Key Points
-
•
Outcomes in older adults with HL have improved in recent decades, likely due to advances in therapy and supportive care.
-
•
Treatment-related toxicity is a significant concern in all patients aged ≥60 years, and bleomycin should be avoided.
Visual Abstract
Abstract
Outcomes in older adults with classic Hodgkin lymphoma (cHL) have traditionally been poor, in part, related to poor tolerance to standard chemotherapy. Herein, we evaluated the survival of patients with cHL aged ≥60 years in British Columbia in a population-based analysis. From 1961 to 2019, 744 patients with newly diagnosed cHL were identified. With a median follow-up of 9 years, 5-year disease-specific survival (DSS) and overall survival (OS) have improved by decade comparison (both P < .001), remaining stable in the past 20 years (DSS, P = .35; OS, P = .26). In the modern management era (2000-present), 361 of 401 patients (90%) received active therapy for cHL and had a 5-year OS of 60%. For those who received curative-intent therapy (n = 327), the 5-year progression-free survival (PFS), OS, and DSS were 60%, 65%, and 76%, respectively, and estimates were superior in those who were 60 to 69 years of age (72%, 77%, and 83%, respectively) compared with those who were 70 to 79 years of age (54%, 57%, and 70%, respectively) and ≥80 years of age (28%, 39%, and 63%, respectively) (P < .05 for all). Overall, pulmonary toxicity occurred in 58 of 279 patients (21%) treated with bleomycin, with 22 of 58 (38%) occurring after cycles 1 or 2, accounting for 8 of 20 (40%) treatment-related deaths. Outcomes in older adults with cHL have improved in recent decades; however, they remain poor for those aged ≥70 years, even in the modern treatment era. Furthermore, treatment-related toxicity remains a significant concern and use of bleomycin should be avoided in most patients.
Introduction
Hodgkin lymphoma (HL) typically affects young adults, though a second peak in incidence occurs later in life, with patients aged ≥60 years representing approximately 20% of all cases.1 Clinically, older patients often present with negative prognostic factors such as advanced stage, B symptoms, and poor performance status (PS), and less commonly with bulky mediastinal disease compared with younger patients.1, 2, 3 There is also a higher frequency of mixed cellularity histology and Epstein-Barr virus (EBV) positivity, which are associated with more aggressive disease.1,4, 5, 6, 7 Coupled with this are frequent comorbidities, which may affect the ability to administer curative-intent combination chemotherapy at standard doses or in a timely fashion.8
Although HL is one of the most curable malignancies, outcomes in older adults have historically been poor.1,2,9 Older patients either have been excluded from prospective studies or have comprised <15% of total enrolled participants, especially if there is integration of dose-intensive regimens or restriction to those with good PS.10, 11, 12 As a result, the optimal therapy in this age group remains ill defined. ABVD (doxorubicin hydrochloride [Adriamycin], bleomycin sulfate, vinblastine sulfate, dacarbazine) has been a standard regimen for younger patients with HL and is an option for select older patients but may be associated with higher rates of treatment toxicity and even mortality, related to biological disease differences and decreased functional reserve due to comorbidities.9,13,14 Brentuximab vedotin (BV) in combination with adriamycin, vinblastin sulfate, dacarbazine (AVD) demonstrated an improved modified progression-free survival (mPFS) over ABVD (P = .035) in stage III/IV classic Hodgkin lymphoma (cHL) in the initial report12 and improved PFS with longer follow-up.15 There was no upper age limit for trial eligibility but only 186 of 1334 patients (14%) were aged ≥60 years, but a benefit of BV + AVD over ABVD was not clearly demonstrated in this older subgroup (5-year PFS, 68% vs 62%, P = .44).16
In British Columbia (BC), era-specific provincial guidelines for older adults with HL have largely followed those for younger patients with modifications. In the modern era (2000 onward), older patients with HL in BC have been managed with dose-modified ABVD or AVD chemotherapy. Since July 2005, the use of radiotherapy (RT) has been guided by a positron emission tomography (PET)–adapted approach.17 Herein, we evaluated the survival of patients with cHL aged ≥60 years over the past 6 decades in BC followed by a more detailed analysis of outcomes in the modern era.
Methods
Patients aged ≥60 years diagnosed with HL from January 1961 to December 2019 in BC were identified in the BC Cancer Lymphoid Cancer database. All diagnoses were confirmed by centralized pathology review by a BC Cancer expert hematopathologist applying pathologic criteria current at the time of diagnosis. Limited stage was defined as nonbulky (<10 cm) stage IA, IB (since 2000), or IIA if within an acceptable radiation field, and the remainder were managed as advanced stage.
Alkylator-based regimens were replaced with ABVD-like chemotherapy in the 1980s. In the modern era, ABVD or AVD has been the mainstay of treatment in fit, eligible patients, with RT to bulky or residual sites routinely used before July 2005 and subsequently guided by a PET-adapted approach (supplemental data).17,18 Individual decisions were made regarding bleomycin omission (AVD) based on age, comorbidities, or underlying lung disease. Following the report of the RATHL trial, bleomycin was omitted after cycle 2 in PET-2–negative cases19 (supplemental data). Etoposide was substituted for doxorubicin if anthracyclines were contraindicated. Curative-intent treatment was defined as use of multiagent chemotherapy or RT alone in doses of >30 Gy in patients with limited-stage radio-encompassible disease.
Detailed information on patient comorbidities, actual chemotherapy dosing, and treatment delays was not available. Given well-described concerns over pulmonary toxicity in patients treated with bleomycin-containing chemotherapy, particularly those aged >60 years,13 a detailed chart review was undertaken in patients treated with bleomycin in the modern era to identify pulmonary events based on clinical, radiographic, and histopathologic findings during treatment. Diagnoses of bleomycin lung toxicity were made by the treating clinicians’ judgment. This study was approved by the University of British Columbia BC Cancer Research Ethics Board and performed according to the Declaration of Helsinki.
Statistical analysis
Overall survival (OS) was calculated from the date of HL diagnosis to the date of last follow-up or death from any cause. PFS was measured from the date of HL diagnosis to the date of last follow-up, progression, any lymphoma relapse, or death from any cause. Disease-specific survival (DSS) was determined from the date of HL diagnosis to date of death from HL, acute toxicity during HL treatment (and including within 30 days of treatment completion), or last follow-up. Survival end points were estimated using the Kaplan-Meier method, and comparisons were made using the log-rank test. Patient characteristics were compared using the χ2 test. All statistical analyses were performed using SPSS version 14.0.
Results
cHL outcomes by decade
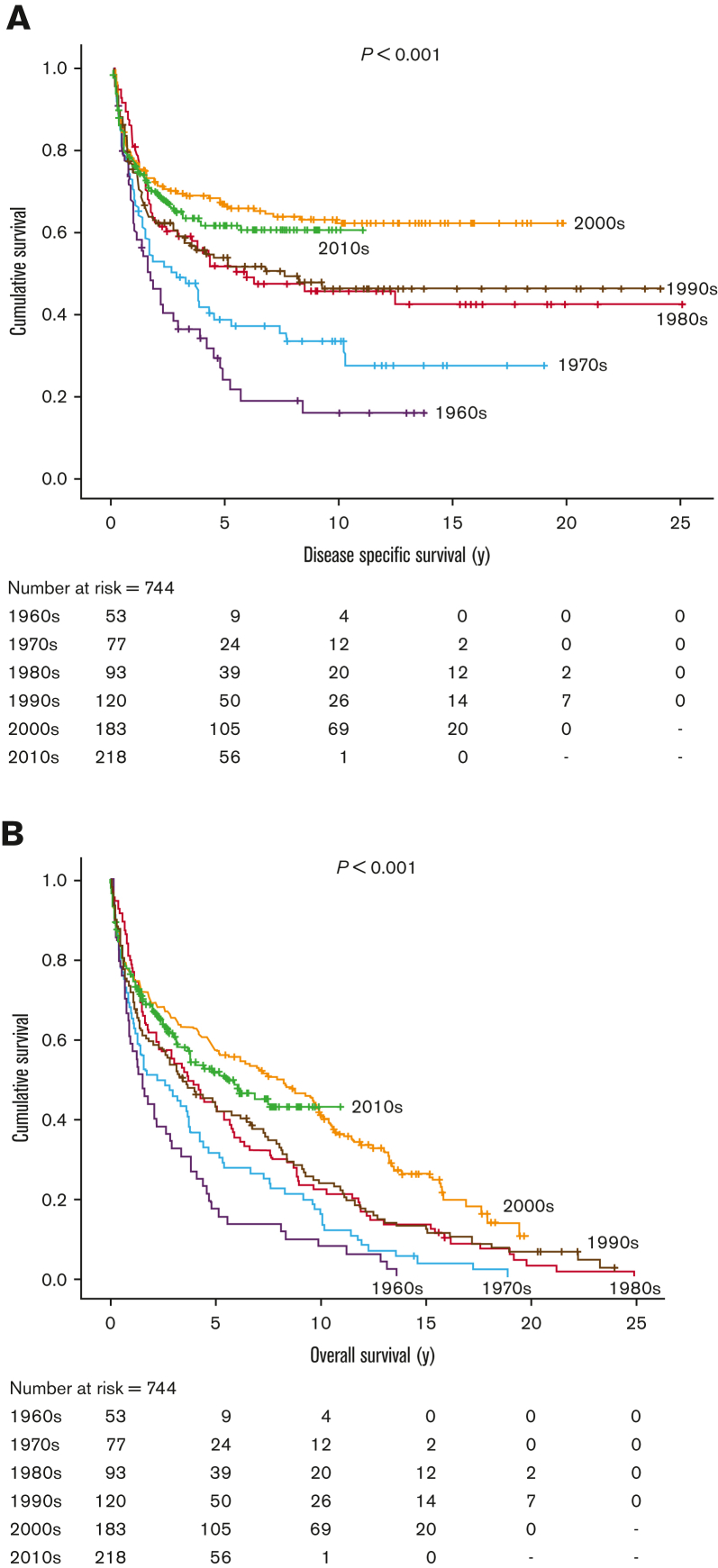
On initial review, 893 cases of newly diagnosed HL were identified in patients aged ≥60 years from January 1961 to December 2019, with follow-up to December 2021. Of these, 149 patients were excluded due to other prior or concurrent lymphoproliferative disease (69 patients excluded due to chronic lymphocytic leukemia/small lymphocytic lymphoma [n = 22] and other non-HL [ n = 47]), nodular lymphocyte–predominant HL (n = 55), insufficient information (n = 21), or HIV-positive (n = 4). The remaining 744 patients were included in this analysis: median age 70 years (60-98 years), 58% male, 67% advanced stage. With a median follow-up of 6.3 years (0.2-24.0 years) in living patients, there have been significant improvements in DSS and OS (both P < .001) by decade comparison (Figure 1), although outcomes have not further improved beyond 2000 (2000-2009 vs 2010-2019, DSS, P = .35; OS, P = .26). Of note, numerically there was a slight improvement in DSS and OS in the 2000s compared with the 2010s, but there was also a greater proportion of patients with high-risk features in the latter era, including stage IV disease (P = .04), anemia (P = .006), lymphopenia (P = .03), and an International Prognostic Score (IPS) ≥4 (P < .001) as well as a trend toward more patients aged ≥80 years (P = .07). Overall, by age group, the 5-year DSS/OS in those aged 60 to 69, 70 to 79, and ≥80 years were 65%/59%, 50%/39%, and 31%/19%, respectively (DSS and OS, P < .001). Survival was worse in those with advanced-stage disease than in those with limited-stage disease (DSS, P < .001; OS, P = .004).
Figure 1.
Historical outcomes in older adults with cHL from 1960 to 2019. (A) DSS; (B) OS.
Outcome of cHL in older adults in the modern management era
To account for advances in diagnosis, staging, therapy, and supportive care in the modern era, we focused specifically on the outcome of patients diagnosed since January 2000. Baseline clinical characteristics of the 401 patients from this period are described in Table 1. Median age was 70 years (60-93 years), 58% were male, 72% had advanced stage, 48% had B symptoms, 30% had extranodal involvement (most commonly bone marrow [13%]), and 44% had PS > 1. Of the 166 patients with known EBV status, 84 (51%) were positive.
Table 1.
Clinical features and outcomes of all older adults with cHL in the modern management era (diagnosis January 2000 to December 2019)
| Clinical features | N = 401 (% or range) | 5-y PFS (%) | P value | 5-y OS (%) | P value | 5-y DSS (%) | P value |
|---|---|---|---|---|---|---|---|
| Age, y | |||||||
| Median (range) | 70 (60-93) | ||||||
| 60-69 | 182 (45) | 68 | <.001 | 72 | <.001 | 78 | <.001 |
| 70-79 | 147 (37) | 45 | 48 | 59 | |||
| ≥80 | 72 (18) | 17 | 22 | 34 | |||
| Sex | |||||||
| Male | 232 (58) | 48 | .25 | 51 | .11 | 61 | .06 |
| Female | 169 (42) | 53 | 58 | 67 | |||
| Stage | |||||||
| Advanced stage | 278/385 (72) | 47 | .011 | 51 | .012 | 59 | <.001 |
| Limited stage | 107/385 (28) | 65 | 70 | 80 | |||
| Extranodal any | 120 (30) | 39 | 41 | 48 | |||
| B symptoms | 187/386 (48) | 46 | .12 | 49 | .048 | 59 | .049 |
| No B symptoms | 199/386 (52) | 57 | 63 | 71 | |||
| Bulky ≥ 10 cm | 35/368 (10) | 59 | .37 | 68 | .16 | 71 | .50 |
| Nonbulky | 333/368 (90) | 53 | 56 | 66 | |||
| PS | |||||||
| PS ≥ 2 | 169/388 (44) | 31 | <.001 | 34 | <.001 | 42 | <.001 |
| PS 0 or 1 | 219/388 (56) | 67 | 71 | 81 | |||
| cHL subtypes | |||||||
| Nodular sclerosis | 182 (45) | 52 | .010 | 56 | .045 | 67 | <.001 |
| Mixed cellularity | 75 (19) | 55 | 58 | 64 | |||
| Lymphocyte rich | 24 (6) | 91 | 91 | 91 | |||
| Lymphocyte depleted | 8 (2) | 25 | 25 | 25 | |||
| Not otherwise specified | 112 (28) | 37 | 42 | 54 | |||
| EBV | |||||||
| Positive | 84/166 (51) | 49 | .13 | 51 | .029 | 62 | .024 |
| Negative | 82/166 (49) | 59 | 63 | 76 |
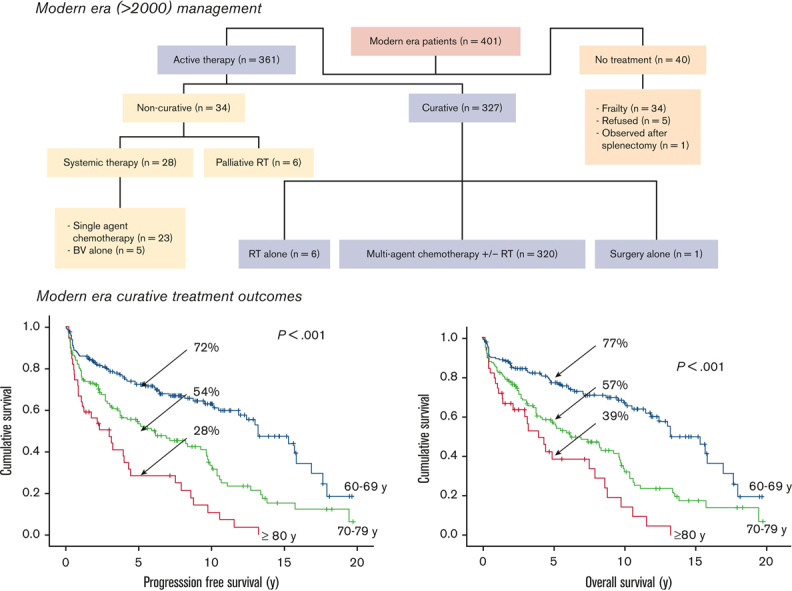
Forty patients (10%) did not receive any active therapy due to frailty. Of the remaining 361 patients who were actively treated, 327 received curative-intent therapy, including 320 (98%) patients treated with multiagent chemotherapy with or without RT, 6 patients who received RT alone for limited-stage disease, and 1 who underwent surgery alone for primary central nervous system cHL (supplemental Figure 1). Most patients treated with curative-intent multiagent chemotherapy received ABVD (n = 252, 79%) or AVD (n = 25, 8%). One patient received BV then sequential AVD (BV-AVD), and the remaining patients received alternate combination regimens that were modified due to comorbidities (supplemental data).
With a median follow-up of 9 years using the reverse Kaplan-Meier method, the 5-year PFS, DSS, and OS for all 401 patients were 50%, 63%, and 54%, respectively. Increasing age was associated with inferior outcomes across all age groups (PFS and OS, P < .001), with 5-year PFS and OS both <50% in patients aged ≥70 years compared with 68% and 72%, respectively, in those aged 60 to 69 years (Table 1). Of interest, bulky disease was more common in the 60- to 69-year cohort compared with the 70- to 79-year and ≥80 year cohorts (13.5% vs 6.5% vs 5.2%, respectively, P = .05); however, all other risk factors were similar across age groups (data not shown). In patients with advanced-stage disease (n = 278), age (PFS and OS, P < .001), anemia (hemoglobin < 105 g/L; PFS and OS, P < 0.001), leukocytosis (white blood cell count > 15 × 109/L; PFS, P = .003; OS, P = .010), lymphopenia (lymphocytes < 0.6 × 109/L and/or <8%; PFS, P = .019; OS, P = .002), and stage IV disease (PFS, P = .031; OS, P = .004) were associated with inferior outcomes. Those with an IPS > 4 had very poor outcomes with 5-year PFS of 34% compared with 57% for those with an IPS of 1 to 3 (P < .001).
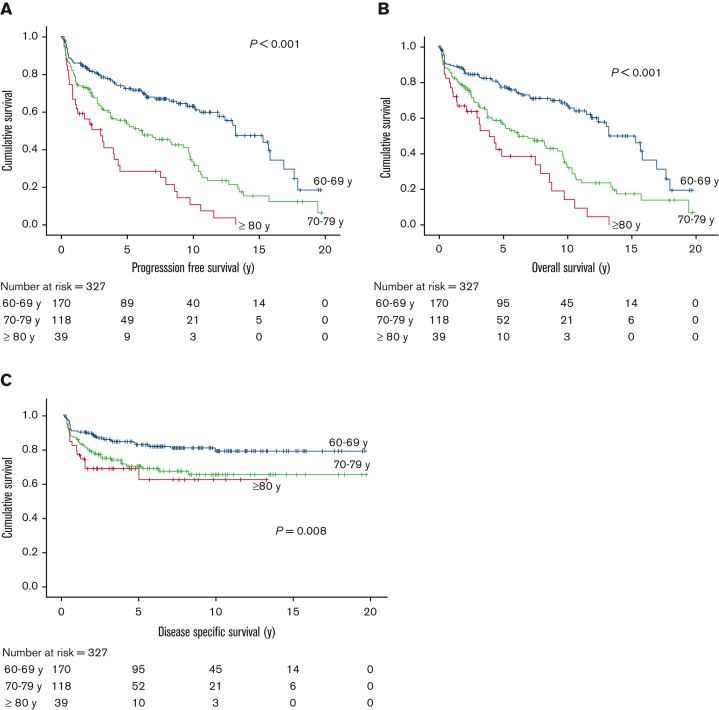
For the 327 patients who received curative-intent therapy, representing 93%, 80%, and 54% of patients in the 60 to 69, 70 to 79, and ≥80 years age groups, respectively, the 5-year PFS was 60%, 5-year OS was 65%, and 5-year DSS was 76%. In patients aged 60 to 69 years, the 5-year PFS and OS were superior at 72% and 77%, respectively, whereas these measures declined to 54% and 57%, respectively, in the 70- to 79-year age group, and 28% and 39%, respectively, in those aged ≥80 (PFS and OS, P < .001) (Figure 2); corresponding 5-year DSS estimates were 83%, 70%, and 63%, respectively (P = .008). There was a trend of inferior outcomes for those with advanced-stage disease compared with those with limited-stage disease (5-year PFS, 57% vs 70%, P = .08; 5-year OS, 61% vs 75%, P = .12); however, the impact of age was seen among patients with limited- or advanced-stage disease (supplemental Table 1).
Figure 2.
Outcome of older adults with cHL in the modern-era curative cohort by age group. (A) PFS; (B) OS; and (C) DSS.
Patients who received ABVD (n = 253) (including BV-AVD in 1 patient) had a superior PFS and OS compared with patients who received other combination curative-intent chemotherapy (5-year PFS, 68% vs 36%, P < .001; 5-year OS, 71% vs 43%, P < .001), including within stratified stage groups in which patients with advanced stage had a 5-year PFS of 61% with ABVD compared with 43% with other regimens (P = .004) and 5-year OS of 64% vs 52%, respectively (P = .010). Patients with limited-stage disease who received ABVD had a 5-year PFS and OS of 86% and 89%, respectively. Similar to the entire cohort, in patients with advanced-stage disease treated with ABVD, age, poor PS stage IV disease, anemia, leukocytosis, and lymphopenia were associated with inferior PFS and/or OS, and those with an IPS ≥ 4 had an inferior prognosis (5-year PFS, 47% vs 71%, P < .001; 5-year OS, 49% vs 75%, P < .001) (Table 2). In multivariate analysis, IPS, age, and treatment with ABVD were associated with PFS and OS (supplemental Table 2).
Table 2.
IPS and other prognostic factors in patients with advanced-stage disease in the modern-era treated with ABVD chemotherapy
| n = 173 (%) | 5-y PFS (%) | P value | 5-y OS (%) | P value | |
|---|---|---|---|---|---|
| Age, y | |||||
| 60-69 | 105 (61) | 68 | .003 | 68 | .005 |
| 70-79 | 60 (35) | 49 | 47 | ||
| ≥80 | 8 (4) | 47 | 47 | ||
| Sex | |||||
| Male | 110/173 (64) | 62 | .54 | 63 | .24 |
| Female | 63/173 (36) | 59 | 66 | ||
| Albumin ≥ 40 g/L | 39/146 (27) | 69 | .39 | 75 | .39 |
| Albumin < 40 g/L | 107/146 (73) | 56 | 57 | ||
| Hemoglobin ≥ 105 g/L | 117/168 (70) | 67 | .021 | 71 | .012 |
| Hemoglobin < 105 g/L | 51/168 (30) | 50 | 52 | ||
| White blood cells < 15 × 109/L | 153/173 (88) | 65 | .001 | 68 | .006 |
| White blood cells ≥5 × 109/L | 15/168 (12) | 32 | 39 | ||
| Lymphocytes ≥ 0.6 × 109/L and/or 8% | 113/162 (60) | 68 | .094 | 74 | .019 |
| Lymphocytes < 0.6 × 109/L and/or 8% | 49/162 (40) | 47 | 46 | ||
| Stage II-III | 102/173 (59) | 64 | .082 | 70 | .025 |
| Stage IV | 71/173 (41) | 57 | 56 | ||
| IPS 1-3 | 98/173 (57) | 71 | <.001 | 75 | <.001 |
| IPS ≥ 4 | 75/173 (43) | 47 | 49 | ||
| PS 0-1 | 94/171 (55) | 73 | .003 | 76 | .009 |
| PS ≥ 2 | 77/171 (45) | 46 | 51 | ||
| No B symptoms | 53/173 (31) | 66 | .75 | 71 | .71 |
| B symptoms | 120/173 (69) | 58 | 61 | ||
| Nonbulky | 140/166 (84) | 60 | .57 | 59 | .53 |
| Bulky | 26/166 (16) | 64 | 64 |
Treatment-related toxicities
In total, 279 of 327 patients (85%) who were treated with curative intent in the modern era received bleomycin, of whom 58 (21%) developed pulmonary toxicity. All cases were diagnosed based on the occurrence of new respiratory symptoms with or without supportive radiography (n = 49, 84%), pulmonary function testing (reduced diffusing capacity of the lungs for carbon dioxide, n = 11 [19%]), or biopsy (n = 1) after starting bleomycin, which led to bleomycin omission in subsequent treatments (n = 46, 79%), treatment discontinuation (n = 4, 7%), steroid administration (n = 17, 29%), or death (n = 8, 14%). Bleomycin toxicity was more frequent in those with advanced stage compared with limited stage (24% vs 13%, P = .038) but no other risk factors were identified (data not shown). Although most cases of pulmonary toxicity occurred after cycle 3 or more (62%), 22 (38%) occurred after cycles 1 and 2, including 11 cases in limited stage, and overall, 8 patients (14%) died as a direct result of pulmonary toxicity. Thirty-one of 58 patients (53%) had concomitant granulocyte colony-stimulating factor use. Although detailed smoking history was not available for most patients, 7 had at least a 15-pack-year history documented, which accounted for 2 cases of early toxicity (within 2 cycles) and 1 death after 5 cycles. Rates of pulmonary toxicity (P = .77) or death due to pulmonary toxicity (P = .55) were not associated with age (supplemental Table 3).
Although there has been a decrease in bleomycin use from 92% of curative-intent–treated patients in the 2000s to 80% in the 2010s (P = .003), there was no difference in the rates of pulmonary toxicity (22% vs 19%, P = .57) or associated deaths (4% vs 2%, P = .42) between these 2 decades. Numerical outcome estimates were lower in those who developed bleomycin toxicity, although results were not significant (5-year OS, 62% vs 71%, P = .17; 5-year PFS, 59% vs 68%, P = .33).
In the whole modern era cohort, there have been 20 treatment-related deaths (5%). In addition to the 8 patients who died of bleomycin pulmonary toxicity, 11 patients died of infectious complications, including 4 due to febrile neutropenia, and 1 death was cardiac related. There was no significant difference in the risk of treatment-related death across age groups (P = .61) (supplemental Table 3). A total of 59 patients in the modern era curative cohort died of disease progression, and the remaining 81 patients died of non–HL-related causes (supplemental Table 3).
Outcomes in older adults with relapsed or refractory (R/R) cHL
Overall, 74 of 327 patients (23%) initially treated with curative intent had R/R disease. In total, 39 patients received systemic therapy, with only 12 patients receiving further multiagent chemotherapy, including 3 who underwent autologous hematopoietic stem cell transplant All 3 patients relapsed within 1 year of transplant. The remaining patients received single-agent chemotherapy or BV. Only 4 patients received PD1 inhibitors, all after BV, given the limited availability during the study period.
Overall, the median OS from first relapse/progression was 9.5 months (0.2-133.6 months), and the corresponding 1-year and 5-year OS was 43.0% and 18.0%, respectively.
Discussion
This study evaluated historical and modern era outcomes in patients with cHL aged ≥60 years in BC. As anticipated, outcomes have improved over the decades, with stabilization in the past 20 years with a 5-year DSS of 63% and a 5-year OS of 54%. This favorable trend has been observed in other studies20 and likely reflects improvements in pathologic diagnosis, introduction of fluorine-18 fluorodeoxyglucose-PET staging, treatment advances, including the shift from MOPP (mechlorethamine hydrochloride, vincristine sulfate [Oncovin], procarbazine hydrochloride, and prednisone) to ABVD, introduction of hematopoietic stem cell transplant for R/R disease, as well as advances in supportive care. In patients treated with curative intent in the modern era, the 5-year PFS and OS were 60% and 65% respectively, and superior in those who received ABVD but also notably better in those in the 60- to 69-year age group compared with those aged >70 years. These outcomes are comparable to more recent analyses of older adults with HL and support that ABVD-type regimens can still be considered in robust, suitable patients.13,21 The importance of comorbidity measures was highlighted in a recent multicenter analysis, which reported higher rates of toxicity-related early treatment discontinuation in older adults with a Cumulative Illness Rating Scale-Geriatric score ≥10 (28% vs 12%, P = .016) or with documented geriatric syndrome (28% vs 13%, P = .02) treated with conventional therapy, as well as higher mortality from causes other than disease or treatment.22
Despite improved outcomes in the modern era, treatment-related toxicity remains an important concern with the use of multiagent chemotherapy in older patients. In particular, high rates of pulmonary toxicity have been reported in patients with HL in this age group treated with bleomycin-containing regimens.9,13,23,24 A PET-adapted approach was evaluated in the RATHL trial to limit use of bleomycin, whereby it is omitted in subsequent cycles if the PET scan after 2 cycles is negative.19 Among the 1203 patients treated with ABVD in the aforementioned study, the rate of grade 3 or 4 pulmonary events was only 1% during cycles 1 and 2, and in PET-negative patients, a lower incidence was reported during the 4 subsequent cycles with bleomycin omitted (AVD) compared with ABVD (1% vs 3%, P < .05). Although the median age of patients included in the RATHL trial was 33 years, with <10% aged ≥60 years, a more recent analysis of bleomycin use in older patients with early-stage favorable HL in the German Hodgkin Study Group HD10 and HD13 trials reported no cases of pulmonary toxicity among all 82 patients randomized to receive 2 cycles of ABVD, compared with 10% in those treated with 4 cycles.25 In this study, we identified much higher rates of early pulmonary toxicity, with more than a third of cases (22/58) developing within the first 2 cycles of bleomycin-containing chemotherapy, including 11 cases in patients treated for limited-stage disease. We note that both previously described studies reported only grade 3 or 4 events, whereas our retrospective analysis used a broader definition of pulmonary toxicity, based mostly on the treating physicians’ clinical judgment. However, of 58 cases, 3 cases (14%) of pulmonary toxicity did result in death, with 3 occurring in patients who had received only 2 cycles of ABVD. Our 3% rate of pulmonary toxicity–related mortality (8 of 279 patients treated with bleomycin) is also consistent with that reported in patients aged ≥60 years in the ABVD arm of the ECHELON-1 study.16 Thus, bleomycin use should be avoided in this age group.
Novel agents including BV and PD1 inhibitors are now being used in older adults with HL. Following encouraging results from earlier studies of BV in R/R HL,26,27 ECHELON-1 demonstrated that BV + AVD was associated with improved mPFS over ABVD in advanced-stage cHL, particularly stage IV disease, and improved PFS in stage III/IV with longer follow-up.12,15 A more recent updated analysis demonstrated an OS benefit of an AVD-BV arm compared with ABVD (6-year OS, 93.9% vs 89.4%, P = .009).28 However, a previous subgroup analysis of the 186 patients age ≥60 years enrolled in the ECHELON-1 trial demonstrated similar mPFS (1-year mPFS, AVD-BV 70.3% vs ABVD 71.4%, P = .99) and PFS (5-year PFS, 67.1% AVD-BV vs 61.6% ABVD, P = .44) in both treatment arms. Similar results were seen in the subgroup analysis of patients aged >60 years for OS (hazard ratio, 0.83; 95% confidence interval, 0.47-1.47), although power was limited. In addition, although there were fewer pulmonary adverse events with BV + AVD compared with ABVD (2% vs 13%) in this age group, rates of peripheral neuropathy were higher (65% vs 43%), including grade 3 and 4 neuropathy (18% vs 3%). Febrile neutropenia was also more common (37% vs 17%) and still occurred in 30% of patients despite primary prophylaxis with granulocyte colony-stimulating factor, highlighting that dose reductions are often also needed in this population.16 Taken together, BV + AVD remains unproven in this age group, and use should be limited to robust patients aged 60 to 69 years. BV and AVD administered in a sequential approach was evaluated in an earlier phase 2 study of patients with stage III/IV cHL aged >60 years. Although cross-trial comparisons are limited, the 2-year PFS of 84% and 2-year OS of 93% compare very favorably and toxicities were more manageable, with lower rates of febrile neutropenia (8%) and less severe peripheral neuropathy (grade 2, 33%; grade 3, 4%) observed than those with AVD-BV given concurrently.29 This approach may be more suitable for older adults, especially those with comorbidities and/or >70 years of age where outcomes with ABVD remain unsatisfactory.
The PD1 inhibitors, nivolumab, pembrolizumab, and tiselizumab have demonstrated high response rates of between 63% and 87% in R/R cHL, with median PFS ranging from 12 to 19 months.30, 31, 32, 33, 34 More recently, pembrolizumab was associated with superior PFS compared with BV in a phase 3 study in the R/R HL setting.31 The aforementioned study included patients who were transplant ineligible, and 16% of the patients enrolled were aged >64 years. Studies involving patients with solid tumors suggest that older adults do not have an increased risk of immune-related adverse events with PD1 inhibitor use.35 Given the synergy between these agents,36 a combination trial of BV and nivolumab in patients who transplant eligible was evaluated, which demonstrated an objective response rate (ORR) of 82% and complete remission rate (CRR) of 61%.37 However, a phase 2 study of the same combination in newly diagnosed older adults (aged ≥60 years, median age 71.5 years) including those with a PS of 2, only induced an ORR of 61% and a CRR of 48%. Neuropathy occurred in 48% (11%, grade 3), and there was 1 death from cardiac arrest that may have been treatment related.38 Although the study did confirm the activity of this combination in older patients, the trial closed early after failure to meet its predefined ORR, and taken together, this approach should not be used in the upfront setting. Nivolumab monotherapy followed by nivolumab plus AVD was assessed in cohort D of the CheckMate 205 trial, which evaluated 51 patients (age ≥18 years), including 6 aged ≥60 years (12%), with untreated advanced-stage cHL. Results were promising with an ORR of 84%, including 67% achieving CRR.39 A clinical trial examining the frontline use of AVD with nivolumab is ongoing (NCT03033914), including a cohort of patients aged ≥60 years, and will provide more insight into the safety and efficacy of this combination in older adults. Furthermore, the ongoing phase 3 cooperative group SWOG1826 study comparing BV-AVD with nivolumab-AVD does not have an upper age limit and does include a PS of 2, which will facilitate enrollment of carefully selected older patients who may be suitable for BV-AVD (NCT03907488).
In our analysis, only ∼20% of all patients with R/R disease who were treated with frontline curative therapy were fit enough to receive potentially curative second-line combination chemotherapy, and only 8% were planned for transplant. Our low transplant rate also highlights the need for effective frontline therapies because most older patients will not be transplant eligible. Overall, the impact of PD1 inhibitors on survival in older patients in this setting is unknown, but given that toxicity is not associated with age and that these agents have been transformative in all-age HL and other cancers, it is likely that they will improve survival in older adults with cHL.
To our knowledge, this study is the largest population-based analysis evaluating the prognosis of older adults with cHL. Although patients in our study were evaluated retrospectively, selection bias was minimized with broad capture of patients in our clinical and pathologic database as well as provincial management guidelines that were uniformly applied. Significant improvement in outcomes has been observed in BC in recent decades, but favorable outcomes are limited to patients aged 60 to 69 years. In all patients, treatment-related toxicity, particularly pulmonary toxicity, is a notable concern with multiagent chemotherapy. Although longer follow-up is required to better characterize the impact of novel therapies on overall outcomes, our findings can serve as a useful historical comparison as these agents become more widely incorporated into frontline therapy in the new era.
Conflict-of-interest disclosure: D.V. received honoraria from/consults for BMS/Celgene, Kite/Gilead, Merck, Kyowa Kirin, Janssen, AstraZeneca, AbbVie, and Roche. A.S.G. received honoraria from/consults for AstraZeneca, AbbVie, Janssen, Celgene, and Sandoz; received research funding from AstraZeneca, AbbVie, and Janssen; and research funding (to the institution) from Roche and AstraZeneca. C.L.F received honoraria from/consults for BMS, Seattle Genetics, Celgene, AbbVie, Sanofi, Incyte, Amgen, and Janssen; and research funding from Teva, Janssen, and Roche/Genentech. J.W.C. consults for Bayer. G.W.S. received honoraria from/consults for Seattle Genetics. L.H.S. received research funding from F. Hoffmann-La Roche Ltd, and Genentech Inc; and received honoraria from/consults for F. Hoffmann-La Roche Ltd, Genentech Inc, AbbVie, Amgen, Apobiologix, Acerta, AstraZeneca, Celgene, Gilead, Janssen, Kite Pharma, Karyopharm, Lundbeck, Merck, MorphoSys, Seattle Genetics, Takeda, Teva, TG Therapeutics, Verastem, and Incyte. K.J.S. received honoraria from/consults for Seattle Genetics, BMS, Merck, Kyowa, Janssen, Novartis, and Incyte; serves on the steering committee of BeiGene; received research funding from Roche and BMS; and serves on the Data and Safety Monitoring Committee of Regeneron. The remaining authors declare no competing financial interests.
Acknowledgment
The authors would like to thank Aixiang Jiang for statistical support.
Authorship
Contribution: P.T.M.C. and K.J.S. designed the study, collected and analyzed data, and wrote the paper; K.J.S. provided clinical care to patients; D.V., A.S.G., C.L.F., D.W.S., J.M.C., and L.H.S. provided clinical materials and reviewed the manuscript; G.W.S., R.D.G., P.F., J.W.C., and B.S. provided pathology reviews and reviewed the paper; and D.W. supervised the PET program and reviewed the paper.
Footnotes
Original data are available on request from the corresponding author, Kerry J. Savage (ksavage@bccancer.bc.ca).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Carter J, David KA, Kritharis A, Evens AM. Current treatment options for older patients with Hodgkin lymphoma. Curr Treat Options Oncol. 2020;21(5):42. doi: 10.1007/s11864-020-00745-9. [DOI] [PubMed] [Google Scholar]
- 2.Engert A, Ballova V, Haverkamp H, et al. German Hodgkin's Study Group Hodgkin's lymphoma in elderly patients: a comprehensive retrospective analysis from the German Hodgkin's Study Group. J Clin Oncol. 2005;23(22):5052–5060. doi: 10.1200/JCO.2005.11.080. [DOI] [PubMed] [Google Scholar]
- 3.Jagadeesh D, Diefenbach C, Evens AM. XII. Hodgkin lymphoma in older patients: challenges and opportunities to improve outcomes. Hematol Oncol. 2013;31(S1):69–75. doi: 10.1002/hon.2070. [DOI] [PubMed] [Google Scholar]
- 4.Keegan TH, Glaser SL, Clarke CA, et al. Epstein-Barr virus as a marker of survival after Hodgkin's lymphoma: a population-based study. J Clin Oncol. 2005;23(30):7604–7613. doi: 10.1200/JCO.2005.02.6310. [DOI] [PubMed] [Google Scholar]
- 5.Stark GL, Wood KM, Jack F, Angus B, Proctor SJ, Taylor PR. Hodgkin’s disease in the elderly: a population-based study. Br J Haematol. 2002;119(2):432–440. doi: 10.1046/j.1365-2141.2002.03815.x. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi MK, Tellam JT, Khanna R. Epstein-Barr virus-associated Hodgkin's lymphoma. Br J Haematol. 2004;125(3):267–281. doi: 10.1111/j.1365-2141.2004.04902.x. [DOI] [PubMed] [Google Scholar]
- 7.Jarrett RF, Stark GL, White J, et al. Scotland and Newcastle Epidemiology of Hodgkin Disease Study Group. Impact of tumor Epstein-Barr virus status on presenting features and outcome in age-defined subgroups of patients with classic Hodgkin lymphoma: a population-based study. Blood. 2005;106(7):2444–2451. doi: 10.1182/blood-2004-09-3759. [DOI] [PubMed] [Google Scholar]
- 8.Brooks E, Connors JM, Sehn LH, et al. Impact of time from diagnosis to initiation of curative-intent chemotherapy on clinical outcomes in patients with Hodgkin lymphoma. Leuk Lymphoma. 2016;57(4):872–879. doi: 10.3109/10428194.2015.1086919. [DOI] [PubMed] [Google Scholar]
- 9.Evens AM, Hong F, Gordon LI, et al. The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: a comprehensive analysis from the North American intergroup trial E2496. Br J Haematol. 2013;161(1):76–86. doi: 10.1111/bjh.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Press OW, Li H, Schöder H, et al. US Intergroup trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: Southwest Oncology Group S0816. J Clin Oncol. 2016;34(17):2020–2027. doi: 10.1200/JCO.2015.63.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallamini A, Tarella C, Viviani S, et al. Early chemotherapy intensification with escalated BEACOPP in patients with advanced-stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two ABVD cycles: long-term results of the GITIL/FIL HD 0607 trial. J Clin Oncol. 2018;36(5):454–462. doi: 10.1200/JCO.2017.75.2543. [DOI] [PubMed] [Google Scholar]
- 12.Connors JM, Jurczak W, Straus DJ, et al. ECHELON-1 Study Group Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2018;378(4):331–344. doi: 10.1056/NEJMoa1708984. [published correction appears in N Engl J Med. 2018;378(9):878] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamatoullas A, Brice P, Bouabdallah R, et al. Outcome of patients older than 60 years with classical Hodgkin lymphoma treated with front line ABVD chemotherapy: frequent pulmonary events suggest limiting the use of bleomycin in the elderly. Br J Haematol. 2015;170(2):179–184. doi: 10.1111/bjh.13419. [DOI] [PubMed] [Google Scholar]
- 14.Straus DJ. Initial Hodgkin treatment of the frail elderly. Blood. 2017;130(26):2813–2814. doi: 10.1182/blood-2017-10-812966. [DOI] [PubMed] [Google Scholar]
- 15.Straus DJ, Długosz-Danecka M, Connors JM, et al. Brentuximab vedotin with chemotherapy for stage III or IV classical Hodgkin lymphoma (ECHELON-1): 5-year update of an international, open-label, randomised, phase 3 trial. Lancet Haematol. 2021;8(6):e410–e421. doi: 10.1016/S2352-3026(21)00102-2. [DOI] [PubMed] [Google Scholar]
- 16.Evens AM, Connors JM, Younes A, et al. Older patients (aged ≥60 years) with previously untreated advanced-stage classical Hodgkin lymphoma: a detailed analysis from the phase III ECHELON-1 study. Haematologica. 2021;102(5):1086–1094. doi: 10.3324/haematol.2021.278438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villa D, Sehn LH, Aquino-Parsons C, et al. Interim PET-directed therapy in limited-stage Hodgkin lymphoma initially treated with ABVD. Haematologica. 2018;10(12):e590–e593. doi: 10.3324/haematol.2018.196782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savage KJ, Connors JM, Villa DR, et al. Advanced stage classical Hodgkin lymphoma patients with a negative PET-scan following treatment with ABVD have excellent outcomes without the need for consolidative radiotherapy regardless of disease bulk at presentation. Blood. 2015;126(23):579. [Google Scholar]
- 19.Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin's lymphoma. N Engl J Med. 2016;374(25):2419–2429. doi: 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bishton MJ, Hubbard R, Witherall R, et al. Era-by-era improvement in survival for elderly patients with Hodgkin lymphoma; outcome data from a large population-based cohort. Ann Oncol. 2015;26(11):2356–2357. doi: 10.1093/annonc/mdv359. [DOI] [PubMed] [Google Scholar]
- 21.Sykorova A, Mocikova H, Lukasova M, et al. Czech Hodgkin’s Lymphoma Study Group. Outcome of elderly patients with classical Hodgkin’s lymphoma. Leuk Res. 2020;90:106311. doi: 10.1016/j.leukres.2020.106311. [DOI] [PubMed] [Google Scholar]
- 22.Orellana-Noia VM, Isaac K, Malecek MK, et al. Multicenter analysis of geriatric fitness and real-world outcomes in older patients with classical Hodgkin lymphoma. Blood Adv. 2021;5(18):3623–3632. doi: 10.1182/bloodadvances.2021004645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evens AM, Helenowski I, Ramsdale E, et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood. 2012;119(3):692–695. doi: 10.1182/blood-2011-09-378414. [DOI] [PubMed] [Google Scholar]
- 24.Martin WG, Ristow KM, Habermann TM, Colgan JP, Witzig TE, Ansell SM. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin’s lymphoma. J Clin Oncol. 2005;23(30):7614–7620. doi: 10.1200/JCO.2005.02.7243. [DOI] [PubMed] [Google Scholar]
- 25.Böll B, Goergen H, Behringer K, et al. Bleomycin in older early-stage favorable Hodgkin lymphoma patients: analysis of the German Hodgkin Study Group (GHSG) HD10 and HD13 trials. Blood. 2016;127(18):2189–2192. doi: 10.1182/blood-2015-11-681064. [DOI] [PubMed] [Google Scholar]
- 26.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gopal AK, Bartlett NL, Forero-Torres A, et al. Brentuximab vedotin in patients aged 60 years or older with relapsed or refractory CD30-positive lymphomas: a retrospective evaluation of safety and efficacy. Leuk Lymphoma. 2014;55(10):2328–2334. doi: 10.3109/10428194.2013.876496. [DOI] [PubMed] [Google Scholar]
- 28.Ansell SM, Radford JA, Connors JM, et al. Overall survival with brentuximab vedotin in stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2022;387(4):310–320. doi: 10.1056/NEJMoa2206125. [DOI] [PubMed] [Google Scholar]
- 29.Evens AM, Advani RH, Helenowski IB, et al. Multicenter phase II study of sequential brentuximab vedotin and doxorubicin, vinblastine, and dacarbazine chemotherapy for older patients with untreated classical Hodgkin lymphoma. J Clin Oncol. 2018;36(30):3015–3022. doi: 10.1200/JCO.2018.79.0139. [DOI] [PubMed] [Google Scholar]
- 30.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuruvilla J, Ramchandren R, Santoro A, et al. KEYNOTE-204 investigators. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study [published correction appears in Lancet Oncol. 2021;22(5):e184.] Lancet Oncol. 2021;22(4):512–524. doi: 10.1016/S1470-2045(21)00005-X. [DOI] [PubMed] [Google Scholar]
- 32.Song Y, Gao Q, Zhang H, et al. Treatment of relapsed or refractory classical Hodgkin lymphoma with the anti-PD-1, tislelizumab: results of a phase 2, single-arm, multicenter study. Leukemia. 2020;34(2):533–542. doi: 10.1038/s41375-019-0545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial [published correction appears in J Clin Oncol. 2018;36(26):2748.] J Clin Oncol. 2018;36(14):1428–1439. doi: 10.1200/JCO.2017.76.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen R, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134(14):1144–1153. doi: 10.1182/blood.2019000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nebhan CA, Cortellini A, Ma W, et al. Clinical outcomes and toxic effects of single-agent immune checkpoint inhibitors among patients aged 80 years or older with cancer: a multicenter international cohort study. JAMA Oncol. 2021;7(12):1856–1861. doi: 10.1001/jamaoncol.2021.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardai SJ, Epp A, Law C.-L. Brentuximab vedotin-mediated immunogenic cell death. [abstract]. In: Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; 2015 Apr 18-22; Philadelphia, PA. Philadelphia (PA): AACR. Cancer Res. 2015;75(suppl 15) Abstract 2469. [Google Scholar]
- 37.Herrera AF, Moskowitz AJ, Bartlett NL, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2018;131(11):1183–1194. doi: 10.1182/blood-2017-10-811224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheson BD, Bartlett NL, LaPlant B, et al. Brentuximab vedotin plus nivolumab as first-line therapy in older or chemotherapy-ineligible patients with Hodgkin lymphoma (ACCRU): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2020;7(11):e808–e815. doi: 10.1016/S2352-3026(20)30275-1. [DOI] [PubMed] [Google Scholar]
- 39.Ramchandren R, Domingo-Domènech E, Rueda A, et al. Nivolumab for newly diagnosed advanced-stage classic Hodgkin lymphoma: safety and efficacy in the phase II CheckMate 205 Study. J Clin Oncol. 2019;37(23):1997–2007. doi: 10.1200/JCO.19.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.