Abstract
Bacterial superantigens (SAgs) have been implicated in inflammatory disease, and SAg-treated mice have increased jejunal T cells. Here we show that T84 cells (a human epithelial cell line) display increased MCP-1 and RANTES mRNA expression and protein production in response to conditioned medium from Staphylococcus aureus enterotoxin B (SEB; a model SAg)-activated immune cells. Also, MCP-1 and RANTES mRNAs were increased in jejunal enterocytes isolated from SEB-treated mice. We suggest that T-cell recruitment to the gut following SAg immune activation could be partially due to epithelium-derived chemokines.
Exposure to bacterial superantigens (SAgs) causes polyclonal T-cell activation (22), and it has been hypothesized that SAgs are involved in the pathophysiology of inflammatory bowel disease (7). Treatment with the prototypic SAg Staphylococcus aureus enterotoxin B (SEB) was found to result in increased CD3+ T cells in the lamina propria of the mouse jejunum with the cells aligned along the epithelial basement membrane and in an intraepithelial location (1). Likewise, Berry et al. have reported increased intraepithelial lymphocytes and lamina propria lymphocytes in the proximal small bowel in SEA-treated rats (3). It has become increasingly apparent that epithelial cells, irrespective of their location, can produce a variety of chemokines (23). Therefore, the present study was designed to assess epithelial chemokine production following SAg immune activation. A variety of chemokines can elicit T-cell and monocyte chemotaxis, and so we focused this investigation by specifically examining the epithelial synthesis of monocyte chemoattractant protein 1 (MCP-1) and regulated on activation, normal T-cell expressed and secreted protein (RANTES); both of which are chemoattractants for immune mononuclear cells.
(This work was presented in part at the 10th International Congress of Mucosal Immunology, Amsterdam, The Netherlands [abstr. 9.4].)
Initially, a reductionistic strategy was adopted by which the effect of conditioned medium from SEB-activated human peripheral blood mononuclear cells (PBMC) (SEB-CM) on chemokine synthesis by the human colonic T84 epithelial cell line was examined. Briefly, PBMC were isolated, resuspended at 106/ml, and treated with 1 μg of SEB per ml for 24 h (8). The cell-free SEB-CM was collected, diluted 1:1 in fresh medium, and added to semiconfluent monolayers of T84 cells. At 3, 6, 9, and 24 h later, total epithelial RNA was extracted by using TRIzol reagent and treated with DNase I (GIBCO, Bio-Rad Laboratories) and 2 μg of RNA was reverse transcribed to cDNA by using 50 U of Expand reverse transcriptase (Boehringer Mannheim) per ml. This was followed by PCRs for MCP-1, RANTES, and the housekeeping gene for glyceraldehyde 3-phosphate dehydrogenase (G3PDH) (Table 1). PCR products were separated on agarose gels and visualized by ethidium bromide staining, and band density was determined by using Kodak 1D image analysis software (Eastman Kodak Co., Rochester, N.Y.). The epithelial response to SEB-CM was compared to that of time-matched T84 cells grown in culture medium only and to that of epithelial preparations treated with human recombinant tumor necrosis factor alpha (TNF-α) only or TNF-α plus gamma interferon (IFN-γ), both at 5 ng/ml (R&D Systems, Minneapolis, Minn.) (these amounts of cytokine are representative of the levels measured in SEB-CM by enzyme-linked immunosorbent assay in this study and in previous studies with this model system [8]). Densitometry analysis of the G3PDH PCR product was used to calculate the dilution factor required to give a constant G3PDH signal. This dilution factor was applied to all of the cDNA samples prior to RANTES and MCP-1 PCRs. Each PCR and gel electrophoresis was performed in duplicate, and the average densitometry value of the resultant bands was used for intergroup comparisons. Data were compared by one-way analysis of variance followed by intergroup comparison with the Newman-Keuls test using WINKS software (Texsoft). T84 cells treated with SEB only (1 μg/ml) and epithelium exposed to conditioned medium from nonactivated immune cells (Fig. 1A) were included as additional controls. Neither treatment significantly affected epithelial RANTES or MCP-1 mRNA expression (SEB; data not shown) (Fig. 1A), and so, T84 cells exposed to culture medium only were used as controls throughout the major portion of this study.
TABLE 1.
Sequences of primers used in this study
| Species and product | Primer sequence | Product size (bp) | No. of PCR cycles | % Agarose gel | Reference |
|---|---|---|---|---|---|
| Human | |||||
| G3PDHa | 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ | 983 | 16 | 1.5 | |
| 5′-CATGTGGGCCATGAGGTCCACCAC-3′ | |||||
| MCP-1 | 5′-CCCAGGGGTAGAACTGTGGTTCAA-3′ | 480 | 34 | 1.1 | 24 |
| 5′-CTCGCTCAGCCAGATGCAATCAAT-3′ | |||||
| RANTES | 5′-CGCTGTCATCCTCATTGCTA-3′ | 197 | 30 | 0.8 | 17 |
| 5′-CACACACTTGGCGGTTCTT-3′ | |||||
| Mouse | |||||
| G3PDH | 5′-CCATGGAGAAGGCTGGGG-3′ | 194 | 25 | 1.5 | 12 |
| 5′-CAAAGTTGTCATGGATGACC-3′ | |||||
| MCP-1 | 5′-AGCCAACTCTCACTGAAGCCA-3′ | 444 | 31 | 1.1 | 12 |
| 5′-CTACACAGAAGTGCTTGAGGTGGT3′ | |||||
| RANTES | 5′-ACCTGCCTCCCCATATGGCT-3′ | 200 | 32 | 2.0 | 6 |
| 5′-GTATTCTTGAACCCACTTCTTC3′ |
Primers purchased from Clontech Laboratories, Palo Alto, Calif.
FIG. 1.
T84 chemokine mRNA expression. (A) Representative gel electrophoresis of PCR products amplified for G3PDH, MCP-1, and RANTES after 6 and 24 h of exposure to medium only (lane 1), 50% conditioned medium from nonactivated PBMC (lane 2), 50% conditioned medium from SEB-activated PBMC (lane 3), TNF-α (5 ng/ml) (lane 4), or TNF-α plus IFN-γ (both at 5 ng/ml) (lane 5). (B and C) Relative change in MCP-1 and RANTES band density (data were normalized and are presented as fold increases compared to time-matched control T84 cells cultured in medium only; mean ± SEM; n = 3 to 6; ∗, P < 0.05 compared to other groups).
Figure 1 shows the time-dependent increase in MCP-1 and RANTES mRNAs in SEB-CM-treated T84 cells. By 3 h posttreatment MCP-1 mRNA was statistically significantly increased (range, 3.5- to 34-fold increase) and remained ∼4-fold higher than that of time-matched controls at 24 h posttreatment (Fig. 1B). In contrast, TNF-α alone evoked only a modest increase in MCP-1 mRNA, and while the combination of TNF-α and IFN-γ did elicit a significant increase in MCP-1 mRNA, the response was consistently less than that elicited by SEB-CM at all time points examined in all experiments. In three to six separate experiments, RANTES expression was always increased (about twofold) in T84 cells treated with SEB-CM (6 to 24 h) compared to that in time-matched control T84 cells (Fig. 1C). This increase in RANTES mRNA expression was statistically significant. Exposure to recombinant TNF-α or TNF-α plus IFN-γ elicited a negligible or only a small RANTES mRNA response from T84 cells. Clearly, analysis of mRNA expression can be of little biological significance in the absence of data relating to the production of functional protein. Figure 2 illustrates MCP-1 and RANTES protein levels in medium from the epithelial cultures as determined by commercial enzyme-linked immunosorbent assays (sensitivity, 50 pg/ml) (since SAg T-cell activation can result in chemokine synthesis [15], the data in Fig. 2 were corrected for chemokine levels in SEB-CM prior to the addition to T84 cells). Essentially, the protein levels paralleled the pattern of mRNA expression: the highest levels of MCP-1 and RANTES were consistently measured in conditioned medium for SEB-CM-treated epithelial cells, which also gave the largest increase in mRNA expression. However, smaller increases in mRNA in response to the recombinant cytokine (compare Fig. 1B and 2A and the 24-h time point for RANTES) were not accompanied by a significant increase in protein. Also noteworthy is the point that a substantially smaller relative increase in RANTES mRNA in response to SEB-CM, compared to MCP-1, resulted in a large increase in RANTES protein; this might imply either better translation efficacy of RANTES mRNA or greater RANTES protein stability in vitro following exposure to the mixed-mediator milieu. The reasons for these disparities are unknown, and further investigations are required to elucidate the factors responsible for the apparent inconsistency between increased mRNA, as assessed by reverse transcription-PCR, and translation to a protein product. Despite this, the data illustrate a significant up-regulation of epithelial RANTES and MCP-1 production in response to SEB immune activation and also highlight the fact that epithelium-derived chemokines are not merely produced en masse but that there is a temporal relationship between the two chemokines, with the level of MCP-1 mRNA and protein peaking before that of RANTES.
FIG. 2.
Bar graphs showing the production of MCP-1 (A) and RANTES (B) proteins by T84 cells in response to treatment with 50% conditioned medium from SEB-activated PBMC (SEB-CM), TNF-α (5 ng/ml), or TNF-α plus IFN-γ (both at 5 ng/ml) (mean ± SEM; n = 3 to 6; ∗, P < 0.05 compared to other groups).
This is the first demonstration that gut epithelial cells can be mobilized to produce chemokines in response to the mixed-mediator milieu produced in response to SAg immune activation. However, numerous investigators have convincingly proven that epithelial cells can either constitutively express MCP-1 and/or RANTES (2, 4, 16) or are induced to do so in response to infectious agents, bacterial products, or recombinant cytokines (19, 23). While there is some variability in the amount and time course of chemokine production, it is clear that epithelial cells can respond to noxious stimuli or proinflammatory cytokines by increasing RANTES and MCP-1 synthesis. Our data extend these observations by showing that low doses of TNF-α (i.e., 5 ng/ml), with or without IFN-γ, have little effect on T84 MCP-1 or RANTES production compared to the physiological milieu created by SAg immune activation. Studies that have shown direct effects of TNF-α and IFN-γ on epithelial chemokine synthesis have typically used the recombinant cytokine at 10 to 100 ng/ml (18, 19, 21, 23), and thus we suggest that the use of physiological supernatants can reveal effects that may be overlooked when single recombinant cytokines are used (9). Furthermore, TNF-α and IFN-γ are important mediators in the modulation of T84 physiology in response to SAg immune activation (8); however, preliminary observations (unpublished data) with neutralizing antibodies against TNF-α and IFN-γ indicate only a small role for these cytokines in SEB-CM-induced epithelial MCP-1 and RANTES mRNA expression (antibody efficacy was confirmed by the ability to prevent signal transduction events in response to recombinant cytokines [unpublished data]). This correlates well with the limited ability of the recombinant cytokines to elicit RANTES and MCP-1 production in this study. Clearly, other factors within the conditioned medium (possibly interleukin-1 or interleukin-6, the levels of which are increased by SEB stimulation [8]) are involved in the modulation of the epithelial chemokine response, acting either alone or in concert with TNF-α with or without IFN-γ (9). Similarly, it is feasible that the activity of these unidentified factors may explain the disparity between chemokine mRNA and protein highlighted above. Also, systemic SEB treatment evoked an increase in lung MCP-1 mRNA that was not significantly different when normal mice were compared with those lacking TNF-α or IFN-γ receptors (13).
Steroids are a mainstay therapy for inflammation, and many studies have documented that steroid treatment of patients with inflammatory disease or in vitro steroid treatment of epithelial cultures can reduce chemokine synthesis (14, 19–21). Also, in in vitro systems analogous to that used here it was shown that both T-cell- and monocyte-driven epithelial pathophysiology could be prevented, at least partially, by the steroid budesonide (11, 25). Thus, T84 monolayers were pretreated with budesonide (10−6 M; Sigma Chemical Co.) for 1 h. The medium was aspirated from the culture well and replaced with 50% SEB-CM plus fresh budesonide, and epithelial RNA was extracted 24 h later and processed for RANTES mRNA expression. Budesonide treatment alone did not significantly affect T84 RANTES mRNA expression. However, pretreatment plus concomitant budesonide treatment did result in a reduction in the increase of RANTES mRNA evoked by exposure to SEB-CM. At 24 h posttreatment (compared to time-matched controls), the fold increases (mean ± the standard error of the mean [SEM]; n = 3) in RANTES mRNA expression in T84 cells were as follows: treatment with 10−6 M budesonide, 0.9 ± 0.1; treatment with 50% SEB-CM, 1.7 ± 0.3 (P < 0.05); treatment with 50% SEB-CM plus budesonide, 1.4 ± 0.06. The reduction in chemokine mRNA expression and, by inference, immune cell chemotaxis represents one of the putative therapeutic benefits of budesonide treatment.
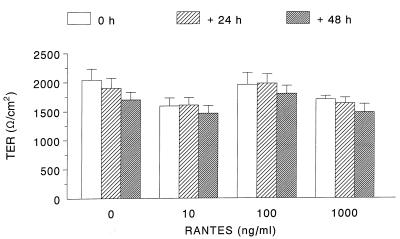
We surmised that RANTES might autocrinely affect epithelial cells. Consequently, T84 cells were treated with RANTES (1 or 10 ng/ml) for 6 or 24 h and MCP-1 and RANTES mRNA expression was examined. Additional epithelial preparations were treated with RANTES (10 ng/ml) plus SEB-CM, and chemokine mRNA was examined 24 h later. Human recombinant RANTES added directly to T84 cells had no significant effect on MCP-1 or RANTES mRNA expression, nor did it consistently alter the ability of SEB-CM to elicit enhanced T84 chemokine mRNA expression (n = 3; data not shown). However, the possibility remained that exposure to RANTES could alter other aspects of epithelial physiology. Thus, transepithelial ion resistance (indicates a barrier to passive ion flux) (Fig. 3) and active ion transport responses stimulated by carbachol or forskolin (data not shown) treatment of T84 cells grown on semipermeable filters were examined in standard Ussing chamber electrophysiology experiments (8). RANTES (10 to 1,000 ng/ml) treatment did not affect any of these functional parameters, and thus no evidence is provided for a direct effect of RANTES on this Cl−-secretory human epithelium.
FIG. 3.
Bar graph showing that treatment with human recombinant RANTES did not significantly affect the transepithelial resistance (TER) of T84 monolayers (mean ± SEM; n = 3 to 6 monolayers). T84 cells were grown to confluence (i.e., >1,000 Ω/cm2) on semipermeable filter supports (Costar Inc.), and then RANTES was added to the basolateral well of the culture plate and transepithelial resistance was subsequently monitored at the specified intervals by using chopstick electrodes and a voltmeter (Millipore, Bedford, Mass.) (11).
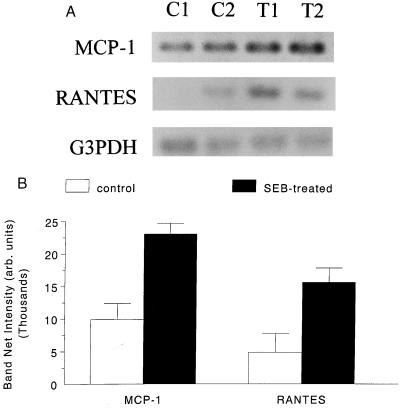
It is critical that in vitro proof-of-principle observations be related to in vivo conditions. Therefore, as a final component of this study, murine epithelial MCP-1 and RANTES mRNA expression was examined (Table 1). Briefly, jejunal epithelial cells (4 × 106) were isolated by mechanical and enzymatic procedures (5) from 10-week-old male BALB/c mice (Charles River Animal Suppliers, St. Constant, Quebec, Canada) treated 4 h previously with a single intraperitoneal injection of 100 μg of SEB (n = 3) (1). (This portion of the study was approved by and conducted under the guidelines of the Animal Care Committee of McMaster University, in compliance with national animal care standards.) Four hours posttreatment was chosen based on the in vitro findings with T84 cells and data illustrating significant structural and functional changes in the jejuna of SEB-treated mice at this time (1, 10). As shown in Fig. 4, and in general accordance with the in vitro findings presented above, small intestinal epithelial cells isolated from SEB-treated mice had a two- to threefold increase in MCP-1 and RANTES mRNAs. These findings add credence to the original postulate that epithelium-derived chemotactic signals might mediate, at least to some degree, the increase in gut T cells observed after systemic SAg treatment (1, 3).
FIG. 4.
(A) Representative PCR electrophoresis for MCP-1, RANTES, and G3PDH of jejunal epithelium isolated from control mice (C1 and C2) and mice treated 4 h previously with 100 μg of SEB (T1 and T2). (B) Densitometry analysis of the electrophoretic gels (n = 3). arb., arbitrary.
In summary, we have shown that (i) SAg immune activation can result in significant and sequential production of MCP-1 and RANTES by gut epithelium; (ii) the use of a conditioned medium is considerably more effective than that of low-dose TNF-α with or without IFN-γ in eliciting a T84 chemokine response, indicating that the use of a mixed-mediator milieu generated in response to immune stimulation can reveal physiological (or pathophysiological) events that may be overlooked when single cytokine preparations are used; and (iii) administration of SEB to mice elicits increased jejunal epithelial expression of RANTES and MCP-1 mRNAs compared to that of enterocytes from sham-treated controls. Collectively, the data add to the study of chemokine production by epithelia and emphasize the potential of these cells to actively participate in immune responses. However, immune cell chemotaxis studies with conditioned medium from the epithelial cells in this model and, indeed, those generated in other model systems are required to precisely assess the full biological significance of epithelial chemokine generation. In conclusion, epithelial chemokine synthesis has been identified as another facet of the integrated immunological-physiological response generated upon exposure to bacterial superantigens and we speculate that this is an important element of the host response to bacterial infections at mucosal surfaces.
Acknowledgments
The technical assistance of J. Lu and Y. Deng is gratefully acknowledged.
This work was supported by operating grants from the Medical Research Council of Canada and the Crohn's and Colitis Foundation of Canada to D. M. McKay. S. Jedrzkiewicz is a student in the Biology and Pharmacology Cooperative Program at McMaster University.
REFERENCES
- 1.Benjamin M A, Lu J, Donnelly G, Dureja P, McKay D M. Changes in murine jejunal morphology evoked by the bacterial superantigen Staphylococcus aureus enterotoxin B are mediated by CD4+ T cells. Infect Immun. 1998;66:2193–2199. doi: 10.1128/iai.66.5.2193-2199.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkman N, Krishnan V L, Gilbey T, Newton R, O'Connor B, Barnes P J, Chung K F. Expression of RANTES mRNA and protein in airways of patients with mild asthma. Am J Respir Crit Care Med. 1996;154:1804–1811. doi: 10.1164/ajrccm.154.6.8970374. [DOI] [PubMed] [Google Scholar]
- 3.Berry J T, Taylor S L, Schlunz L R, Freed R C, Bergdoll M S. Effects of staphylococcal enterotoxin A on the rat gastrointestinal tract. Infect Immun. 1984;44:234–240. doi: 10.1128/iai.44.2.234-240.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casola A, Estes M K, Crawford S E, Ogra P L, Ernst P B, Garofalo R P, Crowe S E. Rotavirus infection of cultured intestinal epithelial cells induces secretion of CXC and CC chemokines. Gastroenterology. 1998;114:947–955. doi: 10.1016/s0016-5085(98)70314-2. [DOI] [PubMed] [Google Scholar]
- 5.Coligon J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1998. pp. 3.19–3.19.11. [Google Scholar]
- 6.Dveksler G S, Basile A A, Dieffenbach C W. Analysis of gene expression: use of oligonucleotide primers for glutaraldehyde-3-phosphate dehydrogenase. PCR Methods Applications. 1992;1:283–285. doi: 10.1101/gr.1.4.283. [DOI] [PubMed] [Google Scholar]
- 7.James S. Potential role of superantigens in gastrointestinal disease. Gastroenterology. 1993;105:1569–1571. doi: 10.1016/0016-5085(93)90169-d. [DOI] [PubMed] [Google Scholar]
- 8.McKay D M, Singh P K. Superantigen-activation of immune cells evokes epithelial (T84) transport and barrier abnormalities via interferon-γ and tumour necrosis factor-α. Inhibition of increased permeability, but not diminished secretory responses by transforming growth factor β2. J Immunol. 1997;159:2382–2390. [PubMed] [Google Scholar]
- 9.McKay D M, Baird A W. Cytokine regulation of epithelial permeability and ion transport. Gut. 1999;44:283–289. doi: 10.1136/gut.44.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKay D M, Benjamin M, Lu J. CD4+ T cells mediate superantigen-induced abnormalities in murine jejunal ion transport. Am J Physiol. 1998;275:G29–G38. doi: 10.1152/ajpgi.1998.275.1.G29. [DOI] [PubMed] [Google Scholar]
- 11.McKay D M, Brattsand R, Wieslander E, Fung M, Croitoru K, Perdue M H. Budesonide inhibits T cell initiated epithelial pathophysiology in an in vitro model of inflammation. J Pharm Exp Ther. 1996;277:403–410. [PubMed] [Google Scholar]
- 12.Natori Y, Sekaguehi M, On Z. Gene expression of CC chemokines in experimental crescentric glomerulonephritis. Clin Exp Immunol. 1997;109:143–148. doi: 10.1046/j.1365-2249.1997.4271321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann B, Emmauilidis K, Stadler M, Holzmann B. Distinct functions of interferon-γ for chemokine expression in models of acute lung inflammation. Immunology. 1998;95:512–521. doi: 10.1046/j.1365-2567.1998.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paine R, Rolfe M, Standiford T, Burdick M, Rollins B, Streiter R. MCP-1 expression by rat type II alveolar epithelial cells in primary culture. J Immunol. 1993;150:4561–4570. [PubMed] [Google Scholar]
- 15.Rahimpour R, Mitchell G, Khandaker M H, Kong C, Singh B, Xu L, Ochi, Feldman R D, Pickering J G, Gill B M, Kelvin D J. Bacterial superantigens induce down-modulation of CC chemokine responsiveness in human monocytes via an alternative chemokine ligand-independent mechanism. J Immunol. 1999;162:2299–2307. [PubMed] [Google Scholar]
- 16.Reinecker H, Loh E Y, Ringler D J, Mehta A, Rombeau J L, MacDermott R P. Monocyte chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology. 1995;108:40–50. doi: 10.1016/0016-5085(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 17.Schall T J, Jougstra J, Dyer B J, Jorgensen J, Clayberger C, Davis M M, Kensky A M. A human T cell specific molecule is a member of a new gene family. J Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- 18.Song F, Ito K, Denniong T L, Kuninger D, Papaconstantinou J, Gourley W, Klimpel G, Balish E, Hokanson J, Ernst P B. Expression of the neutrophil chemokine KC in the colon of mice with enterocolitis and by intestinal epithelial cell lines: effects of flora and proinflammatory cytokines. J Immunol. 1999;162:2275–2280. [PubMed] [Google Scholar]
- 19.Stellato C, Beck L A, Gorgone G A, Proud D, Schall T J, Ono S J, Lichtenstein L M, Schleimer R R. Expression of the chemokine RANTES by a human bronchial epithelial cell line: modulation by cytokines and glucocorticoids. J Immunol. 1995;155:410–418. [PubMed] [Google Scholar]
- 20.Wang J H, Devalia J L, Xia C, Sapsford R J, Davis R J. Expression of RANTES by human bronchial epithelial cells in vitro and in vivo and the effect of corticosteroids. Am J Respir Cell Mol Biol. 1996;14:27–35. doi: 10.1165/ajrcmb.14.1.8534483. [DOI] [PubMed] [Google Scholar]
- 21.Warhurst A C, Hopkins S J, Warhurst G. Interferon-γ induces differential up-regulation of α and β chemokines in colonic epithelial cell lines. Gut. 1998;42:208–213. doi: 10.1136/gut.42.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webb S A, Gascoigne R J. T cell activation by superantigens. Curr Opin Immunol. 1994;6:467–475. doi: 10.1016/0952-7915(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 23.Yang S, Eckmann L, Panja A, Kagnoff M F. Differential and regulated expression of C-X-C, C-C and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113:1214–1223. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura T, Yuhki N, Moore S K, Appella E, Lerman M I, Leonard E J. Human monocyte chemoattractant protein 1 (MCP-1) FEBS Lett. 1989;244:487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- 25.Zareie M, Brattsand R, Sherman P, McKay D M, Perdue M H. Improved effects of novel glucocorticosteroids on immune-induced epithelial pathophysiology. J Pharm Exp Ther. 1999;289:1245–1249. [PubMed] [Google Scholar]