Abstract
Background
Adrenal Cushing syndrome (CS) is usually benign in etiology; however, although rarely, it can be due to adrenocortical carcinoma (ACC); in which case, diagnosis and management are quite complicated.
Case Report
A 34-year-old woman presented with worsening confusion, weight gain, new-onset diabetes, and hypertension. Her history was significant for a 7.4-cm left adrenal mass and CS, which were treated with left adrenalectomy 2 years ago. She received hydrocortisone replacement therapy after the surgery, which was discontinued on admission when evaluation showed hypokalemia, hypercortisolemia, and undetectable adrenocorticotropic hormone. Subsequent testing included 1-mg and 8-mg dexamethasone suppression tests, which did not suppress cortisol; late-night salivary cortisol measurement, which yielded a very high salivary cortisol level; and 24-hour urinary cortisol measurement. The level of 11-deoxycortisol was elevated. A computed tomography scan revealed multiple hepatic lesions, which were fluorodeoxyglucose avid, and a biopsy confirmed metastatic ACC. She received treatment with mitotane, metyrapone (later changed to mifepristone), doxorubicin, cisplatin, and etoposide. Over 8 weeks, mitotane levels became therapeutic at 20 mcg/mL, the hepatic masses decreased in size, and she transitioned to adrenal insufficiency and improved glycemic control. Next-generation sequencing of liver biopsy and germline testing revealed a frameshift loss-of-function allelic variant in the FH gene that encodes the protein fumarate hydratase.
Discussion
We report a case of recurrent CS due to metastatic ACC in a patient with a previously resected adrenal adenoma and FH allelic variant.
Conclusion
Metastatic ACC presenting with severe CS presents a diagnostic and management challenge where combination therapy guided by a multidisciplinary team is essential. FH allelic variant may contribute to ACC progression.
Key words: hypercortisolism, adrenal cancer, adrenal mass, metastases
Abbreviations: ACC, adrenocortical carcinoma; ACTH, adrenocorticotropic hormone; CS, Cushing syndrome; CT, computed tomography; HDDST, high-dose 8 mg dexamethasone suppression test; HLRCC, hereditary leiomyomatosis and renal cell cancer; LDDST, low-dose 1 mg dexamethasone suppression test
Highlights
-
•
Metastatic adrenocortical carcinoma presenting with life-threatening Cushing syndrome is a diagnostic and management challenge
-
•
Early diagnosis and initiation of therapy are critical for the management of severe Cushing syndrome
-
•
Loss-of-function FH allelic variant may be associated with progression of adrenocortical carcinoma
-
•
Patients with resected large adrenal adenomas require close monitoring to identify malignant behavior
Clinical Relevance
This case report demonstrates the challenges in the diagnosis and management of life-threatening Cushing syndrome arising in the setting of metastatic adrenocortical carcinoma in a patient. A frameshift loss-of-function allelic variant in the FH gene found on liver biopsy and germline testing has not been reported typically with adrenocortical carcinoma and, hence, is a novel finding.
Introduction
Adrenal causes of Cushing syndrome (CS) account for approximately 20% of cases in adults, and the majority have benign etiologies.1 Surgery is the first-line treatment option and is nearly 100% curative for patients with CS due to an adrenal adenoma.2 Adrenocortical carcinoma (ACC) is a rare finding, with an estimated incidence of 1 to 2 per million in the United States.3, 4, 5 Differentiation between adrenal adenomas and ACCs can be challenging, with hormone hypersecretion adding an extra layer of complexity to the diagnosis and management. We present a case of CS secondary to an adrenal mass that was completely resected and recurred 2 years later as metastatic ACC.
Case Report
A 34-year-old woman was admitted for worsening confusion. Her original presentation 2 years before the current admission included weight gain, irregular menses, elevated blood pressure, and easy bruising. Laboratory workup demonstrated adrenocorticotropic hormone (ACTH)-independent CS, with serum cortisol failing to suppress after 1-mg dexamethasone suppression, and undetectable ACTH. Her 24-hour urine-free cortisol and midnight salivary cortisol levels were elevated (Table). She had a normal dehydroepiandrosterone-sulfate level of 51 mcg/dL, and she had a normal plasma aldosterone concentration, normal plasma renin activity, and a normal plasma metanephrine level. A computed tomography (CT) scan of the abdomen revealed a 7-cm left adrenal mass, with precontrast Hounsfield units of −8 to 31 (Fig. 1). Postcontrast washout imaging was not performed. The initial impression was a cortisol secreting adrenal mass with a differential diagnosis of benign adrenal adenoma or ACC, for which she underwent a laparoscopic left adrenalectomy, which was uncomplicated. The mass was removed intact and was noted to be grossly encapsulated. There was no gross lymphadenopathy or presence of intraperitoneal metastases. Pathology demonstrated a 7.4-cm × 7.2-cm × 5.0-cm well-circumscribed left adrenal gland consisting of a bright yellow-tan solid, homogeneous cut surface, with no areas of cystic change, hemorrhage, or calcifications identified. There was also a thin rim of normal adrenal gland with red-tan medulla on one side. These were benign features at that time and final pathology was reported as benign adrenal adenoma. Hydrocortisone and fludrocortisone were started after the surgery and slowly weaned. The 6-month follow-up CT scan was deferred because of new pregnancy. Approximately 10 months later, she started gaining weight and developed type 2 diabetes mellitus, which continued to worsen. Within 2 weeks, she was hospitalized for depression, worsening confusion, and inability to perform activities of daily living.
Table.
Laboratory Investigations of the Patient
| Laboratory investigations | Results at the time of the initial presentation of adrenal mass | Results at the time of diagnosis of hepatic metastases | Reference range |
|---|---|---|---|
| Serum potassium | 2.3 mmol/L | 2.3 mmol/L | 3.5-5.1 mmol/L |
| Thyroid-stimulating hormone | 0.45 mcIU/mL | 0.397 mcIU/mL | 0.4-4.3 mcIU/mL |
| Free thyroxine | 1.29 ng/dL | 0.8 ng/dL | 0.6-1.6 ng/dL |
| Hemoglobin A1C | 6.2% (44 mmol/mol) | 8.5% (69 mmol/mol) | 4.0%-6.0% (20-42 mmol/mol) |
| 24-hour urine cortisol | 285 mcg | 3700.2 mcg | <45 ug/d |
| Salivary cortisol | 0.383 mcg/dL | 0.974 mcg/dL | <0.181 ug/dL |
| 8 am serum cortisol after 1 mg of dexamethasone administered overnight | 16.9 mcg/dL | 37.8 mcg/dL | <1.8 mcg/dL; excludes Cushing syndrome |
| 8 am serum cortisol after 8 mg of dexamethasone administered overnight | … | 41.6 mcg/dL | <1.8 mcg/dL; excludes Cushing syndrome |
| Adrenocorticotropic hormone | <5 | 27 ng/mL in LDDST and 19 ng/mL in HDDST | <46 pg/mL |
| Dehydroepiandrosterone sulfate | 51 mcg/dL | 29 mcg/dL | Female premenopausal, 35-430 mcg/dL Postmenopausal, 10-240 mcg/dL |
| Estradiol | … | <20 ng/mL | Midfollicular, 27-123 pg/mL Periovulatory, 96-436 pg/mL Midluteal, 49-294 pg/mL Postmenopausal, 0-40 pg/mL |
| Androstenedione | 4.3 | 0.348 ng/mL | 0.26-2.14 ng/mL |
| Total testosterone | 8.2 ng/dL | 2 ng/dL | Premenopausal, 9-55 ng/dL Postmenopausal, 5-32 ng/dL |
| 17-hydroxyprogesterone | … | 98.6 ng/dL | <206 ng/dL |
| 11-deoxy cortisol | … | 725 ng/dL | <32 ng/dL |
| Chromogranin A | … | 130 ng/mL | <160 ng/mL |
Abbreviations: HDDST: high-dose 8 mg dexamethasone suppression test; LDDST: low-dose 1 mg dexamethasone suppression test.
Fig. 1.
A computed tomography scan of the abdomen demonstrating a 7-cm left adrenal mass, with precontrast Hounsfield units of −8 to 31 due to the heterogeneity of the mass.
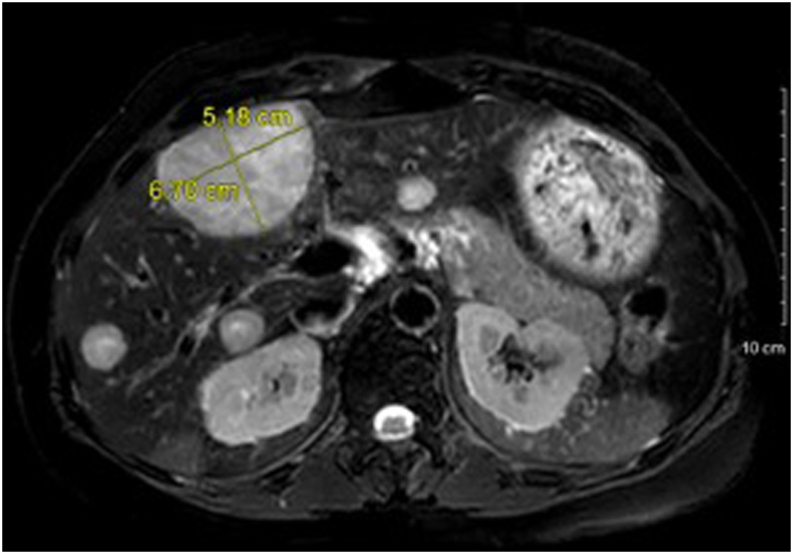
Laboratory workup after discontinuing hydrocortisone and fludrocortisone during hospitalization demonstrated undetectable ACTH with an 8 am cortisol level of 29.9 mcg/dL. Serum cortisol failed to suppress after 1-mg and 8-mg dexamethasone suppression tests. Her 24-hour urine-free cortisol and midnight salivary cortisol levels were elevated (Table). Abdominal magnetic resonance imaging revealed multiple hepatic masses (Fig. 2). There was no residual nodularity in the left adrenalectomy bed, and the right adrenal gland was unremarkable. The liver lesions were fluorodeoxyglucose avid on a positron emission tomography scan (Fig. 3), and a biopsy confirmed metastatic ACC (Fig. 4). Next-generation sequencing studies of the liver biopsy specimen revealed a frameshift loss-of-function allelic variant in the FH gene that encodes the protein fumarate hydratase (c.912_918del p.F305fs; variant allele fraction, 67.8%). Subsequently, a comprehensive panel comprising 77 genes revealed a germline allelic variant in the FH gene (c.912_918delTTTTGTC p.F305Lfs∗22).
Fig. 2.
A magnetic resonance image of the abdomen showing multiple liver lesions in a patient with metastatic adrenocortical carcinoma.
Fig. 3.
A fluorodeoxyglucose–positron emission tomography scan showing fluorodeoxyglucose-avid liver lesions in a patient with metastatic adrenocortical carcinoma.
Fig. 4.
A histopathology slide showing a tissue biopsy sample of a liver lesion with inhibin staining indicative of adrenal origin.
She was started on sulfamethoxazole-trimethoprim for Pneumocystis jirovecii pneumonia prophylaxis and heparin for deep venous thrombosis prophylaxis due to the hypercoagulable state related to severe CS. Spironolactone was initiated to treat hypertension and hypokalemia. Her disease was not surgically resectable or amenable to treatment with local radiation because of extensive hepatic metastases. Mitotane and metyrapone were initially started. Because of issues with insurance coverage, metyrapone was stopped after 9 days and mifepristone (600 mg, daily) was started. She received concurrent chemotherapy with doxorubicin, etoposide, and cisplatin and completed 6 cycles.
Upon achieving the therapeutic mitotane level at 20 mcg/mL, she developed primary adrenal insufficiency, with a serum cortisol level of 3 mcg/dL; hence, mifepristone was stopped and hydrocortisone was initiated, and its dose was titrated up to 30 mg 3 times a day. Her blood pressure and glucose normalized, and she was weaned off all antihypertensives and antihyperglycemic agents. Follow-up imaging over 12 months has continued to demonstrate a decrease in the size of her hepatic lesions. She has not required mineralocorticoid replacement.
Discussion
This case was unique in its diagnostic and management challenges: Metastatic ACC found 2 years after resection of a reportedly benign adenoma presenting with CS and multiple hepatic metastases with no evidence of recurrence in the adrenalectomy bed. The germline and somatic loss-of-function FH allelic variants in this case are unique. Continued case review with a multidisciplinary tumor board, with representation from endocrinology, endocrine surgery, pathology, radiology, oncology, genetics, and radiation oncology, was of particular importance to management decisions.
ACC may be diagnosed as a component of Li-Fraumeni syndrome (TP52 gene) and Lynch syndrome (MSH2, MLH1, PMS2, and MSH6 genes). In general, allelic variants in the FH gene are implicated in hereditary leiomyomatosis and renal cell cancer (HLRCC) and have an autosomal dominant inheritance.6 In HLRCC, the FH gene appears to act as a tumor suppressor because all patients are heterozygous carriers and, at the tumor level, there is a loss of the normal allele.7 A case report suggested a direct involvement of FH germline allelic variant in massive macronodular adrenocortical disease in a patient with HLRCC.7 This finding was validated in later reviews of new genes and molecular pathways associated with adrenocortical tumors.8,9 The germline FH allelic variant in our patient confirms the diagnosis of HLRCC. The loss-of-function FH allelic variant in the metastatic adrenal tissue suggests its potential role in ACC progression, which requires further investigation.
The modified Weiss system is currently the most commonly used method for assessing malignancy.10 Each tumor is graded from 0 to 7 according to the total number of present criteria. The threshold for malignancy is a total score of ≥3. However, it is well known that histologic findings of adrenocortical tumors have poor predictive value in assessing metastatic potential.11 In our case, the CT scan findings at the initial diagnosis of heterogeneity of the adrenal mass did raise concern for possible malignancy; however, the resected adrenal tumor demonstrated a bland tumor cell morphology with only focal hemorrhage/necrosis. In addition, the dehydroepiandrosterone-sulfate level was not elevated, unlike for most ACCs. The absence of the other 4 criteria put this tumor (score 1) below the threshold of malignancy according to the modified Weiss criteria. Hence, even though the radiologic findings were suspicious for ACC, the final pathologic diagnosis was that of a large adrenal adenoma.
ACCs are secretory in approximately 60% of cases,11,12 and hypercortisolism is associated with a worse prognosis.5,11 Severe hypercortisolism can lead to impaired glucose tolerance, significant hypokalemia, elevated blood pressure, worsening of previous or new psychiatric disorders, altered memory and cognitive function, weight gain, fatigue, insomnia, and hirsutism,13 all of which were present in our patient. Other possible consequences include thrombotic and cardiovascular complications, increased risk of infections, reproductive challenges, muscle weakness, and loss of bone density.13 Hence, prompt management is critical. In our patient, the weight gain, type 2 diabetes, and worsening psychiatric symptoms approximately 10 months after adrenal gland resection suggested recurrence of CS but with diagnostic uncertainties because the patient was already receiving hydrocortisone replacement therapy. Measurement of proximal hair cortisol has been suggested as a test in the diagnostic workup of CS but remains to be validated clinically.14 The previous use of hydrocortisone in our patient may have invalidated the findings of this test, if performed. The extensive hepatic involvement made our patient a poor candidate for surgery or radiofrequency ablation. Medications used for CS treatment include ketoconazole, mifepristone, metyrapone, mitotane, and etomidate.2 Ketoconazole inhibits steroidogenesis by inhibiting side-chain cleavage, 17,20-lyase, and 11-beta hydroxylase enzymes. The potential acute hepatic toxicity from ketoconazole2,15 made it a less attractive option for our patient with widespread hepatic metastases. Metyrapone is another 11-beta hydroxylase inhibitor used off-label in the United States. It has a quick onset of action but can worsen hypokalemia.2,16 Etomidate is used in critically ill patients with severe hypercortisolism.2 It requires intensive care monitoring and acts by inhibiting 11-beta hydroxylase and cholesterol side-chain cleavage. Mifepristone is a glucocorticoid receptor antagonist that is approved by the U.S. Food and Drug Administration for patients with CS and glucose intolerance who are not surgical candidates.2,17 The major challenge with mifepristone is the inability to use a biomarker for titration because cortisol levels may increase or remain unchanged.
Mitotane is the only adrenolytic agent approved for the management of ACC2,4,18 and has shown improved recurrence-free and overall survival in patients with resected ACCs and no metastasis.19 In patients with metastases, the option is mitotane monotherapy or various combinations of cisplatin, carboplatin, etoposide, doxorubicin, streptozocin, and mitotane.18 The First International Randomized Trial in Locally Advanced and Metastatic Adrenocortical Carcinoma Treatment clinical trial showed a better response rate and progression-free survival in patients with advanced ACC who received a combination of etoposide, doxorubicin, and cisplatin plus mitotane than in those who received streptozocin plus mitotane as first-line therapy, with similar rates of toxic events.4 Cisplatin plus mitotane is still considered the preferred regimen per the National Comprehensive Cancer Network guidelines.18 Recently, immune checkpoint inhibitors have been evaluated in advanced ACC,20 however, their role in the management of ACC needs further investigation.11 Mitotane causes primary adrenal insufficiency, which can be partial, as suggested by our patient only requiring glucocorticoid replacement. It also increases the metabolism of hydrocortisone; hence, patients require approximately 3 to 4 times the usual physiologic replacement dose of hydrocortisone once they have achieved a therapeutic mitotane level of 14 to 20 mcg/mL.5 The continued improvement in the hepatic metastases of our patient suggests a favorable response to mitotane therapy along with glucocorticoid replacement.
To conclude, we have reported metastatic ACC presenting with life-threatening CS as a diagnostic and management challenge in a patient with FH allelic variant pathogenic for HLRCC, which, to our knowledge, has not been previously reported in ACC. The potential role of this FH allelic variant in the development and spread of ACC needs to be elucidated. The combination of mitotane, glucocorticoid excess treatment, and chemotherapy demonstrated benefit in our patient. Patients with resected large adrenal adenomas require monitoring to identify malignant behavior.
Acknowledgments
Author Contributions
E.S. and N.A are co-first authors.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Stratakis C.A. Cushing syndrome caused by adrenocortical tumors and hyperplasias (corticotropin-independent Cushing syndrome) Endocr Dev. 2008;13:117–132. doi: 10.1159/000134829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nieman L.K., Biller B.M., Findling J.W., et al. Treatment of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807–2831. doi: 10.1210/jc.2015-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C., Sun Y., Wu H., Zhao D., Chen J. Distinguishing adrenal cortical carcinomas and adenomas: a study of clinicopathological features and biomarkers. Histopathology. 2014;64(4):567–576. doi: 10.1111/his.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fassnacht M., Terzolo M., Allolio B., et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 5.Fassnacht M., Dekkers O.M., Else T., et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol. 2018;179(4):G1–G46. doi: 10.1530/EJE-18-0608. [DOI] [PubMed] [Google Scholar]
- 6.Lehtonen H.J., Kiuru M., Ylisaukko-Oja S.K., et al. Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet. 2006;43(6):523–526. doi: 10.1136/jmg.2005.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matyakhina L., Freedman R.J., Bourdeau I., et al. Hereditary leiomyomatosis associated with bilateral, massive, macronodular adrenocortical disease and atypical Cushing syndrome: a clinical and molecular genetic investigation. J Clin Endocrinol Metab. 2005;90(6):3773–3779. doi: 10.1210/jc.2004-2377. [DOI] [PubMed] [Google Scholar]
- 8.Stratakis C.A. New genes and/or molecular pathways associated with adrenal hyperplasias and related adrenocortical tumors. Mol Cell Endocrinol. 2009;300(1-2):152–157. doi: 10.1016/j.mce.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielinska M., Parviainen H., Kiiveri S., Heikinheimo M., Wilson D.B. Review paper: origin and molecular pathology of adrenocortical neoplasms. Vet Pathol. 2009;46(2):194–210. doi: 10.1354/vp.46-2-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aubert S., Wacrenier A., Leroy X., et al. Weiss system revisited: a clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am J Surg Pathol. 2002;26(12):1612–1619. doi: 10.1097/00000478-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Kiseljak-Vassiliades K., Bancos I., Hamrahian A., et al. American Association of Clinical Endocrinology Disease State Clinical Review on the evaluation and management of adrenocortical carcinoma in an adult: a practical approach. Endocr Pract. 2020;26(11):1366–1383. doi: 10.4158/DSCR-2020-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanabria A., Rojas A., Arevalo J., Kowalski L.P., Nixon I. Microscopically positive surgical margins and local recurrence in thyroid cancer. A meta-analysis. Eur J Surg Oncol. 2019;45(8):1310–1316. doi: 10.1016/j.ejso.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Nieman L.K. Recent updates on the diagnosis and management of Cushing’s syndrome. Endocrinol Metab (Seoul) 2018;33(2):139–146. doi: 10.3803/EnM.2018.33.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodes A., Lodish M.B., Tirosh A., et al. Hair cortisol in the evaluation of Cushing syndrome. Endocrine. 2017;56(1):164–174. doi: 10.1007/s12020-017-1231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonino N., Boscaro M., Paoletta A., Mantero F., Ziliotto D. Ketoconazole treatment in Cushing’s syndrome: experience in 34 patients. Clin Endocrinol (Oxf) 1991;35(4):347–352. doi: 10.1111/j.1365-2265.1991.tb03547.x. [DOI] [PubMed] [Google Scholar]
- 16.Daniel E., Aylwin S., Mustafa O., et al. Effectiveness of metyrapone in treating Cushing’s syndrome: a retrospective multicenter study in 195 patients. J Clin Endocrinol Metab. 2015;100(11):4146–4154. doi: 10.1210/jc.2015-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleseriu M., Biller B.M., Findling J.W., et al. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2012;97(6):2039–2049. doi: 10.1210/jc.2011-3350. [DOI] [PubMed] [Google Scholar]
- 18.Shah M.H., Goldner W.S., Benson A.B., et al. Neuroendocrine and adrenal tumors, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(7):839–868. doi: 10.6004/jnccn.2021.0032. [DOI] [PubMed] [Google Scholar]
- 19.Tang Y., Liu Z., Zou Z., Liang J., Lu Y., Zhu Y. Benefits of adjuvant mitotane after resection of adrenocortical carcinoma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018 doi: 10.1155/2018/9362108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raj N., Zheng Y., Kelly V., et al. PD-1 blockade in advanced adrenocortical carcinoma. J Clin Oncol. 2020;38(1):71–80. doi: 10.1200/JCO.19.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]