Abstract
Introduction:
Immune checkpoint inhibitors (ICIs) have become a standard of care in metastatic renal cell carcinoma (mRCC) but are associated with immune-related adverse events (irAEs) including colitis. Growing evidence suggests proton pump inhibitors (PPIs) increase the risk of inflammatory bowel disease (IBD). Given the pathophysiological overlap between IBD and ICI colitis, we sought to evaluate the relationship between PPI use and ICI colitis in mRCC patients.
Patients and Methods:
We performed a retrospective study of adult patients who received ICI therapy for mRCC between 2015 and 2018 at University of Texas Southwestern Medical Center affiliated hospitals. Clinical characteristics, oncological outcomes, ICI colitis details, and PPI use details were collected by manual chart review. The diagnosis of ICI colitis was made via biopsy when available, or by clinical criteria (symptoms and response to immunosuppressive therapy) when biopsy specimens were unavailable or inconclusive. Univariable and multivariable logistic regression analyses were conducted to assess the potential contribution of PPIs to ICI colitis.
Results:
A total of 176 patients received ICI therapy for mRCC, of which 16 (9.1%) were diagnosed with ICI colitis. Patients with ICI colitis presented with elevated stool lactoferritin and calprotectin and a wide range of endoscopic and histologic findings. There were no significant differences between patients with and without ICI colitis in age, gender, medical comorbidities, RCC history, and overall survival. However, exposure to ipilimumab and PPI use were more frequently observed in patients with ICI colitis than those without. In univariable and multivariable logistic regression analyses, exposure to ipilimumab and chronic use of PPIs >8 weeks were significantly associated with ICI colitis.
Conclusion:
In addition to ipilimumab use, chronic use of PPIs may be associated with ICI colitis in patients with mRCC.
Keywords: renal cell carcinoma, immune checkpoint inhibitor, immunotherapy, colitis, proton pump inhibitor
Introduction
Immune checkpoint inhibitors (ICIs), used alone or in combination with tyrosine kinase inhibitors, have improved treatment outcomes of metastatic renal cell carcinoma (mRCC)1–4. The ICIs used in RCC target cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death protein-1 (PD-1), and its ligand programmed death-ligand (PD-L1) and are associated with a wide range of immune-related adverse events (irAEs)5. Across tumor types treated with ICIs, the gastrointestinal (GI) tract is one of the most frequently involved sites of irAEs6. With the increasing use of ICIs, ICI colitis is being increasingly acknowledged7.
To date, several potential risk factors for ICI colitis have been identified. The most robust is exposure to anti-CTLA-4 therapy, as the incidence of ICI colitis has been shown to increase in a dose-dependent manner in prospective studies across tumor types8–10. Additional risk factors have been noted in observational studies. These include Caucasian race11, pre-existing inflammatory bowel disease (IBD)12,13, use of nonsteroidal anti-inflammatory drugs (NSAIDs)14, and tumor type with melanoma appearing to have a greater incidence of ICI colitis relative to mRCC or non-small cell lung carcinoma (NSCLC) in multivariate analyses5,11,15. Interestingly, several studies have implicated the composition of gut microbiome in the development of ICI colitis16–20.
Proton pump inhibitors (PPIs), which have been shown to have dysbiotic effects21,22, are among the most commonly prescribed medications in the United States and frequently overutilized23. Additionaly, PPI use has been associated with the development of microscopic colitis (MC)24,25 and IBD26–28. Of note, the endoscopic and histologic manifestations of ICI colitis are variable, but there is considerable overlap with MC and IBD29,30. We hypothesized that PPI use among mRCC patients may increase the risk of developing ICI colitis following ICI therapy.
Here, we report the incidence, associated risk factors, and clinical outcomes of ICI colitis in mRCC at two tertiary care centers.
Patients and Methods
Study Design and Data Collection
Adult patients with a diagnosis of mRCC who received ICI therapy (nivolumab or ipilimumab or both) at the William P. Clements Jr. University Hospital (CUH) and Parkland Health & Hospital System (PHHS) between 2015 to 2018 were identified. Clinical data, including baseline characteristics, treatment details, irAE details, and oncologic outcomes (including Overall Survival [OS] and Time to Next Treatment [TNT]) were collected by independent chart review (J.Y., R.E., N.L., A.A.). Exposure to ipilimumab was defined as use of ipilimumab at any point in the disease course, including the addition of ipilimumab to nivolumab monotherapy for progressive disease. PPI exposure was defined as an active prescription in the medical record, regardless of duration, at any point from 3 months prior to ICI initiation until ICI colitis, death, or last ICI infusion, whichever occurred first. PPI dose, duration of use, ordering provider, and indication were recorded. All available PPI agents including pantoprazole, omeprazole, esomeprazole, rabeprazole, lansoprazole, and dexlansoprazole were considered.
Patients with suspected ICI colitis were identified by manual chart review by at least two physicians (J.Y., R.E., N.L). The diagnosis of ICI colitis was made by endoscopy with pathologic confirmation, or by the following clinical criteria when pathologic examination was unavailable or inconclusive: (i) consistent clinical presentation and course, including diarrhea following ICI therapy; (ii) discontinuation of immunotherapy and improvement with immunosuppressive agents (steroids with/without biologics); and (iii) exclusion of other causes of colitis, including but not limited to IBD, infectious colitis, radiation-induced colitis. Colitis severity was graded using the common terminology criteria for adverse events (CTCAE) v5.0.
Endoscopic and histologic evaluation
All available endoscopic reports were reviewed by a board-certified gastroenterologist (N.K.) and the gross description was noted. Accompanying colonic biopsy specimens were reviewed by a dedicated GI pathologist (L.P), who commented on the presence or absence of the following features: active inflammation (cryptitis or crypt abscess), basal lymphoplasmacytotic infiltration, crypt architecture changes, increased epithelial apoptosis, and intraepithelial lymphocytosis30,31.
Statistical analysis
Continuous variables were summarized as means with standard deviations or medians with interquartile ranges (IQRs), depending on their distribution pattern assessed by the Shapiro-Wilk test. Accordingly, statistical analyses were performed using the Student’s t-test or Mann-Whitney test. Categorical variables were shown as frequencies with percentages and were analyzed using the Pearson chi-square test. Kaplan-Meier curves were used to assess unadjusted OS time distribution and were compared using the Log-Rank test. Median follow-up time was estimated by the reverse Kaplan-Meier method. Univariable and multivariable exact logistic regression analyses were used to evaluate the association between potential risk factors and ICI colitis. Imputation methods were not used as there were no missing variables in multivariable and univariable analyses. Statistical analysis was carried out using the SPSS software (version 19.0). A P value <0.05 was considered statistically significant.
Results
Patient characteristics
We identified 176 adult patients with mRCC who received ICI therapy during the study period, of which 43 (24.4%) received nivolumab/ipilimumab combination therapy and 133 (75.6%) received nivolumab as the initial ICI therapy (Figure 1). In the latter, ipilimumab was added directly at disease progression in 41 (23.3%) patients at a median of 9.0 (IQR: 3.0-14.0) months after initiation of nivolumab. Thus, a total of 84 (47.7%) patients had any exposure to ipilimumab. Twenty-eight (15.9%) patients were identified with diarrhea or colitis of any cause documented in chart, of which 15 underwent colonoscopy or sigmoidoscopy and 16 were ultimately diagnosed with ICI colitis by either biopsy (n = 10, 5.7%) or pre-defined clinical criteria (n = 6, 3.4%) (Figure 1).
Figure 1.
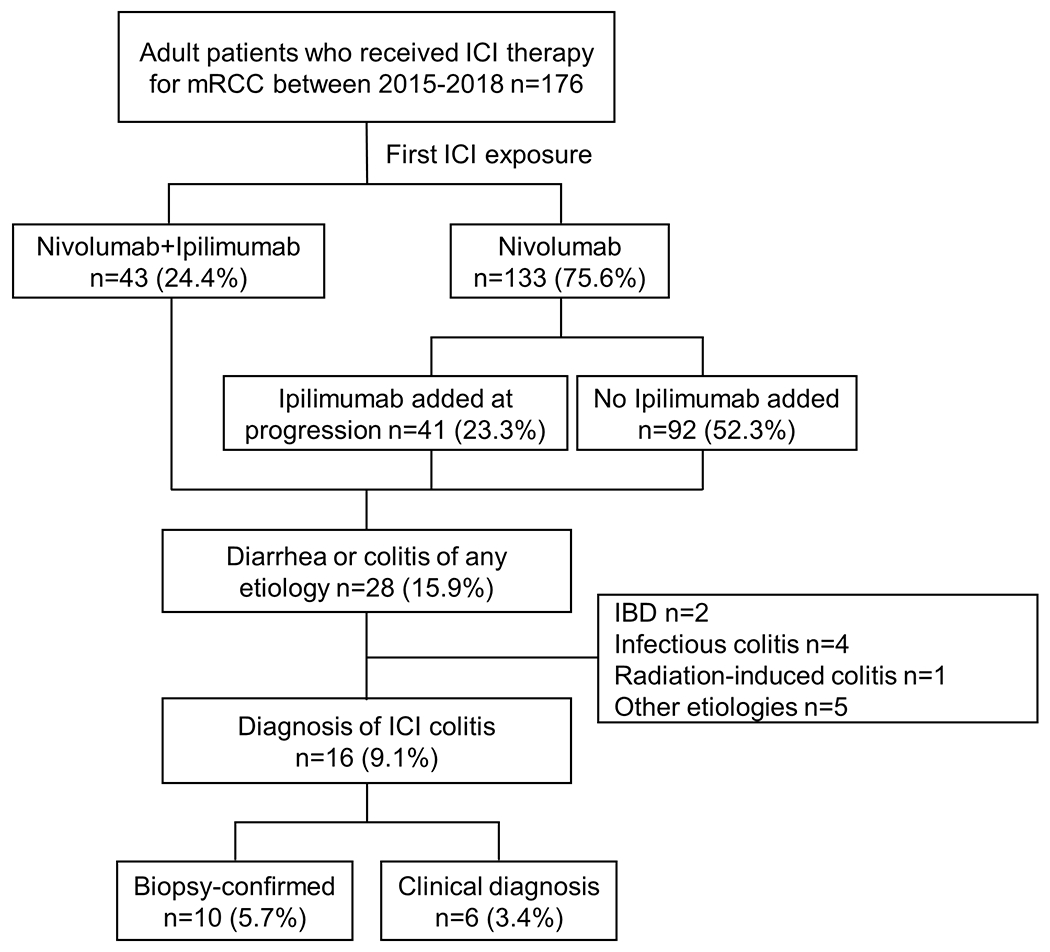
Flowchart showing identification of patients with ICI colitis. Clinical diagnositic criteria for ICI colitis included diarrheal symptoms, consistent stool studies, discontinuation of ICI therapy, treatment with steroids or other immunosuppressive therapy, and exclusion of other colitis etiologies. Nivolumab was used as a single agent in 133 patients, either as a second or later line therapy (n=127) or in the context of a clinical trial (n=6). ICI: immune checkpoint inhibitor, mRCC: metastatic renal cell carcinoma, IBD: inflammatory bowel disease.
As seen in Table 1, no statistically significant differences in age, gender, smoking history, medical comorbidities, RCC history, and IMDC score were noted in patients with and without ICI colitis. All of the patients who developed ICI colitis in this cohort were of Caucasian race, consistent with previous findings that Caucasian race is a risk factor for ICI colitis11. The proportion of patients with any exposure to ipilimumab was higher among patients who developed ICI colitis than those who did not, 14/16 (87.5%) versus 70/160 (43.8%) (P = 0.001), respectively. In addition, patients with ICI colitis were noted to have a higher incidence of non-colitis irAEs (n = 10, 62.5%) than those without ICI colitis (n = 63, 39.4%), although this did not reach statistical significance (P = 0.07). Of note, there were no events of ICI colitis at the PHHS study site (n = 31, 17.6%) despite similar follow up at both sites (Supplementary Table 1). It is worth noting that patients treated at PHHS were more likely to be of non-Caucasian ethnicity (n = 28, 90%) than those treated at CUH (n = 26, 17.6%) (P < 0.001), and less likely to have received ipilimumab during their treatment course, 7 (22.6%) versus 77 (53.1%) (P = 0.002), respectively (Supplementary Table 1).
Table 1.
Patient characteristics
| ICI Colitis Present (n=16) n (%) | ICI Colitis Absent (n=160) n (%) | P value | |
|---|---|---|---|
| Age (year)* | 66 (52-72) | 63 (56-68) | 0.23 |
| Male | 9 (56.3) | 121 (75.6) | 0.09 |
| Race | |||
| Caucasian | 16 (100) | 106 (66.3) | 0.005 |
| Non-Caucasian | 0 (0) | 54 (33.8) | |
| Study site | |||
| PHHS | 0 (0) | 31 (19.4) | 0.052 |
| CUH | 16 (100) | 129 (80.6) | |
| Ever smoker | 9 (56.3) | 71 (44.4) | 0.36 |
| Comorbidities | |||
| Hypertension | 13 (81.3) | 117 (73.1) | 0.48 |
| Type 2 diabetes mellitus | 6 (37.5) | 47 (29.4) | 0.5 |
| Inflammatory bowel disease | 0 (0) | 2 (1.2) | 0.65 |
| Diverticulosis | 6 (37.5) | 87 (54.4) | 0.2 |
| Histological subtype | |||
| ccRCC | 15 (93.8) | 134 (83.8) | 0.29 |
| Non-ccRCC | 1 (6.3) | 26 (16.3) | |
| Nephrectomy | 13 (81.3) | 135 (84.4) | 0.75 |
| Previous radiation therapy | 11 (68.8) | 93 (58.1) | 0.41 |
| Prior Systemic Therapies | |||
| None | 5 (31.3) | 29 (18.1) | 0.45 |
| One | 6 (37.5) | 70 (43.8) | |
| Two or more | 5 (31.3) | 61 (38.1) | |
| IMDC score | |||
| Favorable | 1 (6.3) | 31 (19.4) | 0.28 |
| Intermediate | 12 (75.0) | 84 (52.5) | |
| Poor | 3 (18.8) | 34 (21.3) | |
| Missing | 0 (0) | 11 (6.9) | |
| Initial immunotherapy regimen | |||
| Nivolumab | 7 (43.8) | 126 (78.8) | 0.002 |
| Nivolumab+Ipilimumab | 9 (56.3) | 34 (21.3) | |
| Any exposure to Ipilimumab | 14 (87.5) | 70 (43.8) | 0.001 |
| Non-colitis irAEs | 10 (62.5) | 63 (39.4) | 0.07 |
| Exposure to PPIs | 14 (87.5) | 90 (56.3) | 0.015 |
Data are shown as frequencies (percentages) unless otherwise indicated.
ccRCC: clear cell renal cell carcinoma, CUH: Clements University Hospital, ICI: immune checkpoint inhibitor, IMDC: international metastatic renal cell carcinoma database consortium, irAE: immune-related adverse event, PHHS: Parkland Health & Hospital System, PPI: proton pump inhibitor.
P values were calculated by comparing patients with and without ICI colitis.
Data are shown as medians (interquartile ranges).
Exposure to PPIs
One hundred and four (59.1%) patients had any exposure to PPIs within the study period. PPI use was more frequently observed among patients with ICI colitis than those without, with 14 (87.5%) patients who developed ICI colitis having had PPI exposure versus 90 (56.3%) patients without ICI colitis (P = 0.015) (Table 1). There was no difference in the frequency of PPI exposure between Caucasians and non-Caucasians, 76/122 (62.3%) versus 28/54 (51.9%) (P=0.19). Among all patients who received PPIs, the most frequent indication was gastroesophageal reflux disease (GERD) (n = 53, 60.0%), followed by GI prophylaxis while on steroids for indications excluding ICI colitis (n = 18, 17.3%) (Figure 2A). Of note, the indication for PPI use was either unspecified or for non-specific symptoms (nausea, diarrhea, etc.) in 24 (23.1%) patients. The most common ordering provider among this cohort was at an outside institution or unspecified for 50 (48.1%) patients (Figure 2B). Oncologists were the ordering provider for 28 (26.9%) patients. Of patients with exposure to PPIs, 87 (83.7%) used PPIs for a duration of greater than 8 weeks, which is the duration of empiric therapy suggested for most common indications (Figure 2C)32,33.
Figure 2.
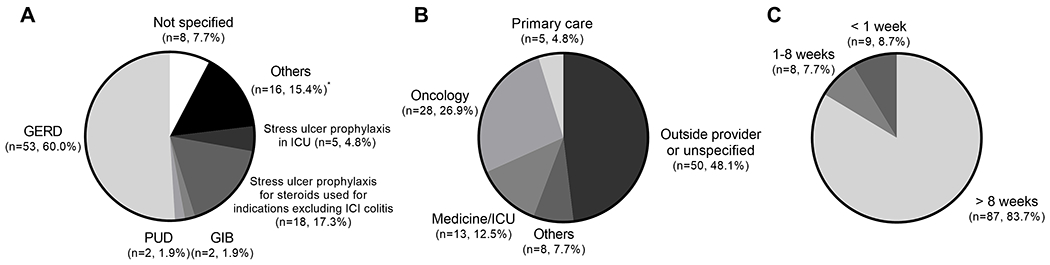
Proton pump inhibitor (PPI) use by indication (A), ordering provider (B), and duration (C) in 104 (59.1%) patients who had PPI exposure from 3 months prior to ICI initiation until ICI colitis, death, or last ICI infusion, whichever occurred first. GERD: gastroesophageal reflux disease, PUD: peptic ulcer disease, GIB: gastrointestinal bleeding, ICI: immune checkpoint inhibitor, ICU: intensive care unit. *Other indications include diarrhea, nausea, abdominal pain, and GI prophylaxis on medical/surgical wards.
Clinical manifestations and outcomes of ICI colitis
Sixteen (9.1%) patients developed ICI colitis (Table 2). The median time of onset of ICI colitis from start of ICI therapy was 3.0 (IQR:1.0-9.0) months. Stool lactoferrin and stool calprotectin were positive in all cases for which they were available, 15 (94%) and 14 (87.5%) patients respectively. Seven (43.8%) patients presented with grade 3 ICI colitis requiring hospitalization, however no deaths related to ICI colitis were observed. ICIs were suspended in all patients who developed ICI colitis. All patients received steroids and 3 (18.8%) patients required infliximab. All patients had resolution of diarrheal symptoms at a median duration of 8.5 (IQR: 5.0 – 14.0) days following onset of symptoms. ICI was restarted in 6 (37.5%) patients at a median of 10.0 (IQR: 7.5-14.5) weeks after the onset of symptoms, of these patients 5 (83.3%) had recurrence of ICI colitis. There was no difference in TNT or OS between patients with and without ICI colitis in this cohort (Figure 3).
Table 2.
Clinical characteristics and outcomes of ICI colitis (n=16)
| Characteristics | Frequency (%) or Median (IQRs) |
|---|---|
| Time from first ICI infusion to colitis (month) | 3.0 (1.0-9.0) |
| PPI Exposure | 14 (87.5%) |
| PPI Exposure Duration (month) | 19.5 (5.3-31.8) |
| Colitis grade | |
| II | 9 (56.3) |
| III | 7 (43.8) |
| Number of bowel movements per day | 5 (3-8) |
| Symptoms duration (day) | 8.5 (5.0 - 14.0) |
| Stool lactoferritin positive (n=15) | 15 (100) |
| Stool calprotectin elevated (n=14) | 14 (100) |
| Endoscopy | 10 (62.5) |
| Time from symptom onset to endoscopy (day) | 8 (4-24) |
| Endoscopic findings (n=10) | |
| Normal | 5 (50) |
| Focal inflammation | 2 (20) |
| Diffuse inflammation | 3 (30) |
| Histologic findings (n=10)a | |
| Normal | 1 (10) |
| Acute inflammation | 9 (90) |
| Chronic inflammation | 3 (30) |
| Lymphocytic pattern | 6 (60) |
| Treatment | |
| ICI suspended | 16 (100) |
| Steroids | 16 (100) |
| Infliximab | 3 (18.8) |
| Outcomes | |
| Hospitalization | 7 (43.8) |
| ICI resumed | 6 (37.5) |
| Time to resumption (week) | 10 (7.5-14.5) |
| Recurrence | 5 (83.3) |
| Concurrent irAEsb | |
| Any | 10 (62.5) |
| Polyarthralgia | 3 (18.8) |
| Pneumonitis | 2 (12.5) |
| Dermatitis | 2 (12.5) |
| Hepatitis | 2 (12.5) |
| Pancreatitis | 2 (12.5) |
| Otherc | 3 (18.8) |
Data are shown as frequencies (percentages) or medians (interquartile ranges).
Some patients had multiple histological findings.
Some patients had multiple irAEs.
Other includes development of Type 1 diabetes and adrenal insufficiency in a single patient, hypophysitis (n = 1), and iritis (n = 1).
ICI: immune-checkpoint inhibitor, irAE: immune-related adverse event, PPI: proton pump inhibitor.
Figure 3.
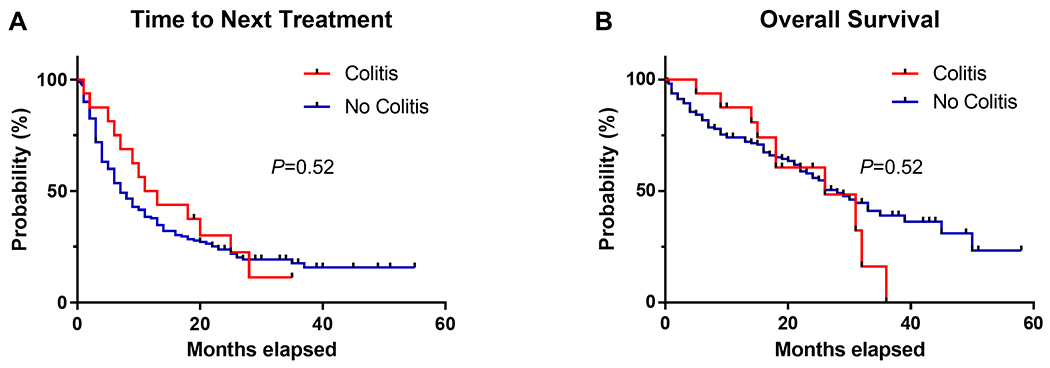
Time to next treatment (A) and overall survival (B) in patients with (n=16) and without (n=160) ICI colitis.
In the 14 patients who developed ICI colitis and had PPI exposure, the most common PPI indication was GERD (n = 12, 85.7%) followed by stress ulcer prophylaxis (n = 2, 14.3%). The duration of PPI exposure varied, but 13 (92.9%) received PPIs for 8 weeks or more, for a median duration of 19.5 (IQR 5.3-31.8) months (Table 2). Two of the 16 (12.5%) patients who developed ICI colitis had no prior PPI exposure. These patients developed grade III colitis and had a similar clinical course to the overall cohort.
Endoscopic and histologic findings
Ten (62.5%) patients with ICI colitis underwent colonoscopy or sigmoidoscopy, with 8 (80%) undergoing endoscopic evaluation within 15 days of symptom onset. Consistent with previous reports31,34, a wide range of endoscopic findings were noted (Table 2, Figure 4A–D). A normal gross appearance was the most common endoscopic finding (n=5, 50%) (Figure 4A). Other endoscopic findings included focal inflammation (n=2, 20%) (Figure 4B–C) and diffuse inflammation (n=3, 30%) (Figure 4D). Of 10 patients with colonic biopsies, the most common patterns of injury were active colitis with increased epithelial apoptosis (n=4, 40%), non-specific colitis (n=4, 40%), and a lymphocytic colitis pattern (n = 1, 10%) (Table 2). One patient had normal histologic findings, but it is worth noting that this patient underwent endoscopy ~60 days post colitis onset and after having received therapy. Neutrophilic cryptitis (n = 7, 70%) (Figure 4E) with concomitant crypt abscess (n = 4, 40%), and mild intraepithelial lymphocytosis (n = 7, 70%) were commonly observed. Basal lymphoplasmacytosis was observed in two cases, with expansion of the lamina propria seen in one case (Figure 4F). Increased apoptosis in crypts was seen in 6 cases (Figure 4G).
Figure 4.
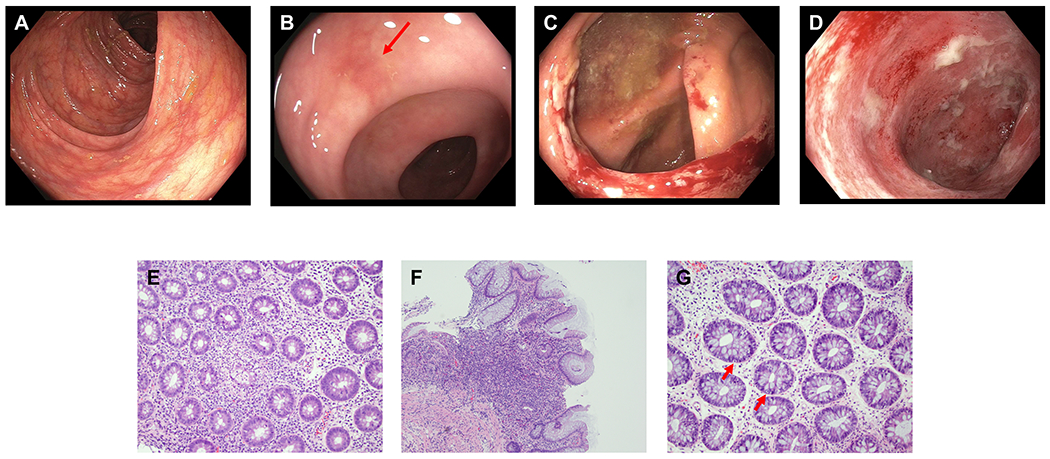
Endoscopic and histologic findings of ICI colitis. A-D: Representative endoscopic images. (A) normal gross appearance; (B) focal erythema (red arrow); (C) focal ulceration; (D) diffuse inflammation. E-G: Representative histologic findings. (E) Active inflammation characterized by cryptitis and crypt abscess, magnification 100x; (F) Expansion of chronic inflammation in lamina propria, magnification 100x; (G) Intraepithelial lymphocytosis and increased crypt epithelial apoptosis (red arrows), magnification 200x.
Logistic regression analysis
We performed univariable analyses in order to identify potential predictors of ICI colitis in this treatment population. This identified Caucasian race (OR: 11.3, 95% CI [2.37-∞], P = 0.004), combination of ipilimumab and nivolumab as the initial regimen (OR: 4.71, 95% CI [1.44-16.08], P = 0.009), exposure to ipilimumab at any time (OR: 8.91, 95% CI [1.95-83.31], P = 0.002), any PPI exposure (OR: 5.4, 95% CI [1.18-50.55], P = 0.024) and chronic PPI exposure >8 weeks (OR: 8.06, 95% CI [1.76-75.33], P = 0.003) as significantly associated with an increased risk of developing ICI colitis (Table 3). Variables with a P < 0.2 in univariable analyses were included in multivariable analyses, inasmuch as collinearity was limited (Table 3). Variables removed due to collinearity in our final model included any PPI exposure (versus chronic >8 weeks) and ipilimumab and nivolumab combination therapy (versus any ipilimumab exposure). In our multivariate model, Caucasian race (OR 8.68, 95% CI [1.71-∞], P = 0.020), exposure to ipilimumab (OR: 7.21, 95% CI [1.46-70.63], P = 0.009), and chronic PPI exposure >8 weeks (OR: 6.9, 95% CI [1.36-68.86], P = 0.013) retained statistical significance (Table 3).
Table 3.
Exact logistic regression analyses for ICI colitis (n=176)
| Variables | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 1.03 | 0.97-1.08 | 0.371 | - | - | - |
| Male | 0.42 | 0.13-1.41 | 0.174 | 0.35 | 0.08-1.49 | 0.179 |
| Caucasian race | 11.3 | 2.37-∞ | 0.004 | 8.68 | 1.71-∞ | 0.020 |
| Ever smoker | 1.61 | 0.50-5.35 | 0.517 | - | - | - |
| Hypertension | 1.59 | 0.41-9.11 | 0.711 | - | - | - |
| Type 2 diabetes mellitus | 1.44 | 0.41-4.67 | 0.678 | - | - | - |
| Diverticulosis | 0.51 | 0.14-1.62 | 0.305 | - | - | - |
| ccRCC | 2.9 | 0.41-127.17 | 0.515 | - | - | - |
| Previous non-ICI systemic therapy | 0.51 | 0.15-2.02 | 0.378 | - | - | - |
| Duration of non-ICI systemic therapy | 1 | 0.97-1.02 | 0.722 | - | - | - |
| Previous radiation therapy | 1.58 | 0.48-6.08 | 0.585 | - | - | - |
| IMDC - Intermediate vs Favorable | 4.39 | 0.60-195.32 | 0.227 | - | - | - |
| IMDC - Poor vs Favorable | 2.7 | 0.20-148.15 | 0.728 | - | - | - |
| Combination therapy as the initial regimen | 4.71 | 1.44-16.08 | 0.009 | - | - | - |
| Any exposure to Ipilimumab | 8.91 | 1.95-83.31 | 0.002 | 7.21 | 1.46-70.63 | 0.009 |
| Non-colitis irAEs | 2.55 | 0.79-8.99 | 0.13 | 2.29 | 0.60-9.62 | 0.278 |
| Any exposure to PPIs | 5.4 | 1.18-50.55 | 0.024 | - | - | - |
| Chronic exposure to PPIs >8 weeks | 8.06 | 1.76-75.33 | 0.003 | 6.9 | 1.36-68.86 | 0.013 |
ccRCC: clear cell renal cell carcinoma, CI: confidence interval, ICI: immune checkpoint inhibitor, IMDC: international metastatic renal cell carcinoma database consortium, irAE: immune-related adverse event, OR: odds ratio, PPI: proton pump inhibitor.
Discussion
In this study we report the incidence, clinical outcomes, and risk factors associated with ICI colitis in mRCC. With the approval of combination immunotherapy with PD-1 and CTLA-4 inhibitors as the first-line therapy in mRCC, the incidence of ICI colitis has predictably increased1,10. The rate of ICI colitis in our cohort was 9% (16/176), similar to rates previously observed given the degree of ipilimumab exposure in our cohort (48%)6. ICI therapy was interrupted in all patients who developed ICI colitis and all received immunosuppressive therapy. Fortunately, there were no deaths attributed to ICI colitis and all patients ultimately had resolution of their symptoms.
Consistent with prior reports, we identified a statistically significant correlation between Caucasian race and ipilimumab use with the development of ICI colitis6,8,11. Furthermore, our results suggest chronic PPI use may be associated with the development of ICI colitis. In the present study, 104 (59%) patients had exposure to PPIs in the study window, of which 87 (83.7%) had chronic exposure to PPIs > 8 weeks. Of note, the indication for PPI use was unknown or inconsistent with professional guidelines in roughly 20% of patients. In multivariate analysis including ipilimumab exposure, race, sex, and development of non-colitis irAEs, chronic PPI exposure retained statistical significance. Our findings extend those from a recent study by Zou et al., which showed PPI use is a risk factor for chronic immune-mediated diarrhea and colitis (defined as persistent or recurrent symptoms, or persistent histologic inflammation >3 months)35. To date, several GI inflammatory diseases have been reported to be associated with PPI use. Early life exposure to PPIs is associated with an increased risk of subsequent IBD26, and PPI use is associated with an increased risk of hospitalization for IBD flare27,28. Furthermore, several large observational studies have noted a correlation between PPI use and the development of MC24,25,36. Pathophysiologically, MC and IBD share some features with ICI colitis as they are all the manifestation of immune deregulation. Moreover, ICI colitis has overlapping endoscopic and histologic features with IBD and MC29,30,37. Of the patients with colonic biopsies in our cohort, two developed a pattern similar to IBD, and a single patient developed a lymphocytic pattern which is a common pattern of injury seen in the lymphocytic colitis subtype of MC. Non-specific inflammatory patterns were seen in most patients. Our findings are consistent with previous reports suggesting histologic overlap of IBD, MC, and ICI colitis.
The precise mechanism of PPI exposure and increased risk of ICI colitis remains unknown, but one possibility is that the dysbiotic effects of PPIs play a role21,22,38. The relationship between the colonic microbiome, response rates to ICI, and development of ICI colitis have begun to be described16,18,20,39. Patients with and without ICI colitis have distinct gut microbiome signatures at baseline20. In animal studies, Bifidobacterium alleviates GI toxicity caused by anti-CTLA4 therapy17, and a recent case study demonstrated that fecal microbiota transplantation may be effective in treating patients with refractory ICI colitis with expansion of Bifidobacterium observed after transplantation18. PPIs are known to alter the composition of gut microbiome, albeit the precise impact on intestinal flora is variable21,22,38. It is possible that PPI-induced decrease in Bifidobacterium species may contribute to ICI colitis38,40. Another plausible mechanism is that PPI use results in a deficiency of micronutrients which could have a protective role. For example, a recent retrospective study of melanoma patients treated with ICI therapy identified vitamin D intake as protective against development of ICI colitis, highlighting the role specific nutrients may play in reducing risk of ICI colitis41. Inasmuch as the present study is retrospective in nature, the underlying diseases for which PPIs were administrated are confounders. While this is a possibility, it is less likely, however, since an inverse relationship between GERD and both MC or IBD has been observed42, and in our cohort, GERD was the most common indication for PPI use among patients who developed ICI colitis.
In addition to the possibility of variables, this study has other important limitations. This is a retrospective study with a relatively limited sample size that evaluates a single tumor type. Thus, it is unclear if our findings can be extrapolated to other tumor types. This can also be a strength, however, as underlying tumor type has been shown to correlate with risk of ICI colitis in several studies6,11,15. By evaluating ICI colitis in RCC only, the potential confounding role of underlying tumor type is reduced. We were also limited by sample size for some of the patient characteristics evaluated, preventing a detailed analysis of these variables and their relationship with ICI colitis. Specifically, there were no events of ICI colitis in non-Caucasian patients in this study or at one of our study sites, PHHS, a local safety-net hospital. This may be the result of treatment pattern differences between the two sites, as the number of patients who received ipilimumab, a known risk factor for ICI colitis, was significantly higher at CUH relative to PHHS (Supplementary Table 1). Additionally, the majority of non-Caucasian patients in our cohort were treated at PHHS, and thus were less likely to have received ipilimumab. Patients at PHHS were noted to have non-ICI-mediated colitis, and both groups had a similar median follow-up duration of over 2 years, so differences in symptom reporting are less likely. Finally, we did not analyze the diet and microbiome of patients at baseline and at the time of ICI colitis, though these would be important analyses to consider in the future.
Despite these limitations, our findings suggest that chronic PPI exposure is associated with an increased risk of ICI colitis. Beyond potential risk of ICI colitis, there are other emerging safety concerns with chronic PPI use, including GI disorders as well as malabsorption of minerals and micronutrients25–27,43,44. Furthermore, increasing evidence suggests that PPI use may have a negative impact on the efficacy of immunotherapy45–47. It is not clear if these adverse effects are dependent on the dose or type of PPI agent, though increasing duration of PPI use (> 8 weeks vs < 8 weeks) appears to carry increased risk of ICI colitis. This study reinforces the notion that clinicians should frequently reassess the risks/benefits of PPI use, avoid unnecessary use, and utilize alternative therapies if possible48.
Conclusion
In conclusion, we identified chronic PPI exposure >8 weeks and anti-CTLA4 therapy as potential risk factors for ICI colitis in mRCC. Additional studies are needed to confirm these findings, however, given the mounting evidence of adverse effects from chronic PPI use, clinicians should frequently reassess the need for PPI use in mRCC patients undergoing ICI therapy.
Supplementary Material
Funding
This sudy was supported by the UTSW Kidney Cancer SPORE grant P50CA196516 (JB). RE receives support from an institutional award from the Burroughs Wellcome Fund.
Disclosure
JB: employee/consultant: Exelixis, Arrowhead; receiver of commercial research grants from Arrowhead; holds ownership interest (including patents) in Peloton Therapeutics/Merck, outside the submitted work.
HH: consulting or advisory role: Bristol Myers Squibb, Pfizer, Exelixis, Bayer, Novartis, Merck, ARMO BioSciences, Corvus Pharmaceuticals, Surface Oncology, Lill; receiver of a grant or contract from Bristol-Myers Squibb and Merck, outside the submitted work.
The remaining authors report no relevant competing interests.
Footnotes
R.E. current address: Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 401 N Broadway, Baltimore, MD 21231
REFERENCES
- 1.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2019;380:1103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2019;380:1116–27. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. The New England journal of medicine 2019;380:1103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Annals of oncology : official journal of the European Society for Medical Oncology 2017;28:2377–85. [DOI] [PubMed] [Google Scholar]
- 6.Soularue E, Lepage P, Colombel JF, et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut 2018;67:2056–67. [DOI] [PubMed] [Google Scholar]
- 7.Dougan M, Wang Y, Rubio-Tapia A, Lim JK. AGA Clinical Practice Update on Diagnosis and Management of Immune Checkpoint Inhibitor Colitis and Hepatitis: Expert Review. Gastroenterology 2020. [DOI] [PubMed] [Google Scholar]
- 8.Tandon P, Bourassa-Blanchette S, Bishay K, Parlow S, Laurie SA, McCurdy JD. The Risk of Diarrhea and Colitis in Patients With Advanced Melanoma Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis. Journal of immunotherapy 2018;41:101–8. [DOI] [PubMed] [Google Scholar]
- 9.Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. The Lancet Oncology 2017;18:611–22. [DOI] [PubMed] [Google Scholar]
- 10.Hammers HJ, Plimack ER, Infante JR, et al. Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35:3851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Abu-Sbeih H, Mao E, et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. Journal for immunotherapy of cancer 2018;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA oncology 2016;2:234–40. [DOI] [PubMed] [Google Scholar]
- 13.Kahler KC, Eigentler TK, Gesierich A, et al. Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. Cancer immunology, immunotherapy : CII 2018;67:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marthey L, Mateus C, Mussini C, et al. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. Journal of Crohn’s & colitis 2016;10:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology 2017;6:e1344805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nature communications 2016;7:10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Yin Q, Chen L, Davis MM. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proceedings of the National Academy of Sciences of the United States of America 2018;115:157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Wiesnoski DH, Helmink BA, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nature medicine 2018;24:1804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Sbeih H, Wang Y. Gut Microbiome and Immune Checkpoint Inhibitor-Induced Enterocolitis. Digestive Diseases and Sciences 2020;65:797–9. [DOI] [PubMed] [Google Scholar]
- 20.Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Annals of oncology : official journal of the European Society for Medical Oncology 2017;28:1368–79. [DOI] [PubMed] [Google Scholar]
- 21.Jackson MA, Verdi S, Maxan M-E, et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nature communications 2018;9:2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Bastard Q, Al-Ghalith GA, Grégoire M, et al. Systematic review: human gut dysbiosis induced by non-antibiotic prescription medications. Alimentary Pharmacology & Therapeutics 2018;47:332–45. [DOI] [PubMed] [Google Scholar]
- 23.Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol 2012;5:219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonderup OK, Nielsen GL, Dall M, Pottegard A, Hallas J. Significant association between the use of different proton pump inhibitors and microscopic colitis: a nationwide Danish case-control study. Alimentary pharmacology & therapeutics 2018;48:618–25. [DOI] [PubMed] [Google Scholar]
- 25.Law EH, Badowski M, Hung YT, Weems K, Sanchez A, Lee TA. Association Between Proton Pump Inhibitors and Microscopic Colitis. The Annals of pharmacotherapy 2017;51:253–63. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz NRM, Hutfless S, Herrinton LJ, et al. Proton Pump Inhibitors, H2 Blocker Use, and Risk of Inflammatory Bowel Disease in Children. The journal of pediatric pharmacology and therapeutics : JPPT : the official journal of PPAG 2019;24:489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juillerat P, Schneeweiss S, Cook EF, Ananthakrishnan AN, Mogun H, Korzenik JR. Drugs that inhibit gastric acid secretion may alter the course of inflammatory bowel disease. Alimentary pharmacology & therapeutics 2012;36:239–47. [DOI] [PubMed] [Google Scholar]
- 28.Shah R, Richardson P, Yu H, Kramer J, Hou JK. Gastric Acid Suppression Is Associated with an Increased Risk of Adverse Outcomes in Inflammatory Bowel Disease. Digestion 2017;95:188–93. [DOI] [PubMed] [Google Scholar]
- 29.Choi K, Abu-Sbeih H, Samdani R, et al. Can Immune Checkpoint Inhibitors Induce Microscopic Colitis or a Brand New Entity? Inflammatory bowel diseases 2019;25:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siakavellas SI, Bamias G. Checkpoint inhibitor colitis: a new model of inflammatory bowel disease? Current opinion in gastroenterology 2018;34:377–83. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Abu-Sbeih H, Mao E, et al. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflammatory bowel diseases 2018;24:1695–705. [DOI] [PubMed] [Google Scholar]
- 32.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. The American journal of gastroenterology 2013;108:308–28; quiz 29. [DOI] [PubMed] [Google Scholar]
- 33.Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology 2017;152:706–15. [DOI] [PubMed] [Google Scholar]
- 34.Verschuren EC, van den Eertwegh AJ, Wonders J, et al. Clinical, Endoscopic, and Histologic Characteristics of Ipilimumab-Associated Colitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2016;14:836–42. [DOI] [PubMed] [Google Scholar]
- 35.Zou F, Abu-Sbeih H, Ma W, et al. Association of Chronic Immune-Mediated Diarrhea and Colitis With Favorable Cancer Response. Journal of the National Comprehensive Cancer Network : JNCCN 2020:1–9. [DOI] [PubMed] [Google Scholar]
- 36.Verhaegh BP, de Vries F, Masclee AA, et al. High risk of drug-induced microscopic colitis with concomitant use of NSAIDs and proton pump inhibitors. Alimentary pharmacology & therapeutics 2016;43:1004–13. [DOI] [PubMed] [Google Scholar]
- 37.Collins M, Soularue E, Marthey L, Carbonnel F. Management of Patients With Immune Checkpoint Inhibitor-Induced Enterocolitis: a Systematic Review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2020. [DOI] [PubMed] [Google Scholar]
- 38.Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruno G, Zaccari P, Rocco G, et al. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World journal of gastroenterology 2019;25:2706–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grover S, Dougan M, Tyan K, et al. Vitamin D intake is associated with decreased risk of immune checkpoint inhibitor-induced colitis. Cancer 2020;126:3758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sonnenberg A, Turner KO, Genta RM. Decreased risk for microscopic colitis and inflammatory bowel disease among patients with reflux disease. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland 2018;20:813–20. [DOI] [PubMed] [Google Scholar]
- 43.Trifan A, Stanciu C, Girleanu I, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World journal of gastroenterology 2017;23:6500–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clinic proceedings 2018;93:240–6. [DOI] [PubMed] [Google Scholar]
- 45.Diao X. Antibiotics and proton pump inhibitors suppress the efficacy of immunotherapy against non-small cell lung cancer. Thoracic cancer 2020;11:1763–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hopkins AM, Kichenadasse G, Karapetis CS, Rowland A, Sorich MJ. Concomitant Proton Pump Inhibitor Use and Survival in Urothelial Carcinoma Treated with Atezolizumab. Clinical Cancer Research 2020;26:5487–93. [DOI] [PubMed] [Google Scholar]
- 47.Qin BD, Jiao XD, Zhou XC, et al. Effects of concomitant proton pump inhibitor use on immune checkpoint inhibitor efficacy among patients with advanced cancer. Oncoimmunology 2021;10:1929727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline. Canadian family physician Medecin de famille canadien 2017;63:354–64. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


