Abstract
Acetic acid is a major component of vinegar and is reported to have beneficial health effects. Notably, it causes oxidative stress and enhances the production of reactive oxygen species (ROS) in gastric cancer cells. ROS play important roles in cellular signal transduction, resulting in the regulation of protein expression and apoptosis. We previously reported that ROS upregulate heme carrier protein 1 (HCP1). Moreover, ROS increase the cellular uptake of porphyrins, which are precursors of heme and substrates for uptake by HCP1. Therefore, we hypothesized that photodynamic therapy (PDT) for cancer treatment using laser irradiation and photosensitizers, such as porphyrin, is enhanced via ROS produced by acetic acid. Herein, we used the rat gastric mucosal cells, RGM1, its cancer-like mutated cells, RGK1, and a manganese superoxide dismutase (MnSOD)-overexpressing RGK cell line, RGK-MnSOD. We confirmed that cancer-specific cellular uptake of porphyrin is increased upon acetic acid treatment and enhances the PDT cytotoxicity in RGK-1, not in RGM-1 and RGK-MnSOD. We believe that this occurs because of the overproduction of ROS and subsequent upregulation of HCP1 in cancerous cells. In conclusion, acetic acid can elevate the effect of PDT by inducing cancer-specific HCP1 expression via ROS production.
Keywords: acetic acid, reactive oxygen species, photodynamic therapy, HCP1, ABCG2
Introduction
Acetic acid is the main component of vinegar and has been reported to have some health benefits. It has been reported to promote calcium absorption and inhibits the renin–angiotensin system to have an antihypertensive effect.(1) It also improves insulin sensitivity and ameliorates type 2 diabetes.(2) Furthermore, acetic acid has been reported to have antitumor effects. It has been reported that “Kibizu”, produced on Amami Oshima Island, Japan, induces apoptosis in leukemia cells and stops the cell cycle in the G1 phase, thereby, suppressing the growth of tumor cells.(3,4) We have previously reported that acetic acid causes cytotoxicity specifically in cancer cells,(5) and increases the levels of reactive oxygen species (ROS) produced by mitochondria (mitROS).(6) ROS are mainly produced by the mitochondrial electron transport chain but are also generated in the endoplasmic reticulum and by the activity of NADPH oxidase. The overproduction of ROS causes redox imbalance, which modulates several signaling pathways and leads to the death of cancer cells.
Photodynamic therapy (PDT) is a relatively less invasive cancer treatment. In PDT, a photosensitizer incorporated into cancer cells is irradiated with light of a specific wavelength, which results in the generation of singlet oxygen and exerts cell-killing effects. Porphyrin, used as a photosensitizer in PDT, accumulates specifically in cancer cells; however, the detailed mechanism through which porphyrin acts as a photosensitizer remains unclear. We have reported that porphyrin is taken up by cells through the heme carrier protein 1 (HCP1), which is highly expressed in cancer cells.(7) Furthermore, we found that the expression of HCP1 is regulated by mitROS and enhances the effect of PDT.(8) Porphyrin is also effluxed out of cells by ATP-binding cassette transporter G2 (ABCG2).(9) We also found that the expression of ABCG2 decreases with increased levels of ROS generation.(10) Thus, we hypothesized that acetic acid-inducible ROS could enhance intracellular porphyrin accumulation and increase the cytotoxicity of PDT toward cancer cells. In this study, we investigated the effect of acetic acid on PDT in terms of ROS production and modulation of the signaling pathway involved in ROS generation in vitro. We evaluated intracellular ROS production, porphyrin accumulation, expression of proteins associated with porphyrin transportation, and the effect of PDT after acetic acid treatment.
Materials and Methods
Cell culture
We previously established and used the following three cell lines: rat normal gastric epithelial cell line, RGM1, its cancer-like mutant cell line, RGK1, and a manganese superoxide dismutase (MnSOD)-overexpressing RGK cell line, RGK-MnSOD.(11,12) MnSOD expresses in the mitochondria and scavenges mitROS, so the effect of mitROS can be investigated by using MnSOD-overexpressing cells. RGM1 was purchased from the Riken Cell Bank (Ibaraki, Japan). The RGM1 was cultured in Dulbecco’s modified Eagle’s medium/Nutrient mixture F-12 with l-glutamine (Life Technologies Co., Carlsbad, CA), and the RGK cells were cultured in DMEM/F12 without l-glutamine (Sigma-Aldrich Co. LLC., St. Louis, MO). The medium was supplemented with 10% fetal bovine serum (Equitech-Bio Inc., Kerrville, TX) and 1% penicillin/streptomycin (Thermo Fisher Scientific, Kanagawa, Japan). The cells were maintained at 37°C in a humidified atmosphere with 5% CO2.
Porphyrin uptake assay
The accumulation of porphyrin in the cells treated with acetic acid was estimated. The cells were cultured overnight in a 12-well cell culture plate at a density of 2 × 105 cells/well. The medium was replaced with fresh medium containing 0, 5, and 10 μM of acetic acid and incubated for 24 h. Thereafter, the cells were washed with phosphate-buffered saline (PBS) twice, and further incubated for 6 h in a fresh medium containing 20 μM of hematoporphyrin dihydrochloride (HpD) (Wako Pure Chem. Ind., Ltd., Osaka, Japan). The cells were then washed with PBS twice and lysed with 100 μl lysis buffer composed of 25 mM Tris-HCl (Sigma-Aldrich Japan K.K., Tokyo, Japan) (pH 7.6), 150 mM NaCl (Wako Pure Chem. Ind., Ltd.), 1% (v/v) Triton X-100 (Sigma-Aldrich Japan K.K.), 0.1% (w/v) sodium dodecyl sulfate (Wako Pure Chem. Ind., Ltd.), and 0.2% (w/v) deoxycholic acid (Wako Pure Chem. Ind., Ltd.). The cell lysate was transferred to a 96-well plate and the fluorescence was measured with a Varioskan microplate reader (Thermo Fisher Scientific K.K.) using excitation/emission wavelengths of 415/625 nm.
Cellular ROS detection by electron spin resonance
The intracellular levels of ROS after treatment with acetic acid were estimated by the ESR method, as described in previous reports.(13,14) Briefly, RGK1 cells were cultured to confluence on small slide cover glasses (49 × 5 × 0.2 mm). These cells were treated with 0, 5, and 10 μM acetic acid-containing medium for 1 h in a 5% CO2 incubator at 37°C. The cover glass was then placed on a quartz flat tissue cell (RDC-60, 60 × 6 × 0.3 mm) (Radical Research Inc., Tokyo, Japan), and 80 μl of a solution for ESR measurement composed of 5 mM each of succinic acid, malic acid, d-glutamic acid, and NADH, and 5 μl 5,5-Dimethyl-1-pyrroline N-oxide was poured onto it. The ESR spectra were measured using a JEOL-TE X-band spectrometer (Jeol, Tokyo, Japan). All ESR spectra were obtained under the following conditions: 10 mW incident microwave power, 0.1 mT modulation width, 8 min sweep time, 7.5 mT sweep width, 0.1 s time contrast, 335.5 mT center field, and 15 mT scan range. ESR measurements and analyses were performed using a Win-Rad Radical Analyzer System (Radical Research).
Western blotting
The cells treated with acetic acid were lysed with the cell lysis buffer mentioned above, and proteins were extracted. Protein samples were mixed with NuPAGE LDS Sample buffer (Life Technologies Japan, Ltd.) and then heated at 70°C for 10 min. The denatured protein samples were applied to the wells of NuPAGE Novex 12% Bis-Tris gels and subjected to SDS-polyacrylamide gel electrophoresis at 100 V for 60 min. The proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore Co., Burlington, MA) by electrophoretic transfer at 2.0 mA/cm2 for 60 min. The membranes were blocked with 15 ml of PVDF Blocking Reagent for Can Get Signal (Toyobo Co. Ltd., Osaka, Japan) for 1 h, and then treated overnight with Can Get Signal Immunoreaction Enhancer Solution 1 (Toyobo Co. Ltd.) containing anti-HCP1 (Aviva Systems Biology, Corp., San Diego, CA), anti-ABCG2 (Cell Signaling Technology, Inc., Danvers, MA), or anti-HIF-1α (Cell Signaling Technology, Inc.) primary antibody at 4°C. The membranes were then washed three times with 15 ml PBS containing 0.1% (v/v) Tween-20 (PBS-T) and subsequently incubated with horseradish peroxidase (HRP)-linked anti-rabbit IgG antibody prepared in Can Get Signal Immunoreaction Enhancer Solution 2 (Toyobo Co. Ltd.) for 60 min at 25°C. Lumina Forte Western HRP Substrate (EMD Millipore, Burlington, MA) was used to develop the blots and images were captured using the ImageQuant LAS 4000 (GE Health Care Japan, Tokyo, Japan). β-Actin was also detected as a control for protein loading.
Cell viability assay after PDT
The cells were cultivated in 96-well plates for 24 h at a concentration of 1 × 105 cells/well. The culture medium was refreshed with a medium containing 0, 5, and 10 μM acetic acid, and the cells were further incubated at 37°C for 24 h. Thereafter, the cells were washed twice with PBS and subsequently cultured in a medium containing 20 μM HpD for 6 h. The cells were then washed twice with PBS and fresh medium without phenol red was added. These cells were irradiated with excimer dye laser (630 nm, 1 J/cm2) using PDT EDL-1 (Hamamatsu Photonics K.K., Hamamatsu, Japan). After irradiation, the cells were incubated for 24 h and the medium was replaced with a medium containing 10% CCK-8 solution (Dojindo Laboratories, Kumamoto, Japan). After incubation at 37°C for 1 h, the absorbance at 450 nm was measured using a DTX 880 multimode microplate reader (Beckman Coulter, Brea, CA).
Statistical analysis
Statistical analysis was performed using the SPSS Statistics 25 software (IBM Corporation, NY). Tukey’s test was used to compare more than two data sets. P<0.05 and p<0.01 were considered as statistically significant differences. All data are represented as means ± SD.
Results
Acetic acid increases the uptake of hematoporphyrin in RGK1
We measured the intracellular levels of porphyrin in cells after treatment with 0, 5, and 10 μM acetic acid for 24 h. The intensity of fluorescence emitted by hematoporphyrin in RGK1 cells increased with the increase in concentration of acetic acid, and significant differences were observed between the control and 10 μM acetic acid-treated cells, while it was unchanged in RGM1 cells and RGK-MnSOD cells (Fig. 1). These results suggest that in cancer cells acetic acid enhances the cellular uptake of hematoporphyrin and/or suppresses its excretion from the cells, and this phenomenon was due to the increase in mitROS.
Fig. 1.
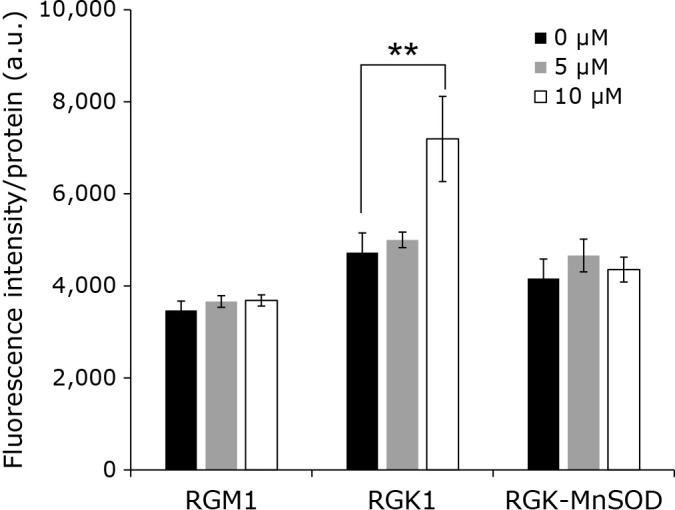
Hematoporphyrin fluorescence intensity. Cells were incubated with various concentrations (0, 5, and 10 μM) of acetic acid for 24 h and 20 μM Hematoporphyrin for 6 h. Error bars indicate SD (n = 8). **p<0.01.
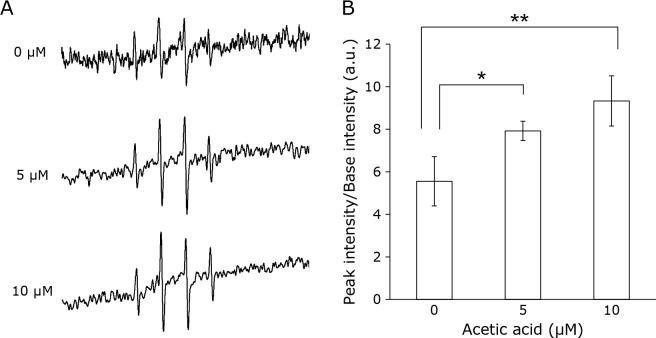
Acetic acid increases the production of ROS in RGK1
We investigated the effect of acetic acid on intracellular ROS production. The levels of ROS in RGK1 cells exposed to 0, 5, and 10 μM acetic acid for 1 h were measured by the electron spin resonance (ESR) method. The ESR signal intensities were increased by acetic acid treatment in a dose-dependent manner (Fig. 2A). The ESR peak signal-to-noise ratio was calculated and was found to be significantly increased in cells treated with 5 and 10 μM compared to that in the untreated cells (Fig. 2B). These results indicate that treatment with acetic acid enhances the intracellular ROS production.
Fig. 2.
Intracellular ROS production in RGK1 cells after acetic acid treatment were measured by ESR. Cells were stimulated with each concentration of acetic acid for 1 h. (A) Representative ESR signals were shown. (B) The relative ratios of ESR signal intensities were shown. Error bars indicate SD (n = 3). *p<0.05, **p<0.01.
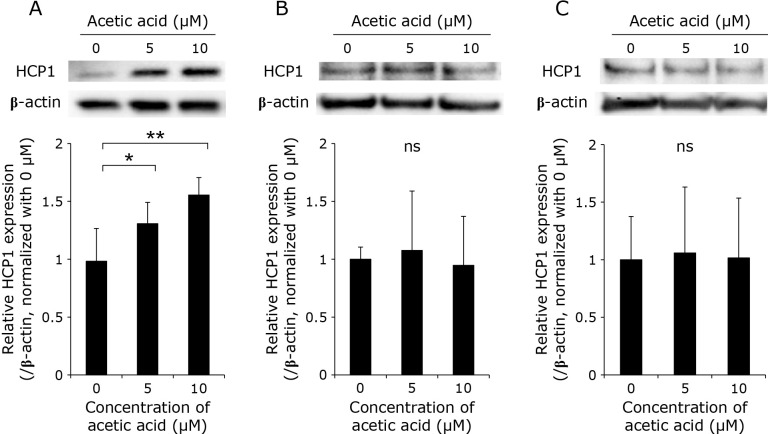
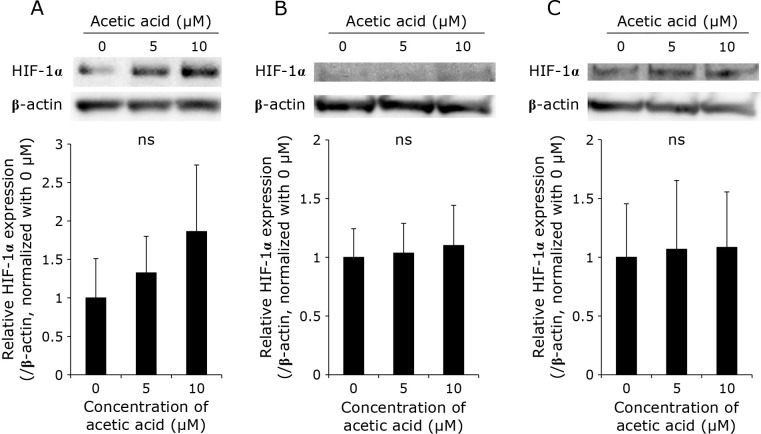
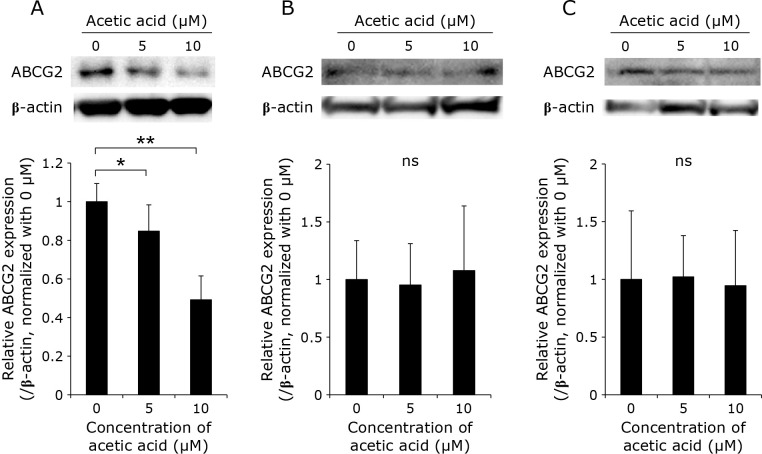
Acetic acid enhances the expression of HCP1 and HIF-1α and attenuates the expression of ABCG2 in RGK1
The expression levels of HCP1 and ABCG2, which are transporters responsible for the uptake and efflux of porphyrin, respectively, as well as of hypoxia inducible factor-1α (HIF-1α), which is a transcription factor induced by hypoxia, in the cells treated with acetic acid were measured by Western blotting. In RGK1, the acetic acid treatment increased the expression of HCP1 and HIF-1α in a dose-dependent manner (Fig. 3A and 5A). Particularly, a significant increase in the expression of HCP1 was observed. On the other hand, the expression of ABCG2 was significantly decreased by the acetic acid treatment (Fig. 4A). In comparison, the expression of these three proteins was almost unchanged in RGM1 (Fig. 3B, 4B, and 5B). Similar results were obtained in RGK-MnSOD (Fig. 3C, 4C, and 5C). These results suggest that acetic acid enhances the intracellular uptake of porphyrin and is difficult to excrete in cancer cells, not in normal gastric mucosal cells and MnSOD-overexpressing cells.
Fig. 3.
Representative Western blotting images of HCP1 in (A) RGK1, (B) RGM1, (C) RGK-MnSOD cells. Cells were stimulated with various concentrations (0, 5, and 10 μM) of acetic acid for 24 h. Relative expression was estimated and shown in graphs. Error bars indicate SD (n = 3). ns, not significant. *p<0.05, **p<0.01.
Fig. 5.
Representative Western blotting images of HIF-1α in (A) RGK1, (B) RGM1, (C) RGK-MnSOD cells. Cells were stimulated with various concentrations (0, 5, and 10 μM) of acetic acid for 24 h. Relative expression was estimated and shown in graphs. Error bars indicate SD (n = 3). ns, not significant.
Fig. 4.
Representative Western blotting images of ABCG2 in (A) RGK1, (B) RGM1, (C) RGK-MnSOD cells. Cells were stimulated with various concentrations (0, 5, and 10 μM) of acetic acid for 24 h. Relative expression was estimated and shown in graphs. Error bars indicate SD (n = 3). ns, not significant. *p<0.05, **p<0.01.
Acetic acid enhances the cytotoxic effect of PDT in RGK1
We evaluated the phototoxic effects of pretreatment with acetic acid and treatment with porphyrin compounds on the cells. Briefly, hematoporphyrin compounds were administered to cells that had been pretreated with acetic acid, and these cells were irradiated with an excimer dye laser. After photo-irradiation, cell viability was measured by the Cell Counting Kit-8 (CCK-8) assay. The viability of RGK1 cells was decreased depending on the acetic acid concentration (Fig. 6). As shown in Fig. 1, 3A, and 4A, the intracellular concentration of porphyrin increased upon pretreatment with acetic acid as a result of the upregulation and downregulation of porphyrin uptake and efflux transporters, respectively. On the other hand, the viability of RGM1 and RGK-MnSOD cells were remained almost unchanged. These results indicate that acetic acid treatment enhances the phototoxicity in cancer cells by increasement of mitROS and contributes to the reduction in cell viability.
Fig. 6.
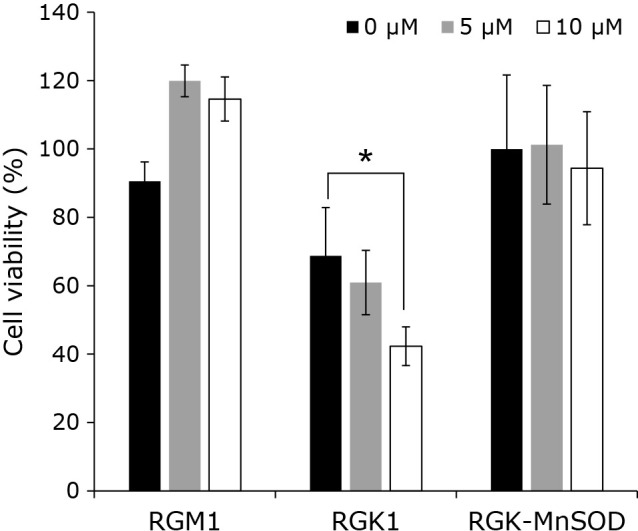
The effect of PDT after acetic acid treatment. Cells were treated with various concentrations (0, 5, and 10 μM) of acetic acid for 24 h, and 20 μM Hematoporphyrin for 6 h. Cells were irradiated with the excimer dye laser light (635 nm, 1 J/cm2). Error bars indicate SD (n = 4). *p<0.05.
Discussion
Acetic acid has been used by humans since ancient times and has been reported to have beneficial effects on health. We previously reported that acetic acid specifically induces the death of cancer cells via the overproduction of ROS in these cells.(6) In addition, induction of intracellular ROS generation enhances porphyrin accumulation and phototoxicity in cancer cells.(7) In this study, we evaluated the effects of acetic acid treatment on PDT. We have shown that acetic acid induces the production of ROS inside the RGK1 cells, and the intracellular level of porphyrin was increased. Moreover, acetic acid was observed to upregulate HCP1, which is a transporter involved in the uptake of porphyrin, whereas it downregulated the expression of ABCG2, which is involved in the efflux of porphyrin. In addition, HIF-1α was also upregulated by acetic acid treatment (although not significantly). We previously reported that HCP1 is involved in the uptake of porphyrin and that its expression is enhanced by mitROS.(7,8) In contrast, the expression of HCP1 and ABCG2 were not changed in RGK-MnSOD cells, and the intracellular level of porphyrin also unchanged. Furthermore, acetic acid did not enhance the effect of PDT in MnSOD-overexpression cells. The results of this study imply that mitROS generated by acetic acid treatment induces the expression of HCP1 and the subsequent intracellular accumulation of porphyrin, thereby, enhancing the efficacy of laser therapy. Moreover, we previously reported that the cytotoxic effect of PDT could be enhanced by induction of mitROS even in cancer cells with different expression of HCP1 or ABCG2.(10) Therefore, it is considered that this PDT-enhancing effect due to the increase of mitROS can be applied to cancer cell lines with different expression levels of them.
HIF-1 is an oxygen-regulated transcriptional activator that plays essential roles in the development, physiology, and pathogenesis in mammals.(15) It is a heterodimer consisting of HIF-1α and HIF-1β. It is reported that ROS production stabilizes HIF-1α.(16) Notably, HIF-1α has been reported to regulate the expression of HCP1.(17) Thus, we suggest that elevation in the expression of HIF-1α by acetic acid contributes to increased expression of HCP1 in RGK1 cells.
ABCG2 is a transporter involved in the efflux of porphyrins, and the molecular mechanism underlying its function has been investigated. Some studies have reported on the relationship between HIFs and ABCG2, and it has been shown in several reports that the activation of HIFs enhances the expression of ABCG2.(18,19) On the contrary, our results indicate that overproduction of ROS increases the expression of HIF-1α and decreases that of ABCG2. We previously reported that the expression of ABCG2 is decreased with the overproduction of ROS.(20) Furthermore, Ogura et al.(21) reported that ROS derived from xanthine oxidase decreases the level of the ABCG2 homodimer. Thus, the expression of ABCG2 is regulated through ROS generation. However, this regulation might be through some other pathway that is different from the signaling mediated by HIF-1α.
ABCG2 is also known as breast cancer resistance protein (BCRP), and is a transporter that is greatly involved in anticancer drug resistance because it causes the efflux of anticancer drugs from the cells.(22) It has been reported that ABCG2 is involved in the excretion of various anticancer agents, such as irinotecan and doxorubicin,(23) and that an inhibitor of ABCG2 enhanced the effect of anticancer drugs.(24) ROS induced by acetic acid decreased the expression of ABCG2, suggesting that the combined use of acetic acid and anticancer drugs can enhance cell damage. Further studies are needed for investigating this premise.
In clinical settings, PDT is approved for esophageal cancer that has recurred after chemoradiation or radiation therapy for gastrointestinal cancer in Japan. However, further expansion of therapeutic indications is being considered. Because of aging, the number of patients with chronic diseases, such as heart and cerebrovascular diseases, and those taking antithrombotic drugs is increasing. PDT is a useful treatment for these cases owing to its low invasiveness. Acetic acid is reported to be beneficial for our health, and some types of vinegars are effective in cancer treatment as mentioned above. In this study, acetic acid did not enhance the cytotoxic effect of PDT in normal gastric mucosal cells. Additionally, acetic acid is routinely used for endoscopic diagnosis to visualize the external structure of the gastrointestinal tract. Therefore, combination therapy with acetic acid and PDT could be a better treatment option for cancer.
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
We have shown the effect of acetic acid on PDT in terms of the modulation of redox signaling pathway in vitro. To investigate and confirm its efficacy in humans, further in vivo studies are under consideration.
Author Contributions
Conceptualization, DM, HI, MT, and HM; methodology, HI and HM; formal analysis, DM, HK, HI, and MT; investigation, DM, HK, HI, and MT; data curation, DM; writing – original draft preparation, DM and MT; writing – review and editing, HK, HI, MT, and HM; visualization, DM; supervision, HM; project administration, HI; funding acquisition, HM. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We appreciate Dr. Shigeru Matsumoto for his continuous support of this work.
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kondo S, Tayama K, Tsukamoto Y, Ikeda K, Yamori Y. Antihypertensive effects of acetic acid and vinegar on spontaneously hypertensive rats. Biosci Biotechnol Biochem 2001; 65: 2690–2694. [DOI] [PubMed] [Google Scholar]
- 2.Johnston CS, Kim CM, Buller AJ. Vinegar improves insulin sensitivity to a high-carbohydrate meal in subjects with insulin resistance or type 2 diabetes. Diabetes Care 2004; 27: 281–282. [DOI] [PubMed] [Google Scholar]
- 3.Mimura A, Suzuki Y, Toshima Y, et al. Induction of apoptosis in human leukemia cells by naturally fermented sugar cane vinegar (kibizu) of Amami Ohshima Island. Biofactors 2004; 22: 93–97. [DOI] [PubMed] [Google Scholar]
- 4.Nanda K, Miyoshi N, Nakamura Y, et al. Extract of vinegar “Kurosu” from unpolished rice inhibits the proliferation of human cancer cells. J Exp Clin Cancer Res 2004; 23: 69–75. [PubMed] [Google Scholar]
- 5.Okabe S, Okamoto T, Zhao CM, Chen D, Matsui H. Acetic acid induces cell death: an in vitro study using normal rat gastric mucosal cell line and rat and human gastric cancer and mesothelioma cell lines. J Gastroenterol Hepatol 2014; 29 Suppl 4: 65–69. [DOI] [PubMed] [Google Scholar]
- 6.Terasaki M, Ito H, Kurokawa H, et al. Acetic acid is an oxidative stressor in gastric cancer cells. J Clin Biochem Nutr 2018; 63: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiyama K, Matsui H, Tamura M, et al. Cancer cells uptake porphyrins via heme carrier protein 1. J Porphyr Phthalocyanines 2012; 17: 36–43. [Google Scholar]
- 8.Ito H, Matsui H, Hirayama A, Indo HP, Majima HJ, Hyodo I. Reactive oxygen species induced by non-steroidal anti-inflammatory drugs enhance the effects of photodynamic therapy in gastric cancer cells. J Clin Biochem Nutr 2016; 58: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnamurthy P, Ross DD, Nakanishi T, et al. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem 2004; 279: 24218–24225. [DOI] [PubMed] [Google Scholar]
- 10.Kurokawa H, Ito H, Terasaki M, Matsui H. Hyperthermia enhances photodynamic therapy by regulation of HCP1 and ABCG2 expressions via high level ROS generation. Sci Rep 2019; 9: 1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi I, Kawano S, Tsuji S, et al. RGM1, a cell line derived from normal gastric mucosa of rat. In Vitro Cell Dev Biol Anim 1996; 32: 259–261. [DOI] [PubMed] [Google Scholar]
- 12.Shimokawa O, Matsui H, Nagano Y, et al. Neoplastic transformation and induction of H+,K+-adenosine triphosphatase by N-methyl-N'-nitro-N-nitrosoguanidine in the gastric epithelial RGM-1 cell line. In Vitro Cell Dev Biol Anim 2008; 44: 26–30. [DOI] [PubMed] [Google Scholar]
- 13.Tamura M, Matsui H, Tomita T, et al. Mitochondrial reactive oxygen species accelerate gastric cancer cell invasion. J Clin Biochem Nutr 2014; 54: 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsui H, Nagano Y, Shimokawa O, et al. Gastric acid induces mitochondrial superoxide production and lipid peroxidation in gastric epithelial cells. J Gastroenterol 2011; 46: 1167–1176. [DOI] [PubMed] [Google Scholar]
- 15.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 2001; 13: 167–171. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida K, Kirito K, Yongzhen H, Ozawa K, Kaushansky K, Komatsu N. Thrombopoietin (TPO) regulates HIF-1α levels through generation of mitochondrial reactive oxygen species. Int J Hematol 2008; 88: 43–51. [DOI] [PubMed] [Google Scholar]
- 17.Kurokawa H, Ito H, Terasaki M, et al. Nitric oxide regulates the expression of heme carrier protein-1 via hypoxia inducible factor-1α stabilization. PLoS One 2019; 14: e0222074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He X, Wang J, Wei W, et al. Hypoxia regulates ABCG2 activity through the activivation of ERK1/2/HIF-1α and contributes to chemoresistance in pancreatic cancer cells. Cancer Biol Ther 2016; 17: 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang L, Liu ZH, Huan Q, et al. Hypoxia-inducible factor-2a is associated with ABCG2 expression, histology-grade and Ki67 expression in breast invasive ductal carcinoma. Diagn Pathol 2012; 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurokawa H, Ito H, Matsui H. The cisplatin-derived increase of mitochondrial reactive oxygen species enhances the effectiveness of photodynamic therapy via transporter regulation. Cells 2019; 8: 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogura J, Kuwayama K, Sasaki S, et al. Reactive oxygen species derived from xanthine oxidase interrupt dimerization of breast cancer resistance protein, resulting in suppression of uric acid excretion to the intestinal lumen. Biochem Pharmacol 2015; 97: 89–98. [DOI] [PubMed] [Google Scholar]
- 22.Staud F, Pavek P. Breast cancer resistance protein (BCRP/ABCG2). Int J Biochem Cell Biol 2005; 37: 720–725. [DOI] [PubMed] [Google Scholar]
- 23.Westover D, Li F. New trends for overcoming ABCG2/BCRP-mediated resistance to cancer therapies. J Exp Clin Cancer Res 2015; 34: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maliepaard M, van Gastelen MA, Tohgo A, et al. Circumvention of breast cancer resistance protein (BCRP)-mediated resistance to camptothecins in vitro using non-substrate drugs or the BCRP inhibitor GF120918. Clin Cancer Res 2001; 7: 935–941. [PubMed] [Google Scholar]