Abstract
We show that the distribution of specific antibodies and antibody-secreting cells in the intestine after oral and rectal immunizations corresponds to the vascularization and lymph drainage patterns of the gut. Oral immunizations induce antibody responses along the parts of the intestine connected to the superior mesenteric vessels and lymph ducts, whereas rectal immunizations induce antibody responses along the parts of the intestine associated with the inferior mesenteric vessels and ducts.
The concept of directing specific effector B cells not only to mucosal tissues in general but to a selected part of the gastrointestinal tract is of potential importance when constructing vaccines against pathogenic microorganisms affecting different parts of the intestine, e.g., Vibrio cholerae, enterotoxigenic Escherichia coli, and rotavirus in the small intestine and Shigella spp. and Clostridium difficile in the large intestine. In this study, we wanted to gain detailed information about the anatomical distribution of antibodies and antibody-secreting cells (ASC) in different parts of the intestinal tract of primates following oral and rectal immunizations compared to that after intradermal immunization. For this purpose, cynomolgus monkeys (Macaca fascicularis) were immunized with cholera toxin (CT) (List Biological Laboratories, Inc., Campbell, Calif.) three to five times, 3 to 6 weeks apart. Intragastric vaccination was performed by administering 50 μg of CT in 5 ml of a 2.8% bicarbonate–1.1% citric acid buffer (pH 7.0) (CT buffer) through a baby-feeding tube into the stomach. Rectal vaccination was performed by instilling 50 μg of CT in 1 ml of CT buffer for 5 min through a tube between two inflated ballons placed in the rectum 2 to 5 cm from the anus. Intradermal injections of 2 μg of CT were given in 0.2 ml of phosphate-buffered saline. Seven days after the final immunization, animals were sacrificed by an intracardiac injection of a lethal dose of thiopental sodium (500 mg) (Pentothal Natrium; Abbott S.p.A.-Campoverde LT.). The small and large intestines were collected and divided into duodenum, ileum, jejunum, ascending colon, transverse colon, descending colon, and rectum. The preparation of mononuclear cell suspensions and the extraction of immunoglobulins from tissue were performed exactly as described previously (4). The numbers of specific ASC were analyzed in a CT-specific enzyme-linked immunospot assay as described previously (3) and are expressed as the means and standard errors of the mean of specific ASC. Specific antibody titers in tissue extracts were analyzed by a GM1-CT enzyme-linked immunosorbent assay (4) and were estimated as the interpolated sample dilution giving an absorbance of 0.4 above the background level (6, 7). Enzyme-linked immunosorbent assay data are expressed as the specific antibody titer in 1 mg of tissue per ml. Pearson's correlation coefficient (r) was determined with Microsoft Excel 97.
Specific ASC in the intestine after oral, rectal, and intradermal immunizations.
We immunized monkeys three to five times orally (three animals), rectally (two animals), or intradermally (three animals) with CT and evaluated the numbers of specific ASC in anatomically distinct parts of the intestine.
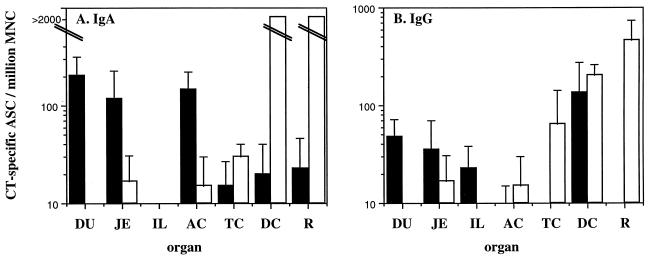
Oral immunization preferentially induced ASC responses in the small intestine and ascending colon, whereas rectal immunization was superior for the induction of ASC responses in the transverse colon, descending colon, and rectum. Intradermal immunization was poor in stimulating intestinal immune responses. In all orally immunized monkeys, the frequency of specific immunoglobulin A (IgA)-ASC was highest in the duodenum, followed by the jejunum and the ileum (Fig. 1A). A similar pattern was observed for CT-specific IgG-ASC (Fig. 1B). Oral immunization also gave rise to high levels of CT-specific IgA-ASC in the ascending colon (Fig. 1A).
FIG. 1.
CT-specific IgA-ASC and IgG-ASC in the intestinal tract following oral and rectal immunizations with CT. Data are expressed as means plus the standard errors of the mean of CT-specific IgA-ASC (A) and IgG-ASC (B) titers measured 7 days after the last booster immunization. Black bars represent oral immunizations (n = 3) and white bars represent rectal immunizations (n = 2). DU, duodenum; JE, jejunum; IL, ileum; AC, ascending colon; TC, transverse colon; DC, descending colon; R, rectum.
In contrast, rectal immunization consistently induced large numbers of specific IgA-ASC and IgG-ASC in the distal part of the large intestine (Fig. 1). CT-specific IgG-ASC, but not IgA-ASC, could also be found in the transverse colon after rectal immunization, whereas low or negligible levels of specific ASC in the ascending colon and in the small intestine (Fig. 1) were observed. When statistical analysis was performed on the results from this small group of animals, there was a significant stepwise correlation, in a distal direction, between the different parts of the intestine (data not shown).
Specific intestinal tissue antibody levels after oral, rectal, or intradermal immunizations.
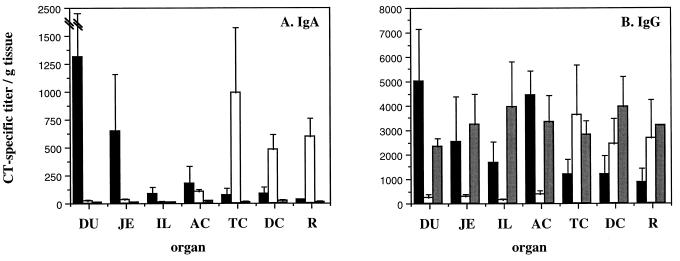
We also analyzed the anti-CT antibody levels in saponin-extracted tissue from the small and large intestines of an additional set of monkeys receiving oral, rectal, or intradermal immunization (three animals per group). As with the ASC responses, oral immunization proved to be the best way of inducing high levels of specific antibodies in the small intestine, and the levels of specific antibodies in different parts of the small intestine followed the same pattern as the CT-specific ASC, with high titers of specific IgA and IgG in the duodenum and lower levels in the jejunum and ileum (Fig. 2). Oral immunization also induced specific IgG (but not IgA) in the ascending colon.
FIG. 2.
CT-specific antibody concentration in the intestinal mucosa after oral, rectal, and intradermal immunizations with CT. Data are expressed as the means plus the standard errors of the mean of the IgA (A) and IgG (B) titers obtained in the protein extract from 1 mg of intestinal tissue per ml taken 7 days after the last immunization. Black bars represent oral immunizations (n = 3), white bars represent rectal immunizations (n = 3), and grey bars represent intradermal immunizations (n = 3). DU, duodenum; JE, jejunum; IL, ileum; AC, ascending colon; TC, transverse colon; DC, descending colon; R, rectum.
Following rectal immunization, the specific antibody titers in intestinal tissue extracts were similar in distribution to the ASC responses, with high levels of specific antibodies in the large intestine but not in the small intestine (Fig. 2). Thus, high levels of both specific IgA and IgG in the transverse colon, the descending colon, and the rectum were detected, whereas only moderate levels of specific IgA and IgG in the ascending colon were found (Fig. 2). The levels of response in the small intestine were negligible.
Intradermal immunization gave rise to high levels of specific IgG, but not IgA, throughout the gastrointestinal tract (Fig. 2). It has previously been shown that such antibodies are most probably derived from transudation from the sera (4).
When the Pearson's correlation coefficient (r) was calculated, there was a statistically significant relationship between the titers of anti-CT IgA in the duodenum, jejunum, ileum, and ascending colon on one hand and between the titers of specific IgA in the transverse colon, descending colon, and rectum on the other hand (Table 1). For IgG, there was a statistically significant relationship between the specific IgG titers in (i) the duodenum, jejunum, and ascending colon and (ii) the transverse colon, descending colon, and rectum (data not shown).
TABLE 1.
Pearson's correlation coefficient (r) for levels of CT-specific IgA in the small and large intestines
| Site |
r for CT-specific IgA levels in:
|
|||||
|---|---|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | Ascending colon | Transverse colon | Descending colon | |
| Jejunum | 0.9998a | |||||
| Ileum | 0.9971a | 0.9982a | ||||
| Ascending colon | 0.9388a | 0.9412a | 0.9473a | |||
| Transverse colon | −0.0847 | −0.0820 | −0.0794 | 0.1967 | ||
| Descending colon | −0.0242 | −0.0213 | −0.0066 | 0.2863 | 0.9403a | |
| Rectum | −0.0242 | −0.2321 | −0.2207 | 0.0932 | 0.9730a | 0.8825a |
P < 0.001.
Relation of immune responses to vascularization.
This distribution of specific antibodies and ASC within the intestinal tract corresponds closely to the vascularization and lymphatic drainage patterns which distinctly separate the small intestine and upper part of the large intestine from the middle and lower parts of the large intestine. In both humans and macaques, the partition between the two systems takes place in the middle of the transverse colon. Thus, the blood supply through the small intestine, the ascending colon, and proximal parts of the transverse colon depends largely on the superior mesenteric artery and vein, whereas the distal part of the transverse colon, the descending colon, and the rectum are vascularized mainly by the inferior mesenteric artery and vein. The lymphatic drainage follows a similar pattern.
The simplest explanation for the difference in ASC and antibody distribution following oral and rectal immunizations would be that specific B cells are located exclusively at sites that can be reached by the antigen. Thus, an antigen delivered at a specific site would reach a given distance in the intestinal tract depending on the dose, the stability of the antigen, the efficiency with which it is taken up, and the concentration threshold necessary to induce an immune response. Consequently, there would be a strong immune response at the site of intestinal immunization, accompanied by a gradually diminishing response away from this site, in this case mostly down the intestine. Two findings contradict this explanation. Firstly, even though the ASC and antibody responses in the small intestine obtained after oral immunization gradually decrease distally, the response obtained in the ascending colon is very strong for specific IgA-ASC and exceeds that in both the jejunum and the ileum. Secondly, even though antigens are not believed to travel from the distal to the proximal part of the gut, rectal immunization induced strong immune responses in both the transverse and descending colons, i.e., at least 0.5 m proximally from the site of antigen delivery; it is noteworthy that the rectal antigen was given between two balloons, which further argues against retrograde migration of the antigen.
The close correlation between the routes of immunization (oral and rectal) and the distribution of CT-specific antibodies and ASC along parts of the intestine associated with either the superior or the inferior mesenteric vessels suggests that there might be distinct mechanisms of distribution mediating extravasation from the respective venules. There are several possible explanations for this finding. (i) There may exist an as-yet-undefined homing receptor or specific combination of adhesion molecules on lymphocytes which distinguishes between endothelial cells in the superior and inferior mesenteric vessels. (ii) There may be a different expression of addressins on the endothelium in the superior and inferior vessels. (iii) Different chemokines directing the recruitment of cells may be secreted in different parts of the intestine. (iv) Vaccine-induced chemokines may affect the extravasation of cells only in the blood vessel where the vaccine was originally encountered but not in any other blood vessel. In this respect, distribution of cells to the intestinal lamina propria is mediated mainly by the mucosal homing receptor integrin α4β7 and its ligand MAdCAM-1, which is expressed on endothelial cells in the intestine and mesenteric lymph nodes (1, 2, 5). Expression of α4β7 alone, however, cannot explain the differences in the migration pattern of cells induced in the upper versus the lower intestinal tract, since peroral and rectal immunization of human volunteers resulted in circulating vaccine-specific ASC having a similar, practically universal expression of α4β7 (8).
In summary, this study demonstrates that there exists a strict anatomical segregation of the intestinal immune response which closely corresponds to and, as we propose, may be dependent on the vascularization and lymph-draining patterns of the intestinal tract. This finding could have important implications for future vaccine development efforts aimed at protection against intestinal pathogens.
Acknowledgments
Kristina Eriksson and Marianne Quiding-Järbrink contributed equally to this work.
The help from Eva Sjögren, Margareta Fredriksson, Annie George-Chandy, Margareta Hedin, Sten Holm, Åke Möller, and Stellan Björk is gratefully acknowledged. We thank Agnes Wold and Pär Bierke for sharing their knowledge of the anatomy of the intestinal tract.
This study was supported by grants from SIDA/SAREC's Special Programme for AIDS and related diseases; NIH grant 1 RO1 A1 35543-02; European Commission (Biomed) contract CT 920272; the Swedish Medical Research Council (MFR) projects 16X-3382 and 16X-8320; the Faculty of Medicine, University of Göteborg; the Swedish Society for Medical Research; and Syntello Inc.
REFERENCES
- 1.Berlin C, Berg E L, Briskin M J, Andrew D P, Kilshaw P J, Holzmann B, Weissman I L, Hamann A, Butcher E. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 2.Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy L M, Butcher E C, Kassam N, Mackay C R, Newman W, Ringler D J. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 3.Eriksson K, Nordström I, Horal P, Jeansson S, Svennerholm B, Vahlne A, Holmgren J, Czerkinsky C. Amplified ELISPOT assay for the detection of HIV-specific antibody-secreting cells in subhuman primates. J Immunol Methods. 1992;153:107–113. doi: 10.1016/0022-1759(92)90312-h. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson K, Quiding-Järbrink M, Osek J, Möller Å, Björk S, Holmgren J, Czerkinsky C. Specific-antibody-secreting cells in the rectums and genital tracts of nonhuman primates following vaccination. Infect Immun. 1998;66:5889–5896. doi: 10.1128/iai.66.12.5889-5896.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamann A, Andrew D P, Jablonski-Westrich D, Holzmann B, Butcher E C. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 6.Jertborn M, Svennerholm A M, Holmgren J. Evaluation of different immunization schedules for oral cholera B subunit–whole cell vaccine in Swedish volunteers. Vaccine. 1993;11:1007–1012. doi: 10.1016/0264-410x(93)90125-h. [DOI] [PubMed] [Google Scholar]
- 7.Jertborn M, Svennerholm A-M, Holmgren J. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J Clin Microbiol. 1986;24:203–209. doi: 10.1128/jcm.24.2.203-209.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quiding-Järbrink M, Nordström I, Granström G, Kilander A, Jertborn M, Butcher E C, Lazarivits A I, Holmgren J, Czerkinsky C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J Clin Investig. 1997;99:1281–1286. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]