Abstract
Objective: Patients with stable coronary artery disease (SCAD) still have a higher risk of adverse cardiovascular events. Shexiang Baoxin Pill (SBP) is widely used as a complementary and alternative treatment for SCAD. This study aimed to further verify the therapeutic effect and safety of SBP on SCAD.
Methods: Seven databases were involved in this meta-analysis as of 1 June 2022. Data was collected from all the randomized controlled trials (RCTs) of the combination of SBP and conventional western medicine (CWM) in treating SCAD which was conducted by two independent authors. Risk of bias was assessed using the Cochrane risk-of-bias 2.0 (RoB2.0) tool, and the meta-analysis was accomplished with Review Manager 5.3. Furthermore, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) profiler 3.2.2 software was selected to grade the current evidence in our findings.
Results: 42 articles, involving 6,694 patients were screened among all the 1,374 records in the analysis. The results demonstrated that the combination therapy was more efficient than CWM alone in lowering the incidence of major adverse cardiovascular events (MACE, RR = 0.50, 95% CI: 0.37 to 0.68, p < 0.00001) and ameliorating the total effective rate of angina symptom improvement (RR = 1.23, 95% CI: 1.19 to 1.28, p < 0.00001), the effective rate of electrocardiogram improvement (RR = 1.34, 95% CI: 1.26 to 1.43, p < 0.00001), the frequency of angina pectoris (MD = −2.83, 95% CI: −3.62 to −2.05, p < 0.00001), and the duration of angina pectoris (MD = −1.32, 95% CI: −2.04 to −0.61, p = 0.0003). We also found that, after SBP treatment, a more positive blood lipid level and left ventricular ejection fraction without the increase in adverse cases were calculated in our meta-analysis. What’s more, Subgroup analysis indicated that treatment duration may be the source of heterogeneity. The certainty of the evidence for MACE, and electrocardiogram improvement exhibited moderate certainty, and the certainty of the evidence for the remaining outcomes was judged as low certainty. The trial sequential analysis further affirmed the clinical efficacy of SBP.
Conclusion: The available evidence indicates that SBP may be an effective therapeutic option in patients with SCAD. However, considering the inferior quality and inconsistent results in the included trials, further rigorous RCTs are required.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero, identifier [CRD42022334529].
Keywords: Shexiang Baoxin Pill, stable coronary artery disease, randomized controlled trials, meta-analysis, grade
1 Introduction
Stable coronary artery disease (SCAD) is the most common type of coronary heart disease (CHD), mainly including stable angina pectoris, stable phase after acute coronary syndrome, and ischemic cardiomyopathy (Montalescot et al., 2013). A report from the American Heart Association (AHA) in 2016 showed that the incidence of SCAD is much higher than that of myocardial infarction, which is twice as high as that of myocardial infarction and is expected to reach 18% of the adult population by 2030 (Mozaffarian et al., 2016). Notably, SCAD can remain stable for a long time or can become unstable at any time due to plaque rupture or erosion leading to acute coronary events. Currently, aspirin and statins are the standard secondary prevention approach in reducing the risk of cardiovascular events in patients with SCAD. However, patients who receive secondary prevention still have a 4%–12% risk of major adverse cardiovascular events (MACE), and there is still a considerable residual cardiovascular risk (Bhatt et al., 2010; Feres et al., 2013). How to further reduce the risk of recurrent cardiovascular events in SCAD remains a hot spot and a challenge for current research.
In recent years, with the increasing clinical evidence of traditional Chinese medicine (TCM) for the treatment of CHD, TCM may become a supplementary and alternative medicine for the primary and secondary prevention of patients with SCAD (Liang and Gu, 2021). Shexiang Baoxin Pill (SBP) is currently one of the most commonly used aromatic medicines for the treatment of cardiovascular diseases in China, which has been widely used to relieve and prevent angina-related symptoms since its marketing in 1981 (Guo et al., 2021; Wei et al., 2021). SBP is a Chinese medicine compound prescription composed of Moschus (the dried preputial secretion of Moschus berezovskii, M. sifanicus or M. moschiferus), Radix Ginseng (Panax ginseng C.A.Mey.), Bovis Calculus Artifactus (the dried gall-stone of Bos taurus domesticus Gmelin), Cinnamomi Cortex (Cinnamomum cassia), Styrax (Liquidambar orientalis Mill.), Bufonis Venenum (Bufo gargarizans), and Borneolum Syntheticum (Dryobalanops aromatica C.F.Gaertn.) (Supplementary Material S1). It has been recommended for the treatment of CHD by the “Guidelines for TCM diagnosis and treatment of stable angina pectoris of coronary heart disease” (China Association of Chinese Medicine Cardiovascular Disease Branch, 2019). Network pharmacology analysis found that SBP and its plasma absorption compounds can dilate blood vessels by upregulating cyclooxygenase-2 and downregulating intercellular adhesion molecule-1 (Fang et al., 2017). Pharmacological studies have shown that SBP has a protective effect on damaged vascular endothelial cells, and can inhibit inflammation of the vascular wall and stabilize atherosclerotic plaques during the atherosclerotic process (Lu et al., 2019; Zhang et al., 2020). Recent studies have found that SBP can affect the endothelial cell signaling pathway to promote the expression of therapeutic angiogenesis-related genes, and it is speculated that this mechanism may be related to the compounds such as ginsenosides and cinnamaldehyde contained in it (Hu et al., 2021). A previous review evaluated the relevant randomized Controlled Trials (RCTs) before December 2017 and showed the efficacy and safety of SBP in the treatment of stable angina (Pan et al., 2019). A subsequent review reported that SBP combined with conventional therapy can improve coronary microvascular function (Wang et al., 2021). In recent years, several research trials have focused on the clinical efficacy and safety of SBP as an additional treatment for SCAD (Wang, 2016; Peng et al., 2017). In particular, a multicenter, double-blind, placebo-controlled phase IV randomized clinical trial in 2021 reaffirmed the clinical value of SBP in patients with SCAD (Ge et al., 2020). Regrettably, there are no relevant systematic reviews to summarize the efficacy and safety of SBP in the treatment of SCAD in terms of both methodological quality and quality of evidence.
Therefore, we conducted a systematic review and meta-analysis based on the available evidence to strictly evaluate the efficacy and safety of SBP for SCAD, and to clarify the strength of the evidence for SBP, to better guide clinical application.
2 Methods
2.1 Program and registration
This report adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement (Page et al., 2021) (Supplementary Material S2). We have already registered our protocol on the PROSPERO (number: CRD42022334529).
2.2 Search strategy
Two investigators independently searched the databases including PubMed, The Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (VIP), WanFang Database, and SinoMed from inception until 1 June 2022. The method for searching was based on subject headings combined with free words. In addition, we also manually retrieved the reference lists of published literature to search for other relevant studies. Unpublished literature was identified by searching the websites of national and international medical specialty societies, clinical trial registration platforms, and clinical practice guideline collections. The search strategies were formulated by physicians (MZ and YW), and statisticians (BL and XW). We have provided detailed search strategies in Supplementary Material S3.
2.3 Inclusion and exclusion criteria
2.3.1 Types of research
Only RCTs of SBP for patients with SCAD were included and not restricted by language or publication type.
2.3.2 Types of participants
All subjects (age ≥18 years) of the included study meet the diagnostic criteria for SCAD established by the AHA, the European Society of Cardiology, or the Chinese Medical Association (Fihn et al., 2012; Montalescot et al., 2013; Interventional Cardiology Group of Cardiovascular Branch of Chinese Medical Association., 2018). No restrictions were made regarding gender, country, or race.
2.3.3 Types of interventions
The experimental group and control group were both treated with antiplatelet drugs, lipid-lowering drugs, vasodilators of nitrate, and other conventional western medicine (CWM) recommended by the guideline (Interventional Cardiology Group of Cardiovascular Branch of Chinese Medical Association., 2018). The experimental group was added with SBP (produced by Shanghai Hutchison Pharmaceuticals Co., Ltd.) for adjuvant therapy.
2.3.4 Types of outcomes
The primary outcomes are as follows: 1) MACE including cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke; 2) the total effective rate of angina symptom improvement (significant effectiveness: symptom basically disappeared, and the number of angina attacks decreased by at least 80%; effective: symptom improved significantly, and the number of angina attacks decreased by 50%–80%; inefficacy: no significant improvement in symptom, less than 50% reduction in the number of angina attacks) (according to “Guiding Principles for Clinical Research of New Chinese Medicines”) (Ministry of health of the people’s republic of China, 2002); 3) electrocardiogram (ECG) improvement (significant effectiveness: ECG returned to the normal range; effective: ST segment was reduced, and after treatment, it rose above 0.05 mV but did not reach the normal level, or the T wave changed from a flat state to an upright state; inefficacy: the ECG had no significant changes compared to before treatment; worsening of disease: the ST segment was reduced by more than 0.05 mV after treatment; the T wave state was completely changed, and ectopic heart rate occurred); 4) adverse events (AEs) including clinical symptoms, signs, laboratory abnormalities.
The secondary outcomes are as follows: 1) angina pectoris frequency: in the unit of times/week; 2) angina pectoris duration: in the unit of min/time; 3) left ventricular ejection fraction (LVEF); 4) blood lipid level including total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C).
2.3.5 Exclusion criteria
The exclusion criteria were as follows: 1) duplicate published literature; 2) the included studies did not report the outcomes of interest of this systematic review; 3) the baseline information of patients was inconsistent; 4) intervention measures combined with other TCM.
2.4 Literature screening and data extraction
The literature retrieved from each database is imported into Endnote software for deduplication, and two researchers screen the literature according to the inclusion and exclusion criteria, extract the information, and recheck each other’s work. If there is any disagreement, the third researcher will be invited to discuss and make a decision. Data were extracted using a standardized data extraction form to extract information including first author, year of publication, sample size, gender, age, disease condition, intervention measures, treatment duration, and outcome indicators.
2.5 Risk of bias assessment
Two reviewers independently assessed the risk of bias in the RCTs using the Cochrane risk-of-bias 2.0 (RoB2.0) tool (Liu et al., 2019). The assessment of ROB includes the following five domains: 1) bias arising from the randomization process; 2) bias due to deviations from intended interventions; 3) bias due to missing outcome data; 4) bias in the measurement of the outcome; 5) bias in the selection of the reported result. Finally, a judgment of the overall risk of bias is generated. The ROB was judged as “low”, “high”, or “some concerns”. Disagreements between the two researchers were resolved through consultation with a third researcher.
2.6 Data analysis
The software Review Manager 5.3 is used for statistical analysis. Cochran’s Q and I 2 are chosen to test for heterogeneity, and if there is statistical heterogeneity among the findings (I 2 ≥ 50%, p < 0.10), a random-effects model will be selected, and conversely, a fixed-effects model will be used. Binary variables were analyzed using relative risk (RR) as the pooled statistic, and continuous variables were analyzed using weighted mean difference (MD) as the pooled statistic, both of which describe the 95% confidence interval (CI). Meta-regression will be performed to find the reasons for heterogeneity, such as publication year, age, course of treatment, and sample size. Further, the statistically significant factors obtained by meta-regression will be used as grouping indicators for subgroup analysis. Sensitivity analyses were performed by omitting each study at a time to assess the consistency and stability of the pooled results. In addition, we examined potential publication bias using Egger’s test method. Finally, the TSA 0.9.5.10 Beta software was used to perform TSA analysis on the associated results.
2.7 Assessing the certainty of evidence
The Grading Recommendations Assessment, Development, and Evaluation (GRADE) technique was used to assess the certainty of the evidence (Goldet and Howick, 2013) following the instructions of the website (https://training.cochrane.org/handbook/current/chapter-14/). RCT evidence is initially classified as high quality, but it can be downgraded due to the risk of bias, inconsistency, indirectness, imprecision, and publication bias. The level of evidence is classified into four categories: “high,” “moderate,” “low,” and “very low”.
3 Results
3.1 Literature search results
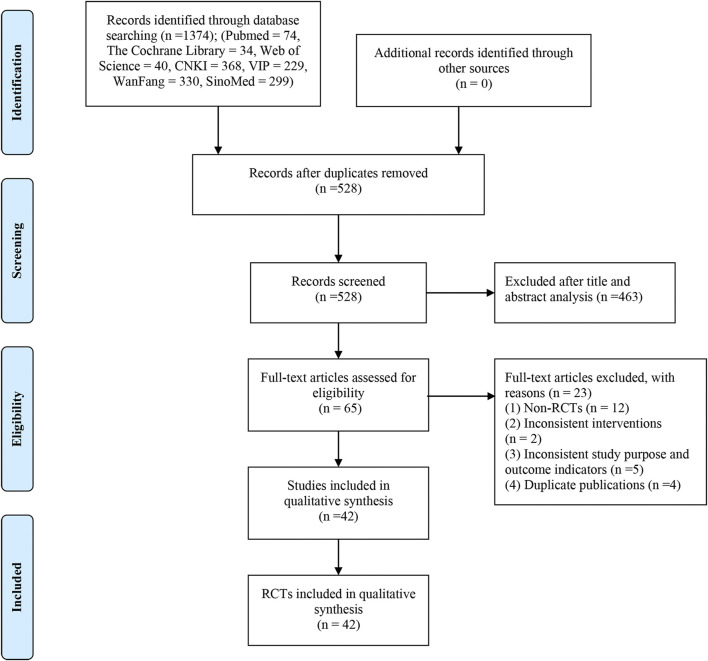
The flowchart of search results is shown in Figure 1 according to the PRISMA study. We included 1,374 records from seven electronic databases. After duplicate studies were removed, we screened 528 titles and abstracts and finally obtained 65 full-text articles. Among them, 23 studies were excluded, of which 12 studies were not RCTs, two studies were inconsistent interventions, five studies did not match the research purpose and outcomes, and four studies were published repeatedly. Supplementary Material S4 contains a list of studies that were excluded by reading the full text. Eventually, 42 eligible studies are included (Sun, 2010; Xu et al., 2010; Shi and Hang, 2011; Guo and Tan, 2012; Jiang, 2012; Wu et al., 2012; Sun, 2013; Yang et al., 2013; Zou et al., 2013; Chen, 2014; Huang et al., 2014; Liu, 2014; Wang, 2014; Zhao, 2014; Hou, 2015; Ji, 2015; Tan, 2015; Wang et al., 2015; Ding, 2016; Miao et al., 2016; Pan, 2016; Wang, 2016; Xia, 2016; Peng et al., 2017; Liu, 2018; Lv, 2018; Wang, 2018; Wang et al., 2018; Zhao, 2018; Zhao et al., 2018; Liao and Huang, 2019; Chen and Chen, 2020; Ge et al., 2020; Wang ang Zhu, 2020; Gao and Chen, 2021; Xia et al., 2021; Xie and Huang, 2021; Zhao and Xie, 2021; Zhou, 2021; Pan et al., 2022; Yang, 2022; Zhao et al., 2022).
FIGURE 1.
Flow diagram of study selection and identification.
3.2 Basic features of literature research
Table 1 shows the basic information of the included studies. All of the trials were double-arm RCTs. All of the studies were operated in China. One study was published in English (Ge et al., 2020), and the others were written in Chinese. A total of 6,694 patients were randomly divided into an SBP group and a control group, including 4,256 men. The mean age of the participants ranged from 51.6 to 81.7 years, who can be defined as middle-aged and elderly patients. Sample sizes range from 30 participants per arm to 1,335 people per arm. In all included studies, the standard type, and dose of CWM in the SBP treatment group were the same as those in the control group. The ingredients of SBP in all studies were the same, and the dosage of SBP in the experimental group was 22.5–67.5 mg three times a day. The shortest intervention period was 2 weeks while the longest was 24 months, and 3 months were the main ones (11/42, 26.19%). Three trials provided specific follow-up after treatment, with a maximum follow-up of 24 months (Peng et al., 2017; Ge et al., 2020) and a minimum follow-up of 6 months (Gao and Chen, 2021). A total of three studies received funding support (Tan, 2015; Ge et al., 2020; Ge et al., 2020; Zhao et al.). All the studies examined the effect of SBP combined with CWM on SCAD, six studies (Yang et al., 2013; Ding, 2016; Peng et al., 2017; Ge et al., 2020; Gao and Chen, 2021; Zhao and Xie, 2021) for MACE, twenty-seven studies (Sun, 2010; Xu et al., 2010; Shi and Hang, 2011; Guo and Tan, 2012; Sun, 2013; Chen, 2014; Huang et al., 2014; Liu, 2014; Wang, 2014; Hou, 2015; Ji, 2015; Tan, 2015; Wang et al., 2015; Ding, 2016; Wang, 2016; Xia, 2016; Liu, 2018; Lv, 2018; Wang et al., 2018; Zhao, 2018; Zhao et al., 2018; Chen and Chen, 2020; Zhu, 2020; Gao and Chen, 2021; Wang ang; Xia et al., 2021; Pan et al., 2022; Zhao et al., 2022) for the total effective rate of angina symptom improvement, sixteen studies (Sun, 2010; Xu et al., 2010; Guo and Tan, 2012; Sun, 2013; Liu, 2014; Wang, 2014; Zhao, 2014; Hou, 2015; Ji, 2015; Xia, 2016; Lv, 2018; Wang et al., 2018; Zhao et al., 2018; Wang ang Zhu, 2020; Gao and Chen, 2021; Pan et al., 2022) for ECG improvement, eighteen studies (Xu et al., 2010; Guo and Tan, 2012; Sun, 2013; Zou et al., 2013; Wang, 2014; Chen, 2014; Zhao, 2014; Hou, 2015; Ji, 2015; Pan, 2016; Xia, 2016; Peng et al., 2017; Lv, 2018; Ge et al., 2020; Wang ang Zhu, 2020; Liu, 2018; Gao and Chen, 2021; Zhao and Xie, 2021) for AEs. Supplementary Material S5 provided follow-up times for all outcome measures.
TABLE 1.
Characteristics of the included trials.
| Study ID | Sample size | Gender | Age (year) | Angina classification(CCS) | Duration | Interventions | SBP dosage | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (T/C) | (M/F) | T | C | T | C | |||||
| Ge et al., 2020 | 1,335/1,327 | 1886/776 | 63.9 ± 9.8 | 63.7 ± 9.9 | Ⅰ∼Ⅲ | 24 months | SBP + CWM | CWM + placebo | 45mg, t.i.d | ①④ |
| Yang, (2022) | 40/40 | 50/30 | 71.53 ± 5.06 | 73.38 ± 5.47 | Ⅰ∼Ⅳ | 3 months | SBP + CWM | CWM | 67.5mg, t.i.d | ⑤⑥⑦ |
| Zhao et al., 2022 | 54/54 | 59/49 | 62.1 ± 10.4 | 62.5 ± 10.8 | Ⅰ∼Ⅲ | 1 month | SBP + CWM | CWM | 45mg, t.i.d | ②⑤⑥⑧ |
| Pan et al., 2022 | 68/65 | 70/63 | 74.23 ± 8.47 | 76.87 ± 7.69 | Ⅰ∼Ⅳ | 1 month | SBP + CWM | CWM | 45mg, t.i.d | ②③⑤⑧ |
| Zhou, (2021) | 35/35 | 41/29 | 71.33 ± 11.20 | 71.23 ± 11.12 | NR | 2 weeks | SBP + CWM | CWM | 67.5mg, t.i.d | ⑤⑥ |
| Zhao and Xie, (2021) | 75/75 | 79/71 | 72.3 ± 11.5 | 72.4 ± 11.9 | Ⅰ∼Ⅳ | 6 months | SBP + CWM | CWM | 45mg, t.i.d | ①④ |
| Xie and Huang, (2021) | 39/39 | 43/35 | 61.38 ± 5.74 | 61.19 ± 5.62 | Ⅰ∼Ⅳ | 2 months | SBP + CWM | CWM | 67.5mg, t.i.d | ⑤⑥ |
| Xia et al., 2021 | 61/61 | 74/48 | 65.53 ± 8.67 | 65.53 ± 8.67 | Ⅰ∼Ⅲ | 3 months | SBP + CWM | CWM | 67.5mg, t.i.d | ②⑤⑥⑧ |
| Gao and Chen, (2021) | 78/78 | 83/73 | 63 ± 12 | 63 ± 12 | Ⅰ∼Ⅳ | 3 months | SBP + CWM | CWM | 45mg, t.i.d | ①②③④ |
| Wang and Zhu, (2020) | 40/40 | 52/28 | 70.5 ± 9.5 | 71.2 ± 9.8 | Ⅰ∼Ⅲ | 2 weeks | SBP + CWM | CWM | 67.5mg, t.i.d | ②③④ |
| Chen and Chen, (2020) | 44/44 | 56/32 | 65.09 ± 7.52 | 65.33 ± 7.40 | Ⅰ∼Ⅲ | 3 months | SBP + CWM | CWM | 22.5–45mg, t.i.d | ②⑤⑥ |
| Zhao et al., 2018 | 40/40 | 41/39 | 58.80 ± 8.38 | 56.53 ± 8.93 | Ⅰ∼Ⅳ | 2 months | SBP + CWM | CWM | 45mg, t.i.d | ②③ |
| Zhao, (2018) | 45/45 | 50/40 | 55.16 ± 5.83 | 54.92 ± 5.08 | Ⅰ∼Ⅲ | 6 months | SBP + CWM | CWM | 45mg, t.i.d | ② |
| Wang et al., 2018 | 30/30 | 24/36 | 55.00 ± 7.53 | 60.00 ± 4.88 | Ⅰ∼Ⅳ | 6 months | SBP + CWM | CWM | 45mg, t.i.d | ②③ |
| Liu, (2018) | 79/79 | 85/73 | 56.4 ± 2.3 | 56.9 ± 2.4 | Ⅰ∼Ⅳ | 2 months | SBP + CWM | CWM | 67.5mg, t.i.d | ②④ |
| Peng et al., 2017 | 60/54 | 65/49 | 60.56 ± 3.53 | 61.65 ± 4.75 | Ⅰ∼Ⅲ | 24 months | SBP + CWM | CWM + placebo | 45mg, t.i.d | ①④⑤ |
| Xia, (2016) | 40/40 | 52/28 | 61.0 ± 7.5 | 60.2 ± 8.7 | NR | 6 months | SBP + CWM | CWM | 45mg, t.i.d | ②③④ |
| Wang, (2016) | 45/45 | 62/28 | 65.46 ± 5.53 | 66.35 ± 6.57 | Ⅰ∼Ⅳ | 12 months | SBP + CWM | CWM | 45mg, t.i.d | ②⑧ |
| Ding, (2016) | 42/42 | 50/34 | 59 ± 2.3 | 60 ± 2.2 | Ⅰ∼Ⅲ | 6 months | SBP + CWM | CWM | 45mg, t.i.d | ①② |
| Wang et al., 2015 | 40/40 | 47/33 | 68.3 ± 5.2 | 68.3 ± 5.2 | NR | 1 month | SBP + CWM | CWM | 45mg, t.i.d | ②⑤ |
| Tang, (2015) | 84/82 | 92/74 | 67.54 ± 4.27 | 67.09 ± 4.45 | NR | 3 months | SBP + CWM | CWM | 45mg, t.i.d | ②⑤ |
| Ji, (2015) | 34/34 | 33/35 | 61.21 ± 9.17 | 62.23 ± 8.87 | Ⅰ∼Ⅲ | 2 months | SBP + CWM | CWM | 45mg, t.i.d | ②③④⑤⑥ |
| Hou, (2015) | 34/34 | 49/19 | 71 ± 1.6 | 72 ± 1.2 | Ⅰ∼Ⅲ | 2 months | SBP + CWM | CWM | 45mg, t.i.d | ②③④ |
| Zhao, (2014) | 60/60 | 66/54 | 63 ± 8.5 | 64 ± 4.5 | Ⅰ∼Ⅳ | 6 months | SBP + CWM | CWM | 45mg, t.i.d | ③④⑧ |
| Wang, (2014) | 55/50 | 61/44 | 81.7 ± 6.03 (total) | Ⅰ∼Ⅳ | 3 months | SBP + CWM | CWM | 45mg, t.i.d | ②③④ | |
| Liu, (2014) | 30/30 | 35/25 | 60.5 (total) | Ⅰ∼Ⅳ | 3 months | SBP + CWM | CWM | 45mg, t.i.d | ②③ | |
| Huang et al., 2014 | 60/60 | 71/49 | 66.31 ± 5.25 | 66.75 ± 6.25 | Ⅱ∼Ⅲ | 1 month | SBP + CWM | CWM | 45mg, t.i.d | ② |
| Chen, 2014 | 45/45 | 52/38 | 57.3 ± 2.1 | 58.1 ± 1.8 | Ⅰ∼Ⅳ | 2 months | SBP + CWM | CWM | 45–67.5mg, t.i.d | ②④ |
| Zou et al., 2013 | 54/54 | 60/48 | 64.5 ± 9.6 (total) | Ⅰ∼Ⅳ | 3 months | SBP + CWM | CWM | 45mg, t.i.d | ④ | |
| Yang et al., 2013 | 45/45 | 46/44 | 62.7 ± 10.4 | 61.4 ± 10.3 | Ⅰ∼Ⅳ | 1 month | SBP + CWM | CWM | 45mg, t.i.d | ① |
| Sun, (2013) | 56/56 | 65/57 | 51.6 | 51.7 | Ⅰ∼Ⅳ | 2 months | SBP + CWM | CWM | 22.5–45mg, t.i.d | ②③④ |
| Lv, (2018) | 32/32 | 38/26 | 56.3 ± 4.9 (total) | Ⅰ∼Ⅳ | 2 months | SBP + CWM | CWM | 45mg, t.i.d | ②③④⑧ | |
| Guo and Tan, (2012) | 58/56 | 99/15 | 76.3 | 74.6 | NR | 6 weeks | SBP + CWM | CWM | 45mg, t.i.d | ②③④⑤⑥ |
| Shi and Hang, (2011) | 65/62 | 79/48 | 56.83 ± 11.89 | 55.26 ± 11.00 | Ⅱ∼Ⅲ | 2 weeks | SBP + CWM | CWM | 45mg, t.i.d | ② |
| Xu et al., 2010 | 42/41 | 46/37 | 67.54 ± 4.27 | 67.09 ± 4.45 | Ⅰ∼Ⅳ | 3 months | SBP + CWM | CWM | 45mg, t.i.d | ②③④⑤⑦⑧ |
| Sun, (2010) | 50/50 | 73/27 | 58.5 | 57.2 | NR | 3 months | SBP + CWM | CWM | 45–67.5mg, t.i.d | ②③ |
| Wang, (2018) | 51/51 | 65/37 | 65.0 ± 6.0 | 65.3 ± 6.2 | NR | 2 months | SBP + CWM | CWM | 45mg, t.i.d | ⑦ |
| Miao et al., 2016 | 40/38 | 42/36 | 67 ± 9 | 65 ± 12 | NR | 2 months | SBP + CWM | CWM | 45mg, t.i.d | ⑦⑧ |
| Liao and Huang (2019) | 47/47 | 61/33 | 70.67 ± 7.95 | 71.24 ± 8.13 | NR | 6 months | SBP + CWM | CWM | 45mg, t.i.d | ⑦ |
| Pan, (2016) | 45/45 | 50/40 | 46.4 ± 5.2 | 43.7 ± 4.5 | NR | 1 month | SBP + CWM | CWM | 45mg, t.i.d | ⑦④ |
| Wu et al., 2012 | 44/44 | 49/39 | 64 | 63 | NR | 3 months | SBP + CWM | CWM | 45mg, t.i.d | ⑦ |
| Jiang, (2012) | 42/42 | 55/29 | 72.6 | 73.5 | NR | 6 months | SBP + CWM | CWM | 45mg, t.i.d | ⑦ |
T, trial group; C, control group; NR, not report; SBP, shexiang baoxin pill; CWM, the conventional western medicine (antiplatelet drugs, lipid-lowering drugs, vasodilators of nitrate, and other conventional western medicine recommended by the guideline); t. i.d., three times a day; CCS, canadian cardiovascular society; Outcomes: ①major adverse cardiovascular events, ②the total effective rate of angina symptom improvement, ③electrocardiogram improvement, ④adverse events,⑤angina pectoris frequency, ⑥angina pectoris duration, ⑦left ventricular ejection fraction, ⑧blood lipid index.
3.3 Literature quality assessment
Supplementary Material S6 shows the risk of bias of the included studies for each outcome from low to high risk. All 42 studies referred to randomization, of which 12 used the random number table (Wang, 2016; Peng et al., 2017; Liu, 2018; Wang, 2018; Liao and Huang, 2019; Chen and Chen, 2020; Wang and Zhu, 2020; Gao and Chen, 2021; Ge, 2021; Xia et al., 2021; Xie and Huang, 2021; Pan et al., 2022), and the remaining 30 studies did not specifically report randomization method. Only one trial (Ge, 2021) adequately reported allocation concealment details. Three studies (Wang, 2016; Peng et al., 2017; Ge, 2021) reported the use of double-blinding, which was considered a low risk of “bias due to deviations from intended interventions”. For the total effective rate of angina symptom improvement, one study (Zhao, 2018) included 90 subjects, but only 88 subjects had outcome data, and no reason was explained, with a high risk of “bias due to miss outcome data”. Due to the objectivity of the outcome indicators, some results have no or little room for judgment, and “bias in the selection of the reported result” of LVEF and blood lipid level should be considered “low risk”. In addition, we found that one of the studies included in this review (Ge, 2021) had a low risk of “bias in the selection of the reported result” because the methods of outcome measurement and analysis were consistent with the prespecified protocol, whereas the remaining 41 studies did not find pre-specified study protocols and were assessed as “some concerns” of “bias in the selection of the reported result”.
3.4 Primary outcome measures
3.4.1 MACE
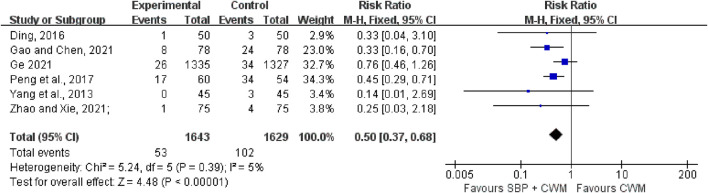
Six trials with 3,272 patients reported the occurrence of MACE. There was little statistical heterogeneity among the studies (I 2 = 5%, p = 0.39), and a fixed-effect model was used for meta-analysis. The results indicated that the experimental group (SBP plus CWM) had better efficacy in lowering the incidence of MACE compared with the control group (RR = 0.50, 95% CI: 0.37 to 0.68, p < 0.00001; Figure 2).
FIGURE 2.
Forest plot of major adverse cardiovascular events.
3.4.2 Angina symptom improvement
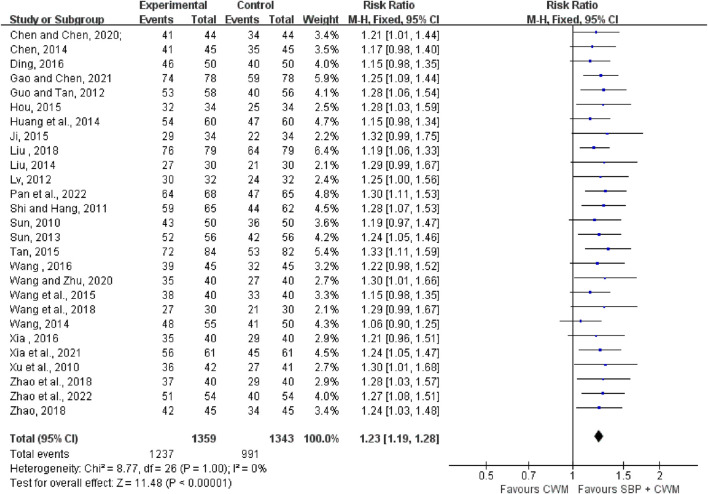
Twenty-seven trials with 2,702 patients reported the total effective rate of angina symptom improvement. The meta-analysis indicated that SBP therapy significantly improved the total effective rate of angina symptom improvement showing a compelling homogeneity (RR = 1.23, 95% CI: 1.19 to 1.28, p < 0.00001; I 2 = 0%; Figure 3).
FIGURE 3.
Forest plot of the total effective rate of angina symptom improvement.
3.4.3 ECG improvement
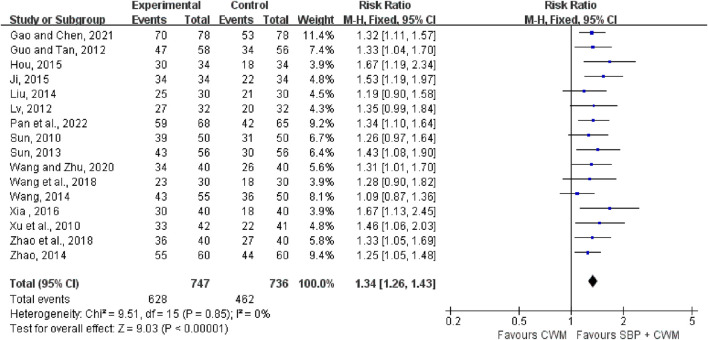
Sixteen trials with 1,483 cases reported the effective rate of ECG improvement. The results showed that compared with the control group, the experimental group could significantly increase the effective rate of ECG improvement (RR = 1.34, 95% CI: 1.26 to 1.43, p < 0.00001; I 2 = 0%), see Figure 4 for details.
FIGURE 4.
Forest plot of electrocardiogram improvement.
3.4.4 AEs
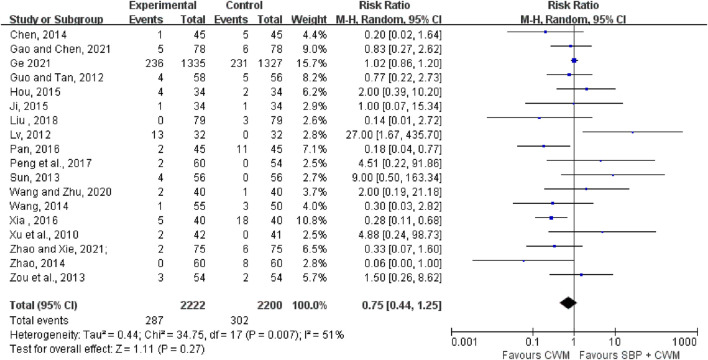
Eighteen studies documented AEs in a total of 4,422 patients. Compared with CWM, the SBP group did not increase the risk of AEs (RR = 0.75, 95% CI: 0.44 to 1.25, p = 0.27; I 2 = 51%; Figure 5) suggesting SBP therapy was safe. Our results revealed that gastrointestinal discomfort, tongue numbness, headache, and rash constitute the most frequently occurring AEs. Remarkable adverse reactions were modest, with no severe adverse impacts, detailed information is shown in Supplementary Material S7.
FIGURE 5.
Forest plot of adverse events.
3.5 Secondary outcome measures
3.5.1 Angina pectoris frequency
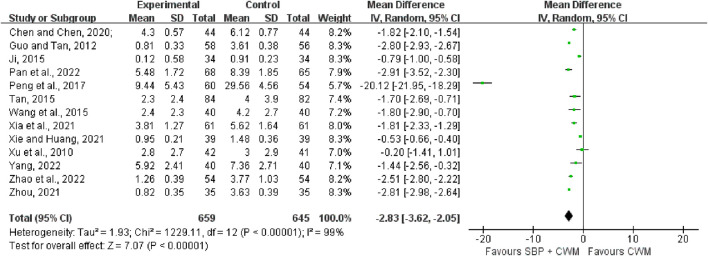
Thirteen studies covering 1,304 patients included angina pectoris frequency as the outcome. The merged data indicated that the combination of SBP was more effective than CWM alone in reducing the frequency of angina pectoris (MD = −2.83, 95% CI: −3.62 to −2.05, p < 0.00001; I 2 = 99%; Figure 6).
FIGURE 6.
Forest plot of angina pectoris frequency.
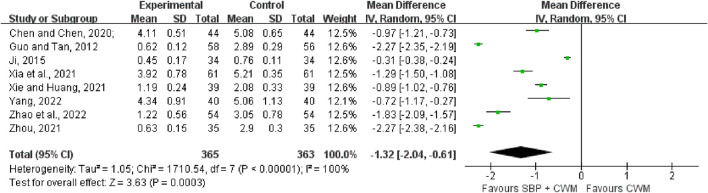
3.5.2 Angina pectoris duration
As shown in Figure 7, eight studies (728 patients) were included to compare the differences between the experimental group and the control group for the duration of angina pectoris. The results of the heterogeneity test showed that I 2 = 100%, p < 0.00001, indicating a high degree of heterogeneity among the studies, so a random-effects model was used for the analysis. The results of the Meta-analysis indicated that compared with the control group, the experimental group could effectively shorten the duration of angina pectoris in patients with SCAD (MD = −1.32, 95% CI: −2.04 to −0.61, p = 0.0003).
FIGURE 7.
Forest plot of angina pectoris duration.
3.5.3 Left ventricular ejection fraction
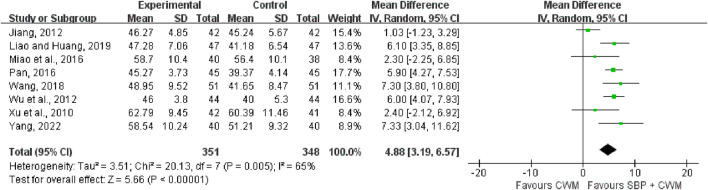
Eight studies covering 699 patients included LVEF (Figure 8) as the outcome. Compared with CWM, the combination of SBP showed a higher increase in LVEF (MD = 4.88, 95% CI: 3.19 to 6.57, p < 0.00001; I 2 = 65%).
FIGURE 8.
Forest plot of left ventricular ejection fraction.
3.5.4 Blood lipid level
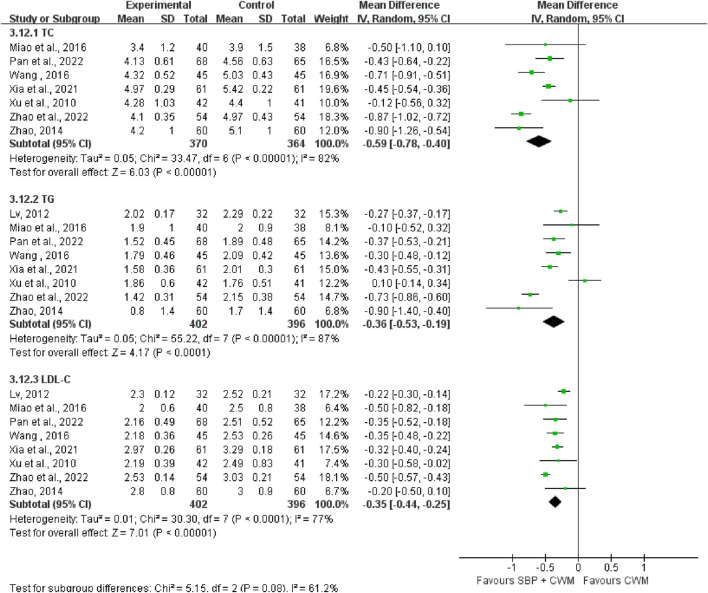
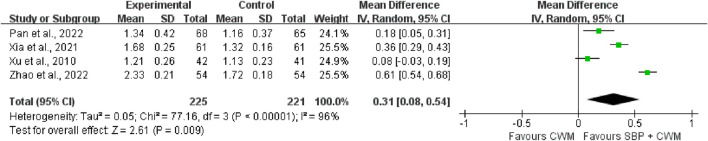
Blood lipid level was measured with TC, TG, LDL-C, and HDL-C. Seven studies covering 734 patients reported SBP therapy reduced TC (MD = −0.59, 95% CI: −0.78 to −0.40, p < 0.00001; I 2 = 82%; Figure 9). Eight studies reported TG, containing 798 patients. Compared with CWM, SBP treatment showed a decrease in TG (MD = −0.36, 95% CI: −0.53 to −0.19, p < 0.00001; I 2 = 87%; Figure 9). LDL-C was reported in eight studies (MD = −0.35, 95% CI: −0.44 to −0.25, p < 0.00001; I 2 = 77%; Figure 9) indicating SBP treatment substantially lowered LDL-C. HDL-C was recorded in four trials with the improvement in the experimental group (MD = 0.31, 95% CI: 0.08 to 0.54, p = 0.009; I 2 = 96%; Figure 10).
FIGURE 9.
Forest plots of total cholesterol, triglyceride, and low-density lipoprotein cholesterol.
FIGURE 10.
Forest plot of high-density lipoprotein cholesterol.
3.6 Meta-regression and subgroup analysis
As results of angina pectoris frequency, angina pectoris duration, LVEF, TC, TG, LDL-C, and HDL-C showed high heterogeneity in our study, we performed a meta-regression analysis based on publication year, mean age, duration of treatment, and sample size. The results of meta-regression analysis showed that the duration of treatment and sample size were significant sources of heterogeneity for angina pectoris duration, and TG, respectively (p < 0.05). In addition, the results revealed that publication year, mean age, treatment duration, and sample size were not remarkable sources of heterogeneity for angina pectoris frequency, LVEF, TC, LDL-C, and HDL-C (all p > 0.05; Supplementary Material S8).
The intervention course of the drug was crucial to the clinical efficacy. We used subgroup analyses to determine whether treatment effects differed by treatment duration. As shown in Table 2 and Supplementary Material S9, the results of subgroup analysis were consistent with the overall study results. Regardless of the length of the course of treatment, SBP combined with CWM could significantly improve the incidence of angina pectoris frequency, angina pectoris duration, LVEF, and blood lipid level. We also found that with the prolongation of the course of treatment, the benefit of some indicators was more obvious, and the combined effect size was larger, such as TC, TG, and LDL-C. In addition, for some outcomes, heterogeneity was reduced after the subgroup analysis, such as angina pectoris frequency, angina pectoris duration, LVEF, TC, and LDL-C, suggesting that treatment duration may be the source of heterogeneity. However, it should be noted that there is still considerable heterogeneity in the remaining subgroups, suggesting that heterogeneity may come from other sources. We believed that the differences in detection equipment and techniques used in different studies may be one of the main sources.
TABLE 2.
Results of subgroup analysis.
| Outcome or subgroup | Studies | Participants | MD/RR (95%CI) | Z | P | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I 2 | P | ||||||
| 1. Adverse Events | 18 | 4,422 | 0.75 (0.44, 1.25) | 1.11 | 0.27 | 51% | 0.007 |
| Treatment duration | |||||||
| ≤2 months | 9 | 844 | 0.99 (0.35, 2.83) | 0.02 | 0.98 | 57% | 0.02 |
| 3 months | 4 | 452 | 0.95 (0.41, 2.21) | 0.12 | 0.91 | 0% | 0.48 |
| 6 months | 3 | 350 | 0.26 (0.12, 0.55) | 3.54 | 0.0004 | 0% | 0.53 |
| 12 months | 2 | 2,776 | 1.02 (0.87, 1.20) | 0.24 | 0.81 | 0% | 0.33 |
| 2. Angina Pectoris Frequency | 13 | 1,304 | −2.83 (−3.62, −2.05) | 7.07 | <0.00001 | 99% | <0.00001 |
| Treatment duration | |||||||
| <2 months | 5 | 505 | −2.74 (−2.89, −2.58) | 34.84 | <0.00001 | 39% | 0.16 |
| 2 months | 2 | 146 | −0.65 (−0.90, −0.39) | 5.00 | <0.00001 | 76% | 0.04 |
| 3 months | 5 | 539 | −1.62 (−2.02, −1.22) | 7.95 | <0.00001 | 42% | 0.14 |
| 24 months | 1 | 114 | −20.12 (−21.95, −18.29) | 21.49 | <0.00001 | — | — |
| 3. Angina Pectoris Duration | 8 | 728 | −1.32 (−2.04, −0.61) | 3.63 | 0.0003 | 100% | <0.00001 |
| Treatment duration | |||||||
| <2 months | 3 | 292 | −2.17 (−2.34, −2.00) | 24.56 | <0.00001 | 81% | 0.005 |
| 2 months | 2 | 146 | −0.60 (−1.17, −0.03) | 2.06 | 0.04 | 98% | <0.00001 |
| 3 months | 3 | 290 | −1.04 (−1.34, −0.73) | 6.68 | <0.00001 | 71% | 0.03 |
| 4. LVEF | 8 | 699 | 4.88 (3.19, 6.57) | 5.66 | <0.00001 | 65% | 0.005 |
| Treatment duration | |||||||
| ≤2 months | 3 | 270 | 5.65 (3.58, 7.72) | 5.34 | <0.00001 | 33% | 0.22 |
| 3 months | 3 | 251 | 5.59 (3.41, 7.77) | 5.03 | <0.00001 | 26% | 0.26 |
| 6 months | 2 | 178 | 3.50 (−1.47, 8.47) | 1.38 | 0.17 | 87% | 0.005 |
| 5. TC | 7 | 734 | −0.59 (−0.78, −0.40) | 6.03 | <0.00001 | 82% | <0.00001 |
| Treatment duration | |||||||
| ≤2 months | 3 | 319 | −0.63 (−0.98, −0.27) | 3.44 | 0.0006 | 83% | 0.003 |
| 3 months | 2 | 205 | −0.36 (−0.65, −0.07) | 2.40 | 0.02 | 52% | 0.15 |
| ≥6 months | 2 | 210 | −0.75 (−0.93, −0.58) | 8.56 | <0.00001 | 0% | 0.36 |
| 6. TG | 8 | 798 | −0.36 (−0.53, −0.19) | 4.17 | <0.0001 | 87% | <0.00001 |
| Treatment duration | |||||||
| 1 month | 2 | 241 | −0.55 (−0.91, −0.20) | 3.07 | 0.002 | 92% | 0.0006 |
| 2 months | 2 | 142 | −0.26 (−0.36, −0.17) | 5.46 | <0.00001 | 0% | 0.44 |
| 3 months | 2 | 205 | −0.18 (−0.69, 0.34) | 0.66 | 0.51 | 93% | <0.0001 |
| ≥6 months | 2 | 210 | −0.55 (−1.13, 0.03) | 1.87 | 0.06 | 79% | 0.03 |
| 7. LDL-C | 8 | 798 | −0.35 (−0.44, −0.25) | 7.01 | <0.00001 | 77% | <0.0001 |
| Treatment duration | |||||||
| 1 month | 2 | 241 | −0.45 (−0.59, −0.31) | 6.22 | <0.00001 | 61% | 0.11 |
| 2 months | 2 | 142 | −0.32 (−0.58, −0.06) | 2.38 | 0.02 | 65% | 0.09 |
| 3 months | 2 | 205 | −0.32 (−0.39, −0.24) | 8.18 | <0.00001 | 0% | 0.89 |
| ≥6 months | 2 | 210 | −0.33 (−0.45, −0.21) | 5.37 | <0.00001 | 0% | 0.37 |
| 8. HDL-C | 4 | 446 | 0.31 (0.08, 0.54) | 2.61 | 0.009 | 96% | <0.0001 |
| Treatment duration | |||||||
| 1 month | 2 | 241 | 0.40 (−0.02, 0.82) | 1.86 | 0.06 | 97% | <0.00001 |
| 3 months | 2 | 205 | 0.22 (−0.05, 0.50) | 1.59 | 0.11 | 94% | <0.00001 |
CI, confidence interval; MD, mean difference; RR, risk ratio; LVEF, left ventricular ejection fraction; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
3.7 Sensitivity analysis and publication bias
The main outcomes, encompassing MACE, the total effective rate of angina symptom improvement, ECG improvement, and AEs were tested by the sensitivity analysis, which involved removing each trial in turn to assess the robustness of the main outcome. The pooled RR values of MACE, the total effective rate of angina symptom improvement, and ECG improvement were relatively stable and reliable, according to Supplementary Material S10. However, one study (Ge, 2021) had a certain impact on the stability of the results of adverse events, which may be related to the study’s high quality, multiple centers, wide population, and long follow-up.
Publication bias was conducted on the outcomes in which trials were over ten. Egger’s test was conducted to confirm the publication bias. As shown in Supplementary Material S11, the results showed ECG improvement (p = 0.073), AEs (p = 0.585), and angina pectoris frequency (p = 0.438) were reliable. Egger’s test of the total effective rate of angina symptom improvement demonstrated that the p-value was less than 0.05 (p = 0.035), which indicated that there is publication bias.
3.8 GRADE assessment
Using GRADE (Table 3), we judged the certainty in our estimates to be low across primary outcomes. For MACE, and ECG improvement, we downgraded the evidence by one level for serious risk of bias, and the evidence was judged as moderate certainty. For the total effective rate of angina symptom improvement, we downgraded the evidence by two levels owing to the serious risk of bias and publication bias, and the evidence was judged as low certainty. For AEs, angina pectoris frequency, angina pectoris duration, LVEF, and blood lipid indicators, we downgraded the evidence by two levels owing to the serious risk of bias and heterogeneity, and the evidence was judged as low certainty.
TABLE 3.
GRADE evidence profile.
| Quality assessment | No of patients | RR/MD (95% CI) | Quality | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. Of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | SBP combined with CWM | CWM | |||
| MACE | ||||||||||
| 6 | serious a | no serious | no serious | no serious | none | 53/1,643 (3.23%) | 102/1,629 (6.26%) | RR = 0.50 (0.37, 0.68) | ⊕⊕⊕O moderate | CRITICAL |
| Angina Symptom Improvement | ||||||||||
| 27 | serious a | no serious | no serious | no serious | Presenceb | 1,237/1,359 (91.02%) | 991/1,343 (73.79%) | RR = 1.23 (1.19, 1.28) | ⊕⊕OO low | CRITICAL |
| ECG Improvement | ||||||||||
| 16 | serious a | no serious | no serious | no serious | none | 628/747 (84.07%) | 462/736 (62.77%) | RR = 1.34 (1.26, 1.43) | ⊕⊕⊕O moderate | CRITICAL |
| Adverse Events | ||||||||||
| 18 | serious a | serious c | no serious | no serious | none | 287/2,222 (12.92%) | 302/2,200 (13.73%) | RR = 0.75 (0.44, 1.25) | ⊕⊕OO low | CRITICAL |
| Angina Pectoris Frequency | ||||||||||
| 13 | serious a | serious c | no serious | no serious | none | 659 | 645 | MD = −2.83 (−3.62, −2.05) | ⊕⊕OO low | CRITICAL |
| Angina Pectoris Duration | ||||||||||
| 8 | serious a | serious c | no serious | no serious | none | 365 | 363 | MD = −1.32 (−2.04, −0.61) | ⊕⊕OO low | IMPORTANT |
| LVEF | ||||||||||
| 8 | serious a | serious c | no serious | no serious | none | 351 | 348 | MD = 4.88 (3.19, 6.57) | ⊕⊕OO low | IMPORTANT |
| TC | ||||||||||
| 7 | serious a | serious c | no serious | no serious | none | 370 | 364 | MD = −0.59 (−0.78, −0.40) | ⊕⊕OO low | IMPORTANT |
| TG | ||||||||||
| 8 | serious a | serious c | no serious | no serious | none | 402 | 396 | MD = −0.36 (−0.53, −0.19) | ⊕⊕OO low | IMPORTANT |
| LDL-C | ||||||||||
| 8 | serious a | serious c | no serious | no serious | none | 402 | 396 | MD = −0.35 (−0.44, −0.25) | ⊕⊕OO low | IMPORTANT |
| HDL-C | ||||||||||
| 4 | serious a | serious c | no serious | no serious | none | 225 | 221 | MD = 0.31 (0.08, 0.54) | ⊕⊕OO low | IMPORTANT |
SBP, shexiang baoxin pill; CWM, the conventional western medicine; CI, confidence interval; MD, mean difference; RR, risk ratio;
Random protocol, blinding, and allocation concealment of some studies were not clear;
Quantitative evaluation of the included data indicated publication bias;
Heterogeneity (I 2 > 50%, p < 0.05) was found.
3.9 Trial sequential analysis
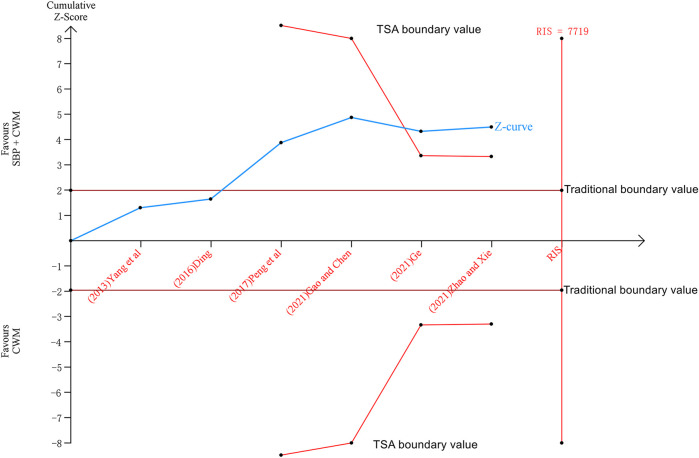
We performed the trial sequential analysis of six trials reporting MACE. The parameters of this study were set as follows: type I error probability α = 5%, statistical power 1-β = 80%, and RR reduced by 20%. The sample size was used as the required information size (RIS) for the two-sided test. The results show that the cumulative Z value after the fifth study (Ge et al., 2020) has crossed the traditional boundary value and the TSA boundary value. Although the cumulative amount of information has not reached the required information size (RIS = 7,719), more experiments are not needed, and a positive conclusion can be obtained in advance, as shown in Figure 11.
FIGURE 11.
Trial sequential analysis of major adverse cardiovascular events.
4 Discussion
Generally, SCAD progresses more slowly and is milder than acute coronary syndrome. Notably, there is a common misunderstanding that the management and treatment of SCAD are mature. The reality is that the rate of misdiagnosis and underdiagnosis of SCAD is high, the quality of life of patients is reduced due to angina pectoris, the application of secondary preventive measures is insufficient, pharmacological treatment is inadequate, the patient benefit is not evident and medical costs increase, resulting in a significant number of patients converting to ACS and there is still a considerable residual cardiovascular risk (De Luca et al., 2019). In the treatment of coronary artery disease, Chinese medicine has played an important role (Liang and Gu, 2021). As a modern Chinese patent medicine, SBP has become the main supplementary drug for secondary prevention of SCAD patients in China, but there has been a lack of high-quality clinical evidence on the effect of SBP on adverse cardiovascular events in patients with SCAD and the safety of long-term use of SBP. In 2018, the “Chinese Expert Consensus on Shexiang Baoxin Pills for the Treatment of Coronary Heart Disease and Angina Pectoris” was officially published (Cardiovascular Diseases Professional Committee of Chinese Association of Integrated Traditional and Western Medicine, 2018), which systematically reviewed and summarized the pharmacological effects, clinical efficacy and safety of SBP, and strongly promoted the clinical application of SBP. With the deepening of clinical practice and research, more and more high-quality research evidence of SBP has been published. Therefore, it is necessary to conduct a systematic review and meta-analysis on the efficacy and safety of SBP in the treatment of SCAD, incorporate more strong evidence, and further clarify the applicable population and the strength of the evidence, to better guide the clinical application of SBP.
4.1 Summary of evidence
Our meta-analysis evaluated the clinical efficacy and safety of SBP in patients with SCAD through the 42 included studies with a total of 6,694 patients, which is the first study focusing on SBP treatment for SCAD patients. The results indicated that SBP combined with CWM could improve the incidence of MACE, the total effective rate of angina symptom improvement and ECG improvement, angina pectoris frequency, angina pectoris duration, and LVEF suggesting the experimental group was superior in clinical efficacy. The ultimate goal of drug therapy for SCAD is to reduce mortality, improve long-term survival, decrease the incidence of important cardiovascular events, and ensure the quality of life. MACE, as the hard endpoint of clinical observation, can objectively and directly reflect drug efficacy and disease prognosis. This study showed that the incidence of MACE was 3.23% (53/1,643) in the experimental group compared with 6.26% (102/1,629) in the control group. The incidence of MACE in the SBP treatment group after treatment was 48.4% lower than that in the control group. The total effective rate of angina symptom improvement and ECG improvement, angina pectoris frequency, and angina pectoris duration can directly assess the severity of the disease and the degree of symptom relief in patients with angina pectoris. LVEF can reflect left ventricular function and provide a certain reference value for the diagnosis and prognosis. Our study found that SBP combined with CWM in the treatment of SCAD has the advantages of improving the total effective rate of angina symptom improvement and ECG improvement, angina pectoris frequency, angina pectoris duration, and LVEF. Dyslipidemia, especially elevated LDL-C, is an important risk factor for cardiovascular morbidity and mortality (Kopin and Lowenstein, 2017). Meta-analysis in this study demonstrated that SBP could remarkably improve the levels of TC, TG, LDL-C, and HDL-C in SCAD patients.
According to the SBP adverse reaction/event report of the National Adverse Drug Reaction Monitoring System (https://www.adrs.org.cn/), the adverse reactions of SBP collected from 2017 to 2021 were 346 cases (about 0.027%), 419 cases (about 0.029%), 479 cases (about 0.035%), 581 cases (about 0.040%), and 775 cases (about 0.048%), belonging to the rare range. Our study also listed AEs in trials to observe the safety of SBP in SCAD patients. Eighteen studies documented AEs in a total of 4,422 patients. According to the report, gastrointestinal discomfort, tongue numbness, headache, and rash are the highest four adverse symptoms. Other adverse cases including red face, liver and kidney damage, arrhythmia, and hypotension were also recorded. Altogether, compared with conventional treatment, SBP therapy did not increase the risk of AEs. What’s more, the results of subgroup analyses according to treatment duration are consistent with the overall study results. Our results also found that treatment duration may be the source of heterogeneity in angina pectoris frequency, angina pectoris duration, LVEF, TC, and LDL-C. Moreover, we determined that the outcomes of MACE, the total effective rate of angina symptom improvement, and ECG improvement were stable and reliable by sensitivity analysis. GRADE is a common method for assessing the certainty of clinical evidence and is widely used in the preparation and revision of guidelines and expert consensus. The overall certainty of the evidence of the outcomes exhibited moderate or low certainty with heterogeneity and methodological problems. Hence, we provide supporting evidence that, to a remarkable extent, SBP can potentially be recommended for planned use for SCAD patients.
4.2 Comparison with previous studies
Multiple systematic reviews and meta-analyses have demonstrated the efficacy and safety of TCM in treating stable coronary artery disease. A meta-analysis constituting high-quality articles involving 824 patients revealed that the application of TCM in the treatment of angina pectoris can improve the therapeutic effect, shorten the attack time, reduce the frequency of angina pectoris, and improve the quality of life (Chen et al., 2021), which was consistent with our results. Nevertheless, their study had no obvious reducing effect on blood lipids. The reason may be the numerous varieties of formulations and ingredients of TCM and insufficient clinical samples. A previous meta-analysis found that corn silk decoction, a Chinese medicine prescription, may improve the levels of TC, TG, and LDL-C (Shi et al., 2019). However, this study did not pay much attention to the clinical endpoints of TCM and could not provide direct evidence of prognosis. The findings of two other meta-analyses indicated that the combination of TCM significantly improved performance compared with CWM solely for the treatment of angina pectoris (Liu et al., 2016; Huiping et al., 2019). Regrettably, they both suffered from methodological quality deficiencies. A prior systematic review demonstrated that in patients with SCAD, revascularization combined with conventional therapy did not lead to an overall survival advantage over medical therapy alone (Laukkanen and Kunutsor, 2021). However, revascularization plus medical therapy may reduce the overall risk of the composite outcome of all-cause death, myocardial infarction, readmission, revascularization, or stroke. This contemporary meta-analysis highlights more effective symptomatic relief of angina pectoris with appropriate adjustment of medical therapy and invasive strategies.
4.3 Limitations
This review is the first attempt to focus on the efficacy and safety of SBP in the treatment of SCAD and has the strength to follow the rigorous review process of Cochrane methodology, reporting standards such as PRISMA, and addressing quality of evidence using the GRADE system. Although we tried to identify all the available evidence, this study has several limitations. Firstly, a high risk of bias existed owing to the lack of blinding and the unclear randomization methods. Secondly, substantial heterogeneity was observed in most outcomes except MACE, the total effective rate of angina symptom improvement, and ECG improvement. Subgroup analysis showed reduced heterogeneity according to treatment duration. Since SCAD was complex, etiology, disease history, nursing treatment, and western treatment strategies may all contribute to the presence of heterogeneity. More research in specific areas is needed to fully assess how these factors play a role in heterogeneity. Thirdly, GRADE evidence quality ratings are mostly low or moderate, and relevant results should be treated with caution.
4.4 Implications for research
We herein reveal important ideas that may advance research in this field. Firstly, it is evident that strategies that improve the methodological quality of RCTs are urgently needed. Going forward, we recommend that more high-quality RCTs should be conducted to improve the strength of the evidence, especially focusing on the implementation of subject-centered randomization, allocation concealment, and blinding. Secondly, RCTs should be reported completely and comprehensively by the CONSORT statement (Cheng, et al., 2017), with particular attention to the reporting of etiology, medical history, nursing treatment, western treatment strategies, and follow-up to find sources of heterogeneity and clarify the prognosis of SCAD patients. Thirdly, despite the revelation that SBP therapy in the analyzed studies was somewhat safe for patients with SCAD, further investigations are needed to confirm the safety of SBP for SCAD. A standard reporting format for adverse drug reactions has been developed (Bian, et al., 2010), and we propose that close attention should be paid to improving the reporting of adverse reactions of SBP. To conclusively understand the long-term safety profile of SBP in patients with SBP, clinical studies incorporating longer follow-up periods are recommended. Our results suggest that SBP combined with CWM can be an alternative treatment for SCAD patients, nonetheless, further large clinical studies should be conducted to explore the long-term safety, efficacy, and optimized dosages of SBP for treating SCAD.
5 Conclusion
In summary, the available evidence indicates that SBP combined with CWM may be effective in the treatment of SCAD to improve the incidence of MACE, the total effective rate of angina symptom improvement and ECG improvement, angina pectoris frequency, angina pectoris duration, LVEF, blood lipid level. However, the risk of bias in the included studies was generally low to high, and the credibility of some results was reduced by heterogeneity. Moreover, the safety of Shexiang Baoxin Pill remains uncertain, more carefully designed large-sample, long-term follow-up RCT should be carried out in the future to provide reliable evidence for SBP in treating SCAD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Author contributions
MZ and YW conceived and designed this study. JW wrote the paper and analyzed the data. TM and CZ performed the literature search, selection, the risk of bias, and data extraction. BL and XW assessed the quality of the study. RY and PH revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The Ministry of Science and Technology of the People’s Republic of China’s Key Projects during the 13th Five-Year Plan Period (2019YFC1710003), the National Natural Science Foundation of China (82074226; 82030120), the Chinese Medicine Evidence-Based Capacity Building Project of State Administration of Traditional Chinese Medicine (2019XZZX-XXG003) supported the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1002713/full#supplementary-material
References
- Bhatt D. L., Eagle K. A., Ohman E. M., Hirsch A. T., Goto S., Mahoney E. M., et al. (2010). Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 304 (12), 1350–1357. 10.1001/jama.2010.1322 [DOI] [PubMed] [Google Scholar]
- Bian Z. X., Tian H. Y., Gao L., Shang H. C., Wu T. X., Li Y. P., et al. (2010). Improving reporting of adverse events and adverse drug reactions following injections of Chinese materia medica. J. Evid. Based. Med. 3 (1), 5–10. 10.1111/j.1756-5391.2010.01055.x [DOI] [PubMed] [Google Scholar]
- Cardiovascular Diseases Professional Committee of Chinese Association of Integrated Traditional and Western Medicine (2018). Chinese expert Consensus on shexiang baoxin pills for the treatment of coronary heart disease and angina pectoris. Chin. J. Integr. Med. 38 (02), 145–153. 10.7661/j.cjim.20170801.307 [DOI] [Google Scholar]
- Chen W., Wang B., Ge Y., Xu H., Jiang C., Yu P., et al. (2021). A systematic review and meta-analysis of clinical research on treating angina pectoris of coronary heart disease with traditional Chinese medicine to promote blood circulation and remove blood stasis. Ann. Palliat. Med. 10 (10), 10506–10514. 10.21037/apm-21-2233 [DOI] [PubMed] [Google Scholar]
- Chen X. Z., Chen C. (2020). Observation of shexiang baoxin pills for stable Angina pectoris of coronary heart disease and its regulating effect on ROS and CT-1 in serum. Chin. J. New Chin. Med. 51 (10), 87–89. 10.13457/j.cnki.jncm.2019.10.025 [DOI] [Google Scholar]
- Cheng C. W., Wu T. X., Shang H. C., Li Y. P., Altman D. G., Moher D., et al. (2017). CONSORT extension for Chinese herbal medicine formulas 2017: Recommendations, explanation, and elaboration (traditional Chinese version). Ann. Intern. Med. 167 (2), W7-W20. W7–W20. 10.7326/IsTranslatedFrom_M17-2977_1 [DOI] [PubMed] [Google Scholar]
- Cheng Z. J. (2014). Shexiang Baoxin Pill combined with Xiaoxintong in the treatment of 45 cases of stable angina pectoris. Chin. Med. Mod. Distance Educ. China 2014 (21), 60–61. 10.3969/j.issn.1672-2779.2014.21.035 [DOI] [Google Scholar]
- China Association of Chinese Medicine Cardiovascular Disease Branch (2019). Guidelines for TCM diagnosis and treatment of stable angina pectoris of coronary heart disease. J. Tradit. Chin. Med. 60 (21), 1880–1890. 10.13288/j.11-2166/r.2019.21.015 [DOI] [Google Scholar]
- De Luca L., Temporelli P. L., Riccio C., Gonzini L., Marinacci L., Tartaglione S., et al. (2019). Clinical outcomes, pharmacological treatment, and quality of life of patients with stable coronary artery diseases managed by cardiologists: 1-year results of the START study. Eur. Heart J. Qual. Care Clin. Outcomes 5 (4), 334–342. 10.1093/ehjqcco/qcz002 [DOI] [PubMed] [Google Scholar]
- Ding H. (2016). Observation on the curative effect of Shexiang Baoxin Pill in the treatment of stable angina pectoris. China Heal Care Nutr. 26 (21), 249–250. [Google Scholar]
- Fang H. Y., Zeng H. W., Lin L. M., Chen X., Shen X. N., Fu P., et al. (2017). A network-based method for mechanistic investigation of Shexiang Baoxin Pill's treatment of cardiovascular diseases. Sci. Rep. 7, 43632. 10.1038/srep43632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feres F., Costa R. A., Abizaid A., Leon M. B., Marin-Neto J. A., Botelho R. V., et al. (2013). Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: The OPTIMIZE randomized trial. JAMA 310 (23), 2510–2522. 10.1001/jama.2013.282183 [DOI] [PubMed] [Google Scholar]
- Fihn S. D., Gardin J. M., Abrams J., Berra K., Blankenship J. C., Dallas A. P., et al. (2012). 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American college of Cardiology foundation/American heart association task force on practice guidelines, and the American college of physicians, American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J. Am. Coll. Cardiol. 60 (24), e44–e164. 10.1016/j.jacc.2012.07.013 [DOI] [PubMed] [Google Scholar]
- Gao T. H., Chen Z. H. (2021). Observation on the curative effect of Shexiang Baoxin Pill in the adjuvant treatment of stable coronary heart disease. Chin. J. Integr. Med. Cardio-/Cerebrovascuiar Dis. 19 (21), 3807–3808. 10.12102/ji.ssn.1672-1349.2021.21.040 [DOI] [Google Scholar]
- Ge J. B., Fan W. H., Zhou J. M., Shi H. M., Ji F. S., Wu Y., et al. (2020). Efficacy and safety of shexiang baoxin pill (MUSKARDIA) in patients with stable coronary artery disease: A multicenter, double-blind, placebo-controlled phase IV randomized clinical trial. Chin. Med. J. 134 (2), 185–192. 10.1097/CM9.0000000000001257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldet G., Howick J. (2013). Understanding GRADE: An introduction. J. Evid. Based. Med. 6 (1), 50–54. 10.1111/jebm.12018 [DOI] [PubMed] [Google Scholar]
- Guo J., Qin Z., He Q., Fong T. L., Lau N. C., Cho W., et al. (2021). Shexiang baoxin pill for acute myocardial infarction: Clinical evidence and molecular mechanism of antioxidative stress. Oxid. Med. Cell. Longev. 2021, 7644648. 10.1155/2021/7644648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Tan B. (2012). Observation of curative effect of shexiang baoxin pill in treating stable Angina pectoris of senile coronary heart disease. Mod. J. Integr. Traditional Chin. West. Med. 21 (22), 2433–2434. 10.3969/j.issn.1008-8849.2012.22.018 [DOI] [Google Scholar]
- Hou J. F. (2015). Observation on the effect of Shexiang Baoxin Pill combined with Xiaoxintong in the treatment of senile stable angina pectoris. Chin. J. Rural Med. Pharm. 2015 (15), 28–29. 10.3969/j.issn.1006-5180.2015.15.018 [DOI] [Google Scholar]
- Hu J., Zhao Y., Wu Y., Yang K., Hu K., Sun A., et al. (2021). Shexiang baoxin pill attenuates ischemic injury by promoting angiogenesis by activation of aldehyde dehydrogenase 2. J. Cardiovasc. Pharmacol. 77 (3), 408–417. 10.1097/FJC.0000000000000967 [DOI] [PubMed] [Google Scholar]
- Huang L., Zhang L., Li G. L. (2014). Shexiang Baoxin Pill combined with aspirin in the treatment of 60 cases of senile blood stasis type stable angina pectoris. Chin. J. Integr. Med. Cardio-/Cerebrovascuiar Dis. 12 (02), 177–178. 10.3969/j.issn.1672-1349.2014.02.025 [DOI] [Google Scholar]
- Huiping W., Yu W., Pei J., Jiao L., Shian Z., Hugang J., et al. (2019). Compound salvia pellet might be more effective and safer for chronic stable angina pectoris compared with nitrates: A systematic review and meta-analysis of randomized controlled trials. Med. Baltim. 98 (9), e14638. 10.1097/MD.0000000000014638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J. H. (2015). Clinical observation of Shexiang Baoxin pills combined with Western medicine in the treatment of 68 cases of stable angina pectoris. China's Naturop. 23 (11), 47–48. [Google Scholar]
- Jiang X. D. (2012). Observation of curative effect of shexiang baoxin pill on ischemic cardiomyopathy. Chin. Med. Mod. Distance Educ. China 10 (13), 50–51. 10.3969/j.issn.1672-2779.2012.13.033 [DOI] [Google Scholar]
- Kopin L., Lowenstein C. (2017). Dyslipidemia. Ann. Intern. Med. 167 (11), ITC81-ITC96–ITC96. 10.7326/AITC201712050 [DOI] [PubMed] [Google Scholar]
- Laukkanen J. A., Kunutsor S. K. (2021). Revascularization versus medical therapy for the treatment of stable coronary artery disease: A meta-analysis of contemporary randomized controlled trials. Int. J. Cardiol. 324, 13–21. 10.1016/j.ijcard.2020.10.016 [DOI] [PubMed] [Google Scholar]
- Liang B., Gu N. (2021). Traditional Chinese medicine for coronary artery disease treatment: Clinical evidence from randomized controlled trials. Front. Cardiovasc. Med. 8, 702110. 10.3389/fcvm.2021.702110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J. Y., Huang E. (2019). Observation of curative effect of shexiang baoxin pill on ischemic cardiomyopathy. Chin. J. Mod. Drug Appl. 13 (08), 131–132. 10.14164/j.cnki.cn11-5581/r.2019.08.076 [DOI] [Google Scholar]
- Liu G. P. (2014). Clinical observation of Shexiang Baoxin Pill combined with Xiaoxintong in the treatment of stable angina pectoris. Inn. Mong. J. Traditional Chin. Med. 33 (23), 6–7. 10.16040/j.cnki.cn15-1101.2014.23.036 [DOI] [Google Scholar]
- Liu H. M. (2018). Therapeutic effect and safety of oral musk Baoxin pill in patients with stable angina pectoris of coronary heart disease. Chin. J. Cardiovasc Rehabil. Med. 27 (06), 684–687. 10.3969/j.issn.1008-0074.2018.06.17 [DOI] [Google Scholar]
- Liu K., Sun D. Q., Liao X., Zhang L. (2019). Interpretation of the risk of bias assessment tool for randomized controlled trials, rev. 2.0. 2016:8565907. Chin. J. Evid. Based Cardiovasc Med. 11 (03), 284–291. 10.3969/j.issn.1674-4055.2019.03.05 [DOI] [Google Scholar]
- Liu W., Xiong X., Yang X., Chu F., Liu H. (2016). The effect of Chinese herbal medicine gualouxiebaibanxia decoction for the treatment of angina pectoris: A systematic review. Evid. Based. Complement. Altern. Med. 2016, 8565907. 10.1155/2016/8565907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Qin Y., Zhang X., Chen C., Xu X., Yu C., et al. (2019). Shexiang baoxin pill alleviates the atherosclerotic lesions in mice via improving inflammation response and inhibiting lipid accumulation in the arterial wall. Mediat. Inflamm. 2019, 6710759. 10.1155/2019/6710759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H. (2018). Clinical observation of shexiang baoxin pill in treating 64 cases of coronary heart disease with stable Angina pectoris. China Mod. Med. 19 (32), 116–117. 10.3969/j.issn.1674-4721.2012.32.058 [DOI] [Google Scholar]
- Miao H. X., Zhu Y. Z., Ye C. X., Zeng J. (2016). Effects of shexiang baoxin pill on ultrasound, inflammatory index and blood lipid in old myocardial infarction. Chin. Foreign Med. Res. 14 (27), 25–27. 10.14033/j.cnki.cfmr.2016.27.012 [DOI] [Google Scholar]
- Montalescot G., Sechtem U., Achenbach S., Andreotti F., Arden C., Budaj A., et al. (2013). 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European society of Cardiology. Eur. Heart J. 34 (38), 2949–3003. 10.1093/eurheartj/eht296 [DOI] [PubMed] [Google Scholar]
- Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., Cushman M., et al. (2016). Executive summary: Heart disease and stroke statistics-2016 update: A report from the American heart association. Circulation 133 (4), 447–454. 10.1161/CIR.0000000000000366 [DOI] [PubMed] [Google Scholar]
- Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 88, 105906. 10.1016/jijsu/2021/105906 [DOI] [PubMed] [Google Scholar]
- Pan F. Q., Xi Y. T., Huang T. F., Cao Y. H., Wu W. (2019). Meta analysis of shexiang baoxin pills in treatment of stable Angina pectoris. Lishizhen Med. Materia Medica Res. 30 (12), 3041–3045. 10.3969/jissn.1008-0805.2019.12.077.012 [DOI] [Google Scholar]
- Pan F. Y., Fan X. T., Huang B. Y. (2022). Clinical study on shexiang baoxin pills combined with metoprolol for stable Angina pectoris of coronary heart disease in senile patients. J. New Chin. Med. 54 (04), 43–46. 10.13457/j.cnki.jncm.2022.04.012 [DOI] [Google Scholar]
- Pan M. Y. (2016). Effect of shexiang baoxin pill on cardiac function of patients with ischemic heart disease. J. North Pharm. 13 (5), 112–113. [Google Scholar]
- Peng B. B., Han Y., Han Q. H. (2017). Observation on the curative effect of Shexiang Baoxin Pill in the treatment of stable angina pectoris. Chin. J. Integr. Med. Cardio-/Cerebrovascuiar Dis. 15 (04), 460–462. 10.3969/j.issn.1672-1349.2017.04.020 [DOI] [Google Scholar]
- Shi B., Hang Y. (2011). Heart pill of musk combined with western medicine treatment of stable Angina pectoris 65 cases of blood stasis. J. Pract. Traditional Chin. Intern. Med. 25 (12), 34–35. 10.3969/j.issn.1671-7813.2011.12.19 [DOI] [Google Scholar]
- Shi S., Yu B., Li W., Shan J., Ma T. (2019). Corn silk decoction for blood lipid in patients with angina pectoris: A systematic review and meta-analysis. Phytother. Res. 33 (11), 2862–2869. 10.1002/ptr.6474 [DOI] [PubMed] [Google Scholar]
- Sun J. H. (2010). Clinical observation of shexiang baoxin pill in treating stable Angina pectoris. Hebei Med. J. 32 (9), 1182. 10.3969/j.issn.1002-7386.2010.09.091 [DOI] [Google Scholar]
- Sun X. L. (2013). Clinical observation of shexiang baoxin pill in treating stable Angina pectoris. Henan Med. Res. 22 (4), 583–584. 10.3969/j.issn.1004-437X2013.04.056 [DOI] [Google Scholar]
- Tan Y. L. (2015). Effect of shexiang baoxin pill on clinical symptoms of elderly patients with stable Angina pectoris. Henan Tradit. Chin. Med. 35 (2), 257–259. 10.16367/j.issn.1003-5028.2015.02.0108 [DOI] [Google Scholar]
- Wang G. Z., Wang H. P., Zhu A. P. (2015). Observation on the curative effect of Shexiang Baoxin Pill in the treatment of stable angina pectoris. Cardiovasc. Dis. J. Integr. traditional Chin. West. Med. 3 (23), 90–91. 10.16282/j.cnki.cn11-9336/r.2015.23.053 [DOI] [Google Scholar]
- Wang H. C. (2014). Clinical observation of Shexiang Baoxin Pill in the treatment of senile stable exertional angina pectoris. Med. Inf. 2014 (21), 278. 10.3969/j.issn.1006-1959.2014.21.327 [DOI] [Google Scholar]
- Wang L. F., Yie J., Yang W. L. (2018). Observation of curative effect of oral administration of Shexiang Baoxin pills in patients with stable angina pectoris. Prev. Treat. Cardio-Cerebral-Vascular Dis. 18 (01), 67–68. 10.3969/jissn.1009_816x.2018.01.022 [DOI] [Google Scholar]
- Wang M., Shan Y., Sun W., Han J., Tong H., Fan M., et al. (2021). Effects of shexiang baoxin pill for coronary microvascular function: A systematic review and meta-analysis. Front. Pharmacol. 12, 751050. 10.3389/fphar.2021.751050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Y. (2016). Long-term curative effect of heart-protecting musk pill on stable Angina pectoris accompanied by hyperlipemia. China Licens. Pharm. 13 (9), 3–6. 10.3969/j.issn.1672-5433.2016.09.001 [DOI] [Google Scholar]
- Wang Y. X., Zhu B. (2020). Clinical efficacy of Shexiang Baoxin Pill combined with diltiazem in the treatment of elderly patients with stable angina pectoris of coronary heart disease. J. Chengdu Med. Coll. 15 (03), 373–375. 10.3969/j.issn.1674-2257.2020.03.022 [DOI] [Google Scholar]
- Wang Z. C. (2018). Clinical therapeutic effects on old myocardial infarction treated with shexiang baoxin pills and trimetazidine hydrochloride tablets in the patients. World J. Integr. Traditional West. Med. 13 (01), 128–130+134. 10.13935/j.cnki.sjzx.180135 [DOI] [Google Scholar]
- Wei J., Liu S., Wang X., Li B., Qiao L., Wang Y., et al. (2021). Efficacy and safety of shexiang baoxin pill for coronary heart disease after percutaneous coronary intervention: A systematic review and meta-analysis. Evid. Based. Complement. Altern. Med. 2021, 2672516. 10.1155/2021/2672516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Jia X. F., He X. L. (2012). Observation and analysis of curative effect of shexiang baoxin pill in treating 88 cases of ischemic cardiomyopathy. Chin. Community Dr. 14 (14), 230–231. 10.3969/j.issn.1007-614x.2012.14.216 [DOI] [Google Scholar]
- Xia X. L. (2016). Influence of oral administration of Shexiang Baoxin Pill for at least 6 months on clinical events in patients with stable angina pectoris. China Prac. Med. 2016 (1), 165–166. 10.14163/j.cnki.11-5547/r.2016.01.124 [DOI] [Google Scholar]
- Xia Z. L., Zhang X., Jin L. T., Chen H. Y. (2021). Clinical effects of Shexiang Baoxin Pills combined with conventional treatment on stable angina pectoris in patients with coronary artery diseases. Chin. Tradit. Pat. Med. 43 (07), 1772–1774. 10.3969/j.issn.1001-1528.2021.07.017 [DOI] [Google Scholar]
- Xie H., Huang R. J. (2021). Effects of Shexiang Baoxin Pill combined with nitroglycerin on angina attack and inflammatory factor levels in patients with stable angina pectoris of coronary heart disease. Prev. Treat. Cardiovasc. Dis. 11 (28), 21–23. [Google Scholar]
- Xu M., Sun N. L., Li J. H., Zhang J., Wei B. Q., et al. (2010). Clinical observation on the efficacy of Shexiang Baoxin pills in the treatment of SAP in the elderly patients. Chin. J. Geriatr. Heart Brain Vessel Dis. 12 (8), 695–698. 10.3969/j.issn.1009-0126.2010.08.008 [DOI] [Google Scholar]
- Yang G. L., Wang S. P., Zhou H. X., Jiang Y. R. (2013). Effect of Shexiang Baoxin Pill on platelet aggregation in patients with stable angina pectoris. Chin. J. Integr. Med. Cardio-/Cerebrovascuiar Dis. 11 (3), 288–289. 10.3969/j.issn.1672-1349.2013.03.018 [DOI] [Google Scholar]
- Yang J., Shi L., Chen H., Wang X., Jiao J., Yang M., et al. (2022). Strategies comparison in response to the two waves of COVID-19 in the United States and India. Int. J. Equity Health 29 (02), 57–59. 10.1186/s12939-022-01666-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Cui Q., Zhao Y., Guo R., Zhan C., Jiang P., et al. (2020). Mechanism of angiogenesis promotion with Shexiang Baoxin Pills by regulating function and signaling pathway of endothelial cells through macrophages. Atherosclerosis 292, 99–111. 10.1016/j.atherosclerosis.2019.11.005 [DOI] [PubMed] [Google Scholar]
- Zhao J. C., Yang Y. J., Tian Y. J. (2022). Efficacy evaluation of Shexiangbaoxin pills in treatment of stable angina pectoris with syndrome of yang deficiency and cold congelation and its effects on inflammatory factors CD31 and CD62P. Hebei Med. J. 44 (04), 516–519. 10.3969/j.issn.1002-7386.2022.04.008 [DOI] [Google Scholar]
- Zhao L. (2018). Efficacy of Shexiang Baoxin Pill in the treatment of elderly patients with stable angina pectoris. Chin. Community Dr. 34 (03), 74+76. 10.3969/j.issn.1007-614x.2018.3.45 [DOI] [Google Scholar]
- Zhao Q., Zhong M., Tang B., Jin Q. S. (2018). Clinical observation of shexiang baoxin pill in adjuvant treatment of chronic stable Angina pectoris. Health Horiz. 2018 (14), 101. 10.3969/j.issn.1005-0019.2018.14.127 [DOI] [Google Scholar]
- Zhao W. X. (2014). Clinical analysis of Shexiang Baoxin Pill in the treatment of stable angina pectoris complicated with carotid atherosclerosis. Hebei Med. J. 2014 (7), 1023–1024. 10.3969/j.issn.1002-7386.2014.07.025 [DOI] [Google Scholar]
- Zhao Y., Xie L. (2021). Clinical efficacy and safety observation of Shexiang Baoxin Pill combined with diltiazem in the treatment of stable angina pectoris in elderly patients with coronary heart disease. Guizhou Med. J. 45 (12), 1934–1935. [Google Scholar]
- Zhou S. Y. (2021). Efficacy of Shexiang Baoxin Pill combined with diltiazem in the treatment of senile coronary heart disease with stable angina pectoris. Health Horiz. 2021 (8), 100. [Google Scholar]
- Zou H. L., Guo H. W., Tang J., Zhang A. J. (2013). Effect of shexiang baoxin pill combined with aspirin on serum adiponectin, endothelin-1, nitric oxide in stable Angina pectoris. Chin. J. Integr. Med. Cardio-/Cerebrovascuiar Dis. 11 (2), 143–144. 10.3969/j.issn.1672-1349.2013.02.008 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.