Abstract
Background
Anti‐melanoma differentiation‐associated gene 5 (MDA5)‐positive dermatomyositis (MDA5+DM) is significantly associated with interstitial lung disease (ILD), especially rapidly progressive ILD (RPILD) due to poor prognosis, resulting in high mortality rates. However, the pathogenic mechanism of MDA5+DM‐RPILD is unclear. Although some MDA5+DM patients have a chronic course of ILD, many do not develop RPILD. Therefore, the related biomarkers for the early diagnosis, disease activity monitoring, and prediction of the outcome of RPILD in MDA5+DM patients should be identified. Blood‐based biomarkers are minimally invasive and can be easily detected.
Methods
Recent relative studies related to blood biomarkers in PubMed were reviewed.
Results
An increasing number of studies have demonstrated that dysregulated expression of blood biomarkers related to ILD such as ferritin, Krebs von den Lungen‐6 (KL‐6), surfactant protein‐D (SP‐D), and cytokines, and some tumor markers in MDA5+DM may provide information in disease presence, activity, treatment response, and prognosis. These studies have highlighted the great potentials of blood biomarker values for MDA5+DM‐ILD and MDA5+DM‐RPILD. This review provides an overview of recent studies related to blood biomarkers, besides highlighted protein biomarkers, including antibody (anti‐MDA5 IgG subclasses and anti‐Ro52 antibody), genetic (exosomal microRNAs and neutrophil extracellular traps related to cell‐free DNA), and immune cellular biomarkers in MDA5+DM, MDA5+DM‐ILD, and MDA5+DM‐RPILD patients, hopefully elucidating the pathogenesis of MDA5+DM‐ILD and providing information on the early diagnosis, disease activity monitoring, and prediction of the outcome of the ILD, especially RPILD.
Conclusions
Therefore, this review may provide insight to guide treatment decisions for MDA5+DM‐RPILD patients and improve outcomes.
Keywords: biomarker, dermatomyositis, interstitial lung disease, MDA5, rapidly progressive interstitial lung disease
Proposed possible pathogenesis of MDA5+DM‐ILD and summary of blood‐based biomarkers for MDA5+DM, MDA5+DM‐ILD, and MDA5+DM‐RPILD.
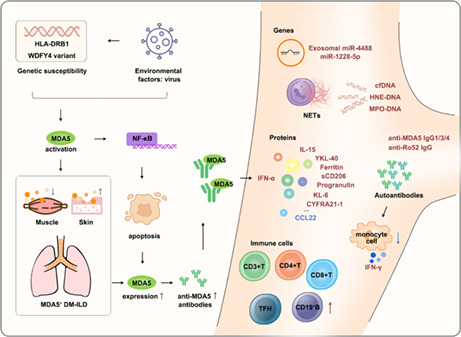
1. INTRODUCTION
Interstitial lung disease (ILD) is among the major extramuscular complications of idiopathic inflammatory myopathies (IIM) and is significantly associated with poor survival. 1 According to a recent meta‐analysis, the global prevalence of ILD in polymyositis (PM) and dermatomyositis (DM), two common clinical subtypes of IIM, is approximately 41%. 2 Myositis‐specific autoantibodies are found in approximately 60% of IIM patients and are closely associated with unique clinical manifestations. 1 Notably, the anti‐melanoma differentiation‐associated gene (MDA5; identified in 13%–30% of IIM patients) is strongly associated with ILD (reported in 90%–95% anti‐MDA5‐positive DM [MDA5+DM]), especially rapidly progressive ILD (RPILD; reported in 50%–80% MDA5+DM), leading to refractory resistance to conventional treatment and high mortality rates. 1 , 3 However, disparities in the proportion of anti‐MDA5 antibodies have been observed among DM patients according to ethnicity, predominantly in the Asian population. 4 Chest X‐ray or high‐resolution CT scan (reticular opacities, ground‐glass opacity, or honeycomb) is widely used to diagnose ILD. RPILD is characterized by worsened radiologic interstitial changes, progressive dyspnea, and hypoxemia within 1 month after respiratory symptom onset. 5 MDA5+DM is typically characterized by the presence of mild or no muscle disease, cutaneous manifestations, arthritis, and notable ILD (especially in East Asian patients). 1
Sato et al. discovered a novel antibody in the serum of clinically amyopathic DM (CADM) patients (“anti‐CADM‐140 antibody”) in 2005, which was significantly associated with RPILD. 6 The CADM‐140 antigen was subsequently identified as MDA5 in 2009. 7 Currently, besides immunoprecipitation [IP (gold standard)], line immunoassay and enzyme‐linked immunosorbent assay are widely used to detect anti‐MDA5 antibodies. 8 , 9 , 10 Although some MDA5+DM patients have a chronic course of ILD, many do not develop RPILD. The level of anti‐MDA5 antibody decreases at RPILD remission and increases at relapse, suggesting that the anti‐MDA5 antibody level is correlated with disease activity and thus can predict RPILD outcomes. 11 , 12 , 13 However, another study reported that the anti‐MDA5 antibody level also decreases in fatal cases, suggesting that this may not reflect the effect of treatment. 14 A low anti‐MDA5 antibody level is reportedly not related to a favorable outcome of the disease. 13 Moreover, the levels of anti‐MDA5 antibodies showed no significant difference between the surviving and non‐surviving groups among MDA5+DM‐ILD patients. 15 However, prospective cohort studies are required to validate these findings.
Blood‐based biomarkers are readily measurable, minimally invasive, and cost‐effective. A large number of blood‐based biomarkers have proven to be linked to ILD, advanced understanding of ILD and informed diagnosis, and prognosis and treatment in ILD patients. 16 , 17 Currently, several studies have also found that these biomarkers are related ILD in MDA5+DM. Emerging evidence focused on MDA5+DM, MDA5+DM‐ILD, and MDA5+DM‐RPILD biomarkers (either alone or in combination with anti‐MDA5 antibodies) for early detection, diagnosis, prognosis, disease activity, and therapeutic monitoring of these diseases. This may further improve the understanding of MDA5+DM‐RPILD pathogenesis and allow determination of an appropriate treatment regimen. This review aimed to summarize progress in emerging roles and potential clinical applications of blood biomarkers, including protein, antibody, gene, and circulating immune cells in MDA5+DM, MDA5+DM‐ILD, and MDA5+DM‐RPILD.
2. PATHOGENESIS OF MDA5 + DM‐ILD
Potential environmental triggers, particularly viral infections causing the autoimmune response and genetic risk for disease susceptibility, may play a role in the pathogenesis of the disease. However, the exact underlying pathogenic mechanisms of MDA5+DM‐ILD are unknown.
MDA5, encoded by the interferon‐induced helicase C domain 1 (IFIH1) gene, is a key RNA sensor belonging to the retinoic acid‐induced gene I (RIG‐I)‐like receptor (RLR) family. 18 It can recognize double‐stranded RNA (dsRNA) virus and then produce type I interferon (IFN), thus activating antiviral immunity. 18 Potential environmental factors related to the dsRNA virus may trigger MDA5+DM‐ILD. Recent studies have also shown that seasonality and geographical distribution can affect the onset of MDA5+DM‐ILD. 19 , 20 , 21 Therefore, respiratory viral infection showing apparent seasonality suggests that a viral influence may induce an immune response in MDA5+DM‐ILD patients. MDA5+DM is characterized by severe skin manifestations. MDA5 is significantly expressed in MDA5+DM skin lesions. 22 Furthermore, the type I IFN signature is upregulated in MDA5+DM skin tissues, suggesting that the type I IFN pathway may be involved in skin damage. 23 , 24 , 25 Some HLA regions, including HLA‐DRB1*0101, *0405, *1201, *0901, *0401, and *1202 are associated with susceptibility to MDA5+DM. 26 , 27 , 28 Furthermore, a genome‐wide association study revealed that a splicing variant of WDFY4 is associated with susceptibility to CADM. 29 The study further demonstrated that a truncated WDFY4 isoform could interact with MDA5 to activate the NF‐κB pathway and promote MDA5‐induced apoptosis. 29 Cell lysis causes MDA5 antigen release into the microenvironment and loss of tolerance to MDA5, thereby leading to the production of MDA5 autoantibodies. 4 Anti‐MDA5 antibody and the RNA‐containing immune complex (IC) (formed by MDA‐5 and anti‐MDA5 antibody) can induce IFN‐α production via Toll‐like receptor 7 (TLR‐7) in an RNA‐dependent manner. 30 MDA5 is expressed in the lung epithelial cells of MDA5+DM‐RPILD patients. 31 CD206 (a marker for the activation of M2 macrophages)‐positive macrophages are found in the lung of severe MDA5+DM‐ILD patients, suggesting that activated macrophages may be involved in MDA5+DM‐ILD pathogenesis. 32 A recent study showed that activated monocytes/macrophages can cause cytokine storm in MDA5+DM‐ILD patients due to the significantly increased expression of IFN‐induced protein with tetratricopeptide repeats 3 (IFIT3), myxovirus resistance 1 (MX1), C‐C motif ligand 2 (CCL2), and clusterin (CLU) mRNAs in circulating monocytes. 33 Notably, coronavirus disease 2019 and MDA5+DM‐ILD have some similarities, indicating that they may have similar underlying pathogenesis. 34 Severe acute respiratory syndrome coronavirus 2 can activate IFN signaling in lung epithelial cells via MDA5‐mediated sensing. 35 Moreover, mild or absent myopathy may be associated with lower expression of IFN‐stimulated genes in the muscles of MDA5+DM patients compared with classic DM. 36 Interestingly, a recent study showed that autoantibodies from B cells of MDA5+DM patients, other than anti‐MDA5 antibodies, could trigger direct IFN‐γ production by peripheral blood mononuclear cells (PBMCs) by a mechanism dependent on monocytes, which unraveled the pathogenesis of dysregulation of IFN‐γ linked to MDA5+DM severity. 37
3. BLOOD‐BASED MOLECULAR BIOMARKERS IN MDA5+DM‐ILD
3.1. Protein biomarkers
Some serum indicators related to macrophage activation may be biomarkers for the diagnosis, prognosis, and monitoring of MDA5+DM‐ILD. Serum ferritin is a key marker of inflammation and macrophage activation. 38 , 39 Elevated serum ferritin levels may detect early diagnosis and prognosis of RPILD patients with DM. 40 , 41 , 42 Serum ferritin levels are increased in MDA5+DM‐ILD patients. High ferritin levels can also predict the severity and poor outcome of MDA5+DM‐ILD. 43 , 44 RPILD usually leads to an unfavorable prognosis. A high serum ferritin level (>1000 μg/L) is found to be closely associated with a high incidence of RPILD and mortality among MDA5+DM patients. 45 Additionally, high ferritin levels (≥828 ng/ml and ≥1000 ng/ml) are a poor prognostic factor in MDA5+DM‐RPILD patients. 46 , 47 Yang et al. also showed that the serum ferritin level of ≥1500 ng/ml [odds ratio (OR) 12.3] is an independent predictor of RPILD in MDA5+DM patients. 48 A multicenter retrospective cohort study reported that the high ferritin level [>2800 pmol/L, hazard ratio (HR) 3.042] is an independent predictor of mortality in MDA5+DM patients. 49 Similarly, a prospective observational study showed that the serum ferritin level of ≥1250 μg/L (HR 10.4) is an independent risk factor for 6‐month all‐cause mortality in MDA5+DM patients. 50 In that study, 12 all‐cause mortality was reported in MDA5+DM patients due to ILD, while 10 patients died of RPILD. 50 A case of hyperferritinemia MDA5+DM‐RPILD autopsy showed that macrophages produce high serum ferritin levels in the lung. 51 Therefore, ferritin concentrations may reflect the efficacy of treatment and the status of ILD in MDA5+DM patients. 46 In summary, serum ferritin levels may predict the outcome of MDA5+DM‐ILD.
Furthermore, elevated serum chitinase‐3‐like‐1 protein (YKL‐40) levels can discriminate RPILD patients among MDA5+DM patients (AUC 0.648, sensitivity 51.2%, and specificity 82.8%). 52 YKL‐40 levels are increased in aggravated ILD. However, YKL‐40 levels decrease after treatment, indicating that YKL‐40 levels can reflect the severity of ILD and facilitate monitoring of the treatment response of ILD among MDA5+DM patients. 52 Cox hazard analysis has also shown that a higher serum YKL‐40 level is an independent risk factor for poor prognosis in MDA5+DM patients. 52 The Kaplan–Meier survival curve has also shown that elevated serum YKL‐40 levels (>80 ng/ml) are associated with a lower 6‐month survival rate in MDA5+DM patients. 52 Serum soluble CD163 (sCD163) levels are higher in patients with anti‐MDA5 antibodies than in those without. 53 Moreover, serum sCD206 levels are higher in MDA5+DM‐ILD patients than in healthy controls. Serum sCD206 levels are also higher in MDA5+DM‐ILD non‐survivors than in survivors. 32 Increased serum sCD206 may be a poor prognostic predictor of MDA5+DM‐ILD. Kaplan–Meier curves have shown that high sCD206 (≥745 ng/ml) is correlated with a lower survival rate of MDA5+DM‐ILD patients, which means the increased serum sCD206 may be a poor prognostic predictor of MDA5+DM‐ILD. 32 Serum neopterin levels are higher in MDA5+DM patients with RPILD than in those without RPILD. 54 However, serum neopterin levels decrease after the treatment of MDA5+DM‐ILD patients, indicating that serum neopterin may be used to monitor response to therapy. 55 Furthermore, serum chitotriosidase levels are elevated in MDA5+DM‐ILD patients. 56 Survival curves have also shown that high chitotriosidase (≥23.5 ng/ml) levels are associated with a higher mortality rate among MDA5+DM‐ILD patients, suggesting that serum chitotriosidase may be a poor prognostic biomarker. 56 Gao et al. reported that high osteopontin levels (≥3000 pg/ml) are correlated with a shorter survival time in MDA5+DM‐ILD patients, indicating that elevated osteopontin may be a poor prognostic biomarker. 57 A recent study also showed that serum progranulin levels are higher in MDA5+DM patients with RPILD than in those without RPILD. 58 Serum progranulin (cutoff 187.26 ng/ml) can distinguish RPILD patients from non‐RPILD patients (AUC 0.715), indicating that progranulin is a potential biomarker for RPILD diagnosis in MDA5+DM patients. 58
Elevated Krebs von den Lungen‐6 (KL‐6) levels in serum (primarily produced by regenerating alveolar type II pneumocytes or impaired alveolar–capillary barriers) can reflect ILD severity and progression. 59 , 60 A prospective study also revealed that serum KL‐6 may predict ILD occurrence and monitor the status and treatment response of ILD in DM/PM patients. 61 Serum KL‐6 levels are higher in MDA5+DM‐ILD patients, especially in MDA5+CADM‐ILD patients, than in healthy controls. 62 For instance, a large‐scale multicenter retrospective cohort study indicated that initial serum KL‐6 levels (≥1000 units/ml, HR 1.8) are an independent risk factor for mortality in MDA5+DM‐ILD patients. 63 High KL‐6 levels are a poor prognostic marker of MDA5+DM‐ILD. 48 , 62 , 64 Zhu et al. also found that serum KL‐6 levels (>500.9 pg/ml, OR 56.38) are an independent risk factor for RPILD. They also showed that serum KL‐6 levels are a potential biomarker for monitoring RPILD progression in MDA5+DM patients. 64 Additionally, elevated KL‐6 levels (in the first 4 weeks of immunosuppressive treatment) in MDA5+DM‐ILD patients may indicate the occurrence of intractable RPILD. 65 In conclusion, serum KL‐6 is a specific biomarker for ILD severity and progression in MDA5+DM patients.
Elevated serum levels of surfactant protein‐D (SP‐D) (secreted from regenerating alveolar type II pneumocytes or impaired alveolar–capillary barrier) are closely correlated with ILD in DM/PM. 66 , 67 , 68 , 69 However, lower SP‐D levels (<100 ng/ml) are a predictor of mortality in DM/PM‐ILD patients. 70 Similarly, Kaieda et al. showed that serum SP‐D levels are lower in non‐survivors than in survivors with MDA5+DM‐ILD, suggesting that lower SP‐D may reflect the occurrence of RPILD in MDA5+DM patients. 71 Serum SP‐D levels are decreased in idiopathic pulmonary fibrosis. 72 Type II alveolar epithelial cells are replaced with regenerated ciliated epithelial cells at the end of fibrotic change, thus reducing SP‐D production. Serum SP‐D levels may also be a predictor of mortality in MDA5+DM‐ILD patients.
Cytokines, as inflammatory mediators, are also potential biomarkers of MDA5+DM‐ILD. For instance, a longitudinal study showed that serum levels of B‐cell activating factor (BAFF) are higher in MDA5+DM than in healthy controls. However, serum BAFF levels decrease after immunosuppressive therapy, suggesting that BAFF is a potential biomarker for monitoring MDA5+DM activity. 73 Anti‐aminoacyl transfer RNA synthetase (ARS) antibodies are associated with chronic ILD in DM/PM patients. Compared with anti‐ARS‐ILD, RPILD, mortality, and poor response to treatment are significantly associated with antiMDA5‐ILD. 67 Serum cytokine interleukin 8 (IL‐8) levels are significantly increased in anti‐MDA5‐ILD patients than in anti‐ARS‐ILD patients. 74 The serum levels of cytokines stromal cell‐derived factor 1‐α (SDF‐1α), IFN‐γ‐inducible 10‐kDa (IP‐10), IL‐7, IL‐17A, RANTES, IFN‐γ, tumor necrosis factor‐α (TNF‐α), macrophage inflammatory protein 1‐β (MIP‐1β), IFN‐α, monocyte chemoattractant protein‐1 (MCP‐1), growth‐regulated oncogene‐α (GRO‐α), and IL‐1α are significantly increased in MDA5+DM‐ILD patients compared with anti‐transcription intermediary factor 1‐γ‐positive DM‐ILD patients. 75 Moreover, a cytokine storm may be involved in MDA5+DM‐RPILD pathogenesis. Gono et al. reported that serum IL‐18 levels are high in dead MDA5+DM‐RPILD patients. 46 They also reported that serum IL‐18 levels decrease after treatment, suggesting that IL‐18 levels can reflect the response to RPILD treatment in MDA5+DM patients. 46 Horai et al. found that serum IFN‐α levels are elevated in MDA5+DM‐RPILD patients. They also showed that the IFN‐α level is positively correlated with the IL‐18 level (r = 0.8139), indicating that IFN‐α may be a biomarker of RPILD. 76 A study reported that serum IFN‐β and CCL2 levels are significantly higher in MDA5+DM‐ILD patients than in ARS+DM‐ILD patients and healthy controls. 33 Moreover, a decrease in IFN‐β and CCL2 levels after the treatment of MDA5+DM‐ILD may reflect the response to the treatment. 33 Furthermore, serum IL‐15 levels in MDA5+DM‐ILD non‐survivors before treatment were significantly higher than those in survivors, indicating that IL‐15 may be involved in MDA5+DM‐ILD progression. 77 Random forest analysis has also shown that increased IL‐15 levels and decreased CCL22 levels can predict RPILD in MDA5+DM patients. 31 Galectin‐9 mRNA levels are associated with mRNA levels of type I IFN‐inducible genes MX1 and IFIH1 in PBMCs from DM patients. 78 Moreover, serum galectin‐9 levels are significantly increased in MDA5+DM patients with RPILD compared with those without RPILD. Serum galectin‐9 levels are also associated with RPILD severity, suggesting that galectin‐9 may be a valuable biomarker for monitoring RPILD severity. 78
A meta‐analysis showed that ILD (risk ratio 0.49) is significantly associated with a reduced risk of cancer in IIM. 79 Interestingly, some tumor markers, including carcinoembryonic antigen (CEA), carbohydrate antigen 153 (CA153), CA724, CA125, CA199, neuron‐specific enolase, and cytokeratin‐19 fragment (CYFRA21‐1), are elevated in DM‐RPILD patients. 80 , 81 , 82 Moreover, increased serum CA125, CA199, and CYFRA21‐1 levels may reflect the severity of lung injury in progressive idiopathic pulmonary fibrosis. 83 , 84 Therefore, lung damage may induce increased levels of tumor markers in ILD patients. Ouyang et al. also showed that serum CEA levels are significantly increased in non‐survivors than in survivors among the MDA5+DM patients. 50 A stepwise multivariate Cox regression has shown that positive CEA (HR 5.2) is an independent risk factor for 6‐month mortality in MDA5+DM. 50 Zuo et al. showed that elevated CEA (OR 5.8) and CA199 (OR 7.8) levels are independent predictors of a lower lung zone consolidation pattern in MDA5+DM patients based on HRCT findings. 85 Serum CA153 levels may also be a predictor of mortality in MDA5+DM patients. Based on cox proportional hazard regression analyses, Tseng et al. showed that CA153 (HR 1.2) is significantly associated with an increased risk of 1‐year mortality in MDA5+DM patients. 86 Kaplan–Meier survival curves have also shown that CA153 levels (>22.2 U/ml) are correlated with increased mortality in MDA5+DM‐ILD patients. 86 Increased serum CYFRA21‐1 levels are associated with a higher risk of acute/subacute interstitial pneumonia (A/SIP). A/SIP is characterized by progressive deterioration of dyspnea and hypoxemia. CYFRA21‐1 levels are higher in dead MDA5+DM‐ILD patients than in survivors, suggesting that CYFRA21‐1 may indicate the occurrence of A/SIP and poor outcomes in MDA5+DM‐ILD. 87
So et al. reported that serum level of the laboratory inflammatory marker lactate dehydrogenase (LDH) > 400 IU/L is an independent predictor of mortality. They also showed that serum LDH level > 300 IU/L (HR 3.189) is an independent risk factor for RPILD in MDA5+DM patients, suggesting that high LDH may reflect ILD severity in MDA5+DM patients. 49 A high erythrocyte sedimentation rate (ESR)‐albumin ratio (EAR) is an independent predictor of mortality in MDA5+DM‐ILD patients. 88 A study also showed that impaired antioxidant function of high‐density lipoprotein (HDL) in the plasma of MDA5+DM patients is associated with higher circulating MPO activity, indicating that HDL may be involved in the vascular inflammation in MDA5+DM. 89
3.2. Antibody biomarkers
Two recent studies have indicated that IgG subclasses of anti‐MDA5 are associated with disease severity and prognosis in DM‐ILD patients. Chen et al. reported that anti‐MDA5 IgG1 (sensitivity 100% and specificity 41.7%), one of the primary isotypes of anti‐MDA5 IgG subclasses, may predict DM‐ILD‐associated mortality. Moreover, the combination of ant‐MDA5 IgG1 and IgG4 has a better specificity (87.5%). 15 Based on the results of multivariate analysis, Xu et al. reported that anti‐MDA5 IgG1 (>13 U/ml) and anti‐MDA5 IgG3 (>11 U/ml) are independent risk factors for mortality in DM‐ILD patients, suggesting that anti‐MDA5 IgG1 and IgG3 may be prognostic biomarkers of DM‐ILD. 90 The anti‐Ro52 antibody, a common myositis‐associated autoantibody, is related to ILD and is an independent risk factor for ILD in DM. 91 , 92 MDA5+DM‐ILD patients with anti‐Ro52 antibody are more likely to develop RPILD than those without anti‐Ro52 antibody. Moreover, these patients are associated with a lower survival rate, indicating that anti‐Ro52 antibody may predict the occurrence of RPILD and a poor outcome in MDA5+DM‐ILD patients. 93 , 94 A recent study found that anti‐Ro52 antibodies are produced in IIM in response to Ro52/IgG/HLA‐DR expressed on the cell surface. 95 Additionally, Miossec et al. found that MDA5+DM patients with a typical indirect immunofluorescence (IIF) MDA5 pattern characterized by a cytoplasmic staining fluorescence profile in rare clustered Hep‐2 cells with a finely granular appearance had a higher risk of RPILD. 37 These autoantibodies in MDA5+DM patients' serum giving the specific cytoplasmic MDA5 pattern on Hep‐2 cells may be autoantibodies other than anti‐MDA5 antibodies, but are potentially involved in the pathogenic process of severity of the disease. 37
3.3. Genetic biomarkers
Exosomal microRNAs (miRNAs) stably circulate in the blood and are biomarkers for various diseases. A study showed that upregulated plasma‐derived exosomal miR‐4488 and miR‐1228‐5p might help diagnose, prevent, and treat MDA5+DM‐ILD based on next‐generation sequencing. 96 Besides miRNA, high serum levels of cell‐free DNA (cfDNA), mainly produced by neutrophil extracellular traps (NETs), may reflect NETs involved in MDA5+DM‐ILD pathogenesis. 97 Circulating NET [both human neutrophil elastase (HNE)‐DNA and myeloperoxidase (MPO)‐DNA complexes] are elevated in MDA5+DM patients, suggesting that anti‐MDA5 antibodies may enhance NET formation, reflecting neutrophil dysregulation. 98
3.4. Cellular biomarkers
Circulating immune cells are also minimally invasive biomarkers for the detection of various diseases. CD4+T and CD8+T cell counts are decreased in MDA5+DM patients compared with MDA5−DM patients, while the CD4+/CD8+ ratio is increased MDA5+DM patients compared with MDA5−DM patients. 99 CD3+T cell counts (AUC 0.688; sensitivity 45%; specificity 88.3%) can discriminate MDA5+DM patients with RPILD from those without RPILD. 52 Cox hazard analysis has also shown that lower CD3+T cell counts are independent factors for poor prognosis in MDA5+DM patients. 52 CD3+CD4+T cell counts are lower in MDA5+DM patients with (A/SIP) than in those with chronic interstitial pneumonia (CIP) 87 and thus may indicate the occurrence of A/SIP. The percentage of T follicular helper (TFH; a subset of CD4+T cells) cells is increased in MDA5+DM patients compared with MDA5−IIM patients or healthy controls. 100 Lower monocyte counts and lymphocyte counts are useful predictors of a poor outcome among MDA5+DM‐ILD patients in the early stage. 101 A high monocyte–lymphocyte ratio (MLR) (MLR >0.604) is an independent predictor of mortality in MDA5+DM‐ILD patients, suggesting that MLR may predict poor prognosis in MDA5+DM‐ILD patients. 88 CD19+B cell counts are higher in MDA5+DM patients than in ARS+IIM patients. 102 Additionally, serum (ESR) levels are higher in the non‐survival group than in the survival group among MDA5+DM patients, indicating that increased ESR may predict poor prognosis in MDA5+DM patients. 103
4. CONCLUSION
This review summarizes the recent articles on blood‐based biomarkers associated with MDA5+DM, MDA5+DM‐ILD, and MDA5+DM‐RPILD. However, most biomarkers are not specific for these three conditions, and information about their sensitivity and specificity as biomarkers is lacking. Moreover, most studies are cross‐sectional cohort studies, lacking prospective cohort studies. These dysregulated biomarkers in the blood may reflect the ILD severity and progression in MDA5+DM and thus provide insights into the pathogenesis of the disease (Figure 1). Therefore, biomarkers for the early recognition of RPILD, treatment response, and predicted outcome may benefit MDA5+DM‐RPILD patients (Table 1) and prevent fatal RPILD. However, further studies with large cohorts, especially prospective cohorts, should assess the biomarkers of MDA5+DM‐RPILD to validate the results. Moreover, emerging evidence suggests that the gut microbiota may also serve as a biomarker for ILD due to the crucial role of the gut–lung axis in respiratory diseases. 104 A recent study has demonstrated that intestinal microbiota dysbiosis could be modified by low‐dose IL‐2 in dermatomyositis. 105 However, there is no evidence that gut microbiota can be used as biomarkers of clinical features and outcomes of MDA5+DM, MDA5+DM‐ILD, and MDA5+DM‐RPILD, which requires to be investigated in the future.
FIGURE 1.
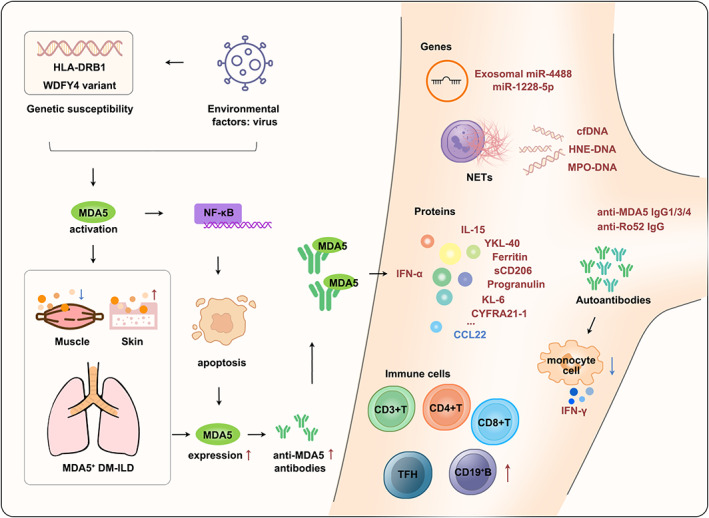
Proposed possible pathogenesis of MDA5+DM‐ILD and summary of blood‐based biomarkers for MDA5+DM, MDA5+DM‐ILD, and MDA5+DM‐RPILD. The potential pathogenesis of MDA5+DM‐ILD is presented both on the left (mainly) and right. Blood biomarkers related to MDA5+DM, MDA5+DM‐ILD, and MDA5+DM‐RPILD are on the right. Bold red and blue texts represent upregulated and downregulated biomarkers, respectively. CCL22, C‐C motif ligand 22; cfDNA, cell‐free DNA; CYFRA21‐1, cytokeratin‐19 fragment; DM, dermatomyositis; HNE‐DNA, human neutrophil elastase‐DNA; IFN, interferon; IL‐15, interleukin 15; ILD, interstitial lung disease; KL‐6, krebs von den Lungen‐6; MDA5, melanoma differentiation‐associated gene 5; miR, microRNA; MPO‐DNA, myeloperoxidase‐DNA; NETs, neutrophil extracellular traps; sCD206, soluble CD206; TFH, T follicular helper; YKL‐40, chitinase‐3‐like‐1 protein.
TABLE 1.
Summary of blood‐based biomarkers for MDA5+DM‐RPILD
| Biomarkers | Pattern of change | Detection Method | Comments |
|---|---|---|---|
| Protein biomarkers | |||
| Ferritin | Up | Chemiluminescence; 50 ELISA 31 | Associated with development of RPILD (single center; retrospective design); 45 poor prognostic factors in RPILD patients (two hospitals; retrospective design) 46 (single center); 47 an independent predictor for RPILD (single center; retrospective design). 48 |
| YKL‐40 | Up | ELISA 52 | Reflect the occurrence of RPILD (single center; cross‐sectional design). 52 |
| Neopterin | Up | ELISA 54 | Associated with RPILD (single center; retrospective design). 54 |
| Progranulin | Up | ELISA 58 | Diagnosis of RPILD (single center; cross‐sectional design). 58 |
| KL‐6 | Up | Latex agglutination test; 64 enzyme immunoassay 69 | An independent risk factor for RPILD and a potential biomarker to monitor RPILD progression (single center; retrospective design); 64 elevated in the first 4 weeks of immunosuppressive treatment in MDA5+DM‐ILD patients may indicate the occurrence of intractable RPILD (single center; retrospective design). 65 |
| IL‐18 | Up | ELISA 46 | Evaluate the response to the treatment of RPILD in MDA5+DM (two hospitals; retrospective design). 46 |
| IFN‐α | Up | ELISA 76 | Reflect the presence of RPILD (two hospitals). 76 |
| IL‐15 | Up |
Bio‐Plex ProTM Human Cytokine assay 31 |
Predict RPILD (Nagasaki University Hospital and related institution; retrospective observational design). 31 |
| CCL22 | Down |
Bio‐Plex ProTM Human Cytokine assay 31 |
Predict RPILD (Nagasaki University Hospital and related institution; retrospective observational design). 31 |
| Galectin‐9 | Up | ELISA 78 | Associated with RPILD; monitor RPILD severity (single center; cross‐sectional and longitudinal design). 78 |
| CYFRA21‐1 | Up | Laboratory tests 87 | Reflect the occurrence of A/SIP (single center; retrospective design). 87 |
| LDH | Up | Laboratory findings 49 | An independent risk factor for RPILD (multicenter; retrospective design). 49 |
| Antibody biomarkers | |||
| Anti‐Ro52 antibody | Positive | Immunoblot assay 94 | More likely to develop RPILD (single center; retrospective design). 94 |
| Autoantibodies (the specific cytoplasmic MDA5 pattern on Hep‐2 cells) | Positive | IIF 37 | A higher risk for RPILD (single center; retrospective design). 37 |
| Cellular biomarkers | |||
| CD3+T cell | Down | Flow cytometry 99 | Diagnosis of RPILD (single center; cross‐sectional design). 52 |
| CD3+CD4+T cell | Down | Flow cytometry 99 | Associated with the occurrence of A/SIP (single center; retrospective design). 87 |
Abbreviations: A/SIP, acute/subacute interstitial pneumonia; CCL22, C‐C motif ligand 22; CYFRA21‐1, cytokeratin‐19 fragment; DM, dermatomyositis; ELISA, enzyme‐linked immunosorbent assay; IFN‐α, interferon‐α; IIF, indirect immunofluorescence; IL‐15, interleukin 15; IL‐18, interleukin 18; KL‐6, krebs von den Lungen‐6; LDH, lactate dehydrogenase; MDA5, melanoma differentiation‐associated gene 5; RPILD, rapidly progressive interstitial lung disease; YKL‐40, chitinase‐3‐like‐1 protein.
AUTHOR CONTRIBUTIONS
Yongzhe Li and Xiaomeng Li are responsible for study conception and design. Yongzhe Li is responsible for study supervision. Xiaomeng Li and Yongmei Liu are responsible for literature search and manuscript writing. Linlin Cheng, Yuan Huang, Songxin Yan, Haolong Li, and Haoting Zhan are responsible for interpretation. All authors contributed to the article and approved the submitted version.
FUNDING INFORMATION
This work was supported by the National Key Research and Development Program of China (2018YFE0207300).
CONFLICT OF INTEREST
The authors have declared no conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Shangqing Yang (Department of Industrial Design, Institute of Art and Architecture, Central South University) for providing guidance on designing the figure and Shulan Zhang (Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital) for assistance with the revised manuscript.
Li X, Liu Y, Cheng L, et al. Roles of biomarkers in anti‐MDA5‐positive dermatomyositis, associated interstitial lung disease, and rapidly progressive interstitial lung disease. J Clin Lab Anal. 2022;36:e24726. doi: 10.1002/jcla.24726
Xiaomeng Li and Yongmei Liu contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lundberg IE, Fujimoto M, Vencovsky J, et al. Idiopathic inflammatory myopathies. Nat Rev Dis Primers. 2021;7:86 (In eng). [DOI] [PubMed] [Google Scholar]
- 2. Sun KY, Fan Y, Wang YX, Zhong YJ, Wang GF. Prevalence of interstitial lung disease in polymyositis and dermatomyositis: a meta‐analysis from 2000 to 2020. Semin Arthritis Rheum. 2021;51:175‐191. (In eng). [DOI] [PubMed] [Google Scholar]
- 3. McPherson M, Economidou S, Liampas A, Zis P, Parperis K. Management of mda‐5 antibody positive clinically amyopathic dermatomyositis associated interstitial lung disease: a systematic review. Semin Arthritis Rheum. 2022;53:151959 (In eng). [DOI] [PubMed] [Google Scholar]
- 4. Nombel A, Fabien N, Coutant F. Dermatomyositis with anti‐mda5 antibodies: bioclinical features, pathogenesis and emerging therapies. Front Immunol. 2021;12:773352 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Romero‐Bueno F, Diaz Del Campo P, Trallero‐Araguás E, et al. Recommendations for the treatment of anti‐melanoma differentiation‐associated gene 5‐positive dermatomyositis‐associated rapidly progressive interstitial lung disease. Semin Arthritis Rheum. 2020;50:776‐790. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140‐kd polypeptide, cadm‐140, in japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571‐1576. (In eng). [DOI] [PubMed] [Google Scholar]
- 7. Sato S, Hoshino K, Satoh T, et al. Rna helicase encoded by melanoma differentiation‐associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60:2193‐2200. (In eng). [DOI] [PubMed] [Google Scholar]
- 8. Mahler M, Betteridge Z, Bentow C, et al. Comparison of three immunoassays for the detection of myositis specific antibodies. Front Immunol. 2019;10:848 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gono T, Okazaki Y, Murakami A, Kuwana M. Improved quantification of a commercial enzyme‐linked immunosorbent assay kit for measuring anti‐mda5 antibody. Mod Rheumatol. 2019;29:140‐145. (In eng). [DOI] [PubMed] [Google Scholar]
- 10. Sato S, Murakami A, Kuwajima A, et al. Clinical utility of an enzyme‐linked immunosorbent assay for detecting anti‐melanoma differentiation‐associated gene 5 autoantibodies. PLoS ONE. 2016;11:e0154285 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sato S, Kuwana M, Fujita T, Suzuki Y. Anti‐cadm‐140/mda5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol. 2013;23:496‐502. (In eng). [DOI] [PubMed] [Google Scholar]
- 12. Matsushita T, Mizumaki K, Kano M, et al. Antimelanoma differentiation‐associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br J Dermatol. 2017;176:395‐402. (In eng). [DOI] [PubMed] [Google Scholar]
- 13. Sakamoto S, Okamoto M, Kaieda S, et al. Low positive titer of anti‐melanoma differentiation‐associated gene 5 antibody is not associated with a poor long‐term outcome of interstitial lung disease in patients with dermatomyositis. Respir Investig. 2018;56:464‐472. (In eng). [DOI] [PubMed] [Google Scholar]
- 14. Abe Y, Matsushita M, Tada K, Yamaji K, Takasaki Y, Tamura N. Clinical characteristics and change in the antibody titres of patients with anti‐mda5 antibody‐positive inflammatory myositis. Rheumatology (Oxford). 2017;56:1492‐1497. (In eng). [DOI] [PubMed] [Google Scholar]
- 15. Chen M, Zhao Q, Diao L, et al. Distribution of anti‐melanoma differentiation associated gene 5 (mda5) igg subclasses in mda5+ dermatomyositis. Rheumatology (Oxford). 2021;61:430‐439. (In eng). [DOI] [PubMed] [Google Scholar]
- 16. Bowman WS, Echt GA, Oldham JM. Biomarkers in progressive fibrosing interstitial lung disease: optimizing diagnosis, prognosis, and treatment response. Front Med. 2021;8:680997 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowman WS, Newton CA, Linderholm AL, et al. Proteomic biomarkers of progressive fibrosing interstitial lung disease: a multicentre cohort analysis. Lancet Respir Med. 2022;10:593‐602. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dias Junior AG, Sampaio NG, Rehwinkel J. A balancing act: Mda5 in antiviral immunity and autoinflammation. Trends Microbiol. 2019;27:75‐85. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toquet S, Granger B, Uzunhan Y, et al. The seasonality of dermatomyositis associated with anti‐mda5 antibody: an argument for a respiratory viral trigger. Autoimmun Rev. 2021;20:102788 (In eng). [DOI] [PubMed] [Google Scholar]
- 20. So H, So J, Lam TT, et al. Seasonal effect on disease onset and presentation in anti‐mda5 positive dermatomyositis. Front Med. 2022;9:837024 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nishina N, Sato S, Masui K, Gono T, Kuwana M. Seasonal and residential clustering at disease onset of anti‐mda5‐associated interstitial lung disease. RMD Open. 2020;6 (In eng):e001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zahn S, Barchet W, Rehkämper C, et al. Enhanced skin expression of melanoma differentiation‐associated gene 5 (mda5) in dermatomyositis and related autoimmune diseases. J Am Acad Dermatol. 2011;64:988‐989. (In eng). [DOI] [PubMed] [Google Scholar]
- 23. Cassius C, Amode R, Delord M, et al. Mda5(+) dermatomyositis is associated with stronger skin type i interferon transcriptomic signature with upregulation of ifn‐κ transcript. J Invest Dermatol. 2020;140:1276‐1279.e7. (In eng). [DOI] [PubMed] [Google Scholar]
- 24. Ono N, Kai K, Maruyama A, et al. The relationship between type 1 ifn and vasculopathy in anti‐mda5 antibody‐positive dermatomyositis patients. Rheumatology (Oxford). 2019;58:786‐791. (In eng). [DOI] [PubMed] [Google Scholar]
- 25. Zhang SH, Zhao Y, Xie QB, Jiang Y, Wu YK, Yan B. Aberrant activation of the type i interferon system may contribute to the pathogenesis of anti‐melanoma differentiation‐associated gene 5 dermatomyositis. Br J Dermatol. 2019;180:1090‐1098. (In eng). [DOI] [PubMed] [Google Scholar]
- 26. Gono T, Kawaguchi Y, Kuwana M, et al. Brief report: association of hla‐drb1*0101/*0405 with susceptibility to anti‐melanoma differentiation‐associated gene 5 antibody‐positive dermatomyositis in the japanese population. Arthritis Rheum. 2012;64:3736‐3740. (In eng). [DOI] [PubMed] [Google Scholar]
- 27. Lin JM, Zhang YB, Peng QL, et al. Genetic association of hla‐drb1 multiple polymorphisms with dermatomyositis in chinese population. HLA. 2017;90:354‐359. (In eng). [DOI] [PubMed] [Google Scholar]
- 28. Chen Z, Wang Y, Kuwana M, et al. Hla‐drb1 alleles as genetic risk factors for the development of anti‐mda5 antibodies in patients with dermatomyositis. J Rheumatol. 2017;44:1389‐1393. (In eng). [DOI] [PubMed] [Google Scholar]
- 29. Kochi Y, Kamatani Y, Kondo Y, et al. Splicing variant of wdfy4 augments mda5 signalling and the risk of clinically amyopathic dermatomyositis. Ann Rheum Dis. 2018;77:602‐611. (In eng). [DOI] [PubMed] [Google Scholar]
- 30. Wang K, Zhao J, Wu W, et al. Rna‐containing immune complexes formed by anti‐melanoma differentiation associated gene 5 autoantibody are potent inducers of ifn‐α. Front Immunol. 2021;12:743704 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shimizu T, Koga T, Furukawa K, et al. Il‐15 is a biomarker involved in the development of rapidly progressive interstitial lung disease complicated with polymyositis/dermatomyositis. J Intern Med. 2021;289:206‐220. (In eng). [DOI] [PubMed] [Google Scholar]
- 32. Horiike Y, Suzuki Y, Fujisawa T, et al. Successful classification of macrophage‐mannose receptor cd206 in severity of anti‐mda5 antibody positive dermatomyositis associated ild. Rheumatology (Oxford). 2019;58:2143‐2152. (In eng). [DOI] [PubMed] [Google Scholar]
- 33. Gono T, Okazaki Y, Kuwana M. Antiviral proinflammatory phenotype of monocytes in anti‐mda5 antibody‐associated interstitial lung disease. Rheumatology (Oxford). 2022;61:806‐814. (In eng). [DOI] [PubMed] [Google Scholar]
- 34. De Lorenzis E, Natalello G, Gigante L, Verardi L, Bosello SL, Gremese E. What can we learn from rapidly progressive interstitial lung disease related to anti‐mda5 dermatomyositis in the management of covid‐19? Autoimmun Rev. 2020;19:102666 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yin X, Riva L, Pu Y, et al. Mda5 governs the innate immune response to sars‐cov‐2 in lung epithelial cells. Cell Rep. 2021;34:108628 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Allenbach Y, Leroux G, Suárez‐Calvet X, et al. Dermatomyositis with or without anti‐melanoma differentiation‐associated gene 5 antibodies: common interferon signature but distinct nos2 expression. Am J Pathol. 2016;186:691‐700. (In eng). [DOI] [PubMed] [Google Scholar]
- 37. Coutant F, Bachet R, Pin JJ, Alonzo M, Miossec P. Monoclonal antibodies from b cells of patients with anti‐mda5 antibody‐positive dermatomyositis directly stimulate interferon gamma production. J Autoimmun. 2022;130:102831 (In eng). [DOI] [PubMed] [Google Scholar]
- 38. Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6:748‐773. (In eng). [DOI] [PubMed] [Google Scholar]
- 39. Ravelli A. Macrophage activation syndrome. Curr Opin Rheumatol. 2002;14:548‐552. (In eng). [DOI] [PubMed] [Google Scholar]
- 40. Gono T, Kawaguchi Y, Hara M, et al. Increased ferritin predicts development and severity of acute interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford). 2010;49:1354‐1360. (In eng). [DOI] [PubMed] [Google Scholar]
- 41. Xu Y, Yang CS, Li YJ, et al. Predictive factors of rapidly progressive‐interstitial lung disease in patients with clinically amyopathic dermatomyositis. Clin Rheumatol. 2016;35:113‐116. (In eng). [DOI] [PubMed] [Google Scholar]
- 42. Lian X, Zou J, Guo Q, et al. Mortality risk prediction in amyopathic dermatomyositis associated with interstitial lung disease: the flair model. Chest. 2020;158:1535‐1545. (In eng). [DOI] [PubMed] [Google Scholar]
- 43. Gono T, Kawaguchi Y, Satoh T, et al. Clinical manifestation and prognostic factor in anti‐melanoma differentiation‐associated gene 5 antibody‐associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford). 2010;49:1713‐1719. (In eng). [DOI] [PubMed] [Google Scholar]
- 44. Fujiki Y, Kotani T, Isoda K, et al. Evaluation of clinical prognostic factors for interstitial pneumonia in anti‐mda5 antibody‐positive dermatomyositis patients. Mod Rheumatol. 2018;28:133‐140. (In eng). [DOI] [PubMed] [Google Scholar]
- 45. Yang Q, Lyu K, Li J, et al. Anti‐melanoma differentiation‐associated 5 gene antibody‐positive dermatomyositis exhibit three clinical phenotypes with different prognoses. Clin Exp Rheumatol. 2022;40:304‐308. (In eng). [DOI] [PubMed] [Google Scholar]
- 46. Gono T, Sato S, Kawaguchi Y, et al. Anti‐mda5 antibody, ferritin and il‐18 are useful for the evaluation of response to treatment in interstitial lung disease with anti‐mda5 antibody‐positive dermatomyositis. Rheumatology (Oxford). 2012;51:1563‐1570. [DOI] [PubMed] [Google Scholar]
- 47. Kurasawa K, Arai S, Namiki Y, et al. Tofacitinib for refractory interstitial lung diseases in anti‐melanoma differentiation‐associated 5 gene antibody‐positive dermatomyositis. Rheumatology (Oxford). 2018;57:2114‐2119. [DOI] [PubMed] [Google Scholar]
- 48. Yang Q, Li T, Zhang X, et al. Initial predictors for short‐term prognosis in anti‐melanoma differentiation‐associated protein‐5 positive patients. Orphanet J Rare Dis. 2021;16:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. So J, So H, Wong V, et al. Predictors of rapidly progressive‐ interstitial lung disease and mortality in patients with autoantibodies against melanoma differentiation‐associated protein 5 dermatomyositis. Rheumatology (Oxford). 2022. 10.1093/rheumatology/keac094. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 50. Ouyang Z, Lin J, Tang A, et al. A matrix prediction model for the 6‐month mortality risk in patients with anti‐melanoma differentiation‐associated protein‐5‐positive dermatomyositis. Front Med (Lausanne). 2022;9:860798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gono T, Miyake K, Kawaguchi Y, Kaneko H, Shinozaki M, Yamanaka H. Hyperferritinaemia and macrophage activation in a patient with interstitial lung disease with clinically amyopathic dm. Rheumatology (Oxford). 2012;51:1336‐1338. (In eng). [DOI] [PubMed] [Google Scholar]
- 52. Jiang L, Wang Y, Peng Q, Shu X, Wang G, Wu X. Serum ykl‐40 level is associated with severity of interstitial lung disease and poor prognosis in dermatomyositis with anti‐mda5 antibody. Clin Rheumatol. 2019;38:1655‐1663. (In eng). [DOI] [PubMed] [Google Scholar]
- 53. Kawasumi H, Katsumata Y, Nishino A, et al. Association of serum soluble cd163 with polymyositis and dermatomyositis, especially in anti‐mda5 antibody‐positive cases. J Rheumatol. 2018;45:947‐955. (In eng). [DOI] [PubMed] [Google Scholar]
- 54. Peng QL, Zhang YM, Liang L, et al. A high level of serum neopterin is associated with rapidly progressive interstitial lung disease and reduced survival in dermatomyositis. Clin Exp Immunol. 2020;199:314‐325. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nishioka A, Tsunoda S, Abe T, et al. Serum neopterin as well as ferritin, soluble interleukin‐2 receptor, kl‐6 and anti‐mda5 antibody titer provide markers of the response to therapy in patients with interstitial lung disease complicating anti‐mda5 antibody‐positive dermatomyositis. Mod Rheumatol. 2019;29:814‐820. (In eng). [DOI] [PubMed] [Google Scholar]
- 56. Fujisawa T, Hozumi H, Yasui H, et al. Clinical significance of serum chitotriosidase level in anti‐mda5 antibody‐positive dermatomyositis‐associated interstitial lung disease. J Rheumatol. 2019;46:935‐942. (In eng). [DOI] [PubMed] [Google Scholar]
- 57. Gao Y, Zhao Q, Xie M, et al. Prognostic evaluation of serum osteopontin in patients with anti‐mda5 antibody‐positive dermatomyositis associated interstitial lung disease. Cytokine. 2020;135:155209 (In eng). [DOI] [PubMed] [Google Scholar]
- 58. Shanshan L, Yamei Z, Ling Z, Xin L, Guochun W. Progranulin correlated with rapid progressive interstitial lung disease in dermatomyositis with anti‐melanoma differentiation‐associated gene 5 antibody. Clin Rheumatol. 2022;41:757‐763. (In eng). [DOI] [PubMed] [Google Scholar]
- 59. Ishikawa N, Hattori N, Yokoyama A, Kohno N. Utility of kl‐6/muc1 in the clinical management of interstitial lung diseases. Respir Investig. 2012;50:3‐13. (In eng). [DOI] [PubMed] [Google Scholar]
- 60. Zhang T, Shen P, Duan C, Gao L. Kl‐6 as an immunological biomarker predicts the severity, progression, acute exacerbation, and poor outcomes of interstitial lung disease: a systematic review and meta‐analysis. Front Immunol. 2021;12:745233 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen F, Lu X, Shu X, Peng Q, Tian X, Wang G. Predictive value of serum markers for the development of interstitial lung disease in patients with polymyositis and dermatomyositis: a comparative and prospective study. Intern Med J. 2015;45:641‐647. (In eng). [DOI] [PubMed] [Google Scholar]
- 62. Ye Y, Fu Q, Wang R, Guo Q, Bao C. Serum kl‐6 level is a prognostic marker in patients with anti‐mda5 antibody‐positive dermatomyositis associated with interstitial lung disease. J Clin Lab Anal. 2019;33:e22978 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gono T, Masui K, Nishina N, et al. Risk prediction modeling based on a combination of initial serum biomarker levels in polymyositis/dermatomyositis‐associated interstitial lung disease. Arthritis Rheumatol. 2021;73:677‐686. (In eng). [DOI] [PubMed] [Google Scholar]
- 64. Zhu Y, Wang L, Sun Y, et al. Serum krebs von den lungen‐6 concentrations reflect severity of anti‐melanoma differentiation‐associated protein 5 antibody positive dermatomyositis associated interstitial lung disease. Clin Exp Rheumatol. 2022;40:292‐297. (In eng). [DOI] [PubMed] [Google Scholar]
- 65. Shirakashi M, Nakashima R, Tsuji H, et al. Efficacy of plasma exchange in anti‐mda5‐positive dermatomyositis with interstitial lung disease under combined immunosuppressive treatment. Rheumatology (Oxford). 2020;59:3284‐3292. (In eng). [DOI] [PubMed] [Google Scholar]
- 66. Chiba H, Otsuka M, Takahashi H. Significance of molecular biomarkers in idiopathic pulmonary fibrosis: a mini review. Respir Investig. 2018;56:384‐391. (In eng). [DOI] [PubMed] [Google Scholar]
- 67. Mimori T, Nakashima R, Hosono Y. Interstitial lung disease in myositis: clinical subsets, biomarkers, and treatment. Curr Rheumatol Rep. 2012;14:264‐274. (In eng). [DOI] [PubMed] [Google Scholar]
- 68. Ihn H, Asano Y, Kubo M, et al. Clinical significance of serum surfactant protein d (sp‐d) in patients with polymyositis/dermatomyositis: correlation with interstitial lung disease. Rheumatology (Oxford). 2002;41:1268‐1272. (In eng). [DOI] [PubMed] [Google Scholar]
- 69. Arai S, Kurasawa K, Maezawa R, Owada T, Okada H, Fukuda T. Marked increase in serum kl‐6 and surfactant protein d levels during the first 4 weeks after treatment predicts poor prognosis in patients with active interstitial pneumonia associated with polymyositis/dermatomyositis. Mod Rheumatol. 2013;23:872‐883. (In eng). [DOI] [PubMed] [Google Scholar]
- 70. Sato S, Masui K, Nishina N, et al. Initial predictors of poor survival in myositis‐associated interstitial lung disease: a multicentre cohort of 497 patients. Rheumatology (Oxford). 2018;57:1212‐1221. (In eng). [DOI] [PubMed] [Google Scholar]
- 71. Kaieda S, Gono T, Masui K, Nishina N, Sato S, Kuwana M. Evaluation of usefulness in surfactant protein d as a predictor of mortality in myositis‐associated interstitial lung disease. PLoS ONE. 2020;15:e0234523 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nishikiori H, Chiba H, Ariki S, et al. Distinct compartmentalization of sp‐a and sp‐d in the vasculature and lungs of patients with idiopathic pulmonary fibrosis. BMC Pulm Med. 2014;14:196 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Matsushita T, Kobayashi T, Kano M, Hamaguchi Y, Takehara K. Elevated serum b‐cell activating factor levels in patients with dermatomyositis: association with interstitial lung disease. J Dermatol. 2019;46:1190‐1196. (In eng). [DOI] [PubMed] [Google Scholar]
- 74. Gono T, Kaneko H, Kawaguchi Y, et al. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology (Oxford). 2014;53:2196‐2203. (In eng). [DOI] [PubMed] [Google Scholar]
- 75. Bai J, Wu C, Zhong D, Xu D, Wang Q, Zeng X. Hierarchical cluster analysis of cytokine profiles reveals a cutaneous vasculitis‐associated subgroup in dermatomyositis. Clin Rheumatol. 2021;40:999‐1008. (In eng). [DOI] [PubMed] [Google Scholar]
- 76. Horai Y, Koga T, Fujikawa K, et al. Serum interferon‐α is a useful biomarker in patients with anti‐melanoma differentiation‐associated gene 5 (mda5) antibody‐positive dermatomyositis. Mod Rheumatol. 2015;25:85‐89. (In eng). [DOI] [PubMed] [Google Scholar]
- 77. Takada T, Ohashi K, Hayashi M, et al. Role of il‐15 in interstitial lung diseases in amyopathic dermatomyositis with anti‐mda‐5 antibody. Respir Med. 2018;141:7‐13. (In eng). [DOI] [PubMed] [Google Scholar]
- 78. Liang L, Zhang YM, Shen YW, et al. Aberrantly expressed galectin‐9 is involved in the immunopathogenesis of anti‐mda5‐positive dermatomyositis‐associated interstitial lung disease. Front Cell Dev Biol. 2021;9:628128 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Oldroyd AGS, Allard AB, Callen JP, et al. A systematic review and meta‐analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:2615‐2628. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang Q, Gao C, Zhang C, et al. Tumor markers are associated with rapidly progressive interstitial lung disease in adult‐dermatomyositis. Clin Rheumatol. 2022;41:1731‐1739. (In eng). [DOI] [PubMed] [Google Scholar]
- 81. Li Y, Gao X, Li Y, et al. Predictors and mortality of rapidly progressive interstitial lung disease in patients with idiopathic inflammatory myopathy: a series of 474 patients. Front Med. 2020;7:363 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhu D, Qiao J, Tang S, et al. Elevated carcinoembryonic antigen predicts rapidly progressive interstitial lung disease in clinically amyopathic dermatomyositis. Rheumatology (Oxford). 2021;60:3896‐3903. (In eng). [DOI] [PubMed] [Google Scholar]
- 83. Maher TM, Oballa E, Simpson JK, et al. An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre profile cohort study. Lancet Respir Med. 2017;5:946‐955. (In eng). [DOI] [PubMed] [Google Scholar]
- 84. Nakayama M, Satoh H, Ishikawa H, et al. Cytokeratin 19 fragment in patients with nonmalignant respiratory diseases. Chest. 2003;123:2001‐2006. (In eng). [DOI] [PubMed] [Google Scholar]
- 85. Zuo Y, Ye L, Liu M, et al. Clinical significance of radiological patterns of hrct and their association with macrophage activation in dermatomyositis. Rheumatology (Oxford). 2020;59:2829‐2837. (In eng). [DOI] [PubMed] [Google Scholar]
- 86. Tseng CW, Wang KL, Fu PK, et al. Gap score and ca‐153 associated with one‐year mortality in anti‐mda‐5 antibody‐positive patients: a real‐world experience. J Clin Med. 2021;10(22):5241. (In eng). 10.3390/jcm10225241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gui X, Ma M, Ding J, et al. Cytokeratin 19 fragment is associated with severity and poor prognosis of interstitial lung disease in anti‐mda5 antibody‐positive dermatomyositis. Rheumatology (Oxford). 2021;60:3913‐3922. (In eng). [DOI] [PubMed] [Google Scholar]
- 88. Jin YZ, Xie MS, Yang C, Wu RL, Zhou YB, Li XM. Prognostic value of peripheral blood markers in patients with myositis‐associated interstitial lung diseases. Scand J Rheumatol. 2021;50:218‐226. (In eng). [DOI] [PubMed] [Google Scholar]
- 89. Bae SS, Lee YY, Shahbazian A, et al. High‐ density lipoprotein function is abnormal in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2020;59:3515‐3525. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Xu YT, Zhang YM, Yang HX, et al. Evaluation and validation of the prognostic value of anti‐mda5 igg subclasses in dermatomyositis‐associated interstitial lung disease. Rheumatology (Oxford, England). 2022; (In eng). 10.1093/rheumatology/keac229. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 91. Wu S, Tang X, Wu L, Lu LJ, Feng X. Association of anti‐ro52 autoantibodies with interstitial lung disease in connective tissue diseases. Ann Rheum Dis. 2021;80:e151 (In eng). [DOI] [PubMed] [Google Scholar]
- 92. Xing X, Li A, Li C. Anti‐ro52 antibody is an independent risk factor for interstitial lung disease in dermatomyositis. Respir Med. 2020;172:106134 (In eng). [DOI] [PubMed] [Google Scholar]
- 93. Gui X, Shenyun S, Ding H, et al. Anti‐ro52 antibodies are associated with the prognosis of adult idiopathic inflammatory myopathy‐associated interstitial lung disease. Rheumatology (Oxford, England). 2022; (In eng). 10.1093/rheumatology/keac090. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 94. Xu A, Ye Y, Fu Q, et al. Prognostic values of anti‐ro52 antibodies in anti‐mda5‐positive clinically amyopathic dermatomyositis associated with interstitial lung disease. Rheumatology (Oxford). 2021;60:3343‐3351. (In eng). [DOI] [PubMed] [Google Scholar]
- 95. Arase N, Tsuji H, Takamatsu H, et al. Cell surface‐expressed ro52/igg/hla‐dr complex is targeted by autoantibodies in patients with inflammatory myopathies. J Autoimmun. 2022;126:102774 (In eng). [DOI] [PubMed] [Google Scholar]
- 96. Zhong D, Wu C, Xu D, Bai J, Wang Q, Zeng X. Plasma‐derived exosomal hsa‐mir‐4488 and hsa‐mir‐1228‐5p: Novel biomarkers for dermatomyositis‐associated interstitial lung disease with anti‐melanoma differentiation‐associated protein 5 antibody‐positive subset. Biomed Res Int. 2021;2021:6676107 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Peng Y, Zhang S, Zhao Y, Liu Y, Yan B. Neutrophil extracellular traps may contribute to interstitial lung disease associated with anti‐mda5 autoantibody positive dermatomyositis. Clin Rheumatol. 2018;37:107‐115. (In eng). [DOI] [PubMed] [Google Scholar]
- 98. Seto N, Torres‐Ruiz JJ, Carmona‐Rivera C, et al. Neutrophil dysregulation is pathogenic in idiopathic inflammatory myopathies. JCI Insight. 2020;5(3):e134189 (In eng). 10.1172/jci.insight.134189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chen F, Wang D, Shu X, Nakashima R, Wang G. Anti‐mda5 antibody is associated with a/sip and decreased t cells in peripheral blood and predicts poor prognosis of ild in chinese patients with dermatomyositis. Rheumatol Int. 2012;32:3909‐3915. (In eng). [DOI] [PubMed] [Google Scholar]
- 100. Zhou M, Li M, Guo C, et al. Circulating tfh cells is correlated with disease activity in anti‐mda5 antibody positive idiopathic inflammatory myopathies. Clin Exp Rheumatol. 2021;39:804‐810. (In eng). [PubMed] [Google Scholar]
- 101. Lv X, Jin Y, Zhang D, et al. Low circulating monocytes is in parallel with lymphopenia which predicts poor outcome in anti‐melanoma differentiation‐associated gene 5 antibody‐positive dermatomyositis‐associated interstitial lung disease. Front Med. 2021;8:808875 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wang Y, Zhu L, Ju B, et al. Alterations of peripheral blood b cell subsets in chinese patients with adult idiopathic inflammatory myopathies. Clin Exp Rheumatol. 2022;40:260‐266. (In eng). [DOI] [PubMed] [Google Scholar]
- 103. Zhou J, Huang W, Ren F, Luo L, Huang D, Tang L. Evaluation of prognostic factors in anti‐mda5 antibody‐positive patients in chongqing, China: a retrospective study. Int J Gen Med. 2021;14:4775‐4781. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chioma OS, Hesse LE, Chapman A, Drake WP. Role of the microbiome in interstitial lung diseases. Front Med. 2021;8:595522 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhufeng Y, Xu J, Miao M, et al. Modification of intestinal microbiota dysbiosis by low‐dose interleukin‐2 in dermatomyositis: a post hoc analysis from a clinical trial study. Front Cell Infect Microbiol. 2022;12:757099 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


