Abstract
Abstract
Cricopharyngeal muscle myotomy (CPM) is a common intervention for relief of dysphagia in patients with Oculo-pharyngeal muscular dystrophy (OPMD). Because of difficulties in approaching and dissecting cricopharyngeal muscle in these patients, we used transillumination for the myotomy (TA-CPM). Transillumination is a simple technique to improve the guidance and navigation of the surgeon in determining the location and depth of myotomy. The purpose of this study is to evaluate the efficacy and safety of transillumination in CPM in OPMD patients. An observational cohort of patients with OPMD who underwent CPM due to dysphagia at one medical center between 2010 and 2019. Two groups of patients are included, according to whether transillumination was used during their surgery. Patients were evaluated before and after surgery (1 week and 1 month) for their dysphagia score with a standardized questionnaire. The surgical team preferences, experience and complexity with and without transillumination were evaluated. Ten OPMD patients (8 heterozygotes, 2 homozygotes for the commonmutation) underwent CPM for relieving dysphagia symptoms at medium size medical center in Israel between 2010 and 2019. Five patients had TA-CPM and the 5 patients had CPM without transillumination. All patients showed an improvement at follow-up examinations, 1 week and 1 month postoperative, including a decrease in dysphagia score and in choking and aspiration events, compared to their preoperative state. TA-CPM improved the surgical approach, reduced the difficulty of CPM and was preferred by the surgical team. From the patients' point of view, TA-CPM was as good as a non-transillumination approach in improving dysphagia. TA-CPM is a cheap, fast and simple technique to improve the surgical outcomes in CPM for patients with OPMD. TA-CPM navigates the surgeon, helps with anatomical orientation, improve the surgeon's comfortable, may shorten the duration of surgery and reduces potential errors. Improvement in dysphagia score was similar in both groups. This technique may improve myotomy procedures for dysphagia of other etiologies.
Level of evidence
IV. Case series (with or without comparison). Endoscopic transillumination assisted myotomy.
Keywords: Oculo-pharyngeal muscular dystrophy, Crico-pharyngeal muscle myotomy, Transillumination, Dysphagia
Introduction
Oculo-Pharyngeal Muscular Dystrophy (OPMD) is a rare genetic disease characterized by muscle weakness that begins in adulthood, usually after age of 50 years. The prevalence of OPMD worldwide varies; In Europe, it is about 1/100,000–1/200,000 people. The highest prevalence rate is 1/600 in Israel's Bukharan Jews and about 1/1,000 in French Canadians in Quebec, Canada [1].
OPMD usually starts in the fifth to sixth decade of life. Early symptoms include eyelid ptosis, limb weakness, dysphagia, tongue weakness and atrophy, proximal upper and lower extremity weakness, facial muscle weakness, dysarthria and dysphonia. About 5–10% of cases the disease is more severe and presents before the age of 45 [1].
OPMD is inherited both autosomal dominantly (in most cases) and recessively. Genetic counseling is possible when a polyadenylate binding protein nuclear 1 (PABPN1) mutation has been identified in a family [2]. Most patients are heterozygotes for one of the expansion mutations of the trinucleotide GCN in the first exon of the gene encoding the PABPN1 on chromosome 14q [2]. Patients with autosomal dominant OPMD have one normal and one expanded allele, in the range of 12–17 GCN repeats. The exceedingly rare patients with autosomal recessive OPMD have two (GCN) 11 alleles [3, 4].
In Israel, OPMD is prevalent among people of central Asian ancestry, commonly referred to as Bukhara Jews [3]. Most of them are heterozygotes sharing a (GCN) 13 founder expansion mutation [3, 4]. Homozygotes for this mutation have a very severe form of the disease, with a markedly reduced life expectancy [5, 6].
In early stages, the best interventions to prevent dysphagia is by means of adopting good eating strategies, dietary modifications and good chewing techniques. However, as disease progress, more active, mechanical interventions to ease swallowing become necessary and surgical treatments provide a descent relief [7]. These include esophageal dilatation procedures, local botulinum toxin injections, and cricopharyngeal myotomies (CPM) [8–13]. CPM may be performed to achieve normal swallowing, but dysphagia usually recurs years after surgery. The intake of dietary supplements and a diet of foods that are soft and easy to swallow are often necessary.
Over the past 22 years, 76 heterozygote and 6 homozygote OPMD patients were treated at our hospital, with follow-up starting from 2 to 30 years after the onset of disease. Of them, ten patients had CPM between 2010 and 2019. This paper presents outcomes of CPM in a small cohort of heterozygote and homozygote OPMD patients and describe the use of transillumination assisted CPM (TA-CPM).
In medicine, transillumination generally refers to the transmission of light through tissues of the body. TA-CPM has never been described. Due to difficulties in dissection of cricopharyngeal muscle from the mucosa in OPMD patients, transillumination-assisted approach may improve surgical and clinical outcomes in patients with OPMD. Furthermore, this simple technique may be used in other myotomies as in Zenker diverticulum surgery.
The purpose of this study is to evaluate TA-CPM in OPMD patients in order to draw conclusions regarding its efficacy and safety of the new surgical technique.
Methods
Patients–Over the past 22 years, 82 OPMD patients were treated at one medical center, which is a regional center for this disease. Patients were treated by a multidisciplinary team of otorhinolaryngologists, neurologists, ophthalmologists, occupational therapist, physiotherapists and dietitians. Analysis was performed on patients who had CPM between 2010 and 2019.
Twenty-eight OPMD patients with moderate to severe dysphagia (score higher than II, according to our dysphagia table score, Table 1) which results in poor quality of life were potential candidates for CPM. They were passed a meticulous physical examination by neurologists and otorhinolaryngologists and had a fiberoptic endoscopic evaluation of their swallowing.
Table 1.
Description of dysphagia scoring
| Description | Dysphagia score |
|---|---|
| No dysphagia: able to eat normal diet | 0 |
| Moderate passage: able to eat some solid foods | I |
| Poor passage: able to eat semi-solid foods | II |
| Very poor passage: able to swallow liquids only | III |
| No passage: unable to swallow anything | IV |
After excluding 18 patients who did not fit for CPM (too frail, terminally ill, mild disease, had good conservative treatment), 10 patients were left and passed CPM.
Of the ten patients who underwent CPM, two were homozygotes to the mutations. In the group of patients who had transillumination during CPM (5 patients), 4 were heterozygotes and 1 was homozygote for the mutation.
The remaining 56 patients were less symptomatic or opted not to undergo the surgery.
Indications for CPM
There are no clear guidelines when to refer OPMD for CPM. The following criteria are common indications (inclusion criteria):
Patients who suffer from moderate to severe (II-IV) dysphagia (according to dysphagia scale score, Table 1).
Patients who lost more than 10% of their body weight in the last several months.
Patients who develop significant dysphonia.
Patients who experience more than two episodes of aspiration pneumonia or chocking.
These symptoms are likely to occur in heterozygotes at the end of the second decade or during the third decade following disease onset, and much earlier in homozygote patients.
In emaciated severely dysphonic or percutaneous endoscopic gastrostomy (PEG) fed patients, this study found CPM to yield, at best, modest to short-term (several months) improvement.
The External Approach to CPM
This approach includes dissection of the lower pharyngeal muscles and identification of the cricopharyngeus muscle [14]. Myotomy of the cricopharyngeus muscle is done by a left cervical oblique incision along the anteromedial border of the sternomastoid muscle. An endotracheal tube No. 7 is introduced in the esophagus and used as a stent. A rigid esophagoscope can also be used in the esophagus for the surgeon's guidance.
In the posterolateral aspect of the pharyngoesophageal junction a 6 cm myotomy is performed. The muscularis along the myotomy is dissected free from the esophageal submucosa, which is thin, and muscle atrophy sometimes presents in OPMD patients (Fig. 1). A nasogastric tube is left in place for gastric decompression until peristalsis has resumed. This is used first to decompress the stomach and to avoid aspiration. A small drain is left at the thoracic inlet level and behind the myotomized zone for 24 h [15].
Fig. 1.
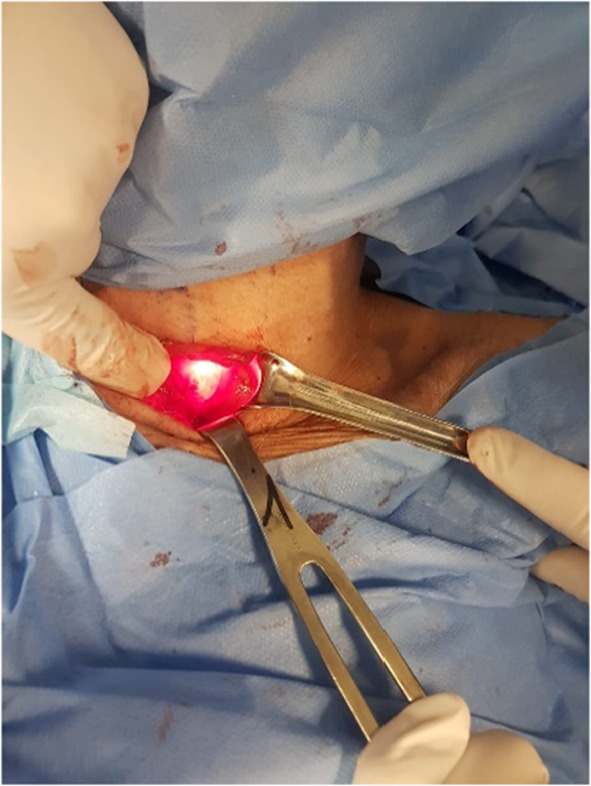
The upper esophageal sphincter is exposed at the left neck, and transillumination is used to dissect the cricopharyngeal muscle
Transillumination Technique in CPM
The patient is intubated under general anesthesia and esophagoscopy is performed to evaluate the upper esophageal region. A tracheal tube is inserted into the upper part of the esophagus as a stent. A fiber optic nasopharyngoscope is inserted into the esophageal tube such that the distal end of the nasopharyngoscope reaches the cricopharyngeus level. A left cervical oblique incision is made along the anteromedial border of the sternomastoid muscle. In the posterolateral aspect of the pharyngoesophageal junction a 6 cm myotomy is performed. The muscularis along the myotomy is dissected during illumination of the upper esophagus by the nasopharyngoscope. The light from inside the esophageal lumen directs the surgeon to the location and depth of the myotomy until it is released from the esophageal submucosa (Fig. 2). A nasogastric tube is left in place for gastric decompression until peristalsis has resumed.
Fig. 2.
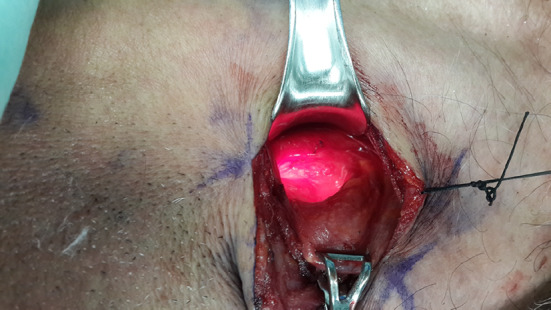
Cricopharyngeal myotomy—the transillumination shows the mucosa of the esophagus and directs the surgeon to cut the cricopharyngeal fibers up to the mucosa
Follow Up
All patients were followed up for at least 12 months after surgery. Several variables were followed, as described below.
Statistical Analysis
Data analysis was done with IBM statistics (SPSS) version 24.
Description analysis of sequential variables was demonstrated using mean, standard deviation and range. When trying to find a difference in dysphagia score before and after CPM, a non-parametric Mann–Whitney test, one-way analysis of variance or one-way ANOVA was conducted. Distributions of scores were similar in both groups as assessed by inspection. A P-value less than 0.05 was considered as statistically significant.
Patient satisfaction—By subjective assessment and by improved in dysphagia score.
Team satisfaction—Only by subjective assessment. Cannot be assessed in this type of study (observational).
Transillumination Efficacy—Cannot be assessed in this type of observational study. Surgery duration was not measured in this study.
Results
There were eight men and two women who passed CPM, mean age 63.8 years (range 49–78, standard deviation 8.5), between 2010 and 2019.
Before surgery, the dysphagia score in all patients was equal or higher than then moderate (II–IV), according to the dysphagia scale scores mentioned in Table 1.
Three men and two women underwent CPM without transillumination; one of the men was OPMD homozygous.
Five men, one homozygous and four heterozygous, underwent CPM with transillumination.
The following chart demonstrated the study methods and conclusions.
Table 2 summarizes the characteristics of the patients who underwent CPM with and without transillumination, and the outcomes in both procedures.
Table 2.
Characteristics of patients with OPMD who underwent CPM and outcomes of the procedures
| Age | Sex | Dysphagia score1 | Transillumination | Demonstration of esophageal mucosa | Post-operative state and dysphagia score1 | OPMD type * | |
|---|---|---|---|---|---|---|---|
| 1 | 62 | F | III | Well, II | Ht | ||
| 2 | 54 | M | IV |
Well, Mild improvement, III–IV. Died two years later from neurologic complication |
HZ | ||
| 3 | 66 | F | III | Very well, I–II | Ht | ||
| 4 | 70 | M | VI | III, Aspirations, pneumonia, died April 2010 | Ht | ||
| 5 | 72 | M | II–III | Well, I–II | Ht | ||
| 6 | 78 | M | III | + | + |
Well, some residual aspirations, II |
Ht |
| 7 | 49 | M | II–III | + | + | Well, I–II | HZ |
| 8 | 65 | M | II | + | + | Well, I | Ht |
| 9 | 55 | M | III | + | + | Well, II | Ht |
| 10 | 67 | M | III | + | + | Well, I | Ht |
* HZ–Homozygote; ht–heterozygote
1 According to Table 1
One week and one-month post-operative, all patients showed markedly improvements and a decrease in their dysphagia score (p < 0.05) (Table 2) as illustrated on Fig. 3.
Fig. 3.
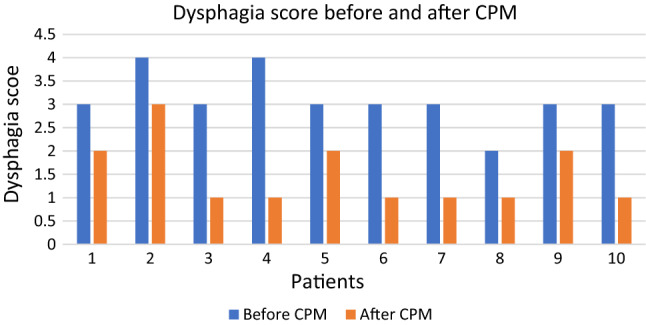
Changes in dysphagia score in patients. Note that patients no. 6–8 had CPM assisted by transillumination
The improvement was measured both objectively and subjectively. All patients felt better and were less symptomatic based on questionnaires; All patients demonstrated a decrease dysphagia score, and in both choking and aspiration events.
No statistical difference was found between the group of patients underwent the surgery with or without transillumination (p value > 0.05). This means that both groups the reduction rates of dysphagia symptoms were similar.
Enteral feeding was delayed for one year in one of the homozygote patients (patient no. 7).
During the follow-up period both patients who had severe preoperative dysphagia passed away: a heterozygote patient (patient no. 4) died within 2 months of the procedure, and a homozygote patient (patient no. 2) died 2 years postoperative.
Discussion
Transillumination is an established technique and a major application of visible light in medicine. It is a component of neurologic evaluation in infants and children. In the field of otolaryngology, transillumination is an old technique especially used in sinus exploration [16, 17], but its use has recently been described in balloon sinuplasty technology with transillumination of the sinuses as LUMA [18], in peroral endoscopic myotomy [19] and to improve the accuracy of percutaneous needle localization in the larynx [20].
This paper describes the usage of transillumination technique to improve the guidance of the surgeon in determining location and depth in CPM. This technique is of great importance in OPMD patients since their muscle fibers are sometimes dystrophic, especially in homozygote patients. Histological changes are common to many muscular dystrophies (loss of muscle fibers, abnormal variation in fiber size, increased number of nuclei, and expanded interstitial fibrous and fatty connective tissue).
In OPMD, CPM is shown to reduce difficulties in transporting food during the swallowing reflex, from the pharynx to the esophagus, especially in cases of refractory oropharyngeal dysphagia.
The myotomy technique was initially described by Montgomery and Lynch [21] and performed on patients with dystrophy. Several subsequent authors have proposed CPM for the management of upper esophageal and pharyngeal dysphagia in the setting of polio, degenerative neurologic disorders, and strokes, and after head and neck surgery [15, 22, 23]. Even though, transillumination has not yet been described in CPM techniques.
Based on research, CPM palliates OPMD dysphagia and can help patients with neurologic disease, who retain proper voluntary deglutition [22].
Improvement after CPM can be expected if there is no deterioration from the basic stage and if there is better swallowing after the surgery. The following criteria are generally important to maintain good swallowing, and they were all preserved in our patients: (1) normal voluntary deglutition (2), adequate tongue movement (3), intact laryngeal function and phonation, and (4) absence of dysarthria.
When combining transillumination during CPM, two aspects should be considered—the patients' outcome and the surgeons' experience and preferences.
From the patient's point of view, using transillumination is non-inferior to the common practice. Both methods decrease symptoms of dysphagia to the same extant, without any statistical difference. Patients in both groups were followed up for one month following surgery and showed improvement in dysphagia score. However, from the surgeon's point of view, using transillumination during CPM has many advantages and little-to no negative influence.
The aid of transillumination during CPM helps to define the area for myotomy and its' main purpose is with adequate muscle cut (to avoid too much or too little cutting). Thus, it reduces chances for esophageal rupture and consequently mediastinitis.
Subjectively, surgeons' preferences were to use the aid of transillumination, ever since they start to use it, in the mid of 2010s. TA-CPM is a new generation, upon surgeon fills more confident. Since it facilitates the orientation in the surgical field, one could compare it to endoscopic sinus surgery with navigation.
Complications can occur in the traditional method of CPM, and they depend on surgical experience and the muscular tissue pathology.
With the aid of transillumination during CPM, there are almost no drawbacks and it makes the procedure to be way more accurate and easier for the surgeon.
In this study we did not measured nor compared the operation time with and without the assistance of transillumination, but to our feeling it may reduce the overall operation time, because surgeons' felt more confident and more familiar with the anatomy variations. Further comparison should be conducted in the future.
Transillumination can facilitate when trying to determine the depth of surgical incision and brightens the entire field. Thus, the dissection is much easier and the surgeon and has less chances to err.
When considering the disadvantages of transillumination, it is worth to mention that the surgical team needs to be familiar with the equipment; Endoscope insertion involves the known risks and may damage the areas where it passes. But, in fact, these drawbacks can be easily overcome with an experienced surgical team.
This study could not demonstrate any statistical difference in patient's dysphagia score between the group underwent CPM with and without transillumination (p value > 0.05). Both groups showed a reduction of both subjective and objective variables and reduction in the dysphagia scores compared to preoperative period. Thus, this study shows that using transillumination technique is not inferior to the common practice and has its advantages in facilitating the surgeon's orientation in the surgical field and may reduce potential adverse events.
This paper presents an innovative technique of TA-CPM for both heterozygote and homozygote OPMD patients. Homozygotes have more rapid deterioration with earlier severe complications; therefore, they should be closely monitored during the first decade of disease.
Long-term assessment of the outcomes and complications of interventions to treat dysphagia associated with OPMD is important. For example, 8-years follow-up of Upper esophageal sphincter (UES) myotomy showed recurrence of swallowing and tracheobronchial symptoms in several cases [11]. A long-term study showed that 3 years after CPM, one-third of the patients who had shown early improvement, exhibited recurrence of dysphagia [24].
The cohort in this study is relatively small and larger numbers of patients should be included in further studies to establish more significant results. However, the rarity of the disease precludes conducting a comparative study. Surgery duration should be investigated as well. Authors suggests investigation of the use of this technique to improve myotomy procedures for dysphagia of other etiologies.
Conclusions
This paper presents the usage of TA-CPM in OPMD patients. TA-CPM is a cheap, fast and simple technique to improve the surgical outcomes in CPM for patients with OPMD. TA-CPM navigates the surgeon, helps with anatomical orientation, improve the surgeon's comfortable, may shorten the duration of surgery and reduces potential errors. Improvement in dysphagia score was similar in both groups. This technique may improve myotomy procedures for dysphagia of other etiologies.
Abbreviations
- CPM
Crico-Pharyngeal Myotomy
- OPMD
Oculo-Pharyngeal Muscular Dystrophy
- UES
Upper Esophageal Sphincter
Funding
This study was not funded by any grant.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Human or Animal Rights
The current publication includes data collection only and did not involve Human Participants and/or Animals.
Informed Consent
Local IRB approved waiving informed consent due to retrospective anonymous data collection.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Galit Avior and Roee Noy have contributed equally.
References
- 1.Trollet C, Gidaro T, Klein P et al (2001) Oculopharyngeal Muscular Dystrophy. In: Adam MP, Ardinger HH, Pagon RA et al (eds) GeneReviews® [Internet]. University of Washington, Seattle, Seattle (WA), pp 1993–2020. https://www.ncbi.nlm.nih.gov/books/NBK1126/
- 2.Brais B, Bouchard JP, Xie YG, et al. Short GCG expansions in the PABPN2 gene cause oculopharyngeal muscular dystrophy. Nat Genet. 1998;18:164–167. doi: 10.1038/ng0298-164. [DOI] [PubMed] [Google Scholar]
- 3.Blumen SC, Nisipeanu P, Sadeh M, et al. Epidemiology and inheritance of oculopharyngeal muscular dystrophy in Israel. NeuromusculDisord. 1997;7(Suppl. 1):S38–40. doi: 10.1016/s0960-8966(97)00080-1. [DOI] [PubMed] [Google Scholar]
- 4.Blumen SC, Korczyn AD, Lavoie H, et al. Oculopharyngeal MD among Bukhara Jews is due to a founder (GCG)9 mutation in the PABP2 gene. Neurology. 2000;55:1267–1270. doi: 10.1212/WNL.55.9.1267. [DOI] [PubMed] [Google Scholar]
- 5.Blumen SC, Brais B, Korczyn AD, et al. Homozygotes for Oculopharyngeal muscular dystrophy have a severe form of the disease. Ann Neurol. 1994;46:115–118. doi: 10.1002/1531-8249(199907)46:1<115::AID-ANA17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 6.Blumen SC, Bouchard J-P, Brais B, Carasso RL, Paleacu D, Drory VE, et al. Cognitive impairment and reduced life span of Oculopharyngeal muscular dystrophy homozygotes. Neurology. 2009;73:596–601. doi: 10.1212/WNL.0b013e3181b388a3. [DOI] [PubMed] [Google Scholar]
- 7.Carter Young E, Durant-Jones L. Gradual onset of dysphagia: a study of patients with oculopharyngeal muscular dystrophy. Dysphagia. 1997;12:196–201. doi: 10.1007/PL00009536. [DOI] [PubMed] [Google Scholar]
- 8.Lacau St Guily J, Perie S, Willing TN, et al. Swallowing disorders in muscular diseases; functional assessment and indications for cricopharyngealmyotomy. Ear Nose Throat J. 1994;73:34–40. doi: 10.1177/014556139407300109. [DOI] [PubMed] [Google Scholar]
- 9.Mathieu J, Lapointe G, Brassard A, et al. A pilot study on upper esophageal dilatation for the treatment of dysphagia in oculopharyngeal muscular dystrophy. NeuromusculDisord. 1997;7:S100–S104. doi: 10.1016/s0960-8966(97)00092-8. [DOI] [PubMed] [Google Scholar]
- 10.Restivo DA, Marchese RR, Staffieri A, De Grandis D. Successful botulinum toxin treatment of dysphagia in oculopharyngeal muscular dystrophy. Gastroenterology. 2000;119:1416. doi: 10.1053/gast.2000.20113. [DOI] [PubMed] [Google Scholar]
- 11.Fradet G, Pouliot D, Robichaud R, St-Pierre S, Bouchard JP. Upper esophageal sphincter myotomy in oculopharyngeal muscular dystrophy: long-term clinical results. NeuromusculDisord. 1997;7:S90–S95. doi: 10.1016/s0960-8966(97)00090-4. [DOI] [PubMed] [Google Scholar]
- 12.Périé S, Eymard B, Laccourreye L, Chaussade S, Fardeau M, Lacau St Guily J. Dysphagia in oculopharyngeal muscular dystrophy: a series of 22 French cases. NeuromusculDisord. 1997;7:S96–S99. doi: 10.1016/s0960-8966(97)00091-6. [DOI] [PubMed] [Google Scholar]
- 13.Gómez-Torres A, Abrante Jiménez A, Rivas Infante E, Menoyo Bueno A, Tirado Zamora I, Esteban Ortega F. Cricopharyngealmyotomy in the treatment of oculopharyngeal muscular dystrophy. ActaOtorrinolaringolEsp. 2012;63:465–469. doi: 10.1016/j.otorri.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Kelly J. External approach to cricopharyngeus muscle (CP) myotomy. Operative Techniques in Otolaryngology Head and Neck Surgery. 2009;8:193–198. doi: 10.1016/S1043-1810(97)80030-9. [DOI] [Google Scholar]
- 15.Poirier NC, Bonavina L, Taillefer R, Nosadini A, Peracchia A, Duranceau A. Cricopharyngealmyotomy for neurogenic oropharyngeal dysphagia. J ThoracCardiovascSurg. 1997;113:233–241. doi: 10.1016/S0022-5223(97)70318-0. [DOI] [PubMed] [Google Scholar]
- 16.Kelly AB. Transillumination of the Antrum of Highmore. Br Med J. 1905;1(2308):650–653. doi: 10.1136/bmj.1.2308.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin N, Prince JM, Kane TD, Goyal A, Mehta D. Congenital cricopharyngeal achalasia in a 4.5-year-old managed by cervical myotomy: a case report. Int J PediatrOtorhinolaryngol. 2011;75:289–292. doi: 10.1016/j.ijporl.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Zeiders JW, Dahya ZJ. Antral lavage using the Lumatransilluminaton wire and vortex irrigator–a safe and effective advance in treating pediatric sinusitis. Int J PediatrOtorhinolaryngol. 2011;75:461–463. doi: 10.1016/j.ijporl.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Baldaque-Silva F, Marques M, Vilas-Boas F, Maia JD, Sá F, Macedo G. New transillumination auxiliary technique for peroral endoscopic myotomy. GastrointestEndosc. 2014;79:544–545. doi: 10.1016/j.gie.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman HT, Dailey SH, Bock JM, Thibeault SL, McCulloch TM. Transillumination for needle localization in the larynx. Laryngoscope. 2015;125:2341–2348. doi: 10.1002/lary.25372. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery WW, Lynch JP. Oculopharyngeal muscular dystrophy treated by inferior constrictor myotomy. Trans Am AcadOphthalmolOtolaryngol. 1971;75:986–993. [PubMed] [Google Scholar]
- 22.Duranceau A. Cricopharyngealmyotomy in the management of neuogenic and muscular dysphagia. NeuromusculDisord. 1997;7:85–89. [Google Scholar]
- 23.Mason RJ, Bremner CG, DeMeester TR, et al. Pharyngeal swallowing disorders: selection for and outcome after myotomy. Ann Surg. 1998;228:598–608. doi: 10.1097/00000658-199810000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coiffier L, Perie S, Laforet P, Eymard B, Lacau St Guily J. Long-term results of crycopharyngealmyotomy in oculopharyngeal muscular dystrophy. Otolaryngol Head Neck Surg. 2006;135:218–222. doi: 10.1016/j.otohns.2006.03.015. [DOI] [PubMed] [Google Scholar]


