Abstract
Background
Urinary crystals are the most diverse forms of urine sediments. Reference images for typical urinary crystals are common, however, but images for interpreting atypical urinary crystals are very rare. The authors reviewed various forms and solubility tests of urine crystals to interpret atypical crystals found in clinical specimens.
Methods
We reviewed textbooks on urinary crystals and articles published in PubMed. Some atypical crystals were confirmed using a solubility test.
Results
The classification, shape, chemical structure, and solubility of the crystals were summarized. In the solubility test, some crystals showed different results; therefore, a new solubility test was proposed based on the literature review. We presented various types of calcium oxalates.
Conclusions
These review articles will be helpful in the examination of atypical crystals found in clinical specimens. The solubility test requires additional studies to discriminate the inconsistent results between the authors.
Keywords: classification, crystal, crystalluria, solubility test, urine
Differential identification of urine crystals with morphologic characteristics and solubility test.
1. INTRODUCTION
The examination of urine sediment remains a long‐standing task in clinical laboratories. With automatic urine sedimentation equipment, it is possible to partially identify urine sediments, but accurate identification of urine crystals is still determined through examination of a microscope with trained personnel. 1 A separate unrefrigerated urine preparation is essential to prevent the precipitation of phosphates and urates, which may occur during refrigeration and affect the analysis of crystals. 2 The accurate identification of urine crystals in clinical laboratories is time‐consuming and difficult. Published photographs and solubility tests were used to identify the crystals. However, photographs that can be utilized for atypical crystals are limited, and solubility tests sometimes show inconsistent results depending on the reference. 3 , 4 , 5
In this article, to help in the differential diagnosis of urine crystals in the clinical laboratory, we present various types of urine crystals and a modified solubility test through a literature review and microscopic examination.
2. CLASSIFICATION OF RENAL STONES
Crystalluria is useful for the diagnosis of lithogenic inherited diseases, the identification of drug crystals, the assessment of metabolic disorders associated with stone formation, and the assessment of the risk of stone recurrence. 6 As urine crystals are derived from renal stones, it is necessary to understand the types of renal stones, as summarized in Table 1. 7 , 8 , 9 As shown in Table 1, normal urine crystals should be identified and reported, as most urine crystals are observed in normal urine. Therefore, it is necessary to familiarize the shapes of various types of normal urine crystals.
TABLE 1.
Classification of renal stones
| Stones | Components | Percentage (%) | Risk factors |
|---|---|---|---|
| CO | 40–60 | Low UV | |
| Whewellite | COM | 20 | Hyperoxaluria |
| Weddellite | COD | 10 | Hypercalciuria |
| Mixed | COM + COD | 20 | |
| CaP | 8–10 | ||
| Carbapatite | CaP carbonate | 6.7 | Hypercalciuria, UTI |
| Brushite | CaP dihydrate | 1.4 | pH >6 and hypercalciuria |
| Mixed CO and CaP | 20.0 | ||
| Struvite | Ammonium magnesium phosphate | 7.2 | Recurrent UTI |
| UA | 5–10 | Low UV | |
| Uricite | Anhydrous UA | 1.6 | Benign prostate hypertrophy |
| UA dihydrate | UA dihydrate | 7–8 | Acidic urine, gout, diabetes |
| Mixed UA‐CO | Uricite + COD | Obese, overeaters | |
| Ammonium urate | <0.4 | ||
| Cystine | <1 | Family history |
Abbreviations: CaP, calcium phosphate; CO, calcium oxalate; COD, calcium oxalate dihydrate; COM, calcium oxalate monohydrate; UA, uric acid; UTI, urinary tract infection; UV, urine volume.
2.1. Urine crystals
2.1.1. Calcium oxalate
Calcium oxalate (CO) crystals are classified into CO monohydrate (COM, whewellite), CO dihydrate (COD, weddellite), and CO trihydrate (COT) crystals. COM and COD are associated with high oxalate concentration and hypercalciuria, respectively. 4 COT or caoxite is rarely found in urine. 10
COM crystals show various morphologies, such as oval spheres, dumbbell shape (Figure 1A), red blood cells (RBCs, Figure 1B), rosettes, 11 , 12 and elongated or narrow hexagons (Figure 1C). We observed club‐shaped, RBC‐sized crystals, which agglomerated to form rosettes (Figure 1D). This crystal was soluble in 30% HCl and insoluble in 30% acetic acid and 10% KOH; therefore, it was determined to be a COM crystal. Because literature does not show a club‐shaped crystal, this crystal is considered to be the first club‐shaped COM crystal reported. Elongated and narrow hexagon‐shaped COM crystals are typically found in the urine of patients with ethylene glycol poisoning, such as ingestion of antifreeze. 13 , 14
FIGURE 1.
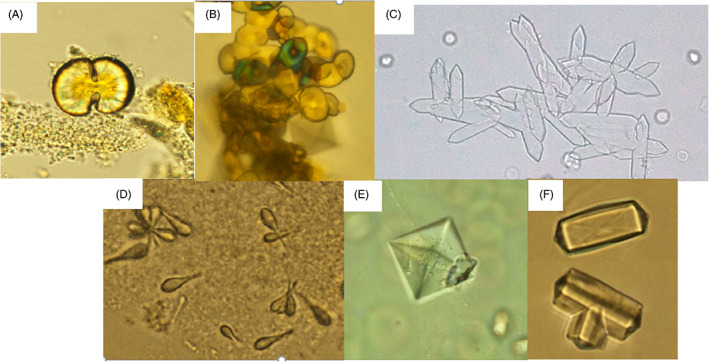
Various shapes of calcium oxalate crystals: (A) dumbbell shape (x400). (B) red blood cell shape (x400). (C) narrow hexagons (x100). (D) club shape or rosette forming crystals (x400). (E) bipyramidal shape (x400) (F) dodecahedral shape (x400).
The COD crystal shows typical bipyramidal crystals (Figure 1E) and rarely dodecahedral crystals (Figure 1F). The dodecahedral COD crystals can be mistaken for triple phosphate crystals but can be distinguished by observing intersecting diagonal lines. COM and COD crystals were observed simultaneously in the urine of one individual.
2.1.2. Calcium phosphate (CaP)
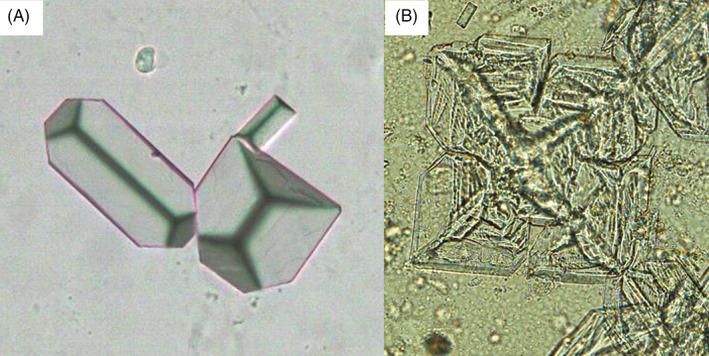
Two types of calcium phosphate (CaP) crystals are usually found in urine: calcium orthophosphates (amorphous carbonated CaP and carbapatites; Figure 2A) and brushite (dicalcium phosphate dihydrate; Figure 2B). Calcium orthophosphates detected by light microscopy (LM) are small granulations with or without large plates. Amorphous carbonate CaP is observed in urine with a high pH (>6.6) and normal calcium and phosphate concentrations. 10 Brushite crystals present as pointed end sticks that often form large aggregates. High concentrations of calcium and phosphate are required for brushite crystal formation. 6
FIGURE 2.

Two calcium phosphate crystals: (A) amorphous calcium phosphate with a large plate (x100). (B) brushite, stick shape calcium phosphate dihydrate (x100).
2.1.3. Triple phosphate (ammonium magnesium phosphate, struvite)
Triple phosphate crystals can be appeared in neutral and alkaline urine and are associated with upper urinary tract infections with urease‐producing organisms. Urease breaks down urea into ammonia and carbon dioxide. Triple phosphate may suggest the presence of urea‐splitting bacteria in the urine specimens. 6 Triple phosphate crystals resemble coffin lids (Figure 3A) and picture frames and sometimes precipitate as large X‐shaped feathery or fernlike crystals (Figure 3B). 6 , 12
FIGURE 3.
Two triple phosphate crystals: (A) coffin lid shape (x200). (B) fernlike X‐shape (x200).
2.1.4. Uric acid (UA) and urates
Five types of uric acid (UA) crystals are found in urine, amorphous UA, crystalline UA, anhydrous UA (uricite), UA monohydrate (UAM), and UA dihydrate (UAD). The formation of UA crystals depends on the UA concentration and acid pH (<5.5). In acidic urine, UAD crystals are formed, whereas amorphous UA is associated with a high urine urate concentration. 6
UA exists as a monoionic urate ion at physiological pH. 15 Amorphous urate (calcium, magnesium, sodium, and potassium urates) was present at LM examination as small yellow‐red granulations (Figure 4A). Crystalline urates (sodium, potassium, and ammonium) appear in various morphologies, such as colorless needles (Figure 4B), thorn apples, and yellow‐brown spheroids without spicules. 12 Ammonium (hydrogen) urate (ammonium biurate; Figure 4C) is formed by combining ammonium and urate ions in alkaline urine or in the pH range of 6.3–7. 10
FIGURE 4.
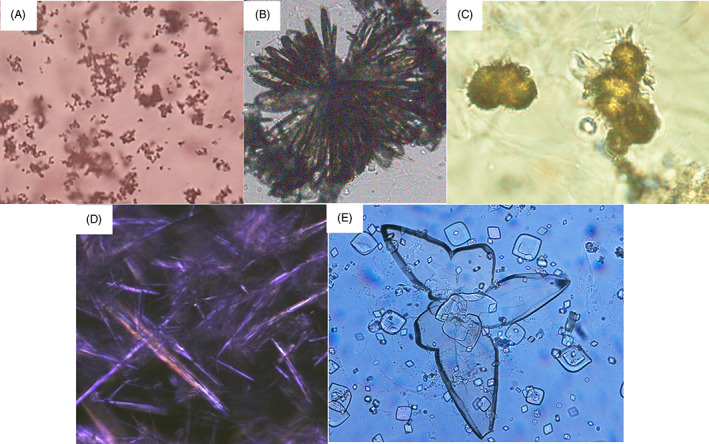
Various shapes of uric acid crystals: (A) amorphous urate (x100). (B) sodium urate showing aggregation of needle shape crystals (x100). (C) ammonium biurate (x400). (D) uric acid monohydrate (x400). (E) uric acid dihydrate (x400).
Anhydrous UA has a rectangular shape 16 but is rarely seen in urine. UAM crystals are rarely seen in urine and are mainly observed as needles in the joint fluid of patients with gout (Figure 4D). 17 UAD crystals are the most common form and appear in a variety of shapes, including rhombic, prisms, and oval forms with pointed ends, wedges, rosettes, and irregular plates (Figure 4E).
2.1.5. 2,8‐Dihydroxyadenine (DHA)
2,8‐Dihydroxyadenine (DHA) crystals can be seen in the urine of patients with 2,8‐dihydroxyadeninuria, which is a rare autosomal recessive disorder of purine metabolism caused by adenine phosphoribosyltransferase (APRT) deficiency. 18 In the absence of APRT activity, adenine is catabolized to DHA by xanthine oxidase, which may lead to crystalluria. DHA crystals in the urine may be considered a pathognomonic finding of APRT deficiency. Some patients with APRT deficiency develop crystal nephropathy, which may lead to chronic kidney disease. 19 Although the prevalence of DHA stones is high in Japan, 20 it has rarely been reported in Korea. 21 The incidence rate in Korea is similar to that in Japan because DHA crystals are occasionally observed in clinical laboratories but have not been reported. DHA crystals appear as round and reddish‐brown tree‐ring patterns with dark outlines and central densities (Figure 5). 20
FIGURE 5.
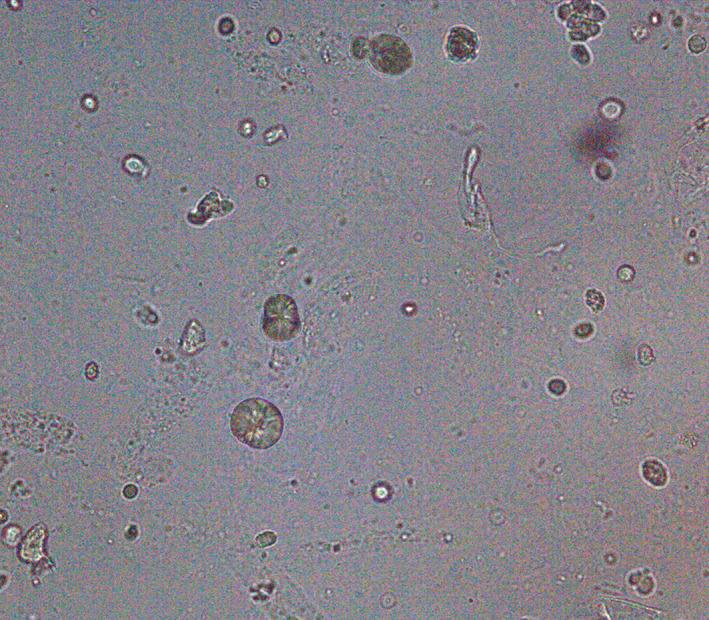
Dihydroxyadenine crystals showing central density with radiating spicules (x400).
2.1.6. Cystine
Cystine crystals are colorless, refractile, hexagonal plates (Figure 6), that occur in the patients with cystinuria and may be associated with cystine calculi. 22 Cystine crystals are rare in urine but must be distinguished from the hexagonal form of UA. While UA crystals are pale yellow, polarized, and insoluble in hydrochloric acid, cystine crystals are colorless, nonpolarized, and soluble.
FIGURE 6.
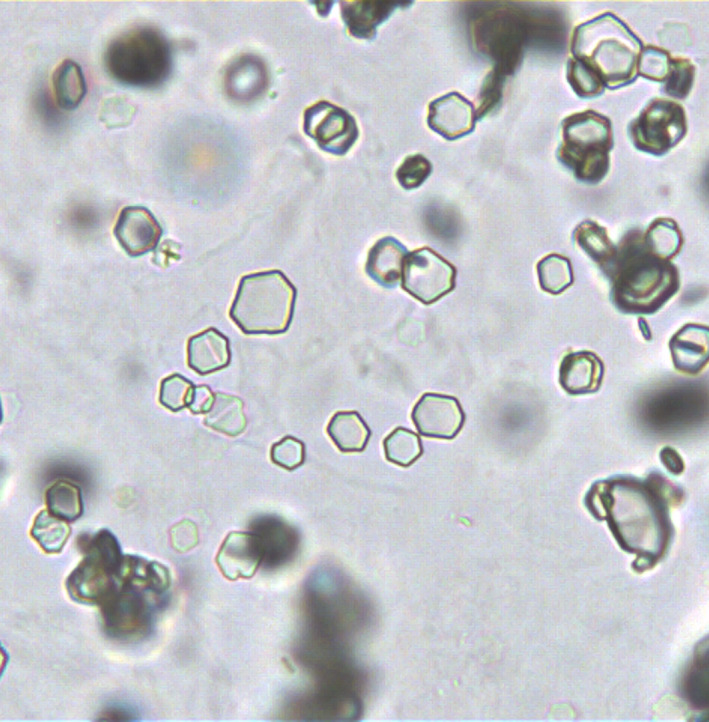
Cystine crystals showing colorless, refractile, and hexagonal plates (x400).
2.2. Crystals of similar shapes
2.2.1. Needle or rectangle with blunt ends
Sodium urate, CaP, and calcium sulfate crystals exhibit this shape. These are very difficult to distinguish through microscopic examination and can be differentiated through the solubility test (Table 2). Sodium and calcium sulfate are usually found in acidic urine, whereas CaP is commonly found in alkaline urine. 12
TABLE 2.
Solubility test for crystals
| Crystals | 60°C Heat | 30% AA | 30% HCl | 10% KOH | Chlo‐roform | Water | Soluble | Insoluble | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Calcium oxalate | − | − | + | − | − | nd | 2, 3 | ||
| Calcium phosphate | − | + | + | − | − | nd | 2, 3 | ||
| Amorphous phosphate | − | + | + | − | − | nd | 2, 3 | ||
| Triple phosphate | − | + | + | − | − | nd | 2, 3 | ||
| Calcium carbonate | − | + | + | − | − | nd | Effervescence after dissolution | 2, 3 | |
| Calcium sulfate | nd | + | nd | nd | nd | + | Water solubility 2.1 g/L | 10, PubChem | |
| Uric acid | − | − | − | + | − | nd | Alcohol | 2, 3 | |
| Amorphous urates | + | + | + | + | − | nd | 50 mM NaOH, HCl, or AA>Uric acid | 2, 3, 21 | |
| Ammonium biurate | + | + | + | + | − | nd | HCl or AA>Uric acid>NaOH>ammonia | 2, 3 | |
| MSU monohydrate | nd | nd | nd | nd | nd | nd | 1 M NaOH | 22 | |
| Bilirubin | − | + | + | + | + | nd | Aceton | 2, 23 | |
| Cholesterol | − | − | − | − | + | nd | Ether and hot alcohol | 2, 3 | |
| Contrast media | nd | nd | nd | nd | nd | nd | 10% NaOH | Ether, chloroform | 2 |
| Cystine | − | − | + | + | − | − | Ammonia | AA, alcohol, ether | 2, 3 |
| DHA | − | − | − | + | − | + | Water solubility 7.11 g/L | 3, HMDB | |
| Hippuric acid | nd | − | nd | nd | nd | + | Hot water and ethanol | 2, Chemicalbook | |
| Leucine | tr | + | + | + | − | + | Water solubility 9.9 g/L | Ether | 3, PubChem |
| Tyrosine | tr | − | + | + | − | tr | Water solubility 0.45 g/L | Alcohol, ether | 2, PubChem |
| Xanthine | nd | − | − | + | nd | nd | 24 | ||
Abbreviations: AA, acetic acid; Chemicalbook, www.chemicalbook.com/ProductIndex_EN.aspx; HMDB—hmdb.ca/metabolites; nd, no data; PubChem, PubChem.ncbi.nlm.nih.gov; tr—trace.
2.2.2. Rectangle with pointed ends
COM, hippuric acid, UA, 12 and needle‐shaped ammonium biurate 10 exhibit this shape. They show very similar microscopic findings and could be distinguished by the solubility test (Table 2). UA and ammonium biurate can also be differentiated using the solubility test.
2.2.3. Dumbbell or spherical shapes
DHA, calcium carbonate, COM, and ammonium biurate without spicules belong to these groups. DHA crystals can be distinguished by tree‐ring patterns with dark outlines and central density. 20 All these crystals can be distinguished by the solubility test (Table 2).
2.2.4. Amorphous granules
Amorphous urate and phosphate appear in the same granular form under a microscope but are formed in acidic and alkaline urine, respectively. 3 , 12 These crystals can be distinguished by the solubility test (Table 2). 23 , 24 , 25 , 26
2.3. Synonyms and chemical formulas
Crystals have been described by different names in the literature, causing confusion. We have presented synonyms and chemical formulas in Table 3 to eliminate confusion and to better understand their composition of crystals. 27 , 28 , 29
TABLE 3.
Synonyms and chemical formulas of urine crystals
| Chemical nomenclature | Synonyms | Chemical formula | Reference |
|---|---|---|---|
| Ammonium hydrogen urate | Ammonium biurate | NH4•C5H3N4O3 | Chemicalbook |
| Ammonium magnesium phosphate hexahydrate | Struvite | MgNH4(PO4)•6H2O | 25 |
| Anhydrous uric acid | Uricite | C5H4N4O3 | 27 |
| Calcium carbonate | Aragonite, Calcite | CaCO3 | Chemicalbook |
| Calcium hydrogen phosphate dihydrate | Brushite | CaHPO4•2H2O | 25 |
| Calcium sulfate | Karstenite | CaSO4 | Chemicalbook |
| Carbonate apatite | Carbapatite | Ca10(PO4O3)6(OHCO3)2 | 25 |
| Calcium oxalate dihydrate | Weddellite | CaC2O4•2H2O | 26 |
| Calcium oxalate monohydrate | Whewellite | CaC2O4•H2O | 26 |
| Cystine | No data | C6H12N2O4S2 | Chemicalbook |
| Hippuric acid | Ammonium hippurate | C9H9NO3 | Chemicalbook |
| Monosodium urate | Sodium urate | C5H3N4NaO3 | PubChem |
| Uric acid dihydrate | No data | C5H4N4O3•2H2O | Chemicalbook |
| Uric acid monohydrate | No data | C5H4N4O3•H2O | Chemicalbook |
| Xanthine | 2,6‐Dihydroxypurine | C5H4N4O2 | Chemicalbook |
| 2,8‐Dihydroxyadenine | 6‐Amino‐2,8‐dihydroxypurine | C5H5N5O2 | Chemicalbook |
Abbreviations: Chemicalbook—www.chemicalbook.com/ProductIndex_EN.aspx; PubChem—PubChem.ncbi.nlm.nih.gov.
2.4. Contaminants that mimic crystals
Free hemosiderin granules appear as coarse, yellow‐brown granules 30 and are confused with amorphous urate and phosphate. Often, these granules agglomerate to become red corpuscles.
Starch crystals are round or oval, are highly refractive, and vary in size. 12 Some of these crystals contain an irregular indentation in the center, resembling molar teeth grooves, and appear as a Maltese cross under polarized light.
2.5. Crystals under polarized microscope
Polarized microscopy (PM) is helpful for crystal identification. Under PM, UA and cholesterol crystals show various colors, whereas cholesterol, leucine, and starch crystals exhibit a typical Maltese cross appearance. 3 , 12
2.6. Solubility test for urine crystals
2.6.1. Summary of solubility test
The solubility test can be performed simply; however, it is an important test that can distinguish crystal types. Solubility tests for differentiating urine crystals have been published in textbooks. However, different results were recorded in some crystals; therefore, the authors propose a new solubility test based on literature and website searches (Table 2).
Amorphous urates were dissolved at 60°C for 90 s or by adding 50 mM NaOH, 23 whereas amorphous phosphate was dissolved in acid. Solubility test studies on monosodium urate and xanthine were very difficult to find, and reports by Molloy et al. 24 and Omokawa et al. 25 are the only ones available. The solubility test for bilirubin crystals was newly presented by referring to the study by Dey et al. 26 because Henry 3 and Sysmex 4 described discrepant results. In the solubility test, HCl for leucine was concluded to be soluble with reference to PubChem's data, because conflicting results were described in the Sysmex and Graff. 12 Tyrosine crystals heated at 60°C were described as relatively soluble in Henry, whereas in Sysmex, it was insoluble. No other reference has been found regarding the solubility of tyrosine under heating.
2.6.2. Example of solubility test
We observed some crystals in the patient's urine (Figure 7A). A solubility test was performed to differentiate these crystals, and they were not dissolved in 10% KOH (Figure 7B). The sharp‐pointed rectangular crystals were dissolved, but the bipyramidal crystals were not dissolved in 30% acetic acid (Figure 7C), and all of them were dissolved in 30% HCl (Figure 7D). Based on the above results, we conclude that CaP and CO crystals had mixed solubilities.
FIGURE 7.
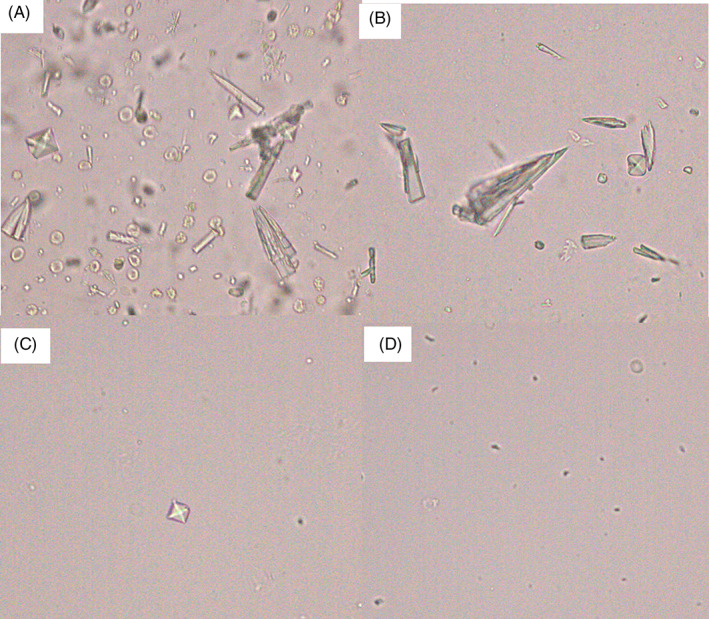
Differentiation of urine crystals by solubility test: (A) Crystals in urine, (B) insoluble crystals with 10% KOH, (C) the sharp‐pointed rectangular crystals were dissolved in 30% acetic acid, (D) bipyramidal crystals were dissolved in 30% acetic acid (all x400).
3. CONCLUSION
We summarized the characteristics of crystals in the urine. Crystals observed in normal urine samples in clinical laboratories tend to be underreported. Reporting normal crystals is not required, and it is not easy to report normal crystals during routine work in a clinical laboratory. However, since normal crystals can be an early finding suggestive of kidney stones, it is desirable to examine and report all crystals. Solubility tests simply and easily distinguish crystals that are difficult to differentiate morphologically. However, the utility of solubility tests in clinical laboratories is low, and the available references are limited. The authors hope that this review and the summary of the solubility test will be helpful in distinguishing urine sediments. The solubility test requires additional studies to discriminate the inconsistent results between the authors.
CONFLICT OF INTEREST
There is no conflict of disclosure.
Lee A‐J, Yoo E‐H, Bae Y‐C, Jung SB, Jeon C‐H. Differential identification of urine crystals with morphologic characteristics and solubility test. J Clin Lab Anal. 2022;36:e24707. doi: 10.1002/jcla.24707
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Cho J, Oh KJ, Jeon BC, Lee SG, Kim JH. Comparison of five automated urine sediment analyzers with manual microscopy for accurate identification of urine sediment. Clin Chem Lab Med. 2019;57:1744‐1753. [DOI] [PubMed] [Google Scholar]
- 2. Kouri T, Fogazzi G, Gant V, Hallander H, Hofmann W, Guder WG. European urinalysis guidelines. Scand J Clin Lab Invest. 2000;231:1‐86. [DOI] [PubMed] [Google Scholar]
- 3. Riley RS, McPherson RA. Basic examination of urine. In: McPherson RA, Pincus MR, eds. Henry's Clinical Diagnosis and Management by Laboratory Methods. 24th ed. Elsevier; 2022:493‐499. [Google Scholar]
- 4. Itoh K, Miyachi H, Asai S, Nozaki T. Atlas of Urinary Sediment. Sysmex Corporation; 2014:85‐98. [Google Scholar]
- 5. Fogazzi GB. Crystalluria: a neglected aspect of urinary sediment analysis. Nephrol Dial Transplant. 1996;11:379‐387. [DOI] [PubMed] [Google Scholar]
- 6. Daudon M, Frochot V. Crystalluria. Clin Chem Lab Med. 2015;53(Suppl 2):s1479‐s1487. [DOI] [PubMed] [Google Scholar]
- 7. Frochot V, Daudon M. Clinical value of crystalluria and quantitative morphoconstitutional analysis of urinary calculi. Int J Surg. 2016;36:624‐632. [DOI] [PubMed] [Google Scholar]
- 8. Viljoen A, Chaudhry R, Bycroft J. Renal stones. Ann Clin Biochem. 2019;56:15‐27. [DOI] [PubMed] [Google Scholar]
- 9. Daudon M, Dessombz A, Frochot V, et al. Comprehensive morpho‐constitutional analysis of urinary stones improves etiological diagnosis and therapeutic strategy of nephrolithiasis. C R Chim. 2016;19:1470‐1491. [Google Scholar]
- 10. Daudon M, Frochot V, Bazin D, Jungers P. Crystalluria analysis improves significantly etiologic diagnosis and therapeutic monitoring of nephrolithiasis. C R Chim. 2016;19:1514‐1526. [Google Scholar]
- 11. Bisaillon S, Tawashi R. Growth of calcium oxalate in gel systems. J Pharm Sci. 1975;64:458‐460. [DOI] [PubMed] [Google Scholar]
- 12. Mundt LA, Kristy S. Graff's Textbook of Urinalysis and Body Fluids. 3rd ed. Wolters Kluwer; 2016. [Google Scholar]
- 13. Hanouneh M, Chen TK. Calcium oxalate crystals in ethylene glycol toxicity. N Engl J Med. 2017;377:1467. [DOI] [PubMed] [Google Scholar]
- 14. Koda R, Watanabe H, Iino N. Calcium oxalate monohydrate crystals in ethylene glycol poisoning. Clin Exp Nephrol. 2017;21:741‐742. [DOI] [PubMed] [Google Scholar]
- 15. Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8‐14. [DOI] [PubMed] [Google Scholar]
- 16. Grases F, Rodriguez A, Costa‐Bauza A. Theobromine inhibits uric acid crystallization. A potential application in the treatment of uric acid nephrolithiasis. PLoS One. 2014;9:e111184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lund‐Iversen M. Uric acid crystals. Tidsskr Nor Laegeforen. 2014;134:2279. [DOI] [PubMed] [Google Scholar]
- 18. Nasr SH, Sethi S, Cornell LD, et al. Crystalline nephropathy due to 2,8‐dihydroxyadeninuria: an under‐recognized cause of irreversible renal failure. Nephrol Dial Transplant. 2010;25:1909‐1915. [DOI] [PubMed] [Google Scholar]
- 19. Verma R, Niraimathi M, Prasad P, Agrawal V. Dihydroxyadenine crystal‐induced nephropathy presenting with rapidly progressive renal failure. Kidney Res Clin Pract. 2018;37:287‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bollée G, Harambat J, Bensman A, Knebelmann B, Daudon M, Ceballos‐Picot I. Adenine phosphoribosyltransferase deficiency. Clin J Am Soc Nephrol. 2012;7:1521‐1527. [DOI] [PubMed] [Google Scholar]
- 21. Jung J, Lee JH, Park YS, et al. Ultra‐rare renal diseases diagnosed with whole‐exome sequencing: utility in diagnosis and management. BMC Med Genet. 2021;14:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sahota A, Tischfield JA, Goldfarb DS, Ward MD, Hu L. Cystinuria: genetic aspects, mouse models, and a new approach to therapy. Urolithiasis. 2019;47:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Behan KJ, Johnston MA. Protocols to dissolve amorphous urate crystals in urine. Lab Med. 2021;53:e63‐e68. [DOI] [PubMed] [Google Scholar]
- 24. Molloy RGE, Sun W, Chen J, Zhou W. Structure and cleavage of monosodium urate monohydrate crystals. Chem Commun. 2019;55:2178‐2181. [DOI] [PubMed] [Google Scholar]
- 25. Omokawa A, Oguma M, Ueki S, Saga T, Hirokawa M. Urine xanthine crystals in tumor lysis syndrome. Urology. 2018;120:e9‐e10. [DOI] [PubMed] [Google Scholar]
- 26. Dey SK, Lightner DA. Toward an amphiphilic bilirubin: the crystal structure of a bilirubin e‐isomer. J Org Chem. 2008;73:2704‐2714. [DOI] [PubMed] [Google Scholar]
- 27. Hesse A, Heimbach D. Causes of phosphate stone formation and the importance of metaphylaxis by urinary acidification: a review. World J Urol. 1999;17:308‐315. [DOI] [PubMed] [Google Scholar]
- 28. Kaloustian J, El‐Moselhy TF, Portugal H. Determination of calcium oxalate (mono‐ and dihydrate) in mixtures with magnesium ammonium phosphate or uric acid: the use of simultaneous thermal analysis in urinary calculi. Clin Chim Acta. 2003;334:117‐129. [DOI] [PubMed] [Google Scholar]
- 29. Grases F, Villacampa AI, Costa‐Bauzá A, Söhnel O. Uric acid calculi: types, etiology and mechanisms of formation. Clin Chim Acta. 2000;302:89‐104. [DOI] [PubMed] [Google Scholar]
- 30. Rous P. Urinary siderosis. J Exp Med. 1918;28:645‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


