Abstract
Background
Pain after caesarean sections (CS) can affect the well‐being of the mother and her ability with her newborn. Conventional pain‐relieving strategies are often underused because of concerns about the adverse maternal and neonatal effects. Complementary alternative therapies (CAM) may offer an alternative for post‐CS pain.
Objectives
To assess the effects of CAM for post‐caesarean pain.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register, LILACS, PEDro, CAMbase, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (6 September 2019), and checked the reference lists of retrieved articles.
Selection criteria
Randomised controlled trials (RCTs), including quasi‐RCTs and cluster‐RCTs, comparing CAM, alone or associated with other forms of pain relief, versus other treatments or placebo or no treatment, for the treatment of post‐CS pain.
Data collection and analysis
Two review authors independently performed study selection, extracted data, assessed risk of bias and assessed the certainty of evidence using GRADE.
Main results
We included 37 studies (3076 women) which investigated eight different CAM therapies for post‐CS pain relief. There is substantial heterogeneity among the trials. We downgraded the certainty of evidence due to small numbers of women participating in the trials and to risk of bias related to lack of blinding and inadequate reporting of randomisation processes. None of the trials reported pain at six weeks after discharge.
Primary outcomes were pain and adverse effects, reported per intervention below. Secondary outcomes included vital signs, rescue analgesic requirement at six weeks after discharge; all of which were poorly reported, not reported, or we are uncertain as to the effect
Acupuncture or acupressure
We are very uncertain if acupuncture or acupressure (versus no treatment) or acupuncture or acupressure plus analgesia (versus placebo plus analgesia) has any effect on pain because the quality of evidence is very low. Acupuncture or acupressure plus analgesia (versus analgesia) may reduce pain at 12 hours (standardised mean difference (SMD) ‐0.28, 95% confidence interval (CI) ‐0.64 to 0.07; 2 studies; 130 women; low‐certainty evidence) and 24 hours (SMD ‐0.63, 95% CI ‐0.99 to ‐0.26; 2 studies; 130 women; low‐certainty evidence).
It is uncertain whether acupuncture or acupressure (versus no treatment) or acupuncture or acupressure plus analgesia (versus analgesia) has any effect on the risk of adverse effects because the quality of evidence is very low.
Aromatherapy
Aromatherapy plus analgesia may reduce pain when compared with placebo plus analgesia at 12 hours (mean difference (MD) ‐2.63 visual analogue scale (VAS), 95% CI ‐3.48 to ‐1.77; 3 studies; 360 women; low‐certainty evidence) and 24 hours (MD ‐3.38 VAS, 95% CI ‐3.85 to ‐2.91; 1 study; 200 women; low‐certainty evidence). We are uncertain if aromatherapy plus analgesia has any effect on adverse effects (anxiety) compared with placebo plus analgesia.
Electromagnetic therapy
Electromagnetic therapy may reduce pain compared with placebo plus analgesia at 12 hours (MD ‐8.00, 95% CI ‐11.65 to ‐4.35; 1 study; 72 women; low‐certainty evidence) and 24 hours (MD ‐13.00 VAS, 95% CI ‐17.13 to ‐8.87; 1 study; 72 women; low‐certainty evidence).
Massage
We identified six studies (651 women), five of which were quasi‐RCTs, comparing massage (foot and hand) plus analgesia versus analgesia. All the evidence relating to pain, adverse effects (anxiety), vital signs and rescue analgesic requirement was very low‐certainty.
Music
Music plus analgesia may reduce pain when compared with placebo plus analgesia at one hour (SMD ‐0.84, 95% CI ‐1.23 to ‐0.46; 2 studies; 115 women; low‐certainty evidence), 24 hours (MD ‐1.79, 95% CI ‐2.67 to ‐0.91; 1 study; 38 women; low‐certainty evidence), and also when compared with analgesia at one hour (MD ‐2.11, 95% CI ‐3.11 to ‐1.10; 1 study; 38 women; low‐certainty evidence) and at 24 hours (MD ‐2.69, 95% CI ‐3.67 to ‐1.70; 1 study; 38 women; low‐certainty evidence). It is uncertain whether music plus analgesia has any effect on adverse effects (anxiety), when compared with placebo plus analgesia because the quality of evidence is very low.
Reiki
We are uncertain if Reiki plus analgesia compared with analgesia alone has any effect on pain, adverse effects, vital signs or rescue analgesic requirement because the quality of evidence is very low (one study, 90 women).
Relaxation
Relaxation may reduce pain compared with standard care at 24 hours (MD ‐0.53 VAS, 95% CI ‐1.05 to ‐0.01; 1 study; 60 women; low‐certainty evidence).
Transcutaneous electrical nerve stimulation
TENS (versus no treatment) may reduce pain at one hour (MD ‐2.26, 95% CI ‐3.35 to ‐1.17; 1 study; 40 women; low‐certainty evidence). TENS plus analgesia (versus placebo plus analgesia) may reduce pain at one hour (SMD ‐1.10 VAS, 95% CI ‐1.37 to ‐0.82; 3 studies; 238 women; low‐certainty evidence) and at 24 hours (MD ‐0.70 VAS, 95% CI ‐0.87 to ‐0.53; 1 study; 108 women; low‐certainty evidence).
TENS plus analgesia (versus placebo plus analgesia) may reduce heart rate (MD ‐7.00 bpm, 95% CI ‐7.63 to ‐6.37; 108 women; 1 study; low‐certainty evidence) and respiratory rate (MD ‐1.10 brpm, 95% CI ‐1.26 to ‐0.94; 108 women; 1 study; low‐certainty evidence).
We are uncertain if TENS plus analgesia (versus analgesia) has any effect on pain at six hours or 24 hours, or vital signs because the quality of evidence is very low (two studies, 92 women).
Authors' conclusions
Some CAM therapies may help reduce post‐CS pain for up to 24 hours. The evidence on adverse events is too uncertain to make any judgements on safety and we have no evidence about the longer‐term effects on pain.
Since pain control is the most relevant outcome for post‐CS women and their clinicians, it is important that future studies of CAM for post‐CS pain measure pain as a primary outcome, preferably as the proportion of participants with at least moderate (30%) or substantial (50%) pain relief. Measuring pain as a dichotomous variable would improve the certainty of evidence and it is easy to understand for non‐specialists. Future trials also need to be large enough to detect effects on clinical outcomes; measure other important outcomes as listed lin this review, and use validated scales.
Plain language summary
Complementary and alternative therapies for post‐caesarean pain
Background
Pain after caesarean sections (CS) can affect the well‐being of the mother and her interaction with her baby. To manage pain relief during this period, most women receive analgesic drugs. However, these medications can potentially cause side effects in the mother and her baby. Complementary and alternative therapies (CAM) may be a safe way of reducing pain after a CS without adverse effects.
What is the question?
What are the effects of CAM in the treatment of post‐caesarean pain?
Why is this important?
The findings of this review will be useful to help inform women, midwives and doctors about the potential benefits and disadvantages of CAM for pain relief after CS.
What evidence did we find?
We searched the literature in September 2019 and found 37 studies that evaluated eight different types of CAM. The certainty of the evidence from the studies ranged from low to very low, which means that we cannot be confident in the findings. The key reasons for this were that results were not always completely or clearly reported, the studies had serious limitations, and the results lacked precision.
Acupuncture or acupressure
We are uncertain if acupuncture or acupressure (versus no treatment) or acupuncture or acupressure plus analgesia (versus placebo plus analgesia) has any effect on pain because the quality of evidence is very low. Acupuncture or acupressure plus analgesia (versus analgesia) may reduce pain at 12 hours and 24 hours.
It is uncertain whether acupuncture or acupressure (versus no treatment) or acupuncture or acupressure plus analgesia (versus analgesia) has any effect on the risk of adverse effects because the quality of evidence is very low.
Aromatherapy
Aromatherapy may reduce pain at 12 and 24 hours when compared with placebo plus analgesia. It is uncertain if aromatherapy compared with placebo plus analgesia has any effect on adverse effects (anxiety).
Electromagnetic therapy
Electromagnetic therapy may reduce pain at 12 and 24 hours and may reduce rescue analgesic requirement compared with placebo plus analgesia.
Massage therapy
We are uncertain if hand and foot massage plus analgesia, compared with analgesia, has any effect on pain, adverse effects (anxiety) heart rate and respiratory rate because the quality of evidence is very low.
Music therapy
Music plus analgesia, compared with placebo plus analgesia, may reduce pain at one hour and 24 hours. It is uncertain if music plus analgesia, compared with placebo plus analgesia, has any effect on the risk of adverse effects (anxiety) or on heart rate.
Music plus analgesia compared with analgesia may reduce pain at one hour and 24 hours.
Reiki
It is uncertain if Reiki, compared with analgesia has any effect on pain at either one hour or 24 hours, adverse effects (anxiety) or vital signs because the quality of evidence is very low.
Relaxation
It is uncertain if relaxation, compared with standard care, has any effect on pain at 12 hours but it may reduce pain at 24 hours after the intervention.
Transcutaneous electrical nerve stimulation (TENS)
TENS may reduce pain at one hour after the intervention, compared with no treatment.
TENS plus analgesia, compared with placebo plus analgesia, may reduce pain, heart rate and respiratory rate.
It is uncertain if TENS plus analgesia, compared with analgesia, has any effect on pain at six or 24 hours after the intervention or on vital signs or on rescue analgesic requirement.
What does this mean?
There may be some benefit of acupuncture or acupressure, aromatherapy, electromagnetic therapy, massage, music therapy, relaxation, and TENS in the management of pain in women undergoing CS. From these trials, the evidence on harmful effects of CAM are lacking or are very uncertain.
Since pain control is the most relevant outcome for post‐CS women and their clinicians, it is important that future studies of CAM for post‐CS pain measure pain, preferably as the proportion of participants with at least moderate (30%) or substantial (50%) pain relief. Future trials also need to have be large enough to detect effects on clinical outcomes; measure other important outcomes as listed lin this review, and use validated scales.
Summary of findings
Summary of findings 1. Acupuncture or acupressure versus no treatment for post‐caesarean pain.
| Acupuncture versus no treatment for post‐caesarean pain | ||||||
|
Patient or population: post‐caesarean pain
Settings: maternity unit
Intervention: acupressure Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with Acupuncture or acupressure | |||||
|
Abdominal pain assessed with: VAS Scale from 0 to 10 Followup: 24 hours |
The mean abdominal pain in the no treatment group was 4.18 |
MD 0.82 lower (1.74 lower to 0.10 higher) | ‐ | 50 (1 RCT) | ⊕⊝⊝⊝ very low1,2 | |
|
Adverse effects – back pain assessed with: VAS Scale from 0 to 10 Follow‐up: 24 hours |
The mean back pain score in the no treatment group was 2.84 | MD 0.88 lower (1.94 lower to 0.18 higher) | ‐ | 50 (1 RCT) | ⊕⊝⊝⊝ very low1,2 | |
| Vital signs | Not reported | |||||
| Rescue analgesic requirement up to 24 hours | Not reported | |||||
| Pain at six weeks after discharge | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; VAS: visual analogue scale. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level due to risk of high risk of selection, performance and reporting bias
2 Downgraded two levels due to imprecision: few participants and 95% CI consistent with possible benefit and possible harm
Summary of findings 2. Acupuncture or acupressure plus analgesia versus placebo plus analgesia for post‐caesarean pain.
| Acupuncture plus analgesia versus placebo plus analgesia for post‐caesarean pain | ||||||
|
Patient or population: post‐caesarean pain
Settings: maternity unit
Intervention: acupressure plus analgesia Comparison: placebo plus analgesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo plus analgesia |
Risk with Acupuncture or acupressureplus analgesia |
|||||
|
Pain assessed with VAS Scale from 0 to 10 Follow‐up: 12 hours |
The mean pain score in the placebo plus analgesia group was 4.42 | MD 0.01 higher (0.74 lower to 0.76 higher) | ‐ | 108 (1 RCT) | ⊕⊝⊝⊝ very low1,2 | |
| Adverse effects | Not reported | |||||
| Vital signs | Not reported | |||||
| Rescue analgesic requirement (number of analgesics consumed) | The mean number of analgesics consumed in the placebo plus analgesia group was 0.96 | MD 0.00 [0.16 lower to 0.16 higher] | ‐ | 108 (1 RCT) | ⊕⊝⊝⊝ very low1,2 | |
| Pain at six weeks after discharge | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; VAS: visual analogue scale. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level due to high risk of selection, performance and reporting bias
2 Downgraded two levels due to imprecision: few participants and 95% CI consistent with possible benefit and possible harm
Summary of findings 3. Acupuncture or acupressure plus analgesia versus analgesia for post‐caesarean pain.
| Acupuncture plus analgesia versus analgesia for post‐caesarean pain | ||||||
|
Patient or population: post‐caesarean pain
Settings: maternity unit
Intervention: acupuncture or acupressure plus analgesia Comparison: analgesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with analgesia | Risk with Acupuncture or acupressureplus analgesia | |||||
|
Pain Follow‐up: 12 hours |
SMD 0.28 SD lower (0.64 lower to 0.07 higher) | ‐ | 130 (2 RCTs) | ⊕⊕⊝⊝ low1,2 | Acupuncture plus analgesia may reduce pain slightly compared with analgesia (SMD between 0.20 and 0.39 indicates a small effect) | |
|
Pain Follow‐up: 24 hours |
SMD 0.63 SD lower (0.99 lower to 0.26 lower) | ‐ | 130 (2 RCTs) | ⊕⊕⊝⊝ low1,3 | Acupuncture plus analgesia may reduce pain compared with analgesia (SMD between 0.5 and 0.79 indicates a moderate effect) | |
| Adverse effects (pruritus) Follow‐up: up to 24 hours | Study population | RR 0.50 (0.08 to 3.29) | 60 (1 RCT) | ⊕⊝⊝⊝ very low4,5 | ||
| 100 per 1,000 | 50 per 1,000 (8 to 329) | |||||
| Vital signs | Not reported | |||||
| Rescue analgesic requirement (cumulative dose) assessed with: mg | The mean rescue analgesic requirement (cumulative dose) in the control group was 15.28 mg | MD was 5 mg lower (7.67 lower to 2.34 lower) | ‐ | 60 (1 RCT) | ⊕⊕⊝⊝ low3,4 | |
| Pain at six weeks after discharge | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level due to high risk of performance bias and unclear risk of selection and detection bias
2 Downgraded one level due to imprecision: 95% CI spans possible benefit and possible harm
3 Downgraded one level for imprecision: few participants
4 Downgraded one level due to high risk of performance bias and unclear risk of selection bias
5 Downgraded one level due to imprecision: few participants and 95% CI spans possible benefit and possible harm
Summary of findings 4. Aromatherapy plus analgesia versus placebo plus analgesia for post‐caesarean pain.
| Aromatherapy plus analgesia versus placebo plus analgesia for post‐caesarean pain | ||||||
|
Patient or population: post‐caesarean pain
Settings: maternity unit
Intervention: aromatherapy plus analgesia Comparison: placebo plus analgesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo plus analgesia | Risk with Aromatherapy plus analgesia | |||||
|
Pain assessed with: VAS Scale from 0 to 10 Follow‐up: 12 hours |
The mean pain score in the placebo plus analgesia group ranged from 4.58 to 5.77 | MD 2.63 lower (3.48 lower to 1.77 lower) | ‐ | 360 (3 RCTs) | ⊕⊕⊝⊝ low1 | |
|
Pain assessed with: VAS Scale from: 0 to 10 Follow‐up: 24 hours |
The mean pain score in the placebo plus analgesia group was 4.05 | MD 3.38 lower (3.85 to 2.91 lower) | ‐ | 200 (1 RCT) | ⊕⊕⊝⊝ low2,3 | |
|
Adverse effects (anxiety) Assessed with: State‐Trait Anxiety Inventory Scale from 20 to 80 (higher score = greater anxiety) Follow‐up: 12 hours |
The mean adverse effects (anxiety) score in the placebo group was 49.02 | MD 19.87 lower (22.11 to 17.63 lower) | ‐ | 80 (1 RCT) |
⊕⊝⊝⊝ verylow1,3 | |
|
Vital signs: heart rate Assessed with beats per minute |
The mean heart rate in the placebo plus analgesia group was 82.85 beats per minute | MD MD 0.6 beats per minute higher | ‐ | 80 (1 RCT) | ⊕⊝⊝⊝ very low1,4 | |
| Rescue analgesic requirement | Study population | RR 0.69 (0.19 to 2.49) | 220 (3 RCTs) | ⊕⊝⊝⊝ very low1,4,5 | ||
| 900 per 1,000 | 621 per 1,000 (171 to 1,000) | |||||
| Pain at six weeks after discharge | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded two levels due to high risk of selection, performance, detection, attrition and reporting bias
2 Downgraded one level due to unclear risk of selection and detection bias
3 Downgraded one level due to imprecision: few participants
4 Downgraded two levels due to imprecision: few participants and 95% CI spans possible benefit and possible harm
5 Downgraded one level due to inconsistency: heterogeneity in effect size
Summary of findings 5. Electromagnetic therapy plus analgesia versus placebo plus analgesia for post‐caesarean pain.
| Eletromagnetic therapy plus analgesia versus placebo plus analgesia for post‐caesarean pain | ||||||
|
Patient or population: post‐caesarean pain
Settings: maternity unit
Intervention: eletromagnetic therapy plus analgesia Comparison: placebo plus analgesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo plus analgesia | Risk with Electromagnetic therapy plus analgesia | |||||
|
Pain assessed with VAS Scale from 0 to 100 Follow‐up 12 hours |
The mean pain score in the placebo plus analgesia group was 38 | MD 8 lower (11.65 lower to 4.35 lower) | ‐ | 72 (1 RCT) | ⊕⊕⊝⊝ low1 | |
|
Pain assessed with VAS Scale from: 0 to 100 Follow‐up: 24 hours |
The mean pain score in the placebo plus analgesia group was 36 | MD 13 lower (17.13 lower to 8.87 lower) | ‐ | 72 (1 RCT) | ⊕⊕⊝⊝ low1 | |
| Adverse effects | Not reported | |||||
| Vital signs | Not reported | |||||
|
Rescue analgesic requirement Assessed with: mean suppository counts Follow‐up: 24 hours |
The mean suppository counts in the placebo plus analgesia group was 3.1 | MD 1.5 lower (1.95 lower to 1.05 lower) | ‐ | 72 (1 RCT) | ⊕⊕⊝⊝ low1 | |
| Pain at six weeks after discharge | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded two levels for imprecision: very few participants
Summary of findings 6. Massage (foot and hand) plus analgesia versus analgesia for post‐caesarean pain.
| Massage (foot and hand) plus analgesia versus analgesia for post‐caesarean pain | ||||||
|
Patient or population: patients with post‐caesarean pain
Settings: maternity unit
Intervention: massage (foot and hand) plus analgesia Comparison: analgesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with analgesia | Risk with Massage (foot and hand) plus analgesia | |||||
|
Pain assessed with VAS Scale from 0 to 10 Follow‐up: 12 hours |
The mean pain score in the analgesia group ranged from 3.75 to 6.23 | MD 2.03 lower (2.48 lower to 1.59 lower) | ‐ | 651 (6 RCTs) | ⊕⊝⊝⊝ verylow1,2 | |
|
Pain assessed with VAS Scale from 0 to 10 Follow‐up: 24 hours |
The mean pain score in the analgesia group ranged from 3.52 to 7.4 | MD 1.51 lower (1.78 lower to 1.24 lower) | ‐ | 230 (3 RCTs) | ⊕⊝⊝⊝ verylow1,3 | |
|
Adverse effects (anxiety) assessed with VAS (scale from 0 to 10) and STAI (scale from 20 to 80) Follow‐up: 90 minutes |
SMD 0.45 lower (0.70 lower to 0.19 lower) | ‐ | 266 (2 RCTs) |
⊕⊝⊝⊝ verylow3,4 | Massage (foot and hand) plus analgesia may reduce anxiety slightly compared with analgesia. (SMD between 0.2 and 0.49 indicates a small effect). |
|
| Vital signs ‐ heart rate assessed with: beats per minute | The mean heart rate in the analgesia group ranged from 82.48 to 87.20 beat per minute | MD 1.78 lower (4.28 lower to 0.72 higher) | ‐ | 231 (2 RCTs) | ⊕⊝⊝⊝ verylow4,5 | |
| Vital signs ‐ respiratory rate assessed with: breaths per minute | The mean respiratory rate in the analgesia group ranged from 20.19 to 21.40 breaths per minute | MD 0.52 lower (0.91 lower to 0.12 lower) | ‐ | 231 (2 RCTs) | ⊕⊝⊝⊝ verylow3,4 | |
| Rescue analgesic requirement | Study population | RR 0.19 (0.09 to 0.41) | 236 (2 RCTs) | ⊕⊝⊝⊝ verylow1,6 | ||
| 337 per 1,000 | 64 per 1,000 (30 to 138) | |||||
| Pain at six weeks after discharge | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; STAI: State‐Trait Anxiety Inventory; VAS: visual analogue scale. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded two levels due to risk of high selection, performance and reporting bias, and unclear risk of detection bias
2 Downgraded one level due to inconsistency: heterogeneity in effect size
3 Downgraded one level due to imprecision: few participants
4 Downgraded two levels due to high risk of selection and performance bias
5 Downgraded one level due to imprecision: wide 95% CI spans possible benefit and possible harm
6 Downgraded one level due to imprecision: few events
Summary of findings 7. Music plus analgesia versus placebo plus analgesia for post‐caesarean pain.
| Music plus analgesia versus placebo plus analgesia for post‐caesarean pain | ||||||
|
Patient or population: post‐caesarean pain
Settings: maternity unit
Intervention: music plus analgesia Comparison: placebo plus analgesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo plus analgesia | Risk with Music plus analgesia | |||||
|
Pain Follow up: 1 hour |
SMD 0.84 lower (1.23 lower to 0.46 lower) | ‐ | 115 (2 RCTs) | ⊕⊕⊝⊝ low1,2 | Music plus analgesia may result in a large reduction in pain compared with placebo plus analgesia. (SMD 0.8 or greater indicates a large effect). |
|
|
Pain assessed with: VAS Scale: 0‐10 Follow‐up: 24 hours |
The mean pain score in the placebo plus analgesia group was 3.3 | MD 1.79 lower (2.67 lower to 0.91 lower) | ‐ | 38 (1 RCT) | ⊕⊕⊝⊝ low2,3 | |
|
Adverse effects (anxiety) assessed with: VAS Scale from 0 to 100 Follow‐up: 30 minutes |
The mean adverse events (anxiety) in the placebo plus analgesia group was 13 | MD 2 lower (7.83 lower to 3.83 lower) | ‐ | 77 (1 RCT) |
⊕⊝⊝⊝ very low1,4 | |
| Vital signs‐ heart hate assessed with beats per minute | The mean heart rate in the placebo plus analgesia group was 83 | MD 4 higher (2.48 lower to 10.48 higher) | ‐ | 77 (1 RCT) | ⊕⊝⊝⊝ very low1,4 | |
|
Rescue analgesic requirement (dose ‐ morphine) assessed with: mg |
The mean rescue analgesic requirement (dose) in the placebo plus analgesia group was 2.5 mg | MD 0.9 lower (1.70 lower to 0.10 lower) | ‐ | 77 (1 RCT) | ⊕⊕⊝⊝ low1,2 | |
| Pain at six weeks after discharge | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; SMD: standardised mean difference; VAS: visual analogue scale. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level due to high risk of performance bias and unclear risk of selection bias
2 Downgraded one level due to imprecision: few participants
3 Downgraded one level due to risk of performance and reporting bias
4 Downgraded two levels for imprecision: few participants and wide 95% CI spanning possible benefit and possible harm
Summary of findings 8. Music plus analgesia versus analgesia for post‐caesarean pain.
| Music plus analgesia versus analgesia for post‐caesarean pain | ||||||
| Patient or population: post‐caesarean pain Setting: maternity unit Intervention: music plus analgesia Comparison: analgesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with analgesia | Risk with Music plus analgesia | |||||
| Pain assessed with: VAS Scale from: 0 to 10 Follow‐up: 1 hour | The mean pain score in the analgesia group was 5.2 | MD 2.11 lower (3.11 lower to 1.10 lower) | ‐ | 38 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
| Pain assessed with: VAS Scale from: 0 to 10 Follow‐up: 24 hours | The mean pain score in the analgesia group was 4.2 | MD 2.69 lower (3.67 lower to 1.70 lower) | ‐ | 38 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
| Adverse effects | Not reported | |||||
| Vital signs | Not reported | |||||
|
Rescue analgesic requirement (cumulative dose) – Tramadol assessed with: mg |
The mean rescue analgesic requirement (cumulative dose) in the analgesia group was 352.57 mg | MD 45.14 mg lower (86.77 lower to 3.51 lower) | ‐ | 70 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
|
Rescue analgesic requirement (cumulative dose) – Diclofenac assessed with: mg |
The mean rescue analgesic requirement (cumulative dose) in the analgesia group was 72.86 mg | MD 21.43 mg lower (41.65 lower to 1.21 lower) | ‐ | 70 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
| Pain at 6 weeks after discharge | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;MD: mean difference; RCT: randomised controlled trial; VAS: visual analogue scale. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level due to high risk of performance and unclear risk of selection bias
2 Downgraded one level for imprecision: few participants
Summary of findings 9. Reiki plus analgesia versus analgesia for post‐caesarean pain.
| Reiki plus analgesia versus analgesia for post‐caesarean pain | ||||||
|
Patient or population: post‐caesarean pain
Settings: maternity unit
Intervention: Reiki plus analgesia Comparison: analgesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with analgesia | Risk with Reiki plus analgesia | |||||
| Pain assessed with: VAS Scale from: 0 to 10FFollow up: one hour | The mean pain score in the analgesia group was 4.26 | MD 2.2 lower (2.87 lower to 1.53 lower) | ‐ | 90 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | |
| Pain assessed with: VAS Scale from: 0 to 10 Follow‐up: 24 hours | The mean pain score in the analgesia group was 3.76 | MD 2.52 lower (3.07 lower to 1.97 lower) | ‐ | 90 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | |
|
Adverse effects (anxiety)
assessed with: STAI Scale from: 20 to 80 Follow‐up: 24 hours |
The mean adverse effects (anxiety) in the analgesia group was 32.87 | MD 9 lower (11.12 lower to 6.88 lower) | ‐ | 90 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | |
| Vital signs ‐ heart rate assessed with: beats per minute | The mean heart rate in the analgesia group was 89.71 beats per minute | MD 3.58 beats per minute lower (8.26 lower to 1.1 higher) | ‐ | 90 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | |
| Vital signs ‐ respiratory rate assessed with: breaths per minute | The mean respiratory rate in the analgesia group was 19.04 breaths per minute | MD 0.68 breaths per minute lower (1.27 lower to 0.09 lower) | ‐ | 90 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | |
| Rescue analgesic requirement | Not reported | |||||
| Pain at six weeks after discharge | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; STAI: State‐Trait Anxiety Inventory; VAS: visual analogue scale | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded two levels for high risk of selection, performance and detection bias
2 Downgraded one level for imprecision: few participants
Summary of findings 10. Relaxation versus standard care for post‐caesarean pain.
| Relaxation versus standard care for post‐caesarean pain | ||||||
|
Patient or population: post‐caesarean pain
Settings: maternity unit
Intervention: relaxation Comparison: standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard care | Risk with Relaxation | |||||
| Pain assessed with: VAS Scale from: 0 to 10 Follow‐up: 12 hours | The mean pain score in the standard care group was 4.27 | MD 0.04 lower (0.62 lower to 0.54 higher) | ‐ | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | |
| Pain assessed with: VAS Scale from: 0 to 10 Follow‐up: 24 hours | The mean pain score in the standard care group was 4.1 | MD 0.53 lower (1.05 lower to 0.01 lower) | ‐ | 60 (1 RCT) | ⊕⊕⊝⊝ LOW 1,3 | |
| Adverse effects | Not reported | |||||
| Vital signs | Not reported | |||||
| Rescue analgesic requirement | Not reported | |||||
| Pain at six weeks after discharge | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; VAS: visual analogue scale. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level for unclear risk of selection bias and high risk of performance and detection bias
2 Downgraded two levels for imprecision: few participants and wide 95% CI spanning possible benefit and possible harm
3 Downgraded one level for imprecision: few participants
Summary of findings 11. TENS versus no treatment for post‐caesarean pain.
| TENS versus no treatment for post‐caesarean pain | ||||||
|
Patient or population: post‐caesarean pain
Settings: maternity unit
Intervention: TENS Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with TENS | |||||
| Pain assessed with: NAS Scale from: 0 to 10 Follow‐up: 1 hour | The mean pain score in the no treatment group was 3.56 | MD 2.26 lower (3.35 lower to 1.17 lower) | ‐ | 40 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
| Adverse effects | Not reported | |||||
| Vital signs | Not reported | |||||
| Rescue analgesic requirement | Not reported | |||||
| Pain at six weeks after discharge | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NAS: numerical analogue scale; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level for unclear risk of selection bias and high risk of performance bias
2 Downgraded one level for imprecision: few participants
Summary of findings 12. TENS plus analgesia versus placebo plus analgesia for post‐caesarean pain.
| TENS plus analgesia versus placebo plus analgesia for post‐caesarean pain | ||||||
|
Patient or population: post‐caesarean pain
Settings: maternity unit
Intervention: TENS plus analgesia Comparison: placebo plus analgesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo plus analgesia | Risk with TENS plus analgesia | |||||
| Pain assessed with: VAS Follow‐up: 1 hour | SMD 1.1 lower (1.37 lower to 0.82 lower) | ‐ | 238 (3 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | TENS plus analgesia may result in a large reduction in pain compared with placebo plus analgesia (SMD 0.8 or greater indicates a large effect. | |
| Pain assessed with: VAS Scale from: 0 to 10 Follow‐up: 24 hours | The mean pain score in the placebo plus analgesia group was 1.2 | MD 0.7 lower (0.87 lower to 0.53 lower) | ‐ | 108 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
| Adverse effects | Two studies specifically reported that none of the women had any adverse effects (234 women). | |||||
|
Vital signs ‐ heart rate
assessed with: beats per minute Follow‐up: 30 minutes |
The mean heart rate in the placebo plus analgesia group was 77 beats per minute | MD 7 beats per minute lower (7.63 lower to 6.37 lower) | ‐ | 108 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
|
Vital signs: respiratory rate
assessed with: breaths per minute Follow‐up: 30 minutes |
The mean respiratory rate in the placebo plus analgesia group was 18 breaths per minute | MD 1.1 breaths per minute lower (1.26 lower to 0.94 lower) | ‐ | 108 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
|
Rescue analgesic requirement (cumulative dose) ‐ Diclofenac
assessed with: mg Follow‐up 24 hours |
The mean rescue analgesic requirement (cumulative dose) in the placebo plus analgesia group was 147.2 mg | MD 58.4 mg lower (67.11 lower to 49.69 lower) | ‐ | 108 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | |
| Pain at six weeks after discharge | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NAS: numerical analogue scale; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level due to unclear risk of selection, performance and detection bias
2 Downgraded one level due to imprecision: few participants
Summary of findings 13. TENS plus analgesia versus analgesia for post‐caesarean pain.
| TENS plus analgesia versus analgesia for post‐caesarean pain | ||||||
|
Patient or population: post‐caesarean pain
Settings: maternity unit
Intervention: TENS plus analgesia Comparison: analgesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with analgesia | Risk with TENS plus analgesia | |||||
| Pain Follow‐up: 6 hours | SMD 0.04 higher (0.37 lower to 0.45 higher) | ‐ | 92 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,2 | TENS plus analgesia may result in little to no difference in pain compared with analgesia. (SMD smaller than 0.2 indicates trivial or no effect). |
|
| Pain assessed with: VAS Scale from: 0 to 10 Follow‐up: 24 hours | The mean pain score in the analgesia group was 31.4 | MD 1.73 lower (11.57 lower to 8.11 higher) | ‐ | 42 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2,3 | |
| Adverse effects | Not reported | |||||
| Vital signs ‐ heart rate assessed with: beats per minute | The mean heart rate in the analgesia group was 80 beats per minute | MD 3 beats per minute lower (6.51 lower to 0.51 higher) | ‐ | 50 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2,4 | |
| Vital signs ‐ respiratory rate assessed with: breaths per minute | The mean respiratory rate in the analgesia group was 19 breaths per minute | MD 0 breaths per minute (1.11 lower to 1.11 higher) | ‐ | 50 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 4 | |
| Rescue analgesic requirement (cumulative dose) ‐ dipyrone and morphine up to four hours assessed with: mg | The mean rescue analgesic requirement in the analgesia group ranged from 6.2 to 1,600 | MD ‐487.55 mg (1463.19 lower to 488.09 higher) | ‐ | 92 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,2 | |
| Pain at six weeks after discharge | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; SMD: standardised mean difference; TENS: Transcutaneous electrical nerve stimulation; VAS: visual analogue scale. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level for high risk of performance and detection bias, and unclear risk of selection bias
2 Downgraded one level for imprecision: few participants and 95% CI spans possible harm and possible benefit
3 Downgraded one level for high risk of performance and detection bias
4 Downgraded one level for unclear risk of selection, performance and detection bias
Background
Description of the condition
A caesarean section (CS) is the most frequent major surgery currently performed in the world, with an estimated 18.5 million procedures being performed each year (Parra 2016; WHO 2005; WHO 2013). According to estimates of the World Health Organization (WHO), 15% of all deliveries are by CS, with large differences between and within countries (WHO 2013). While CSs account for over 50% of all deliveries in several Latin American countries and in China, the CS rate is less than 5% in several regions of Africa (Althabe 2006; Betrán 2007; Ronsmans 2006). Over the last four decades the rates of CS have been steadily increasing in most high‐ as well as in many low‐ and middle‐income countries, for reasons that are not yet completely understood (Cavallaro 2013; WHO 2013).
It is estimated that 50% to 71% of all patients who undergo any type of surgery will experience moderate to intense pain (Apfelbaum 2003; Sousa 2009b). As after any major surgery, postoperative pain is inevitable after a CS. The intensity of this pain is variable and influenced by several factors including individual sensitivity, age, psychological, social and environmental factors (Msee 2006; Pan 2006).
There are compelling reasons for adequate pain relief after CS, including women's satisfaction and comfort, as well as the reduction of long‐term adverse effects for mothers and infants. Higher pain scores after CS are associated with the development of chronic pain three months after the surgery (Cançado 2012; Msee 2006; Pan 2006). Compared to women with mild pain, women with severe pain in the first day after a CS are 2.5 to three times more likely to develop postpartum depression and persistent pain eight weeks after delivery (Eisenach 2008). Moreover, persistent pain associated with depression can lead to negative maternal behaviour and affect the cognitive development of the infants (Grace 2003). Compared to other postoperative patients, women in the postpartum period following CS are at higher risk of thromboembolism and this risk can be further exacerbated by immobility related to pain or excessive sedation induced by opioids (Pan 2006).
Women who have a caesarean delivery present unique challenges in the treatment of postoperative pain. While the women need medication to reduce the pain associated with having to sit, rise and walk relatively soon after their surgery to care for their infants, they also need to remain alert and energetic enough to interact with and breastfeed their newborns (Sousa 2009b). To achieve these goals, the ideal analgesic for a woman who delivers by CS should produce minimal maternal side effects, minimal or no interference with caring for her newborn or discharge from hospital and also have minimal transfer in breast milk, and consequently little or no effect on neonates (Pan 2006).
Description of the intervention
The term complementary and alternative medicine (CAM) refers to a group of medical and healthcare systems, practices and products that are not generally considered to be part of conventional medicine (Committee CAM 2005; WHO 2001; WHO 2002; WHO 2013b; Wieland 2011). Complementary medicine used for postoperative analgesia includes acupuncture or acupressure, aromatherapy, massage, music therapy and transcutaneous electrical nerve stimulation (TENS); all included in the CAM operational definition (Wieland 2011). These practices can be used alone or to complement other forms of pain relief, including analgesic drugs (Dowswell 2009; Good 2001; Good 2002; Kim 2006; Smith 2011; Smith 2018b).
The CAM practices are currently divided into five domains, or types of therapies: energy medicine, manipulative and body‐based practices, mind‐body medicine, natural product‐based therapies and whole medical systems. Although there is some overlap among these categories, it is the most acceptable CAM categorisation (Wieland 2011). CAM practices used for the relief of post‐caesarean pain include the following.
Acupuncture or acupressure is a therapeutic modality involving the insertion of fine needles through the skin (Schulenburg 2015; White 2009). It has been extensively studied in the management of acute and chronic pain (Garcia 2009; Lee 2014; Manyanga 2014; Vickers 2012; Wang 2008).
Aromatherapy is the therapeutic use of essential oils extracted from plants (Bikmoradi 2015; Stevensen 1994). Aromatherapy oils can be inhaled or rubbed on the patient's skin to alleviate stress and pain (Kim 2006).
Electromagnetic is the therapeutic use of magnetic fields that surround and penetrate the human body and is classified as an energy medicine (Wieland 2011).
Massage produces body relaxation, deeper respiration, improved quality of sleep and pain reduction (Bauer 2010; Hattan 2002).
Music therapy has been shown to reduce postoperative pain, anxiety and stress (Good 2001; Good 2002).
Reiki is a Japanese technique that aims to help in restoring the body's energy system, by stimulating the natural healing processes of the body. Reiki practitioners use light manual touch manual to facilitate the opening of the practitioner’s own energy channels and also the energy channels of patients (Salles 2014). The use of Reiki as complementary therapy has grown rapidly and is used in hospitals in the USA and Europe to help relieve pain and improve the patient recovery process (Teixeira 2009).
Relaxation is a technique that reduces stress through impact on mental and physical conditions, mood, anxiety, and self‐esteem (Heidari Gorji 2014). The instruction of Benson's relaxation technique includes the following steps: quote: "stay in confidence position; close your eyes; calm down and relax your body, relax from your toes to the top of your head; take a breath from your nose and keep your awareness; do this for 15 minutes, try to keep your body and muscles relaxed. Then open your eyes slowly and do not move for some minutes, do not worry it is not important to which level of relaxation you have reached leave your body and let it happen itself. Do not care about interfering thoughts and let them go" (Heidari Gorji 2014).
Transcutaenous electrical nerve stimulation (TENS) involves delivering small electrical impulses from a battery‐operated device via electrode pads attached to the skin close to the area affected by pain (Vance 2014).
How the intervention might work
Acupuncture or acupressure
There are different hypotheses to explain how acupuncture or acupressure leads to pain reduction. Some studies indicate that its analgesic effects are due to the release of beta‐endorphins in the lumbar spine and increased 5‐hydroxy tryptophan levels in the cerebrum. Other explanations include the overriding of the pain stimulus by the biochemical lines of acupuncture or acupressure in the transmitting process of the central nervous system, and the more traditional explanation is that acupuncture or acupressure frees a blockage of "Qi" or energy flow (Green 2002; Zhang 2014).
Aromatherapy
Each oil is believed to have different therapeutic properties according to its chemical composition and lavender is one of the most used for pain relief (Bagetta 2010). The soothing effects of lavender oil are due to its lipophilic monoterpenes which react with cell membranes and cause changes in the activity of ion channels, carriers and nervous receptors (Kim 2006).
Electromagnetic therapy
Electromagnectic therapy has been evaluated for different purposes regarding pain treatment. A systematic review postulates that when compared to placebo, electromagnetic therapy has a beneficial effect on pain, stiffness, and physical function in patients with osteoarthritis (Yang 2020).
Massage
Local massage may have systemic pain‐modulating effects due to stimulation of the 'nonpainful' nerve fibres that interfere with pain transmission in the spinal cord. The feet and hands are good massage areas because they have abundant mechanoreceptors that stimulate nonpainful nerve fibres, resulting in pain inhibition (Kimber 2008; Wang 2004).
Music therapy
The commonly accepted theory is that music acts as a distracter, focusing the patient’s attention away from negative stimuli to something pleasant and encouraging. By occupying the mind with something familiar and soothing, music would allow the patient to escape and relax into his or her “own world” (Nilsson 2008).
Reiki
This complementary therapy has its roots in Eastern traditions, seeking equilibrium between body and mind, focusing on the 'chakras', which are energy centres of the human body (Freitag 2015). These processes can be used to induce relaxation and treatment of health problems.
Relaxation
Benson's relaxation technique is easy to learn and administer and it could be used for relieving pain intensity and to improve quality of life in haemodialysis patients (Rambod 2014).
Transcutaneous electrical nerve stimulation (TENS)
TENS has been extensively investigated for several types of pain relief, including postoperative and CS pain (Bjordal 2003; Paula 2006; Sluka 2001).
Studies typically refer to the gate control theory of pain to explain the effects of high‐frequency TENS. The stimulation of large diameter afferent fibres inhibits the input from small diameter afferent fibres in the substantia gelatinosa of the spinal cord. This is thought to be a segmental inhibition that does not involve descending inhibitory pathways. On the other hand, low‐frequency TENS would relieve pain by activating endogenous opioid pathways (Desantana 2008; Kocyigit 2012; Sluka 2001)
Why it is important to do this review
Pain after CS can affect the well‐being of the mother and her ability to care for, breastfeed and interact with her newborn infant, and can have significant long‐term adverse effects (Eisenach 2008). Postoperative CS pain is often inadequately managed because conventional pain‐relieving strategies are underused often because of concerns about the adverse maternal and neonatal effects of the most commonly used drugs (Gadsden 2005; Pan 2006).
There are systematic reviews which have assessed the use of CAM as alternative or complimentary forms of treatment for pain in childbirth (Barragán 2011; Jones 2012; Smith 2006; Smith 2020; Smith 2011; Smith 2018b) and there are several trials on CAM for postoperative pain after CS. However, there are no systematic reviews of the literature which assess the effects of CAM compared with other forms of treatment for postoperative pain relief in CS. The findings of this review will be useful to help inform women and healthcare professionals about the potential benefits and disadvantages of CAM for pain relief after CS.
Objectives
To assess the effects of complementary alternative therapies for post‐caesarean pain.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) (including those using a cluster‐randomised design) and quasi‐randomised trials comparing complementary alternative medicine (CAM) therapies, alone or associated with other forms of pain relief, versus other treatments or placebo or no treatment, in the treatment of post‐caesarean section (CS) pain. Studies using a cross‐over design were not eligible for inclusion. Studies available only in abstract form were not included in the review.
Types of participants
Women in the postpartum period after a CS.
Types of interventions
All types of CAM according to the WHO criteria (Committee CAM 2005; WHO 2001; WHO 2002; WHO 2013b; Wieland 2011), including acupuncture or acupressure, aromatherapy, massage, music therapy, Reiki, relaxation and transcutaneous electrical nerve stimulation (TENS) for the treatment of postoperative pain in women submitted to CS. We compared the following.
Type of CAM versus placebo.
Type of CAM versus no treatment.
Type of CAM plus analgesia versus placebo plus analgesia.
Type of CAM plus analgesia versus analgesia.
Types of outcome measures
Primary outcomes
Pain measured by a validated instrument or scoring system (such as the visual analogue scale (VAS)) up to discharge
Adverse effects (worsening of pain, anxiety, backache, pruritus (itching of the skin), sedation)
Secondary outcomes
Vital signs (heart rate, respiration rate, systolic and diastolic artery pressure) in the early postoperative period
Rescue analgesic requirement, assessed by dose or frequency of postoperative analgesic. We considered the 'frequency' as how often women receive analgesia (e.g. four times a day).
Pain at six weeks after discharge (VAS)
Women's satisfaction measured up to discharge (verbal satisfaction questionnaire)
Breasfeeding at discharge (verbal questionnaire)
Interaction with the baby measured up to discharge (verbal questionnaire)
Walking at discharge (verbal questionnaire)
Length of hospitalisation (days of hospital stay)
Search methods for identification of studies
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (6 September 2019)
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
We searched these databases: Latin American and Caribbean Health Science Information database (LILACS) (Appendix 1), the Physiotherapy Evidence Database (PEDro) (Appendix 2) and CAMbase (Complementary and Alternative Medicine CAM) (Appendix 3) (6 September 2019).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (6 September 2019) (Appendix 4).
Searching other resources
We checked reference lists of the included studies manually to identify any additional studies. We contacted specialists in the field and authors of the included trials for unpublished data.
We did not apply any language or date restrictions.
Data collection and analysis
The data collection and analysis methods in this section were based on the Cochrane Pregnancy and Childbirth Group's standard methods text.
Selection of studies
After merging the search results and removing duplicate records, three review authors (Sandra Zimpel (SAZ), Gustavo Porfírio (GJMP) and Ronald Flumignan (RLGF)) independently screened the references identified by the literature search. Titles and abstracts were assessed to select potentially relevant reports which were retrieved for full‐text reading. We retrieved and examined the full text of selected studies for compliance with eligibility criteria. The reasons for exclusion of individual trials were documented. The review authors' team (SAZ, GJMP, RLGF, Maria Torloni (MRT) and Edina Silva (EMKS)) was consulted in case of disagreements during this process. We present the process of study identification and selection using the PRISMA flow chart diagram (Figure 1).
1.
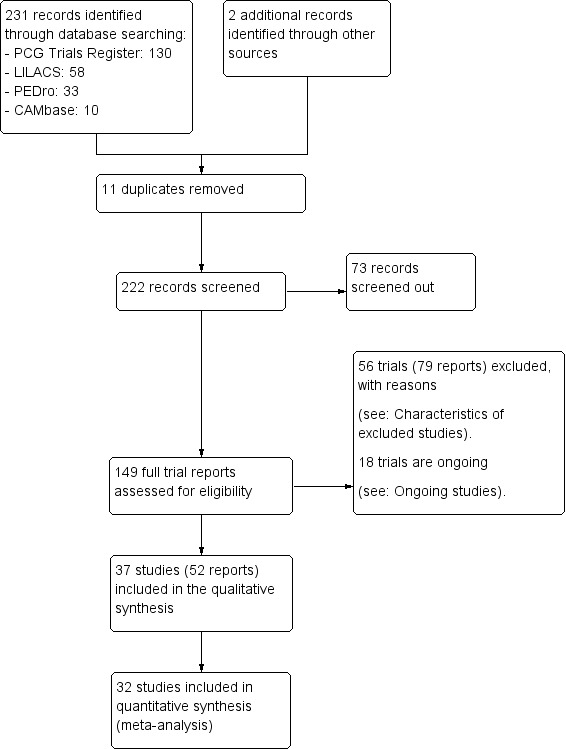
Study flow diagram.
Data extraction and management
We created a specific data abstraction form to extract relevant information from each of the included studies. Three review authors (SAZ, GJMP and RLGF) independently extracted the data using this form. We resolved discrepancies through review authors' team discussion. We entered data into Review Manager software (RevMan 2014) and checked them for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details. When data were reported only in graphs we extracted data of interest such as mean, standard deviation (SD) or standard error (SE) using software such as graphreader.com and the RevMan. We tried to identify translators for all foreign languages with which we were unfamiliar.
Assessment of risk of bias in included studies
Three review authors (SAZ, GJMP and RLGF) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We resolved any disagreement by review authors' team discussion.
(1) Random sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence and assessed whether it was reported in sufficient detail to allow an assessment of whether it should produce comparable groups.
We categorised the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We categorised the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We categorised the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We categorised methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We categorised methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We categorised the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study’s pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We categorised whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
Measures of treatment effect
Dichotomous data
For dichotomous variables, we calculated the risk ratio (RR) and 95% confidence intervals (CIs).
Continuous data
For continuous data, mean differences (MD) and 95% CIs between treatment groups were calculated where studies reported exactly the same outcomes. Where similar outcomes were reported on different scales, the standardised mean difference (SMD) and 95% CIs were calculated. To interpret SMD we used the following thresholds.
SMD < 0.2 = trivial or no effect
SMD ≥ 0.2 and < 0.5 = small effect
SMD ≥ 0.5 and < 0.8 = medium effect
SMD ≥ 0.8 = large effect
Time‐to‐event data
We did not include any time‐to‐event data. In future updates, if we need to include these data, since the most appropriate way of summarising them is to use methods of survival analysis and express the intervention effect as a hazard ratio, these data will be taken directly from the results of the studies. If estimates of log hazard ratios and standard errors can be obtained from results of the studies, these data will be combined using the generic inverse‐variance method (Higgins 2019).
Unit of analysis issues
The unit of analysis was the individual participant (unit to be randomised for interventions to be compared), i.e. the number of observations in the analysis matched the number of individuals randomised. In the case of studies with multiple intervention groups, we combined groups to create a single pair‐wise comparison, combining all relevant intervention or control groups into a single group (Higgins 2019).
Cluster‐randomised trials
We did not identify any cluster‐randomised trials. In future updates we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We consider it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
In future updates, we will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials were not eligible for inclusion.
Dealing with missing data
For included studies, we noted levels of attrition. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominators for each outcome in each trial were the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We quantified inconsistencies among the pooled estimates using the I2 statistic. This illustrates the percentage of variability in effect estimates resulting from heterogeneity rather than sampling error (Higgins 2019).
As strict thresholds for interpretation of I2 are not recommended, we used the guide to interpretation in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
When an I² lay in an area of overlap between two categories (e.g. between 50% and 60%), we considered differences in participants and interventions among the trials contributing data to the analysis (Deeks 2017).
Assessment of reporting biases
If we had included 10 or more studies in the meta‐analysis, we would have investigated reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually. If asymmetry was suggested by visual assessment, we would have performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% CIs, and the estimates of I².
Subgroup analysis and investigation of heterogeneity
If cases of substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses:
multiparous versus primiparous women;
first caesarean versus repeat caesarean;
elective caesarean versus emergency caesarean.
In this review we did not have enough data for subgroup analyses. In future updates, if possible, subgroup analyses will be restricted to the review's primary outcomes. We would have assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We would have reported the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
We have used subgroups to display data at different time points or different drugs for relevant outcomes as needed, but have not performed subgroup interaction tests on these data.
Sensitivity analysis
If the number of studies had been sufficient, we planned to perform sensitivity analysis according to risk of bias on the primary outcome. This would have been done by excluding the trials with inadequate randomisation sequence generation, allocation concealment, high levels of post‐randomisation losses or exclusions and uncertain or unblinded outcome assessment (Deeks 2001). We also planned to perform sensitivity analysis for primary outcomes by removing quasi‐randomised trials to examine the effect of excluding such trials as well as sensitivity analysis to explore the impact of including studies with high levels of missing data. In this version of the review, there were not enough studies per meta‐analysis to perform meaningful sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
For this review we assessed the certainty of the evidence using the GRADE approach as outlined in the GRADE handbook in order to determine the certainty of the body of evidence relating to post‐CS pain and the following interventions.
Acupuncture or acupressure versus no treatment
Acupuncture or acupressure plus analgesia versus placebo plus analgesia
Acupuncture or acupressure plus analgesia versus analgesia
Aromatherapy plus analgesia versus placebo plus analgesia
Electromagnetic therapy plus analgesia
Massage (foot and hand) plus analgesia versus analgesia
Music plus analgesia versus placebo plus analgesia
Music plus analgesia versus analgesia
Reiki plus analgesia versus analgesia
Relaxation versus standard care
TENS versus no treatment
TENS plus analgesia versus placebo plus analgesia
TENS plus analgesia versus analgesia
We reported on the following outcomes in the ’Summary of findings’ tables.
Pain measured by a validated instrument or scoring system (such as the visual analogue scale (VAS)) up to discharge (up to one hour; up to 24 hours)
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation)
Vital signs (heart rate and respiratory rate)
Rescue analgesic requirement (dose and frequency of postoperative analgesic)
Pain at six weeks after discharge (VAS)
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the interventions´ effect and a measure of certainty for each of the interventions was produced using the GRADE approach. The GRADE approach uses five aspects (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each intervention. The evidence can be downgraded from 'high’ by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
A total of 231 records were retrieved from electronic databases and two additional records were identified through other sources. After the exclusion of 11 duplicate records, 222 unique records were screened. A total of 73 records were considered not relevant at this stage and 149 selected for full‐text reading. Thrty‐seven studies (52 reports) are included. Fifty‐six studies (79 reports) were excluded (see Characteristics of excluded studies). Eighteen trials are ongoing (see Characteristics of ongoing studies).
Included studies
The 37 studies (3076 participants) tested eight different types of complementary and alternative medicine (CAM) therapies for the relief of pain after a caesarean section (CS).
Four trials did not report any data that we could use in our analysis (Alves 2015; Bonabi 2018; Gamermann 2015; Yang 2019). Two of these studies are non‐English language and we have not yet been able to obtain translations of the data (Bonabi 2018; Yang 2019).
For details of the included studies, see the Characteristics of included studies table.
Design
Out of the 37 included randomised trials, 10 were classified as quasi‐randomised trials because the authors used a simple randomisation procedure (Abbaspoor 2014; Ahn 2017; Degirmen 2010; Hanan 2011; Hassani 2015; Irani 2015; Midilli 2015; Midilli 2016; Saatsaz 2016; Solehati 2015), and 15 did not provide details of the method used for randomisation (Alves 2015; Bonabi 2018; Dong 2015; Ebneshahidi 2008; Hadi 2011; Jaafarpour 2008; Melo de Paula 2006; Najafi 2017; Navarro Nunez 2000; Olapour 2013; Ramezani 2016; Sharma 2019; Smith 1986; Varghese 2014; Yang 2019).
One study was triple‐blinded, i.e. participants, personnel and outcome assessors, (Lima 2014), two were double‐blinded (Davies 1982; Gamermann 2015), 11 studies were single‐blinded (Alves 2015; Ebneshahidi 2008; Farzaneh 2019; Hadi 2011; Irani 2015; Melo de Paula 2006; Saatsaz 2016; Simonelli 2018; Smith 1986; Sousa 2009a; Wu 2009), 17 studies were unclear about blinding (Abbaspoor 2014; Ahn 2017; Bonabi 2018; Degirmen 2010; Dong 2015; Hanan 2011; Hassani 2015; Kayman‐Kose 2014; Midilli 2015; Navarro Nunez 2000; Olapour 2013; Ramezani 2016; Sen 2010; Sharifipour 2015a; Sharma 2019; Varghese 2014; Yang 2019), and five studies were not blinded (Binder 2011; Jaafarpour 2008; Midilli 2016; Najafi 2017; Solehati 2015).
Settings
The 37 studies were conducted in 13 different countries: 14 in Iran (Abbaspoor 2014; Bonabi 2018; Ebneshahidi 2008; Farzaneh 2019; Hadi 2011; Hassani 2015; Irani 2015; Jaafarpour 2008; Khooshideh 2017; Najafi 2017; Olapour 2013; Ramezani 2016; Saatsaz 2016; Sharifipour 2015a), five in Turkey (Degirmen 2010; Kayman‐Kose 2014; Midilli 2015; Midilli 2016; Sen 2010), five in Brazil (Alves 2015; Gamermann 2015; Lima 2014; Melo de Paula 2006; Sousa 2009a), three China (Dong 2015; Wu 2009; Yang 2019), one in Canada (Smith 1986), two in India (Sharma 2019; Varghese 2014), and one each in Egypt (Hanan 2011), Indonesia (Solehati 2015), Korea (Ahn 2017), Mexico (Navarro Nunez 2000), Sweden (Binder 2011), the UK (Davies 1982), and the USA (Simonelli 2018). All included studies were conducted in hospital settings.
Participants
The age of the women included in the 37 trials ranged from 16 to 45 years. The majority of trials included women from 18 to 35 years of age. Some trials did not report the age of participants.
Five studies were conducted with women undergoing CS under general anaesthesia involved 392 women (Ebneshahidi 2008; Kayman‐Kose 2014; Midilli 2015; Midilli 2016; Sen 2010). Three studies used general anaesthesia and spinal cord anaesthesia, and these involved a total of 161 women (Davies 1982; Ramezani 2016; Smith 1986). Seventeen studies used solely spinal anaesthesia and they tested 1325 women (Abbaspoor 2014; Ahn 2017; Binder 2011; Farzaneh 2019; Gamermann 2015; Hadi 2011; Irani 2015; Jaafarpour 2008; Khooshideh 2017; Lima 2014; Najafi 2017; Olapour 2013; Sharifipour 2015a; Sharma 2019; Solehati 2015; Sousa 2009a; Wu 2009). Six studies did not describe the type of anaesthesia used in their 1202 patients (Alves 2015; Degirmen 2010; Dong 2015; Hanan 2011; Irani 2015; Navarro Nunez 2000).
Six studies included only multiparous women (Abbaspoor 2014; Binder 2011; Gamermann 2015; Irani 2015; Kayman‐Kose 2014; Smith 1986) and eight studies included only primiparous women (Dong 2015; Jaafarpour 2008; Khooshideh 2017; Najafi 2017; Saatsaz 2016; Sen 2010; Simonelli 2018; Yang 2019). The other studies either did not describe the parity of the women or included both primiparous and multiparous women.
Sample size
The number of participants included in each of the 37 studies ranged from 18 (Smith 1986) to 200 (Hadi 2011; Kayman‐Kose 2014). However, most of the studies had small sample sizes.
Funding
The majority of trials did not report their funding sources (Abbaspoor 2014; Bonabi 2018; Davies 1982; Degirmen 2010; Dong 2015; Ebneshahidi 2008; Hadi 2011; Hanan 2011; Hassani 2015; Jaafarpour 2008; Kayman‐Kose 2014; Lima 2014; Melo de Paula 2006; Midilli 2015; Midilli 2016; Najafi 2017; Navarro Nunez 2000; Ramezani 2016; Saatsaz 2016; Sen 2010; Solehati 2015; Varghese 2014; Yang 2019). Three trials reported not having any funding sources (Ahn 2017; Alves 2015; Gamermann 2015). The remaining trials were self‐funded, funded by government grants or host hospitals and universities (Binder 2011; Farzaneh 2019; Irani 2015; Khooshideh 2017; Olapour 2013; Sharifipour 2015a; Sharma 2019; Simonelli 2018; Smith 1986; Sousa 2009a; Wu 2009).
Conflicts of interest
Most of the trials did not mention conflicts of interest (Abbaspoor 2014; Ahn 2017; Davies 1982; Degirmen 2010; Dong 2015; Ebneshahidi 2008; Gamermann 2015; Hadi 2011; Hanan 2011; Hassani 2015; Jaafarpour 2008; Kayman‐Kose 2014; Khooshideh 2017; Lima 2014; Melo de Paula 2006; Midilli 2015; Navarro Nunez 2000; Sen 2010; Sharifipour 2015a; Sharma 2019; Smith 1986; Solehati 2015; Sousa 2009a; Varghese 2014; Wu 2009; Yang 2019); and the remaining trials stated they had no conflicts of interest (Alves 2015; Binder 2011; Bonabi 2018; Farzaneh 2019; Irani 2015; Midilli 2016; Najafi 2017; Olapour 2013; Ramezani 2016; Saatsaz 2016; Simonelli 2018).
Interventions
The 37 included studies tested eight different types of CAMs for the relief of post‐CS pain: acupuncture or acupressure, aromatherapy, electromagnetic therapy, massage, music therapy, Reiki, relaxation, and TENS.
Acupuncture or acupressure
Seven randomised trials (Ahn 2017; Bonabi 2018; Dong 2015; Gamermann 2015; Ramezani 2016; Wu 2009; Yang 2019), three of which did not contribute data (Bonabi 2018; Gamermann 2015; Yang 2019), tested acupuncture or acupressure for post‐CS. Ahn 2017 randomised 52 Korean women for hand acupressure discs intervention for 24 hours onto 12 acupressure points; the control group was not detailed. Bonabi 2018 randomised 90 women in three groups: acupressure L4 point, acupressure SP6 point and control. The participants received acupressure for 10 seconds, followed by 2 seconds of rest until 20 minutes per section. Both acupressure groups would have been analysed together in this review, but unfortunately the full data are not available at this moment (published manuscript in Persian). Dong 2015 randomised 108 parturients, divided into three treatment groups: patient‐controlled intravenous analgesia (PCIA), auricular‐plaster therapy (APT) and combination therapy (APT with PCIA), but we included only the PCIA and APT with PCIA groups for analysis. Pressure was applied with the index finger and thumb, causing temporary swelling and pain, three times per day. Gamermann 2015 randomised a total of 58 women after elective CS to receive acupuncture at two points (P6 and Ll4) or sham acupuncture in the same points, for 20 minutes. Ramezani 2016 randomised 108 women for acupressure LI4 or only touch (control group). Wu 2009 randomised 60 Chinese women into three groups: acupuncture plus analgesia, electro‐acupuncture plus analgesia versus analgesia. Acupuncture and electro‐acupuncture were performed on the point Sp6, bilaterally, during 30 minutes, and were analysed together in this review. Yang 2019 randomised 120 women for auricular acupuncture or standard care.
Aromatherapy
Four trials (Hadi 2011; Najafi 2017; Olapour 2013; Sharifipour 2015a) compared the effects of aromatherapy for the relief of post‐CS pain. The 200 participants in Hadi 2011 inhaled a single dose of lavender essence (or artificial aromatic material similar to lavender essence) through an oxygen mask for three minutes, three hours after receiving intravenous analgesic. Pain was assessed 30 minutes, eight hours and 16 hours after the intervention using a visual analogue scale (VAS). Najafi 2017 randomised 80 women to chamomile essence or a placebo essence. Both groups received analgesia with diclofenac 100 mg via rectal suppository. Olapour 2013 delivered the intervention four, eight and 12 hour after the onset of postoperative pain, using three drops (lavender essence oil or placebo oil) poured on cotton placed inside a cast container. The 60 participants were asked to inhale from the container from a distance of 10 cm, for five minutes. Sharifipour 2015a randomised 120 women to three drops of Citrus arantium (experimental) and three drops of saline (control).
Electromagnetic therapy
Khooshideh 2017 randomised 72 women to receive pulsed electromagnetic field (36 participants) or a sham intervention (36 participants) for pain relief. The intervention was consisted by an elliptical coil that was 12 cm in size and a radiofrequency energy generator powered by battery that had an emission frequency of 27.1 MHz, a pulse rate of 1000 pulses per second, a 100 µs pulse duration, and a peak spatial power density of 75 µW/cm2. Both groups received diclofenac 100 mg suppositories, once a day.
Massage
Nine randomised trials tested massage for pain relief (Abbaspoor 2014; Degirmen 2010; Hanan 2011; Hassani 2015; Irani 2015; Saatsaz 2016; Sharma 2019; Simonelli 2018; Varghese 2014). However, Saatsaz 2016 did not provide data for meta‐analysis.
Degirmen 2010 and Saatsaz 2016 randomised participants in three groups: foot and hand massage (20 minutes duration), foot massage alone (10 minutes) and control (no massage). In one study (Degirmen 2010) massage was performed on average 2.5 hours after spinal anaesthesia and the outcomes were pain and vital signs while the other study did not provide further intervention details (Saatsaz 2016).
Hassani 2015 randomised 20 women in three groups: foot reflexology, foot massage or standard care for five minutes. Both experimental groups were analysed together in this review.
Simonelli 2018 randomised participants for three groups: massage, standard care and individualised attention. The standard care and individualised attention were analysed together in this review.
Abbaspoor 2014, Hanan 2011, Irani 2015 and Sharma 2019 randomised participants into two groups: foot plus hand massage (during 20 minutes) and control. Abbaspoor 2014 delivered the intervention 1.5 to 2 hours after the administration of analgesics post CS and assessed pain and the need for additional analgesia. Hanan 2011 performed three separate massage sessions 5:40, 11:40, 17:40 hour after delivery and assessed only pain. Irani 2015 measured pain and anxiety four hours following the surgery, and then did the massage intervention in experimental group. Sharma 2019 performed massage for 20 minutes, two times a day, for three days in the experimental group.
Varghese 2014 randomised 60 women to 15 minutes of reflexology once a day for five days or standard care (control group). No details were provided about the local of massage (e.g. foot, hand).
Music therapy
Three trials assessed the effects of music therapy for the relief of post‐CS pain (Ebneshahidi 2008; Farzaneh 2019; Sen 2010).
Ebneshahidi 2008's music therapy group listened to music for 30 minutes in the postoperative period and all participants were evaluated for pain, analgesia requirement and anxiety. The control group used silent headphones for the same period and both groups received PCA. Farzaneh 2019 randomised 57 women in three groups: nature‐based music sounds, sham (silent) headphones and standard care. All groups received the interventions for 20 minutes and also received PCA. We analysed the sham and standard care groups together in this review. Sen 2010 exposed the experimental participants to music one hour after CS and assessed pain and the amount of analgesics used by all women. The control group was exposed to silence and both groups also received PCA.
Reiki
Two randomised trials (Midilli 2015; Midilli 2016) compared the effect of Reiki sessions versus routine care in the relief of post‐CS pain assessed by the VAS. Midilli 2015 performed sessions 24 and 48 hours after surgery and each session lasted three minutes per day. Midilli 2016 randomised 45 women into three groups: Reiki, sham and standard care. All received the intervention for 15 minutes during 24 hours to 48 hours post‐CS period. The sham and standard care groups were analysed together in this review.
Relaxation
Solehati 2015 evaluated the effect of Benson relaxation technique for 10 minutes for four days after CS for pain relief. The control groups received standard care.
TENS
Ten studies evaluated TENS, with or without analgesia, for pain relief in women in the post‐CS period (Alves 2015; Binder 2011; Davies 1982; Jaafarpour 2008; Kayman‐Kose 2014; Lima 2014; Melo de Paula 2006; Navarro Nunez 2000; Smith 1986; Sousa 2009a), although four did not contribute any data to the meta‐analyses (Alves 2015; Davies 1982; Lima 2014; Smith 1986). Of the six studies contributing data, three compared the use of TENS plus analgesia versus placebo plus analgesia; both groups received analgesia as routine prescription (Jaafarpour 2008; Kayman‐Kose 2014; Melo de Paula 2006), Sousa 2009a compared TENS versus no treatment analgesia, although analgesia was used in both groups when requested; and Navarro Nunez 2000 and Binder 2011 compared TENS plus analgesia versus analgesia (1 g of dipyrone or morphine in 24 hours).
The studies in this comparison were very heterogeneous in relation to the dose and the duration of the intervention. Eight studies (Alves 2015; Binder 2011; Jaafarpour 2008; Kayman‐Kose 2014; Melo de Paula 2006; Navarro Nunez 2000; Smith 1986; Sousa 2009a), which include all the studies that contributed data to this review, used high‐frequency TENS ranging from 66 Hz to 100 Hz; Davies 1982 used low‐dose TENS (25 Hz) and Lima 2014 compared high‐dose (100 Hz) and low‐dose (4 Hz) TENS. Smith 1986 used TENS for three days; Binder 2011; Davies 1982; Jaafarpour 2008 used TENS for 24 hours; Navarro Nunez 2000 and Lima 2014 used TENS for four hours; Sousa 2009a used TENS for 45 minutes; AND finally, Alves 2015, Kayman‐Kose 2014 and Melo de Paula 2006 used TENS for only 30 minutes.
Kayman‐Kose 2014 used TENS for women after vaginal or CS delivery; we included the data related only to the women in the post‐CS group. The scales used to assess pain also differed. Most studies used a VAS (Alves 2015; Binder 2011; Jaafarpour 2008; Lima 2014; Melo de Paula 2006; Navarro Nunez 2000; Kayman‐Kose 2014), but others used a numerical scale (Lima 2014; Sousa 2009a) along with an analogue scale (Davies 1982), or a McGill questionnaire (Smith 1986) and a cross‐modality registration (Navarro Nunez 2000).
Outcomes
In most of the studies included in this review, the outcomes were similar. The main outcome measures were pain, analgesic doses and vital signs. However, these outcomes were assessed at different time periods, ranging from 20 minutes to three days after surgery, which made it difficult to perform quantitative analyses for each intervention.
Primary outcomes
Thirty‐three studies reported our primary outcome of pain (Abbaspoor 2014; Ahn 2017; Alves 2015; Binder 2011; Davies 1982Degirmen 2010; Dong 2015; Ebneshahidi 2008; Farzaneh 2019; Gamermann 2015; Hadi 2011; Hanan 2011; Hassani 2015; Jaafarpour 2008; Kayman‐Kose 2014; Khooshideh 2017; Lima 2014; Melo de Paula 2006; Midilli 2015; Midilli 2016; Najafi 2017; Navarro Nunez 2000; Olapour 2013; Ramezani 2016; Saatsaz 2016; Sharifipour 2015a; Sharma 2019; Simonelli 2018; Smith 1986; Solehati 2015; Sousa 2009a; Varghese 2014; Wu 2009).
Seven studies reported adverse effects (Ahn 2017; Ebneshahidi 2008; Kayman‐Kose 2014; Lima 2014; Midilli 2015; Saatsaz 2016; Simonelli 2018).
Secondary outcomes
Nine studies reported vital signs (Degirmen 2010; Ebneshahidi 2008; Jaafarpour 2008; Midilli 2015; Midilli 2016; Navarro Nunez 2000; Olapour 2013; Saatsaz 2016; Sharifipour 2015a).
Seventeen studies reported rescue analgesic requirement (Ahn 2017; Binder 2011; Davies 1982; Gamermann 2015; Jaafarpour 2008; Kayman‐Kose 2014; Olapour 2013; Midilli 2015; Midilli 2016; Najafi 2017; Navarro Nunez 2000; Ramezani 2016; Saatsaz 2016; Sen 2010; Sharifipour 2015a; Smith 1986; Wu 2009).
Three studies reported women's satisfaction (Gamermann 2015; Olapour 2013; Sen 2010).
Two studies reported breastfeeding at discharge (Hanan 2011; Saatsaz 2016).
None of the included studies reported the following secondary outcomes.
Pain at six weeks after discharge
Interaction with the baby
Walking at discharge
Length of hospitalisation
Acupuncture or acupressure
Ahn 2017 evaluated the effect of acupuncture on pain using VAS at 0.5; 1; 2 and 24 hours after intervention. They also evaluated nausea and vomiting repercussions. Bonabi 2018 evaluated the effects of acupressure on pain using VAS, but did not provide the time points on the available abstract. Three studies evaluated the effect of acupuncture plus analgesia on pain at various postoperative moments using VAS at 6, 12, 24 and 48 hours after intervention (Dong 2015; Gamermann 2015; Wu 2009). The demand for analgesic (PCA) and morphine doses after surgery were evaluated for two studies (Dong 2015; Wu 2009). Gamermann 2015 also assessed the satisfaction of postpartum women about the treatment received and performed assessments 24 and 48 hours postoperatively. Wu 2009 also evaluated dizziness and itching, and all their outcomes were assessed at 1, 4 and 24 hours after surgery. Ramezani 2016 evaluated the effect of acupressure on pain using VAS at 0; 1 and 2 hours after interventions. Yang 2019 evaluated the effects of auricular acupuncture or acupressure on pain and other outcomes such as anus exhaust time, incidence of postpartum haemorrhage, urinary retention and constipation, and postpartum average hospitalisation day, but did not provide the specific method on the available abstract.
Aromatherapy
Hadi 2011 evaluated pain 30 minutes, 8 hours and 16 hours after the intervention. Najafi 2017 evaluated pain by a mean of VAS scores at 0 and 15 minutes after the intervention. This study also assessed vital signs and analgesic consumption. Olapour 2013 and Sharifipour 2015a assessed pain in four different periods: in the immediate postoperative period and after 4, 8 and 12 hours. Olapour 2013 also assessed the amount of analgesics used, blood pressure, heart rate and patient satisfaction. Sharifipour 2015a also evaluated anxiety, pulse rate, blood pressure, nausea, vomiting, and headache. All studies used a VAS to assess pain.
Electromagnetic therapy
Khooshideh 2017 evaluated pain at 24 hours and one week after intervention by VAS. This study also evaluated analgesic use, surgical site inflammation, patient satisfaction and return to daily activities.
Massage
All the studies that tested massage assessed pain using a numerical range scale (NRS). Abbaspoor 2014, Degirmen 2010 and Irani 2015 evaluated the participants immediately after the massage and 90 minutes after the intervention. Hanan 2011 assessed pain after each of the sessions (6, 12 and 18 hours after delivery) using the McGill questionnaire. Hassani 2015 evaluated pain by a not detailed numeric scale and also evaluated vital signs. Abbaspoor 2014 also assessed the demand for more analgesics and Degirmen 2010 also assessed vital signs (blood pressure, respiratory rate and pulse). Saatsaz 2016 assessed pain using VAS immediately and 90 minutes after the intervention. This study also evaluated anxiety level, haemodynamic indicators levels, and breastfeeding frequency. Sharma 2019 assessed pain using numeric scale (0‐10) at one, two and three days after CS. This study also evaluated the vital signs. Simonelli 2018 assessed pain using numeric scale (0‐10) at one and 60 minutes after CS. This study also evaluated stress and relaxation. Varghese 2014 assessed pain using VAS at five days after intervention. This study also evaluated the quality of sleep by Pittsburgh sleep quality index.
Music therapy
Pain after music therapy was assessed using a VAS in all studies but at different times. Ebneshahidi 2008 assessed pain 30 minutes and six hours after the intervention. Farzaneh 2019 assessed only pain at 15 minutes and one hour after each intervention and the interventions were performed every eight hours for until 48 hours after CS, i.e. they assessed pain in six time points. Sen 2010 evaluated pain at 1, 4, 12 and 24 hours after CS.
The request for additional analgesics was assessed by Ebneshahidi 2008 and Sen 2010. Ebneshahidi 2008 also assessed anxiety, and the effects of the intervention on the vital signs of the participants (blood pressure, respiratory and heart rates).
Reiki
Pain was assessed using VAS in both studies which tested Reiki (Midilli 2015; Midilli 2016). These studies also assessed the dose of analgesics consumed and changes in the vital signs (blood pressure, respiratory rate and heart rate) of the participants. Midilli 2015 also investigated the anxiety of the participants.
Relaxation
Solehati 2015 evaluated the effect of Benson relaxation technique in reducing pain intensity 12, 24 and 48 hours after CS using VAS.
TENS
The time points for measuring pain varied: Alves 2015 10, 30, 60 minutes; Binder 2011 1, 3, 6, 9, 12 and 24 hours postpartum.; Davies 1982 24 hours; Jaafarpour 2008 24 hours; Kayman‐Kose 2014 eight hours; Lima 2014 20, 40, 60 minutes; Melo de Paula 2006 30 minutes; Navarro Nunez 2000 240 minutes; Smith 1986 24 hours, 72 hours; Sousa 2009a 105 minutes.
In addition to pain, four studies also assessed the amount of analgesics consumed by the women (Binder 2011; Davies 1982; Jaafarpour 2008; Navarro Nunez 2000). Four of these studies performed assessment 24 hours after the surgery (Binder 2011; Davies 1982; Jaafarpour 2008; Smith 1986), one study (Navarro Nunez 2000) assessed outcomes after four hours, two studies (Alves 2015; Lima 2014) assessed after 60 minutes, Kayman‐Kose 2014 after eight hours and Sousa 2009a after 105 minutes. Vital signs (heart rate, respiratory rate and blood pressure) were evaluated by Jaafarpour 2008 and Navarro Nunez 2000 and the time at the first feeding was described only Jaafarpour 2008. Most studies used VAS to assess pain (Alves 2015; Binder 2011; Jaafarpour 2008; Kayman‐Kose 2014; Lima 2014; Melo de Paula 2006; Sousa 2009a). Besides a VAS, Davies 1982, Smith 1986 and Sousa 2009a also used a McGill questionnaire, and Navarro Nunez 2000 used a cross‐modality registration. Kayman‐Kose 2014, Lima 2014, Melo de Paula 2006, Smith 1986 and Sousa 2009a provided pain in different numerical scales. Four studies involving TENS, could not be included in meta‐analyses for pain. We contacted the authors of these studies but only two sent additional information. These studies are presented in the qualitative analyses (narrative description) (Binder 2011; Davies 1982; Lima 2014; Smith 1986).
Excluded studies
Fifty‐six studies were excluded for at least one reason (Characteristics of excluded studies). Nineteen studies did not evaluate CAM (Allameh 2013; Amin‐Hanjani 1992; Beiranvand 2014; Cal 2016; Chaowalit 2018; Charoenkwan 2017; Citak 2012; Ghana 2017; Gillier 2016; Gist 2018; Gursen 2016; Gustafson 2018; Krum 2006; Mahishale 2014; Mohseni 2018; Myers 2014; Norouzi 2013; Robinson 2017; Sharifi 2013), as defined by Cochrane (Wieland 2011). Twenty‐four studies were not performed for the treatment of post‐caesarean pain, i.e. CAM was used for prevention of the postoperative pain (when used in the pre‐ and intra‐operative periods), or CAM was used for the treatment of other disturbances (e.g. anxiety, flatulency) (Abadi 2018; Abu Bakar 2015; Agah 2007; Ali 2017; Blackburn 2011; Chang 2005; Fazel 2017; Ho 1996; Khezri 2017; Khoshtarash 2012; Kuo 2016; Kurdi 2018; Kushnir 2012; Li 2012a; Li 2012b; Mousavi 2017; Rasuli 2017; Razmjoo 2012; Reza 2007; Sadeghi 2019; Sen 2009; Shabanian 2017; Sharifipour 2015b; vanderVaart 2011). Five studies were excluded because they were not randomised (Chen 2005; Hollinger 1986; Mokhtari 2010; Reynolds 1987; Xue 2016). Four studies were excluded because the type of comparison was not consistent with our protocol (Hong 2003; Houshyar 2015; Kerai 2011; Keshavarz 2010). Five studies were excluded because only abstracts were available (Blackburn 2011; Henkel 2018; Ohashi 2012; Saberkari 2009; Tarasov 1995). One study was retrospective (Hollinger 1986).
Ongoing studies
We identified 18 ongoing studies, evaluating seven different CAM therapies for post‐CS pain relief:
aromatherapy (Jahdi 2015; Mobaraki 2019; Mojalli 2017; Pakseresht 2016; Zardosht 2016);
TENS (Balachandran 2019; Klinger 2018; Maassarani 2018; Santana 2014; Shahoei 2017);
reflexology (Bagherzadeh 2019; Joghataei 2015; Kazemi 2019; Oberbaum 2008);
herbal supplement (Hakimi 2018);
acupuncture or acupressure (Kim 2015);
massage (Latifi 2012);
electromagnetism (Phillibert 2015).
All ongoing studies plan to report data on pain relief. Bagherzadeh 2019, Latifi 2012, and Pakseresht 2016 plan to report data on vital signs. Bagherzadeh 2019 plans to report breastfeeding data. Balachandran 2019, Oberbaum 2008, and Zardosht 2016 plan to report quality of life (QoL) data. Hakimi 2018, Joghataei 2015, Mobaraki 2019, and Mojalli 2017 plan to report data on adverse effects. Oberbaum 2008 and Phillibert 2015 plan to report data on rescue analgesic requirement.
We tried to contact trial authors; we also searched by the trial number of registration and by the title of the study on all databases of interest for this review. However, there are no additional data for all these ongoing studies.
Risk of bias in included studies
Two review authors assessed independently the risk of bias of the 37 included studies in accordance with the 'Risk of bias' tables in RevMan.
When there were not enough data to assess the risk of bias, we contacted the authors by email. If they did not reply, we classified this assessment as unclear. Only two authors responded to our emails and provided additional information (Sousa 2009a; Wu 2009).
A graphical summary of the results of the 'Risk of bias' assessment for the included studies is provided in Figure 2 and Figure 3.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eleven studies were classified as low risk of bias for the random sequence generation domain because they described adequate randomisation methods (Davies 1982; Farzaneh 2019; Gamermann 2015; Kayman‐Kose 2014; Khooshideh 2017; Lima 2014; Sen 2010; Sharifipour 2015a; Solehati 2015; Sousa 2009a; Wu 2009).
Sixteen studies were judged as having an unclear risk of bias because they did not adequately describe the method used for randomisation (Alves 2015; Binder 2011Bonabi 2018; Dong 2015; Ebneshahidi 2008; Hadi 2011; Melo de Paula 2006; Najafi 2017; Navarro Nunez 2000; Olapour 2013; Ramezani 2016; Sharma 2019; Simonelli 2018; Smith 1986; Varghese 2014; Yang 2019).
Ten studies were judged as having a high risk of bias because they were described as quasi‐randomised (Abbaspoor 2014; Ahn 2017; Degirmen 2010; Hanan 2011; Hassani 2015; Irani 2015; Jaafarpour 2008; Midilli 2015; Midilli 2016; Saatsaz 2016).
The allocation concealment was judged as adequate in six studies, classified as low risk (Binder 2011; Gamermann 2015; Kayman‐Kose 2014; Khooshideh 2017; Lima 2014; Simonelli 2018). Twenty‐five studies were classified as unclear risk bias because they did not clearly describe the allocation concealment (Abbaspoor 2014; Ahn 2017; Alves 2015; Bonabi 2018; Davies 1982; Dong 2015; Ebneshahidi 2008; Farzaneh 2019; Hadi 2011; Hanan 2011; Hassani 2015; Jaafarpour 2008; Melo de Paula 2006; Navarro Nunez 2000; Olapour 2013; Saatsaz 2016; Sen 2010; Sharifipour 2015a; Sharma 2019; Smith 1986; Solehati 2015; Sousa 2009a; Varghese 2014; Wu 2009; Yang 2019). Six studies were judged as high risk of bias because they described an inadequate allocation method (Degirmen 2010; Irani 2015; Midilli 2015; Midilli 2016; Najafi 2017; Ramezani 2016).
Blinding
Six studies provided a clear description of blinding of the participants and personnel and were classified as low risk for this domain (Davies 1982; Hadi 2011; Khooshideh 2017; Lima 2014; Smith 1986). Ten studies were unclear about the blinding of participants and personnel (Alves 2015; Bonabi 2018; Dong 2015; Jaafarpour 2008; Kayman‐Kose 2014; Melo de Paula 2006; Navarro Nunez 2000; Olapour 2013; Sharma 2019; Varghese 2014) and were classified as having an unclear risk for this domain. Twenty studies were classified as having a high risk of bias because the women and/or intervention providers could not be blinded (e.g. involving massage, music, relaxation) or were not blinded by the trialists (Abbaspoor 2014; Ahn 2017; Binder 2011; Degirmen 2010; Ebneshahidi 2008; Farzaneh 2019; Gamermann 2015; Hanan 2011; Hassani 2015; Irani 2015; Midilli 2015; Midilli 2015; Najafi 2017; Ramezani 2016; Saatsaz 2016; Sen 2010; Sharifipour 2015a; Simonelli 2018; Solehati 2015; Sousa 2009a; Wu 2009; Yang 2019)
The blinding of outcome assessors was not reported in 21 studies which were classified as having an unclear risk of bias for this domain (Abbaspoor 2014; Ahn 2017; Bonabi 2018; Davies 1982; Degirmen 2010; Dong 2015; Hadi 2011; Hanan 2011; Hassani 2015; Jaafarpour 2008; Kayman‐Kose 2014; Melo de Paula 2006; Navarro Nunez 2000; Olapour 2013; Ramezani 2016; Sen 2010; Sharifipour 2015a; Sharma 2019; Smith 1986; Varghese 2014; Yang 2019). Eleven studies were classified as having a low risk of bias because the outcome assessors were blinded to the intervention and control groups (Alves 2015; Ebneshahidi 2008; Farzaneh 2019; Gamermann 2015; Irani 2015; Khooshideh 2017; Lima 2014; Saatsaz 2016; Simonelli 2018; Sousa 2009a; Wu 2009). Five studies were classified as having a high risk of bias because they clearly stated that the outcome assessors were not blinded (Binder 2011; Midilli 2015; Midilli 2016; Najafi 2017; Solehati 2015).
Incomplete outcome data
Three studies were judged as unclear risk of bias for this domain because there were losses, but these were not clearly described (Bonabi 2018; Hassani 2015; Lima 2014). Only one study was judged as high risk of bias because the trial authors stated that there were no losses in one report, but a related publication with the same identification number (14N201402215912) had 40 more participants (Sharifipour 2015a). The remaining 33 studies were judged to be at low risk of bias for this domain (Abbaspoor 2014; Ahn 2017; Alves 2015; Binder 2011; Davies 1982; Degirmen 2010; Dong 2015; Ebneshahidi 2008; Farzaneh 2019; Gamermann 2015; Hadi 2011; Hanan 2011; Irani 2015; Jaafarpour 2008; Kayman‐Kose 2014; Khooshideh 2017; Melo de Paula 2006; Midilli 2015; Midilli 2016; Najafi 2017; Navarro Nunez 2000; Olapour 2013; Ramezani 2016; Saatsaz 2016; Sen 2010; Sharma 2019; Simonelli 2018; Smith 1986; Solehati 2015; Sousa 2009a; Varghese 2014; Wu 2009; Yang 2019).
Selective reporting
Five studies were categorised as having a high risk of reporting bias because they did not describe in their results the outcomes proposed in their methods (Abbaspoor 2014; Farzaneh 2019; Irani 2015; Ramezani 2016; Sharifipour 2015a). The other 32 studies reported all pre‐specified outcomes and were therefore classified as having low risk of bias (Ahn 2017; Alves 2015; Binder 2011; Bonabi 2018; Davies 1982; Degirmen 2010; Dong 2015; Ebneshahidi 2008; Gamermann 2015; Hadi 2011; Hanan 2011; Hassani 2015; Jaafarpour 2008; Kayman‐Kose 2014; Khooshideh 2017; Lima 2014; Melo de Paula 2006; Midilli 2015; Midilli 2016; Najafi 2017; Navarro Nunez 2000; Olapour 2013; Saatsaz 2016; Sen 2010; Sharma 2019; Simonelli 2018; Smith 1986; Solehati 2015; Sousa 2009a; Varghese 2014; Wu 2009; Yang 2019).
Other potential sources of bias
Six included studies had other potential sources of risk of bias as following: five studies did not describe clearly their use of analgesic medication associated with the intervention and did not provide sufficient details about the control groups (Ahn 2017; Alves 2015; Hadi 2011; Hanan 2011; Hassani 2015), and the trial registration number (14N201402215912) is not in the Iranian Registry of Clinical Trials as stated by trialists on the publications (Sharifipour 2015a). We identified no other potential sources of risk of bias in the other 31 studies (Abbaspoor 2014; Binder 2011; Bonabi 2018; Davies 1982; Degirmen 2010; Dong 2015; Ebneshahidi 2008; Farzaneh 2019; Gamermann 2015; Irani 2015; Jaafarpour 2008; Kayman‐Kose 2014; Khooshideh 2017; Lima 2014; Melo de Paula 2006; Midilli 2015; Midilli 2016; Najafi 2017; Navarro Nunez 2000; Olapour 2013; Ramezani 2016; Saatsaz 2016; Sen 2010; Sharma 2019; Simonelli 2018; Smith 1986; Solehati 2015; Sousa 2009a; Varghese 2014; Wu 2009; Yang 2019).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13
1. Acupuncture or acupressure
1.1 Acupuncture or acupressure versus placebo
Bonabi 2018 compared acupuncture or acupressure versus placebo (the points were touched with the same pattern without pressure) but no data were available for analysis.
1.2 Acupuncture or acupressure versus no treatment
Two studies compared acupuncture or acupressure (Korean hand acupressure discs and auricular acupuncture) versus no treatment, but only Ahn 2017 had available data for analysis and reported results as mean ± standard deviation (SD) (Ahn 2017; Yang 2019).
We could not use data from Yang 2019 because we are still waiting a translation of the Chinese article. The data in the abstract are available only in percentages.
Primary outcomes
Pain
It is uncertain whether acupuncture or acupressure has any effect on pain compared with no treatment because the certainty of evidence is very low (mean difference (MD) ‐0.82, 95% confidence interval (CI) ‐1.74 to 0.10; 1 study; 50 participants; very low‐certainty evidence; Table 1; Analysis 1.1)
1.1. Analysis.

Comparison 1: Acupuncture versus no treatment, Outcome 1: Abdominal pain up to 24 hours (VAS)
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation)
It is uncertain whether acupuncture or acupressure has any effect on back pain because the certainty of evidence is very low (MD ‐0.88, 95% CI ‐1.94 to 0.18; 1 study; 50 participants; very low‐certainty evidence; Table 1; Analysis 1.2)
1.2. Analysis.

Comparison 1: Acupuncture versus no treatment, Outcome 2: Back pain up to 24 hours (VAS)
Secondary outcomes
Vital signs
There are no available data for this outcome.
Rescue analgesic requirement
One study (Ahn 2017) reported rescue analgesic requirement but the authors did not describe clearly the unit of this analysis so we could not use the data in analysis.
Pain at six weeks after discharge
There are no available data for this outcome.
Women's satisfaction
There are no available data for this outcome.
Breastfeeding at discharge
There are no available data for this outcome.
Interaction with the baby
There are no available data for this outcome.
Walking at discharge
There are no available data for this outcome.
Length of hospitalisation
There are no available data for this outcome.
1.3 Acupuncture or acupressure plus analgesia versus placebo plus analgesia
See Table 2.
Primary outcomes
Pain measured up to 12, 24 and 48 hours, by a validated instrument or scoring system (such as the VAS)
Ramezani 2016 reported pain at two hours after the intervention (acupressure) by VAS (0 to 10 cm). It is uncertain whether acupressure plus analgesia has any effect on pain because the certainty of evidence is very low (MD 0.01, 95% CI ‐0.74 to 0.76; 1 study; 108 participants; very low‐certainty evidence) (Analysis 2.1).
2.1. Analysis.

Comparison 2: Acupuncture plus analgesia versus placebo plus analgesia, Outcome 1: Pain
Gamermann 2015 did not find any difference between the experimental (acupuncture plus analgesia) and control group (sham acupuncture plus analgesia) for resting or motion pain evaluated by VAS at 24 hours and 48 hours after CS. See Table 14. Although Gamermann 2015 planned to report mean and SD, they reported data as a position value and an interval without sufficient information to confirm if it corresponds to a CI, quartiles values or minimum and maximum values. Additional clarification was sought but not obtained for inclusion in the meta‐analysis.
1. Acupuncture group (A) versus sham acupuncture group (S) for post‐CS pain and satisfaction.
| group A | group S | P value1 | |
| 24‐hour post‐CS | |||
| Morphine (mg) | 24 | 33 | 0.49 |
| Resting VAS | 3 (0.5 to 5) | 0 (0 to 4.5) | 0.71 |
| Motion VAS | 5 (3 to 8) | 4 (1.5 to 5) | 0.67 |
| Satisfaction (0–10) | 10 (9; 10) | 10 (9 to 10) | 0.62 |
| 48‐hour post‐CS | |||
| Morphine (mg) | 60 | 63 | 0.91 |
| Resting VAS | 2 (0; 3.5) | 0 (0 to 1.5) | 0.029 |
| Motion VAS | 5 (0.5–6.5) | 4 (1.5 to 5) | 0.67 |
| Satisfaction (0–10) | 10 (9.5–10) | 10 (9 to 10) | 0.79 |
Adapted from Gamermann 2015.
CS: caesarean section mg: milligrams VAS: visual analogue scale
1: Mann‐Whitney test
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation)
There are no available data for this outcome.
Secondary outcomes
Vital signs
There are no available data for this outcome.
Rescue analgesic requirement
Gamermann 2015 did not find any difference between the experimental (acupuncture plus analgesia) and control group (sham acupuncture plus analgesia) for Rescue analgesic requirement at 24 hours and 48 hours after CS but they did not provide data for meta‐analysis (see Table 14).
Ramezani 2016 reported the rescue analgesic requirement the mean (SD) number of analgesics consumed (MD 0.00, 95% CI ‐0.16 to 0.16; 108 women; studies = 1; Analysis 2.2)
2.2. Analysis.

Comparison 2: Acupuncture plus analgesia versus placebo plus analgesia, Outcome 2: Rescue analgesic requirement (number of analgesic)
Pain at six weeks after discharge
There are no available data for this outcome.
Women's satisfaction
Gamermann 2015 did not find a difference between experimental (acupuncture plus analgesia) and control group (sham acupuncture plus analgesia) for participant satisfaction evaluated by a scale (0 to 10 points) at 24 hours and 48 hours after CS. See Table 14.
Ramezani 2016 planned to measure participant satisfaction but they did not report this outcome in the final report.
Breastfeeding at discharge
There are no available data for this outcome.
Interaction with the baby
There are no available data for this outcome.
Walking at discharge
There are no available data for this outcome.
Length of hospitalisation
There are no available data for this outcome.
1.4 Acupuncture or acupressure plus analgesia versus analgesia
See Table 3.
Primary outcomes
Pain measured up to 12, 24 and 48 hours, by a validated instrument or scoring system (such as the VAS)
Two studies (Dong 2015; Wu 2009) showed acupuncture plus analgesia may reduce pain slightly when compared to analgesia at 12 hours (standardised mean difference (SMD) ‐0.28, 95% CI ‐0.64 to 0.07; 2 studies; 130 participants; low‐certainty evidence); may reduce pain at 24 hours (SMD ‐0.63, 95% CI ‐0.99 to ‐0.26; 2 studies; 130 participants; low‐certainty evidence) (Analysis 3.1), and it is uncertain if acupuncture plus analgesia has any effect on pain at 48 hours because the certainty of evidence is very low (MD ‐0.06, 95% CI ‐0.48 to 0.36; 70 participants; 1 study; very low‐certainty evidence) (Dong 2015) (Analysis 3.2).
3.1. Analysis.

Comparison 3: Acupuncture plus analgesia versus analgesia, Outcome 1: Pain ‐ up to 12 and 24 hours
3.2. Analysis.

Comparison 3: Acupuncture plus analgesia versus analgesia, Outcome 2: Pain ‐ up to 48 hours
Yang 2019 planned to report pain after CS but the data are not available because the full text is in Chinese.
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation)
It is uncertain whether acupuncture plus analgesia has any effect on pain because the certainty of evidence is very low (risk ratio ((RR) 0.50, 95% CI 0.08 to 3.29; 1 study; 60 participants; very low‐certainty evidence) (Wu 2009) (Analysis 3.3).
3.3. Analysis.

Comparison 3: Acupuncture plus analgesia versus analgesia, Outcome 3: Adverse effects (pruritus)
Secondary outcomes
Vital signs
There are no available data for this outcome.
Rescue analgesic requirement
Wu 2009 reported rescue analgesic requirement at 24 hours after intervention in two different forms: 1) the mean (SD) dose of analgesics taken and 2) the mean (SD) number of analgesics consumed. Acupuncture plus analgesia may slightly decrease rescue analgesic requirement at 24 hours after intervention, measured with dose of morphine (MD ‐5.00 mg, 95% CI ‐7.67 to ‐2.34; 1 study; 60 participants; low‐certainty evidence) (Analysis 3.4) and by the number of analgesics consumed (MD ‐20.45, 95% CI ‐30.92 to ‐9.98; 1 study; 60 participants) (Analysis 3.5).
3.4. Analysis.

Comparison 3: Acupuncture plus analgesia versus analgesia, Outcome 4: Rescue analgesic requirement (cumulative dose)
3.5. Analysis.

Comparison 3: Acupuncture plus analgesia versus analgesia, Outcome 5: Rescue analgesic requirement (number of analgesic)
Pain at six weeks after discharge
There are no available data for this outcome.
Women's satisfaction
There are no available data for this outcome.
Breastfeeding at discharge
There are no available data for this outcome.
Interaction with the baby
There are no available data for this outcome.
Walking at discharge
There are no available data for this outcome.
Length of hospitalisation
Yang 2019 planned to report average hospitalisation days after CS but the data are not available because the full text is in Chinese.
2. Aromatherapy
2.1 Aromatherapy versus placebo
There are no included studies for this comparison.
2.2 Aromatherapy versus no treatment
There are no included studies for this comparison.
2.3 Aromatherapy plus analgesia versus placebo plus analgesia
See Table 4.
Primary outcomes
Pain measured up to 12 and 24 hours, by a validated instrument or scoring system (such as the VAS)
The effect of aromatherapy in post‐CS pain was evaluated in four studies (Hadi 2011; Najafi 2017; Olapour 2013; Sharifipour 2015a), but not all of the studies reported data we could use in our analysis.
Aromatherapy plus analgesia may slightly decrease pain at 12 hours compared with placebo plus analgesia (MD ‐2.63 VAS, 95% CI ‐3.48 to ‐1.77; 3 studies; 360 participants; low‐certainty evidence) and at 24 hours (MD ‐3.38 VAS, 95% CI ‐3.85 to ‐2.91; 1 study; 200 participants; low‐certainty evidence) (Hadi 2011; Najafi 2017; Sharifipour 2015a) (Analysis 4.1).
4.1. Analysis.

Comparison 4: Aromatherapy plus analgesia versus placebo plus analgesia, Outcome 1: Pain
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation, nausea and vomiting)
Sharifipour 2015a measured anxiety at 12 hours after intervention, assessed by State‐Trait Anxiety Inventory (STAI) which ranges from 20 to 80 points and a higher score means more severe anxiety. It is uncertain is aromatherapy plus analgesia has any effect on anxiety compared with placebo plus analgesia (MD ‐19.87, 95% CI ‐22.11 to ‐17.63; 1 study; 80 participants; very low‐certainty evidence) (Analysis 4.2).
4.2. Analysis.

Comparison 4: Aromatherapy plus analgesia versus placebo plus analgesia, Outcome 2: Anxiety
There are no other available data regarding adverse events.
Secondary outcomes
Vital signs
It is uncertain if there is any difference between aromatherapy plus analgesia and control groups regarding heart rate (MD 0.60 beats per minute (bpm), 95% CI ‐1.60 to 2.80; 1 study; 80 participants; very low‐certainty evidence) (Analysis 4.3) and systolic blood pressure (MD ‐3.62 mm Hg, 95% CI ‐7.71 to 0.47; 1 study; 80 participants; very low‐certainty evidence) (Analysis 4.5) (Sharifipour 2015a).
4.3. Analysis.

Comparison 4: Aromatherapy plus analgesia versus placebo plus analgesia, Outcome 3: Heart rate (bpm)
4.5. Analysis.

Comparison 4: Aromatherapy plus analgesia versus placebo plus analgesia, Outcome 5: Systolic blood pressure (mm Hg)
There was lower diastolic blood pressure in the experimental group than in the control group (MD ‐3.62 mm Hg, 95% CI ‐6.97 to ‐0.27; 1 study; 80 participants (Analysis 4.4).
4.4. Analysis.

Comparison 4: Aromatherapy plus analgesia versus placebo plus analgesia, Outcome 4: Diastolic blood pressure (mm Hg)
None of the other included studies provided data for this outcome.
Rescue analgesic requirement
Olapour 2013, Najafi 2017 and Sharifipour 2015a reported the frequency of additional analgesic requirement.
It is uncertain whether aromatherapy plus analgesia decreases rescue analgesic requirement compared with placebo plus analgesia because the certainty of the evidence is very low (RR 0.69, 95% CI 0.19 to 2.49; 3 studies; 220 participants; I2 = 99%) (Analysis 4.6).
4.6. Analysis.

Comparison 4: Aromatherapy plus analgesia versus placebo plus analgesia, Outcome 6: Rescue analgesic requirement
Since Sharifipour 2015a reported that all participants required analgesic supplementation, there was substantial heterogeneity of results. Therefore, we carried out another analysis including only Najafi 2017 and Olapour 2013 data, which found less analgesic requirement in the aromatherapy plus analgesia group (RR 0.58, 95% CI 0.45 to 0.75; 2 studies; 140 participants; I2 = 0%).
Pain at six weeks after discharge
There are no available data for this outcome.
Women's satisfaction
Only one study (Olapour 2013) assessed the level of satisfaction of mothers, noting that 90% of them declared satisfaction with the use of aromatherapy, while in the placebo group, the satisfaction was 50%. Aromatherapy plus analgesia may slightly increase women's satisfaction compared with aromatherapy plus analgesia (RR 1.80, 95% CI 1.23 to 2.62; 1 study; 60 participants) (Analysis 4.7).
4.7. Analysis.

Comparison 4: Aromatherapy plus analgesia versus placebo plus analgesia, Outcome 7: Satisfaction
Breastfeeding at discharge
There are no available data for this outcome.
Interaction with the baby
There are no available data for this outcome.
Walking at discharge
There are no available data for this outcome.
Length of hospitalisation
There are no available data for this outcome.
2.4 Aromatherapy plus analgesia versus analgesia
There are no included studies for this comparison.
3. Electromagnetic therapy
3.1 Electromagnetic therapy versus placebo
There are no included studies for this comparison.
3.2 Electromagnetic therapy versus no treatment
There are no included studies for this comparison.
3.3 Electromagnetic therapy plus analgesia versus placebo plus analgesia
See Table 5.
Primary outcomes
Pain measured up to 12, 24 and 48 hours, by a validated instrument or scoring system (such as the VAS)
Khooshideh 2017 assessed pain by VAS with scale from 0 to 100 mm, where higher scores mean more severe pain.
Electromagnetic therapy plus analgesia may reduce pain compared with placebo plus analgesia at 12 hours (MD ‐8.00, 95% CI ‐11.65 to ‐4.35; 1 study; 72 participants; low‐certainty evidence), and at 24 (MD ‐13.00, 95% CI ‐17.13 to ‐8.87; 1 study; 72 participants; low‐certainty evidence) and 48 hours (MD ‐8.00, 95% CI ‐11.52 to ‐4.48; 1 study; 72 participants) after the intervention (Analysis 5.1).
5.1. Analysis.

Comparison 5: Electromagnetic therapy plus analgesia versus placebo plus analgesia, Outcome 1: Pain (VAS)
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation, nausea and vomiting)
There are no available data for this outcome.
Secondary outcomes
Vital signs
There are no available data for this outcome.
Rescue analgesic requirement
Khooshideh 2017 measured rescue analgesic requirement by mean suppository counts. Electromagnetic therapy plus analgesia may decrease rescue analgesic requirement compared with placebo plus analgesia at 24 hours (MD ‐1.50, 95% CI ‐1.95 to ‐1.05; 1 study; 72 participants; low‐certainty evidence) and seven days (MD ‐2.00, 95% CI ‐2.43 to ‐1.57; 1 study; 72 participants) (Analysis 5.2).
5.2. Analysis.

Comparison 5: Electromagnetic therapy plus analgesia versus placebo plus analgesia, Outcome 2: Rescue analgesic requirement
Pain at six weeks after discharge
There are no available data for this outcome.
Women's satisfaction
Khooshideh 2017 assessed the level of satisfaction of mothers, noting that 50% of them declared high satisfaction with the use of electromagnetic therapy, while in the placebo group, high satisfaction was 25% (RR 2.00, 95% CI 1.04 to 3.84; 1 study; 72 participants) (Analysis 5.3).
5.3. Analysis.

Comparison 5: Electromagnetic therapy plus analgesia versus placebo plus analgesia, Outcome 3: Satisfaction
Breastfeeding at discharge
There are no available data for this outcome.
Interaction with the baby
There are no available data for this outcome.
Walking at discharge
There are no available data for this outcome.
Length of hospitalisation
There are no available data for this outcome.
3.4 Electromagnetic therapy plus analgesia versus analgesia
There are no included studies for this comparison.
4. Massage
4.1 Massage versus placebo
There are no included studies for this comparison.
4.2 Massage versus no treatment
There are no included studies for this comparison.
4.3 Massage plus analgesia versus placebo plus analgesia
There are no included studies for this comparison.
4.4 Massage plus analgesia versus analgesia
See Table 6.
Primary outcomes
Pain measured up to 12, 24, 48 and 120 hours, by a validated instrument or scoring system (such as the VAS)
Nine included studies compared massage plus analgesia versus analgesia for pain after CS Abbaspoor 2014; Degirmen 2010; Hanan 2011; Hassani 2015; Irani 2015; Saatsaz 2016; Sharma 2019; Simonelli 2018; Varghese 2014).
We are uncertain if massage plus analgesia has any effect on pain compared with analgesia alone at 12 hours (MD ‐2.03, 95% CI ‐2.48 to ‐1.59; 6 studies; 651 participants; I2 = 86%; very low‐certainty evidence), 24 hours (MD ‐1.51, 95% CI ‐1.78 to ‐1.24; 3 studies; 230 participants;I2 = 0%; very low‐certainty evidence), 48 hours (MD ‐1.86, 95% CI ‐2.18 to ‐1.54; 2 studies; 80 participants; I2 = 0%) and 120 hours (MD ‐2.09, 95% CI ‐2.38 to ‐1.79; 2 studies; 120 participants; I2 = 0%) (Analysis 6.1).
6.1. Analysis.

Comparison 6: Massage (foot and hand) plus analgesia versus analgesia, Outcome 1: Pain
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation, nausea and vomiting)
Two included studies reported anxiety assessed by different scores with the use of massage therapy at 90 minutes after the intervention (Saatsaz 2016) and at 60 minutes after the intervention (Simonelli 2018). Saatsaz 2016 used the State‐Trait Anxiety Inventory (STAI), scale from 20 to 80, Simonelli 2018 used a VAS, scale from 0 to 10, and both considered higher values as more severe anxiety.
It is uncertain if massage plus analgesia has any effect on anxiety compared with analgesia alone at 90 minutes (SMD ‐0.45, 95% CI ‐0.70 to ‐0.19; 2 studies; 266 participants; I2 = 82%; very low‐certainty evidence) (Analysis 6.2; SMD between 0.2 and 0.49 indicates a small effect).
6.2. Analysis.

Comparison 6: Massage (foot and hand) plus analgesia versus analgesia, Outcome 2: Adverse effects (anxiety)
Secondary outcomes
Vital signs
Three included studies evaluated the effect of massage on the vital signs of the women (Degirmen 2010; Hassani 2015; Saatsaz 2016). However, Hassani 2015 evaluated vital signs by the difference between the follow‐up assessment and the baseline data. Therefore, we could not use Hassani 2015 data in meta‐analysis.
It is uncertain if there is any difference between massage plus analgesia compared with analgesia in terms of heart rate (MD ‐1.78 bpm, 95% CI ‐4.28 to 0.72; 2 studies; 231 participants; I2 = 0%; very low‐certainty evidence) (Analysis 6.3) or respiratory rate(MD ‐0.52 breaths per minute (brpm), 95% CI ‐0.91 to ‐0.12; 2 studies; 231 participants; I2 = 74%; very low‐certainty evidence) (Analysis 6.4).
6.3. Analysis.

Comparison 6: Massage (foot and hand) plus analgesia versus analgesia, Outcome 3: Heart rate
6.4. Analysis.

Comparison 6: Massage (foot and hand) plus analgesia versus analgesia, Outcome 4: Respiratory rate (breaths per minute)
It is unclear if there is any difference between massage plus analgesia compared with analgesia in terms of systolic blood pressure (MD ‐2.10 mm Hg, 95% CI ‐4.83 to 0.64; 2 studies; 231 participants; I2 = 33%;) (Analysis 6.5) and diastolic blood pressure (MD ‐0.10 mm Hg, 95% CI ‐2.09 to 1.89; 2 studies; 231 participants; I2 = 53%) (Analysis 6.6).
6.5. Analysis.

Comparison 6: Massage (foot and hand) plus analgesia versus analgesia, Outcome 5: Systolic blood pressure
6.6. Analysis.

Comparison 6: Massage (foot and hand) plus analgesia versus analgesia, Outcome 6: Diastolic blood pressure
Rescue analgesic requirement
Two included studies reported dichotomous data of analgesic requirement after massage for post‐CS women (Abbaspoor 2014; Saatsaz 2016), but Saatsaz 2016 reported no events. There was a lower number of participants in the massage group who required additional diclofenac compared to placebo group: 15% versus 70%. Massage plus analgesia may slightly educe rescue analgesic requirement compared with analgesia (RR 0.19, 95% CI 0.09 to 0.41; 1 study; 80 participants; very low‐certainty evidence) (Analysis 6.7).
6.7. Analysis.

Comparison 6: Massage (foot and hand) plus analgesia versus analgesia, Outcome 7: Rescue analgesic requirement
Simonelli 2018 reported that 'there were fewer requests for opioids on postoperative days 1 and 2 among participants in the massage group than among those in the other study groups'. They also reported that there was no significant differences in non‐steroidal anti‐inflammatories (NSAIDs) use among the groups, but this study did not provide any data that we could use in analysis.
Pain at six weeks after discharge
There are no available data for this outcome.
Women's satisfaction
There are no available data for this outcome.
Breastfeeding at discharge
Two included studies reported the number of women breastfeeding at discharge (Hanan 2011; Saatsaz 2016). Fewer women were breastfeeding in the massage plus analgesia group than in the analgesia only group (RR 0.65, 95% CI 0.44 to 0.95; participants = 306; studies = 2; I2 = 15%) (Analysis 6.8).
6.8. Analysis.

Comparison 6: Massage (foot and hand) plus analgesia versus analgesia, Outcome 8: Breastfeeding
Interaction with the baby
There are no available data for this outcome.
Walking at discharge
There are no available data for this outcome.
Length of hospitalisation
There are no available data for this outcome.
5. Music therapy
5.1 Music therapy versus placebo
There are no included studies for this comparison.
5.2 Music therapy versus no treatment
There are no included studies for this comparison.
5.3 Music therapy plus analgesia versus placebo plus analgesia
See Table 7.
Primary outcomes
Pain measured up to one, 24 and 48 hours, by a validated instrument or scoring system (such as the VAS)
Ebneshahidi 2008 and Farzaneh 2019 measured the effect of music therapy on post‐caesarean pain by VAS (Ebneshahidi 2008 scale: 0 to 100 and Farzaneh 2019 scale: 0 to 10; in both, higher values mean more severe pain). Music therapy plus analgesia may result in a large reduction in pain at one hour compared with placebo plus analgesia (SMD ‐0.84, 95% CI ‐1.23 to ‐0.46; 115 participants; 2 studies; low‐certainty evidence; SMD 0.8 or greater indicates a large effect ) (Analysis 7.1). Music therapy plus analgesia may also decrease pain, compared with placebo plus analgesia, at 24 hours (MD ‐1.79, 95% CI ‐2.67 to ‐0.91; 38 participants; 1 study; low‐certainty evidence) and at 48 hours after the intervention (MD ‐1.21, 95% CI ‐1.67 to ‐0.75; 38 participants; 1 study) (Analysis 7.2).
7.1. Analysis.

Comparison 7: Music plus analgesia versus placebo plus analgesia, Outcome 1: Pain ‐ up to 1 hour
7.2. Analysis.

Comparison 7: Music plus analgesia versus placebo plus analgesia, Outcome 2: Pain ‐ up to 24 and 48 hours
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation)
Ebneshahidi 2008 measured anxiety levels by VAS (0 to 100 mm), where a higher level means more severe pain. It is uncertain whether music therapy plus analgesia has any effect on anxiety compared with placebo plus analgesia because the certainty of the evidence is very low (MD ‐2.00, 95% CI ‐7.83 to 3.83; 1 study; 77 participants) (Analysis 7.3).
7.3. Analysis.

Comparison 7: Music plus analgesia versus placebo plus analgesia, Outcome 3: Adverse effects (anxiety)
Secondary outcomes
Vital signs
Ebneshahidi 2008 measured vital signs 30 minutes after the intervention. It is uncertain whether music therapy plus analgesia has any effect on heart rate compared with placebo plus analgesia because the certainty of evidence is very low (MD 4.00 bpm, 95% CI ‐2.48 to 10.48; 1 study; 77 participants; very low‐certainty evidence) (Analysis 7.4), systolic blood pressure (MD ‐3.00 mm Hg, 95% CI ‐10.38 to 4.38; 1 study; 77 participants) ( Analysis 7.5).
7.4. Analysis.

Comparison 7: Music plus analgesia versus placebo plus analgesia, Outcome 4: Heart hate (bpm)
7.5. Analysis.

Comparison 7: Music plus analgesia versus placebo plus analgesia, Outcome 5: Systolic blood pressure (mm Hg)
It is unclear if there is any difference between music therapy plus analgesia and placebo plus analgesia in terms of diastolic blood pressure (MD ‐2.00 mm Hg, 95% CI ‐7.59 to 3.59; 1 study; 77 participants) (Analysis 7.6).
7.6. Analysis.

Comparison 7: Music plus analgesia versus placebo plus analgesia, Outcome 6: Diastolic blood pressure (mm Hg)
Rescue analgesic requirement
Ebneshahidi 2008 measured rescue analgesic requirement by dose of morphine (mg) 30 minutes after the intervention. Music therapy plus analgesia may decrease rescue analgesic requirement compared with placebo plus analgesia (MD ‐0.90, 95% CI ‐1.70 to ‐0.10; 1 study; 77 participants; low‐certainty evidence) (Analysis 7.7).
7.7. Analysis.

Comparison 7: Music plus analgesia versus placebo plus analgesia, Outcome 7: Rescue analgesic requirement (dose)
Pain at six weeks after discharge
There are no available data for this outcome.
Women's satisfaction
There are no available data for this outcome.
Breastfeeding at discharge
There are no available data for this outcome.
Interaction with the baby
There are no available data for this outcome.
Walking at discharge
There are no available data for this outcome.
Length of hospitalisation
There are no available data for this outcome.
5.4 Music therapy plus analgesia versus analgesia
See Table 8.
Primary outcomes
Pain measured up to one, 24 and 48 hours, by a validated instrument or scoring system (such as the VAS)
Two studies compared the effects of music therapy with standard analgesic treatment in post‐caesarean pain (Farzaneh 2019; Sen 2010), but Sen 2010 did not provide data for meta‐analysis because they presented it only in graphics. Our attempts to contact the trialists to obtain the missing data were unsuccessful.
Music therapy plus analgesia may decrease pain compared with analgesia at one hour (MD ‐2.11, 95% CI ‐3.11 to ‐1.10; 1 study; 38 participants; low‐certainty evidence), 24 hours (MD ‐2.69, 95% CI ‐3.67 to ‐1.70; 1 study; 38 participants; low‐certainty evidence) and at 48 hours after the intervention (MD ‐1.79, 95% CI ‐2.40 to ‐1.18; 1 study; 38 participants) (Farzaneh 2019) (Analysis 8.1).
8.1. Analysis.

Comparison 8: Music plus analgesia versus analgesia, Outcome 1: Pain
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation)
Sen 2010 reported a similar number of nausea and vomiting between the groups at 24 hours after the intervention (experimental group: 4/35, control group: 6/35). Since nausea and vomiting are not an adverse effect of interest for this review, we did not input this in the meta‐analysis. There are no other available data for this outcome.
Secondary outcomes
Vital signs
Sen 2010 reported that there was no difference between experimental and control groups for HR, mean arterial blood pressure, O2 saturation and respiratory ratio with P > 0.05, but the trial authors did not provided the numerical details for meta‐analysis.
Rescue analgesic requirement
Music therapy plus analgesia may decrease rescue analgesic requirement compared with analgesia for both cumulative consumption of tramadol (total amount in mg) (MD ‐45.14, 95% CI ‐86.77 to ‐3.51; 1 study; 70 participants; low‐certainty evidence) and with diclofenac (total amount in mg) (MD ‐21.43, 95% CI ‐41.65 to ‐1.21; 1 study; 70 participants; low‐certainty evidence) at 24 hours after the intervention (Sen 2010) (Analysis 8.2).
8.2. Analysis.

Comparison 8: Music plus analgesia versus analgesia, Outcome 2: Rescue analgesic requirement (cumulative dose)
Women's satisfaction
Sen 2010 measured patient satisfaction by VAS (0 to 10 cm) at 24 hours after the intervention, where higher scores mean more satisfaction. There was greater satisfaction in the music therapy plus analgesia compared with the analgesia group (MD 0.63, 95% CI 0.20 to 1.06; 1 study; 70 participants; ) (Analysis 8.3).
8.3. Analysis.

Comparison 8: Music plus analgesia versus analgesia, Outcome 3: Satisfaction
Pain at six weeks after discharge
There are no available data for this outcome.
Breastfeeding at discharge
There are no available data for this outcome.
Interaction with the baby
There are no available data for this outcome.
Walking at discharge
There are no available data for this outcome.
Length of hospitalisation
There are no available data for this outcome.
6. Reiki
6.1 Reiki versus placebo
There are no included studies for this comparison.
6.2 Reiki versus no treatment
There are no included studies for this comparison.
6.3 Reiki plus analgesia versus placebo plus analgesia
Primary outcomes
Pain measured up to 24 and 48 hours, by a validated instrument or scoring system (such as the VAS)
Midilli 2016 evaluated the use of Reiki compared to analgesia only and to sham Reiki (placebo group) in the relief of post‐caesarean pain, but we could not pool their results because its individual groups results were not described. The authors reported that quote: "Mean VAS measurement values for the Reiki group were significantly lower than those of the control and sham Reiki groups".
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation)
Midilli 2016 did not report any adverse effects of interest for this review.
Secondary outcomes
Vital signs
Midilli 2016 assessed vital signs (mean breathing rates, pulse rates, and systolic and diastolic blood pressure measurement) but did not provide the experimental and control groups data separately for analysis.
Rescue analgesic requirement
Midilli 2016 assessed the number of analgesics taken by patients but separate data by group are not available. The trial authors reported that quote: "the difference between the number of analgesics taken by patients in the Reiki group, the sham Reiki group, and the control group after the application on the first day until the next application day was found to be statistically insignificant (P = 0.58)".
Pain at six weeks after discharge
There are no available data for this outcome
Women's satisfaction
There are no available data for this outcome.
Breastfeeding at discharge
There are no available data for this outcome.
Interaction with the baby
There are no available data for this outcome.
Walking at discharge
There are no available data for this outcome.
Length of hospitalisation
There are no available data for this outcome.
6.4 Reiki plus analgesia versus analgesia
See Table 9.
Primary outcomes
Pain measured up to one, 24 and 48 hours, by a validated instrument or scoring system (such as the VAS)
Midilli 2015 and Midilli 2016 evaluated the use of Reiki compared to analgesia in the relief of post‐caesarean pain, but we could not pool their results because one study did not describe results separately by groups (Midilli 2016).
It is uncertain whether Reiki plus analgesia decreases pain compared with analgesia at one hour after the intervention in 24 hours after CS (MD ‐2.20, 95% CI ‐2.87 to ‐1.53; 1 study; 90 participants; very low‐certainty evidence) and at 24 hours, in 48 hours after CS (MD ‐2.52, 95% CI ‐3.07 to ‐1.97; 1 study; 90 participants; very low‐certainty evidence) (Midilli 2015) (Analysis 9.1).
9.1. Analysis.

Comparison 9: Reiki plus analgesia versus analgesia, Outcome 1: Pain
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation)
Midilli 2015 measured anxiety with the State‐Trait Anxiety Inventory (STAI), which is a 20‐question test that allows individuals to respond on a 4‐point Likert‐type scale (20 to 80 points; higher number means more anxiety). It is uncertain whether Reiki plus analgesia decreases anxiety, compared with analgesia, at 24 hours (MD ‐8.20, 95% CI ‐10.67 to ‐5.73; 1 study; 90 participants; very low‐certainty evidence) and 48 hours (MD ‐9.00, 95% CI ‐11.12 to ‐6.88; 1 study; 90 participants) after CS (Analysis 9.2).
9.2. Analysis.

Comparison 9: Reiki plus analgesia versus analgesia, Outcome 2: Adverse effects (anxiety)
Midilli 2016 did not report any adverse effects of interest for this review.
Secondary outcomes
Vital signs
It is uncertain whether Reiki plus analgesia, compared with analgesia, has any effect on heart rate at one hour after the intervention, 48 hours after CS (MD ‐3.58 bpm, 95% CI ‐8.26 to 1.10; 1 study; 90 participants; very low‐certainty evidence) (Analysis 9.3). The same study also measured heart rate at one after the intervention, 24 hours after CS and there was no clear difference between the groups (MD ‐4.49 bpm, 95% CI ‐9.85 to 0.87; 1 study; 90 participants) (Analysis 9.3)
9.3. Analysis.

Comparison 9: Reiki plus analgesia versus analgesia, Outcome 3: Heart rate
It is uncertain whether Reiki plus analgesia, compared with analgesia, has any effect on respiratory rate at one hour after the intervention, 48 hours after CS (MD ‐0.68 brpm, 95% CI ‐1.27 to ‐0.09; 1 study; 90 participants; very low‐certainty evidence) (Analysis 9.4). The same study also measured heart rate at one after the intervention, 24 hours after CS and there was no clear difference between the groups (MD ‐0.74 brpm, 95% CI ‐1.32 to ‐0.16; 1 study; 90 participants; Analysis 9.4).
9.4. Analysis.

Comparison 9: Reiki plus analgesia versus analgesia, Outcome 4: Respiratory rate
It is unclear if there is any difference between Reiki plus analgesia, compared with analgesia, in terms of systolic blood pressure at hour after the intervention, 24 hours after CS (MD ‐2.18, 95% CI ‐7.45 to 3.09; 1 study; 90 participants) or at one hour after the intervention, 48 hours after CS (MD ‐1.71, 95% CI ‐6.21 to 2.79; 1 study; 90 participants) (Analysis 9.5).
9.5. Analysis.

Comparison 9: Reiki plus analgesia versus analgesia, Outcome 5: Systolic blood pressure (mm Hg)
It is unclear if there is any difference between Reiki plus analgesia, compared with analgesia, in terms of diastolic blood pressure at hour after the intervention, 24 hours after CS (MD ‐0.62, 95% CI ‐5.09 to 3.85; 1 study; 90 participants) or at one hour after the intervention, 48 hours after CS (MD ‐0.58, 95% CI ‐4.10 to 2.94; 1 study; 90 participants) (Analysis 9.6).
9.6. Analysis.

Comparison 9: Reiki plus analgesia versus analgesia, Outcome 6: Diastolic blood pressure (mm Hg)
Midilli 2016 assessed vital signs (mean breathing rates, pulse rates, and systolic and diastolic blood pressure measurement) but did not provide the experimental and control groups data separately for analysis.
Rescue analgesic requirement
Midilli 2015 evaluated the number of analgesics required after Reiki application, but reported data as median and interquartile range. Therefore, no meta‐analysis was possible.
Midilli 2016 assessed the number of analgesic taken by patients but separately data by group are not available. The trial authors reported that quote:"the difference between the number of analgesics taken by patients in the Reiki group, the sham Reiki group, and the control group after the application on the first day until the next application day was found to be statistically insignificant (P = 0.58)".
Pain at six weeks after discharge
There are no available data for this outcome.
Women's satisfaction
There are no available data for this outcome.
Breastfeeding at discharge
There are no available data for this outcome.
Interaction with the baby
There are no available data for this outcome.
Walking at discharge
There are no available data for this outcome.
Length of hospitalisation
There are no available data for this outcome.
7. Relaxation
7.1 Relaxation versus placebo
There are not included studies for this comparison.
7.2 Relaxation versus no standard care
See Table 10.
Primary outcomes
Pain measured up to 12, 24, 48 and 84 hours, by a validated instrument or scoring system (such as the VAS)
Solehati 2015 assessed the effect of Benson relaxation compared to standard care on post‐CS pain by VAS, scale from 0 to 10, where higher values mean more severe pain. It is uncertain whether relaxation, compared with standard care, has any effect on pain at 12 hours after the intervention (MD ‐0.04 VAS, 95% CI ‐0.62 to 0.54; 1 study; 60 participants; very low‐certainty evidence). Relaxation, compared with standard care, may reduce pain at 24 hours compared with standard care(MD ‐0.53, 95% CI ‐1.05 to ‐0.01; 1 study; 60 participants; low‐certainty evidence), at 48 hours (MD ‐1.16, 95% CI ‐1.62 to ‐0.70; 1 study; 60 participants), and at 84 hours after the intervention (MD ‐0.88 VAS, 95% CI ‐1.31 to ‐0.45; 1 study; 60 participants) (Analysis 10.1).
10.1. Analysis.

Comparison 10: Relaxation versus standard care, Outcome 1: Pain
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation)
There are no available data for this outcome.
Secondary outcomes
No data are available for any of our pre‐specified secondary outcomes.
7.3 Relaxation plus analgesia versus placebo plus analgesia
There are no included studies for this comparison.
7.4 Relaxation plus analgesia versus analgesia
There are no included studies for this comparison.
8. TENS
8.1 TENS versus placebo
There are no included studies for this comparison.
8.2 TENS versus no treatment
See Table 11.
Primary outcomes
Pain measured up to one and two hours, by a validated instrument or scoring system (such as the VAS)
Alves 2015 and Sousa 2009a compared TENS versus no treatment (usual care) for the treatment of post‐CS pain. Alves 2015 planned to evaluate pain in mean but reported the data of interest in median and interquartile range. Therefore, Alves 2015 data are not available for meta‐analysis.
TENS, compared with no treatment, may reduce pain at one hour (MD ‐2.26, 95% CI ‐3.35 to ‐1.17; 1 study; 40 participants; low‐certainty evidence) and two hours after the intervention (MD ‐2.55, 95% CI ‐3.64 to ‐1.46; 1 study; 40 participants) when analysed by a numerical analogue scale (NAS) where 0 represents no pain and 10 represents the highest level of pain (Sousa 2009a) (Analysis 11.1).
11.1. Analysis.

Comparison 11: TENS versus no treatment, Outcome 1: Pain (NAS)
One study also measured pain using the McGill questionnaire at one hour after the intervention. There was no clear difference between TENS and no treatment (MD ‐1.95, 95% CI ‐3.95 to 0.05; 1 study; 40 participants) (Sousa 2009a) (Analysis 11.2).
11.2. Analysis.

Comparison 11: TENS versus no treatment, Outcome 2: Pain (measured with McGill pain questionnaire: higher score = more pain)
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation)
There are no available data for this outcome.
Secondary outcomes
There are no data available for any of our pre‐specified secondary outcomes.
8.3 TENS plus analgesia versus placebo plus analgesia
See Table 12.
Primary outcomes
Pain measured up to one, six, 12, 24, 48 and 72 hours after the intervention by a validated instrument or scoring system (such as the VAS)
Six studies (Davies 1982Jaafarpour 2008; Kayman‐Kose 2014; Lima 2014; Melo de Paula 2006; Smith 1986) evaluated the effect of TENS plus analgesia, compared to placebo plus analgesia for post‐CS pain. Davies 1982 presented their results only in graphs and could not be used in the meta‐analyses because the SDs were not available. Lima 2014 and Smith 1986 reported pain data only in graphs but we could extract the mean and SD of pain evaluated by a NRS using the graphreader.com and the RevMan calculator. The NRS uses numbers from 0 (means no pain) to 10 of pain (the highest level of pain). Lima 2014 reported data at one hour and Smith 1986 reported data at 24, 48 and 72 hours after the intervention. Jaafarpour 2008, Kayman‐Kose 2014 and Melo de Paula 2006 provided data of pain as VAS up to one, six, 12 and 24 hours after the intervention. Kayman‐Kose 2014, Lima 2014 and Smith 1986 provided data of pain as NRS up to one, 24, 48 and 72 hours after the intervention.
TENS plus analgesia, compared with placebo plus analgesia, may result in a large reduction in pain at one hour after the start of the intervention (SMD ‐1.10 VAS, 95% CI ‐1.37 to ‐0.82; 3 studies; 238 participants; I2 = 0%; low‐certainty evidence) (SMD 0.8 or greater indicates a large effect) (Analysis 12.1), and may also reduce pain at 24 hours after the start of the intervention (MD ‐0.70 VAS, 95% CI ‐0.87 to ‐0.53; participants = 108; studies = 1; low‐certainty evidence) (Analysis 12.2).
12.1. Analysis.

Comparison 12: TENS plus analgesia versus placebo plus analgesia, Outcome 1: Pain ‐ up to one hour (VAS)
12.2. Analysis.

Comparison 12: TENS plus analgesia versus placebo plus analgesia, Outcome 2: Pain ‐ 6, 12, 24 hours
TENS plus analgesia, compared with placebo plus analgesia, may reduce pain at one hour when assessed by NRS (MD ‐2.26, 95% CI ‐2.85 to ‐1.67; 2 studies; 134 participants; I2 = 88%) (Analysis 12.3).
12.3. Analysis.

Comparison 12: TENS plus analgesia versus placebo plus analgesia, Outcome 3: Pain (NRS)
TENS plus analgesia may reduce pain when evaluated by VAS at six hours (MD ‐1.10 VAS, 95% CI ‐1.34 to ‐0.86; 108 participants; 1 study) at 12 hours (MD ‐1.40 VAS, 95% CI ‐1.58 to ‐1.22; 108 participants; 1 study; low‐certainty evidence). TENS plus analgesia may slightly reduce pain when assessed by NRS at 24 hours (MD ‐0.94, 95% CI ‐1.63 to ‐0.24; 1 study; 18 participants; low‐certainty evidence), and at 48 hours (MD ‐0.90, 95% CI ‐1.41 to ‐0.40; 1 study; 18 participants; low‐certainty evidence) after the start of the intervention (Analysis 12.3). It is uncertain if TENS plus analgesia have any effect on pain, when assessed by NRS, at 72 hours after the intervention (MD ‐0.37, 95% CI ‐0.96 to 0.22; 1 study; 18 participants) (Analysis 12.3).
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation)
Four of the studies did not report adverse effects (Davies 1982Jaafarpour 2008; Melo de Paula 2006; Smith 1986). Two studies specifically reported that none of the women had any adverse effects (Kayman‐Kose 2014; Lima 2014).
Secondary outcomes
Vital signs
Jaafarpour 2008 assessed vital signs at 30 minutes of the intervention. TENS plus analgesia may slightly reduce heart rate (MD ‐7.00 bpm, 95% CI ‐7.63 to ‐6.37; 1 study; 108 participants; low‐certainty evidence; Analysis 12.4) and respiratory rate (MD ‐1.10 brpm, 95% CI ‐1.26 to ‐0.94; 1 study; 108 participants; low‐certainty evidence; Analysis 12.5), compared with placebo plus analgesia.
12.4. Analysis.

Comparison 12: TENS plus analgesia versus placebo plus analgesia, Outcome 4: Heart rate
12.5. Analysis.

Comparison 12: TENS plus analgesia versus placebo plus analgesia, Outcome 5: Respiratory rate
TENS plus analgesia, compared with placebo plus analgesia, may also reduce systolic blood pressure (MD ‐4.00, 95% CI ‐6.26 to ‐1.74; 1 study; 108 participants; Analysis 12.6) and diastolic blood pressure (MD ‐4.00, 95% CI ‐5.57 to ‐2.43; 1 study; 108 participants; Analysis 12.7).
12.6. Analysis.

Comparison 12: TENS plus analgesia versus placebo plus analgesia, Outcome 6: Systolic blood pressure [mm Hg]
12.7. Analysis.

Comparison 12: TENS plus analgesia versus placebo plus analgesia, Outcome 7: Diastolic blood pressure
Rescue analgesic requirement
Four studies evaluated the effect of TENS on the need for analgesia during the postoperative period (Davies 1982; Jaafarpour 2008; Kayman‐Kose 2014; Smith 1986). TENS plus analgesia, compared with placebo plus analgesia, may reduce rescue analgesic requirement, when assessed by cumulative doses of diclofenac, up to 24 hours after the intervention (MD ‐58.40 mg, 95% CI ‐67.11 to ‐49.69; 1 study; 108 participants; low‐certainty evidence) (Jaafarpour 2008) (Analysis 12.8).
12.8. Analysis.

Comparison 12: TENS plus analgesia versus placebo plus analgesia, Outcome 8: Rescue analgesic requirement (cumulative dose)
TENS plus analgesia, compared with placebo plus analgesia may reduce the risk of requiring rescue analgesic at eight hours after the intervention (RR 0.79, 95% CI 0.66 to 0.94; 1 study; 100 participants) (Kayman‐Kose 2014) (Analysis 12.9).
12.9. Analysis.

Comparison 12: TENS plus analgesia versus placebo plus analgesia, Outcome 9: Rescue analgesic requirement (patients after 8 hours)
TENS plus analgesia may reduce rescue analgesic requirement, when assessed by the number of additional analgesics used, at 72 hours after the intervention (MD ‐0.64, 95% CI ‐1.01 to ‐0.28; 2 studies; 53 participants = 53; I2 = 0%) (Davies 1982; Smith 1986 ) (Analysis 12.10).
12.10. Analysis.

Comparison 12: TENS plus analgesia versus placebo plus analgesia, Outcome 10: Rescue analgesic requirement (number of analgesic)
Pain at six weeks after discharge
There are no available data for this outcome.
Women's satisfaction
There are no available data for this outcome.
Breasfeeding at discharge
Neither study reported breastfeeding at discharge, but Jaafarpour 2008 compared the mean length of the first breastfeed. Jaafarpour 2008 reported shorter first feeds of the babies in the TENS group compared to the control group: 52.8 (SD 3.8) versus 63.7 (SD 2.3) minutes (P < 0.001) ‐ data not shown.
Interaction with the baby
There are no available data for this outcome.
Walking at discharge
There are no available data for this outcome.
Length of hospitalisation
There are no available data for this outcome.
8.4 TENS plus analgesia versus analgesia
See Table 13.
Primary outcomes
Pain measured up to one, three, six, 12 and 24 hours after the intervention by a validated instrument or scoring system (such as the VAS)
Binder 2011 and Navarro Nunez 2000 evaluated TENS plus analgesia compared to analgesia in the relief of post‐caesarean pain. Binder 2011 reported their results in graphs and numbers by VAS (0 to 100 mm). It is uncertain if TENS plus analgesia has any effect on pain at six hours compared with analgesia (SMD 0.04, 95% CI ‐0.37 to 0.45; 2 studies; 92 participants; Analysis 13.1; very low‐certainty evidence) (SMD smaller than 0.2 indicates trivial or no effect).
13.1. Analysis.

Comparison 13: TENS plus analgesia versus analgesia, Outcome 1: Pain (VAS) ‐ up to six hours
We are uncertain if there is any difference between TENS plus analgesia compared to analgesia in the relief of at 24 hours after the start of intervention (MD ‐1.73, 95% CI ‐11.57 to 8.11; 1 study; 42 participants; very low‐certainty evidence) (Analysis 13.2).
13.2. Analysis.

Comparison 13: TENS plus analgesia versus analgesia, Outcome 2: Pain (VAS)
It is unclear if TENS plus analgesia, compared with analgesia, has any effect on pain at one hour (MD 4.93, 95% CI ‐1.47 to 11.33; 1 study; 42 participants), three hours (MD 1.64, 95% CI ‐8.80 to 12.09; 1 study; 42 participants), 12 hours (MD 7.87, 95% CI ‐1.77 to 17.51; 1 study; 42 participants).
Adverse effects (worsening of pain, anxiety, backache, pruritus, sedation)
There are no available data for this outcome.
Secondary outcomes
Vital signs
It is uncertain whether TENS plus analgesia, compared with analgesia has any effect on heart rate (MD ‐3.00, 95% CI ‐6.51 to 0.51; 1 study; 50 participants; very low‐certainty evidence) (Analysis 13.3), respiratory rate (MD 0.00 brpm, 95% CI ‐1.11 to 1.11; 1 study; 50 participants; very low‐certainty evidence) (Analysis 13.4), systolic blood pressure (MD 2.00, 95% CI ‐1.70 to 5.70; 1 study; 50 participants) (Analysis 13.5) or diastolic blood pressure (MD 1.00, 95% CI ‐1.99 to 3.99; 1 study; 50 participants) (Analysis 13.6) (Navarro Nunez 2000).
13.3. Analysis.

Comparison 13: TENS plus analgesia versus analgesia, Outcome 3: Heart rate (bpm)
13.4. Analysis.

Comparison 13: TENS plus analgesia versus analgesia, Outcome 4: Respiratory rate
13.5. Analysis.

Comparison 13: TENS plus analgesia versus analgesia, Outcome 5: Systolic blood pressure [mm Hg]
13.6. Analysis.

Comparison 13: TENS plus analgesia versus analgesia, Outcome 6: Diastolic blood pressure
Rescue analgesic requirement
Binder 2011 and Navarro Nunez 2000 assessed rescue analgesic requirement by dose of morphine in mg (Binder 2011) and by dose of dipyrone in g (Navarro Nunez 2000).
It is uncertain whether TENS plus analgesia, compared with analgesia, reduces rescue analgesic requirement at four hours after the intervention (MD ‐487.55, 95% CI ‐1463.19 to 488.09; 92 participants; 2 studies; very low‐certainty evidence) (Binder 2011; Navarro Nunez 2000) (Analysis 13.7).
13.7. Analysis.

Comparison 13: TENS plus analgesia versus analgesia, Outcome 7: Rescue analgesic requirement (cumulative dose)
It is uncertain if TENS plus analgesia, compared with analgesia, reduces rescue analgesic requirement in terms of cumulative dose of morphine at 24 hours after the intervention (MD ‐16.90, 95% CI ‐27.47 to ‐6.33; 42 participants; 1 study; low‐certainty evidence) (Binder 2011) (Analysis 13.7).
Pain at six weeks after discharge
There are no available data for this outcome.
Women's satisfaction
There are no available data for this outcome.
Breastfeeding at discharge
There are no available data for this outcome.
Interaction with the baby
There are no available data for this outcome.
Walking at discharge
There are no available data for this outcome.
Length of hospitalisation
There are not available data for this outcome.
Discussion
Summary of main results
This review assessed the effects of complementary and alternative medicine (CAM) in the postoperative period of caesarean section (CS). We included 37 randomised controlled trials (RCTs) or quasi‐RCTs, which used eight different types of CAM in 3076 women. Due to the large variation between studies regarding doses, outcome measures and follow‐up periods, pooling data was not always appropriate, and many analyses only contain data from one or two studies.
We found little data relating to adverse effects and vital signs. No studies reported pain at six weeks after discharge
Acupuncture or acupressure
It is uncertain if acupuncture or acupressure, compared with no treatment, reduces pain at 12 hours after the intervention, reduces the risk of adverse effects (back pain), or reduces rescue analgesic requirement compared with no treatment (Table 1).
It is uncertain if acupuncture or acupressure plus analgesia, compared with placebo plus analgesia, reduces pain at 12 hours after the intervention, or if it reduces rescue analgesic requirement (Table 2).
Acupuncture or acupressure plus analgesia, compared with analgesia, may reduce pain at 12 hours and 24 hours after the intervention and may reduce rescue analgesic requirement. It is uncertain if acupuncture or acupressure plus analgesia, compared with analgesia, has any effect on the risk of adverse effects (pruritis) (Table 3).
Aromatherapy
Aromatherapy may reduce pain at 12 and 24 hours when compared with placebo plus analgesia. It is uncertain if aromatherapy compared with placebo plus analgesia has any effect on the risk of adverse effects (anxiety), vital signs or rescue analgesic requirement (Table 4).
Electromagnetic therapy
Electromagnetic therapy may reduce pain at 12 and 24 hours and may reduce rescue analgesic requirement compared with placebo plus analgesia (Table 5).
Massage therapy
It is uncertain if massage plus analgesia, compared with analgesia, has any effect on pain at either 12 or 24 hours, adverse effects (anxiety), vital signs or rescue analgesic requirement (Table 6).
Music therapy
Music plus analgesia, compared with placebo plus analgesia, may reduce pain at one hour and 24 hours, and may reduce rescue analgesic requirement. It is uncertain if music plus analgesia, compared with placebo plus analgesia, has any effect on the risk of adverse effects (anxiety) or on heart rate (Table 7).
Music plus analgesia compared with analgesia may reduce pain at one hour and 24 hours and may reduce rescue analgesic requirement (Table 8).
Reiki
It is uncertain if Reiki, compared with analgesia has any effect on pain at either one hour or 24 hours, adverse effects (anxiety), vital signs or rescue analgesic requirement (Table 9)
Relaxation
It is uncertain if relaxation, compared with standard care, has any effect on pain at 12 hours, but it may reduce pain at 24 hours after the intervention (Table 10).
Transcutaneous electrical nerve stimulation (TENS)
TENS may reduce pain at one hour after the intervention, compared with no treatment (Table 11).
TENS plus analgesia, compared with placebo plus analgesia, may reduce pain, heart rate, respiratory rate and rescue analgesic requirement (Table 12).
It is uncertain if TENS plus analgesia, compared with analgesia, has any effect on pain at six or 24 hours after the intervention or on vital signs or on rescue analgesic requirement (Table 13).
Overall completeness and applicability of evidence
While most of the studies reported our primary outcome of pain up to discharge, we identified very little evidence relating to adverse effects of CAM interventions. It is also noteworthy that none of the studies measured our secondary outcomes related to pain at six weeks after discharge, interaction with the baby, walking at discharge and length of hospitalisation.
There was substantial heterogeneity in the methods of the included studies and many of them did not provide complete and clear information about their data. This hindered the quantitative analyses and the assessment of the risk of bias of many studies.
The number of trials for each of the eight specific types of CAM was small, ranging from one to eight studies. Moreover, the included studies had small primary sample sizes. The largest study involved 200 women treated with aromatherapy. Another issue is the poor reporting quality of most of these trials, which directly affects data extraction and judgment of risk of bias.
There was considerable variation in the use of the same intervention (e.g. dosages, time of application, characteristic frequency (TENS)). The variation of assessment for similar outcomes as rescue analgesic requirement (e.g. by total dosage, number of additional usage, difference from baseline) impaired the results. In many cases, this prevented the summarisation of evidence in quantitative analyses and contributed to the high heterogeneity seen in several meta‐analyses.
It is noteworthy that the studies included in this review were conducted in 14 different countries and most of which (75%) were low‐ or middle‐income countries. Social and cultural aspects of the evaluated interventions can also interfere with their acceptability and effectiveness for the relief of post‐CS pain. Therefore, the external validity of the overall evidence presented in this review should be considered with caution.
Another issue was the fact that we had six trials, which fulfilled our selection criteria but could not be included in the analyses because their data were incompletely described and we were unable to obtain full data, despite contacting the trialists.
Quality of the evidence
Despite the increasing number of RCTs on CAM in the past decades, few of them have a low risk of bias, and the risk of bias varied throughout the included trials. Fourteen of 37 included studies were judged as at low risk of bias. Blinding of staff, participants and outcome assessors was judged as unclear or high risk in the majority of trials.
The certainty of evidence is very low to low. We downgraded the certainty of evidence due to risk of bias, particularly with regard to random sequence generation and lack of blinding of participants, which could have an impact on self‐reported pain outcomes. We also downgraded the certainty of evidence due to imprecision resulting from low numbers of participants and wide 95% confidence intervals that are consistent with possible benefit and possible harm.
Potential biases in the review process
We conducted a sensitive search of the literature and we believe that we identified all the relevant trials that met our inclusion criteria. However, the possibility remains that we may have missed some trials, particularly in the grey literature. We adhered to the inclusion and exclusion criteria prespecified in the protocol in order to limit subjectivity (Zimpel 2014). We made efforts to obtain additional relevant data from study authors, but were unable to do so for some of them. If we can source supplementary data, we will consider them in future updates. The selection, data extraction and 'Risk of bias' assessment of the included studies were performed in duplicate by two independent review authors to reduce potential bias of the review process.
Agreements and disagreements with other studies or reviews
There are a number of Cochrane Reviews that address different CAMs in the labour management, but none of them have evaluated post‐CS pain (Smith 2006; Smith 2020; Smith 2011; Smith 2018a; Smith 2018b).
Although we did not find any other systematic review on the effects of CAM for post‐CS pain, Ferraz 2017 evaluated Reiki or prayer for controlling pain among women undergoing CS. There are some differences between our methods, beyond our different objectives. They aimed to follow Cochrane guidance but imposed language limits and evaluated only data in English or Portuguese. The authors included three of our identified RCTs in their quantitative and qualitative analysis and concluded that quote: "low‐certainty evidence suggested that use of Reiki and prayer meditation might be associated with pain reduction" (Midilli 2015; Beiranvand 2014; vanderVaart 2011). Following Cochrane standards, we were more sensitive and did not impose any language restrictions. We included Midilli 2016, who analysed 'Reiki plus analgesia versus placebo plus analgesia' in this review. Ferraz 2017 did not analyse Midilli 2016. We excluded Beiranvand 2014 – religion and spirituality are not considered as CAM ‐ and vanderVaart 2011 ‐ the CAM started in the preoperative period to prevent postoperative pain after CS ‐ from our analysis. Moreover, our conclusions are more circumspect.
Authors' conclusions
Implications for practice.
At 12 hours after the intervention, there is low‐certainty evidence to support the use of acupuncture or acupressure, aromatherapy, electromagnetic therapy, massage, music.
There is low‐certainty evidence to suggest that the effect of acupuncture or acupressure, aromatherapy, electromagnetic therapy, massage, music on pain is sustained at 24 hours after the intervention. Relaxation and transcutaneous electrical nerve stimulation (TENS) may also reduce post‐CS pain for up to 24 hours. We are uncertain about the effects of Reiki on post‐CS pain.
It may be that aromatherapy reduces the risk of anxiety compared with placebo at 90 minutes. Similarly, massage plus analgesia may reduce anxiety compared with analgesia alone, also at 90 minutes. Evidence about the risk of adverse effects with other forms of complementary and alternative medicine (CAM) is very uncertain.
Data on vital signs, rescue analgesic requirement, pain at six weeks after discharge, women's satisfaction, breastfeeding at discharge, interaction with the baby, walking at discharge, length of hospitalisation, are either lacking or the evidence is of such low certainty that no conclusions can be drawn.
Implications for research.
Since pain control is the most relevant outcome for post‐CS women and their clinicians, it is important that future studies of CAM for post‐CS pain measure pain as a primary outcome, preferably as the proportion of participants with at least moderate (30%) or substantial (50%) pain relief. The measure of pain as a dichotomous variable would improve the certainty of evidence and it is easy to understand for non‐specialists. Future trials need to be large enough to detect effects on clinical outcomes; they should include not only the main clinical outcome of pain, but also measure impairment or disability, vital signs, rescue analgesic requirement, pain at six weeks after discharge, women's satisfaction, breastfeeding at discharge, interaction with the baby, walking at discharge, length of hospitalisation, and use validated scales. All the foreseen outcomes must to be reported at the end of trial. Finally, studies need to be of at least six weeks' duration to assess the long‐term effects of CAM during the post‐CS period. Six weeks may be long enough to provide additional data on rare adverse events following CAM treatment, and assess its effects during the puerperal period. Future trials should include participants with none or more previous deliveries, and provide individual data by type of anaesthesia during the CS. Continuous outcome data need to be uniform, with use of similar scales, especially for pain and rescue analgesic requirement.
Further studies, with the characteristics suggested above, comparing CAM with a placebo control, remain necessary to evaluate CAM for wider clinical use in post‐CS women. The 17 ongoing studies that we identified, which aim to recruit over 1500 women altogether, will add further to the evidence presented here relating to acupressure, aromatherapy, electromagnetic therapy, massage and TENS, as well as providing new evidence about the effects of reflexology and herbal extract ointments on post‐CS pain.
What's new
| Date | Event | Description |
|---|---|---|
| 4 September 2020 | Amended | We have edited the abstract to remove a repeated phrase and made other minor formatting edits. |
History
Protocol first published: Issue 7, 2014 Review first published: Issue 9, 2020
Notes
Parts of this review are based on a standard template established by Cochrane.
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
The review authors wish to thank Cochrane Pregnancy and Childbirth, Fiona Stewart, from the Cochrane Children and Families Network, Cochrane Brazil and the Division of Vascular and Endovascular Surgery, Universidade Federal de São Paulo, Brazil, for methodological support. We would also like to thank Aidan Tan for translating Li 2012 and Bita Mesgarpour for translating Persian language papers.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), members of our international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. LILACS search strategy via Bireme: 56 (september 2th 2018)
#1 Mh:(Cesarean Section) OR Mh:(E04.520.252.500*)
#2 Mh:(Pain) OR Mh:(E04.520.252.500*)
#3 Mh:(C10.597.617*) OR Mh:(C23.888.592.612*) OR Mh:(F02.830.816.444*) OR Mh:(G11.561.600.810.444*)
#4 #1 AND #2 AND #3
Appendix 2. PEDro search strategy: 16 (september 2th 2018)
#1 cesarean section*
#2 pain*
#3 clinical trial*
#4 #1 AND #2 AND #3
Appendix 3. CAM base search strategy: 10 (september 2th 2018)
#1 "Cesarean Section" or "cesarian section"
#2 "Pain"
#3 #1 AND #2
Appendix 4. ICTRP and Clinicaltrials.gov ‐ search methods
Each line was run separately
ICTRP
pain AND cesarean
pain and caesarean
analgesia and cesarean
analgesia and caesarean
ClinicalTrials.gov
Advanced search
cesarean | Interventional Studies | Pain
cesarean | Interventional Studies | Analgesics
Data and analyses
Comparison 1. Acupuncture versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Abdominal pain up to 24 hours (VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Up to 24 hours | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐1.74, 0.10] |
| 1.2 Back pain up to 24 hours (VAS) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐1.94, 0.18] |
| 1.2.1 Up to 24 hours | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐1.94, 0.18] |
Comparison 2. Acupuncture plus analgesia versus placebo plus analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Up to 12 hours | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.74, 0.76] |
| 2.2 Rescue analgesic requirement (number of analgesic) | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.16, 0.16] |
Comparison 3. Acupuncture plus analgesia versus analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Pain ‐ up to 12 and 24 hours | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 Up to 12 hours | 2 | 130 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.64, 0.07] |
| 3.1.2 Up to 24 hours | 2 | 130 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐0.99, ‐0.26] |
| 3.2 Pain ‐ up to 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.2.1 Up to 48 hours | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.48, 0.36] |
| 3.3 Adverse effects (pruritus) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.08, 3.29] |
| 3.4 Rescue analgesic requirement (cumulative dose) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐5.00 [‐7.67, ‐2.34] |
| 3.5 Rescue analgesic requirement (number of analgesic) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐20.45 [‐30.92, ‐9.98] |
Comparison 4. Aromatherapy plus analgesia versus placebo plus analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Pain | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 Up to 12 hours | 3 | 360 | Mean Difference (IV, Random, 95% CI) | ‐2.63 [‐3.48, ‐1.77] |
| 4.1.2 Up to 24 hours | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐3.38 [‐3.85, ‐2.91] |
| 4.2 Anxiety | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐19.87 [‐22.11, ‐17.63] |
| 4.3 Heart rate (bpm) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐1.60, 2.80] |
| 4.4 Diastolic blood pressure (mm Hg) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐3.62 [‐6.97, ‐0.27] |
| 4.5 Systolic blood pressure (mm Hg) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐3.62 [‐7.71, 0.47] |
| 4.6 Rescue analgesic requirement | 3 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.19, 2.49] |
| 4.7 Satisfaction | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.23, 2.62] |
Comparison 5. Electromagnetic therapy plus analgesia versus placebo plus analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Pain (VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 Up to 12 hours | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐8.00 [‐11.65, ‐4.35] |
| 5.1.2 Up to 24 hours | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐13.00 [‐17.13, ‐8.87] |
| 5.1.3 Up to 48 hours | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐8.00 [‐11.52, ‐4.48] |
| 5.2 Rescue analgesic requirement | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 Up to 24 hours | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐1.95, ‐1.05] |
| 5.2.2 Up to 7 days | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐2.43, ‐1.57] |
| 5.3 Satisfaction | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.04, 3.84] |
Comparison 6. Massage (foot and hand) plus analgesia versus analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Pain | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1.1 Up to 12 hours | 6 | 651 | Mean Difference (IV, Random, 95% CI) | ‐2.03 [‐2.48, ‐1.59] |
| 6.1.2 Up to 24 hours | 3 | 230 | Mean Difference (IV, Random, 95% CI) | ‐1.51 [‐1.78, ‐1.24] |
| 6.1.3 Up to 48 hours | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐2.18, ‐1.54] |
| 6.1.4 More than 48 up to 120 hours | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐2.09 [‐2.38, ‐1.79] |
| 6.2 Adverse effects (anxiety) | 2 | 266 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.70, ‐0.19] |
| 6.3 Heart rate | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐1.78 [‐4.28, 0.72] |
| 6.4 Respiratory rate (breaths per minute) | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.91, ‐0.12] |
| 6.5 Systolic blood pressure | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐4.83, 0.64] |
| 6.6 Diastolic blood pressure | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐2.09, 1.89] |
| 6.7 Rescue analgesic requirement | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.09, 0.41] |
| 6.8 Breastfeeding | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.44, 0.95] |
Comparison 7. Music plus analgesia versus placebo plus analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Pain ‐ up to 1 hour | 2 | 115 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.23, ‐0.46] |
| 7.1.1 Up to 1 hour | 2 | 115 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.23, ‐0.46] |
| 7.2 Pain ‐ up to 24 and 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.2.1 Up to 24 hours | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐1.79 [‐2.67, ‐0.91] |
| 7.2.2 Up to 48 hours | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐1.21 [‐1.67, ‐0.75] |
| 7.3 Adverse effects (anxiety) | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐7.83, 3.83] |
| 7.4 Heart hate (bpm) | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | 4.00 [‐2.48, 10.48] |
| 7.5 Systolic blood pressure (mm Hg) | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐10.38, 4.38] |
| 7.6 Diastolic blood pressure (mm Hg) | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐7.59, 3.59] |
| 7.7 Rescue analgesic requirement (dose) | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.70, ‐0.10] |
Comparison 8. Music plus analgesia versus analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1.1 Up to 1 hour | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐2.11 [‐3.11, ‐1.10] |
| 8.1.2 Up to 24 hours | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐2.69 [‐3.67, ‐1.70] |
| 8.1.3 Up to 48 hours | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐1.79 [‐2.40, ‐1.18] |
| 8.2 Rescue analgesic requirement (cumulative dose) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.2.1 Tramadol | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐45.14 [‐86.77, ‐3.51] |
| 8.2.2 Diclofenac | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐21.43 [‐41.65, ‐1.21] |
| 8.3 Satisfaction | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [0.20, 1.06] |
Comparison 9. Reiki plus analgesia versus analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1.1 Up to one hour | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐2.87, ‐1.53] |
| 9.1.2 Up to 24 hours | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐2.52 [‐3.07, ‐1.97] |
| 9.2 Adverse effects (anxiety) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.2.1 Up to one hour ‐ 24 hours after CS | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐8.20 [‐10.67, ‐5.73] |
| 9.2.2 Up to one hour ‐ 48 hours after CS | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐9.00 [‐11.12, ‐6.88] |
| 9.3 Heart rate | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.3.1 Up to one hour ‐ 24 hours after CS | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐4.49 [‐9.85, 0.87] |
| 9.3.2 Up to one hour ‐ 48 hours after CS | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐3.58 [‐8.26, 1.10] |
| 9.4 Respiratory rate | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.4.1 Up to one hour ‐ 24 hours after CS | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐1.32, ‐0.16] |
| 9.4.2 Up to one hour ‐ 48 hours after CS | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐1.27, ‐0.09] |
| 9.5 Systolic blood pressure (mm Hg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.5.1 Up to one hour ‐ 24 hours after CS | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐2.18 [‐7.45, 3.09] |
| 9.5.2 Up to one hour ‐ 48 hours after CS | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐1.71 [‐6.21, 2.79] |
| 9.6 Diastolic blood pressure (mm Hg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.6.1 Up to one hour ‐ 24 hours after CS | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐5.09, 3.85] |
| 9.6.2 Up to one hour ‐ 48 hours after CS | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐4.10, 2.94] |
Comparison 10. Relaxation versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1.1 Up to 12 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.62, 0.54] |
| 10.1.2 Up to 24 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.05, ‐0.01] |
| 10.1.3 Up to 48 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐1.62, ‐0.70] |
| 10.1.4 Up to 84 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐1.31, ‐0.45] |
Comparison 11. TENS versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Pain (NAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1.1 Up to one hour | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐2.26 [‐3.35, ‐1.17] |
| 11.1.2 Up to two hours | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐2.55 [‐3.64, ‐1.46] |
| 11.2 Pain (measured with McGill pain questionnaire: higher score = more pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.2.1 Up to one hour | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.95 [‐3.95, 0.05] |
Comparison 12. TENS plus analgesia versus placebo plus analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Pain ‐ up to one hour (VAS) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1.1 Up to one hour | 3 | 238 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.37, ‐0.82] |
| 12.2 Pain ‐ 6, 12, 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.2.1 Up to six hours | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.34, ‐0.86] |
| 12.2.2 Up to 12 hours | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐1.58, ‐1.22] |
| 12.2.3 Up to 24 hours | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐0.87, ‐0.53] |
| 12.3 Pain (NRS) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.3.1 Up to one hour | 2 | 134 | Mean Difference (IV, Fixed, 95% CI) | ‐2.26 [‐2.85, ‐1.67] |
| 12.3.2 Up to 24 hours | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐1.63, ‐0.24] |
| 12.3.3 Up to 48 hours | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.41, ‐0.40] |
| 12.3.4 Up to 72 hours | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.96, 0.22] |
| 12.4 Heart rate | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐7.00 [‐7.63, ‐6.37] |
| 12.5 Respiratory rate | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.26, ‐0.94] |
| 12.6 Systolic blood pressure [mm Hg] | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐4.00 [‐6.26, ‐1.74] |
| 12.7 Diastolic blood pressure | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐4.00 [‐5.57, ‐2.43] |
| 12.8 Rescue analgesic requirement (cumulative dose) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.8.1 Diclofenac | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐58.40 [‐67.11, ‐49.69] |
| 12.9 Rescue analgesic requirement (patients after 8 hours) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.66, 0.94] |
| 12.10 Rescue analgesic requirement (number of analgesic) | 2 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐1.01, ‐0.28] |
| 12.10.1 Up to six hours | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐1.00, ‐0.16] |
| 12.10.2 Up to 24 hours | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐1.99, 0.56] |
| 12.10.3 Up to 48 hours | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐2.37, 0.95] |
| 12.10.4 Up to 72 hours | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.97, 0.10] |
Comparison 13. TENS plus analgesia versus analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Pain (VAS) ‐ up to six hours | 2 | 92 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.37, 0.45] |
| 13.1.1 Up to six hours | 2 | 92 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.37, 0.45] |
| 13.2 Pain (VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.2.1 Up to one hour | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 4.93 [‐1.47, 11.33] |
| 13.2.2 Up to three hours | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 1.64 [‐8.80, 12.09] |
| 13.2.3 Up to 12 hours | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 7.87 [‐1.77, 17.51] |
| 13.2.4 Up to 24 hours | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐1.73 [‐11.57, 8.11] |
| 13.3 Heart rate (bpm) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐6.51, 0.51] |
| 13.4 Respiratory rate | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.11, 1.11] |
| 13.5 Systolic blood pressure [mm Hg] | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐1.70, 5.70] |
| 13.6 Diastolic blood pressure | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐1.99, 3.99] |
| 13.7 Rescue analgesic requirement (cumulative dose) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.7.1 Dipyrone and Morphine up to four hours | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐487.55 [‐1463.19, 488.09] |
| 13.7.2 Morphine up to 24 hours | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐16.90 [‐27.47, ‐6.33] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbaspoor 2014.
| Study characteristics | ||
| Methods | Quasi‐randomised controlled study, Iran. | |
| Participants | 80 women submitted to elective CS, 18 to 35 years old, 37 to 42 weeks’ gestational age, in their second pregnancy with previous CS, estimated birthweight 2500 g to 4000 g, and transverse incision on uterus and abdomen in the previous CS (group massage therapy n: 40 and control group n: 40). | |
| Interventions | The intervention group received 20 minutes' foot and hand massage including petrissage (massage technique), kneading and friction (5 minutes in each hand and foot) initiated 1.5 to 2 hours after spinal anaesthesia. The control group received standard care and the investigator stood near the participant's bed and talked to her for 20 minutes without any other intervention. In both groups, at the request of a participant for pain relief, analgesics were used and the analgesic name, dosage and times of using were recorded. | |
| Outcomes | Pain relief, analgesics use, analgesic name, dosage and times evaluated before and 90 minutes after intervention. | |
| Notes | Funding sources: not mentioned. Setting: Obstetrics ward of Mustafa Khomeini Hospital, Iran. Conflicts of interest: not mentioned. Dates of trial: 1st April to 30th July 2011. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random sampling method (evenly ordered and assigned to 1 of 2 treatment arms). |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | High risk | One of the proposed outcomes (vital signs) was not presented in results. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Ahn 2017.
| Study characteristics | ||
| Methods | Quasi‐randomised controlled study, Korea | |
| Participants | 52 women submitted to elective CS, ASA I–II, haemoglobin levels of ≥10 g/dL, stable vital signs, singleton pregnancy without obstetric complications, the ability to communicate, and agreeing to use IV‐PCA after the caesarean delivery under spinal anaesthesia (group acupressure n: 26 and control group n: 26). One acupressure participant dropped out of the study because she could not keep the Seoambong on her skin and one control participant was excluded after being transferred to the intensive care unit. | |
| Interventions | Intervention: Korean hand acupressure discs were applied for 24 hours onto 12 acupressure points (K‐9, F‐4 for nausea and vomiting; M‐3, M‐4, L‐4, H‐2, H‐3, H‐7 for abdominal pain; and I‐38, J‐2 for back pain). Control: not described |
|
| Outcomes | Nausea and vomiting incidences and pain scores were analysed. | |
| Notes | Funding sources: did not receive any specific grant. Setting: Korean women’s hospital, Korea. Conflicts of interest: none declared. Dates of trial: not mentioned, published in 2017. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐experimental design, randomly assigned. No detail provided. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were few and balanced losses between groups (one lost in each group of 25 participants). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | High risk | The trial authors did not describe the intervention details of the control group.. |
Alves 2015.
| Study characteristics | ||
| Methods | Randomised controlled single‐blind trial, Brazil. | |
| Participants | Sixty women in the postpartum period of CS participated in the study. Participants had to be aged between 18 and 42 years, within 8 to 24 hours of the postpartum period, having pain at the incision site and admitted to hospital. Participants were either primiparous or multiparous mothers. The women were equally distributed between intervention group (n: 30) and control group (n: 30). |
|
| Interventions | The intervention group has received TENS continuously for 30 minutes, with frequency of 100 Hz and pulse width of 100 ms, intensity according to the participant's pain threshold. Placement of electrodes across the board and crossing a incision, 2 cm above and below the incision. The study did not describe what was accomplished in the control group. | |
| Outcomes | Age, weight and pain pre and post intervention (VAS). | |
| Notes | Funding sources: none. Setting: Maternidade Nossa Senhora de Lourdes, Aracaju, SE, Brazil. Conflicts of interest: authors declare no competing interests. Dates of trial: January to March 2015. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment blind as intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not described losses. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | High risk | The trial authors did not describe the intervention details of the control group. They also did not describe if there was additional use of analgesics in some of the groups. |
Binder 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial, Sweden. | |
| Participants | 50 multiparous women (27 to 37 years) with a healthy, singleton pregnancy at term and an anticipated elective (scheduled) CS under spinal anaesthesia were randomised. Divided into 2 groups: the control group (n: 25) received analgesia (PCA) alone and the intervention group (n: 25) received PCA in combination with Hi‐TENS. | |
| Interventions | The control group received PCA with 5 mL morphine (10 mg/mL) and 45 mL NaCl and the intervention group received PCA in combination with TENS immediately after surgery. The stimulator was set to give high‐frequency stimulation at 70 Hz, and was used continuously for at least 24 hours. | |
| Outcomes | Levels of morphine consumed, pain and sedation (VAS) at 1, 3, 6, 9, 12 and 24 hours postpartum. | |
| Notes | Dropouts: 8 women (5 control group‐ PCA alone and 3 intervention group ‐ PCA and TENS). Funding sources: Skaraborg Institute for Research and Development, the Scientific Committee at Central Hospital, Skovde, Sweden, the Foundation for the Masonic Orphanage in Stockholm (Stiftelsen Frimurare Barnhuset) and the Swedish Research Council, K2001‐ 27P‐13085‐036. Setting: county hospital in South‐west Sweden. Conflicts of interest: authors declare no competing interests. Dates of trial: 2001 to 2003. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The participants were randomly assigned by a person who was independent in relation to the study. |
| Allocation concealment (selection bias) | Low risk | Use of sealed and opaque envelope. Quote: "The assignment to each identity number was placed in to a sealed, opaque envelope and delivered one‐by‐one." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design did not control for a blind usage of the TENS apparatus. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The observer was not blinded to the different treatments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were few and balanced losses between groups (3 women dropped out in the PCA‐TENS group and 5 women dropped out in the PCA‐m group). |
| Selective reporting (reporting bias) | Low risk | Presents the results of the proposed analysis methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Bonabi 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial, Iran | |
| Participants | 90 women referred to the maternity hospitals of Rafsanjan, Iran. | |
| Interventions | Three groups: acupressure on LI4 point, SP6 point and control group. Intervention was performed bilaterally, in 10 seconds of pressure and 2 seconds of rest for 20 minutes sequentially. In the control group, the points were touched with the same pattern without pressure. | |
| Outcomes | Post cesarean pain. | |
| Notes | Full data in Persian are not available. Only the English abstract is available at the moment. Funding sources: not described. Setting: maternity hospitals of Rafsanjan, Iran Conflicts of interest: authors declare no competing interests Dates of trial: 2017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses not described. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the proposed analysis methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Davies 1982.
| Study characteristics | ||
| Methods | Randomised controlled study, UK. | |
| Participants | 35 women who were delivered by elective CS under general or epidural anaesthesia received TENS active or control (18 general anaesthesia: control 8 and TENS 10; 17 epidural analgesia: control 6 and TENS 11). The intervention group n: 22 and the control group n: 14. | |
| Interventions | All participants received conventional analgesia (15 mg of papaveretum intramuscularly or Distalgesie 2 tablets‐ paracetamol 650 mg, dextropropoxphene 65 mg); the intervention group received in addition the TENS active, 25 Hz, 200 ms, 0‐40V, intervals of 15 minutes for 24 hours. | |
| Outcomes | Pain scores (linear analogue scale) and the analgesic requirement 24 hours after finalised surgery. | |
| Notes | Funding sources: not mentioned. Setting: UK, no details. Conflicts of interest: not mentioned. Dates of trial: none mentioned, published 1982. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomised by a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The control groups received the stimulator unit without batteries. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the proposed analysis methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Degirmen 2010.
| Study characteristics | ||
| Methods | Quasi‐randomised controlled experimental study, Turkey. | |
| Participants | 75 conscious women were divided into 3 groups: a control group (n: 25), a foot and hand massage group (n: 25), and a foot massage group (n: 25). | |
| Interventions | The foot and hand massage group received 20 minutes' massage include petrissage, kneading and friction; the foot massage group received 10 minutes' massage; the control group did not receive any massage. The massage intervention was applied 2.5 ± 1.0 hours after the administration of analgesics in the intervention groups. | |
| Outcomes | The pain intensity and vital findings of the participants were measured 1 to 4 hours after a dose of pain medication in both control and massage groups. Measurements were recorded on the Premassage–Postmassage Postoperative Pain and Vital Findings Follow‐up Form in 60 to 90 minutes after the intervention. | |
| Notes | Funding sources: not mentioned. Setting: obstetric intensive care units and services of all the public and university hospitals in the province of Eskisehir, Turkey, namely, Eskisehir Public Hospital, Gynaecology and Obstetrics Hospital, and Osmangazi University Education and Training Hospital. Conflicts of interest: not mentioned. Dates of trial: 1st January and 30th April 2006. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random sampling method. The participants were randomised according to their order of presentation and evenly divided into 3 groups. |
| Allocation concealment (selection bias) | High risk | The participants were randomised according to their order of presentation and evenly divided into 3 groups ‐ no attempt to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the outcomes proposed in methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Dong 2015.
| Study characteristics | ||
| Methods | Randomised controlled study, China. | |
| Participants | 108 puerperae in the Hangzhou Chinese Medical Hospital, primipara; 21 to 34 years of age; 37 to 42 weeks of gestation; implementation of CS; and informed consent were randomly divided into 3 treatment groups (36 participants/group): PCIA, APT and combination therapy (APT with PCIA). | |
| Interventions | The PCIA group used the PCIA pump which was turned on within 0.5 hours after the participant returned to the obstetrics ward. The pump contained 100 mL of saline with 100 ug of sufentanil and 10 mg of tropisetron. The flow rate was 2 mL/hour. 1 press of the pump delivered 0.5 mL, and the participant had to wait 15 minutes before receiving another delivery. In the APT group, trained researchers implemented the therapy within 0.5 hours after the participant returned to the obstetrics ward. After the ear was disinfected with 75% alcohol, vaccaria seeds were positioned on acupoints of the ear, including “zi gong”, “pen qiang”, “shen men,” and “pi zhi xia”. Pressure was applied with the index finger and thumb, causing temporary swelling and pain. The combination therapy group received both treatments. All treatments lasted 2 days, i.e. until 48 hours after CS. |
|
| Outcomes | General information, degree of pain (pain at rest and when turning) 6, 12, 24, and 48 hours were recorded at after surgery, uterine pain recorded within 15 minutes after intravenous infusion of oxytocin was started on the first and second days after surgery. and concentrations of serum cortisol and IL‐6 was collected at 7:30 to 8:00 on the day of surgery and the second day after surgery. | |
| Notes | 4 participants were removed from the study. Funding sources: not mentioned. Setting: Hangzhou Chinese Medical Hospital obstetrics ward, China. Conflicts of interest: not mentioned. Dates of trial: July 2012 to May 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants were removed from the stud: PCIA group: 1, APT group: 2 and combination therapy group: 1. Total dropout rate was 3.7%. |
| Selective reporting (reporting bias) | Low risk | The outcomes proposed in the methods were described in the results. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Ebneshahidi 2008.
| Study characteristics | ||
| Methods | Randomised controlled study, Iran. | |
| Participants | 77 women aged 18 to 36 years, ASA I–II, received general anaesthesia and elective CS. music group n: 38 women, silence group n: 39 women. | |
| Interventions | The intervention group was exposed to 30 minutes of music, 15 minutes after arrival at the recovery room and the control group was exposed to silence by using headphones.Both groups received morphine administered in the recovery room and via the PCA for the first postoperative hour. | |
| Outcomes | Pain (VAS), anxiety (VAS), vital signs (blood pressure and heart rate) and morphine consumption 30 minutes after finalised surgery. | |
| Notes | 2 participants from the music group were excluded because of technical problems with cassette players at the recovery. Another participant from the control group was identified as an outlier for extreme anxiety and was dropped from the analyses. Funding sources: not mentioned. Setting: Sadi Hospital, Iran. Conflicts of interest: not mentioned. Dates of trial: not mentioned, published 2008. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | After 30 minutes of intervention, each VAS scale was presented to the participants individually by an instructed nurse who was unaware of assignments. After 30 minutes of intervention, an attending nurse who was unaware of assignments measured heart rate and noninvasive blood pressure 2 times with a 5‐minute interval. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The losses were few and balanced between groups. |
| Selective reporting (reporting bias) | Low risk | The outcomes proposed in the methods were described in the results. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Farzaneh 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial, Iran | |
| Participants | 57 women undergoing CS under spinal anaesthesia. The inclusion criteria were mothers interested in participating in the study, with no cancer and chronic pains, hearing or speech impairment, and no addiction to drugs, sedatives, and alcohol, psychological and mental health, haemodynamic stability, a minimum education level of primary school, and consent to participate. | |
| Interventions | 19 participants in each group: music therapy (headphones with nature‐based sounds), sham (headphones without sound) and control without headphones (standard care). Time of intervention 20 minutes. All groups received standard care with PCA as the analgesic treatment. | |
| Outcomes | Pain (VAS) after CS. | |
| Notes | Funding sources: the study was financially supported by the Research Deputy of Jahrom University of Medical Sciences, Jahrom, Iran. Setting: Motahari Hospital, Jahrom, Iran. Conflicts of interest: the authors declare no conflict of interests. Dates of trial: April 2015 to February 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised trial. Quote: "Randomization numbers were created from the Randomizer website of the Social Psychology Network". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind all participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"'The investigator recorded pain intensity every eight hours after the surgery. The investigator was not aware of group allocation to limit bias in the recording of parameters." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | High risk | 1 of the proposed outcomes (vital signs) was not presented in results. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Gamermann 2015.
| Study characteristics | ||
| Methods | Randomised controlled study. Brazil. | |
| Participants | A total of 58 women undergoing elective CS were randomised. Divided into 2 groups: the treatment group (n: 28) received standard anaesthesia plus acupuncture and the control group (n: 28) received standard anaesthesia and sham acupuncture. | |
| Interventions | The treatment group (n: 28) received standard anaesthesia for CS and plus acupuncture at 2 points: P6 and LI4, soon after spinal anaesthesia. The control group (n: 28) received standard anaesthesia for CS and sham acupuncture, soon after spinal anaesthesia. The needles where held in place for 20 minutes. | |
| Outcomes | Pain in rest and motion, vomiting. nausea, morphine consumed, women satisfaction 24 and 48 hours after surgery. | |
| Notes | Funding sources: no "outside" sources. Setting: Hospitalde Clínicas de Porto Alegre, Brazil. Conflicts of interest: not mentioned. Dates of trial: August 2011 and March 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table was used to generate randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was accomplished by using sealed envelopes. Only the doctor who inserted the acupuncture needles had knowledge of the contents of the envelopes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluators of outcomes and people involved in the data analysis did not have access to this information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | All outcomes proposed in the methods were reported. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Hadi 2011.
| Study characteristics | ||
| Methods | Randomised controlled single‐blind trial, Iran. | |
| Participants | 200 women after term, planned elective CS using spinal anaesthesia, no concurrent operation and duration of operation less than 90 minutes. Divided into 2 groups: the intervention group received aromatherapy n:100 women and the control group received placebo n: 100 women. | |
| Interventions | The intervention group inhaled a single dose of lavender essence through an oxygen mask during 3 minutes, 3 hours after receiving intravenous analgesic. The control group inhaled artificial aromatic material similar to lavender essence through an oxygen mask during 3 minutes, 3 hours after receiving intravenous analgesic. |
|
| Outcomes | Pain was assessed 30 minutes, 8 hours and 16 hours after the intervention using a VAS. | |
| Notes | Funding sources: not mentioned. Setting: Tabriz Taleghani teaching centre, Iran. Conflicts of interest: not mentioned. Dates of trial: June 2010 to June 2011. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo: artificial aromatic substance similar to lavender essence. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | The outcomes proposed in the methods were reported. |
| Other bias | High risk | The study does not state clearly about the use of analgesic medication associated with the intervention. |
Hanan 2011.
| Study characteristics | ||
| Methods | Quasi‐randomised controlled study, Egypt. | |
| Participants | A total of 150 women conscious, with intact hand and foot skin and free from arthritis, phlebitis, burn wound, injury, inflammation, eczema, cardiovascular and respiratory disease, undergoing CS were randomised, 75 participants in each group. | |
| Interventions | The intervention group received foot and hand massage for 20 minutes, 5 minutes for each hand then 5 minutes for each foot. The massage was applied 3 times at 5:40, 11:40, 17:40 hour after delivery. The control group received routine analgesics for pain relief. | |
| Outcomes | Level of pain (numerical rating scale), conditions aggravating pain, pain characteristic (Modified McGill pain questionnaire, short form). Outcomes assessed 6 hours, 12 hours and 18 hours after surgery. | |
| Notes | Funding sources: not mentioned. Setting: Ain Shams Maternity University Hospital. Conflicts of interest: not mentioned. Dates of trial: January 2011 to September 2011. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The sample was a systematic random sample. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | All outcomes proposed in the methods were reported. |
| Other bias | High risk | The trial authors did not clearly state about the use of analgesic medication associated with the intervention, and about the general characteristics of the sample. |
Hassani 2015.
| Study characteristics | ||
| Methods | Quasi‐randomised controlled study, Iran. | |
| Participants | 20 women were selected by" convenient sampling" from those hospitalised for CS and who did not have acute orthopaedic ankle problems, had no history of foot reflexology treatment, had no addiction to drugs, painkillers and alcohol and also had were consciousness and did not have a history of diabetes. | |
| Interventions | Foot reflexology (foot massage in ankle area downward for 5 and 2.5 minutes for each foot) and control group (not detailed). | |
| Outcomes | Vital signs and pain | |
| Notes | Funding sources: not mentioned. Setting: Iman Reza Hospital, Kermanshah, Iran Conflicts of interest: not mentioned. Dates of trial: not mentioned, published in 2015. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were selected by convenient sampling and were randomly divided into two groups. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Low risk | All outcomes proposed in the methods were reported. |
| Other bias | High risk | The study does not state clearly about the use of analgesic medication associated with the intervention and does not provide details about the control group. |
Irani 2015.
| Study characteristics | ||
| Methods | Quasi‐randomised clinical trial, single‐blinded, Iran. | |
| Participants | 80 women referred to the maternity ward for electiveCS under spinal anaesthesia. Participants having basic education, full‐term pregnancy, having 2 or 3 parities, healthy baby, first minute Apgar score of above 7, healthy skin in the massage area, full consciousness after the surgery, willingness to receive massage, no addiction, not having medical conditions such as diabetes, cardiovascular diseases, psychological, sensory and motor disorders, visual or hearing impairment, not having healthy feet and hands or history of severe emotional crisis such as death, migration or divorce during the last 6 months. Participants were randomly assigned to a control (n: 40) and intervention group (n: 40). | |
| Interventions | In the intervention group, the massage was performed for 20 minutes on participant’s extremities (5 minutes for each). The main specialised massage techniques included rotational friction movements, stretching, grasping and flexing on different parts of hands and feet from wrist to toes without focusing on a certain point. In the control group, in the other group, the researcher went to the participants’ bedside for 20 minutes, and had an informal chat with them. | |
| Outcomes | Pain, anxiety and vital signs of participants after CS. We employed demographic, observation and examination checklists and VAS for measuring pain and anxiety (with zero representing no pain and anxiety and 10 (100 mm) indicating intense and unbearable pain and anxiety). Four hours following the surgery, the massage intervention was given to the experimental group, by the researcher who them measured pain and anxiety 60 and 90 minutes after the massage. | |
| Notes | Funding sources: this study was extracted from a research project, approved by Mashhad University of Medical Sciences and Health Services (code: 910071), and was sponsored by the Research Department of the university. Setting: Omolbanin Hospital, Mashhad, Iran. Conflicts of interest: authors declare no conflicts of interest. Dates of trial: July to September of 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Selected through convenience sampling method. |
| Allocation concealment (selection bias) | High risk | Participants were randomly assigned to a control and intervention group using colour cards. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The levels of pain and anxiety were assessed by the researcher’s co‐worker, who was blindfolded during the randomisation process. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses after randomisation. |
| Selective reporting (reporting bias) | High risk | 1 of the proposed outcomes (vital signs) was not presented in results. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Jaafarpour 2008.
| Study characteristics | ||
| Methods | Randomised control study, Iran. | |
| Participants | 108 women aged 18 to 35, 50 kg to 75 kg body weight and 150 cm to 70 cm of height, term pregnancy, and same dose of spinal anaesthesia drugs, primipara, transverses CS and patients of a particular surgeon (54 in each group). | |
| Interventions | The author stated in methods quote: “58 study subjects were randomly allocated to two study arms (TENS‐ON i.e. Intervention group and TENS‐ OFF i.e. Control group)”. Therefore we included the study. The intervention group received routine palliative (analgesics) drugs similar to control group and the transcutaneous electrical stimulation to (TENS‐ON) with bi channel pulse, frequency of 100 Hz with a current intensity of 30 mA and pulse duration of 100 μs. The TENS device was continuously used for first 24 hours except temporary breaks for walking, using toilets, etc. The control group received routine palliative (analgesics) drugs TENS‐ OFF continuously used for first 24 hours except temporary breaks for walking, using toilets, etc. |
|
| Outcomes | Questionnaire included the demographic data, severity of pain was assessed using VAS before surgery and after surgery at different time intervals viz 0.5, 1, 1.5, 2, 4, 8, 12, 16, 20 and 24 hours in both the groups. Additionally, dosage of analgesics used, time of starting breastfeeding were also documented, perception about reduction in pain, satisfaction, rate and vital signs were assessed 1, 12 and 24 hours after finalised surgery. | |
| Notes | Funding sources: not mentioned. Setting: Ilam Shahid Mustafa Khomeini hospital, Iran. Conflicts of interest: not mentioned. Dates of trial: 2006 to 2007. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described. Reports to be quote: "quasi‐experimental" but women also "randomly assigned" to groups and similar baseline characteristics are reported between the groups. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis methods proposed. |
| Other bias | Low risk | This study is free from other risks of bias. |
Kayman‐Kose 2014.
| Study characteristics | ||
| Methods | Prospective, randomised, placebo‐controlled and participant‐blinded study, Turkey. | |
| Participants | 200 healthy women who gave birth to healthy newborns (n: 100 women who gave birth by CS and n: 100 women who delivered by vaginal route). Included only the 100 women who had CS under general anaesthesia were randomly assigned to the placebo group (Group 1) or the treatment group (Group 2) | |
| Interventions | The treatment group received TENS 100Hz,which was performed once for 30 minutes after childbirth was completed. The high frequency equipment with 2 channel output and 4 electrodes was adopted for the TENS. No electrical current was transmitted in the placebo group. A non‐opioid analgesic (75 mg of diclofenac sodium) was administered intramuscularly in the both group. | |
| Outcomes | The alteration in pain intensity (VAS and numerical scale) and the requirement for analgesics at eighth hour of childbirth. | |
| Notes | Funding sources: not mentioned. Setting: Afyonkarahisar Kocatepe University Medical School Hospital, Turkey. Conflicts of interest: authors have no conflicts of interest. Dates of trial: January 2010 and July 2010. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly allocated to the placebo group or the treatment group from a random number table. |
| Allocation concealment (selection bias) | Low risk | The randomisation by using sequentially‐numbered, sealed and opaque envelopes constructed from a random number table. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Khooshideh 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial, Iran | |
| Participants | 72 women: 36 were assigned to the intervention (PEMF treated) group, and 36 were allocated in the placebo (sham‐PEMF treated) group. Inclusion criteria: 20 to 35 years of age, singleton uncomplicated pregnancy, a gestational age of 37 to 42 weeks, and not having a history of > 1 CS. Exclusion criteria: having any underlying medical disease, having a history of any abdominal surgery other than CS, having a history of any drug or opium dependency, and refusing to give an informed consent to participate in the study. | |
| Interventions | PEMF and sham‐PEMF plus diclofenac 100 mg suppositories, once a day Intervention consisted of an elliptical coil that was 12 cm in size and a radiofrequency energy generator powered by battery that had an emission frequency of 27.1 MHz, a pulse rate of 1000 pulses per second, a 100‐microseconds pulse duration, and a peak spatial power density of 75 microwatts/cm2 |
|
| Outcomes | Pain, analgesic use, surgical site inflammation, participant satisfaction and return to daily activities | |
| Notes | Funding sources: Research Deputy of the Tehran University of Medical Sciences. Setting: Arash Women Hospital (a tertiary referral centre), of the Tehran University of Medical Sciences, Tehran, Iran. Conflicts of interest: authors declared no conflicts of interest. Dates of trial: August 2014 to December 2014. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial authors used a quote: "computerized random number generator" |
| Allocation concealment (selection bias) | Low risk | Trial authors used quote: "consecutive opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The healthcare providers, the participants, and the data collectors were all blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The healthcare providers, the participants, and the data collectors were all blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis proposed in the methods, and an outcome (participant satisfaction) that was not described in the registered protocol. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Lima 2014.
| Study characteristics | ||
| Methods | Randomised controlled single‐blind study, Brazil. | |
| Participants | 34 women undergoing caesarean delivery (primiparous or multiparous), aged more than 18 years (18 to 42 years), with pain greater than 3, undergoing spinal anaesthesia and incision P‐fannestiel, literate, oriented and absence of pathology genitourinary. There were three treatment groups: TENS High frequency (n: 13), TENS low frequency (n: 12), and one placebo group (n: 9). | |
| Interventions | 13 women received TENS high frequency 100 Hz (G100), 12 women in the TENS low frequency 4 Hz (G4) and 9 women in the placebo group (GP) (appliance off), the pulse duration of 100 μs with a current intensity of according to the threshold of each participant. The TENS device was continuously used for 30 minutes in each participant, only 1 section, 8 hours after the CS. Participants who received drug prescription inflammatory or analgesic were submitted to the assessment and intervention after 6 and 8 hours, respectively, to minimise possible interactions between effects of drugs and TENS. | |
| Outcomes | Pain score and adverse effect were evaluated immediately after, 20, 40 and 60 minutes after finalised treatment, using numerical rating scale (0‐10). | |
| Notes | Funding sources: not mentioned. Setting: Santa Casa de Misericórdia and Hospital Estadual Dirceu Arcoverde (HEDA), Brazil. Conflicts of interest: not mentioned. Dates of trial: not mentioned, published 2014. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly allocated into 3 groups according to software. |
| Allocation concealment (selection bias) | Low risk | The randomisation and allocation, hidden in opaque envelopes and numbered, were performed by a researcher no research participant. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants were unaware of the treatment protocol to which each participant was allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessor was unaware in treatment protocol to which each participant was allocated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were losses which were not described. We contacted the author in 24 June 2015 with no response. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Melo de Paula 2006.
| Study characteristics | ||
| Methods | Randomised controlled study, Brazil. | |
| Participants | 30 participants who were undergoing elective caesarean delivery (15 in each group), between the ages of 16 to 35 years, with pain intensity greater than zero. First pregnancy or have undergone at most 2 previous deliveries. | |
| Interventions | The intervention group received TENS using conventional TENS current (F = 100Hz and T = 50μs) with asymmetric bipolar pulse applied after the end of the anaesthesia for 50 minutes. The control group received placebo treatment being adopted the same procedures, differing only in respect to the regulation of the intensity of the current, getting the power turned off. routine procedures adopted by the health team, including intravenous injection of about 400 mg of meperidine, remained similar in both groups. | |
| Outcomes | Pain score (VAS and verbal numeric scale) evaluated before and 30 minutes after intervention. | |
| Notes | Funding sources: not mentioned. Setting: Maternidade Augusta Gomes Bastos, Rio Verde‐GO, Brazil. Conflicts of interest: not mentioned. Dates of trial: not mentioned, published 2006. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Midilli 2015.
| Study characteristics | ||
| Methods | Quasi‐randomised controlled clinical trial, Turkey. | |
| Participants | 100 women planned or unplanned caesarean delivery, Turkish nationality, with ability to speak Turkish, age between 18 to 45 years, hospital length of stay of at least 2 days, orientation to place and time, operation performed under general anaesthesia, only using a non opioid analgesic drug prescribed by a doctor: diclofenac 75 mg/3 mL, intramuscular. The experimental group (Reiki) n: 45 and the group without Reiki n: 45. |
|
| Interventions | The experimental group (Reiki group) received Reiki for 30 minutes, 10 identified regions of the body for 3 minutes each once a day for 2 days (in the first 24 and 48 hours). The group without Reiki application (control group) was merely given a rest for 30 minutes. | |
| Outcomes | Participants’ demographic information, pain (VAS), Anxiety (SAI) and haemodynamic parameters (blood pressure, breathing rate, and pulse rate) 1 and 2 days after finalised surgery. | |
| Notes | Dropouts: 10 women (5 of each group). Funding sources: not mentioned Setting: obstetric unit in Turkey Conflicts of interest: not mentioned Dates of trial: September to December 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The groups were selected by age and number of births by using a random group assignment method and simple randomisation technique. |
| Allocation concealment (selection bias) | High risk | Quote: "At the start of the study, when a participant was selected for the experimental group, a participant of the same age and with the same number of births was included in the control group." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The same researcher performed all data collection and Reiki applications. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small loss and balanced between groups (5 participants in each group). |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Midilli 2016.
| Study characteristics | ||
| Methods | Quasi‐randomised controlled trial, Turkey | |
| Participants | 45 women hospitalised in an obstetric unit of an Hospital in Turkey, by convention sampling. The women underwent a CS under general anaesthesia. | |
| Interventions | Reiki (n = 16), sham Reiki (n = 16), and control (n = 16) groups. Interventions were applied for 15 minutes to the incision area of body in the first 24 and 48 hours after the operation within 4 to 8 hours of the application of standard analgesics. | |
| Outcomes | Pain and vital signs | |
| Notes | Dropouts: 3 women (1 of each group). Funding sources: not mentioned. Setting: obstetric unit on Turkey. Conflicts of interest: declared that there is no conflict of interest. Dates of trial: September to December 2012. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "when a patient was selected for the experimental group, a participant of the same age and number of births as the one assigned to the experimental group was included in the control groups" |
| Allocation concealment (selection bias) | High risk | There was no allocation concealment for the staff. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small loss and balanced between groups (1 participant in each group). |
| Selective reporting (reporting bias) | Low risk | Pesents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Najafi 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial, Iran | |
| Participants | 80 women who were candidates for elective CS under spinal anaesthesia in primiparous women. | |
| Interventions | Chamomile flower essence (experimental, n = 40) and saline water (control, n = 40). All participants received 100 mg sodium diclofenac rectal suppository after CS. | |
| Outcomes | Pain, vital signs, analgesic consumption | |
| Notes | Dropouts: none. Funding sources: not mentioned. Setting: Besat Hospital, Sanandaj, Iran. Conflicts of interest: declared that there is no conflict of interest. Dates of trial: 2016. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | High risk | There was no allocation concealment for the staff. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | The trial presents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Navarro Nunez 2000.
| Study characteristics | ||
| Methods | Randomised controlled study, Mexico. | |
| Participants | 50 healthy women with an average of 67 kg, who have received identical anaesthesia, were undergoing elective caesarean delivery, allocated in 2 groups: intervention group TENS n: 25 and control group n: 25. | |
| Interventions | The intervention group received TENS continuous for 4 hours, initiated 5 minutes after surgery, with a frequency of 100 Hz with a current intensity of 30 mA and pulse duration of 100 μs. The control group received 1 g dipyrone intravenously. | |
| Outcomes | Pain, duration of pain, drug consumption, blood pressure, heart and breath rate (before and up to 4 hours after surgery). The pain was assessed cross‐modality registration balloon connected to a graduated scale in millimetres of mercury. |
|
| Notes | Funding sources: not mentioned. Setting: Mexico. Conflicts of interest: not mentioned. Dates of trial: trials lasted 14 months, published 2000. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Olapour 2013.
| Study characteristics | ||
| Methods | Randomised controlled triple blind study, Iran. | |
| Participants | 60 women, ASA class I and II, without hypertension, coagulation disorders, migraines and chronic headaches, no history of allergies to medicinal plants, no history of anosmia (loss of smell), were allocated to 2 groups: the intervention group (n: 30) and the control group (n: 30). | |
| Interventions | The intervention group received 3 drops of an aromatherapy blend containing lavender essence 10% (provided by The Barij Essence Pharmaceutical Compnay) poured on cotton in cast containers and the participant was asked to inhale it for 5 minutes from a distance of 10 cm. The inhalation aromatherapy was performed 4, 8 and 12 hours after the onset of postoperative pain. The control group received 3 drops of placebo with similar smell and appearance to the lavender oil essence, in an identical delivery device, in the same period and at the same time as the intervention group. If the VAS was greater than 3, analgesic was given in accordance with the hospital routine protocol. |
|
| Outcomes | Pain scores were measured using the VAS postoperative period end before and after the aromatherapy, heart rate, blood pressure, participant's satisfaction, time of first request of analgesia, completing analgesia were recorded before and after the aromatherapy based on the questionnaire. | |
| Notes | Funding sources: Ahvaz Jundishapur University of Medical Sciences, Vice Chancellor for Research and Technology. Setting: Ahvaz, Iran. Conflicts of interest: authors declare they have no financial disclosure. Dates of trial: published 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | The outcomes proposed in the methods were described in the results. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Ramezani 2016.
| Study characteristics | ||
| Methods | Cluster (block of 4) randomised controlled trial, Iran | |
| Participants | 108 women who had undergone CS (general or spinal anaesthesia) were randomised. Primiparous or multiparous. | |
| Interventions | Experimental group (acupressure on LI4) and control group (touch on the same point). | |
| Outcomes | Pain, vital signs and analgesic consumption | |
| Notes | Funding sources: not described. Setting: Fatemiyeh Hospital, Shahroud, Iran. Conflicts of interest: authors declare they have no financial disclosure. Dates of trial: not described, published in 2016. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | High risk | There was no allocation concealment for the staff. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of personnel is not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses identified or described. |
| Selective reporting (reporting bias) | High risk | One of the proposed outcomes (satisfaction) was not presented in results. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Saatsaz 2016.
| Study characteristics | ||
| Methods | Quasi‐randomised controlled trial, Iran | |
| Participants | 156 primiparous women undergoing elective CS, aged 20 to 35 years and were primiparous. | |
| Interventions | Foot massage (n = 52), hand and foot massage (n = 52) and control (n = 52, standard care). Over the 90‐minute duration of the assessment, none of the groups received any analgesics. | |
| Outcomes | Pain, anxiety level, haemodynamic indicators levels, breastfeeding frequency | |
| Notes | Full data in Persian are not available. Only the English abstract is available at the moment. Funding sources: not described. Setting: Imam Ali teaching hospital of Amol, Iran. Conflicts of interest: authors declare they have no financial disclosure. Dates of trial: July 2014 to June 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The card drawing technique was used to randomize the assignment of subjects to the groups. A total of 156 identical cards were first prepared, and 52 were labelled “foot massage”, 52 “hand and foot massage” and 52 “no interventions”. A card was randomly drawn for each participant who entered the study |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of personnel is not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the mean intensity of pain was measured by an assistant researcher who was blinded to the group allocation procedures and was not involved in performing the massages" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses identified or described. |
| Selective reporting (reporting bias) | Low risk | The trial presents the results of the outcomes proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Sen 2010.
| Study characteristics | ||
| Methods | Randomised prospective single‐blind study, Turkey. | |
| Participants | 70 women (ASA‐I), between the ages of 20 to 40 years, with uncomplicated singleton pregnancies of at least 36 weeks of gestation, who were planned to undergo elective CS via a Pfannenstiel incision under general anaesthesia were enrolled (35 participants in each group). | |
| Interventions | In Group 1,participants listened to music through a headphone (whatever she liked) for 1 hour, after surgery (as the Aldrete scores ≥ 9). In Group 2, participants did not listen to any music during the same period. In the postanaesthesia care unit; all participants were connected to PCA (tramadol 3 mg/mL). | |
| Outcomes | The participant’s level of satisfaction with perioperative care was assessed using VAS at 24 hours post‐surgery. The severity of postoperative pain during sitting and lying were assessed with VAS. Postoperative mean arterial blood pressure, heart rate, respiratory rate peripheral oxygen saturation (SpO2), end‐tidal carbon dioxide concentration (EtCO2), verbal rating scores, VAS (sitting and lying); consumption, demand and delivery of tramadol were recorded at 4, 8, 12, 16, 20 and 24 hours. The presence and intensity of any side effects were assessed at 4, 8, 12, 16, 20 and 24 hours after surgery, as well as sedation verbal rating scores, nausea and vomiting. |
|
| Notes | Funding sources: not mentioned. Setting: Turkey Conflicts of interest: not mentioned. Dates of trial: published 2010. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly allocated according to computer‐generated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | All outcomes proposed in methods were reported in the results. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Sharifipour 2015a.
| Study characteristics | ||
| Methods | Randomised controlled trial, Iran | |
| Participants | 80 women refereed to CS (primiparous or multiparous). Inclusion criteria were as follows: 1) age range of 18 to 35 years; 2) no prior history of hypertension, coagulopathy, migraine, allergies to plants, olfactory dysfunction or known anxiety disorders; 3) non‐use of addictive drugs or psychotropic medications; 4) birth of a healthy neonate; 5) use of spinal anaesthesia for CS; and 6) absence of respiratory failure during surgery. | |
| Interventions | Citrus aurantium fragrance (3 drops) and control (3 drops of saline). | |
| Outcomes | Pain, anxiety, pulse rate, blood pressure, nausea, vomiting, and headache | |
| Notes | Funding sources: Tehran University of Medical Sciences. Setting: Motazedi Hospital of Kermanshah, Iran. Conflicts of interest: not mentioned. Dates of trial: conducted in 2015. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Numbers 1 and 3 were written on two identical cards and a colleague, who was unaware of the content of each card, was asked to choose one of the cards" |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Interventions were performed in separate rooms; one room was filled with the aromatic essence of Citrus aurantium and another with the fragrance of normal saline." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although the trial authors stated that there were no losses, the other publication with the same identification number (14N201402215912) had 40 more participants. |
| Selective reporting (reporting bias) | High risk | One outcome was reported from 120 participants and all other outcomes from only 80 participants. All data referred to be from the same registered trial (14N201402215912). |
| Other bias | High risk | The registration number (14N201402215912) is not in the Iranian Registry of Clinical Trials as informed by authors on both related publications. |
Sharma 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial, India | |
| Participants | 60 women undergoing CS under spinal anaesthesia (30 experimental and 30 control), age 21 to 35 years, primiparous or multiparous. | |
| Interventions | Foot and hand massage (experimental) and standard care (control) | |
| Outcomes | Pain and vital signs | |
| Notes | Funding sources: self‐funded. Setting: hospital of Greater Noida, India. Conflicts of interest: none declared. Dates of trial: published in 2019. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | All outcomes proposed in the methods were reported in the results. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Simonelli 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial, USA. | |
| Participants | 165 primiparous women and who had experienced unplanned cesarean births, under spinal anaesthesia, age 17 to 46 years. | |
| Interventions | Massage (n = 55), standard care (n ‐ 55), or individualised attention (n = 55). | |
| Outcomes | Birth pain, stress, and relaxation | |
| Notes | Funding sources: Yvonne L. Munn Center of Nursing Research. Setting: Massachusetts General Hospital, USA. Conflicts of interest: the authors report no conflict of interest. Dates of trial: published in 2018. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Ssealed‐envelope technique was used to randomise participants. |
| Allocation concealment (selection bias) | Low risk | Quote; "The envelopes were sealed, shuffled, and stacked to ensure randomisation of group assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of personnel was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Study staff collected these data from the electronic charting system to remain blinded to study group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Smith 1986.
| Study characteristics | ||
| Methods | Randomised single‐blind experimental study, Canada. | |
| Participants | 18 multiparous women, each having undergone an elective caesarean delivery under general or epidural anaesthesia, aged 21 years or more and English‐speaking. There were 9 women in the experimental group and 9 in the placebo group. | |
| Interventions | The intervention group received TENS current, consisting of spike wave form impulses of 80 µs duration, delivered at a frequency of 85 Hz. The amplitude was adjustable, ranging from 0 to 75 mA. The placebo group received stimulator that was identical to the real one, but the current activated only the indicator light and not the electrode leads. The electrodes were placed by the surgeon after the surgery, still in the operating room, but TENS unit was only connected in the recovery room, the participant remained for 3 days. | |
| Outcomes | Pain scores (McGill Pain Questionnaire with consists of 2 main indices, Pain Rating Index (PRI) and Present Pain Intensity (PPI)) end the analgesic requirement 24 and 72 hours after finalised surgery. | |
| Notes | Funding sources: supported in part by NHRDP Grant No. 6605‐2108‐47. Setting: large metropolitan hospital, Canada. Conflicts of interest: not mentioned. Dates of trial: not clear, published 1986. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Immediately following electrode placement each participant was assigned randomly to the experimental or control group. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants assigned to the control group were given the same instructions regarding the use of the TENS machine but their machines delivered no current. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Solehati 2015.
| Study characteristics | ||
| Methods | Quasi‐experiment, prospective, unblinded, randomised study, Indonesia. | |
| Participants | 30 participants were recruited, who met the inclusion criteria, first birth by CS, using ketoprofen therapy, using spinal anaesthesia, awareness compos mentis and had never experienced the Benson relaxation technique. In the intervention group (IG), Benson relaxation technique was performed (respondents in the Cibabat hospital) (n: 30); whereas, those who were not given the intervention Benson relaxation were considered as the control group (CG) (respondents in the Sartika Asih hospital) (n: 30). | |
| Interventions | The Benson relaxation was performed for participants: they were suggested to take a particular form of expression in the names of God or a word that has a calming sense to the participants, repeatedly spoken with a regular rhythm with resignation, they were suggested to take deep breath through nose and exhale with the lips while saying the names of God or the word that has a calming sense. The Benson relaxation method was presented to IG and continued after the operation for 10 minutes to 4 days (84 hours): then the second day, third, and fourth every 12 hours at 6 AM and 6 PM. In the CG, Benson relaxation was not performed and regular care as room procedure was performed. | |
| Outcomes | Demographic characteristics and score pain using scale VAS pain before and after the intervention. | |
| Notes | Funding sources: issues were supported by author. Setting: Bandung, Indonesia. Conflicts of interest: not mentioned. Dates of trial: not clear, published 1986. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned into 2 groups of 30 by a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study is described as quote: "not blind". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study is described as quote: "not blind". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Sousa 2009a.
| Study characteristics | ||
| Methods | Randomised single‐blind experimental study, Brazil. | |
| Participants | 40 women undergoing caesarean delivery, aged more than 18 years (18 to 45 years), primiparous or multiparous, not obese, undergoing spinal anaesthesia and incision P‐fannestiel,with pain, literate and understand the pain scale. There were 20 women in the experimental group and 20 in the placebo group. | |
| Interventions | The intervention group received TENS current initiate 24 hours postoperatively, consisting of spike waveform impulses of 75 µs duration, delivered at a frequency of 100 Hz, remaining in postpartum women for 45 minutes. The control group was accompanied by the researcher for the same 45 minutes. The women remained without medication during the study period, being excluded if they needed it. | |
| Outcomes | Pain scores (McGill Pain Questionnaire with consists of 2 main indices, Pain Rating Index (PRI) and Present Pain Intensity (PPI) end the numerical categorical scale (VAS) after intervention and 1 hour after intervention. | |
| Notes | Results sent by author. Funding sources: National Council for Scientific and Technological Development. Setting: maternity hospital in Brazil. Conflicts of interest: not mentioned. Dates of trial: April to May 2007. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly allocated according to site www.randomization.com. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The lead researcher conducted the evaluations of 2 groups without knowing what the postpartum group received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses after randomisation. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Varghese 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial, India | |
| Participants | 60 post‐CS mothers, primiparous or multiparous. | |
| Interventions | Experimental (n = 30): 15‐minute foot reflexology session at the same time each evening for five consecutive days. Control (n = 30): standard care |
|
| Outcomes | Pain and quality of sleep | |
| Notes | Funding sources: not described. Setting: India. Conflicts of interest: not mentioned. Dates of trial: published in 2014. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Wu 2009.
| Study characteristics | ||
| Methods | Randomised controlled study, China. | |
| Participants | 60 women (ASAI‐II) with the first time pregnancy having CS for childbirth. Allocated in 3 groups: acupuncture group (n: 20 participants), electro‐acupuncture group (n: 20 participants) and control group (n: 20participants). | |
| Interventions | Acupuncture group (20 participants): acupuncture point San Yin Jiao (Sp6) was applied bilaterally until feeling De‐Qi sensation, needles were applied for 30 minutes, and then the PCA was applied. Electro‐acupuncture group (20 participants): after needles were placed in the acupuncture points bilaterally and participants reported De‐Qi sensation, a low frequency of 2 Hz with a suitable current, based on the degree of the muscle twitching, was connected. The points were stimulated for 30 minutes before the PCA was applied. Control group (20 participants): only PCA, for 30 minutes. |
|
| Outcomes | The vital signs (such as blood pressure, heart rates and blood oxygen level), the pain intensity (VAS), the dosage of PCA morphine demand, the frequency of PCA intake and the opioid‐related side effects, such as nausea, vomiting, dizziness and pruritus were also documented 1, 4 and 24 hours after finalised surgery. | |
| Notes | Additional data provided by the authors. Funding sources: fund from Kaohsiung Medical University Hospital, Taiwan, China. Setting: China Medical University hospital. Conflicts of interest: not mentioned. Dates of trial: not mentioned, published 2009. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | To ensure concealment of group assignment, the research associate contacted the research assistant in the hospital with the participant’s information for randomisation. Previously determined computer‐generated the randomised number sequence in blocks of 4 or 6 were used. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | We considered that was not possible to blind participants from electro‐acupuncture. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All data were collected by another well‐trained doctor who was double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Yang 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial, China | |
| Participants | 120 women who underwent CS. | |
| Interventions | Experimental: auricular acupuncture or acupressure plus standard care Control: standard care |
|
| Outcomes | Pain, anus exhaust time, incidence of postpartum haemorrhage, urinary retention and constipation, and postpartum average hospitalisation day. | |
| Notes | Full data are in Chinese; only the English abstract is available, awaiting translation (July 2020). Funding sources: not described. Setting: China. Conflicts of interest: not mentioned. Dates of trial: not mentioned, published 2019. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not possible to blind the personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | Presents the results of the analysis proposed in the methods. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
APT: Auricular‐plaster therapy ASA: American Society Anesthesiology physical status CS: caesarean section IV: intravenous NaCl: sodium chloride PCA: patient‐controlled analgesia PCIA: patient‐controlled intravenous analgesia PEMF: pulsed electro magnetic fields TENS: transcutaneous electric nerve stimulation VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abadi 2018 | The CAM was used in the preoperative period to prevent postoperative pain after CS. |
| Abu Bakar 2015 | CAM was not used for the treatment of post‐caesarean pain. |
| Agah 2007 | CAM was used in the preoperative period to prevent postoperative pain after CS. |
| Ali 2017 | CAM was used in the preoperative period to prevent postoperative pain after CS. |
| Allameh 2013 | Quran reading is not considered a CAM. |
| Amin‐Hanjani 1992 | Cold therapy is not considered a CAM. |
| Beiranvand 2014 | Religion and spirituality are not considered a CAM. |
| Blackburn 2011 | Only the abstract is available. CAM was used in the preoperative period to prevent postoperative pain after CS. |
| Cal 2016 | Hand and foot bathing are not considered as CAM. |
| Chang 2005 | CAM was used in the preoperative and intra‐operative periods to prevent postoperative pain after CS. |
| Chaowalit 2018 | Cold therapy is not considered as CAM. |
| Charoenkwan 2017 | Abdominal binders is not considered as CAM. |
| Chen 2005 | The study was not a randomised trial. Quote: "the study used a quasi‐experimental design and convenience sampling". |
| Citak 2012 | Physiotherapy is not considered as CAM. |
| Fazel 2017 | CAM was not used for the treatment of post‐cesarean pain. |
| Ghana 2017 | Abdominal binders is not considered as CAM. |
| Gillier 2016 | Abdominal binders is not considered as CAM. |
| Gist 2018 | Cold therapy is not considered as CAM. |
| Gursen 2016 | Kinesio taping is not considered as CAM. |
| Gustafson 2018 | Abdominal binders is not considered as CAM. |
| Henkel 2018 | Only the abstract is available. |
| Ho 1996 | CAM was used in the preoperative period to prevent postoperative pain after CS. |
| Hollinger 1986 | This study was retrospective. Quote: "reviewed the medical charts of 72 women retrospectively". |
| Hong 2003 | There is not a valid comparison because there is not a control group without CAM. |
| Houshyar 2015 | There is not a valid comparison because there is not a control group without CAM. |
| Kerai 2011 | There is not a valid comparison because there is not a control group without CAM. |
| Keshavarz 2010 | There is not a valid comparison because there is not a control group without CAM. |
| Khezri 2017 | CAM was used in the preoperative period to prevent postoperative pain after CS. |
| Khoshtarash 2012 | Reflexology was used in the preoperative period to prevent postoperative pain after CS. |
| Krum 2006 | Physical therapy is not considered as CAM. |
| Kuo 2016 | CAM was not used for the treatment of post‐cesarean pain. |
| Kurdi 2018 | CAM was used in the intra‐operative period to prevent postoperative pain after CS. |
| Kushnir 2012 | CAM was used in the preoperative period to prevent postoperative pain after CS. |
| Li 2012a | CAM was used in the preoperative period to prevent postoperative pain after CS. |
| Li 2012b | CAM was used in the preoperative period to prevent postoperative pain after CS. |
| Mahishale 2014 | An abdominal corset is not considered as CAM. |
| Mohseni 2018 | Amniotic membrane dressing is not considered as CAM. |
| Mokhtari 2010 | Non‐randomised clinical trial. Quote: "A quasi‐experimental time series design and clinical trial was used. Method of sampling was convenience non probability". |
| Mousavi 2017 | CAM was used in the preoperative period to prevent postoperative pain after CS. |
| Myers 2014 | Abdominal binders is not considered as CAM. |
| Norouzi 2013 | Kangaroo care is not considered as CAM. |
| Ohashi 2012 | Only the abstract is available. |
| Rasuli 2017 | Music therapy was used in the preoperative period to prevent postoperative pain after CS. |
| Razmjoo 2012 | Reflexology was used in the preoperative period to prevent postoperative pain after CS. |
| Reynolds 1987 | The study was not randomised trial. Quote: "the study was not randomised in that odd and even hospital numbers were used to divide patients into and control groups". |
| Reza 2007 | CAM was used in the intra‐operative period to prevent postoperative pain after CS. |
| Robinson 2017 | Counselling is not considered a CAM and the trial was withdrawn. |
| Saberkari 2009 | Only the abstract is available. |
| Sadeghi 2019 | CAM was used in the preoperative period to prevent postoperative pain after CS. |
| Sen 2009 | CAM was used in the preoperative period to prevent postoperative pain after CS. |
| Shabanian 2017 | CAM was used in the preoperative period to prevent postoperative pain after CS. |
| Sharifi 2013 | The Quran is not considered as CAM. The intervention was used to prevent postoperative pain after CS (intervention used in the preoperative period). |
| Sharifipour 2015b | CAM was not used for the treatment of post‐cesarean pain. |
| Tarasov 1995 | Only the abstract is available. |
| vanderVaart 2011 | CAM was started in the preoperative period to prevent postoperative pain after CS. |
| Xue 2016 | The study was not a randomised trial. |
CAM: complementary and alternative medicine CS: caesarean section TENS: transcutaneous electrical nerve stimulation
Characteristics of ongoing studies [ordered by study ID]
Bagherzadeh 2019.
| Study name | Investigating the effect of reflexology and relaxation of Benson on pain, physiological symptoms, lactation and weight of newborn in women undergoing cesarean section |
| Methods | Randomised clinical trial; not blinded |
| Participants | Inclusion criteria: women submitted to an elective CS, under spinal anaesthesia; age 18 to 35 years; first or second pregnancy. Exclusion criteria: rupture of membrane; mental or physical disorder; pain score below 3 at the beginning of intervention; the hospitalisation of newborns in the intensive care units; any mother or infant disorder interfering with the infant feeding Target sample size: 135 women |
| Interventions | Two experimental groups (Benson's reflexology and relaxation) and one other control group (standard care). |
| Outcomes | Primary outcomes: pain and physiological symptoms (pulse, blood pressure and O2 saturation) Secondary outcomes: breast feeding and baby weight |
| Starting date | 21 April 2019 |
| Contact information | Razieh Bagherzadeh Bushehr University of Medical Sciences, Salman Farsi St., Sabze Abad Blvd., Bushehr, Boushehr, Iran Postal code 7518759577 | +98 77 3345 0236 | r.bagherzadeh@bpums.ac.ir |
| Notes | IRCT20190122042453N1 | No data provided |
Balachandran 2019.
| Study name | To study and evaluate the effectiveness of treatment by Percutaneous Electrical NeuroStimulation (PENS) for post‐operative pain in cesarean section patients using primary relief v 2.0 |
| Methods | Randomised controlled trial; double‐blinded (participant and care provider) |
| Participants | Inclusion criteria
Exclusion criteria
Target sample size: 22 women |
| Interventions | All interventions will be provided after CS Experimental: primary relief v 2.0 device Placebo comparator: paracetamol |
| Outcomes | Primary outcome: pain using VAS score Secondary outcome: quality of life |
| Starting date | 2 January 2019 |
| Contact information | V Balachandran MD Warangal, Telangana, India, 506002 +91 9946452707 | v.balachandran@dyansys.com |
| Notes | NCT03829774 | No data provided |
Hakimi 2018.
| Study name | Effect of Eremostachys Laciniata suppository on post caesarean section pain and distress triple blind controlled clinical trial |
| Methods | Randomised controlled trial; triple‐blinded |
| Participants | Women with a non‐emergency cesarean section referral to Al‐Zahra Hospital will be included, and if the duration of surgery increases more than an hour, they will be excluded Target sample size: 86 |
| Interventions | Control group: diclofenac rectal suppository 50 mg every 8 hours to 3 doses. Intervention group: Eremostachys rectal suppository (Chelledaghi herbal extract) every 8 hours to 3 doses. In case of severe pain and anxiety in both groups, the patient will receive additional analgesic that will be recorded in the checklist of the medications received. |
| Outcomes | Pain and distress |
| Starting date | 31 October 2018 |
| Contact information | Sevil Hakimi PhD Nursing and Midwifery Faculty, Tabriz University of Medical Sciences, Shariati Avenue. Tabriz, Tbabriz, East Azarbaijan Postal code 5138947977 | +98 41 3475 3907 | hakimis@tbzmed.ac.ir |
| Notes | IRCT20150424021917N9 | No data provided |
Jahdi 2015.
| Study name | The effect of Calendula ointment on healing and pain localized wound cesarean section in nulliparous women |
| Methods | Randomised clinical trial with placebo; triple‐blinded |
| Participants | Inclusion criteria: primiparous women with age from 20 to 37 years; gestational age 37 to 42 weeks; ability to read and write; not having history of allergies to any topical medicine; no addiction to tobacco, drugs and psychotropic; avoiding use of anticoagulants, antidepressants, anticonvulsants and drugs that weaken the immune system; no history of previous surgical incision cesarean section (above the symphysis pubis); lack premature rupture of membranes > 18 hours; not having a BMI > 35; lateral‐lower uterine incision and Pfannenstiel technique skin incision; no accumulation of fat in the abdomen; lack of abnormal vaginal bleeding; Spinal anaesthesia; the same type of stitches; presence of attendant with patient; using chromic suture to the mucous and silk for skin. Exclusion criteria: lack of improper use of marigold ointment or Vaseline ointment based on form was developed according to participants; unwillingness to continue to participate in the study; using other drugs or methods of healing; using medications affecting wound healing; occurring any infection or bleeding at the wound that requires medical intervention Target sample size: 108 |
| Interventions | Intervention: Calendula ointment 2 times a day until 10 days after birth Intervention: placebo ointment 2 times a day until 10 days after birth Control: follow the routine of the hospital |
| Outcomes | Primary: wound healing Secondary: caesarean wound severity pain |
| Starting date | 04 August 2015 |
| Contact information | Fereshteh Jahdi Tehran University of Medical Sciences, Iran +982188773073 | +982182471404 | f.jahdi@iums.ac.ir |
| Notes | IRCT201507252248N18 | No data provided |
Joghataei 2015.
| Study name | Comparison of the effect of foot reflexology and auriculotherapy on pain and anxiety in women following elective cesarean section |
| Methods | Randomised controlled trial; double‐blinded |
| Participants | Inclusion criteria: singleton pregnancy; labor pains not started; the elective cesarean section planned before the onset of labour; no medical condition in mother (cardiac, respiratory, hepatic or neurologic); using spinal analgesia for CS; no addiction to drugs, sedatives and alcohol in mother. Exclusion criteria: uncomfortable feel in ear acupressure or foot massage; complications during and after surgery such as prolonged duration of surgery, excessive bleeding and fetal death. Target sample size: 132 women |
| Interventions | Intervention: reflexology intervention performed once for 20 minutes in both legs 2 to 3 hours after injecting anaesthetic for spinal anaesthesia Intervention: auriculo therapy performed once for 20 minutes in both ears Control: standard care |
| Outcomes | Primary: pain and anxiety Secondary: nausea‐bloodshed and bloodshed |
| Starting date | 23 September 2015 |
| Contact information | Razieh Joghataei Mashhad University of Medical Sciences, Iran +98 51 4562 2849 | joghataeir911@mums.ac.ir |
| Notes | IRCT2014122920475N1 | No data provided |
Kazemi 2019.
| Study name | Comparative study of the effects of reflexology and auriculo therapy on the pain after cesarean section |
| Methods | Randomised controlled trial; double‐blinded |
| Participants | Inclusion criteria: single pregnancy; low‐risk pregnancy; not having special disease history; lack of starting labour pain; having a transverse incision cesarean section surgery; belonging to the first class of anaesthesia risk Exclusion criteria: discomfort in ear or pain while massaging or putting pressure on feet; complications during and after CS |
| Interventions | Intervention: reflexology intervention takes place for 20 minutes in both 6 hours and 30 hours after the CS Intervention: auriculo therapy intervention; the auricular seed are attached for 24 hours Control: standard care; the researcher is present at the mother's bedside for 20 minutes doing nothing twice |
| Outcomes | Pain |
| Starting date | 23 August 2018 |
| Contact information | NameMajid Kazemi Rafsanjan University of Medical Sciences, Iran +98 34 3425 7663 | dr.kazemi.n@rums.ac.ir |
| Notes | IRCT20131228015965N15 |No data provided |
Kim 2015.
| Study name | Battlefield auricular acupuncture for control of post‐partum pain |
| Methods | Randomised controlled trial; single‐blinded |
| Participants | Inclusion criteria
Exclusion criteria
Target sample size 90: women |
| Interventions | Intervention: standard of care plus battlefield auricular acupuncture Control: standard of care only |
| Outcomes | Primary: decrease in overall pain Secondary: decrease in amount of pain medicine used |
| Starting date | February 2016 |
| Contact information | Michael J Kim MD Mike O'Callaghan Federal Medical Center, Nellis Air Force Base, Nevada, United States, 89191 None phone or email provided |
| Notes | NCT02526186 | No data provided |
Klinger 2018.
| Study name | Extent of analgesic effects of transcutaneous electrical nerve stimulation in patients after caesarean section |
| Methods | Randomised controlled trial; double‐blinded |
| Participants | Inclusion criteria: all women older than 18 years of age and undergoing elective CS are considered study participants. Exclusion criteria: women under the age of 18 years. At the time of intervention the women are no longer 'pregnant'. Women who need emergency surgery and/or intensive care monitoring are also excluded. If complications occur intraoperatively (e.g. delivery of a 'sick' newborn, haemorrhagic shock, etc.) and/or during hospitalisation (e.g. embolism with intensive care unit stay), the participants are also excluded from the study. Participants who can not explain or explain their consent (for example, incompliant or dementia) or who indicate in the preoperative examination to suffer from a psychiatric illnesses and / or chronic pain are also excluded from the study. |
| Interventions | Arm 1: TENS device postoperatively in addition to the standard pain medication. ('verum‐ TENS') Arm 2: TENS device postoperatively in addition to the standard medication. They also receive augmented care/attention. ('verum‐ TENS' ‐ augmented) Arm 3: TENS device postoperatively in addition to standard medication. This TENS does not perform any analgesic function ('placebo TENS'). Arm 4: TENS device postoperatively in addition to standard pain pharmacotherapy. This TENS does not perform any analgesic function. Participants also receive augmented care/attention. ('placebo‐TENS'‐ augmented) Arm 5: Standard pain medication (therapy as usual). Arm 6: Standard medication and augmented intensive care/attention (therapy as usual, augmented) |
| Outcomes | Primary: postoperative pain intensity (related to the CS surgical wound) Secondary: subjective mood (general depression scale), catastrophism (questionnaire for pain treatment in pain situations), functional capacity (pain and mood inventory including postoperative functional capacity) |
| Starting date | 27 August 2018 |
| Contact information | Dr Regine Klinger Bereich Schmerzmedizin und Schmerzpsychologie Universitätsklinikum Hamburg‐Eppendorf (UKE) Zentrum für Anästhesiologie und Intensivmedizin Klinik und Poliklinik für Anästhesiologie Martinistraße 52, 20246 Hamburg, Germany 040 741052837 | 040 741044963 | r.klinger at uke.de | uke.de/kliniken‐institute/kliniken/anästhesiologie/index.html |
| Notes | DRKS00013123 | No data provided |
Latifi 2012.
| Study name | The Impact of foot and hand massage on cesarean postoperative pain |
| Methods | Randomised controlled trial; double‐blinded |
| Participants | Inclusion: elective Caesarean operation; consciousness; literacy; spinal anaesthesia; 18 to 40 years old Exclusion: phlebitis; eczema; arthritis; burn wound; injury on their hands or feet and cardiovascular; respiratory diagnosis; or psychological problems like depression Target sample size: 90 women |
| Interventions | Intervention: hand massage Intervention: hand and foot massage Control: standard care |
| Outcomes | Primary: pain Secondary: vital findings |
| Starting date | 21 January 2012 |
| Contact information | Shahrbanoo Latifi Babol University of Medical Sciences, Iran +98 11 1223 4142 | latifinursing@yahoo.com |
| Notes | IRCT201112248498N1 | No data provided |
Maassarani 2018.
| Study name | Pulsed short wave therapy in Cesarean section |
| Methods | Randomised controlled trial; masking: quadruple (participant, care provider, investigator, outcomes assessor) |
| Participants | Inclusion criteria
Exclusion criteria
Target sample size: 250 women |
| Interventions | Intervention: standard protocol for the control of postoperative pain as well as an active RecoveryRx Pulsed Short Wave Therapy device. Control: standard protocol for the control of postoperative pain as well as a sham RecoveryRx Pulsed Short Wave Therapy device. |
| Outcomes | Change in pain VAS Time to patient mobility Wound closure at day 7 Wound complications |
| Starting date | 01 April 2018 |
| Contact information | Mahmoud Maassarani PhD New Mazloum Hospital, Tripoli, Lebanon +96179156547 | m.maassarani@outlook.fr |
| Notes | NCT03604068 | No data provided |
Mobaraki 2019.
| Study name | The examining of the effect of foot massage with orange essence on grade pain and anxiety women under cesarean section |
| Methods | Randomised controlled trial; not blinded |
| Participants | Inclusion criteria: nulliparous mothers with CS; no medical condition in mother; mothers with no tissue damage on their feet; using spinal analgesia for CS; have GCS of 15. Exclusion criteria: mothers who have serious health disorder; mothers who have tissue damage on their feet. Target sample size: 80 women |
| Interventions | Intervention: foot massage with orange essence Control: foot massage will be performed without orange essence |
| Outcomes | Pain and anxiety |
| Starting date | 09 April 2019 |
| Contact information | Fatemeh Mobaraki Shahroud University of Medical Sciences 3614773947 Tehran Street, Shahrood, Semnan, Iran +98 23 3239 5054 | fatemehmobaraki96@yahoo.com |
| Notes | IRCT20181226042137N1 | No data provided |
Mojalli 2017.
| Study name | Chammomil fragrance impact on anxiety and pain after cesarean section in nulliparous women |
| Methods | Randomised controlled trial; double‐blinded |
| Participants | Inclusion criteria: Iranian race women, from 18 to 35 years, submitted to spinal anaesthesia; CS time less than 90 minutes; single pregnancy; the absence of anxiety disorders Exclusion criteria: ileus inertia during or after operation Target sample size: 98 women |
| Interventions | Intervention group: seven drops of chamomile fragrance for 10 minutes inhalation Control group: seven drops of still water for 10 minutes |
| Outcomes | Primary: pain; time point 10 minutes; method of measurement 'VAS' Secondary: anxiety; time point 10 minutes; method of measurement 'Spilberg anxiety inventory' |
| Starting date | 01 July 2017 |
| Contact information | Dr Mohammad Mojalli PhD Gonabad University Of Medical Sciences, Gonabad, Iran +98 51 5722 3028 | mmojali@yahoo.com |
| Notes | IRCT2017052834169N1 | No data provided |
Oberbaum 2008.
| Study name | Homeopathy for post‐operative (C. section) recovery |
| Methods | Randomised clinical trial; double‐blind |
| Participants | Women hospitalised for elective CS, from the 1st to 3rd pregnancies, 18 years and older, age < 50 years, body weight < 100 kg, signing of informed consent form Target sample size: 90 women |
| Interventions | The women were divided in 3 different groups: Active comparator: A Bellis perennis and Staphysagria (C6), Active comparator: B Bellis perennis and Staphysagria (C30) and Placebo comparator: C placebo remedy |
| Outcomes | Pain, analgesic use, duration of hospital stay, blood loss, postoperative complications, quality of life assessment, adverse effects of treatment |
| Starting date | August 2008 |
| Contact information | Dr Menachem Oberbaum MD Shaare Zedek Medical Center, Jerusalem, Israel None phone or email provided |
| Notes | NCT00725569 | No data provided |
Pakseresht 2016.
| Study name | The survey effect of lavender aromatherapy on the pain level after cesarean section among women referred to Al‐Zahra hospital in Rasht in 2016‐ 2017 |
| Methods | Randomised controlled trial; double‐blinded |
| Participants | Inclusion criteria: pain VAS score higher than 3; using spinal anaesthesia; use of a type of anaesthetic (bupivacaine 0.05); no concurrent operation; duration of operation less than 90 minutes; full term pregnancy; no history of allergies to medicinal plants, anosmia, migraines, chronic headaches, coagulation disorders, polyhydramnios, diabetes, preeclampsia, twin pregnancy. Exclusion criteria: nausea, vomiting; allergy or absence of patients satisfaction after first dose of aromatherapy. Target sample size: 110 women |
| Interventions | Intervention: 5 drops (100%) lavender essence on a cotton swab and the swab will be placed for 3 minutes in the oxygen mask 4 hours after the surgery Control: 5 drops of placebo will be applied on a cotton swab and the swab will be placed for 3 minutes in the oxygen mask |
| Outcomes | Primary: pain (VAS) Secondary: respiratory rate, pulse rate and blood pressure |
| Starting date | 22 August 2016 |
| Contact information | Dr Sedigheh Pakseresht PhD Shahid Beheshti School of Nursing and Midwifery, Daneshjoo St., Shahid Beheshti Highway, Rasht, 39841‐41469, Iran +98 911 331 5015 | +98 13 3355 0097 | paksersht@yahoo.com |
| Notes | IRCT2016072529063N1 | No data provided |
Phillibert 2015.
| Study name | Effect of pulsed electromagnetic field therapy on pain after cesarean delivery |
| Methods | Randomised controlled trial; masking: triple (participant, care provider, investigator) |
| Participants | Inclusion criteria
Exclusion Criteria
Target sample size: 84 women |
| Interventions | Intervention: the PEMF device is placed over the incision after caesarean delivery and then turned on. Device appears to be operational and functions correctly. Control: the PEMF is placed over the incision after caesarean delivery and then turned on. The device only appears to be function correctly because the lights turn on, but does not emit a pulsed electromagnetic frequency. |
| Outcomes | Primary: pain after caesarean delivery Secondary: assessment of the amount of narcotics uses for pain control after caesarean delivery |
| Starting date | January 2015 |
| Contact information | Donald Phillibert New York City Health and Hospitals Corporation None phone or email provided |
| Notes | NCT02365753 | No data provided |
Santana 2014.
| Study name | Use of TENS for reducing pain after caesarean |
| Methods | Placebo‐controlled randomised clinical trial; double‐blind study with 5 arms (three interventions groups and two control groups) |
| Participants | Women undergoing caesarean delivery with incisional pain intensity greater than 3 on a numeric scale with 15 years or more, the period between 8 and 12 hours postpartum. ASA I or II. Absence of hearing impairment or visual communication, or even have no cognitive disorder/psychiatric impairment. Target sample size: 125 women |
| Interventions | Treatment group: TENS for pain relief will be applied at a frequency of 100 Hz, for 20 minutes. In the treatment group 'A' in 25 women, the electrodes are placed 2.5 cm above and below the incision with sensory threshold intensity. In the treatment group 'B' in 25 women, electrodes are placed in the paravertebral region at the level of T8 and L5 with sensory threshold intensity. In the treatment group 'C' in 25 women, electrodes are placed in the same location of the treatment group 'B', however with motor threshold intensity. Placebo (control) group: the electrodes will be placed 2.5 cm above and below the incision in the placebo group 'D' in 25 women, and paravertebral the level of T8 and L5 in the placebo group 'E' in 25 women, but in these groups the chain will be emitted only during the first 30 seconds. |
| Outcomes | Reduction in pain intensity at rest and in motion. |
| Starting date | 05 April 2014 |
| Contact information | Josimari Melo de Santana. |
| Notes | RBR‐459y54 | No data provided |
Shahoei 2017.
| Study name | Effect of Transcutaneous Electrical Nerve Stimulation in post‐cesarean pain |
| Methods | Randomised controlled trial; not blinded |
| Participants | Inclusion criteria: singleton pregnancy, elective caesarean with transverse incision, same anaesthesia, same gynaecologist, same narcotic dose, newborn with Apgar score more than 7, having pain in incision site, do not have medical complications, do not use drugs. Exclusion criteria: having pace maker, skin irritation, sensitivity to electrodes, fever and haemorrhage during 24 hour after CS. Target sample size: 90 women |
| Interventions | Intervention: TENS electrodes will be insert 5 cm below and top of incision Sham intervention: TENS will be insert at the same place as intervention group but device will be turned off Control: there is no intervention, only routine care will be done but evaluation will be the same. |
| Outcomes | Pain severity after CS |
| Starting date | 28 February 2017 |
| Contact information | Roonak Shahoei Kurdistan University of Medical Sciences, Iran +98 87 3366 1120 | roonak.shahoei@muk.ac.ir |
| Notes | IRCT2017020314556N4 | No data provided |
Zardosht 2016.
| Study name | Compare the effectiveness of essential oils of chamomile or placebo on intensity and quality of pain after cesarean section |
| Methods | Randomised controlled trial; double‐blinded |
| Participants | Inclusion criteria: consent for participation in the study; ability to speak Farsi; the same type of anaesthesia (spinal anaesthesia) and anaesthetic used; 38 to 42 weeks of gestational age; nulliparous. Exclusion criteria: reluctance to continue participating in the exercises; use powerful hypnotics or analgesics drugs; having allergy and breathing problems; history of warfarin use Target sample size: 128 women |
| Interventions | Intervention: essential oil of chamomile 5% on the pad (small gas) dropped Control: placebo‐treated pad |
| Outcomes | Primary: pain Intensity (VAS) Secondary: pain quality (modified Short Form McGill Pain Questionnaire) |
| Starting date | 22 June 2015 |
| Contact information | Roqiyeh Zardosht Sabzevar University of Medical Sciences, School of Paramedics, SHahroud road, Sabzevar +98 51 4426 4430 | zardoshtr911@mums.ac.ir |
| Notes | IRCT2016042427558N1 | No data provided |
ASA: American Society Anesthesiology physical status BMI: body mass index CS: caesarean section GCS: Glasgow Coma Scale NICU: neonatal intensive care unit TENS: transcutaneous electrical nerve stimulation VAS: visual analogue scale
Differences between protocol and review
We amended the objectives from 'To assess the effectiveness and safety of' in the protocol to 'To assess the effects of' in the review to be more in accordance with the purpose of our research (Zimpel 2014).
We have added an additional search of ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP), LILACS, PEDro and CAM base.
Comparisons previously defined in the protocol were analysed in the review, separately for each type of CAM.
We created a 'Summary of findings' table for the comparison of relaxation versus standard care as this is a clinically important comparison.
We amended the interpretation of I2 following the guidance for interpretation in the Cochrane Handbook for Systematic Reviews of Interventions.
In accordance with sections 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions, we amended how we used fixed‐effect model and random‐effects model, with the evaluation of homogeneity's included studies, considering population, interventions, comparators and outcomes characteristics. This section of the Handbook states "The choice between a fixed‐effect and random‐effects meta‐analysis should never be made on the basis of a statistical test for heterogeneity."
Contributions of authors
SAZ is guarantor for the review. She co‐ordinated the contributions from the co‐authors. SAZ and RLGF wrote the final draft of the review. EMKS and GP contributed to writing the methods and statistical analysis sections of the review. SAZ and MRT drafted the clinical sections of the background. SAZ, RLGF and MRT answered to the comments of the referees. All authors contributed to the writing this review.
Sources of support
Internal sources
No sources of support provided, Other
External sources
No sources of support supplied
Declarations of interest
Sandra Zimpel: none known.
Maria Regina Torloni: none known.
Gustavo JM Porfirio: none known.
Ronald LG Flumignan: none known.
Edina MK da Silva: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Abbaspoor 2014 {published data only}
- Abbaspoor Z, Akbari M, Najar S. Effect of foot and hand massage in post cesarean section pain control: a randomized control trial. Pain Management Nursing 2014;15(1):132-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- IRCT201105226546N1. The effect of hand and foot massage on the pain after cesarean section surgery. en.irct.ir/trial/6996 (first received 16 July 2011).
Ahn 2017 {published data only}
- Ahn NY, Park HJ. Effects of Korean hand acupressure on opioid-related nausea and vomiting, and pain after caesarean delivery using spinal anaesthesia. Complementary Therapies in Clinical Practice 2017;28:101-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Alves 2015 {published data only}
- Alves EM, Rabêlo TN, Santos MG, Souza IG, Lima PA, Santana LS. Transcutaneous electric nerve stimulation for post-cesarean section analgesia [Eletroestimulação nervosa transcutânea para analgesia pós-operatória em cesariana]. Revista Dor 2015;16(4):263-6. [DOI: 10.5935/1806-0013.20150053] [DOI] [Google Scholar]
Binder 2011 {published data only}
- Binder P, Gustafsson A, Uvnas-Moberg K, Nissen E. Hi-TENS combined with PCA-morphine as post caesarean pain relief. Midwifery 2011;27(4):547-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bonabi 2018 {published data only}
- Bonabi TN, Jaberi AA, Esmaeilzadeh S, Ravizi AH. Comparison of the effect of acupressure in Li4 and SP6 points on the severity of pain after Cesarean section. Iranian Journal of Obstetrics, Gynecology and Infertility 2018;21(6):9-17. [DOI: 10.22038/IJOGI.2018.11628] [DOI] [Google Scholar]
- IRCT20171113037426N1. Comparison the effect of acupressure in LI4 and SP6 points on post-cesarean section pain severity in women. en.irct.ir/trial/27694 (first received 13 January 2018).
Davies 1982 {published data only}
- Davies JR. Ineffective transcutaneous nerve stimulation following epidural anaesthesia. Anaesthesia 1982;37:453-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Degirmen 2010 {published data only}
- Degirmen N, Ozerdogan N, Sayiner D, Kosgeroglu N, Ayranci U. Effectiveness of foot and hand massage in postcesarean pain control in a group of Turkish pregnant women. Applied Nursing Research 2010;23(3):153-8. [DOI: 10.1016/j.apnr.2008.08.001] [DOI] [PubMed] [Google Scholar]
Dong 2015 {published data only}
- Dong LN, Yu Q, Ye JE, Pei JX, Qian X, Feng Y. Effects of auricular-plaster therapy on pain and serum levels of cortisol and IL-6 after cesarean section. International Journal of Nursing Sciences 2015;2(3):273-8. [DOI: 10.1016/j.ijnss.2015.08.005] [DOI] [Google Scholar]
Ebneshahidi 2008 {published data only}
- Ebneshahidi A, Mohseni M. The effect of patient-selected music on early postoperative pain, anxiety, and hemodynamic profile in cesarean section surgery. Journal of Alternative and Complementary Medicine 2008;14(7):827-31. [DOI: 10.1089/acm.2007.0752] [DOI] [PubMed] [Google Scholar]
Farzaneh 2019 {published data only}
- Farzaneh M, Abbasijahromi A, Saadatmand V, Parandavar N, Dowlatkhah HR, Bahmanjahromi A. Comparative effect of nature-based sounds intervention and headphones intervention on pain severity after cesarean section: a prospective double-blind randomized trial. Anesthesiology and Pain Medicine 2019;9(2):e67835. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRCT2015020114049N2. Clinical trial the effect of sound therapy on severity of pain in women after cesarean section, by spinal anesthesia compared with patients with routine care. en.irct.ir/trial/13751 (first received 7 July 2015).
Gamermann 2015 {published data only}
- Gamermann PW, Martins AL, Rosa L, Ribeiro HD, Borba DL, Antoniazi V, et al. Acupuncture as a complement to the pharmacological management if pain, nausea and vomiting after cesarean section: a randomized clinical trial. Acupuncture and Related Therapies 2015;3:11-4. [DOI: 10.1016/j.arthe.2014.12.002] [DOI]
Hadi 2011 {published data only}
- Hadi N, Hanid AA. Lavender essence for post-cesarean pain. Pakistan Journal of Biological Sciences 2011;14(11):664-7. [DOI: 10.3923/pjbs.2011.664.667] [DOI] [PubMed] [Google Scholar]
Hanan 2011 {published data only}
- Hanan A, Kamilia R, Ahmed R, Amina M. Investigate the itilization of natural measures on relieving post cesarean incision pain. Asian Journal of Nursing Education and Research 2014;4(4):388-93. [ajner.com/AbstractView.aspx?PID=2014-4-4-2] [Google Scholar]
- Hanan A, Kamilia R, Ahmed R, Amina M. Investigate the utilization of natural measures on relieving post cesarean incision pain. Egyptian Journal of Medical Science 2011;32:667-80. [ejmsonline.org/abstracts/682] [Google Scholar]
Hassani 2015 {published data only}
- Hassani S, Hassani K. The effect of foot reflexology on physiologic indices and pain severity following cesarean delivery. Research Journal of Medical Sciences 2015;9(3):114-7. [Google Scholar]
Irani 2015 {published data only}
- Irani M, Kordi M, Tara F, Bahrami HR, Nejad KS. The effect of hand and foot massage on pain and anxiety. Avicenna Journal of Phytomedicine 2015;5(Suppl 1):63. [Google Scholar]
- Irani M, Kordi M, Tara F, Bahrami HR, Nejad KS. The effect of hand and foot massage on post-cesarean pain and anxiety. Journal of Midwifery and Reproductive Health 2015;3(4):465-71. [Google Scholar]
- Kordi M, Irani M, Bahrami HR, Sardasht FG. Effect of hand and foot massage on vital signs of women after caesarean section. Iranian Journal of Obstetrics, Gynecology and Infertility 2016;19(15):8-15. [DOI: 10.22038/IJOGI.2016.7397] [DOI] [Google Scholar]
Jaafarpour 2008 {published data only}
- Jaafarpour M, Khani A, Javadifar N, Taghinejad H, Mahmoudi R, Saadipour KH. The analgesic effect of transcutaneous electrical nerve stimulation (TENS) on caesarean under spinal anaesthesia. Journal of Clinical and Diagnostic Research 2008;2(3):815-9. [Google Scholar]
Kayman‐Kose 2014 {published data only}
- Kayman-Kose S, Arioz DT, Toktas H, Koken G, Kanat-Pektas M, Kose M, et al. Transcutaneous electrical nerve stimulation (TENS) for pain control after vaginal delivery and cesarean section. Journal of Maternal-Fetal and Neonatal Medicine 2014;27(15):1572-5. [DOI: 10.3109/14767058.2013.870549] [DOI] [PubMed] [Google Scholar]
Khooshideh 2017 {published data only}
- IRCT2014070711020N3. Comparison of the effect of pulse electro magnetic field with placebo on the amount of post cesarean section pain in pregnant women admitted in Arash hospital. en.irct.ir/trial/11382 (first received 2 August 2014).
- Khooshideh M, Latifi Rostami SS, Sheikh M, Ghorbani Yekta B, Shahriari A. Pulsed electromagnetic fields for postsurgical pain management in women undergoing cesarean section: a randomized, double-blind, placebo-controlled trial. Clinical Journal of Pain 2017;33(2):142-7. [DOI: 10.1097/AJP.0000000000000376] [DOI] [PubMed] [Google Scholar]
Lima 2014 {published data only (unpublished sought but not used)}
- Lima LE, Lima AS, Rocha CM, dos Santos GF, Bezerra AJR, Hazime FA, et al. High and low frequency transcutaneous electrical nerve stimulation in post-cesarean pain intensity [Estimulação elétrica nervosa transcutânea de alta e baixa frequência na intensidade da dor pós-cesárea]. Fisioterapia e Pesquisa 2014;21(3):243-8. [DOI: 10.590/1809-2950/65021032014] [DOI] [Google Scholar]
- NCT01897311. Effect of modulation of tens in pain after cesarean section. clinicaltrials.gov/show/NCT01897311 (first received 24 May 2013).
Melo de Paula 2006 {published data only}
- Melo de Paula G, Molinero de Paula VR, Dias RO, Mattei K. Transcutaneous electric nerve stimulation (TENS) in postoperative cesarean [Estimulação elétrica nervosa transcutânea (TENS) no pós-operatório de cesariana]. Revista Brasileira de Fisioterapia 2006;10(2):219-24. [Google Scholar]
Midilli 2015 {published data only}
- Midilli TS, Eser I. Effects of Reiki on post-cesarean delivery pain, anxiety, and hemodynamic parameters: a randomized, controlled clinical trial. Pain Management Nursing 2015;16(3):388-400. [DOI: 10.1016/j.pmn.2014.09.005] [DOI] [PubMed] [Google Scholar]
Midilli 2016 {published data only}
- Midilli TS, Gunduzoglu NC. Effects of reiki on pain and vital signs when applied to the incision area of the body after cesarean section surgery: A single-blinded, randomized, double-controlled study. Holistic Nursing Practice 2016;30(6):368-78. [DOI: 10.1097/HNP.0000000000000172] [DOI] [PubMed] [Google Scholar]
Najafi 2017 {published data only}
- IRCT2016081726861N2. The comparison of the effect of lavender essence and chamomile essence on pain severity after cesarean section under spinal anesthesia: a blind placebo-controlled randomized clinical trial. en.irct.ir/trial/22158 (first received 27 September 2016).
- Najafi B, Mojab F, Ghaderi L, Farhadifar F, Rroshani D, Seidi J. The effect of chamomile flower essence on pain severity after elective caesarean section under spinal anaesthesia: a randomized clinical trial. Journal of Clinical and Diagnostic Research 2017;11(11):UC01-4. [DOI: 10.7860/JCDR/2017/29689.10836] [DOI] [Google Scholar]
Navarro Nunez 2000 {published data only}
- Navarro Nunez C, Pacheco Carrasco M. Transcutaneous electric stimulation (TENS) to reduce pain after cesarean section. Ginecologia y Obstetricia de Mexico 2000;68:60-3. [PMID: ] [PubMed] [Google Scholar]
Olapour 2013 {published data only}
- IRCT201204249552N1. Comparison of the effectiveness of inhalation of aromatherapy blend containing lavender essential oil and placebo on cesarean post operative pain relief in pregnant women. en.irct.ir/trial/10117 (first received 16 September 2012).
- Olapour A, Behaeen K, Akhondzadeh R, Soltani F, Al Sadat Razavi F, Bekhradi R. The effect of inhalation of aromatherapy blend containing lavender essential oil on cesarean postoperative pain. Anesthesiology Pain Medicine 2013;3(1):203-7. [DOI: 10.5812/aapm.9570] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramezani 2016 {published data only}
- IRCT2015080722407N3. The effectiveness of acupressure on pain relief after cesarean. en.irct.ir/trial/19331 (first received 19 December 2015).
- Ramezani S, Hamidzadeh A, Abdollahpour S, Khosravi A. Effects of LI4 acupressure on post-cesarean section pain. International Journal of Health Studies 2016;2(2):23-6. [DOI: 10.22100/ijhs.v2i2.138] [DOI] [Google Scholar]
Saatsaz 2016 {published data only}
- Rezaei R, Beheshti Z, Saatsaz S, Alipour A. Massage-therapy and post cesarean pain control. Iranian Journal of Obstetrics, Gynecology and Infertility 2017;20(4):34-43. [DOI: 10.22038/IJOGI.2017.8979] [DOI] [Google Scholar]
- Saatsaz S, Rezaei R, Alipour A, Beheshti Z. Massage as adjuvant therapy in the management of post-cesarean pain and anxiety: a randomized clinical trial. Complementary Therapies in Clinical Practice 2016;24:92-8. [DOI: 10.1016/j.ctcp.2016.05.014] [DOI] [PubMed] [Google Scholar]
Sen 2010 {published data only}
- Sen H, Yanarates O, Sizlan A, Kilic E, Ozkan S, Dagli G. The efficiency and duration of the analgesic effects of musical therapy on postoperative pain. Agri 2010;22(4):145-50. [PubMed] [Google Scholar]
Sharifipour 2015a {published data only}
- IRCT201402215912N14. Comparison the impact of Salvia officinalis and Citrus arantium aroma on post cesarean anxiety and pain. en.irct.ir/trial/6383 (first received 18 April 2014).
- Sharifipour F, Ali MM, Hashemzadeh M. Comparison of the effect of citrus arantium and salvia officinalis aroma on post-cesarean section pain. Iranian Journal of Obstetrics, Gynecology and Infertility 2017;20(2):41-9. [DOI: 10.22038/IJOGI.2017.8713] [DOI] [Google Scholar]
- Sharifipour F, Sohail Baigi S, Mirmohammad M. The aromatic effects of citrus arantium on pain and vital signs after caesarean section. International Journal of Biology, Pharmacy and Allied Sciences 2015;4(7):5063-72. [Google Scholar]
- Sharifipour F, Sohailbaigi S, Dastmozd L. Comparison of the citrus arantium and salvia officinalis aroma impacts on post cesarean anxiety. Acta Medica Mediterranea 2016;32:977-81. [Google Scholar]
Sharma 2019 {published data only}
- Sharma K, Kumari R. Study to assess the effectiveness of foot and hand massage on reducing pain among post natal mothers who had undergone caesarean section. International Journal of Nursing Education 2019;11(1):79-84. [DOI: 10.5958/0974-9357.2019.00017.5] [DOI] [Google Scholar]
Simonelli 2018 {published data only}
- Simonelli MC, Doyle LT, Columbia M, Wells PD, Benson KV, Lee CS. Effects of connective tissue massage on pain in primiparous women after cesarean birth. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2018;47(5):591-601. [DOI: 10.1016/j.jogn.2018.07.006] [DOI] [PubMed] [Google Scholar]
Smith 1986 {published data only}
- Smith CM, Guralnick MS, Gelfand MM, Jeans ME. The effects of transcutaneous electrical nerve stimulation on post-cesarean pain. Pain 1986;27:181-93. [DOI: 10.1016/0304-3959(86)90209-5] [DOI] [PubMed] [Google Scholar]
Solehati 2015 {published data only}
- Solehati T, Rustina Y. Benson relaxation technique in reducing pain intensity in women after cesarean section. Anesthesiology and Pain Medicine 2015;5(3):e22236. [DOI: 10.5812/aapm.22236v2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sousa 2009a {published data only}
- Sousa L, Gomes FA, Pitangui AC, Nakano AM. Assessment of transcutaneous electrical nerve stimulation for pain relief after cesarean section: a randomized clinical trial [Avaliação da estimulação elétrica transcutânea do nervo para o alívio de dor após cesareana: ensaio clínico randomizado]. Revista Brasileira de Saúde Maternal Infantil 2009;9(1):49-57. [Google Scholar]
Varghese 2014 {published data only}
- Varghese J, George J, Gowda YS. A randomized control trial to determine the effect of foot reflexology in intensity of pain and quality of sleep in post caesarean mothers. IOSR Journal of Nursing and Health Science 2014;3(1):39-43. [Google Scholar]
Wu 2009 {published data only}
- Wu HC, Liu YC, Ou KL, Chang YH, Hsieh CL, Tsai AH, et al. Effects of acupuncture on post-cesarean section pain. Chinese Medical Journal 2009;122(15):1743-8. [PMID: ] [PubMed] [Google Scholar]
Yang 2019 {published data only}
- Yang GY, Chen QZ, Fu HY, Chen CH. Effect of auricular acupuncture on postpartum rehabilitation of primipara with cesarean. Zhongguo Zhen Jiu = Chinese Acupuncture & Moxibustion 2019;39(7):717-20. [DOI: 10.13703/j.0255-2930.2019.07.010 ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abadi 2018 {published data only}
- Abadi F, Abadi F, Fereidouni Z, Amirkhani M, Karimi S, Najafi Kelyani M. Effect of acupressure on preoperative cesarean section anxiety. Journal of Acupuncture and Meridian Studies 2018;11(6):361-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- IRCT20171015036784N3. The effect of acupressure on anxiety before cesarean section and nausea, vomiting and pain after cesarean section in woman admitted to hospital. en.irct.ir/trial/27409 (first received 29 January 2018).
Abu Bakar 2015 {published data only}
- Abu Bakar MR, Abdul Kadir A, Abdul Wahab SZ, Abdul Karim AH, Nik Hussain NH, Mohd Noor N, et al. Randomized controlled trial on the effect of channa striatus extract on measurement of the uterus, pulsatility index, resistive index of uterine artery and superficial skin wound artery in post lower segment caesarean section women. PLOS One 2015;10(7):e0133514. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Agah 2007 {published data only}
- Agah M, Sadeghi H, Roodneshin F. The efficacy of favorite music on pain after cesarean section. Regional Anesthesia and Pain Medicine 2008;33(5 Suppl 1):183. [Google Scholar]
- Agah M, Sadeghi H. The efficacy of favourite music on pain after cesarean section. Iranian Journal of Obstetrics, Gynecology and Infertility 2007;10(1):41-6. [Google Scholar]
Ali 2017 {published data only}
- Ali AJ, IRCT2017092814049N7. Comparing effectiveness of Damask rose and lavender scent on severity of pain and anxiety after Cesarean section, through women. en.irct.ir/trial/13755 2017.
Allameh 2013 {published data only}
- Allameh T, JabalAmeli M, Lorestani K, Akbari M. The efficacy of quran sound on anxiety and pain of patients under cesarean section with regional anesthesia: A randomized case-controlled clinical trial. Journal of Isfahan Medical School 2013;31(235):601-10. [Google Scholar]
Amin‐Hanjani 1992 {published data only}
- Amin-Hanjani S, Corcoran J, Chatwani A. Cold therapy in the management of postoperative cesarean section pain. American Journal of Obstetrics and Gynecology 1992;167(1):108-9. [DOI] [PubMed] [Google Scholar]
Beiranvand 2014 {published data only}
- Beiranvand S, Noparast M, Eslamizade N, Saeedikia S. The effects of religion and spirituality on postoperative pain, hemodynamic functioning and anxiety after cesarean section. Acta Medica Iranica 2014;52(12):909-15. [PubMed] [Google Scholar]
Blackburn 2011 {published data only}
- Blackburn K, Scott P, Wilson C, Lanier K, Borges A, Curtis E, et al. The effects of music therapy on women's anxiety before and during cesarean delivery. Reproductive Sciences 2011;18(4):374A. [Google Scholar]
- Denney JM, Blackburn KL, Bleach CC, Martinez AR, Philips JB, Lanier K, et al. The effects of music intervention on women's anxiety before and after cesarean delivery: a Randomized Controlled Trial. Music and Medicine 2018;10(4).
- NCT01049477. The effects of music therapy on women's anxiety before and during cesarean delivery. clinicaltrials.gov/ct2/show/record/NCT01049477 (first received 14 Janaury 2010).
Cal 2016 {published data only}
- Cal E, Cakiroglu B, Kurt AN, Hartiningsih SS, Suryani, Dane S. The potential beneficial effects of hand and foot bathing on vital signs in women with caesarean section. Clinical & Investigative Medicine - Medecine Clinique et Experimentale 2016;39(6):S86-8. [PubMed] [Google Scholar]
Chang 2005 {published data only}
- Chang SC, Chen CH. Effects of music therapy on women's physiologic measures, anxiety, and satisfaction during cesarean delivery. Research in Nursing & Health 2005;28(6):453-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chaowalit 2018 {published data only}
- Chaowalit M, TCTR20190605007. Cold therapy for pain relief in postoperative cesarean section ; randomized controlled trial. http://www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=4798 (first received 18 December 2018).
Charoenkwan 2017 {published data only}
- Charoenkwan K, NCT03080506. Effect of elastic abdominal binder on pain and functional recovery after cesarean delivery: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT03080506 (first received 15 March 2017).
Chen 2005 {published data only}
- Chen HM, Chang FY, Hsu CT. Effect of acupressure on nausea, vomiting, anxiety and pain among post-cesarean section women in Taiwan. Kaohsiung Journal of Medical Sciences 2005;21(8):341-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Citak 2012 {published data only}
- Citak Karakaya I, Yuksel I, Akbayrak T, Demirturk F, Karakaya MG, Ozyuncu O, et al. Effects of physiotherapy on pain and functional activities after cesarean delivery. Archives of Gynecology and Obstetrics 2012;285(3):621-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fazel 2017 {published data only}
- Fazel N, Pejhan A, Tabarraei Y, Taghizadeh M, Sharifi N. Effects of anethum graveolens L. (dill) essential oil on the intensity of retained intestinal gas, flatulence and pain after cesarean section: a randomized, double-blind placebo-controlled trial. Journal of Herbal Medicine 2017;8:8-13. [DOI: 10.1016/j.hermed.2017.01.002] [DOI] [Google Scholar]
- IRCT2013061212438N3. The effect of oral dill drop on flatulence in patient post cesarean in Sabzevar Mobini hospital. en.irct.ir/trial/12491 (first received 07 July 2013).
Ghana 2017 {published data only}
- Ghana S, Hakimi S, Mirghafourvand M, Abbasalizadeh F, Behnampour N. Randomized controlled trial of abdominal binders for postoperative pain, distress, and blood loss after cesarean delivery. International Journal of Gynaecology and Obstetrics 2017;37(3):271-6. [DOI] [PubMed] [Google Scholar]
- Ghana S, Hakimi S, Mirghafourvand M, Abbasalizadeh F, Behnampour N. The effects of abdominal binder on wound healing and consumed pain medications after cesarean section: a randomized control trial. Iranian Red Crescent Medical Journal 2017;19(4):e44119. [DOI: 10.5812/ircmj.44119] [DOI] [Google Scholar]
- IRCT2015042521917N2. Comparison of the effects using abdominal binder and routine medical care on clinical outcomes of cesarean section: a randomized controlled trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015042521917N2 2015.
Gillier 2016 {published data only}
- Gillier CM, Sparks JR, Kriner R, Anasti JN. A randomized controlled trial of abdominal binders for the management of postoperative pain and distress after cesarean delivery. International Journal of Gynaecology and Obstetrics 2016;133:188-91. [DOI] [PubMed] [Google Scholar]
- NCT02129894. The use of abdominal binders in patients undergoing cesarean sections: a randomized controlled trial. clinicaltrials.gov/ct2/show/record/NCT02129894 (first received 2 May 2014).
Gist 2018 {published data only}
- Gist W, NCT03711994. Cold therapy for pain control following caesarean section. Available at clinicaltrials.gov/ct2/show/NCT03711994 (first received 19 October 2018).
Gursen 2016 {published data only}
- Gursen C, Inanoglu D, Kaya S, Akbayrak T, Baltaci G. Effects of exercise and Kinesio taping on abdominal recovery in women with cesarean section: a pilot randomized controlled trial. Archives of Gynecology and obstetrics 2016;293(3):557-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gustafson 2018 {published data only}
- Gustafson JL, Dong F, Duong J, Kuhlmann ZC. Elastic abdominal binders reduce cesarean pain postoperatively: a randomized controlled pilot trial. Kansas Journal of Medicine 2018;11(2):1-19. [PMC free article] [PubMed] [Google Scholar]
- Gustafson JL, Kuhlmann ZC, Frazier LM, Duong J. Do elastic abdominal binders reduce postoperative pain and blood loss? A randomized controlled trial.. Obstetrics & Gynecology 2015;125(5 Suppl):51S. [Google Scholar]
- NCT01786330. Do elastic abdominal binders reduce post operative pain and blood loss? clinicaltrials.gov/ct2/show/NCT01786330 (first received 7 February 2013).
Henkel 2018 {published data only}
- Henkel B, Hesse T, Klausenitz C, Hahnenkamp K, Mustea A, Usichenko TI. Acupuncture for pain control after cesarean section - a randomized, placebo-controlled investigation. Journal of Acupuncture & Meridian Studies 2018;11(4):248. [DOI: 10.1016/j.jams.2018.08.172] [DOI]
- NCT02364167. Acupuncture vs. placebo acupuncture and vs. standard therapy for pain control after elective caesarean section - a randomized controlled trial. Available at clinicaltrials.gov/ct2/show/NCT02364167 (first received 16 February 2015).
Ho 1996 {published data only}
- Ho CH, Hseus SS, Lee T, Lee, TY. Effect of P-6 acupressure on prevention of nausea and vomiting after epidural morphine for post-cesarean section pain relief. Acta Anaesthesiologica Scandinavica 1996;40:372-5. [DOI: 10.1111/j.1399-6576.1996.tb04448.x] [DOI] [PubMed] [Google Scholar]
Hollinger 1986 {published data only}
- Hollinger JL. Transcutaneous electrical nerve stimulation after cesarean birth. Physical Therapy 1986;66(1):36-8. [DOI] [PubMed] [Google Scholar]
Hong 2003 {published data only}
- Hong JY, Yoon HJ. The effects of preoperative information and hand massage on postoperative satisfaction score after cesarean section under combined spinal-epidural anesthesia. Korean Journal of Anesthesiology 2003;45(4):492-7. [Google Scholar]
Houshyar 2015 {published data only}
- Houshyar AE, Rezaie HH, Jahani Y, Kazemi M, Monfared S. Comparison of two methods of aromatherapy with lavender essence and Transcutaneous Electrical Nerve Stimulation (TENS) on cesarean postoperative pain. Iranian Journal of Obstetrics, Gynecology and Infertility 2015;18(146):6-12. [DOI: 10.22038/IJOGI.2015.4422] [DOI] [Google Scholar]
Kerai 2011 {published data only}
- Kerai S, Saxena KN, Anand R, Dali JS, Taneja B. Comparative evaluation of transversus abdominis plane block with transcutaneous electrical nerve stimulation for postoperative analgesia following lower segment caesarean section. Journal of Obstetric Anaesthesia and Critical Care 2011;1(1):30-4. [Google Scholar]
Keshavarz 2010 {published data only}
- Keshavarz M. Comparison the effect of skin to skin contact and music during skin to skin contact on maternal state anxiety in cesarean section unit. Available at irct.ir/trial/1959 (first received: 31 October 2009).
Khezri 2017 {published data only}
- IRCT2014040617138N1. Comparison of gabapentin plus vitamin B - complex and gabapentin alone on pain after spinal anesthesia for cesarean section. Available at en.irct.ir/trial/15834 (first received 21 September 2014).
- Khezri MB, Nasseh N, Soltanian G. The comparative preemptive analgesic efficacy of addition of vitamin B complex to gabapentin versus gabapentin alone in women undergoing cesarean section under spinal anesthesia: a prospective randomized double-blind study. Medicine 2017;96(15):e6545. [DOI: 10.1097/MD.0000000000006545] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khoshtarash 2012 {published data only}
- IRCT138812021174N4. The effect of foot reflexology on pain and physiological parameters after cesarean section in patients referring to Alzahra educational center in Rasht. en.search.irct.ir/view/2414 (first received 10 April 2011).
- Khoshtarash M, Ghanbari A, Yeganeh MR, Kazemnejhad E, Rezasoltani P. Effects of foot reflexology on pain and physiological parameters after cesarean section. Koomesh 2012;14(1):109-16. [Google Scholar]
- Khoshtarash M, Ghanbari A, Yeganeh MR, Kazemnejhad E, Rezasoltani P. Survey the effect of foot reflexology on pain and physiological parameters after cesarean section in patients referring to Alzahra Educational Center in Rasht. Holistic Nursing and Midwifery 2010;20(2):27-33. [Google Scholar]
Krum 2006 {published data only}
- Krum LL, Fin M, Ford R, Badger T, Wesley M. Effect of conservative physical therapy management status post cesarean section: a randomized control group design. Journal of Women’s Health Physical Therapy 2006;30(2):27-8. [DOI: 10.1097/01274882-200630020-00010] [DOI] [Google Scholar]
Kuo 2016 {published data only}
- Kuo SY, Tsai SH, Chen SL, Tzeng YL. Auricular acupressure relieves anxiety and fatigue, and reduces cortisol levels in post-caesarean section women: a single-blind, randomised controlled study. International Journal of Nursing Studies 2016;53:17-26. [DOI: 10.1016/j.ijnurstu.2015.10.006] [DOI] [PubMed] [Google Scholar]
Kurdi 2018 {published data only}
- Kurdi MS, Gasti V. Intraoperative meditation music as an adjunct to subarachnoid block for the improvement of postoperative outcomes following cesarean section: a randomized placebo-controlled comparative study. Anesthesia, Essays and Researches 2018;12(3):618-24. [PMID: ] [DOI] [PMC free article] [PubMed]
Kushnir 2012 {published data only}
- Kushnir J, Friedman A, Ehrenfeld M, Kushnir T. Coping with preoperative anxiety in cesarean section: physiological, cognitive, and emotional effects of listening to favorite music. Birth 2012;39(2):121-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2012a {published data only}
- Li Y, Dong Y. Preoperative music intervention for patients undergoing cesarean delivery. International Journal of Gynecology and Obstetrics 2012;119(1):81-3. [DOI: 10.1016/j.ijgo.2012.05.017] [DOI] [PubMed] [Google Scholar]
Li 2012b {published data only}
- Li JZ, Li XZ, Wang MS, Li JP, Shi F, Yu HF. Effects of transcutaneous electrical stimulation of auricular Shenmen point on postoperative nausea and vomiting and patient-controlled epidural analgesia in cesarean section. Chinese Medical Journal 2012;92(27):1892-5. [PMID: ] [PubMed] [Google Scholar]
Mahishale 2014 {published data only}
- Mahishale A, CTRI/2014/06/004662. Effect of post maternity abdominal corset on pain and functional independence in early postpartum women undergone caesarean section- a randomized controlled trial. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=7701 (first received 10 June 2014).
Mohseni 2018 {published data only}
- IRCT2016040516110N3. Clinical trial of the effect of amniotic membrane on post cesarean pain. Available at irct.ir/trial/15170 (first received 30 June 2016).
- Mohseni F, Saem J, Sekhavati E, Molazem Z, Tabrizi R. Amniotic membrane for pain control after cesarean section. Crescent Journal of Medical and Biological Sciences 2018;5(3):198-202. [cjmb.org/uploads/pdf/pdf_CJMB_65.pdf]
Mokhtari 2010 {published data only}
- IRCT138904124299N1. Comparison of impact of foot reflexology massage and bensone relaxation on severity of pain after cesarean section. Available at en.irct.ir/trial/4553 (first received: 29 August 2010).
- Mokhtari NJ, Sirati NM, Sadegui SM, Ghanbari Z, Babatabar DH, Mahmoudi H. Comparison of impact of foot reflexology massage and bensone relaxation on severity of pain after cesarean section. Payesh 2010;9(3):289-98. [payeshjournal.ir/article-1-567-en.html] [Google Scholar]
Mousavi 2017 {published data only}
- IRCT20180526039845N1. Study of the effect of auriculotherapy on nausea and vomiting in women following elective cesarean section. Available at irct.ir/trial/33603 (firste received 27 June 2019).
- Mousavi FS, Golmakani N, Taghanaki HR, Saki A, Akhlaghi F. Effects of auriculotherapy on post cesarean anxiety. Iranian Journal of Obstetrics, Gynecology and Infertility 2017;20(6):50-60. [DOI: 10.22038/ijogi.2017.9325] [DOI] [Google Scholar]
Myers 2014 {published data only}
- Myers JR, Gillier CM, Kriner RM, Constant AD, Wetzel LA, Anasti JN. The use of abdominal binders in patients undergoing cesarean delivery: a prospective randomized controlled trial. Obstetrics & Gynecology 2014;123(5 Suppl):160S. [DOI: 10.1097/01.aog.0000447160.43489.c7] [DOI] [Google Scholar]
Norouzi 2013 {published data only}
- Norouzi F, Keshavarz M, SeyedFatemi N, Montazeri A. The impact of kangaroo care and music on maternal state anxiety. Complementary Therapies in Medicine 2013;21(5):468-72. [DOI: 10.1016/j.ctim.2013.07.006] [DOI] [PubMed] [Google Scholar]
Ohashi 2012 {published data only}
- NCT01383122. The efficacy of pulsed electromagnetic field therapy for management of post-operative pain following cesarean delivery. Available at clinicaltrials.gov/show/NCT01383122 (first received 24 June 2011). [ClinicalTrials.gov: NCT01383122]
- Ohashi Y, Downey K, Berstein P, Guest S, Carvalho JC. The efficacy of pulsed electromagnetic field therapy for management of postoperative pain following cesarean section: a randomized, double blind, placebo controlled study. In: Society for Obstetric Anesthesia and Perinatology (SOAP) 44th Annual Meeting; 2015 May 2-5; Monterey, USA. 2012:S-1.
Rasuli 2017 {published data only}
- Rasuli S, IRCT2017041216325N5. Clinical trials of checking the effect of music therapy during cesarean section on serum ß endorphin, Vital Sign and postoperative pain on pregnant women. Available at en.irct.ir/trial/15310 (first received 28 July 2017).
Razmjoo 2012 {published data only}
- Razmjoo N, lotfabadi LH, Yousefi F, Esmaeeli H, Azizi H, Lotfalizadeh M. Effect of foot reflexology on pain and anxiety in women following elective cesarean section. Iranian Journal of Obstetrics, Gynecology and Infertility 2012;15(1):8-16. [DOI: 10.22038/ijogi.2012.5746] [DOI] [Google Scholar]
Reynolds 1987 {published data only}
- Reynolds RA, Gladstone N, Ansari AH. Transcutaneous electrical nerve stimulation for reducing narcotic use after cesarean section. Journal of Reproductive Medicine 1987;32:843-6. [PMID: ] [PubMed] [Google Scholar]
Reza 2007 {published data only}
- Reza N, Ali SM, Saeed K, Abul-Qasim A, Reza TH. The impact of music on postoperative pain and anxiety following cesarean section. Middle East Journal of Anesthesiology 2007;19(3):573-86. [PMID: ] [PubMed] [Google Scholar]
Robinson 2017 {published data only}
- Robinson JN, NCT03359798. The effect of a counseling intervention on post-cesarean section narcotic use: a randomized controlled trial. Available at clinicaltrials.gov/ct2/show/NCT03359798 (first recevied 19 September 2019).
Saberkari 2009 {published data only}
- Saberkari MR, Nia KN. Transcutaneous electrical nerve stimulation (TENS) on postcesarean section pain. Pain Practice 2009;9(Suppl 1):150. [DOI: 10.1111/j.1533-2500.2009.00267.x] [DOI] [Google Scholar]
Sadeghi 2019 {published data only}
- Sadeghi T, IRCT20150713023190N8. Investigating the effect of auriculotherapy on shoulder pain after cesarean section. Available at en.irct.ir/trial/36298 (first received 20 January 2019).
Sen 2009 {published data only}
- Sen H, Sizlan A, Yanarates O, Kilic E, Ozkan S, Dagli G. The effect of musical therapy on postoperative analgesia. Pain Practice 2009;9:151. [Google Scholar]
- Sen H, Sizlan A, Yanarates O, Kilic E, Ozkan S, Dagli G. The effect of musical therapy on postoperative analgesia. TAF Preventive Medicine Bulletin 2009;8(2):107-12. [Google Scholar]
Shabanian 2017 {published data only}
- Shabanian G, IRCT2016110112101N2. Evaluating the effect of Dill (Anethum graveolens L.) seed essence on pain after cesarean delivery: a clinical trial. Available at en.irct.ir/trial/12228 (first received 23 March 2017).
Sharifi 2013 {published data only}
- Sharifi A, Alipour A, Baharloei S. Comparison of the effect of instrumental music and voices of Holy Quran on anxiety of woman before cesarean. Journal of Urmia Nursing and Midwifery Faculty 2013;106:841-7. [Google Scholar]
Sharifipour 2015b {published data only}
- Sharifipour F, Bakhteh A, Mirmohammad M. Effects of citrus aurantium aroma on post-cesarean anxiety. Iranian Journal of Obstetrics, Gynecology and Infertility 2015;18(169):12-20. [DOI: 10.22038/IJOGI.2015.6131] [DOI] [Google Scholar]
Tarasov 1995 {published data only}
- Tarasov A, Alahuhta S, Salomaki T, Nuutinen L. Acupuncture for the premedication and postoperative pain control in obstetric surgery. Acta Anaesthesiologica Scandinavica 1995;39:164. [DOI: 10.1111/j.1399-6576.1995.tb04298.x] [DOI] [Google Scholar]
vanderVaart 2011 {published data only}
- ISRCTN79265996. The effect of reiki on pain in women undergoing non-emergency caesarean sections. isrctn.com/ISRCTN79265996 (first received 27 May 2009). [ISRCTN79265996]
- Vandervaart S, Berger H, Tam C, Goh I, Gijsen V, Wildt SN, et al. The effect of reiki on pain in women after elective caesarean section-a double blinded randomized controlled trial. Canadian Journal of Clinical Pharmacology 2010;17(1):e221. [Google Scholar]
- Vandervaart S, Berger H, Tam C, Goh I, Gijsen VM, Wildt SN, et al. The effect of distant reiki on pain in women after elective caesarean section: a double-blinded randomised controlled trial. BMJ Open 2011;1(1):e000021. [DOI: 10. 1136/bmjopen-2010- 000021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervaart S. A Double-Blinded Randomized Controlled Trial on the Effect of Distant Reiki on Pain after Non-Emergency Caesarean Section and the Effect of CYP2D6 Variation on Codeine Analgesia [thesis]. Toronto: University of Toronto, 2011. [Google Scholar]
Xue 2016 {published data only}
- Xue M, Fan L, Ge LN, Zhang Y, Ge JL, Gu J, et al. Postoperative foot massage for patients after caesarean delivery. Zeitschrift fur Geburtshilfe und Neonatologie 2016;220(4):173-8. [DOI: 10.1055/s-0042-104802] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Bagherzadeh 2019 {published data only}
- Bagherzadeh R, IRCT20190122042453N1. Investigating the effect of reflexology and relaxation of Benson on pain, physiological symptoms, lactation and weight of newborn in women undergoing cesarean section. n.irct.ir/trial/38249 (first received 5 April 2019).
Balachandran 2019 {published data only}
- Balachandran V, NCT03829774. To study and evaluate the eEffectiveness of treatment by Percutaneous Electrical NeuroStimulation (PENS) for post-operative pain in cesarean section patients using primary relief v 2.0. clinicaltrials.gov/ct2/show/NCT03829774 (first received 4 February 2019).
Hakimi 2018 {published data only}
- Hakimi S, IRCT20150424021917N9. Effect of eremostachys laciniata suppository on post secarean section pain and distress. en.irct.ir/trial/32929 (first received 31 October 2018).
Jahdi 2015 {published data only}
- Jahdi F, IRCT201507252248N18. The effect of calendula ointment on healing and pain localized wound cesarean section in nulliparous women. en.irct.ir/trial/1890 (first received 5 November 2015).
Joghataei 2015 {published data only}
- Joghataei R, IRCT2014122920475N1. Comparison of the effect of foot reflexology and auriculotherapy on pain and anxiety in women following elective cesarean section. en.irct.ir/trial/18154 (first received 6 November 2015).
Kazemi 2019 {published data only}
- Kazemi M, IRCT20131228015965N15. Comparative study of the effects of reflexology and auriculotherapy on the pain after cesarean section. en.irct.ir/trial/34483 (first received 30 July 2019).
Kim 2015 {published data only}
- Kim MJ, NCT02526186. Battlefield auricular acupuncture for control of post-partum pain. clinicaltrials.gov/ct2/show/record/NCT02526186 (first received 18 August 2015).
Klinger 2018 {published data only}
- Klinger R, DRKS00013123. Extent of analgesic effects of transcutaneous electrical nerve stimulation in patients after caesarean section. drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00013123 (first received 2 May 2018).
Latifi 2012 {published data only}
- Latifi S, IRCT201112248498N1. The impact of foot and hand massage on cesarean postoperative pain. en.irct.ir/trial/8957 (first received 24 January 2012).
Maassarani 2018 {published data only}
- Maassarani M, NCT03604068. Protocol for the randomized controlled trial of pulsed short wave therapy in cesarean section. clinicaltrials.gov/ct2/show/NCT03604068 (first received 27 July 2018).
Mobaraki 2019 {published data only}
- Mobaraki F, IRCT20181226042137N1. The examining of the effect of foot massage with orange essence on grade pain and anxiety women under cesarean section. en.irct.ir/trial/38076 (first received 27 April 2019).
Mojalli 2017 {published data only}
- Mojalli M, IRCT2017052834169N1. Chammomil fragrance impact on anxiety and pain after cesarean section in nulliparous women. en.irct.ir/trial/26178 (first recevied 5 September 2017).
Oberbaum 2008 {published data only}
- Oberbaum M, NCT00725569. Homeopathy for post-operative (c. section) recovery. clinicaltrials.gov/show/NCT00725569 (first received 29 July 2008).
Pakseresht 2016 {published data only}
- Pakseresht S, IRCT2016072529063N1. The survey effect of lavender aromatherapy on the pain level after cesarean section among women referred to Al-Zahra hospital in Rasht in 2016- 2017. en.irct.ir/trial/23447 (first received 19 September 2016).
Phillibert 2015 {published data only}
- Phillibert D, NCT02365753. Effect of pulsed electromagnetic field therapy on pain after cesarean delivery. clinicaltrials.gov/ct2/show/NCT02365753 (first received 19 February 2015).
Santana 2014 {published data only}
- Santana JM, Lotti RC, RBR-459y54. Effect of TENS in pain after cesarean. ensaiosclinicos.gov.br/rg/RBR-459y54/ (first recevied 15 September 2014).
Shahoei 2017 {published data only}
- Shahoei R, IRCT2017020314556N4. Effect of transcutaneous electrical nerve stimulation in post-cesarean pain. en.irct.ir/trial/14170 (first received 10 February 2017).
Zardosht 2016 {published data only}
- Zardosht R, IRCT2016042427558N1. Compare the effectiveness of essential oils of chamomile or placebo on intensity and quality of pain after cesarean section. en.irct.ir/trial/22551 (first received 9 October 2016).
Additional references
Althabe 2006
- Althabe F, Belizán JM. Caesarean section: the paradox. Lancet 2006;368:1472-3. [DOI: 10.1016/S0140-6736(06)69616-5] [DOI] [PubMed] [Google Scholar]
Apfelbaum 2003
- Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be under managed. Anesthesia & Analgesia 2003;97(2):534–40. [DOI: 10.1213/01.ANE.0000068822.10113.9E] [DOI] [PubMed] [Google Scholar]
Bagetta 2010
- Bagetta G, Morrone LA, Rombola L, Amantea D, Russo R, Berliocchi L. Neuropharmacology of the essential oil of bergamot. Fitoterapia 2010;81:453-61. [DOI: 10.1016/j.fitote.2010.01.013]] [DOI] [PubMed] [Google Scholar]
Barragán 2011
- Barragán Loayza IM, Solà I, Juandó Prats C. Biofeedback for pain management during labour. Cochrane Database of Systematic Reviews 2011, Issue 6. Art. No: CD006168. [DOI: 10.1002/14651858.CD006168.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bauer 2010
- Bauer BA, Cutshall SM, Wentworth LJ, Engen D, Messner PK, Wood CM, et al. Effect of massage therapy on pain, anxiety and tension after cardiac surgery: a randomized study. Complementary Therapies in Clinical Practice 2010;16(2):70-5. [DOI: 10.1016/j.ctcp.2009.06.012] [DOI] [PubMed] [Google Scholar]
Betrán 2007
- Betrán AP, Merialdi M, Lauer JA, Bing-Shun W, Thomas J, Van Look P, et al. Rates of caesarean section: analysis of global, regional and national estimates. Paediatric and Perinatal Epidemiology 2007;21(2):98-113. [DOI: 10.1111/j.1365-3016.2007.00786.x] [DOI] [PubMed] [Google Scholar]
Bikmoradi 2015
- Bikmoradi A, Seifi, Z, Poorolajal J, Araghchian M, Safiaryan R, Oshvandi, K. Effect of inhalation aromatherapy with lavender essential oil on stress and vital signs in patients undergoing coronary artery bypass surgery: A single-blinded randomized clinical trial. Complementary Therapies in Medicine 2015;23(3):331-8. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Bjordal 2003
- Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. European Journal of Pain 2003;7(2):181–8. [DOI: 10.1016/S1090-3801(02)00098-8] [DOI] [PubMed] [Google Scholar]
Cançado 2012
- Cançado TO, Omais M, Ashmawi HA, Torres ML. Chronic pain after cesarean section. Influence of anesthetic/surgical technique and postoperative analgesia [Dor crônica pós-cesareana. Influência da técnica cirúrgica/anestésica e analgesia pós-operatória]. Revista Brasileira de Anestesiologia 2012;62(6):762-74. [DOI: 10.1590/S0034-70942012000600002] [DOI] [PubMed] [Google Scholar]
Cavallaro 2013
- Cavallaro FL, Cresswell JA, França GV, Victora CG, Barros AJ, Ronsmans C. Trends in caesarean delivery by country and wealth quintile: cross-sectional surveys in southern Asia and sub-Saharan Africa. Bulletin of the World Health Organization 2013;91(12):897-972. [DOI: 10.2471/BLT.13.117598] [DOI] [PMC free article] [PubMed] [Google Scholar]
Committee CAM 2005
- Bondurant S. Complementary and Alternative Medicine in the United States. Institute of Medicine of the National Academies Press edition. Vol. 1. Washington, DC: Committee on the Use of Complementary and Alternative Medicine by the American Public, 2005. [DOI: 10.17226/11182] [DOI] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-analysis in Context. 2nd edition. London (UK): BMJ Publication Group, 2001. [DOI: 10.1002/14651858.] [DOI] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors). In: Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook (accessed 24 September 2019). [Google Scholar]
Desantana 2008
- DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of Transcutaneous Electrical Nerve Stimulation for treatment of hyperalgesia and pain. Current Rheumatology Reports 2008;10(6):492–9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowswell 2009
- Dowswell T, Bedwell C, Lavender T, Neilson JP. Transcutaneous electrical nerve stimulation (TENS) for pain management in labour. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD007214. [DOI: 10.1002/14651858.CD007214.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenach 2008
- Eisenach JC, Pan PH, Smiley R, Lavand’homme P, Landau R, Houle TT. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain 2008;140(1):87–94. [DOI: 10.1016/j.pain.2008.07.011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferraz 2017
- Ferraz GA, Rodrigues MR, Lima SA, Lima MA, Maia GL, Pilan CA Neto, et al. Is reiki or prayer effective in relieving pain during hospitalization for cesarean? A systematic review and meta-analysis of randomized controlled trials. Sao Paulo Medical Journal 2017;135(2):123-32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freitag 2015
- Freitag VL, Andrade A, Badke MR. Reiki as therapeutically in health care: a narrative review of the literature [El Reiki como forma terapéutica en el cuidado de la salud: una revisión narrativa de la literatura]. Enfermería Global 2015;38:335-45. [ISSN 1695-6141] [Google Scholar]
Gadsden 2005
- Gadsden J, Hart S, Santos AC. Post-cesarean delivery analgesia. Anesthesia and Analgesia 2005;101(5):62-9. [PMID: 16334493] [DOI] [PubMed] [Google Scholar]
Garcia 2009
- Garcia MK, Driver L, Haddad R, Lee R, Palmer JL, Moshe QIW. Acupuncture for treatment of uncontrolled pain in cancer patients: a pragmatic pilot study. Integrative Cancer Therapies 2009;20(10):1-8. [DOI: 10.1177/1534735413510558] [DOI] [PubMed] [Google Scholar]
Good 2001
- Good M, Stanton-Hicks M, Grass JA. Relaxation and music to reduce postsurgical pain. Journal of Advanced Nursing 2001;33:208-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Good 2002
- Good M, Anderson GC, Stanton-Hicks M, Grass JA, Makii M. Relaxation and music reduce pain after gynecologic surgery. Pain Management Nursing 2002;3:61-70. [DOI: 10.1053/jpmn.2002.123846] [DOI] [PubMed] [Google Scholar]
Grace 2003
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: A review and critical analysis of the literature. Archives of Women's Mental Health 2003;6:263–74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Green 2002
- Green S, Buchbinder R, Barnsley L, Hall S, White M, Smidt N, et al. Acupuncture for lateral elbow pain. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No: CD003527. [DOI: 10.1002/14651858.CD003527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hattan 2002
- Hattan J, King L, Griffiths P. The impact of foot massage and guided relaxation following cardiac surgery: a randomized controlled trial. Journal of Advanced Nursing 2002;37(2):199-207. [DOI: 10.1046/j.1365-2648.2002.02083.x] [DOI] [PubMed] [Google Scholar]
Heidari Gorji 2014
- Heidari Gorji MA, Davanloo AA, Heidarigorji AM. The efficacy of relaxation training on stress, anxiety, and pain perception in hemodialysis patients. Indian Journal of Nephrology 2014;24(6):356-61. [DOI: 10.4103/0971-4065.132998] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Available from www.training.cochrane.org/handbook (accessed 06 January 2020).
Jones 2012
- Jones L, Othman M, Dowswell T, Alfirevic Z, Gates S, Newburn M, et al. Pain management for women in labour: an overview of systematic reviews. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No: CD009234. [DOI: 10.1002/14651858.CD009234.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2006
- Kim JT, Wajda M, Cuff G, Serota D, Schlame M, Axelrod DM, et al. Evaluation of aromatherapy in treating postoperative pain: pilot study. Pain Practice 2006;6(4):273–7. [DOI: 10.1111/j.1533-2500.2006.00095.x] [DOI] [PubMed] [Google Scholar]
Kimber 2008
- Kimber L, McNabb M, Mc Court C, Haines A, Brocklehurst P. Massage or music for pain relief in labour: a pilot randomised placebo controlled trial. European Journal of Pain 2008;12(8):961-9. [DOI: 10.1016/j.ejpain.2008.01.004] [DOI] [PubMed] [Google Scholar]
Kocyigit 2012
- Kocyigit F, Akalin E, Gezer NS, Orbay O, Kocyigit A, Ada E. Functional magnetic resonance imaging of the effects of low-frequency transcutaneous electrical nerve stimulation on central pain modulation: a double-blind, placebo-controlled trial. Clinical Journal of Pain 2012;28(7):581–8. [DOI: 10.1097/AJP.0b013e31823c2bd7] [DOI] [PubMed] [Google Scholar]
Lee 2014
- Lee MS, Ernst E. Acupuncture for surgical conditions: an overview of systematic reviews. International Journal of Clinical Practice 2014;68(6):783-9. [DOI: : 10.1111/ijcp.12372] [DOI] [PubMed] [Google Scholar]
Manyanga 2014
- Manyanga T, Froese M, Zarychanski R, Abou-Setta A, Friesen C, Tennenhouse M, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complementary and Alternative Medicine 2 2014;14(312):1-9. [DOI: 10.1111/ijcp.12372] [DOI] [PMC free article] [PubMed] [Google Scholar]
Msee 2006
Nilsson 2008
- Nilsson U. The anxiety and pain-reducing effects of music interventions: a systematic review. AORN Journal 2008;87(4):780-807. [DOI: 10.1016/j.aorn.2007.09.01] [DOI] [PubMed] [Google Scholar]
Pan 2006
- Pan PH, Coghill R, Houle TT, Seid MH, Lindel WM, Parker RL, et al. Multifactorial preoperative predictors for postcesarean section pain and analgesic requirement. Anesthesiology 2006;104:417–25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parra 2016
- Parra J. The abuse of cesarean and the principle of beneficence [Abuso de la operación cesárea y el principio de beneficencia]. Revista Latinoamericana de Bioética 2016;16(1):60-71. [DOI: 10.18359/rlbi.1441] [DOI] [Google Scholar]
Paula 2006
- Paula GM, Paula VR, Dias RO, Mattei K. Transcutaneous Electrical Nerve Stimulation (TENS) following cesarean surgery. Brazilian Journal of Physical Therapy 2006;10(2):219-24. [DOI: 10.1590/S1413-35552006000200013] [DOI] [Google Scholar]
Rambod 2014
- Rambod M, Sharif F, Pourali-Mohammadi N, Pasyar N, Rafii F. Evaluation of the effect of Benson's relaxation technique on pain and quality of life of haemodialysis patients: a randomized controlled trial. International Journal of Nursing Studies 2014;51(7):964–73. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ronsmans 2006
- Ronsmans C, Holtz S, Stanton C. Socioeconomic differentials in caesarean rates in developing countries: a retrospective analysis. Lancet 2006;368(9546):1516–23. [DOI: 10.1016/S0140-6736(06)69639-6] [DOI] [PubMed] [Google Scholar]
Salles 2014
- Salles LF, Vannucci L, Salles A, Silva MJ. The effect of Reiki on blood hypertension [Efeito do Reiki na hipertensão arterial]. Acta Paulista de Enfermagem 2014;27(5):479-84. [DOI: ] [Google Scholar]
Schulenburg 2015
- Schulenburg J. Considerations for complementary and alternative Interventions for pain. AORN Journal 2015;101(3):319-26. [DOI: 10.1016/j.aorn.2015.01.013] [DOI] [PubMed] [Google Scholar]
Sluka 2001
- Sluka KA, Walsht D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. Journal of Pain 2003;4(3):109-21. [DOI: 10.1054/jpai,2003.434] [DOI] [PubMed] [Google Scholar]
Smith 2006
- Smith CA, Collins CT, Cyna AM, Crowther CA. Complementary and alternative therapies for pain management in labour. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD003521. [DOI: 10.1002/14651858.CD003521.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2011
- Smith CA, Collins CT, Crowther CA. Aromatherapy for pain management in labour. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD009215. [DOI: 10.1002/14651858.CD009215] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2018a
- Smith CA, Levett KM, Collins CT, Armour M, Dahlen HG, Suganuma M. Relaxation techniques for pain management in labour. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No: CD009514. [DOI: 10.1002/14651858.CD009514.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2018b
- Smith CA, Levett KM, Collins CT, Dahlen HG, Ee CC, Suganuma M. Massage, reflexology and other manual methods for pain management in labour. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No: CD009290. [DOI: 10.1002/14651858.CD009290.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2020
- Smith CA, Collins CT, Levett KM, Armour M, Dahlen HG, Tan AL, Mesgarpour B. Acupuncture or acupressure for pain management during labour. Cochrane Database of Systematic Reviews 2020, Issue 2. Art. No: CD009232. [DOI: 10.1002/14651858.CD009232.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sousa 2009b
- Sousa L, Pitangui AR, Gomes FA, Nakano AM, Ferreira CH. Measurement and characteristics of post-cesarean section pain and the relationship to limitation of physical activities [Mensuração e características de dor após cesárea e sua relação com limitação de atividades]. Acta Paulista de Enfermagem 2009;22(6):741-7. [DOI: 10.1111/j.1447-0756.2011.01824.x] [Google Scholar]
Stevensen 1994
- Stevensen CJ. The psychophysiological effects of aromatherapy massage following cardiac surgery. Complementary Therapies in Medicine 1994;2:21-35. [DOI: 10.1016/0965-2299(94)90156-2] [DOI] [Google Scholar]
Teixeira 2009
- Teixieira N. Reiki: religion or therapeutic practice? [Reiki: religião ou prática terapêutica?]. Belo Horizonte 2009;7(15):142-56. [DOI: ] [Google Scholar]
Vance 2014
- Vance CG, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Management 2014;4:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vickers 2012
- Vickers AJ, Cronin AM, Maschino AC, Lewith G, MacPherson H, Foster NE, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Archives of Internal Medicine 2012;172(19):1444-53. [DOI: 10.1001/archinternmed.2012.3654.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2004
- Wang H, Keck JF. Foot and hand massage as an intervention for postoperative pain. Pain Management Nursing 2004;5(2):59-65. [DOI: 10.1016/j.pmn.2004.01.002] [DOI] [PubMed] [Google Scholar]
Wang 2008
- Wang C, Pablo P, Chen X, Schmid C, Alindon TM. Acupuncture for pain relief in patients with rheumatoid arthritis: a systematic review. Arthritis and Rheumatism 2008;59(9):1249–56. [DOI: 10.1002/art.24009] [DOI] [PubMed] [Google Scholar]
White 2009
- White A, Editorial Board of Acupuncture in Medicine. Western medical acupuncture: a definition. Acupuncture in Medicine 2009;27(1):33-5. [10.1136/aim.2008.000372] [DOI] [PubMed] [Google Scholar]
WHO 2001
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, et al. Trends in alternative medicine use in the United States, 1990—1997: results of a follow-up national survey. In: World Health Organization: Legal Status of Traditional Medicine and Complementary Alternative Medicine: a Worldwide Review. Geneva: World Health Organization, 2001. [DOI: 10.1001/jama.280.18.1569] [DOI] [Google Scholar]
WHO 2002
- World Health Organization. National Policy on Traditional Medicine and Complementary/Alternative Medicine. Geneva: World Health Organization, 2002. [ISBN 978 92 4 150] [Google Scholar]
WHO 2005
- World Health Organization. Facts and Figures from the World Health Report. http://www.who.int/whr/2005/media_centre/facts_en.pdf (accessed 30 June 2014) 2005.
WHO 2013
- World Health Organization. Global Health Observatory Data Repository. Maternal Health Services: births by caesarean section. http://apps.who.int/gho/data/node.main.HE-1571?lang=en (accessed 14 April 2016) 2013.
WHO 2013b
- World Health Organization. Traditional Medicine Strategy 2014–2023. World Health Organization 2013.
Wieland 2011
- Wieland LS, Manheimer E, Berman BM. Development and classification of an operational definition of complementary and alternative medicine for the Cochrane Collaboration. Alternative Therapies in Health and Medicine 2011;17(2):50-9. [PMID: 21717826 ] [PMC free article] [PubMed] [Google Scholar]
Yang 2020
- Yang X, He H, Ye W, Perry TA, He C. Effects of pulsed electromagnetic field therapy on pain, stiffness, physical function, and quality of life in patients with osteoarthritis: a systematic review and meta-analysis of randomized placebo-controlled trials. Physical therapy 2020;6:pzaa054. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhang 2014
- Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture–electroacupuncture on persistent pain. Anesthesiology 2014;120(2):1-22. [DOI: 10.1097/ALN.0000000000000101] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Zimpel 2014
- Zimpel SA, Torloni MR, Porfirio G, da Silva EM. Complementary and alternative therapies for post‐caesarean pain. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No: CD011216. [DOI: 10.1002/14651858.CD011216] [DOI] [PMC free article] [PubMed] [Google Scholar]


