Abstract
Background
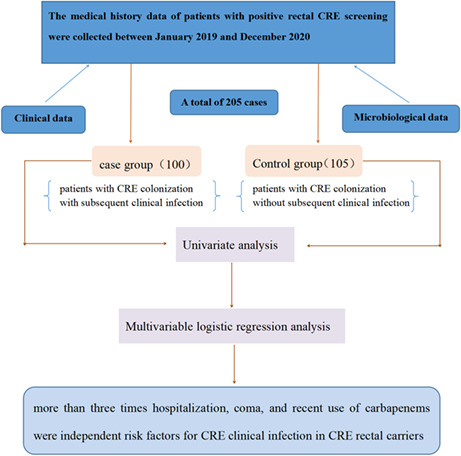
We aimed to identify the risk factors for subsequent carbapenem‐resistant Enterobacterales (CRE) infections in patients with initial rectal colonization with CRE.
Methods
We conducted a retrospective case–control study on inpatients with rectal CRE colonization between January 2019 and December 2020. Clinical and microbiological data were extracted from hospital patients' medical records and the clinical microbiology laboratory. Risk factors were assessed and compared between patients with CRE colonization who had subsequent infections and those who did not have infections.
Results
Among 1064 patients screened for CRE, we enrolled 205 patients with rectal CRE colonization. Among the 205 colonized bacteria, 78.5% were Klebsiella pneumoniae, with 62.9% of them producing Klebsiella pneumoniae carbapenemase (KPC). Multivariate logistic regression analysis revealed that more than three times hospitalization (p = 0.026), being in a coma (p = 0.019), and exposure to carbapenems (p = 0.015) were independent risk factors for CRE clinical infection among CRE rectal carriers.
Conclusion
This is the first study to report that more than three times hospitalization is an independent risk factor for subsequent CRE clinical infection in CRE intestinal carriers. Carbapenem‐resistant Klebsiella pneumoniae is the most important species isolated from hospitalized CRE rectal carriers and is the most common cause of subsequent infections.
Keywords: carbapenem‐resistant Enterobacterales, rectal colonization, risk factors, subsequent infection
To analyze the risk factors for subsequential clinical infection in hospitalized patients with rectal carbapenem‐resistant Enterobacterales (CRE) carriage and to provide reference for clinical prevention CRE infections. Univariate analysis was conducted to detect the risk factors of subsequent infection. Through multivariable logistic regression analysis of these factors, it was found that more than three times hospitalization, coma, and recent use of carbapenems were independent risk factors for CRE clinical infection in CRE rectal carriers.
1. INTRODUCTION
In the past decade, carbapenem‐resistant Enterobacterales (CRE) has become a public health concern worldwide. 1 CRE is highly resistant to most available antimicrobial agents; moreover, they are associated with high morbidity and costs. 2 CRE infection is associated with a higher 30‐day mortality rate than other infections (63.8% vs. 33.4%). 3 Rectal CRE carriers considerably contribute to the transmission of these microorganisms in hospital settings, 4 with the rate of CRE infections among asymptomatic CRE carriers ranging from 0.3% to 69.5%. 5 , 6 , 7 , 8 CRE colonization is an independent risk factor for CRE infections, with intensive care unit (ICU) patients with CRE colonization having at least a twofold increased risk of CRE infection. 9 CRE infections are more among patients with previous CRE colonization (65%) than in those without (27%, p < 0.0001). 10 Approximately 50% of ICU patients with intestinal CRE colonization from a hospital in Northern Manhattan developed CRE infection within 30 days, with the CRE infection rate being 10.8 times higher than that in non‐colonized patients. 10 Other risk factors for CRE include exposure to certain antimicrobials (fluoroquinolones and cephalosporins) and invasive procedures involving a scope device. 11 We previously reported that 8.5% of hospitalized patients had rectal CRE colonization, with the dominant clone strain being Klebsiella pneumoniae carbapenemase (KPC)‐producing Klebsiella pneumoniae ST11. 12 Among rectal carriers of the carbapenem‐resistant Klebsiella pneumoniae (CRKP), 37.1% of the cases developed subsequent CRKP clinical infection. 13 Colonization is considered a prerequisite for infection; however, the extent to which colonized patients develop CRE infections remains unclear. Moreover, there is insufficient clinical evidence regarding the risk factors for subsequent CRE infections in patients with rectal CRE colonization. 5 , 6 , 7 , 8 Sufficient clinical evidence could inform decision‐making regarding infection‐control interventions, including screening and contact precautions, for colonized patients. Therefore, we aimed to establish risk factors for subsequent clinical CRE infections among CRE rectal carriers to identify high‐risk inpatients and prevent CRE infections.
2. MATERIALS AND METHODS
2.1. Study design and definitions
This was a single‐center, cross‐sectional, retrospective study performed at Xiangya hospital of Central South University in China, which is a 3500‐bed teaching hospital with an annual admission of >130,000 inpatients. Hospitalized patients who were admitted to intensive care units and hematologic department with stool samples submitted for routine analysis were screened for CRE. We enrolled cases with positive CRE screening results and without prior CRE infections from January 2019 to December 2020. Previous colonization defined as CRE rectal colonization prior to clinical infection. The CRE case group (patients with subsequent infection) was defined as CRE rectal carriers who developed subsequential CRE infections with identical species as colonized CRE after 48 h of positive screening test. 14 , 15 The isolation of CRE strains before or within 48 h after admission was excluded. Cases without complete medical records were also excluded. The control group comprised CRE rectal carriers without subsequent CRE infections.
Carbapenem‐resistant Enterobacterales infections included bloodstream infection, pneumonia, peritonitis, wound infection, and urinary tract infection. Moreover, subsequent CRE infection was defined as CRE isolated in relevant infection sites and presenting signs and symptoms. 16
2.2. Data collection
We collected the following clinical data from electronic medical records of patients with CRE colonization: general information (sex, age, department, duration of hospital stay, previous hospitalization, alcohol drinking history, smoking history, hospital transfer and sickbed change, and ICU admission), underlying conditions (hypertension, heart disease, diabetes mellitus, hematopathy, lung disease, renal disease, liver disease, pancreatitis, enteritis, gastritis, craniocerebral trauma, and solid tumor), invasive procedures and devices (arterial catheter, central venous catheter, endotracheal intubation, tracheotomy, mechanical ventilation, urinary catheter and nasogastric tube, and previous surgery in 1 and 3 months), coma condition (the Glasgow Coma Scale score ≤8), 17 antibiotic exposure (penicillin, cephalosporins, carbapenems, aminoglycosides, fluoroquinolones, β‐lactam/β‐lactamase inhibitors, vancomycin, tigecycline, metronidazole, and anti‐fungal agents).
2.3. Microbiological procedure and resistance gene detection
Stool samples of hospitalized patients were screened for CRE using MacConkey agar and 10 μg meropenem discs as aforementioned. 12 All isolated bacteria were identified using MALDI Biotype systems (Bruker). The sensitivity of imipenem and meropenem was detected using the Kirby‐Bauer method. Escherichia coli strain ATCC 25922 was used for quality control. The results were interpreted using the Clinical and Laboratory Standards Institute M100 breakpoints. 18 Polymerase chain reaction (PCR) was performed as previously described, 12 targeting the genes encoding bla KPC, bla NDM, bla VIM, bla IMP, and bla OXA‐48 to confirm carbapenemase.
2.4. Statistical analysis
Statistical analysis was performed using SPSS version 22.0 software (IBM Corporation). The normality of data distribution was analyzed using the Shapiro–Wilk test. Numerical values were expressed as mean ± SD (for normal distribution) or as the median and percentiles (for non‐normally distribution). Between‐group comparisons of continuous variables were performed using Student's t test (for normal distribution) or the Wilcoxon rank‐sum test (for non‐normally distribution). Categorical variables were compared using the chi‐square test or Fisher's exact test. We calculated the p value, odds ratio (OR) value, and 95% confidence intervals (CI) of the variables. Variables with a two‐tailed p value ≤0.05 in the univariate analysis were included in the logistic regression model for the multivariate analysis. Statistical significance was set at p ≤ 0.05. The independent risk factors were checked for multicollinearity.
3. RESULTS
3.1. Patient cohort and clinical characteristics
Among 1064 patients screened for CRE, we enrolled 205 patients with rectal CRE colonization. The cases of rectal CRE colonization were mainly distributed in the central ICU (66%, 32.2%), respiratory ICU (51%, 24.9%), and hematology department (26%, 12.7%). Overall, 100 (48.8%) carriers who developed subsequent CRE clinical infections were included in the case group. The main clinical infection was pneumonia (61.0%), followed by bloodstream (26.0%), urinary tract infection (10.0%), and wound infections (3.0%). The other 105 CRE carriers without subsequent CRE infections were included in the control group.
3.2. Microbial characteristics
Table 1 shows microbial characteristics. Among 205 colonized bacteria, 161 (78.5%), 24 (11.7%), 8 (3.9%), 5 (2.4%), 3 (1.5%), 3 (1.5%), and 1 (0.5%) were K. pneumoniae, E. coli, Enterobacter cloacae, Citrobacter freundii, Klebsiella aerogenes, Klebsiella oxytoca, and Raoultella planticola, respectively. K. pneumoniae was the most important species among hospitalized rectal CRE carriers and was the most common cause of subsequent infections. Escherichia coli was more common in the case group than in the control group (17.0% vs 6.7%; p = 0.021).
TABLE 1.
Species distribution of positive rectal carbapenem‐resistant Enterobacteriaceae screening cases
| Species | Case group (n = 100, %) | Control group (n = 105, %) | Total (n = 205, %) | p |
|---|---|---|---|---|
| Klebsiella pneumoniae | 76 (76.0) | 85 (81.0) | 161 (78.5) | 0.388 |
| Escherichia coli | 17 (17.0) | 7 (6.7) | 24 (11.7) | 0.021* |
| Enterobacter cloacae | 2 (2.0) | 6 (5.7) | 8 (3.9) | 0.312 |
| Citrobacter freundii | 1 (1.0) | 4 (3.8) | 5 (2.4) | 0.395 |
| Klebsiella aerogenes | 1 (1.0) | 2 (1.9) | 3 (1.5) | 1.000 |
| Raoultella planticola | 0 (0.0) | 1 (1.0) | 1 (0.5) | 1.000 |
| Klebsiella oxytoca | 3 (3.0) | 0 (0.0) | 3 (1.5) | 0.228 |
p < 0.05.
All rectal CRE isolates were carbapenemase‐producing Enterobacterales, 129 (62.9%), 44 (21.5%), 24 (11.7%), and 5 (2.4%) produced KPC, New Delhi metallo‐beta‐lactamase, verona Integron‐encoded MBL, and imipenemase. Moreover, three isolates carried two types of carbapenemase genes (bla NDM and bla VIM in two isolates as well as bla KPC and bla NDM in one isolate).
3.3. Risk factors analysis
The length of hospital stay was significantly longer in the case group than in the control group (interquartile range, 27 vs. 21 days, p = 0.020). A total of 43 patients had been previously hospitalized more than three times, including 28 (28.0%) and 15 (14.3%) in the case and control groups, respectively, with a significant between‐group difference (p = 0.016). Univariate analysis revealed that compared with patients in the control group, those in the case group were more likely to have undergone central venous catheter insertion (62.0% vs. 42.9%, p = 0.006), tracheotomy (34.0% vs. 18.1%, p = 0.009), nasogastric tube insertion (68.0% vs. 50.5%, p = 0.011); been in a coma condition (21.0% vs. 7.6%, p = 0.006); and received carbapenems (72.0% vs. 54.3%, p = 0.009) and amikacin (13.0% vs. 3.8%, p = 0.017). There was no statistically significant difference in sex, age, history of smoking or alcohol use, history of surgery, and ICU admission. Furthermore, there was no statistically significant difference in most comorbidities, including solid tumor, hypertension, diabetes mellitus, hematopathy, and organ transplantation (Table 2).
TABLE 2.
Univariate analysis of risk factors associated with subsequent carbapenem‐resistant Enterobacterales (CRE) clinical infection among CRE carriers
| Variables | Case group (n = 100) | Control group (n = 105) | p |
|---|---|---|---|
| General information | |||
| Male sex, n (%) | 76 (76.0%) | 80 (76.2%) | 0.975 |
| Median age (range), years | 51 (31–66) | 53.5 (41.5–67) | 0.109 |
| Age ≥65 years, n (%) | 37 (37.0%) | 30 (28.6%) | 0.198 |
| Length of stay (days), IQR (range) | 21 (13–34) | 27 (15.25–47) | 0.020* |
| Previous hospitalization, n (%) | 47 (47.0%) | 41 (39.0%) | 0.250 |
| Previous hospitalizations >1, n (%) | 41 (41.0%) | 33 (31.4%) | 0.154 |
| Previous hospitalizations >2, n (%) | 28 (28.0%) | 20 (19.0%) | 0.130 |
| Previous hospitalizations >3, n (%) | 28 (28.0%) | 15 (14.3%) | 0.016* |
| History of smoking, n (%) | 30 (30.0%) | 35 (33.3%) | 0.608 |
| History of alcohol, n (%) | 16 (16.0%) | 17 (16.2%) | 0.970 |
| Comorbid conditions, n (%) | |||
| Solid organ tumor | 10 (10.0%) | 13 (12.4%) | 0.589 |
| Lung disease | 30 (30.0%) | 25 (24.0%) | 0.337 |
| Hypertension | 28 (28.0%) | 25 (23.8%) | 0.493 |
| Heart disease | 12 (12.0%) | 11 (10.5%) | 0.730 |
| Diabetes mellitus | 15 (15.0%) | 18 (17.1%) | 0.676 |
| Hematopathy | 15 (15.0%) | 27 (25.7%) | 0.057 |
| Renal disease | 11 (11.0%) | 6 (5.7%) | 0.170 |
| Liver disease | 14 (14.0%) | 12 (11.4%) | 0.580 |
| Biliary tract disease | 3 (3.0%) | 3 (2.9%) | 1.000 |
| Pancreatitis | 5 (5.0%) | 7 (6.7%) | 0.611 |
| Enteritis | 6 (6.0%) | 2 (1.9%) | 0.249 |
| Being in coma condition (Gcs score ≤8) | 21 (21.0%) | 8 (7.6%) | 0.006* |
| Organ transplantation | 2 (2.0%) | 3 (2.9%) | 0.548 |
| Pyohemia | 3 (3.0%) | 3 (2.9%) | 1.000 |
| Invasive procedures, n (%) | |||
| Central venous catheter | 62 (62.0%) | 45 (42.9%) | 0.006* |
| Arterial catheter | 60 (60.0%) | 54 (51.4%) | 0.217 |
| Endotracheal intubation | 44 (44.0%) | 39 (37.1%) | 0.317 |
| Tracheotomy | 34 (34.0%) | 19 (18.1%) | 0.009* |
| Mechanical ventilation | 50 (50.0%) | 46 (43.8%) | 0.375 |
| Nasogastric tube | 68 (68.0%) | 53 (50.5%) | 0.011* |
| Urinary catheter | 65 (65.0%) | 56 (53.3%) | 0.090 |
| Other clinical parameters, n (%) | |||
| Previous surgery (in a month) | 20 (20.0%) | 22 (21.0%) | 0.866 |
| Artificial prosthesis | 0 (0.0%) | 3 (2.9%) | 0.262 |
| Use proton pump inhibitors | 55 (55.0%) | 49 (46.7%) | 0.233 |
| Using chemotherapeutic drugs | 15 (15.0%) | 14 (13.3%) | 0.732 |
| Admission to ICU, n (%) | 60 (60.0%) | 55 (52.4%) | 0.272 |
| Transfer from another hospital, n (%) | 16 (16.2%) | 13 (12.4%) | 0.440 |
| Antimicrobial exposure, n (%) | |||
| Penicillin | 1 (1.0%) | 5 (4.8%) | 0.237 |
| Cephalosporins | 23 (23.0%) | 18 (17.1%) | 0.295 |
| Carbapenems | 72 (72.0%) | 57 (54.3%) | 0.009* |
| Aztreonam | 3 (3.0%) | 1 (1.0%) | 0.579 |
| Latamoxef | 2 (2.0%) | 2 (1.9%) | 1.000 |
| β‐lactam/β‐lactamase inhibitors | 71 (71.0%) | 65 (61.9%) | 0.168 |
| Vancomycin | 34 (34.0%) | 31 (29.5%) | 0.491 |
| Teicoplanin | 11 (11.0%) | 11 (10.5%) | 0.904 |
| Amikacin | 13 (13.0%) | 4 (3.8%) | 0.017* |
| Gentamicin | 6 (6.0%) | 4 (3.8%) | 0.687 |
| Tobramycin | 0 (0.0%) | 1 (1.0%) | 1.000 |
| Fluoroquinolones | 37 (37.0%) | 38 (36.2%) | 0.904 |
| Linezolid | 23 (23.0%) | 31 (29.5%) | 0.289 |
| Tigecycline | 15 (15.2%) | 10 (9.5%) | 0.221 |
| Colistin | 15 (15.2%) | 9 (8.6%) | 0.145 |
| Tetracycline | 14 (14.0%) | 9 (8.6%) | 0.218 |
| Metronidazole | 7 (7.0%) | 2 (1.9%) | 0.150 |
| Sulfamethoxazole | 4 (4.0%) | 2 (1.9%) | 0.635 |
| Azithromycin | 2 (2.0%) | 4 (3.8%) | 0.723 |
| Antifungal agents | 50 (50.0%) | 47 (44.8%) | 0.453 |
| The use of antibiotics ≥3, n (%) | 69 (69.0%) | 59 (56.2%) | 0.058 |
p < 0.05.
Multivariate logistic regression analysis revealed that more than three times previous hospitalization (OR 2.438, 95% CI 1.115–5.333, p = 0.026), being in a coma condition (OR 3.137, 95% CI 1.203–8.178, p = 0.019), and carbapenems exposure (OR 1.571, 95% CI 0.174–4.488, p = 0.015) were independent risk factors for CRE clinical infection among CRE rectal carriers (Table 3).
TABLE 3.
Multivariable logistic regression for factors associated with subsequent carbapenem‐resistant Enterobacteriaceae infections
| Variables | OR (95% CI) | p |
|---|---|---|
| Length of stay (days) | 1.007 (0.996–1.018) | 0.225 |
| Previous hospitalizations >3 | 2.438 (1.115–5.333) | 0.026* |
| Being in coma condition | 3.137 (1.203–8.178) | 0.019* |
| Central venous catheter | 1.579 (0.830–3.071) | 0.161 |
| Tracheotomy | 1.488 (0.683–3.245) | 0.317 |
| Nasogastric tube | 1.571 (0.799–3.091) | 0.191 |
| Carbapenems exposure | 2.295 (1.174–4.488) | 0.015* |
| Amikacin exposure | 2.758 (0.785–9.686) | 0.113 |
p < 0.05.
In the group of patients with more than three times previous hospitalization, there was no statistically significant difference regarding age, sex, and comorbidities between the patients with subsequent infections and those without (p > 0.05; Table S1).
4. DISCUSSION
Carbapenem‐resistant Enterobacterales colonization is an important risk factor for infection. 9 , 19 We found that CRKP was the most important strain among cases with rectal CRE colonization and clinical infections (78.6% and 76.0%, respectively), which is consistent with the results of other domestic studies. 4 , 5 A previous study showed that CRE isolates in Miami‐Dade County were predominantly K. pneumoniae. 20 Consistent with previous studies, the main carbapenem‐resistant bacteria in bloodstream infections among rectal carriers was K. pneumoniae (73.8% of the cases). 21 A recent study on 702 patients suggested that carriers of carbapenemase‐producing K. pneumoniae may have an increased likelihood of prolonged carriage. 22
A previous Chinese study found that 37.1% of intestinal CRKP carriers developed clinical CRKP infections; additionally, ICU admission was an independent risk factor for subsequent clinical infections among intestinal CRKP carriers. 13 We observed no statistically significant difference in terms of hospitalization in the ICU. This could be attributed to a large proportion of patients being from the ICUs. The proportion of clinical CRE infections in our study is consistent with that reported in a tertiary medical center in Israel (48.8% vs. 47%). 23
Previous exposure to fluoroquinolones and carbapenems is independent risk factors for CRE infection. 5 , 24 We found that prior exposure to carbapenems was an independent risk factor for clinical CRE infection in rectal CRE carriers, which is consistent with previous study. 22 However, prior exposure to fluoroquinolones was not associated with CRE subsequent infection among rectal CRE carriers in this study. Notably, we found that more than three times hospitalization and being in a coma condition were independent risk factors. Being in coma might be associated with receiving more invasive procedures such as tracheal intubation, urinary catheter insertion, or venous catheter insertion, which may increase the risk of subsequent infection when the patient colonized CRE. Patients with multiple admissions may increase the risk of exposure to colonized CRE. Additionally, repeated admissions indicate underlying disease, weakened immunity, long‐term antibiotic use, and repeated invasive procedures, which may increase the risk of subsequent infection among CRE colonizer.
This study has several limitations. First, this was a single‐center study, which limits the generalizability of our findings. Second, since this was a retrospective study, we could not implement a comorbidity index for measuring the severity of comorbid conditions, which increased the risk of selection bias. Third, the genetic relatedness between the colonization and subsequent infection strains was not analyzed, although we enrolled patients with subsequent CRE infections caused by the same species as the colonized CRE. Molecular analysis need be performed to confirm this in the future. Nonetheless, this study provides findings that could inform infection control in this population.
5. CONCLUSION
In conclusion, we found that K. pneumoniae was the dominant strain responsible for rectal CRE colonization and subsequent clinical infections in hospitalized patients. Further, we found that more than three times hospitalization were an independent risk factor for clinical CRE infections in intestinal CRE carriers. Additionally, prior carbapenems use and being under coma conditions were independent risk factors for clinical CRE infections among rectal CRE carriers. Our findings may inform clinicians to take effective measures for preventing subsequent nosocomial infections in patients at a high risk of rectal CRE colonization.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. XC, JZJ, QY, and WEL collected and analyzed the patient data, MZ written the first draft of the article and HLL proofread the final draft. All authors read and approved the final article.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Table S1
Chen X, Zhou M, Yan Q, Jian Z, Liu W, Li H. Risk factors for carbapenem‐resistant Enterobacterales infection among hospitalized patients with previous colonization. J Clin Lab Anal. 2022;36:e24715. doi: 10.1002/jcla.24715
Xia Chen and Mao Zhou contributed equally.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. World Health Organization . Global priority list of antibiotic‐resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization; 2017. [Google Scholar]
- 2. Chotiprasitsakul D, Srichatrapimuk S, Kirdlarp S, Pyden AD, Santanirand P. Epidemiology of carbapenem‐resistant Enterobacteriaceae: a 5‐year experience at a tertiary care hospital. Infect Drug Resist. 2019;12:461‐468. doi: 10.2147/IDR.S192540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sabino S, Soares S, Ramos F, et al. A cohort study of the impact of carbapenem‐resistant Enterobacteriaceae infections on mortality of patients presenting with sepsis. mSphere. 2019;4(2):e00052‐19. doi: 10.1128/mSphere.00052-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan L, Sun J, Xu X, Huang S. Epidemiology and risk factors of rectal colonization of carbapenemase‐producing Enterobacteriaceae among high‐risk patients from ICU and HSCT wards in a university hospital. Antimicrob Resist Infect Control. 2020;9(1):155. doi: 10.1186/s13756-020-00816-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yin L, He L, Miao J, et al. Carbapenem‐resistant Enterobacterales colonization and subsequent infection in a neonatal intensive care unit in Shanghai, China. Infect Prev Pract. 2021;3(3):100147. doi: 10.1016/j.infpip.2021.100147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khawcharoenporn T, Laichuthai W. Subsequent carbapenem‐resistant Enterobacteriaceae (CRE)‐associated infections among hospitalized patients with CRE colonization: impact of antibiotic use and other factors. Infect Control Hosp Epidemiol. 2020;41(9):1084‐1089. doi: 10.1017/ice.2020.220 [DOI] [PubMed] [Google Scholar]
- 7. Tischendorf J, de Avila RA, Safdar N. Risk of infection following colonization with carbapenem‐resistant Enterobactericeae: a systematic review. Am J Infect Control. 2016;44(5):539‐543. doi: 10.1016/j.ajic.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang L, Zhai W, Lin Q, et al. Carbapenem‐resistant Enterobacteriaceae in hematological patients: outcome of patients with carbapenem‐resistant Enterobacteriaceae infection and risk factors for progression to infection after rectal colonization. Int J Antimicrob Agents. 2019;54(4):527‐529. doi: 10.1016/j.ijantimicag.2019.06.023 [DOI] [PubMed] [Google Scholar]
- 9. Dickstein Y, Edelman R, Dror T, Hussein K, Bar‐Lavie Y, Paul M. Carbapenem‐resistant Enterobacteriaceae colonization and infection in critically ill patients: a retrospective matched cohort comparison with non‐carriers. J Hosp Infect. 2016;94(1):54‐59. doi: 10.1016/j.jhin.2016.05.018 [DOI] [PubMed] [Google Scholar]
- 10. Mcconville TH, Sullivan SB, Gomez‐Simmonds A, et al. Carbapenem‐resistant Enterobacteriaceae colonization (CRE) and subsequent risk of infection and 90‐day mortality in critically ill patients, an observational study. PLoS One. 2017;12(10):e0186195. doi: 10.1371/journal.pone.0186195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Predic M, Delano JP, Tremblay E, Iovine N, Brown S, Prins C. Evaluation of patient risk factors for infection with carbapenem‐resistant Enterobacteriaceae. Am J Infect Control. 2020;48(9):1028‐1031. doi: 10.1016/j.ajic.2019.11.025 [DOI] [PubMed] [Google Scholar]
- 12. Liu Q, Liu L, Li Y, Chen X, Yan Q, Liu WE. Fecal carriage and epidemiology of carbapenem‐resistant Enterobacteriaceae among hospitalized patients in a University hospital. Infect Drug Resist. 2019;12:3935‐3942. doi: 10.2147/IDR.S233795 eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen X, Liu Q, Liu WE, Yan Q. Risk factors for subsequential carbapenem‐resistant Klebsiella pneumoniae clinical infection among rectal carriers with carbapenem‐resistant Klebsiella pneumoniae . Infect Drug Resist. 2020;13:1299‐1305. doi: 10.2147/IDR.S247101 eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alon D, Mudrik H, Chowers M, Shitrit P. Control of a hospital‐wide outbreak of carbapenem‐resistant acinetobacter baumannii (CRAB) using the israeli national carbapenem‐resistant Enterobacteriaceae (CRE) guidelines as a model. Infect Control Hosp Epidemiol. 2020;41(8):926‐930. doi: 10.1017/ice.2020.158 [DOI] [PubMed] [Google Scholar]
- 15. Juan CH, Chou SH, Chen IR, Yang CI, Lin YT, Chen L. Intestinal colonisation with hypervirulent or third‐generation cephalosporin‐resistant Klebsiella pneumoniae strains upon hospital admission in a general ward in Taiwan. Int J Antimicrob Agents. 2022;60(2):106624. doi: 10.1016/j.ijantimicag.2022.106624 [DOI] [PubMed] [Google Scholar]
- 16. Tian X, Sun S, Jia X, Zou H, Li S, Zhang L. Epidemiology of and risk factors for infection with extended‐spectrum beta‐lactamase‐producing carbapenem‐resistant Enterobacteriaceae: results of a double case‐control study. Infect Drug Resist. 2018;11:1339‐1346. doi: 10.2147/IDR.S173456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The glasgow coma scale at 40 years: standing the test of time. Lancet Neurol. 2014;13(8):844‐854. doi: 10.1016/S1474-4422(14)70120-6 [DOI] [PubMed] [Google Scholar]
- 18. Clinical and Laboratory Standards Institute U . Performance standards for antimicrobial susceptibility testing, M100 30th edition. Accessed June 18, 2020. https://clsi.org/standards/products/microbiol.2020
- 19. Sun Y, Yu L, Gao W, et al. Investigation and analysis of the colonization and prevalence of carbapenem‐resistant Enterobacteriaceae in pediatric liver transplant recipients. Infect Drug Resist. 2021;14:1957‐1966. doi: 10.2147/IDR.S304998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jimenez A, Trepka MJ, Munoz‐Price LS, et al. Epidemiology of carbapenem‐resistant Enterobacteriaceae in hospitals of a large healthcare system in Miami, Florida from 2012 to 2016: five years of experience with an internal registry. Am J Infect Control. 2020;48(11):1341‐1347. doi: 10.1016/j.ajic.2020.04.013 [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Lin Q, Chen Z, et al. Construction of a risk prediction model for subsequent bloodstream infection in intestinal carriers of carbapenem‐resistant Enterobacteriaceae: a retrospective study in hematology department and intensive care unit. Infect Drug Resist. 2021;14:815‐824. doi: 10.2147/IDR.S286401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim YK, Chang IB, Kim HS, Song W, Lee SS. Prolonged carriage of carbapenemase‐producing Enterobacteriaceae: clinical risk factors and the influence of carbapenemase and organism types. J Clin Med. 2021;10(2):310. doi: 10.3390/jcm10020310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Debby BD, Ganor O, Yasmin M, et al. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis. 2012;31(8):1811‐1817. doi: 10.1007/s10096-011-1506-5 [DOI] [PubMed] [Google Scholar]
- 24. Kim YK, Song SA, Lee JN, et al. Clinical factors predicting persistent carriage of Klebsiella pneumoniae carbapenemase‐producing carbapenem‐resistant Enterobacteriaceae among patients with known carriage. J Hosp Infect. 2018;99(4):405‐412. doi: 10.1016/j.jhin.2017.10.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


