Abstract
Synovial cell sarcoma (SS) is a rare soft tissue sarcoma which can occur at any site in the body. SS arise from pluripotent mesenchymal cells and can occur in the head and neck region. We present a case of primary SS of the thyroid gland in a 47- year- old male and discuss the diagnostic and management challenge. A literature review of this rare entity was done.
Keywords: Thyroid cancer, Synovial sarcoma, Pathology, Cytology
Introduction
Synovial cell sarcoma (SS) is a rare soft tissue sarcoma which can occur at any site in the body. Most SS arise in the extremities, near the large joints, with a predilection to the lower extremities. SS arise from pluripotent mesenchymal cells, not from synovial tissues, [1] however the name given for their appearance and not their origin [2]. Therefore, they can also occur in any other anatomical location, including the head and neck [3]. Sarcomas of the head and neck account for approximately 1% of all head and neck malignancies. SS represents less than 10% of all primary sarcomas of the head and neck [4].
SS is classified into 2 main subtypes: biphasic and monophasic. Monophasic SS is the more common subtype and is formed of only spindle cells. Biphasic SS consist of both spindle and epithelial components. SS is characterized by a specific t(X; 18) (p11.2; q11.2) translocation leading to generation of SYT-SSX fusion gene transcripts [4]. The median age of diagnosis of SS is in the late thirties. The thyroid is an exceptionally rare site, and to our knowledge there have only been 12 reported cases of SS of the thyroid gland in the literature [[5]]. We present a case of SS of the thyroid gland in a 47 year-old male. This study was approved by The Hospital ethics' committee.
Case report
A 47-year-old male presented with progressively enlarging lower neck mass. Clinical manifestations included mild dyspnea, progressive dysphagia but without weight loss. Neck Ultrasound showed a diffuse heterogeneous echo pattern with micro-calcific foci and areas of vascularity. Fine needle aspiration Cytology (FNCA) revealed oncocytic neoplasm with atypical cells (Bethesda IV). Indirect laryngoscopy demonstrated bilateral freely mobile vocal cords and no palpable lymph node. CT with contrast (Fig. 1) showed lower neck and superior mediastinal mass encroaching upon the trachea. After discussion by tumour board, decision was done to do total thyroidectomy. Informed consent was obtained from the patient. Histopathological examination revealed a 5.5 cm lobulated mass with nodular surface. Microscopic examination revealed an invasive tumorous growth with biphasic epithelial and mesenchymal elements. Immunohistochemical testing (IHC) was negative for CD117, TTF-1, and SMA and positive for Bcl2, CD99 and TLE-1 antibody. (Figs. 2, 3) Fluorescence in situ hybridization (FISH)) technique demonstrated the characteristic t(X; 18) (p11; q11) translocation. Thus, the definitive diagnosis as primary SS of the thyroid gland was made. The patient received postoperative CRT and he is currently disease free for 12 months.
Fig. 1.
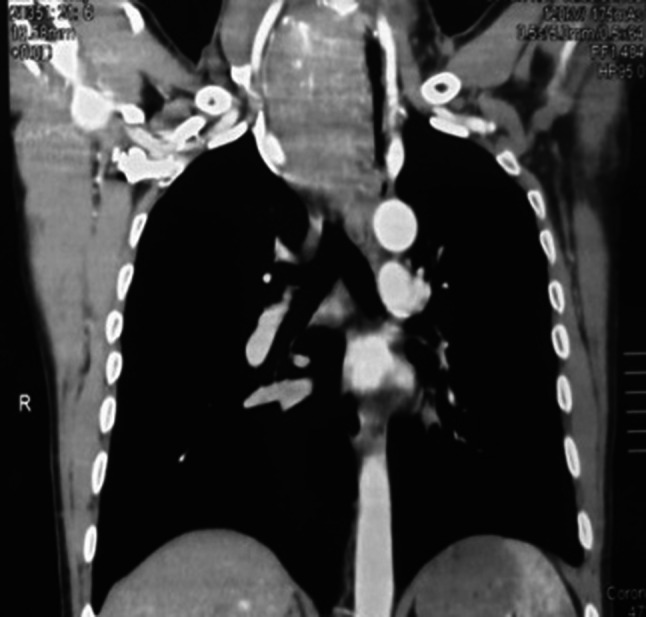
C.T neck with contrast in the coronal plane demonstrating the extent of the mass
Fig. 2.
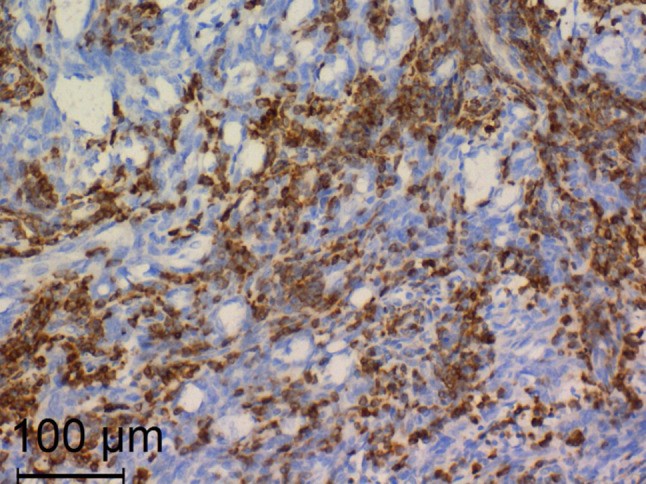
Positive immunostaining for Bcl2. (× 200, Anti Bcl2)
Fig. 3.
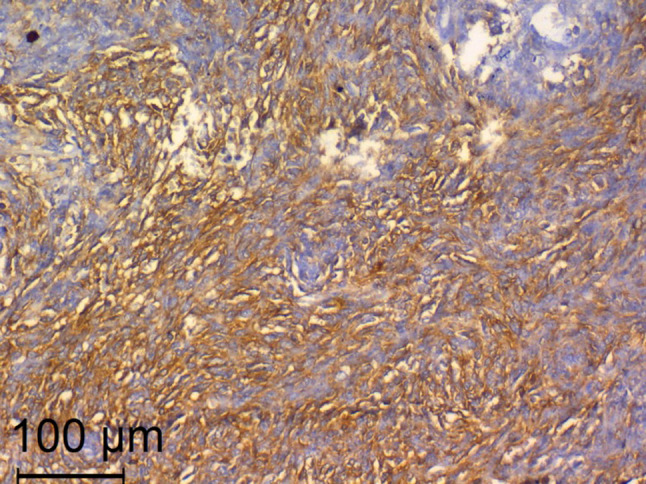
Positive immunostaining for CD99. (× 200, Anti CD99)
Discussion
SS of the thyroid gland is an extremely rare, with only few published studies (Table 1). SS of the thyroid gland typically presents with a rapidly enlarging neck mass, which may be associated with compression symptoms. Unfortunately, these clinical features are not pathognomonic and present in other types of thyroid cancer. Histologically, SS can be monophasic or biphasic type [5] and in our case, the tumour was biphasic in nature.
Table 1.
Review of literature
| Study | Number of cases | Age/sex | Clinical presentation | Management |
|---|---|---|---|---|
| Roth [6] | 1 | 15/M | Excessive salivation/otalgia | Total thyroidectomy and cobalt therapy |
| Kikuchi [7] | 1 | 60/M | Hoarseness/palpable mass | Total thyroidectomy + neck dissection + partial resection of thyroid and cricoid cartilages |
| Jang [8] | 1 | 15/M | Palpable mass | Total thyroidectomy + left Neck dissection |
| Ryu [9] | 1 | 72/F | Dysphagia/hoarseness/palpable mass | Total thyroidectomy + tracheal fenestration |
| Ghafouri [10] | 1 | 44/F | Rapidly growing mass | Incomplete excision |
| Boudin [11] | 1 | 55/M | Rapidly growing mass | Thyroidectomy with Incomplete excision, CRT + complete excision |
| Bansal [12] | 1 | 28/F | Rapidly growing mass/ dysphagia/weight loss | Total thyroidectomy + resection of retrosternal tumour + RT |
| Murro [13] | 1 | 41/M | Dysphonia/ left neck mass | Total thyroidectomy + subsequent total laryngopharyngoesophagectomy |
| Shi [14] | 1 | 31/M | Asymptomatic mass | Total Thyroidectomy with Incomplete excision/ Chemotherapy and neck dissection at relapse |
| Owen [[5]] | 5 | 37/M | Not recorded | Total Thyroidectomy with RT |
| 47/M | Left thyroid nodule | Total thyroidectomy + bilateral lymph node dissection | ||
| 42/M | Recurrent neck mass | Local excision | ||
| 36/M | Neck mass | Irresectable/ chemotherapy then RT and ATR inhibitor | ||
| 30/F | Right thyroid mass | Local excision |
The initial work up for any patient presenting with thyroid mass is ultrasound-guided FNAC [3]. Primary SS of thyroid gland should be differentiated from other spindle cell neoplasms as well as from other common thyroid neoplasms. Thyroid carcinomas are more common and have their characteristic immunoprofile (such as thyroglobulin or calcitonin) that is not present in SS. Other spindle cell neoplasms such as Ewing sarcoma rarely occur in the thyroid gland [13,5].
SS can be easily differentiated from the other more common thyroid neoplasms. Papillary carcinomas are characterized by their oval nuclei, with nuclear grooves, intranuclear cytoplasmic inclusions and fine chromatin. Papillary carcinomas often demonstrate true papillary formations. Medullary carcinoma consists of spindle cells with neuroendocrine chromatin and variable cytoplasm; lympho/plasmacytoid cells and atypical cells. Anaplastic carcinoma demonstrate necrotic and hemorrhagic background, giant or fusiform cells, pleomorphic nuclei, prominent nucleoli, frequent and atypical mitotic figures [13].
The main differential diagnosis of Thyroid SS is Spindle epithelioid tumor with thymus like differentiation (SETTLE). SETTLE constitutes the greatest challenge for both the head and neck surgeon and the pathologist. Both entities share similar cytomorphologic features. The aspirates are usually highly cellular and show spindle cells with scanty cytoplasm, uniform oval nuclei and indistinct nucleoli. Moreover, both SETTLE and SS share similar immunohistochemical (IHC) profile. Both conditions revealed immunoreactivity to pan-CK, CD99, Bcl-2, and EMA. Positive staining for TLE-1 antibody favors SS, since SETTLE is rarely reactive for this marker [13,5].
In our case, IHC testing was negative for CD117, TTF-1, and SMA and positive for Bcl2, CD99 and TLE-1 antibody. SS was differentiated from SETTLE based on the negative staining for SMA and positive staining for TLE-1 antibody. In addition, Fluorescence in situ hybridization (FISH)) technique demonstrated the characteristic t(X; 18) (p11; q11) translocation. Thus, the definitive diagnosis as primary SS of the thyroid gland was made.
No management consensus exists for primary thyroid SS. Generally, the treatment entails wide local excision with safety margins. The role of postoperative adjuvant chemotherapy and radiotherapy (CRT) is controversial. The patients received postoperative CRT in some studies but data related to recurrence or survival advantage was not reported. Other studies reported no survival or control benefit [11,12]. In our case, total thyroidectomy was sufficient to completely excise the tumour and postoperative CRT was given. Radical surgery in the form of total thyroidectomy with partial resection of thyroid and cricoid cartilages was necessary in some reports [7].
Overall, the prognosis of thyroid SS is very unfavorable possibly due to the rare location, rapid growth, and difficult complete excision. As the published studies are limited, data related to prognosis cannot be made. Studies reported loco regional recurrence and metastatic relapse after surgery and survival was limited 3 or 36 months. Our patient did not suffer any local or distant recurrence for 12 months after surgery [7,9]. The need for follow-up surveillance for any local or distant recurrence is important [5].
Conclusion
SS is a rare clinical entity that should be always considered when dealing with thyroid mass. Identification of the t(X; 18) (p11; q11) translocation by fluorescence in situ hybridization and/or RT-PCR is essential to establish the definitive diagnosis. Generally, the treatment entails wide local excision with safety margins. The role of postoperative adjuvant chemotherapy and radiotherapy (CRT) is controversial.
Acknowledgements
We extend our thanks to our colleagues from the Pathology Department, Faculty of Medicine; University of Alexandria: Prof. Dr. Nagwa Oweiss; Professor of Pathology, Prof. Dr. Hanan Tayel; Professor of Pathology and Dr.Maram Allam; Assistant lecturer.
Author contributions
MZ: Corresponding author; design of the study, data collection and interpretation, final approval of the manuscript; AH: data interpretation, manuscript revision and approval; SA: drafting the manuscript and data collection; AY: data collection and interpretation, final approval of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carrillo R, Rodriguez-Peralto JL, Batsakis JG. Synovial sarcomas of the head and neck. Ann Otol Rhinol Laryngol. 1992;101:367–370. doi: 10.1177/000348949210100415. [DOI] [PubMed] [Google Scholar]
- 2.O’Sullivan PJ, Harris AC, Munk PL. Radiological features of synovial cell sarcoma. Br J Radiol. 2008;81:346–356. doi: 10.1259/bjr/28335824. [DOI] [PubMed] [Google Scholar]
- 3.Chan JA, McMenamin ME, Fletcher CD. Synovial sarcoma in older patients: clinicopathological analysis of 32 cases with emphasis on unusual histological features. Histopathology. 2003;43(1):72–83. doi: 10.1046/j.1365-2559.2003.01643.x. [DOI] [PubMed] [Google Scholar]
- 4.Sturgis EM, Potter BO. Sarcomas of the head and neck region. Curr Opin Oncol. 2003;15:239–252. doi: 10.1097/00001622-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Owen C, Constantinidou A, Miah AB, Thway K, Fisher C, Benson C, et al. Synovial Sarcoma of the thyroid gland, diagnostic pitfalls and clinical management. Anticancer Res. 2018;38:5275–5282. doi: 10.21873/anticanres.12853. [DOI] [PubMed] [Google Scholar]
- 6.Roth JA, Enzinger FM, Tannenbaum M. Synovial sarcoma of the neck: a followup study of 24 cases. Cancer. 1975;35(4):1243–1253. doi: 10.1002/1097-0142(197504)35:4<1243::AID-CNCR2820350432>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi I, Anbo J, Nakamura S, Sugai T, Sasou S, Yamamoto M, Oda Y, Shiratsuchi H, Tsuneyoshi M. Synovial sarcoma of the thyroid. Report of a case with aspiration cytology findings and gene analysis. Acta Cytol. 2003;47(3):495–500. doi: 10.1159/000326558. [DOI] [PubMed] [Google Scholar]
- 8.Jang KS, Min KW, Jang SH, Paik SS, Tae K, Jang SJ, Park MH. Primary synovial sarcoma of the thyroid gland. J Korean Med Sci. 2007;22(Suppl):S154–158. doi: 10.3346/jkms.2007.22.S.S154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryu CH, Cho KJ, Choi SH. Synovial sarcoma of the thyroid gland. Clin Exp Otorhinolaryngol. 2011;4(4):204–206. doi: 10.3342/ceo.2011.4.4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghafouri A, Anbara T, Mir A, Lashkari M, Nazari M. Thyroid synovial sarcoma: a case report. Acta Med Iran. 2013;51(1):69–72. [PubMed] [Google Scholar]
- 11.Boudin L, Fakhry N, Chetaille B, Perrot D, Nguyen AT, Daidj N, et al. Primary synovial sarcoma of the thyroid gland: case report and review of the literature. Case Rep Oncol. 2014;7(1):6–13. doi: 10.1159/000357913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bansal N, Ranade RS, Mishra A. Synovial sarcoma mimicking thyroid carcinoma. ANZ J Surg. 2017;87(11):E214–E215. doi: 10.1111/ans.13113. [DOI] [PubMed] [Google Scholar]
- 13.Murro D, Slade JM, Syed S, Gattuso P. Fine needle aspiration of secondary synovial sarcoma of the thyroid gland. Diagn Cytopathol. 2015;43(11):928–932. doi: 10.1002/dc.23327. [DOI] [PubMed] [Google Scholar]
- 14.Shi RL, Qu N, Gao LL, Lu ZW, Sun GH, Ji QH. Primary synovial sarcoma of the thyroid with locally repeated relapses in short periods: a case report. Biomed Rep. 2016;5(1):79–82. doi: 10.3892/br.2016.670. [DOI] [PMC free article] [PubMed] [Google Scholar]


