Abstract
Mucosal immunization with Helicobacter heilmannii urease B or Helicobacter pylori urease, given nasally with cholera toxin, protects BALB/c mice against H. heilmannii infection and significantly reduces a preexisting infection. However, immunization aggravates gastric corpus atrophy. Our results underline the necessity of defining immunization regimens that do not enhance mucosal damage.
Helicobacter heilmannii (previously known as Gastrospirillum hominis) infects the human gastric mucosa. The infection can lead to chronic active gastritis (16, 17), gastric erosions (1, 3, 8, 15), and cancers (25, 31). Several lines of evidence suggest an animal-to-human transmission of H. heilmannii; human and cat strains share morphological and genomic similarities (9, 16), and most infected people have had contact with domestic animals (20, 29).
Urease is a highly conserved protein among the gastric Helicobacter species and was successfully used to vaccinate different animals against Helicobacter pylori (18, 19) or Helicobacter felis (6, 14, 21) infections. Our aims were to determine whether mucosal immunization with recombinant H. heilmannii urease B can prevent or cure H. heilmannii infection and to compare the efficies of H. pylori and H. heilmannii ureases as immunogens.
Recombinant H. heilmannii urease B.
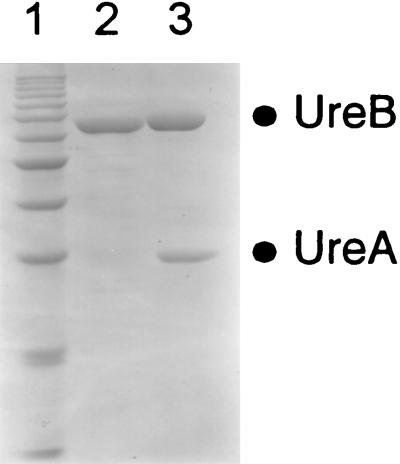
The gene encoding the B subunit of H. heilmannii urease (ureB) was amplified from DNA extracted from a gastric biopsy of an H. heilmannii-infected cat. Primers flanked with unique restriction sites and corresponding to positions 930 to 946 and 2636 to 2619 (EMBL L25079) (28) were used for amplification. The amplified fragment was cloned into pQE9 (Qiagen AG, Basel, Switzerland), and the recombinant protein was expressed in Escherichia coli and purified on nickel nitrilotriacetic acid-agarose as previously described (6, 14, 21). Upon analysis on a Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel (12% polyacrylamide), the resulting urease B protein (H. heilmannii UreB) displayed an apparent molecular mass of 62 kDa, similar to that of H. pylori urease B (Fig. 1).
FIG. 1.
Analysis of purified recombinant H. heilmannii urease B subunit by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Lanes: 1, molecular mass markers (15 μl of the 10-kDa protein ladder [Gibco, BRL]); 2, H. heilmannii urease B (5 μg); 3, H. pylori urease (5 μg).
Immunization and gastric tissue analysis.
BALB/c mice were nasally immunized four times weekly under light anesthesia with H. heilmannii UreB or H. pylori urease (UreAB; kindly provided by OraVax Ltd., Cambridge, Mass.) together with 5 μg of cholera toxin (CT; Calbiochem, La Jolla, Calif.). Control mice were immunized with 5 μg of CT. Twenty days after the last immunization or before the first immunization, mice were orally infected once with gastric tissue from H. heilmannii-infected mice. Groups of mice immunized similarly were infected intragastrically with 5 × 107 H. felis cells for validation and comparison. One month later, animals were killed by cervical dislocation. One-half of the stomach was used for a semiquantitative urease test (UT), as previously described (6, 14, 21). The other half of the stomach was fixed in neutral buffered 10% formalin and routinely processed for histology. Five-micrometer-thick sections were stained with hematoxylin and eosin and cresyl violet. Mice were considered protected or cured from Helicobacter infection when both UT and histological analysis were negative. All slides were evaluated by a pathologist blinded to the study code.
Prophylactic immunization.
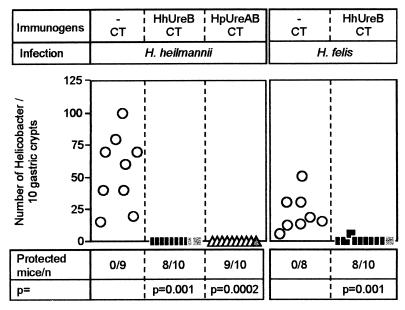
One hundred percent (9 of 9) of the CT-immunized animals were infected by H. heilmannii, as reflected by high numbers of bacteria (median no./10 crypts = 65; range, 15 to 100 [Fig. 2]). In contrast, 80% (8 of 10) of the mice immunized with 30 μg of H. heilmannii UreB were protected from H. heilmannii infection. Similar results were obtained when the immunogen was 30 μg of H. pylori UreAB: 90% (9 of 10) of the animals were protected from H. heilmannii infection. Immunization with H. heilmannii UreB also prevented H. felis infection in 80% (8 of 10) of the animals (Fig. 2).
FIG. 2.
Assessment of Helicobacter colonization in prophylactically immunized mice. The mean number of Helicobacter cells counted in 10 gastric crypts on three different slides was determined for each mouse. Mice were considered protected when both the histology and the UT (data not shown) were negative. Mice with a positive UT are represented by grey symbols. Statistical analysis was performed with Fisher's exact test versus the CT-immunized group. Hh, H. heilmannii; Hp, H. pylori.
Taken together, these data demonstrate that H. heilmannii infection can be prevented by both H. heilmannii UreB and H. pylori UreAB, confirming that protective immunity against Helicobacter infections can be elicited by homologous, as well as heterologous, Helicobacter urease or subunits thereof (21).
Therapeutic immunization.
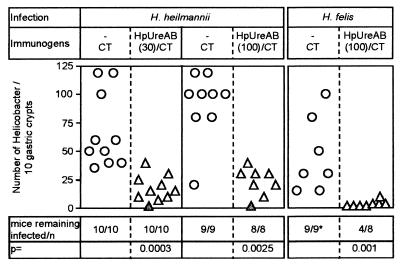
To determine whether nasal immunization with H. pylori UreAB could treat H. heilmannii infection, H. heilmannii-infected mice were immunized with 30 or 100 μg of H. pylori UreAB plus 5 μg of CT in two independent experiments. CT-immunized mice remained colonized by large numbers of H. heilmannii bacteria located deep in the gastric pit. Median numbers of 55 (range, 35 to 120) and 100 (range, 20 to 120) per 10 crypts were observed, respectively. None of the H. pylori UreAB-immunized mice cleared the infection, but a three- to fourfold reduction of the bacterial burden was recorded (Fig. 3). Similar observations have been reported when Helicobacter mustelae-infected ferrets (7), H. pylori-infected monkeys (11), and H. pylori-infected humans (22) were mucosally immunized with urease. It has to be noted, though, that mucosal delivery of 100 μg of H. pylori UreAB given to a group of H. felis-infected mice (in parallel to H. heilmannii-infected mice) led to cure of the H. felis infection in 50% (4 of 8) of the animals (Fig. 3). The use of H. heilmannii recombinant UreB plus CT did not improve the therapeutic capacity of the immunization regimen (data not shown). These results indicate that the treatment of H. heilmannii infection is more difficult to achieve than the treatment of H. felis or H. pylori infection in the murine model (6).
FIG. 3.
Assessment of Helicobacter colonization in therapeutically immunized mice. The presence of Helicobacter in gastric tissues was assessed as in Fig. 2. The dose of immunogen is expressed in micrograms and indicated in parentheses. Statistical analysis was performed with the Wilcoxon-Kruskall-Wallis rank sum test versus the CT-immunized group. An asterisk denotes that two of the mice infected with H. felis and immunized with CT had a positive UT but were not analyzed by histology. Hp, H. pylori.
Histological analysis of the gastric mucosa.
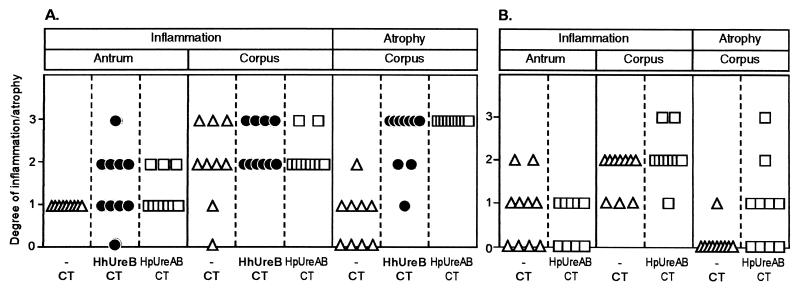
As depicted in Fig. 4A and 5A, H. heilmannii infection elicited, in CT-immunized animals, a mild infiltration of polymorphonuclear cells and lymphocytes in the antrum (grade 1), a moderate to severe infiltration in the corpus (grades 2 and 3), and a mild corpus atrophy in 4 of 9 animals. Unimmunized mice responded like CT-immunized animals upon challenge (data not shown). When H. heilmannii UreB or H. pylori UreAB was added to the prophylactic immunization regimen, the degree of inflammation observed in the antrum or the corpus of immunized mice was not significantly different from that of CT-immunized animals, but corpus atrophy was severely aggravated in 70% (7 of 10) of the H. heilmannii UreB-immunized animals (Wilcoxon-Kruskall-Wallis rank sum test; P = 0.0005) (Fig. 5B) and in 100% (10/10) of the H. pylori UreAB-immunized animals (Wilcoxon-Kruskall-Wallis rank sum test; P = 0.0001).
FIG. 4.
Histopathological changes in the gastric mucosa of prophylactically (A) and therapeutically (B) immunized mice challenged or infected with H. heilmannii. Mice were immunized with 30 μg of H. heilmannii (Hh) UreB or H. pylori (Hp) UreAB together with 5 μg of CT or with 5 μg of CT alone. The degree of inflammation was defined as the absence (grade 0) or the presence (grade 1, mild; grade 2, moderate; grade 3, severe) of polymorphonuclear and lymphocytic cells in the antrum or the corpus of the gastric mucosa. Corpus atrophy was characterized by the loss of parietal cells and a decrease in the mucosal thickness and graded according to the extent of atrophic surface: grade 1, less than 30%; grade 2, 30 to 60%; grade 3, more than 60%.
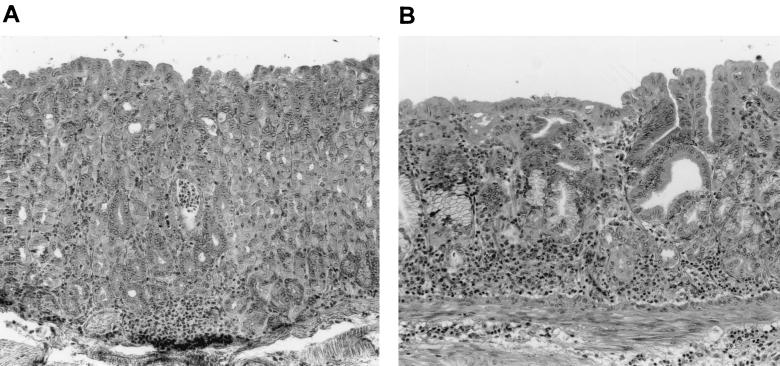
FIG. 5.
Histological analysis of the corpus mucosa of immunized mice subsequently infected with H. heilmannii on hematoxylin-and-eosin-stained sections. (A) Moderate inflammation in CT-immunized mice. (B) Chronic inflammation in H. heilmannii UreB-immunized mice with severe atrophy.
Therapeutically CT-immunized mice (Fig. 4B) displayed the same level of antral and corpus inflammation as prophylactically immunized mice, but had less pronounced corpus atrophy (Wilcoxon-Kruskall-Wallis rank sum test; P = 0.035). This atrophy was significantly increased upon H. pylori UreAB immunization (Wilcoxon-Kruskall-Wallis rank sum test; P = 0.02), but was not as severe as the one observed in mice prophylactically immunized with H. pylori UreAB (Wilcoxon-Kruskall-Wallis rank sum test; P = 0.0002). Similar to what was observed in the prophylactic setting, the inflammation present in the antrum and corpus of therapeutically H. pylori UreAB-immunized mice was not significantly different from that of CT-immunized animals.
Aggravation of inflammation status has been documented in prophylactically immunized mice challenged with H. felis (14, 21, 26) and has been described as infiltration of immune cells, in particular polymorphonuclear cells and lymphocytes (6). It has been correlated with the presence of residual bacteria and has been shown to partially resolve upon antibiotic treatment (12). In the case of H. heilmannii-infected mice, the inflammation status is not significantly aggravated by immunization, but corpus atrophy is.
The protective immune mechanisms triggered by mucosal immunization are not fully elucidated. Recent data suggest that protection of mice against Helicobacter infection by immunization with urease is dependent on major histocompatibility complex class II-restricted, cell-mediated mechanisms and that antibody responses are not required (13). Immunization activates both Th1 and Th2 Helicobacter-specific responses (23, 27), and it has been hypothesized that these T helper cell responses may be responsible for enhanced gastric inflammation (Th1) and reduced bacterial colonization (Th2) (24). Besides T-cell infiltration, we and others have observed a significant recruitment of neutrophils and macrophages in immunized mice (13). One could speculate that these cells once recruited and activated might produce cytokines, nitric oxide, and oxy-radicals and enhance mucosal damage (2, 30). Alternatively, immunization might potentiate the autoimmune reactions that can occur upon bacterial infection (4, 5, 10). These reactions might depend on the Helicobacter species, since urease-immunized animals that are challenged or infected with H. felis do not develop corpus atrophy (data not shown).
In summary, the present study shows that nasal immunization with recombinant homologous or heterologous urease prevents H. heilmannii infection in mice and reduces the bacterial load of a preexisting infection. These results confirm the value of urease as a vaccine antigen for the induction of cross-reactive immune responses against different Helicobacter species. However, an issue that is very important in Helicobacter vaccine development is being raised: immunized challenged mice and, to a lesser extent, therapeutically immunized animals develop corpus atrophy. Despite recent encouraging results in humans with immunization against H. pylori (22), this observation should prompt caution and underlines the importance of understanding immune and inflammatory responses in the gastric mucosa. This knowledge will be required for defining immunization regimens and/or antigens containing T epitopes able to elicit the appropriate protective or curative immune response without enhancing the inflammation.
Acknowledgments
This work was supported by the Swiss National Foundation (grants 31.46858.96 and 31-53771.98 to I.C.T. and 31.43240.95 to A.B.).
We thank P. Michetti and L. Hathaway for critical reading of the manuscript and N. Porta and D. Bachmann for assistance.
REFERENCES
- 1.Akin O Y, Tsou V M, Werner A L. Gastrospirillum hominis-associated chronic active gastritis. Pediatr Pathol Lab Med. 1995;15:429–435. doi: 10.3109/15513819509026978. [DOI] [PubMed] [Google Scholar]
- 2.Albina J E, Reichner J S. Role of nitric oxide in mediation of macrophage cytotoxicity and apoptosis. Cancer Metastasis Rev. 1998;17:39–53. doi: 10.1023/a:1005904704618. [DOI] [PubMed] [Google Scholar]
- 3.al-Himyary A J, Zabaneh R I, Zabaneh S S, Barnett S. Gastrospirillum hominis in acute gastric erosion. South Med J. 1994;87:1147–1150. doi: 10.1097/00007611-199411000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Appelmelk B J, Faller G, Claeys D, Kirchner T, Vandenbroucke-Grauls C M. Bugs on trial: the case of Helicobacter pylori and autoimmunity. Immunol Today. 1998;19:296–299. doi: 10.1016/s0167-5699(98)01281-x. [DOI] [PubMed] [Google Scholar]
- 5.Claeys D, Faller G, Appelmelk B J, Negrini R, Kirchner T. The gastric H+, K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology. 1998;115:340–347. doi: 10.1016/s0016-5085(98)70200-8. [DOI] [PubMed] [Google Scholar]
- 6.Corthesy-Theulaz I, Porta N, Glauser M, Saraga E, Vaney A C, Haas R, Kraehenbuhl J P, Blum A L, Michetti P. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology. 1995;109:115–121. doi: 10.1016/0016-5085(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 7.Cuenca R, Blanchard T G, Czinn S J, Nedrud J G, Monath T P, Lee C K, Redline R W. Therapeutic immunization against Helicobacter mustelae in naturally infected ferrets. Gastroenterology. 1996;110:1770–1775. doi: 10.1053/gast.1996.v110.pm8964402. [DOI] [PubMed] [Google Scholar]
- 8.Debongnie J C, Donnay M, Mairesse J, Lamy V, Dekoninck X, Ramdani B. Gastric ulcers and Helicobacter heilmannii. Eur J Gastroenterol Hepatol. 1998;10:251–254. doi: 10.1097/00042737-199803000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Dieterich C, Wiesel P, Neiger R, Blum A, Corthésy-Theulaz I. Presence of multiple “Helicobacter heilmannii” strains in an individual suffering from ulcers and in his two cats. J Clin Microbiol. 1998;36:1366–1370. doi: 10.1128/jcm.36.5.1366-1370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon M F. Autoimmune reactions in type A and H. pylori gastritis. Helicobacter. 1998;3:222. doi: 10.1046/j.1523-5378.1998.08le1.x. [DOI] [PubMed] [Google Scholar]
- 11.Dubois A, Lee C K, Fiala N, Kleanthous H, Mehlman P T, Monath T. Immunization against natural Helicobacter pylori infection in nonhuman primates. Infect Immun. 1998;66:4340–4346. doi: 10.1128/iai.66.9.4340-4346.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ermak T H, Ding R, Ekstein B, Hill J, Myers G A, Lee C K, Pappo J, Kleanthous H K, Monath T P. Gastritis in urease-immunized mice after Helicobacter felis challenge may be due to residual bacteria. Gastroenterology. 1997;113:1118–1128. doi: 10.1053/gast.1997.v113.pm9322506. [DOI] [PubMed] [Google Scholar]
- 13.Ermak T H, Giannasca P J, Nichols R, Myers G A, Nedrud J, Weltzin R, Lee C K, Kleanthous H, Monath T P. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:1–12. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrero R L, Thiberge J-M, Huerre M, Labigne A. Recombinant antigens prepared from the urease subunits of Helicobacter spp.: evidence of protection in a mouse model of gastric infection. Infect Immun. 1994;62:4981–4989. doi: 10.1128/iai.62.11.4981-4989.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goddard A F, Logan R P, Atherton J C, Jenkins D, Spiller R C. Healing of duodenal ulcer after eradication of Helicobacter heilmannii. Lancet. 1997;349:1815–1816. doi: 10.1016/S0140-6736(05)61696-0. [DOI] [PubMed] [Google Scholar]
- 16.Heilmann K L, Borchard F. Gastritis due to spiral shaped bacteria other than Helicobacter pylori: clinical, histological, and ultrastructural findings. Gut. 1991;32:137–140. doi: 10.1136/gut.32.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilzenrat N, Lamoureux E, Weintrub I, Alpert E, Lichter M, Alpert L. Helicobacter heilmannii-like spiral bacteria in gastric mucosal biopsies. Prevalence and clinical significance. Arch Pathol Lab Med. 1995;119:1149–1153. [PubMed] [Google Scholar]
- 18.Kleanthous H, Myers G A, Georgakopoulos K M, Tibbitts T J, Ingrassia J W, Gray H L, Ding R, Zhang Z-Z, Lei W, Nichols R, Lee C K, Ermak T H, Monath T P. Rectal and intranasal immunizations with recombinant urease induce distinct local and serum immune responses in mice and protect against Helicobacter pylori infection. Infect Immun. 1998;66:2879–2886. doi: 10.1128/iai.66.6.2879-2886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchetti M, Rossi M, Giannelli V, Giuliani M M, Pizza M, Censini S, Covacci A, Massari P, Pagliaccia C, Manetti R, Telford J L, Douce G, Dougan G, Rappuoli R, Ghiara P. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–37. doi: 10.1016/s0264-410x(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 20.Meining A, Kroher G, Stolte M. Animal reservoirs in the transmission of Helicobacter heilmannii. Results of a questionnaire-based study. Scand J Gastroenterol. 1998;33:795–798. doi: 10.1080/00365529850171422. [DOI] [PubMed] [Google Scholar]
- 21.Michetti P, Corthesy-Theulaz I, Davin C, Haas R, Vaney A C, Heitz M, Bille J, Kraehenbuhl J P, Saraga E, Blum A L. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 22.Michetti P, Kreiss K, Kotloff K, Porta N, Blanco J L, Bachmann D, Herranz M, Saldinger P F, Corthésy-Theulaz I, Losonsky G, Nichols R, Simon J, Stolte M, Ackerman S, Monath T P, Blum A L. Oral immunization with urease and E. coli heatlabile enterotoxin is safe and immunogenic in H. pylori infected adults. Gastroenterology. 1999;116:804–812. doi: 10.1016/s0016-5085(99)70063-6. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 24.Mohammadi M, Nedrud J, Redline R, Licke N, Czinn S. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;133:1846–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 25.Morgner A, Bayerdorffer E, Meining A, Stolte M, Kroher G. Helicobacter heilmannii and gastric cancer. Lancet. 1995;346:511–512. doi: 10.1016/s0140-6736(95)91364-5. [DOI] [PubMed] [Google Scholar]
- 26.Pappo J, Thomas W D, Jr, Kabok Z, Taylor N S, Murphy J C, Fox J G. Effect of oral immunization with recombinant urease on murine Helicobacter felis gastritis. Infect Immun. 1995;63:1246–1252. doi: 10.1128/iai.63.4.1246-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saldinger P F, Porta N, Launois P, Louis J A, Waanders G A, Bouzourene H, Michetti P, Blum A L, Corthesy-Theulaz I E. Immunization of BALB/c mice with Helicobacter urease B induces a T helper 2 response absent in Helicobacter infection. Gastroenterology. 1998;115:891–897. doi: 10.1016/s0016-5085(98)70261-6. [DOI] [PubMed] [Google Scholar]
- 28.Solnick J V, O'Rourke J, Lee A, Tompkins L S. Molecular analysis of urease genes from a newly identified uncultured species of Helicobacter. Infect Immun. 1994;62:1631–1638. doi: 10.1128/iai.62.5.1631-1638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stolte M, Wellens E, Bethke B, Ritter M, Eidt H. Helicobacter heilmannii (formerly Gastrospirillum hominis) gastritis: an infection transmitted by animals? Scand J Gastroenterol. 1994;29:1061–1064. doi: 10.3109/00365529409094888. [DOI] [PubMed] [Google Scholar]
- 30.Ward P A, Duque R E, Sulavik M C, Johnson K J. In vitro and in vivo stimulation of rat neutrophils and alveolar macrophages by immune complexes. Production of O-2 and H2O2. Am J Pathol. 1983;110:297–309. [PMC free article] [PubMed] [Google Scholar]
- 31.Yang H, Li X, Xu Z, Zhou D. “Helicobacter heilmannii” infection in a patient with gastric cancer. Dig Dis Sci. 1995;40:1013–1014. doi: 10.1007/BF02064190. [DOI] [PubMed] [Google Scholar]