (per DSM-5 criteria above), and the threshold for rapid cycling disorder is only four or more discrete mood episodes in a single year.17 For the screening of PTSD, we recommend the use of the 5-question Primary Care PTSD Screen for DSM-5 (PC-PTSD-5).31 For the screening of BPD, we recommend the use of the 10-item McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD).32 Recent research has shown the MSI-BPD sub-items for self-harm and/or previous suicide attempts to be predictive of BPD (and absence of bipolar disorder) in psychiatric inpatient adults.33
Psychotic disorders, including schizophrenia and schizoaffective disorder, are less common in primary care but may represent an important differential diagnosis for bipolar 1 disorder. Schizoaffective disorder is characterized by at least 2 weeks of delusions or hallucinations without prominent mood symptoms following or preceding the mood episode, whereas in bipolar 1 psychotic symptoms only occur during the mood episode.17 Unlike bipolar disorder, patients with schizophrenia spectrum illness have predominantly psychotic symptoms and demonstrate significant psychosocial impairment over time.
As bipolar disorder is a chronic illness, ongoing symptom assessment is important to detect response, remission, and recurrence. The PHQ-9 can be used for ongoing assessment of depressive symptoms, while the Patient Mania Questionnaire (PMQ-9) can be considered for tracking the severity of manic symptoms over time.34
Medical Evaluation
There is no laboratory test or imaging required for diagnosing bipolar disorder. Labs and imaging should be utilized when one needs to exclude causes of symptoms that mimic bipolar depression and mania/hypomania. Initial tests to consider include a complete blood count (evaluating for infection, anemia), liver function tests, a basic metabolic panel (noting baseline renal function), a urine toxicology screen, and thyroid function studies. Broad laboratory studies such as these allow the clinician to assess for some of the most common causes of altered mental status, such as infections, metabolic or electrolyte derangements, endocrine abnormalities, and frank intoxication. In patients with signs of psychosis, an EEG, MRI brain (preferred), or CT head can be useful. In some patients, testing may be performed for less common causes of altered mental status, including heavy metal toxicity (urine studies for heavy metals), syphilis, HIV, hepatitis C, and urine porphyrins.
Many medical conditions can cause depression or induce mania. Obstructive sleep apnea and age-appropriate cancer screening is appropriate in certain populations to consider as possible causes of mood symptoms. Secondary mania should be considered when the first manic episode occurs before puberty or after the age of 40.35 Possible neurologic causes of secondary mania include multiple sclerosis, Huntington’s disease, autoimmune encephalitis, anti-NMDA-receptor encephalitis, Wilson’s disease, cancers, and traumatic brain injuries. Cerebrovascular disease and the various dementias may elicit both depression or manic symptoms, as well as endocrinopathies such as Cushing’s disease, hypothyroidism, or hyperthyroidism. Infectious etiologies include neurosyphilis, herpes encephalitis, and influenza. Additionally, vitamin B12, niacin, folate, and thiamine deficiencies may have an etiologic role.
Many medications can induce secondary mania, including corticosteroids, levodopa, methylphenidate and amphetamines, antidepressants, and disulfiram.35 The role of antidepressants in treating patients with bipolar disorder is discussed further below. Antiepileptic medications, anti-Parkinson medications, flunarizine, efavirenz, amiodarone, and IFN-alpha may cause depression in patients. The role of beta blockers in the development of depression remains controversial. Evidence suggests long-term opioid use may be associated with new-onset depression and sedatives can mimic depression.36
Treatment and Management – General Approach
Psychoeducation
Psychoeducation empowers the patient, allowing them to develop skills that will help detect and manage disease exacerbation. Important topics to cover include the chronicity and waxing-waning course of bipolar disorder, recognition of symptoms and triggers, evidence-based treatments available, the use of maintenance medications to prevent manic and depressive episodes, and the use of acute medications when such episodes arise.
Level of Care
Patients who are experiencing manic or severe major depressive episodes or are psychotic, suicidal, aggressive, having thoughts of hurting others, or so severely ill that they are unable to care for themselves should be evaluated emergently for hospitalization. We recommend that every primary care clinic develop a mental health emergency protocol that physicians and nursing staff can refer to when patients are in times of crisis. Partial hospitalization programs should be considered for moderately ill patients. When acute safety concerns are not present, outpatient care is often appropriate. Frequency of follow-up will be guided by patient acuity (eg, weekly to biweekly for acutely ill patients, monthly for active medication adjustment, or 2 to 3 times a year when stable). A team-based approach involving psychiatric specialty care is ideal. However, this often is not possible given the shortage of psychiatrists in the United States.37
Treatment Strategies
The goal of treatment for acute episodes, whether depression or mania/hypomania, is sustained remission and improved function. This is especially important because patients with residual symptoms are at increased risk of recurrence.38 While rapid response to initial treatment strategies is desirable, full remission often takes weeks or months.39 One study found that 25% of manic episodes resolve within 4 weeks, 50% within 7 weeks, and 75% within 15 weeks of onset. The same study found that 25% of depressed episodes resolve within 6 weeks, 50% within 15 weeks, and 75% within 35 weeks. Given this, stabilization is often an initial goal on the way to symptom resolution and recovery of baseline functioning.
Pharmacology is the mainstay of treatment for bipolar disorder. Adjunctive targeted psychotherapy can be offered to patients as second line treatment for acute bipolar depression. Psychotherapies with evidence for use in bipolar depression include psychoeducation, cognitive behavioral therapy, functional remediation group, family focused psychotherapy, and interpersonal and social rhythm therapy.40 No evidence exists for psychotherapy in the treatment of acute mania.
Treatment and Management: General Approach to Medication Management
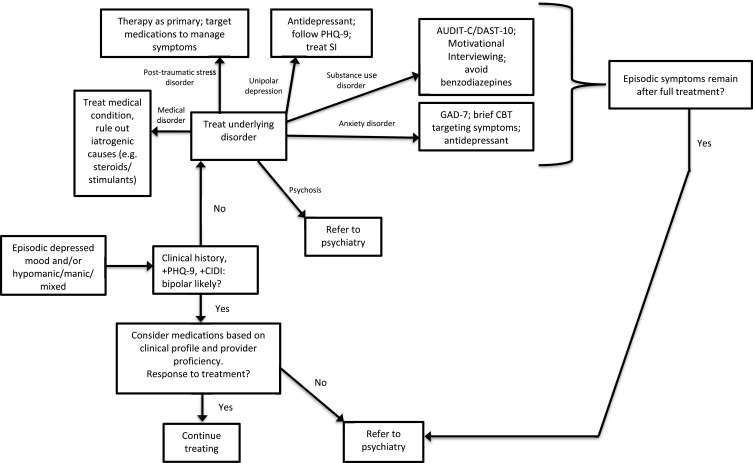
Figure 1 shows the treatment pathways for patients presenting with mood episodes in the primary care setting. Pharmacologic management of bipolar disorder includes management of both acute bipolar disorder (mania and depression) and maintenance treatment. For the purposes of management in the primary care setting, our review is exclusively of monotherapy, with a focus on 1st and 2nd line agents. Medications recommended below are supported by clinical research and Canadian Network for Mood and Anxiety Treatments (CANMAT) treatment guidelines,41 are relatively easy to use, and have at least one formulation approved by the Food and Drug Administration for the relevant phase of illness. It is important to be aware that the majority of patients with bipolar disorder will require combination therapy to adequately control their symptoms, much like in patients with chronic hypertension. For more information on combination therapy, we recommend referring to the CANMAT guidelines.41
Figure 1.
Bipolar disorder treatment pathway in primary care.
Onset of Action
Although the full effects of medication adjustment typically take 6-8 weeks, improvement is often noted within the first 2 weeks. One meta-analysis found that less than a 10% improvement of manic symptoms at week 1 warranted a change in treatment. However, patients with a 10% or greater improvement at week 1 needed a minimum trial of 2 weeks to assess if medication changes were warranted. If at week 2, patients did not achieve at least a 50% improvement in symptoms, it was recommended that they have adjustments made to the medication. Changes at either week could include increasing the dose, switching the medication or adding a second medication. In our experience, titrating medication to the maximally tolerated dose is an appropriate first step. Should the patient have minimal to no response at the maximally tolerated dose, it is reasonable to consider switching medications.42 When patients experience a significant improvement in symptoms during the first 2 weeks of treatment, it is appropriate to wait a full 4–6 weeks before considering dose modification or an alternative medication.
Reassessment
Depending on the severity and acuity of the patient’s symptoms, we recommend re-evaluating the patient in 1–4 weeks by assessing for symptoms both subjectively through patient report and objectively through rating scales, such as the PHQ-9 and/or Patient Mania Questionnaire-9 (PMQ-9).
Treatment Adherence
As clinicians’ impressions of patient adherence in bipolar disorder are often inaccurate,43 periodic and careful assessment of medication adherence and motivation to adhere to treatment is recommended. Several factors have been associated with lower adherence, including younger patient age, first year of treatment, poor medication tolerability, and more severe depressive symptoms.44,45 Lower medication adherence has been associated with adverse outcomes, including greater risk of suicidality and hospitalization.46
Treatment and Management: Acute Manic/Hypomanic Episodes
Special consideration should be given to safety during evaluation. Manic episodes that involve psychotic, suicidal, or homicidal symptoms require hospitalization, as well as patients so ill they lack the judgement required to care for themselves or their dependents. Electroconvulsive therapy may be indicated in this population.
We recommend treating acute episodes with lithium, quetiapine, risperidone, paliperidone, or aripiprazole (Table 1). Patients exhibiting mixed affective states or rapid cycling are at higher risk of developing emergent depression and becoming at risk of harm to self. For these patients, certain second-generation antipsychotics, such as aripiprazole, quetiapine, and risperidone have been shown to be effective.
Table 1.
Psychopharmacologic Management of Bipolar Disorder by Phase of Illness
| Mania | Depression | Maintenance | |
|---|---|---|---|
| First-line treatment | Lithium* Quetiapine* Risperidone, Paliperidone* Aripiprazole* Asenapine Divalproex Cariprazine |
Quetiapine* Lurasidone* Lithium* Lamotrigine |
Lithium* Quetiapine* Lamotrigine* Divalproex Asenapine Aripiprazole |
| Second-line treatment | Olanzapine Carbamazepine Ziprasidone Haloperidol ECT |
Divalproex Cariprazine ECT |
Olanzapine Risperidone Carbamazepine Paliperidone |
Notes: *Our first-line recommendations based on evidence supporting their use, CANMAT guideline recommendations, and/or FDA approved indications.
Treatment and Management: Acute Depressive Episodes
Bipolar depression has been demonstrated to be more treatment resistant than unipolar depression18 and the associated suicidality has been demonstrated to be of higher lethality.47 Treating these episodes to remission is crucial since the presence of residual bipolar depressive symptoms are associated with long-term disability and functional impairment.38 Once bipolar depression is appropriately identified, we recommend treating acute episodes with lithium, quetiapine, or lurasidone. The side effect profiles can guide medication selection.
Antidepressants
Treatment of bipolar depression with antidepressants is complex and controversial.48–50 Several principles are important to consider. Antidepressants should not be used as monotherapy in bipolar disorder. Antidepressant monotherapy is associated with an increased risk of mania. Switching to mania may occur more often in patients on tricyclic antidepressants and venlafaxine.51 Combining an antidepressant with an antimanic medication reduces the risk of switching to mania. Finally, if an antidepressant is used to treat bipolar depression, there are no guidelines discussing how long the antidepressant should be continued.
Treatment and Management: Maintenance Treatment
As a general principle, every patient with bipolar disorder should be on a maintenance medication regimen. Some will be on this regimen for years, while others for life. Maintenance treatment is crucial to the long-term management of the patient with bipolar disorder as nearly 50% of patients who recover from a mood episode will have a recurrent episode within 2 years.52 The purpose of maintenance treatment is to prevent or delay further exacerbations because bipolar exacerbations are associated with more suicide attempts,53 neurodegeneration,54 and worse social and occupational outcomes.55 Effective maintenance treatment can improve cognitive impairment and minimize illness progression.56,57
Although most studies evaluating the effectiveness of maintenance treatment focus on patients with bipolar 1 disorder, in practice it is common to generalize these findings to those with bipolar 2. We recommend monotherapy with either lithium, quetiapine, or lamotrigine (Table 1). Lithium is effective at preventing both mania and depression, with a greater ability to prevent manic over depressive exacerbations. It is the only medication shown to decrease suicidality in bipolar disorder.58 Lamotrigine is effective at preventing bipolar depression and has some effectiveness in preventing mania. Lamotrigine is appropriate in patients with a predominant history of bipolar depressive symptoms. Quetiapine has been shown to be effective in preventing manic, depressive, and mixed episodes.
Treatment and Management: Medication Titration
This section reviews dosing, side effects, and common considerations for medications recommended above. Highlights of this information is also summarized in Table 2, along with information on other medications considered 1st or 2nd line for bipolar disorder.
Table 2.
Pharmacotherapy for the Treatment of Bipolar Disorder
| Drug | Dose | Advantages | Considerations | Monitoring |
|---|---|---|---|---|
| Aripiprazole | Start 5–15mg daily. Can increase by 5–10mg every two weeks. NTE 30 mg daily. | Low QTc prolongation risk, quick onset for mania, may lower prolactin, lower MetS risk, LAAI. | Long half-life, dose adjust for drug interactions (2D6, 3A4) | Akathisia, TD, weight gain, suicidal thoughts/behaviors |
| Asenapine | Start 5–10mg twice daily, NTE 20 mg. Transdermal: Start 3.8mg/24 hours, after 1 week may increase to 5.7mg/24 hours or 7.6mg/24 hours. |
SL/transdermal formulations, good option in gastric bypass or other diseases that affect GI absorption of drugs. | Potential metabolic side effects, orthostasis, induced by smoking (1A2), cost. | Weight gain, EPS, akathisia, TD, drowsiness, oral hypoesthesia, suicidal thoughts Topical: application site reactions/rash. |
| Carbamazepine | Start 100–200 mg once or twice daily, increase by 200 mg/day every 1–4 days. Target dose 800–1200mg/day. Effective dose may range 200–1800 mg/day. Requires dose adjustment 3–4 weeks after initiation due to autoinduction (3A4). |
Effective for comorbid seizures and trigeminal neuralgia. | HLA allele testing for those of Asian ancestry, SJS, hyponatremia, agranulocytosis, cardiac side effects, causes significant drug interactions. | Sodium, TFT, renal function, suicidal thoughts/behaviors. Therapeutic serum levels are not established for treating BP. Some target seizure disorder treatment levels, 4–12 mcg/mL. |
| Cariprazine | Mania: 1.5 mg on day one, 3 mg on day two, then increase by 1.5–3 mg every two weeks, usual dose range 3–6mg Depression: 1.5 mg for two weeks, increase to 3 mg based on efficacy and tolerability |
Lower risk metabolic side effects, may lower prolactin, low QTc prolongation risk. | Long half-life, initial dose titration orthostasis, dose adjust for drug interactions (3A4), cost. | EPS, akathisia, TD, insomnia, nausea, suicidal thoughts/behaviors. |
| Divalproex | Load dose: Initiate 25 mg/kg for three days, draw trough serum concentration on fourth day and adjust dose based on result. Fixed dose: initiate at 250–500mg daily and adjust based on tolerability. Usual maintenance dose for either dose initiation method: 15mg/kg/day. |
Numerous dosage forms, preferred in history of TBI, rapid onset when load dosed. | Avoid in women of child-bearing potential (teratogenic, PCOS). | Weight gain, sedation, suicidal thoughts/behaviors. Target serum concentration 50–125 mcg/mL (85–125 mcg/mL for ER formulation). |
| Haloperidol | Start 2–5 mg daily, titrate as tolerated. NTE 15mg. | Cost, LAAI. | Hyperprolactinemia, adjust for drug interactions (2D6, 3A4). | EPS, akathisia, TD. |
| Lamotrigine | 25 mg daily weeks 1 and 2, 50 mg daily weeks 3 and 4, 100 mg week 5, 200 mg week 6. Patients taking valproate: 25 mg every other day weeks 1 and 2, 25 mg daily weeks 3 and 4, 50 mg daily, Week 5 onwards, increase by 25–50 mg every 1–2 weeks. Max daily dose = 200 mg daily. In patients taking carbamazepine, phenytoin, phenobarbital or primidone: 50 mg daily weeks 1 and 2, 100 mg daily weeks 3 and 4, 200 mg daily week 5, 300 mg daily week 6, 400mg daily week 7. Patients may require 500mg daily. |
Benefits for depression, relatively well tolerated, low weight gain potential. | Delayed effect due to slow titration, SJS risk, class 1B antiarrhythmic. | Rash, drug interactions, suicidal thoughts/behaviors. |
| Lithium | Start 300 mg daily with dose adjustment every 5–7 days according to serum trough concentration, tolerability or symptoms. | Neuroprotective, decreased suicide risk, efficacy for mania and depression. | GI side effects, polyuria/polydipsia, diabetes insipidus, drug interactions. | Renal function, tremor, acne. Target serum concentration 0.6–0.8 mEq/L (0.8–1.2mEq/L in acute mania). |
| Lurasidone | Start 20 mg daily, increase by 20 mg weekly. NTE 120 mg/day. Doses >80 mg of limited benefit. | Quick onset for depression, lower risk of metabolic side effects. | Take with >350 kcal, dose adjust for Child-Pugh class B, C & CrCl<50mL/min, cost. | EPS, akathisia, TD, suicidal thoughts/behaviors. |
| Olanzapine | Start 5–15mg daily, may increase by 5 mg increments to a typical dose up to 20 mg. | Quick onset for mania. | Significant MetS, induced by cigarette smoking (1A2). | Sedation, weight gain, constipation, orthostatic hypotension, EPS, TD. |
| Paliperidone | 6mg daily, increase by 3 mg every 5 days, NTE 12 mg, most patients require >6mg. | Quick onset for mania, LAAI. | MetS, renal dose adjustment, hyperprolactinemia. | Weight, EPS, TD, renal function. |
| Quetiapine | Start 25–50 mg nightly. Increase dose by 50 mg weekly up to a maximum total daily dose of 800mg. Typical depression target dose = 200–300 mg daily. For quetiapine XR : 300mg on day 1, increase to 400-600mg on day 2; target dose of 400-800mg daily. | Quick onset, possibly anxiolytic, efficacy for mania and depression. | MetS, orthostatic hypotension, available as once daily formulation. | Weight gain, dizziness, sedation, constipation, TD, suicidal thoughts/behaviors. |
| Risperidone | Start 1–2mg daily or in 2 divided doses; can increase by 1 mg daily to a usual dose of 4mg per day. NTE 6mg. | Quick onset for mania, LAAI. | MetS, orthostasis and tachycardia, hyperprolactinemia, consider dose adjustment with renal impairment. | Weight gain, EPS, TD, headache, renal function. |
| Ziprasidone | Start 20–40 mg twice daily with meals, can increase dose on second day to typical dose of 40 mg-80 mg twice daily. | Low MetS. | Take with >/=500 calories, QTc prolongation. | EPS, akathisia, TD, dizziness, headache, drowsiness, suicidal thoughts/behaviors |
Abbreviations: EPS, extrapyramidal side effects; GI, gastrointestinal; LAAI, long acting antipsychotic injection; MetS, metabolic side effects; NTE, not to exceed; SJS, Stevens-Johnson Syndrome; TD, tardive dyskinesia; TFT, thyroid function tests.
Quetiapine
When initiating quetiapine, we typically start at a dose of 50 mg nightly and increase the total daily dose by 50 mg every 5–7 days. Target doses are often around 200–300 mg nightly, with some patients benefiting from as low as 100 mg and as high as 800mg nightly. We typically dose quetiapine at night as sedation is a common adverse effect. Both the immediate release and extended release can be dosed once daily, although a slower dose titration may be needed with immediate release. Alternately, a twice daily dose titration can be used with the immediate release and then the medication can be consolidated to once daily at nighttime after the target dose is achieved. We use the extended release formulation when covered by the patient’s insurance as it minimizes orthostasis and sedation. However, for patients with pronounced insomnia, the immediate release formulation would be preferred as it peaks at 1.5 hours rather than 6 hours for the extended release product.59 For patients who are highly sensitive to quetiapine’s sedating effects, we favor the extended release product, but may start the immediate release formulation at 25 mg nightly as 25 mg doses are not available in the extended release formulation. Common side effects of quetiapine include fatigue, drowsiness, dizziness, and headache. Other possible side effects to monitor for include blood pressure changes, serum cholesterol changes (decreased HDL, increased total cholesterol and triglycerides), weight gain, increased appetite, dry mouth, and constipation. Quetiapine has not been associated with an increased risk of major malformations of infants with first trimester of pregnancy. Although low birth weight is more common in women exposed to second generation antipsychotics, the differences may be due to other between group differences and is of questionable clinical significance.60,61
Lithium
We typically start with the extended release formulation at 300 mg daily and increase the total daily dose to 600mg in 5–7 days if tolerated. The goal is to reach a therapeutic serum concentration of 0.8–1.2 mEq/L for acute mania. This is often achieved at doses between 900–1800 mg per day. A lower serum concentration of 0.6–0.8 mEq/L may be an adequate and practical target for routine maintenance;62 this target is also recommended by the UK National Institute for Health and Clinical Excellence (NICE) for both treatment initiation and maintenance.63 Since the average half-life of lithium is just over 24 hours, levels should be drawn at least 5 days after dose adjustment. Serum concentration is measured as a 12-hour trough, as lithium levels fluctuate within the first 12 hours of dose administration but remain relatively stable between 12 and 24 hours. We typically begin monitoring serum concentrations when a dose of 900 mg nightly has been achieved and repeat testing with each dose adjustment.
For simplicity, we often dose lithium in the evening and, one week after dose adjustment, ask patients to have blood levels checked in the morning (12 hours after their last dose). This provides an opportunity for simultaneous metabolic screening and monitoring for dyslipidemia and diabetes. While data are inconclusive, some data suggest once daily dosing and use of extended-release lithium products may preserve renal function. Given no studies have reported a downside to this practice, we recommend once daily dosing to minimize renal injury.64–66
Lithium toxicity is a relatively common phenomenon, with around 7% of patients experiencing at least one episode of elevated lithium levels during their treatment.67 If lithium toxicity is suspected, it can be confirmed with serum lithium levels. Initial symptoms of toxicity can include tremor, polyuria and polydipsia, gastrointestinal distress, and weakness. Mild toxicity can be seen at a lithium serum concentration around 1.5 mEq/L and a serum concentration of 2.5 mEq/L is considered a medical emergency.
Several factors impact lithium levels: renal function, salt and fluid balance, and certain other medications. Sodium and volume depletion may occur in renal insufficiency, vomiting, diarrhea, excessive exercise or sweating, water restriction, and low salt diets. Patients are educated to monitor for symptoms of toxicity if these situations arise, notify the clinic, and obtain a serum concentration if indicated.
Commonly prescribed medications that can increase lithium concentration include diuretics (with the exception of potassium-sparing diuretics), angiotensin-receptor blockers, angiotensin-converting enzyme inhibitors, and non-steroidal anti-inflammatory medications. While these medications are not contraindicated, if patients require use of them, we recommend prophylactic dose adjustments and more frequent monitoring of serum concentration. We advise patients taking lithium to avoid alcohol and excessive caffeine intake as these may decrease lithium’s efficacy or serum concentration. Lithium use in the first trimester of pregnancy is associated with a small increased risk of major cardiac malformation. This risk is dose related, with doses greater than 900 mg daily associated with a three-fold greater risk than doses less than 600mg daily.68
Lamotrigine
Lamotrigine is typically initiated with a prescribed titration schedule of 25 mg daily for 2 weeks, 50 mg daily for 2 weeks, 100 mg daily for 1 week, and then 200 mg daily. In our experience, patients typically begin to report symptom improvement at a total daily dose of 50–200 mg. This improvement may fluctuate initially as lamotrigine experiences autoinduction, with about a 20% reduction in steady-state serum concentration in the first few weeks of treatment. Autoinduction typically stabilizes by week 4 of treatment. While doses above 200 mg daily may be indicated in certain individuals, in the authors’ experience this can be associated with greater neurocognitive adverse effects.
Lamotrigine has many clinically significant drug–drug interactions, with medications both increasing and decreasing its serum levels. Valproate reduces lamotrigine metabolism and increases serum levels. Patients who must be on both medications should follow an adjusted lamotrigine titration schedule (Table 2). Estradiol-containing oral contraceptives significantly reduce lamotrigine levels. Maintenance doses of lamotrigine when a patient is also on estradiol-containing oral contraceptives can range from 300 to 500 mg/day. In general, lamotrigine has a more favorable side effect profile than lithium. Common side effects include nausea, rash, dizziness, and fatigue. Benign rashes may occur in the first 1–2 months of treatment in up to 10% of patients. Life-threatening rashes associated with lamotrigine, such as Stevens-Johnson syndrome (SJS, 0.08–0.3% of adults) or toxic epidermal necrolysis (TEN, ~1 in 1000 adults), almost always occur within the first 8 weeks of treatment.69,70 The risk of SJS is higher in children, those with a history of anticonvulsant-associated rash, patients with higher starting doses of lamotrigine, and patients with rapid escalation of lamotrigine dose.71 All patients should be educated on the common possibility of a benign rash, the rare instance of SJS/TEN, to contact the clinic if they develop a rash, and that lamotrigine should be re-titrated if they miss more than 4 days.
Increasing estrogen levels during pregnancy can cause lamotrigine serum concentration to drop over the course of pregnancy and return to baseline within 4 weeks of delivery. No routine adjustment of dose is currently recommended, but some have proposed adjusting dose based on serum concentration monitoring over the course of pregnancy.72,73 Prenatal exposure to lamotrigine is not associated with an increased risk of major malformations.
Aripiprazole
We typically start aripiprazole for bipolar disorder between 5–15mg daily. The half-life of aripiprazole and its active metabolite, dehydro-aripiprazole, are 75 and 94 hours, respectively. Due to its prolonged half-life, dose adjustments should be made at intervals of 2 weeks or more. The total daily dose may be increased by 5 mg every 2 weeks up to a maximum of 30 mg daily. Aripiprazole is available as several long-acting antipsychotic injections administered every 4, 6 or 8 weeks.
Approximately 18% of patients experience insomnia, thus it is often dosed in the morning. Akathisia is dose related; rapid titration should be avoided to improve tolerability. Other common side effects include constipation, nausea, sedation, and dizziness. Aripiprazole does not appear to significantly increase the risk for major congenital malformation.74
Risperidone
The usual starting dose of risperidone in bipolar disorder is 1–2 mg daily. The total daily dose can be increased by 1 mg daily. In clinical practice, risperidone is usually divided twice daily during initiation to minimize orthostatic hypotension and then converted to once daily dosing when the titration is complete. Most patients respond to 4mg daily, although total daily doses of up to 6mg may be used. It is available as a long-acting injection administered intramuscularly every 2 weeks or subcutaneously every 4 weeks.
Risperidone has a moderate risk for metabolic side effects. It can be associated with hyperprolactinemia and resulting gynecomastia/sexual dysfunction. While tachycardia is often transient, it typically resolves with a lower dose. Other possible side effects include Parkinsonism, akathisia, somnolence, and dizziness.
In one Medicaid database study, risperidone was associated with a small increase in non-cardiac congenital malformations. Other studies have not replicated this finding, including a recent large study.75 In general, risperidone is considered safe for use in pregnancy.76
Paliperidone
As the active metabolite of risperidone, all the same information about the side effects of risperidone applies to paliperidone.77 The initiation dose is 6mg daily. The total daily dose can be increased in 3 mg increments every 5 days to a maximum of 12 mg daily. Typically, doses greater than 6mg are needed. For patients with renal impairment, the starting dose is 3 mg with a maximum total daily dose of 6 mg for impairment in the range of CrCl 50 to <80mL/min and a starting dose of 1.5mg with a maximum daily dose of 3mg for those with impairment in the range of CrCl 10 to <50mL/min.41 Paliperidone is available as a long-acting injection administered monthly, quarterly or twice annually. Paliperidone does not appear to be a risk factor for major congenital malformations.75
Lurasidone
The usual starting dose of lurasidone is 20 mg daily administered with at least 350 kcal of food. This level of caloric intake is necessary for lurasidone absorption; if a patient cannot consistently take this medication with the necessary 350 kcal, another agent is required. The total daily dose can be increased by 20 mg weekly as needed up to 120 mg daily. However, clinical trials have not demonstrate increased efficacy at doses of greater than 80 mg daily.78 For patients with moderate hepatic impairment, the maximum total daily dose is 80mg, while the maximum total daily dose is 40mg for those with severe hepatic impairment. The maximal daily dose is 80mg for patients with renal impairment (CrCl < 50mL/min).
Lurasidone is generally well tolerated with a relatively low risk of metabolic side effects. Adverse reactions are dose related and include Parkinsonism, akathisia, somnolence, nausea, and diarrhea. Lurasidone does not appear to be associated with an increased risk of major congenital malformations.75
Treatment and Management: Medical Comorbidity and Metabolic Monitoring
Patients with bipolar disorder have an increased risk of comorbid medical diagnoses, including hypertension, dyslipidemia, diabetes, CVD (angina pectoris, atherosclerosis, and myocardial infarction), metabolic syndrome and obesity, chronic obstructive pulmonary disease, asthma, chronic bronchitis, migraines, and gastric ulcer. There appears to be a bidirectional relationship between bipolar symptoms and cardiovascular, endocrine, and metabolic illness.79
Many of the medications used to treat bipolar disorder have metabolic side effects. Although psychiatric medications can be associated with weight gain, studies have found that over 50% of patients with bipolar illness meet criteria for being overweight or obese independent of psychiatric medication use.79–82
These factors contribute to an estimated loss of 10–20 potential life years, with CVD being the leading cause of early mortality in patients with bipolar disorder.83–85 For these reasons, we recommend routine metabolic monitoring in this patient population with yearly measurement of blood pressure, weight/BMI, fasting glucose or hemoglobin A1C, and lipid profile.
Treatment of Co-Occurring Psychiatric Disorders
When treating psychiatric symptoms in the context of bipolar disorder, it is important to distinguish which of these symptoms are the result of bipolar disorder itself and which represent a separate psychiatric diagnosis. This allows the clinician to determine whether or not a psychiatric disorder is comorbid with bipolar disorder or if all reported symptoms are a result of bipolar disorder. In treating patients with bipolar disorder, maintaining a clear diagnostic hierarchy86 is critically important in directing treatment.
For example, inattention and hyperactivity are symptoms common to bipolar disorder and ADHD. Like bipolar disorder, ADHD can be correctly diagnosed and effectively treated in the primary care setting.87 Accurate assignment of the diagnostic hierarchy can be challenging when as many as 25% of bipolar 1 or 2 patients meet criteria for ADHD.28 If resulting from bipolar disorder, these symptoms will improve when the bipolar illness is fully treated. However, if a comorbid diagnosis of ADHD is inappropriately made, treatment with stimulants may exacerbate symptoms of bipolar disorder. Treating comorbid symptoms is recommended while targeting bipolar symptoms as long as the treatment does not risk exacerbating the underlying bipolar illness (e.g., providing patients with strategies for organizational skills but not using stimulant medications).
Anxiety is another common bipolar symptom that will improve by treating the underlying bipolar disorder, but assignment of the diagnostic hierarchy can be difficult when up to 75% of individuals with bipolar 1 or 2 have comorbid anxiety disorders.28 According to a regression analysis of structured clinical interviews in 739 patients with bipolar illness, alcohol and cannabis misuse are the most common psychiatric comorbidities in men, while anxiety and eating disorders are most common in women.88
Patients often use substances to self-medicate around sleep or mood symptoms, and those with bipolar illness have higher rates of comorbid SUD than those with several other common psychiatric illnesses.89 Understanding the sequence of symptoms from childhood to present including the onset of mood symptoms and the initiation of substance use can be helpful to determine diagnostic hierarchy. A recent meta-analysis identified factors associated with higher-risk SUD in bipolar disorder, including number of manic episodes, suicidality, and male gender.90 Higher frequency and intensity of bipolar treatment is recommended for patients with at least one of these characteristics.90 Given the prevalence of these comorbid disorders, more research is needed to better understand optimal treatments in this area.91
Lastly, patients with bipolar disorder and a history of trauma have a more challenging clinical course.92 As noted above, there is often difficulty distinguishing between PTSD and bipolar symptoms; as a result, concurrent treatment, targeting both diseases, can be helpful (e.g., trauma-focused psychotherapy and mood stabilizing medications).
Collaborative Care Models for Bipolar Disorder
Patients with bipolar disorder have traditionally been treated in specialty psychiatric clinics.12 However, effective models have been developed that allow patients with mental health conditions to be successfully treated in primary care. The Collaborative Care model (CoCM) is an evidence-based method of treating mental health conditions within primary care, demonstrated to improve clinical outcomes.93 The CoCM team consists of a primary care provider, a behavioral health care manager, and a psychiatric consultant.94 The intervention utilizes a registry to track and follow a population of patients, delivering measurement-based care to target specific outcomes.
Evidence supporting the use of CoCM is most robust for the treatment of depression and anxiety disorders in primary care. As noted above, existing literature shows that bipolar disorders frequently present in the primary care setting.95,96 One focus group study revealed that CoCM psychiatrists have high confidence in using the CoCM model to treat bipolar disorder in primary care.97 A recent effectiveness trial showed significantly and substantially improved clinical outcomes in patients with bipolar disorder and/or PTSD who enrolled in a telepsychiatry CoCM program.98 As primary care providers will encounter patients with bipolar disorder, implementation of the Collaborative Care model can position the clinic to successfully identify and treat this population.
Conclusion
Bipolar disorder is a chronic and impairing condition, characterized by periods of depression and mania/hypomania and often comorbid with other psychiatric diagnoses. When symptomatic, patients are more likely to experience depressive than manic/hypomanic symptoms. Bipolar depression can be more clinically severe and is associated with higher rates of suicidality than unipolar depression, and up to 40% of cases of treatment-resistant depression may represent undiagnosed bipolar disorder. Screening for bipolar disorder can be aided by use of the PHQ-9 and CIDI. Treatment of bipolar disorder should always involve assessment of safety and take into consideration phase of illness (i.e., depressive, manic/hypomanic, mixed, or maintenance). Lithium should be considered as a first line treatment for bipolar disorder given its neuroprotective properties, association with decreased suicide risk, and effectiveness across the different phases of the disorder. Medical comorbidity, particularly metabolic and cardiovascular diseases, is common in individuals with bipolar disorder, and regular screening for and close management of such conditions is critical given high mortality and morbidity burden.
Disclosure
Christopher T. Lim has received consulting fees from Aetna Inc., CVS Health, and Lyra Health and research funding from Humana Inc. He also reports equity from Cartwheel Care. Hsiang Huang has received research funding from Humana Inc. The authors report no other conflicts of interest in this work.
References
- 1.McIntyre RS, Berk M, Brietzke E, et al. Bipolar disorders. Lancet. 2020;396(10265):1841–1856. doi: 10.1016/S0140-6736(20)31544-0 [DOI] [PubMed] [Google Scholar]
- 2.Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1–3):101–104. doi: 10.1016/j.schres.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 3.Crump C, Sundquist K, Winkleby MA, Sundquist J. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931–939. doi: 10.1001/jamapsychiatry.2013.1394 [DOI] [PubMed] [Google Scholar]
- 4.Kodesh A, Goldshtein I, Gelkopf M, Goren I, Chodick G, Shalev V. Epidemiology and comorbidity of severe mental illnesses in the community: findings from a computerized mental health registry in a large Israeli health organization. Soc Psychiatry Psychiatr Epidemiol. 2012;47(11):1775–1782. doi: 10.1007/s00127-012-0478-9 [DOI] [PubMed] [Google Scholar]
- 5.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- 6.Cloutier M, Greene M, Guerin A, Touya M, Wu E. The economic burden of bipolar I disorder in the United States in 2015. J Affect Disord. 2018;226:45–51. [DOI] [PubMed] [Google Scholar]
- 7.Judd LL, Schettler PJ, Akiskal HS, et al. Long-term symptomatic status of bipolar I vs. bipolar II disorders. Int J Neuropsychopharmacol. 2003;6(2):127–137. doi: 10.1017/S1461145703003341 [DOI] [PubMed] [Google Scholar]
- 8.Na KS, Kang JM, Cho SE. Prevalence of DSM-5 mixed features: a meta-analysis and systematic review. J Affect Disord. 2021;282:203–210. doi: 10.1016/j.jad.2020.12.149 [DOI] [PubMed] [Google Scholar]
- 9.Tondo L, Vázquez GH, Pinna M, Vaccotto PA, Baldessarini RJ. Characteristics of depressive and bipolar disorder patients with mixed features. Acta Psychiatr Scand. 2018;138(3):243–252. doi: 10.1111/acps.12911 [DOI] [PubMed] [Google Scholar]
- 10.Culpepper L. Pathways to the diagnosis of bipolar disorder. J Fam Pract. 2015;64(6 Suppl):S4–S9. [PubMed] [Google Scholar]
- 11.Wang PS, Berglund P, Olfson M, Pincus HA, Wells KB, Kessler RC. Failure and delay in initial treatment contact after first onset of mental disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):603–613. doi: 10.1001/archpsyc.62.6.603 [DOI] [PubMed] [Google Scholar]
- 12.Cerimele JM, Fortney JC, Unützer J. Bipolar disorder and population health. Psychiatr Serv. 2017;68(2):192–194. doi: 10.1176/appi.ps.201600011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daveney J, Panagioti M, Waheed W, Esmail A. Unrecognized bipolar disorder in patients with depression managed in primary care: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2019;58:71–76. doi: 10.1016/j.genhosppsych.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 14.Daigneault A, Duclos C, Saury S, Paquet J, Dumont D, Beaulieu S. Diagnosis of bipolar disorder in primary and secondary care: what have we learned over a 10-year period? J Affect Disord. 2015;174:225–232. doi: 10.1016/j.jad.2014.10.057 [DOI] [PubMed] [Google Scholar]
- 15.Manning JS. Bipolar disorder, bipolar depression and comorbid illness. J Fam Pract. 2015;64(6 Suppl):S10–S15. [PubMed] [Google Scholar]
- 16.Kupfer DJ, Frank E, Grochocinski VJ, Cluss PA, Houck PR, Stapf DA. Demographic and clinical characteristics of individuals in a bipolar disorder case registry. J Clin Psychiatry. 2002;63(2):120–125. doi: 10.4088/JCP.v63n0206 [DOI] [PubMed] [Google Scholar]
- 17.Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013. [Google Scholar]
- 18.Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1. doi: 10.1186/s40345-019-0160-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldessarini RJ, Vieta E, Calabrese JR, Tohen M, Bowden CL. Bipolar depression: overview and commentary. Harv Rev Psychiatry. 2010;18(3):143–157. doi: 10.3109/10673221003747955 [DOI] [PubMed] [Google Scholar]
- 20.Perugi G, Pacchiarotti I, Mainardi C, et al. Patterns of response to antidepressants in major depressive disorder: drug resistance or worsening of depression are associated with a bipolar diathesis. Eur Neuropsychopharmacol. 2019;29(7):825–834. doi: 10.1016/j.euroneuro.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 21.Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US preventive services task force recommendation statement. JAMA. 2016;315(4):380–387. doi: 10.1001/jama.2015.18392 [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB, The PHQ-9. validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessler RC, Akiskal HS, Angst J, et al. Validity of the assessment of bipolar spectrum disorders in the WHO CIDI 3.0. J Affect Disord. 2006;96(3):259–269. doi: 10.1016/j.jad.2006.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phelps J, Bale J, Squires K 3rd, Pipitone O. Bipolarity in a collaborative care model variation: detection, prevalence, and outcomes. Psychiatr Serv. 2020;71(11):1098–1103. doi: 10.1176/appi.ps.202000024 [DOI] [PubMed] [Google Scholar]
- 25.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craddock N, Sklar P. Genetics of bipolar disorder. Lancet. 2013;381(9878):1654–1662. doi: 10.1016/S0140-6736(13)60855-7 [DOI] [PubMed] [Google Scholar]
- 27.Calabrese JR, Gao K, Sachs G. Diagnosing Mania in the Age of DSM-5. Am J Psychiatry. 2017;174(1):8–10. doi: 10.1176/appi.ajp.2016.16091084 [DOI] [PubMed] [Google Scholar]
- 28.Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241–251. doi: 10.1001/archgenpsychiatry.2011.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerullo MA, Strakowski SM. The prevalence and significance of substance use disorders in bipolar type I and II disorder. Subst Abuse Treat Prev Policy. 2007;2:29. doi: 10.1186/1747-597X-2-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunderson JG, Choi-Kain LW. Medication management for patients with borderline personality disorder. Am J Psychiatry. 2018;175(8):709–711. doi: 10.1176/appi.ajp.2018.18050576 [DOI] [PubMed] [Google Scholar]
- 31.Prins A, Bovin MJ, Smolenski DJ, et al. The primary care PTSD screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206–1211. doi: 10.1007/s11606-016-3703-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanarini MC, Vujanovic AA, Parachini EA, Boulanger JL, Frankenburg FR, Hennen J. A screening measure for BPD: the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). J Pers Disord. 2003;17(6):568–573. doi: 10.1521/pedi.17.6.568.25355 [DOI] [PubMed] [Google Scholar]
- 33.Palmer BA, Pahwa M, Geske JR, et al. Self-report screening instruments differentiate bipolar disorder and borderline personality disorder. Brain Behav. 2021;11(7):e02201. doi: 10.1002/brb3.2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerimele JM, Russo J, Bauer AM, et al. The Patient Mania Questionnaire (PMQ-9): a Brief scale for assessing and monitoring manic symptoms. J Gen Intern Med. 2021;37(7):1680–1687. doi: 10.1007/s11606-021-06947-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price AL, Marzani-Nissen GR. Bipolar disorders: a review. Am Fam Physician. 2012;85(5):483–493. [PubMed] [Google Scholar]
- 36.Scherrer JF, Salas J, Copeland LA, et al. Prescription opioid duration, dose, and increased risk of depression in 3 large patient populations. Ann Fam Med. 2016;14(1):54–62. doi: 10.1370/afm.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas KC, Ellis AR, Konrad TR, Holzer CE, Morrissey JP. County-level estimates of mental health professional shortage in the United States. Psychiatr Serv. 2009;60(10):1323–1328. doi: 10.1176/ps.2009.60.10.1323 [DOI] [PubMed] [Google Scholar]
- 38.Simon GE, Bauer MS, Ludman EJ, Operskalski BH, Unützer J. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237–1245. doi: 10.4088/JCP.v68n0811 [DOI] [PubMed] [Google Scholar]
- 39.Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339–347. doi: 10.1001/archgenpsychiatry.2010.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swartz HA, Swanson J. Psychotherapy for bipolar disorder in adults: a review of the evidence. Focus Am Psychiatr Publ. 2014;12(3):251–266. doi: 10.1176/appi.focus.12.3.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170. doi: 10.1111/bdi.12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welten CC, Koeter MW, Wohlfarth TD, et al. Early nonresponse in the antipsychotic treatment of acute mania: a criterion for reconsidering treatment? Results from an individual patient data meta-analysis. J Clin Psychiatry. 2016;77(9):e1117–e1123. doi: 10.4088/JCP.15r10051 [DOI] [PubMed] [Google Scholar]
- 43.Martin KB. Accuracy of psychiatrists’ assessment of medication adherence in an outpatient setting. Cureus. 2020;12(12):e11847. doi: 10.7759/cureus.11847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoue T, Sano H, Kojima Y, Yamada S, Shirakawa O. Real-world treatment patterns and adherence to oral medication among patients with bipolar disorders: a retrospective, observational study using a healthcare claims database. Neuropsychiatr Dis Treat. 2021;17:821–833. doi: 10.2147/NDT.S299005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Consoloni JL, M’Bailara K, Perchec C, et al. Trajectories of medication adherence in patients with Bipolar Disorder along 2 years-follow-up. J Affect Disord. 2021;282:812–819. doi: 10.1016/j.jad.2020.12.192 [DOI] [PubMed] [Google Scholar]
- 46.Prajapati AR, Dima A, Mosa G, et al. Mapping modifiable determinants of medication adherence in bipolar disorder (BD) to the theoretical domains framework (TDF): a systematic review. Psychol Med. 2021;51(7):1082–1098. doi: 10.1017/S0033291721001446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong M, Lu L, Zhang L, et al. Prevalence of suicide attempts in bipolar disorder: a systematic review and meta-analysis of observational studies. Epidemiol Psychiatr Sci. 2019;29:e63. doi: 10.1017/S2045796019000593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGirr A, Vöhringer PA, Ghaemi SN, Lam RW, Yatham LN. Safety and efficacy of adjunctive second-generation antidepressant therapy with a mood stabiliser or an atypical antipsychotic in acute bipolar depression: a systematic review and meta-analysis of randomised placebo-controlled trials. Lancet Psychiatry. 2016;3(12):1138–1146. doi: 10.1016/S2215-0366(16)30264-4 [DOI] [PubMed] [Google Scholar]
- 49.Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156–167. doi: 10.4088/JCP.09r05385gre [DOI] [PubMed] [Google Scholar]
- 50.Gijsman HJ, Geddes JR, Rendell JM, Nolen WA, Goodwin GM. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry. 2004;161(9):1537–1547. doi: 10.1176/appi.ajp.161.9.1537 [DOI] [PubMed] [Google Scholar]
- 51.Melhuish Beaupre LM, Tiwari AK, Gonçalves VF, et al. Antidepressant-associated mania in bipolar disorder: a review and meta-analysis of potential clinical and genetic risk factors. J Clin Psychopharmacol. 2020;40(2):180–185. doi: 10.1097/JCP.0000000000001186 [DOI] [PubMed] [Google Scholar]
- 52.Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry. 2006;163(2):217–224. doi: 10.1176/appi.ajp.163.2.217 [DOI] [PubMed] [Google Scholar]
- 53.Slama F, Bellivier F, Henry C, et al. Bipolar patients with suicidal behavior: toward the identification of a clinical subgroup. J Clin Psychiatry. 2004;65(8):1035–1039. doi: 10.4088/JCP.v65n0802 [DOI] [PubMed] [Google Scholar]
- 54.Vieta E, Reinares M, Rosa AR. Staging bipolar disorder. Neurotox Res. 2011;19(2):279–285. doi: 10.1007/s12640-010-9197-8 [DOI] [PubMed] [Google Scholar]
- 55.Nolen WA, Luckenbaugh DA, Altshuler LL, et al. Correlates of 1-year prospective outcome in bipolar disorder: results from the Stanley Foundation Bipolar Network. Am J Psychiatry. 2004;161(8):1447–1454. doi: 10.1176/appi.ajp.161.8.1447 [DOI] [PubMed] [Google Scholar]
- 56.Kozicky JM, Torres IJ, Silveira LE, Bond DJ, Lam RW, Yatham LN. Cognitive change in the year after a first manic episode: association between clinical outcome and cognitive performance early in the course of bipolar I disorder. J Clin Psychiatry. 2014;75(6):e587–e593. doi: 10.4088/JCP.13m08928 [DOI] [PubMed] [Google Scholar]
- 57.Berk M, Berk L, Dodd S, et al. Stage managing bipolar disorder. Bipolar Disord. 2014;16(5):471–477. doi: 10.1111/bdi.12099 [DOI] [PubMed] [Google Scholar]
- 58.Cipriani A, Pretty H, Hawton K, Geddes JR. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805–1819. doi: 10.1176/appi.ajp.162.10.1805 [DOI] [PubMed] [Google Scholar]
- 59.Quetiapine. Lexi-drugs. Riverwoods, IL: Wolters Kluwer Health, Inc. Available from: http://online.lexi.com. Accessed October 7, 2022. [Google Scholar]
- 60.Cohen LS, Góez-Mogollón L, Sosinsky AZ, et al. Risk of major malformations in infants following first-trimester exposure to quetiapine. Am J Psychiatry. 2018;175(12):1225–1231. doi: 10.1176/appi.ajp.2018.18010098 [DOI] [PubMed] [Google Scholar]
- 61.Damkier P, Videbech P. The safety of second-generation antipsychotics during pregnancy: a clinically focused review. CNS Drugs. 2018;32(4):351–366. [DOI] [PubMed] [Google Scholar]
- 62.Malhi GS, Tanious M, Das P, Berk M. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192–211. doi: 10.1177/0004867412437346 [DOI] [PubMed] [Google Scholar]
- 63.NICE. Bipolar disorder: assessment and management NICE Guideline 185 updated edition February; 2020. Available from: https://www.nice.org.uk/guidance/cg185/chapter/1-Recommendations#managing-bipolar-disorder-in-adults-in-the-longer-term-in-secondary-care-2. Accessed November 11, 2022.
- 64.Carter L, Zolezzi M, Lewczyk A. An updated review of the optimal lithium dosage regimen for renal protection. Can J Psychiatry. 2013;58(10):595–600. doi: 10.1177/070674371305801009 [DOI] [PubMed] [Google Scholar]
- 65.Durbano F, Mencacci C, Dorigo D, Riva M, Buffa G. The long-term efficacy and tolerability of carbolithium once a day: an interim analysis at 6 months. Clin Ter. 2002;153(3):161–166. [PubMed] [Google Scholar]
- 66.Wallin L, Alling C. Effect of sustained-release lithium tablets on renal function. Br Med J. 1979;2(6201):1332. doi: 10.1136/bmj.2.6201.1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ott M, Stegmayr B, Salander Renberg E, Werneke U. Lithium intoxication: incidence, clinical course and renal function - a population-based retrospective cohort study. J Psychopharmacol. 2016;30(10):1008–1019. doi: 10.1177/0269881116652577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fornaro M, Maritan E, Ferranti R, et al. Lithium exposure during pregnancy and the postpartum period: a systematic review and meta-analysis of safety and efficacy outcomes. Am J Psychiatry. 2020;177(1):76–92. doi: 10.1176/appi.ajp.2019.19030228 [DOI] [PubMed] [Google Scholar]
- 69.Arif H, Buchsbaum R, Weintraub D, et al. Comparison and predictors of rash associated with 15 antiepileptic drugs. Neurology. 2007;68(20):1701–1709. doi: 10.1212/01.wnl.0000261917.83337.db [DOI] [PubMed] [Google Scholar]
- 70.Hirsch LJ, Arif H, Nahm EA, Buchsbaum R, Resor SR Jr, Bazil CW. Cross-sensitivity of skin rashes with antiepileptic drug use. Neurology. 2008;71(19):1527–1534. doi: 10.1212/01.wnl.0000334295.50403.4c [DOI] [PubMed] [Google Scholar]
- 71.Hirsch LJ, Weintraub DB, Buchsbaum R, et al. Predictors of Lamotrigine-associated rash. Epilepsia. 2006;47(2):318–322. doi: 10.1111/j.1528-1167.2006.00423.x [DOI] [PubMed] [Google Scholar]
- 72.Clark CT, Klein AM, Perel JM, Helsel J, Wisner KL. Lamotrigine dosing for pregnant patients with bipolar disorder. Am J Psychiatry. 2013;170(11):1240–1247. doi: 10.1176/appi.ajp.2013.13010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pariente G, Leibson T, Shulman T, Adams-Webber T, Barzilay E, Nulman I. Pregnancy outcomes following in utero exposure to lamotrigine: a systematic review and meta-analysis. CNS Drugs. 2017;31(6):439–450. doi: 10.1007/s40263-017-0433-0 [DOI] [PubMed] [Google Scholar]
- 74.Freeman MP, Viguera AC, Góez-Mogollón L, et al. Reproductive safety of aripiprazole: data from the Massachusetts general hospital National pregnancy registry for atypical antipsychotics. Arch Womens Ment Health. 2021;24(4):659–667. doi: 10.1007/s00737-021-01115-6 [DOI] [PubMed] [Google Scholar]
- 75.Viguera AC, Freeman MP, Góez-Mogollón L, et al. Reproductive safety of second-generation antipsychotics: updated data from the Massachusetts general hospital national pregnancy registry for atypical antipsychotics. J Clin Psychiatry. 2021;82(4). doi: 10.4088/JCP.20m13745 [DOI] [PubMed] [Google Scholar]
- 76.Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic Use in Pregnancy and the Risk for Congenital Malformations. JAMA Psychiatry. 2016;73(9):938–946. doi: 10.1001/jamapsychiatry.2016.1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Invega Sustenna. U.S. prescribing information. Available from: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVEGA+SUSTENNA-pi.pdf. Accessed November 11, 2022.
- 78.Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160–168. doi: 10.1176/appi.ajp.2013.13070984 [DOI] [PubMed] [Google Scholar]
- 79.Kemp DE, Gao K, Chan PK, Ganocy SJ, Findling RL, Calabrese JR. Medical comorbidity in bipolar disorder: relationship between illnesses of the endocrine/metabolic system and treatment outcome. Bipolar Disord. 2010;12(4):404–413. doi: 10.1111/j.1399-5618.2010.00823.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elmslie JL, Silverstone JT, Mann JI, Williams SM, Romans SE. Prevalence of overweight and obesity in bipolar patients. J Clin Psychiatry. 2000;61(3):179–184. doi: 10.4088/JCP.v61n0306 [DOI] [PubMed] [Google Scholar]
- 81.McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry. 2002;63(3):207–213. doi: 10.4088/JCP.v63n0306 [DOI] [PubMed] [Google Scholar]
- 82.Maina G, Salvi V, Vitalucci A, D’Ambrosio V, Bogetto F. Prevalence and correlates of overweight in drug-naïve patients with bipolar disorder. J Affect Disord. 2008;110(1–2):149–155. doi: 10.1016/j.jad.2007.12.233 [DOI] [PubMed] [Google Scholar]
- 83.Staudt Hansen P, Frahm Laursen M, Grøntved S, Puggard Vogt Straszek S, Licht RW, Nielsen RE. Increasing mortality gap for patients diagnosed with bipolar disorder-A nationwide study with 20 years of follow-up. Bipolar Disord. 2019;21(3):270–275. doi: 10.1111/bdi.12684 [DOI] [PubMed] [Google Scholar]
- 84.Kessing LV, Vradi E, McIntyre RS, Andersen PK. Causes of decreased life expectancy over the life span in bipolar disorder. J Affect Disord. 2015;180:142–147. doi: 10.1016/j.jad.2015.03.027 [DOI] [PubMed] [Google Scholar]
- 85.Hällgren J, Ösby U, Westman J, Gissler M. Mortality trends in external causes of death in people with mental health disorders in Sweden, 1987–2010. Scand J Public Health. 2019;47(2):121–126. doi: 10.1177/1403494818758912 [DOI] [PubMed] [Google Scholar]
- 86.Ghaemi N. Clinical psychopharmacology. In: The Concept of a Diagnostic Hierarchy. Oxford, UK: Oxford University Press; 2019. [Google Scholar]
- 87.Huang H, Huang H, Spottswood M, Ghaemi N. Approach to evaluating and managing adult attention-deficit/hyperactivity disorder in primary care. Harv Rev Psychiatry. 2020;28(2):100–106. doi: 10.1097/HRP.0000000000000248 [DOI] [PubMed] [Google Scholar]
- 88.Loftus J, Scott J, Vorspan F, et al. Psychiatric comorbidities in bipolar disorders: an examination of the prevalence and chronology of onset according to sex and bipolar subtype. J Affect Disord. 2020;267:258–263. doi: 10.1016/j.jad.2020.02.035 [DOI] [PubMed] [Google Scholar]
- 89.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518. doi: 10.1001/jama.1990.03450190043026 [DOI] [PubMed] [Google Scholar]
- 90.Messer T, Lammers G, Müller-Siecheneder F, Schmidt RF, Latifi S. Substance abuse in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Res. 2017;253:338–350. doi: 10.1016/j.psychres.2017.02.067 [DOI] [PubMed] [Google Scholar]
- 91.Tolliver BK, Anton RF. Assessment and treatment of mood disorders in the context of substance abuse. Dialogues Clin Neurosci. 2015;17(2):181–190. doi: 10.31887/DCNS.2015.17.2/btolliver [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Agnew-Blais J, Danese A. Childhood maltreatment and unfavourable clinical outcomes in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3(4):342–349. doi: 10.1016/S2215-0366(15)00544-1 [DOI] [PubMed] [Google Scholar]
- 93.Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:Cd006525. doi: 10.1002/14651858.CD006525.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.University of Washington, psychiatry and behavioral sciences, division of population health: principles of collaborative care; 2021. Available from: https://aims.uw.edu/collaborative-care/principles-collaborative-care. Accessed November 11, 2022.
- 95.Smith DJ, Griffiths E, Kelly M, Hood K, Craddock N, Simpson SA. Unrecognised bipolar disorder in primary care patients with depression. Br J Psychiatry. 2011;199(1):49–56. doi: 10.1192/bjp.bp.110.083840 [DOI] [PubMed] [Google Scholar]
- 96.Cerimele JM, Chwastiak LA, Dodson S, Katon WJ. The prevalence of bipolar disorder in general primary care samples: a systematic review. Gen Hosp Psychiatry. 2014;36(1):19–25. doi: 10.1016/j.genhosppsych.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cerimele JM, Halperin AC, Spigner C, Ratzliff A, Katon WJ. Collaborative care psychiatrists’ views on treating bipolar disorder in primary care: a qualitative study. Gen Hosp Psychiatry. 2014;36(6):575–580. doi: 10.1016/j.genhosppsych.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fortney JC, Bauer AM, Cerimele JM, et al. Comparison of teleintegrated care and telereferral care for treating complex psychiatric disorders in primary care: a pragmatic randomized comparative effectiveness trial. JAMA Psychiatry. 2021;78(11):1189. doi: 10.1001/jamapsychiatry.2021.2318 [DOI] [PMC free article] [PubMed] [Google Scholar]