Abstract
A 60‐year‐old woman underwent polycaprolactone‐based filler injection in her midface. Four months later, she developed progressive swelling of midface after a self‐limiting viral gastroenteritis. The diagnosis was “delayed‐onset immune‐mediated nodule formation triggered by a virus.” Ultrasonography enabled us to efficiently treat her with a tailored intralesional corticosteroid injection method.
Keywords: allergy and immunology; dermatology; ear, nose and throat; general surgery; pharmacology
Short abstract
Evidence supports the safety of polycaprolactone (PCL)‐based fillers. However, we have faced PCL‐related delayed‐onset nodules triggered by viruses. Such nodules can be efficiently treated by ultrasound‐assisted intralesional corticosteroid injection.
1. INTRODUCTION
Practice of esthetic medicine has a long history but recent introduction of various types of medical products—especially injectable fillers—and their approval by authorized regulatory agencies, has flourished this field. 1 , 2 Hyaluronic acid (HA)‐based fillers are main choices in esthetic field that have been widely used for years. They are safe, biocompatible, and can be easily resolved by hyaluronidase. 1 , 3 , 4 , 5 Polycaprolactone (PCL)‐based fillers are a new generation that have been introduced in 2000 s. 2 , 6 This group of fillers are collagen stimulators and have shown acceptable safety and satisfactory immediate and long‐term results in limited available studies. 2 , 6 , 7 PCLs are biodegradable and have been well‐received because of the perception and claims of their long‐lasting effects but their main drawback is that they do not respond to hyaluronidase and may need invasive removal procedures. 2 , 7
In general, perceived safety and reversibility of fillers as well as their ease of application by minimally invasive procedures have made these products popular among clients and physicians. 4 , 8 However, like any other medical practice, esthetic procedures are not free of complications and with the increasing prevalence of the use of injectable fillers, we expect to see related complications more than ever. 4 , 5 , 8 These complications may be due to natural body responses to the filler's components, technical injection issues, using inappropriate products or some unexpected events (like infection or migration) and could happen early or even years after the procedure. 3 , 4
Various imaging modalities including plain X‐ray, CT scan, MRI, PET scan, and ultrasonography (USG) have been proposed for the diagnosis of filler‐related complications. 9 To get the best and safest results in esthetic medicine, many practitioners have recently been utilizing ultrasonography (USG) in their daily practice which is a dramatic advancement in this field. Using USG with Doppler properties enables esthetic physicians to safely inject in appropriate tissue layers, minimizes vascular and other adverse events and facilitates diagnosis and treatment of complications. 3 , 10 , 11
In this report, we present a late PCL filler‐related complication (nodule formation triggered by viral infection) which could not be easily treated without the use of USG. This case underscores the indispensable role of USG in esthetic treatments and management of filler complications.
2. CASE DESCRIPTION
A 60‐year‐old woman was referred to our clinic with chief complaint of progressive and expanding swelling and pain in cheeks and under eyes (around the sites of previously injected filler). According to the history of previous injection plan obtained from her former esthetic physician, a total amount of 6 cc PCL filler had been placed in her “zygomatic arch and eminence,” “medial and lateral sub‐orbicularis oculi fat (SOOF),” “superficial malar fat pad,” and “nasolabial fold” bilaterally, 6 months prior to our visit. 27G needle was used for zygomatic region injections and other parts were injected with a 25G cannula. In past medical history, she reported hypertension, hyperlipidemia, and pre‐diabetic condition and there was no recent history of COVID‐19 or its immunization. No trauma, dental and sinus problems, and autoimmune diseases were reported. The trigger of her swelling was a simple and self‐limited viral infection (gastroenteritis), 4 months after filler injection. The swelling had been progressively continuing even 2 months after recovery of virus so that reached periorbital areas and caused pain, and discomfort.
In her inspection, deformity and diffuse edema of face and malar edema were observed. We found multiple palpable firm and tender nodules with the biggest diameters of 70 × 50 and 60 × 40 millimeters (mm) in right and left midface areas, respectively. In physical examination, we did not detect local fluctuation and/or abscess or any sign indicative of systemic infection. Based on past medical history and patient's condition, our diagnosis was “delayed‐onset nodules, triggered by a viral infection”. We began the treatment with local injection of triamcinolone (1 mg/cm2) in nodules by canalization and injection method using a 70 mm 18G cannula and under direct observation. This treatment is accepted for late‐onset immune‐mediated nodules. 2 , 8 Due to patient's medical background and involvement of T lymphocytes in delayed type hypersensitivity reactions, neither systemic corticosteroid nor antihistamine were prescribed. 8 , 12
Unfortunately, our treatment at first step was not successful and 15 days later, she again presented with persistent symptoms. At her second presentation, we studied her face by handheld 20 MHz ultrasound device and found soft tissue thickness of 20 mm in midface areas and tissue reaction in deep fat pads of preorbital regions (Figure 1). With the assistance of ultrasound, we found out that the cause of failure of our first treatment was deep placement of filler where we did not reach in first local corticosteroid injection. Based on the deep disposition of filler and extent of its spread, we planned for a targeted second step treatment to enable us to reach all involved areas. We drew a grid in her midface (on the nodules) to determine points of injection and ensure complete coverage during treatment (Figure 2). Each square of the grid with dimensions of 1 × 1 cm became one unit for corticosteroid injection (Figure 2). Ultrasound helped us to consider each square as a cube with 20 mm depth and have a 3D perspective of the involved area to plan for deep therapeutic corticosteroid injection. In this regard, we first horizontally canalized the involved areas in each side with a 70 mm 18G cannula and then vertically injected 0.1 cc of diluted 10 mg/cc triamcinolone at two various depths with a 20 mm 27G needle in each unit: first injection was at 20 mm depth and then we dragged back the needle to reach the other point of injection at 10 mm depth. In addition, 1 mg/cm3 of the triamcinolone with same dilution was injected at both infraorbital regions (SOOFs) using 18G cannula under guidance of ultrasound.
FIGURE 1.
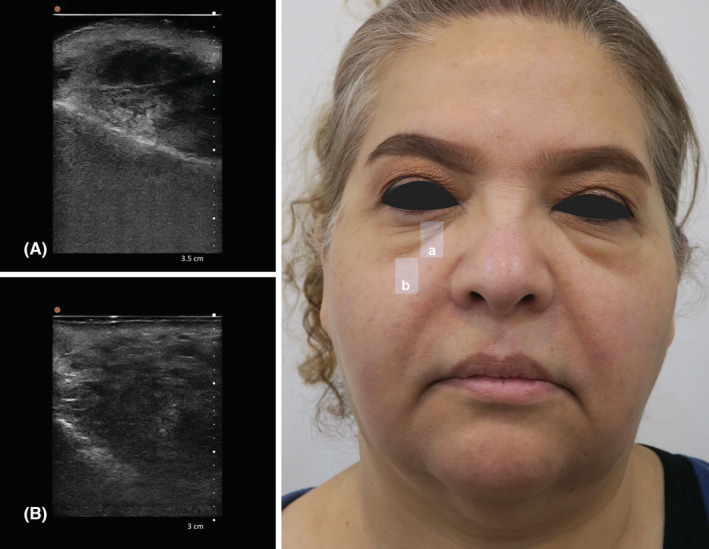
(A) Ultrasonography of the right tear trough, B mode, sagittal view by handheld 20 MHz ultrasound; (B) Ultrasonography of the mid cheek in midpupillary line inferior to mid cheek groove, B mode, sagittal view, by handheld 20 MHz ultrasound. (A) It shows edema in periorbital area and hyper echogenicity in suborbicularis oculi fat (SOOF). (B) A mass about 20 mm in length has filled the whole space between the maxillary bone and skin and multiple bright hyperechoic spots with mini‐comet‐tail artifact in hypoechoic matrix are indicative of polycaprolactone deposits.
FIGURE 2.
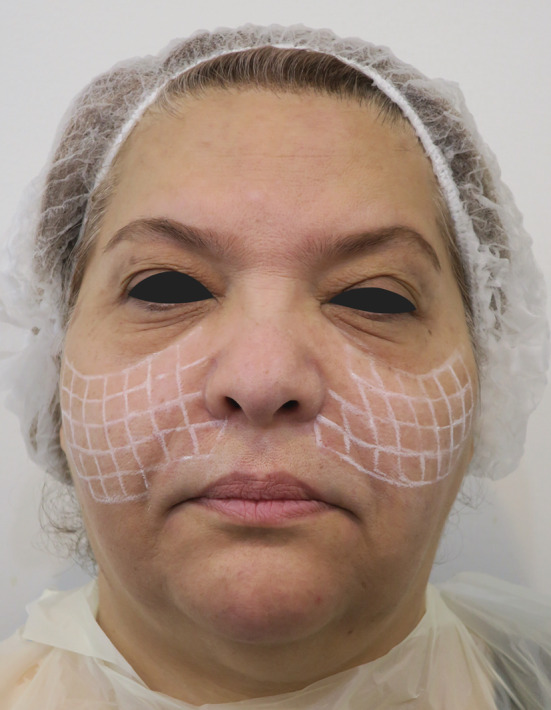
Targeted treatment design for patient to reach all involved areas by nodules and inject corticosteroid efficiently
To achieve the desired dilution of our medication, we mixed 0.5 cc of triamcinolone 40 mg/cc with 0.5 cc lidocaine 2% and 1 cc normal saline solution. 7 Patient was discharged with oral minocycline for 4 weeks and the results after 4 weeks of follow‐up were satisfactory (Figure 3).
FIGURE 3.
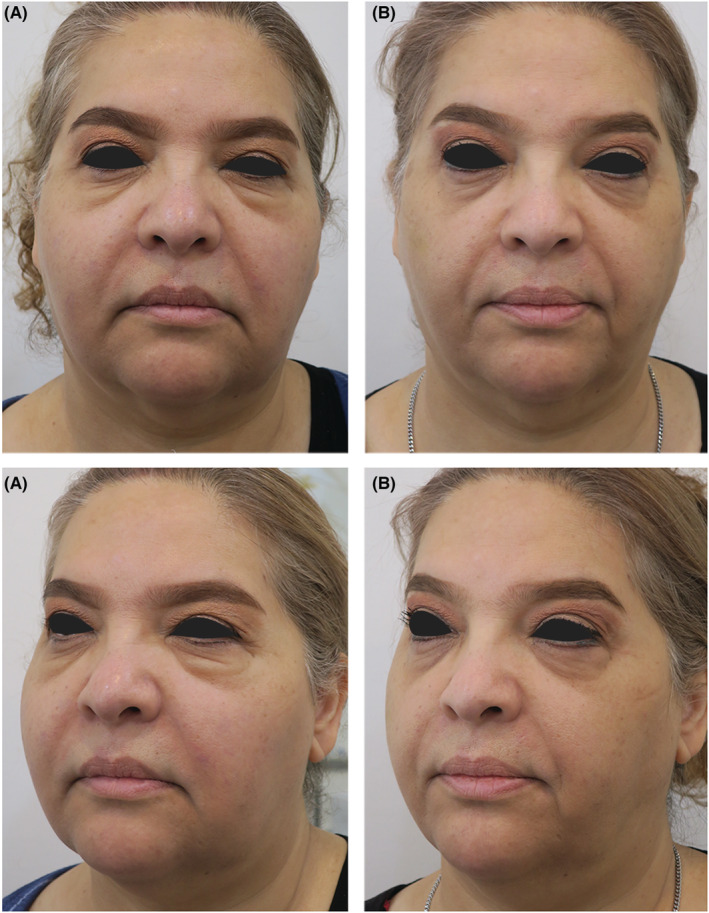
(A) Patient in her first referral to our clinic. (B) 4 weeks after treatment with intralesional corticosteroid injection under guidance of ultrasound
3. DISCUSSION
Expansion and popularity of esthetic medicine means more procedures, involvement of more physicians in this field and consequently, facing more complications and adverse effects (4, 5, 8). While filler‐related complications can be minimized through knowledge of facial anatomy, injection training, and having enough information about dermal filler products, facing these complications seems inevitable even for the best esthetic practitioners. 3 , 4 , 8 , 12 Our knowledge about complications of esthetic procedures is also limited because it is still a new field, and many issues are not well‐known or reported; thus, sharing our experiences adds to the body of evidence and assists other practitioners to diagnose and manage these complications in a timely manner. 4 , 5
Like all medical consultations, our first measures for this patient were thorough history taking and clinical examination. In esthetic field, knowledge about previously done procedures and the type and amount of injected fillers is important to decide for the best management of complications. 8 If we have no clue of the filler type and site, USG can reveal them. 3 , 8 In this case, available data and our findings were in favor of late‐onset hypersensitivity reaction and affirmed the diagnosis of “delayed‐onset nodule formation, triggered by a viral infection.”
Nodule formation can be an early or delayed complication of all fillers and its cause may be infection, hypersensitivity reactions or mixed so that would not be easily distinguished. 4 , 5 , 8 The probability of nodule formation with PCLs has not been reported more than other fillers 2 , 7 but it should be kept in mind that PCLs have been newly launched 2 and we still lack evidence about their probable complications. 6 There are few reports of delayed nodules with HA fillers triggered by viruses or immunization. 4 , 8 Sporadic reports of histologically confirmed hypersensitive delayed‐onset nodules in PCL fillers with unknown underlying causes are available. 6 , 13 Kalantari et al. have recently reported the first case of delayed hypersensitivity reaction of PCL filler to COVID‐19 vaccine 14 and to our knowledge, the present report is the second case of delayed immunologic reaction of PCL‐based fillers to virus exposure.
Regardless of the cause, the definite treatment for filler‐related delayed hypersensitivity reactions is filler removal. 2 , 5 , 8 , 12 For HA fillers we can choose removal as the first treatment by using hyaluronidase. However, removal of non‐HA fillers needs invasive or surgical procedures that is not always accepted and/or applicable. Considering the site and extent of the involvement in the current case, the surgical filler removal could have resulted in significant disfiguration and scars. Besides cosmetic issues, facial surgeries in the mid‐face can result in temporary or even permanent palsies of facial nerve branches. 15 A recent systematic review of the anatomical variations of facial nerve distribution patterns in the mid‐face revealed that more than 50% of the population lack adequate anastomosis between the facial nerve branches in this region. Findings of this study show considerable risk of mid‐face post‐operative nervous complications. 15 Thus, it is safer to regard surgery as the last treatment in such cases. 2
Recommended first line treatment for PCL‐related delayed‐onset immunologic nodules is intralesional corticosteroid (may be combined with 5FU for better response) with or without oral corticosteroid. This local treatment may regularly continue until the filler absorbs gradually and symptoms subside. 2 , 5 , 8 Due to our patient's underlying conditions, prescription of systemic corticosteroid was risky; so, intralesional treatment was chosen. The reason for failure of our first step treatment was deep deposition of filler that was found out by USG on the patient's second visit. If USG had not been used, she may have undergone repeated ineffective superficial intralesional injections or even surgical removal would have been chosen. 5 , 8 Malar edema was another problem in this case. It is a complication of under‐eye filler injections and may be due to deposition of filler superficial to malar septum or direct pressure on lymphatic system that disturbs lymphatic drainage. 5 , 16 As mentioned, we also treated her malar edema by local corticosteroid injection at infraorbital regions under guidance of ultrasound. Prescription of minocycline in this patient was for coverage of probable bacterial agents that may have a role in delayed‐onset nodules 5 and its anti‐inflammatory properties as well. 17
Based on our knowledge, this article is the second report of delayed‐onset nodule formation in a PCL‐based filler triggered by virus exposure. As PCL‐based fillers are new, probability of this phenomenon should be addressed by the clinicians in future studies. Ultrasound application by providing a 3D perspective through adding the third axis to the 2D x‐y axes of the surface gives a more comprehensive anatomical insight of the site to the practitioners. Using this feature guided us in doing therapeutic injections and resulted in the most effective treatment for this patient. We also tried to design an innovative and effective tailored treatment method as the second step: determining therapeutic units with assistance of USG enabled us to spread medication to all affected areas with the least injection points in a short time. Discussion about the primary rejuvenation esthetic procedures is not the aim of this report but it is worth mentioning that every person is unique and for correction of facial imperfections, one method does not fit all. Tailored cosmetic treatment based on the analysis of client's characteristics and imperfections, contributes to the most efficient and natural results, and minimizes complications.
4. CONCLUSION
Combination of comprehensive physical examination, patient's past medical history and USG provides a great package for the treatment of filler‐related complications. All esthetic procedures—for cosmetic issues, rejuvenation, and treatment of complications—should be targeted based on patient's condition and characteristics. USG enables the practitioners to have a thorough anatomical perspective of the site of action and tailor procedures to each client to minimize complications or treat them efficiently. Another important point is that no product is risk‐free and esthetic physicians should always expect undetermined complications of products—especially newly launched ones—and be ready to manage them.
AUTHOR CONTRIBUTIONS
Pantea Shekarriz: Conceptualization; data curation; methodology; project administration; supervision; validation; writing – review and editing. Pardis Shojaee: Conceptualization; investigation; methodology; validation; visualization; writing – original draft; writing – review and editing.
CONFLICT OF INTEREST
None.
CONSENT
Written informed consent was obtained from the patient for publishing this paper.
ACKNOWLEDGMENTS
None.
Shekarriz P, Shojaee P. Ultrasound‐assisted management of filler‐related complications: Report of a successful treatment of delayed‐onset nodules related to polycaprolactone‐based filler. Clin Case Rep. 2022;10:e06646. doi: 10.1002/ccr3.6646
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the Figures 1, 2, 3 of the manuscript.
REFERENCES
- 1. Kontis TC, Rivkin A. The history of injectable facial fillers. Facial Plast Surg. 2009;25(2):67‐72. [DOI] [PubMed] [Google Scholar]
- 2. Christen MO, Vercesi F. Polycaprolactone: or how a well‐known and futuristic polymer has become an innovative collagen‐stimulator in esthetics. Clin Cosmet Investig Dermatol. 2020;13:31‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schelke LW, Decates TS, Velthuis PJ. Ultrasound to improve the safety of hyaluronic acid filler treatments. J Cosmet Dermatol. 2018;17(6):1019‐1024. [DOI] [PubMed] [Google Scholar]
- 4. Fitzgerald R, Bertucci V, Sykes JM, Duplechain JK. Adverse reactions to injectable fillers. Facial Plast Surg. 2016;32(5):532‐555. [DOI] [PubMed] [Google Scholar]
- 5. Urdiales‐Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42(2):498‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skrzypek E, Górnicka B, Skrzypek DM, Krzysztof MR. Granuloma as a complication of polycaprolactone‐based dermal filler injection: ultrasound and histopathology studies. J Cosmet Laser Ther. 2019;21(2):65‐68. [DOI] [PubMed] [Google Scholar]
- 7. Lin S‐L, Christen M‐O. Polycaprolactone‐based dermal filler complications: a retrospective study of 1111 treatments. J Cosmet Dermatol. 2020;19(8):1907‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones DH, Fitzgerald R, Cox SE, et al. Preventing and treating adverse events of injectable fillers: evidence‐based recommendations from the American Society for Dermatologic Surgery Multidisciplinary Task Force. Dermatol Surg. 2021;47(2):214‐226. [DOI] [PubMed] [Google Scholar]
- 9. Haneke E. Managing complications of fillers: rare and not‐so‐rare. J Cutan Aesthet Surg. 2015;8(4):198‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jaguś D, Skrzypek E, Migda B, Woźniak W, Mlosek RK. Usefulness of doppler sonography in aesthetic medicine. J Ultrason. 2021;20(83):e268‐e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polańska A, Dańczak‐Pazdrowska A, Jałowska M, Żaba R, Adamski Z. Current applications of high‐frequency ultrasonography in dermatology. Postepy Dermatol Alergol. 2017;34(6):535‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vedamurthy M. Beware what you inject: complications of injectables‐dermal fillers. J Cutan Aesthet Surg. 2018;11(2):60‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiang CH, Peng JH, Peng HP. Filler‐induced granuloma from polycaprolactone‐based collagen stimulator injection in the tear trough area: a case report. J Cosmet Dermatol. 2021;20(5):1529‐1531. [DOI] [PubMed] [Google Scholar]
- 14. Kalantari Y, Sadeghzadeh‐Bazargan A, Aryanian Z, Hatami P, Goodarzi A. First reported case of delayed‐type hypersensitivity reaction to non‐hyaluronic acid polycaprolactone dermal filler following COVID‐19 vaccination: a case report and a review of the literature. Clin Case Rep. 2022;10(2):e05343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poutoglidis A, Paraskevas GK, Lazaridis N, et al. Extratemporal facial nerve branching patterns: systematic review of 1497 cases. Laryngol Otol. 2022;26:1‐7. [DOI] [PubMed] [Google Scholar]
- 16. Anido J, Fernández JM, Genol I, Ribé N, Pérez SG. Recommendations for the treatment of tear trough deformity with cross‐linked hyaluronic acid filler. J Cosmet Dermatol. 2021;20(1):6‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garrido‐Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169(2):337‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in the Figures 1, 2, 3 of the manuscript.


