Abstract
The prevalence of Helicobacter pylori (H. pylori) has decreased during several decades due to improvements in the sanitary environment in Japan. Consequently, a relative increase in the incidence of H. pylori-uninfected gastric cancer is expected. We analyzed the trends in H. pylori-uninfected gastric cancer. Two hundred fifty-eight patients with gastric cancer were retrospectively analyzed. The study was divided into four periods: 2008–2011 (first period), 2012–2014 (second period), 2015–2017 (third period), and 2018–2021 (fourth period). The status of H. pylori infection was divided into four categories: uninfected, successful eradication, spontaneous eradication, and persistent infection. Gastric mucosal atrophy was divided into six grades according to the Kimura–Takemoto classification. The proportion of H. pylori infections significantly changed over the study period (p = 0.007). In particular, the rate of H. pylori-uninfected gastric cancer tended to increase over time (0%, 2.9%, 4.9%, and 13.4% in the first, second, third, and fourth periods, respectively; p = 0.0013). The rate of no atrophy (C-0) in gastric cancer tended to increase over time (0%, 2.9%, 4.9%, and 11.0% in the first, second, third, and fourth periods, respectively; p = 0.0046). In conclusion, the rate of H. pylori-uninfected gastric cancer without gastric atrophy tended to increase over time.
Keywords: Helicobacter pylori, uninfected, gastric cancer, trend
Introduction
Gastric cancer is firmly associated with Helicobacter pylori (H. pylori).(1) A prospective, long-term study showed that there were no cases of gastric cancer in H. pylori un-infected group.(2) However, in clinical practice, we occasionally encounter a small number of gastric cancers in H. pylori un-infected cases.(3) Besides H. pylori, there are other carcinogenic factors for gastric cancer such as Epstein–Barr virus infection, high salt intake, genetic factors and so on.(4–6)
The prevalence of H. pylori has decreased during several decades due to improvements in the sanitary environment and widespread eradication of H. pylori in Japan.(7,8) Therefore, a relative increase is expected in the incidence of H. pylori-uninfected gastric cancer. In this study, the trends in H. pylori-uninfected gastric cancer were analyzed.
Methods
Patients
Two hundred seventy-six patients with gastric cancer were retrospectively analyzed using an endoscopic database and clinical charts. Gastric cancers were diagnosed at the Toyoshima Endoscopy Clinic between January 2008 and July 2021. Patients underwent esophagogastroduodenoscopy for symptoms, surveillance for upper gastrointestinal disorders, abnormal findings on upper gastrointestinal Barium X-ray, or screening.(9) Biopsy specimens were taken from lesions suspected to be gastric cancer, and the final diagnosis of gastric cancer was pathologically confirmed.(10) Because the Toyoshima Endoscopic Clinic is an outpatient clinic, patients with gastric cancer were treated at other hospitals. The pathological reports of the resected specimens on patients treated for gastric cancer from other hospitals were also used in this study. The exclusion criteria are as follows: (1) esophagogastric junction carcinoma (n = 11) and (2) unknown H. pylori status (n = 7). Finally, 258 patients with gastric cancer were analyzed. The study was divided into four periods: 2008–2011 (first period), 2012–2014 (second period), 2015–2017 (third period), and 2018–2021 (fourth period).
Clinicopathological assessment
Clinicopathological findings, such as age, sex, gastric mucosal atrophy, and progression of early or advanced cancer, were reviewed. Gastric mucosal atrophy was divided into six grades (C-I, C-II, C-III, O-I, O-II, and O-III) according to the Kimura–Takemoto classification.(11–13) No atrophy was defined as C-0. Early gastric cancer was defined as adenocarcinoma confined to the mucosa and/or submucosa. Advanced gastric cancer was defined as gastric cancer that invaded deeper than the submucosal layer.
H. pylori infection status
The H. pylori infection status was divided into four categories at the time of gastric cancer diagnosis. The four categories included uninfected, successful eradication, spontaneous eradication, and persistent infection.(14) H. pylori uninfected was defined as satisfying the following three criteria: (1) endoscopic findings showed C-0 or C-1 in the status of gastric mucosal atrophy; (2) clinical findings were negative for at least 1 of the 3 following tests: urea breath test, H. pylori stool antigen test, or serum H. pylori antibodies; and (3) no history of H. pylori eradication therapy. Successful eradication was confirmed by a 13C-urea breath test after H. pylori eradication treatment.(15) Spontaneous eradication was defined as satisfying the following three criteria: (1) endoscopic findings showed gastric mucosal atrophy from C-2 to O-3 according to the Kimura–Takemoto Classification; (2) clinical findings were negative for at least 1 of the 3 following tests: 13C-urea breath test, H. pylori stool antigen test, or H. pylori-specific immunoglobulin G antibodies in the serum; and (3) no history of H. pylori eradication therapy.(16) H. pylori infection was defined as H. pylori-positive infection, including eradication failure. H. pylori infection was defined when one of the following tests was positive: urea breath test, stool antigen test, or histology of the background mucosa. If there was a discrepancy in test results, urea breath test results were prioritized. Eradication failure was confirmed by a urea breath test.
Ethics
This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki. This study was approved by the Certificated Review Board of Yoyogi Mental Clinic on July 16, 2021 (approval no. RKK227).
Statistical analysis
Categorical data were compared among the four groups using the χ2 test. The Cochran-Armitage test was used to evaluate trends. We calculated with the Stat Mate IV software (ATOMS, Tokyo, Japan). A p value of less than 0.05 was considered to be statistically significant.
Results
The characteristics of the 258 patients included in this study are shown in Table 1. Among the 258 patients, 47, 68, 61, and 82 were diagnosed with gastric cancer between 2008–2011 (first period), 2012–2014 (second period), 2015–2017 (third period), and 2018–2021 (fourth period), respectively. There were no significant differences among the four groups in terms of age, sex, and the ratio of early gastric cancer to advanced gastric cancer.
Table 1.
Characteristics of gastric cancer by period
| Period | 2008–2011 | 2012–2014 | 2015–2017 | 2018–2021 |
|---|---|---|---|---|
| Patient number | 47 | 68 | 61 | 82 |
| Mean age ± SD | 64.4 ± 12.6 | 65.5 ± 11.6 | 65.3 ± 13.4 | 68.2 ± 11.7 |
| Male:Female | 22:25 | 45:23 | 38:23 | 47:35 |
| Early cancer:Advanced cancer | 38:9 | 59:9 | 49:12 | 68:14 |
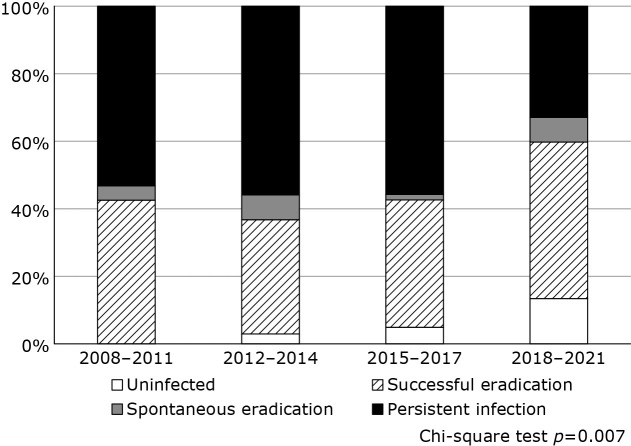
Figure 1 shows the trend in H. pylori status in gastric cancer patients over time. The proportion of H. pylori infection significantly changed over the study period (p = 0.007). In particular, the rate of H. pylori-uninfected gastric cancer tended to increase over time (0%, 2.9%, 4.9%, and 13.4% in the first, second, third, and fourth periods, respectively; p = 0.0013).
Fig. 1.
Trend of H. pylori status in gastric cancer by period.
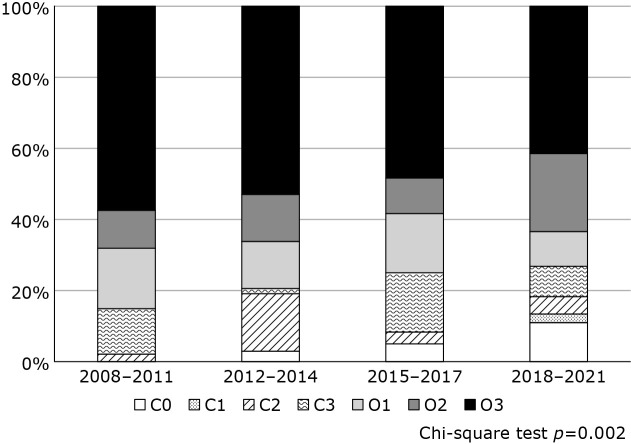
Figure 2 shows the trend of the gastric atrophy status in gastric cancer over time. The proportion of patients with gastric atrophy also changed significantly over the study period (p = 0.002). Particularly, the rate of no atrophy (C-0) in gastric cancer tended to increase over time (0%, 2.9%, 4.9%, and 11.0% in the first, second, third, and fourth periods, respectively; p = 0.0046).
Fig. 2.
Trend of gastric atrophy status in gastric cancer by period.
Table 2 shows clinicopathological features of H. pylori-uninfected gastric cancers. All H. pylori-uninfected gastric cancers were early gastric cancers and were treated by endoscopic resection. Histological types included signet ring cell carcinoma (12.5%), well-differentiated adenocarcinoma (37.5%), papillary adenocarcinoma (25%), and fundic gland type adenocarcinoma (25%).
Table 2.
Clinicopathological features of H. pylori un-infected gastric cancers (n = 16)
| Location | |
| Upper part | 5 (31.2%) |
| Middle part | 7 (43.8%) |
| Lower part | 4 (25%) |
| Tumor size (range; mm) | 5.6 (2–14) |
| Morphology | |
| 0-I (protruding) | 2 (12.5%) |
| 0-IIa (superficial elevated) | 9 (56.2%) |
| 0-IIb (superficial flat) | 2 (12.5%) |
| 0-IIc (superficial depressed) | 3 (18.8%) |
| Depth of invasion | |
| M | 12 (75%) |
| SM | 4 (25%) |
| Histological type | |
| Signet ring cell carcinoma | 2 (12.5%) |
| Well-differentiated adenocarcinoma | 6 (37.5%) |
| Papillary adenocarcinoma | 4 (25%) |
| Fundic gland type adenocarcinoma | 4 (25%) |
Discussion
This study showed that the rates of H. pylori-uninfected gastric cancer and no atrophy in gastric cancer tended to increase over time. This is the first report on the increasing trend of H. pylori-uninfected gastric cancers without gastric atrophy.
This phenomenon is due to the decline in the H. pylori infection rate. A recent systematic review showed decreasing trends in the prevalence of H. pylori infection in Japan.(7) The predicted prevalence rates of H. pylori infection were 64.1%, 59.1%, 49.1%, 34.9%, 24.6%, 15.6%, and 6.6% among those born in 1940, 1950, 1960, 1970, 1980, 1990, and 2000, respectively. The prevalence of H. pylori infection is estimated to decrease by approximately 10% every 10 years across all age groups.
In another study, Kawai et al.(17) reported the cumulative incidence risks for gastric cancer during a lifetime. The risks were 17.0% for males and 7.7% for females in the H. pylori-infected population, and 1.0% for males and 0.5% for females in the uninfected population. While the H. pylori infection rate changes dramatically, a change in the ratio of H. pylori-infected to uninfected gastric cancers would be natural.
The reported incidence of H. pylori-uninfected gastric cancer is in the range of 0.4–8.4%.(18–21) It ranged from 0.4–2.0% before the early 2010s and 1.2–8.4% after the late 2010s. Recent reports have indicated a relatively high incidence. Our study also showed an increasing trend in each period.
Histological types of H. pylori-uninfected gastric cancers included signet ring cell carcinoma (12.5%) and fundic gland-type adenocarcinoma (25%). The endoscopic features of signet ring cell carcinoma include pale-colored, superficial, flat or depressed lesions located in the lower-to-middle third of the stomach.(22) The endoscopic features of fundic gland type adenocarcinoma include flat elevated lesions similar to a submucosal tumor, with a whitish color, located in the middle-to-upper third of the stomach, and without atrophy in the background mucosa.(23) It is necessary to consider these characteristics during endoscopic screening.
A case of fundic gland type adenocarcinoma was reported in 2007.(24) Thereafter, 10 cases were reported by Ueyama et al.,(25) who proposed a novel disease concept based on endoscopic and immunohistochemical findings. Since then, reports on fundic gland type adenocarcinoma have been frequent.(26,27) This lesion has become well-recognized by endoscopists and may have contributed to an increase in the ratio of uninfected gastric cancer.
The present study had some limitations including a small sample size. Furthermore, this was a single-center, retrospective study, although the endoscopic database was well managed. Larger, multicenter studies are recommended for future studies.
In conclusion, the rate of H. pylori-uninfected gastric cancer without gastric atrophy tended to increase over time.
Author Contributions
Conceptualization: OT; Data curation: KS; Formal analysis: TN; Investigation: SY and TM; Writing: TN; Review & editing: AT, JK, HE, HS, MF, and OT.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Nishizawa T, Suzuki H. Gastric carcinogenesis and underlying molecular mechanisms: Helicobacter pylori and novel targeted therapy. Biomed Res Int 2015; 2015: 794378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345: 784–789. [DOI] [PubMed] [Google Scholar]
- 3.Kishikawa H, Kimura K, Ito A, et al. Predictors of gastric neoplasia in cases negative for Helicobacter pylori antibody and with normal pepsinogen. Anticancer Res 2015; 35: 6765–6771. [PubMed] [Google Scholar]
- 4.Matsuo T, Ito M, Takata S, Tanaka S, Yoshihara M, Chayama K. Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter 2011; 16: 415–419. [DOI] [PubMed] [Google Scholar]
- 5.Toyoshima O, Nishizawa T, Sekiba K, et al. A single nucleotide polymorphism in Prostate Stem Cell Antigen is associated with endoscopic grading in Kyoto classification of gastritis. J Clin Biochem Nutr 2021; 68: 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goto A, Nishikawa J, Ito S, et al. Estimation of salt intake from spot urine may assist the risk assessment of gastric cancer. J Clin Biochem Nutr 2020; 66: 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C, Nishiyama T, Kikuchi S, et al. Changing trends in the prevalence of H. pylori infection in Japan (1908–2003): a systematic review and meta-regression analysis of 170,752 individuals. Sci Rep 2017; 7: 15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori H, Suzuki H, Omata F, et al. Current status of first- and second-line Helicobacter pylori eradication therapy in the metropolitan area: a multicenter study with a large number of patients. Therap Adv Gastroenterol 2019; 12: 1756284819858511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishizawa T, Suzuki H, Arita M, et al. Pethidine dose and female sex as risk factors for nausea after esophagogastroduodenoscopy. J Clin Biochem Nutr 2018; 63: 230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishizawa T, Suzuki H, Arano T, et al. Characteristics of gastric cancer detected within 1 year after successful eradication of Helicobacter pylori. J Clin Biochem Nutr 2016; 59: 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969; 1: 87–97. [Google Scholar]
- 12.Takahashi K, Sugimoto M, Kawai Y, et al. Association between dyspeptic symptoms and endoscopic findings based on the Kyoto classification of gastritis in Japanese male. J Clin Biochem Nutr 2022; 70: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishizawa T, Toyoshima O, Kondo R, et al. The simplified Kyoto classification score is consistent with the ABC method of classification as a grading system for endoscopic gastritis. J Clin Biochem Nutr 2021; 68: 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakitani K, Nishizawa T, Arita M, et al. Early detection of gastric cancer after Helicobacter pylori eradication due to endoscopic surveillance. Helicobacter 2018; 23: e12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishizawa T, Suzuki H, Fujimoto A, et al. Effects of patient age and choice of antisecretory agent on success of eradication therapy for Helicobacter pylori infection. J Clin Biochem Nutr 2017; 60: 208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishikawa H, Ojiro K, Nakamura K, et al. Previous Helicobacter pylori infection-induced atrophic gastritis: a distinct disease entity in an understudied population without a history of eradication. Helicobacter 2020; 25: e12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai S, Wang C, Lin Y, Sasakabe T, Okuda M, Kikuchi S. Lifetime incidence risk for gastric cancer in the Helicobacter pylori-infected and uninfected population in Japan: a Monte Carlo simulation study. Int J Cancer 2022; 150: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato S, Matsukura N, Tsukada K, et al. Helicobacter pylori infection-negative gastric cancer in Japanese hospital patients: incidence and pathological characteristics. Cancer Sci 2007; 98: 790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono S, Kato M, Suzuki M, et al. Frequency of Helicobacter pylori-negative gastric cancer and gastric mucosal atrophy in a Japanese endoscopic submucosal dissection series including histological, endoscopic and serological atrophy. Digestion 2012; 86: 59–65. [DOI] [PubMed] [Google Scholar]
- 20.Shibagaki K, Fukuyama C, Mikami H, et al. Gastric foveolar-type adenomas endoscopically showing a raspberry-like appearance in the Helicobacter pylori-uninfected stomach. Endosc Int Open 2019; 7: E784–E791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueyama H, Iwano T, Uchida R, et al. Trend of H. pylori-uninfected gastric cancer: post H. pylori era. Japanese Journal of Helicobacter Research 2022; 23: 114–123. [Google Scholar]
- 22.Kim GH. Systematic endoscopic approach to early gastric cancer in clinical practice. Gut Liver 2021; 15: 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueyama H, Yao T, Akazawa Y, et al. Gastric epithelial neoplasm of fundic-gland mucosa lineage: proposal for a new classification in association with gastric adenocarcinoma of fundic-gland type. J Gastroenterol 2021; 56: 814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukamoto T, Yokoi T, Maruta S, et al. Gastric adenocarcinoma with chief cell differentiation. Pathol Int 2007; 57: 517–522. [DOI] [PubMed] [Google Scholar]
- 25.Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy 2014; 46: 153–157. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Wang S, Zhang Y, Ye F, Wang C. Clinicopathological features of early stage gastric adenocarcinoma of fundic gland type: case series. Medicine (Baltimore) 2022; 101: e28469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishibashi F, Fukushima K, Ito T, Kobayashi K, Tanaka R, Onizuka R. Influence of Helicobacter pylori infection on endoscopic findings of gastric adenocarcinoma of the fundic gland type. J Gastric Cancer 2019; 19: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]