Abstract
Purpose
Conventional treatment suffers from a dilemma of poor efficacy. The clinical use of Janus kinase (JAK) inhibitors in the treatment of steroid-induced rosacea has rarely been explored.
Patients and Methods
We present a case of steroid-induced rosacea successfully treated with JAK inhibitor tofacitinib, with no adverse effects.
Results
This case report of successful treatment shows a good clinical efficacy of JAK inhibitors tofacitinib in the treatment of SIR.
Conclusion
JAK inhibitor tofacitinib may be a promising agent for the treatment with steroid-induced rosacea, especially for patients who have failed to conventional therapy.
Keywords: steroid-induced rosacea, JAK inhibitors, tofacitinib
Introduction
Steroid-induced rosacea (SIR) is a severe withdrawal reaction that occurs after long-term and excessive topical steroids application on face is discontinued, resembling rosacea that can present erubescence, angiotelectasis and pustule. Avoidance of application of steroids to the face and the use of topical antibiotics, tetracycline antibiotics, and antihistamines have traditionally been the treatment options1, which turns out to be insufficient. Recent progress in the scientific understanding of SIR suggests that tofacitinib may be a promising therapeutic agent. We report a case of SIR treated with the JAK 1/3 inhibitors tofacitinib.
Case Presentation
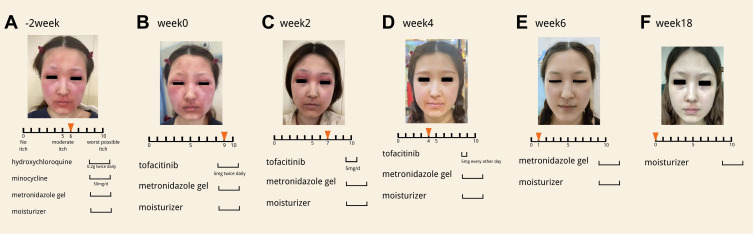
A 28-year-old woman presented with “rosacea” of 2 years’ duration for which her previous physician had prescribed daily use of triamcinolone 0.1% cream. She complained of swelling and a worsening situation of her rosacea each time she discontinued it. She went to visit another doctor who prescribed tacrolimus and crisaborole ointment to treat her. She insisted the prescription was making the symptoms worse. Increasing involvement of the face and suffering feelings were causing her significant pain. When she first came to our visit, she reported intermittent itching (Figure 1A) (peak pruritus was 6 of 10 on a numeric rating scale). She was given the hydroxychloroquine (0.2g twice daily), minocycline (50mg/d) and topical metronidazole gel for 2 weeks. However, the patient reported severe itching (peak pruritus was 9 of 10 on a numeric rating scale). Her face was more swollen and irritable (Figure 1B). Given the progressive disease, the ineffective routine plan and the patient’s associated concern, we decided to pursue a more aggressive strategy. We were looking for a new anti-inflammatory drug. An article2 published on JAAD which referred to JAK/STAT signaling pathway contributing to multiple inflammatory dermatoses inspired us to treat her with JAK1/3 inhibitors tofacitinib after she had undertook blood routine examination, biochemistry analysis, coagulation function and chest CT to exclude severe infection, coagulation disorder, hepatic failure, renal disfunction and tuberculosis. Treatment with tofacitinib was initiated at a dosage of 5 mg twice daily. After 2 weeks symptoms relieved a lot, swelling on her face reduced (Figure 1C). The dosage was decreased to 5 mg/d for the next 2 weeks. As we can see, only perioral erythema remained and the patient reported middle itching (Figure 1D) (peak pruritus was 4 of 10 on a numeric rating scale. Then, the dosage was decreased to 5mg every other day for 2 weeks. She kept on using the topical metronidazole gel during the whole process. Finally, the skin lesions almost disappeared completely (Figure 1E) with occasionally itching (peak pruritus was 1 of 10 on a numeric rating scale and her mental health improved. Course of drug treatment suspended. She discontinued tofacitinib for 12 weeks without relapse of the eruption (Figure 1F). No adverse effects were detected.
Figure 1.
Changes of skin lesions and itching scale during tofacitinib treatment.(A) Image of skin lesions and itching scale before patient started oral tofacitinib. (B) Image of skin lesions and itching scale when patient started oral tofacitinib. (C–E) Images of skin lesions and itching scale at week 2,4,6. (F) Image of skin lesions and itching scale at week 18.
Discussion
Steroids augment toll-like receptor 2 (TLR2) expression in human Keratinocytes,3 which enhances kallikrein-related peptidase 5 production and activity, turning cathelicidin into the activated form LL-37.4 LL-37 contributes to the angiectasis and angiogenesis in epidermis and aggravates inflammatory response with emergence of pro-inflammatory cytokines (eg, TNF-α, IL-6 and IL-8). These pro-inflammatory cytokines can further stimulate the TLR pathway expression. A vicious cycle comes into being. Steroids can attenuate the pro-inflammatory cytokines, so the activated innate immune system is suppressed when using steroids. After steroid is suspended, pro-inflammatory cytokines are still produced via the loop. The accumulation of pro-inflammatory cytokines is possibly the pathogenesis of SIR. The JAK/STAT signaling pathway is a signal transduction pathway stimulated by cytokines5, which has a wide range of function on immune responses and immunoregulations. The JAK/STAT pathway is known to have a cross-talk with TLR signaling pathway.6 They share some mutual inflammatory cytokines such as TNF-α, IL-6, IL-8. The JAK inhibitors suppress them via the deactivation of JAK/STAT signaling pathway that acts upon. Steroid-induced rosacea is refractory inflammatory dermatosis. Inflammatory response plays an important role in the process and recurrence of disease. The reduction of inflammatory cytokines is the reason why the drug takes effect and avoids recurrence after discontinuation of the drug.
Conclusion
To our knowledge, this report is the first one to demonstrate effective therapy for patients with SIR by the JAK1/3 inhibitors. SIR is a refractory disease which is easy to attack repeatedly. This case treated with tofacitinib that responds rapidly is noteworthy. Further investigation of the efficacy and safety of tofacitinib in the treatment of patients with SIR, including those for whom the condition has been more long-standing, will be crucial.
Acknowledgments
We thank the patient for granting permission to publish this information. The patient in this manuscript has given written informed consent to publication of her case details.
Funding Statement
There is no funding to report.
Author contributions
Tianhao Li, Henghong Wang and Caiying Wang contributed equally as co-first authors. Henghong Wang, as the submitting author has been authorised by all co-authors to submit the research article. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Ethics Correlations
Signed consent was obtained from the patient for the publication of the case details and accompanying images. Institutional approval was not required to publish the case details.
Consent Statement
Written informed consent was provided by the patient for publication of images and information.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
- 1.Ljubojeviae S, Basta-Juzbasiae A, Lipozeneiae J. Steroid dermatitis resembling rosacea: aetiopathogenesis and treatment. J Eur Acad Dermatol Venereol. 2002;16(2):121–126. doi: 10.1046/j.1468-3083.2002.00388_2.x [DOI] [PubMed] [Google Scholar]
- 2.Shreberk-Hassidim R, Ramot Y, Zlotogorski A. Janus kinase inhibitors in dermatology: a systematic review. J Am Acad Dermatol. 2017;76(4):745–753.e19. doi: 10.1016/j.jaad.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 3.Shibata M, Katsuyama M, Onodera T, Ehama R, Hosoi J, Tagami H. Glucocorticoids enhance Toll-like receptor 2 expression in human keratinocytes stimulated with Propionibacterium acnes or proinflammatory cytokines. J Invest Dermatol. 2009;129(2):375–382. doi: 10.1038/jid.2008.237 [DOI] [PubMed] [Google Scholar]
- 4.Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131(3):688–697. doi: 10.1038/jid.2010.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36(4):542–550. doi: 10.1016/j.immuni.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luu K, Greenhill CJ, Majoros A, Decker T, Jenkins BJ, Mansell A. STAT1 plays a role in TLR signal transduction and inflammatory responses. Immunol Cell Biol. 2014;92(9):761–769. doi: 10.1038/icb.2014.51 [DOI] [PubMed] [Google Scholar]