Abstract
Eosinophilic chronic rhinosinusitis with nasal polyps (eCRSwNP) and non-eosinophilic chronic rhinosinusitis with nasal polyps (neCRSwNP) are two distinct endotypes of chronic rhinosinusitis with nasal polyps (CRSwNP). The aim of the study was to find the prevalence of eCRSwNP and neCRSwNP, their clinical comparison and to establish predictive values for clinical and diagnostic factors to differentiate between eCRSwNP and neCRSwNP in Indian population with CRSwNP. This study was a prospective cohort, multi- institutional study. A total of 162 patients who were diagnosed with nasal polyps at different military hospitals in India during the period from 2011 to 2020 were selected for study. They were diagnosed in accordance with EPOS guidelines. They were randomly divided into two groups as eCRSwNP and neCRSwNP based on the response to oral corticosteroids for 2 weeks duration and the prevalence of eCRSwNP was established. Blood samples were collected and endoscopic sinus surgery was performed in all patients after atleast 2 months of last steroid dose. Preop CT scan scores, preop nasal endoscopy scores, preop blood eosinophil counts, preop tissue eosinophil counts were compared between the groups. Postop followup was done at 6 months by comparing CT scan scores and nasal endoscopy scores. Predictive values for clinical and diagnostic factors were established to diagnose eCRSwNP in Indian population. Out of a total 162 patients, 121 (74.6%) patients were classified into eCRSwNP and 41 (23.6%) into neCRSwNP out of a total of 162 patients with CRSwNP. CRSwNP was seen in the 4th decade. eCRSwNP was seen in the later part and neCRSwNP was seen in the early part. eCRSwNP was more common in males and neCRSwNP was more common in females. Smoking, asthma and aspirin intolerance were more commonly seen in eCRSwNP than neCRSwNP, p < 0.001, p = 0.020 respectively. Preop total CT scan score, preop bood absolute eosinophil count,preop blood eosinophil percentage, tissue eosinophil percentage, postop nasal endoscopy score, postop CT scan score were stastically significant in eCRSwNP, p < 0.001 except preop total nasal endoscopic score. Tissue absolute eosinophil count had best predictive accuracy plotted with receiver operating characteristic (ROC) curve analysis, area under curve (AUC) 0.923(95% CI, 0.876–0.970). The cutoff points determined to diagnose eCRSwNP were ≥ 15 for preop total CT scan score, ≥ 378 × 106/L for preop absolute blood eosinophil count, ≥ 6.5% for preop blood eosinophil percentage, ≥ 14% for tissue eosinophil percentage, ≥ 16 for absolute tissue eosinophil count, ≥ 1 for 6 months postop total nasal endoscopy score, ≥ 2 for 6 months postop total CT scan score. eCRSwNP and neCRSwNP are two distinct endotypes of nasal polyps present in Indian population with CRSwNP. Two thirds of the patients with nasal polyps were eCRSwNP and the prevalence in Indian population is more than the East Asian population but less than the Western population. There is a high chance of recurrence and treatment failures for eCRSwNP than neCRSwNP. The cutoff points for various non invasive diagnostic predictors are useful to diagnose the patients with eCRSwNP during the outpatient visits and hence plan for better treatment strategies.
Keywords: Chronic rhinosinusitis with nasal polyps, Eosinophic chronic rhinosinusitis with nasal polyps, Non-eosinophilic chronic rhinosinusitis with nasal polyps
Introduction
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a chronic sinonasal inflammatory condition with symptoms lasting for more than 12 weeks [1]. Nasal polyps are more frequently observed with chronic rhinosinusitis, they are the inflammatory out growths of the sinonasal tissues originating from the ethmoid sinuses and typically originate bilaterally [1]. Nasal polyps is a multifactorial disease due to infectious,non infectious inflammation, anatomic and genetic abnormalities but most theories consider conditions leading to chronic inflammation in the nasal cavity to cause nasal polyps [2]. It is also observed that nasal polyps itself comprises with several disorders such as AFS, AERD cystic fibrosis and with disorders having distinct etiology [2]. Mucosal eosinophilia is known as one of the widely accepted causes for chronic inflammation. The presence of eosinophilis is associated with more aggrasive form of the disease and recurrence of nasal polyps after surgery [3]. CRS with Nasal polyps are classified as eosinophilic chronic rhinosinusitis with nasal polyps (eCRSwNP)and non eosinophilic chronic rhinosinusitis with nasal polyps (neCRSwNP) based on the different degrees of inflammation [4]. T-cell derived interleukin 5(IL-5) and autosecretion of IL-5 from activated eosinophils release a wide range of cytoxic proteins and transforming growth factors which has the pathogenesis to polyp formation and recurrence [5]. neCRSwNP can be more easily controlled by endoscopic sinus surgery (ESS) and long term low dose macrolide therapy whereas eCRSwNP has high recurrence following surgery, responds to steroid therapy and unresponsive to macrolide therapy [6, 7].
The pattern of inflammation is predominantly eosinophilic dominant in western population but in Japan and east asian population it is less than 50% present [8, 9]. Hence there may also be a role of ethnic and genetic causes for the etiology of polyps. Various previous studies have differentiated CRSwNP using multivariate regression analysis and odds ratios (ORs) by analyzing clinical features, endoscopic scores, CT scan scores, tissue and blood eosinophilia [10–13] into eCRSwNP and neCRSwNP.
Although the treatment strategies are different for eCRSwNP and neCRSwNP, there are few studies available on Indian population with CRSwNP that describe the prevalence, clinical, radiological and other diagnostic criteria to differentiate between eCRSwNP and neCRSwNP.
Methods
This was a prospective cohart study to know the prevalence of eCRSwNP and neCRSwNP.The study period was from Jun 2010 to Jan 2020 at tertiary care level military hospitals situated at different parts of India. Hyderabad/Secunderabad from 2011 to 2013, Bhopal from 2013 to 2016, Visakhapatnam from 2016 to 2019, Jammu from 2019 to 2020. Local residents such as veterans, military personnel and their dependants reporting to ENT dept at different military hospitals in India were enrolled in the study. A total of 162 patients with nasal polyps were recruited into the study. The diagnostic criteria for CRSwNP patients included in the study were in accordance with EPOS guidelines [14]. Patients who had other associated conditions such as cystic fibrosis, primary ciliary dyskinesis, AFRS, unilateral nasal polyps,cysts,antrochoanal polyps were excluded from the study. All patients enrolled into the study were free from use of any medication during the last one month.
Thorough history was taken for other risk factors of asthma, intolerance to aspirin,smoking, other systemic disease and family history of nasal polyps. During the opd visit the patients included in the study were randomized into two groups as eCRSwNP and neCRSwNP. If the polyps reduced in size and subjective symptoms improved after a systemic course of steroid tab prednisolone 50 mg per day for one week followed by one week tapering, a diagnosis of eCRSwNP was made, if not neCRSwNP was made [3].
Blood examination and surgery were done atleast two months after the last steroid dose. Nasal endoscopy was done preoperatively in all patients and documented as per Lund and Kennedy [15] endoscopy scoring system. CT scan scoring was documented as per Lund Mackay [16] CT scan score. Blood samples were drawn from all patients and a complete peripheral eosinophil percentage and absolute blood eosinophil count were done by pathologist before surgery.
ESS was performed in all patients with nasal polyps by the same surgeon. Complete removal of polyps and opening of the natural ostia were done based on the findings of the preoperative CT scan. The polyp tissue removed during the ESS was sent for HPE by pathologist.
Paraffin embedded samples were sectioned at 4 µm thickness, and after staining with H&E were observed under light microscopy at 400 × magnification. The tissue eosinophils were counted for 10 non overlapping fields and mean value was noted. Eosinophils were counted both as absolute numbers per hpf and as percentage to the total inflammatory cells.
Postoperative followup was done weekly for first month, at 3 months and at 6 months. Maximum followup was done for 6 months period. Endoscopic assessment and CT scan assessment were documented for analysis at 6 months followup period.
The aim of the present study is to find the prevalence of eCRSwNP and neCRSwNP and compare them by nasal endoscopy, CT scan imaging, blood eosinophil counts, tissue eosinophil counts and to establish diagnostic cutoff values to differentiate eCRSwNP from neCRSwNP in Indian population with CRSwNP.
The study was approved by the medical ethics committee of the respective military hospitals. A written informed consent was obtained from all the patients for the study and before being operated in the operation theatre.
Statistical Analysis:
All Statistical analysis were performed using IBM SPSS Statistics Version 26. The diagnostic risk factors and odds ratios (ORs) for CRSwNP to differentiate into eCRSwNP and neCRSwNP were analyzed using multivariate logistic regression analysis.Chi-square test was done to caliculate the frequencies of risk factors. Student t-test, Mann–Whitney U test, were performed for comparison of two independent groups (eCRSwNP and neCRSwNP) where applicable and Wilcoxon rank test was performed for comparision of two dependent groups (Before and after ESS). Clinical parameters that were significantly different between eCRSwNPand neCRSwNP were used in the subsequent evaluation as possible predictors of eCRSwNP. ROC curve plotted and diagnositic accuracy of each predictor was caliculated by knowing the area under the curve (AUC). Best cutoff values were caliculated by plotting sensitivity and specificity.
Results
The demographic and risk factors for eCRSwNP are shown in the Table 1. 162 patients were included in the study. 104 (64%) were male patients and 58 (36%) were female patients. 121 patients were classified as eCRSwNP and 41 patients as neCRSwNP.
Table 1.
The demographic and risk factors for eCRSwNP and neCRSwNP
| Demographics and risk factors | eCRSwNP (n = 121, 74.6%) | neCRSwNP (n = 41, 23.4%) | P value |
|---|---|---|---|
| Age, median (min–max) | 39 (17–71) | 32 (21–68) | 0.002 |
| Gender | |||
| Male | 81 (66.9%) | 23 (56.1%) | < 0.001 |
| Female | 40 (33.1%) | 18 (43.9%) | 0.004 |
| Duration of disease (mean) | 2.49 (95% CI 2.00–2.99) | 2.85 (95% CI 2.57–3.13) | 0.135 |
| Risk factors | |||
| Asthma | 18 (14.8%) | 3 (7.3%) | 0.002 |
| Aspirin hypersensitivity | 7 (5.8%) | 2 (4.8%) | 0.564 |
| Family history | 9 (7.4%) | 5 (12.1%) | 0.083 |
| Systemic disease | 4 (3.3%) | 2 (4.8%) | 0.414 |
| Smoking | 16 (13.2%) | 8 (19.5%) | < 0.001 |
| Asthma and aspirin intolerance | 8 (6.6%) | 1 (2.4%) | 0.020 |
The median age for eCRSwNP is 37 (17–71) mean 39.72 ± SD 12.17 (95% CI 37.53–41.91) and median age for neCRSwNP is 32 (21–68) mean age 34.22 ± 10.53 (95% CI 30.89–37.55) p = 0.002. eCRSwNP was more common in males 81 (66%), p < 0.001 and neCRSwNP was more common in females 18 (43.9%), p = 0.004. We found no significance with regard to duration of the disease, aspirin hypersensitivity, family history, systemic disease; p = 0.135, p = 0.564, p = 0.083, p = 0.414.,respectively. However in eCRSwNP smoking patients, combination of asthma and aspirin hypersensitivity were commonly seen. p < 0.001, p = 0.020.
The comparision of diagnostic factors with mean ± SD and 95% between the two groups are shown in the Table 2. There was a significant difference between the two groups for all factors except preop nasal endoscopy. Postop followup with nasal endoscopy and CT scan at 6 months showed a significant recurrence of polyps in the eCRSwNP group.
Table 2.
Comparision of clinical characteristics between eCRSwNP and neCRSwNP
| Diagnostic factor | eCRSwNP | neCRSwNP | P value | ||
|---|---|---|---|---|---|
| Mean ± SD | 95% CI, SE | Mean ± SD | 95%CI, SE | ||
| Preop total nasal endoscopy score | 9.34 ± 2.12 | 8.96, 9.72, 0.19 | 9.05 ± 1.38 | 8.61–9.48, 0.22 | 0.224 |
| Preop total CT scan score | 16.21 ± 4.06 | 15.48–16.95, 0.37 | 13.44 ± 2.78 | 12.56–14.32,0.43 | < 0.001 |
| Preop bood eosinophil percentage | 11.37 ± 6.38 | 10.22–12.52, 0.58 | 6.20 ± 3.52 | 5.09–7.30, 0.55 | < 0.001 |
| Preop blood absolute eosinophil count (× 106/L) | 937.5 ± 17.2 | 845.4–1031.3, 46.97 | 379.5 ± 305.1 | 283.2–476.2, 47.71 | < 0.001 |
| Tissue eosinophil percentage | 21.01 ± 8.64 | 19.45–22.56, 0.79 | 10.24 ± 4.40 | 8.86–11.63, 0.69 | < 0.001 |
| Tissue absolute eosinophil count | 31.60 ± 15.05 | 28.89–34.30, 1.37 | 14.44 ± 4.92 | 12.89–15.99, 0.77 | < 0.001 |
| Postop nasal endoscopy score | 0.86 ± 0.76 | 0.72–1.00, 0.07 | 0.54 ± 0.60 | 0.35–0.72, 0.09 | 0.014 |
| Postop total CT scan score | 3.12 ± 1.86 | 2.78–3.45, 0.17 | 2.17 ± 1.67 | 1.64–2.70, 0.26 | 0.004 |
CT scan scores before and after 6 months followup were 15.51 ± SD 3.96 (95% CI 14.90–16.13 SE 0.31), 2.88 ± SD 1.85 (95% CI 2.59–3.16, SE 0.15) p < 0.001 showed better surgery and treatment outcomes.
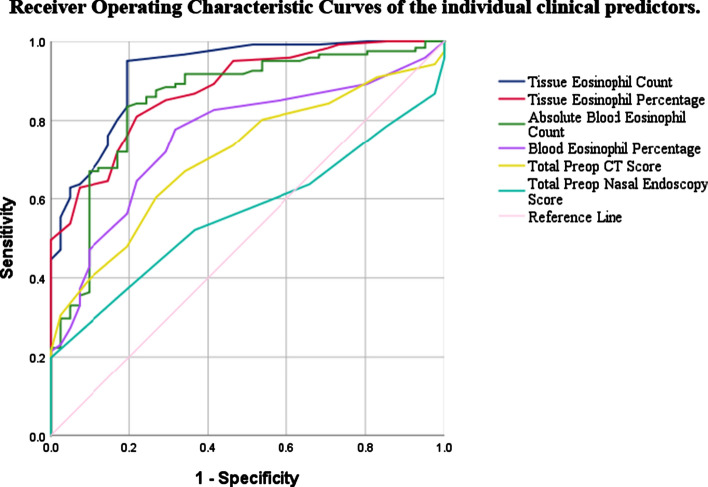
Receiver Operating Characteristic (ROC) curve plotted to assess the predictive values for eCRSwNP (Fig. 1). The curve was plotted by sensitivity and specificity of the predictor to find the best cut off value.The diagnositic ability of each predictor was caliculated based on the area under curve (AUC). AUC of each parameter are presented in Table 3. Absolute tissue eosinophil count showed high accuracy as a predictor for eCRSwNP with AUC 0.923.
Fig. 1.
Receiver operating characteristic curves of the individual clinical predictors
Table 3.
ROC curve analysis of clinical factors associated with eCRSwNP
| Predictors | AUC | 95% CI | |
|---|---|---|---|
| Lower | Upper | ||
| Tissue absolute eosinophil count | 0.923 | 0.876 | 0.970 |
| Tissue eosinophil percentage | 0.878 | 0.823 | 0.933 |
| Blood absolute eosinophil count | 0.852 | 0.781 | 0.922 |
| Blood eosinophil percentage | 0.758 | 0.679 | 0.837 |
| Preop total CT scan score | 0.709 | 0.627 | 0.791 |
| Preop total nasal endoscopy score | 0.563 | 0.475 | 0.651 |
The cutoff point for each predictor and corresponding sensitivity,specificity (1-sensitivity)2 + (1-specificity)2 at different thresholds is shown in Table 4. A highest predictive accuracy 0.040 [lowest (1-sensitivity)2 + (1-specificity)2] with cut off value of ≥ 16 with sensitivity of 81% and specificity of 78% was shown for total tissue eosinophil count. A lowest predictive accuracy was found for postop nasal endoscopy score at 6 months.
Table 4.
Determination of cut-off points to predict eCRSwNP in Indian population
| Diagnostic predictor | Cutoff value | Sensitivity | Specificity | (1-Sensitivity)2 + (1-specificity)2 |
|---|---|---|---|---|
| Total preop CT scan score | ≥ 15 | 66.9% | 65.9% | 0.226 |
| Total preop blood absolute eosinophil count (× 106/L) | ≥ 378 | 84.3% | 78.0% | 0.245 |
| Total preop blood eosinophil percentage | ≥ 6.5% | 77.7% | 68.3% | 0.150 |
| Total tissue eosinophil percentage | ≥ 14% | 81% | 78% | 0.084 |
| Total tissue eosinophil count | ≥ 16 | 95% | 80.5% | 0.040 |
| Postop total CT score at 6 months followup | ≥ 2 | 61.2% | 82.9% | 0.303 |
| Postop total endoscopy score at 6 months followup | ≥ 1 | 65.3% | 51.2% | 0.330 |
The cutoff points based on the lowest value of (1-sensitivity)2 + (1-specificity)2
Discussion
The clinically defined phenotypes (CRS without nasal polyps and CRS with nasal polyps) were not adequate for treatment, hence the search for new endotypes or subtypes began. Endotypes could be differentiated on the types of cells involved, such as abundance of eosinophils, neutrophils, TH cell population, levels of IgE or cytokines [17]. It was demonstrated that the inflammatory mediators TH2 based cytokines IL4, IL 5 and IL13 are key feature for eCRSwNP whereas neCRSwNP are by predominantly TH1/TH17 cell patterns [4]. Steroids especially glucocorticoids reduce the polyps associated with eosinophils by size and new formation affecting the inflammatory pathways in various ways [18]. The presence of mucosal eosinophilia is assosciated with severe form of disease recurrence after surgery. In our study during the OPD visit all patients diagnosed to have nasal polyps in accordance with EPOS guidelines [14] were given 50 mg of tab prednisolone for one week and and tapered over next one week. The symptom score and endoscopic scores were re-evaluated after 2 weeks. Those who had improvement in the nasal endoscopy score and symptom score [15] were classified into eCRSwNP and others into neCRSwNP.
Nasal polyps do not show the same pattern of eosinophilic inflammation in different parts of the world. Eosinophilic inflammation is common in western population as about 80% of the nasal polyps are eosinophilic [19]. Whereas non eosinophilic or neutrophilic inflammation is common in China,Korea and Japanese patients [8]. Our present study was done to find the inflammatory pattern of nasal polyps in Indian population as there are no other studies available till date.
In our study we found 121 (74.6%) 2/3rd patients classified into eCRSwNP and rest 1/3rd, 41 (24.3%) patients, classified into neCRSwNP. The mean age of the patient when reported with symptoms for eCRSwNP was in the fourth decade at 39 years and for neCRSwNP it was 34 years. The mean age for all patients was 38.3 y. Minimum age 17 and maximum age was 71 y. 64% of male and 36% female patients were affected with nasal polyps. The incidence in male is more than female and increases considerably after the age of 40 y, our results are comparable [20]. Mean duration of the disease when presented to OPD was 2.49 y for eCRSwNP and 2.85 y for neCRSwNP. CRSwNP is known to occur in 7% of the patients with asthma whereas asthma is known to occur in 26% to 48% of the patients with polyps [14] but in our study we found 13% of the total patients with asthma. The patients with asthma were significantly high in the eCRSwNP group than neCRSwNP group. p = 0.002. AERD is seen in approximately in 10% patients with nasal polyps. The exact prevalence is not known. 5.5% of the total CRSwNP patients had AERD in our study. However not significant in either groups. Smokers were more common in the group with eCRSwNP. Family history and systemic disesase had similar risk in both the groups. 9 (5.5%) patients out of 162 patients had combination of Aspirin and AERD (Aspirin triad), out of which, 1 patient belonged to neCRSwNP group.
Nasal polyps are smooth gelatinous, round structure arising from the ethmoid sinuses or middle meatus. They are easily found during rhinoscopic examination. Nasal endoscopy is done to visualize various stages of hyperaemia, polyp size,edema and origin of the nasal polyps. The two groups were compared base on the polyp size, edema and discharge as per Lund and Kennedy [15] endoscopic scoring system. There was no stastistically significant difference between the two groups (p = 0.224). The mean endoscopic scrores for neCRSwNP and eCRSwNP groups were 9.05 ± 1.378 and 9.34 ± 2.12 respectively. This is comparable to other studies [21]. Total CT scan score was caliculated as per Lund Mackay [16] scoring system in both the groups. Gargi Rai et al. [22] showed that total CT score is higher in eCRSwNP as it is severe form of disease and involves bilateral sinuses where as Tecimer et al. [21] showed no significant difference between the total CT scan score. In our study the mean total preop scores for neCRSwNP and eCRSwNP were 13.44 ± 2.78 and 16.21 ± 4.06 and p < 0.001. The patients with recurrent group of polyps were more aggressive with more total CT scores. Some of the CT scan based studies described about individual CT scores, PE/AE ratio, E/M ratio. We have not discussed the same in our present study.
The gold standard for diagnosing the eCRSwNP is by histopathological assessment. Varoius studies from all over the world describe a wide range of mean eosinophils values which range from 3 to 60% and counts from 6 to 300/hpf. In our study from Indian population we showed tissue absolute counts with mean value of 31.60 ± 15.05 and 14.44 ± 4.92 in eCRSwNP and neCRSwNP groups respectively. Tissue eosinophil percentage showed mean value of 21.01% ± 8.64% and 10.24% ± 4.40% resply. The mean values were stastically significant.
Patients with recurrent nasal polyps are known to have higher blood eosinophils than patients without recurrence [23]. We studied preop blood eosinophil percentage and preop blood absolute counts(× 106//L).The mean preop blood eosinophil percentage and absolute counts were significanty higher in the recurrent group eCRSwNP than neCRSwNP group. A positive correlation was seen between tissue eosinophil counts, blood eosinophil percentages and counts. Pearson correlation coefficient r = 1, r = 0.617, r = 0.602 respectively. A similar positive correlation was found in other studies [13, 24].
All 162 patients were operated by a single surgeon. All patients operated were followed up weekly during the first month, at 3 months and at 6 months. Endoscopic staging score and CT scan staging score were compared in both the groups at 6 months to find the recurrence of polyps. The group with recurrence (eCRSwNP) had high mean scores than the group without recurrence (neCRSwNP) in both total postop nasal endoscopy scores and total CT scan scores. eCRSwNP mean 0.86 (95% CI 0.72–1.00, SD 0.756). p = 0.014 and 3.12 (95% CI 2.78–3.45, SD 1.858). P = 0.004 for total postop nasal endoscopy and total CT scan scores resply. The group with more severe polyp score preoperatively eCRSwNP had significant post op recurrence at 6 months. Deconde et al. [25] showed that the mean total post op nasal endoscopy improved at 6 months only. There was a significant difference between the preop nasal endoscopy score and postop score at 6 months. There was no significant difference between 6 and 12 months or between 12 months or 18 months. Hence in our study we decided to follow up to 6 months. Total CT scan scores before surgery and at 6 months followup 15.27 (95%CI 14.62–15.91, SD 4.157) and 2.77, (95% CI 2.59–3.16, SD1.854) p < 0.001, showed better surgery outcomes.
ROC curve analysis of the predictive diagnostic factors associated with eCRSwNP group of nasal polyps and AUC of each parameter are presented in Table 3.The tissue absolute count was found to be the best predictor with AUC 0.923 (95% CI, 0.876–0.970).
The best cutoff values of individual predictors for Indian population are shown in Table 4. The smallest value of [(1-sesitivity)2 + (1-specificity)2] is used for diagnostic cutoff value. The tissue absolute count was found to have a cutoff value of ≥ 16 eosinophils/hpf. Cutoff values are very useful as it will be easy to evaluate the clinical and diagnostic factors in the outpatient department, to predict eCRSwNP or neCRSwNP and to plan a better treatment strategy before the patient is actually intervened with invasive procedures. The chance of recurrence can be predicted without actually going into surgical procedures which some patients may be unwilling. The options of medical management or endoscopic sinus surgery, combination of medical and ESS and the chance of recurrence can be easily evaluated during the outpatient visits.
Conclusion
The prevalence of eosinophilic nasal polyps in the Indian population with CRSwNP is less than the western population and more than the East Asian population. About 2/3 rd of the idiopathic bilateral nasal polyps are eosinophilic in nature. Eosinophilic nasal polyposis is more severe form of disease and presents with recurrence of the disease. The various preoperative diagnositic investigations and clinical outcomes differentiates eCRSwNP and neCRSwNP group with good accuracy for better treatment strategy.
Acknowledgement
None.
Funding
This research did not receive any specific grant from any funding agencies.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Availability of the Data and Material
Data collected and documented while individual patients included in the study were treated at respective military hospitals.
Ethical Approval
All medical and surgical interventions performed in this study involving the human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to Participate
Written informed consent was obtained from all individual patients included in the study.
Consent for Publication
NA.
Footnotes
Mohan Raghav Guthikonda previously at INHS Kalyani, Naval Base, Gandhigram Post, Visakhapatnam-Andhra Pradesh, 530005, India. Military Hospital Bhopal, Bairagarh, Cantonment, Bhopal- Madhya Pradesh, PIN-462030, India. Military Hospital Secunderabad, Trimulgherry, Secunderabad-Telangana, PIN-530015, India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohan Raghav Guthikonda, Email: gm2463@gmail.com.
Aswini Gude, Email: aish.ashwini@gmail.com.
Aditya Nutakki, Email: nutakki.aditya@gmail.com.
References
- 1.Stevens WW, Schleimer RP, Kern RC. Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2016;4(4):565–572. doi: 10.1016/j.jaip.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirtsreesakul V. Update on nasal polyps: etiopathogenesis. J Med Association Thail. 2005;88(12):7. [PubMed] [Google Scholar]
- 3.Newton JR, Ah-See KW. A review of nasal polyposis. Ther Clin Risk Manag. 2008;4(2):507. doi: 10.2147/TCRM.S2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama T, et al. Mucosal eosinophilia and recurrence of nasal polyps—new classification of chronic rhinosinusitis. Rhinology. 2011;49(4):392. doi: 10.4193/Rhino10.261. [DOI] [PubMed] [Google Scholar]
- 5.Fan G-K, Wang H, Takenaka H. Eosinophil infiltration and activation in nasal polyposis. Acta Otolaryngol (Stockh) 2007;127(5):521–526. doi: 10.1080/00016480600951368. [DOI] [PubMed] [Google Scholar]
- 6.Becker SS. Surgical management of polyps in the treatment of nasal airway obstruction. Otolaryngol Clin North Am. 2009;42(2):377–385. doi: 10.1016/j.otc.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Cervin A, Wallwork B. Macrolide therapy of chronic rhinosinusitis. Rhinology. 2007;45(4):259. [PubMed] [Google Scholar]
- 8.Ishitoya J, Sakuma Y, Tsukuda M. Eosinophilic chronic rhinosinusitis in Japan. Allergol Int. 2010;59(3):239–245. doi: 10.2332/allergolint.10-RAI-0231. [DOI] [PubMed] [Google Scholar]
- 9.Sakuma Y, et al. New clinical diagnostic criteria for eosinophilic chronic rhinosinusitis. Auris Nasus Larynx. 2011;38(5):583–588. doi: 10.1016/j.anl.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Kim J-W, Hong S-L, Kim Y-K, Lee CH, Min Y-G, Rhee C-S. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Neck Surg. 2007;137(6):925–930. doi: 10.1016/j.otohns.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 11.Czerny MS, Namin A, Gratton MA, Antisdel JL. Histopathological and clinical analysis of chronic rhinosinusitis by subtype: chronic rhinosinusitis subtype histopathology. Int Forum Allergy Rhinol. 2014;4(6):463–469. doi: 10.1002/alr.21304. [DOI] [PubMed] [Google Scholar]
- 12.Meng Y, Lou H, Wang C, Zhang L. Predictive significance of computed tomography in eosinophilic chronic rhinosinusitis with nasal polyps: diagnosis of eosinophilic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(8):812–819. doi: 10.1002/alr.21749. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Cao P-P, Liang G-T, Cui Y-H, Liu Z. Diagnostic significance of blood eosinophil count in eosinophilic chronic rhinosinusitis with nasal polyps in Chinese adults. Laryngoscope. 2012;122(3):498–503. doi: 10.1002/lary.22507. [DOI] [PubMed] [Google Scholar]
- 14.Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl. 2007;20:1–136. [PubMed] [Google Scholar]
- 15.Lund VJ, Kennedy DW. Quantification for staging sinusitis. Ann Otol Rhinol Laryngol. 1995;104(10_suppl):17–21. doi: 10.1177/000348949510410s02. [DOI] [PubMed] [Google Scholar]
- 16.Lund V, Kennedy D. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3):S35–S40. doi: 10.1016/S0194-5998(97)70005-6. [DOI] [PubMed] [Google Scholar]
- 17.Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol. 2015;136(6):1431–1440. doi: 10.1016/j.jaci.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Norlander T, Brönnegaard M, Stierna P. The relationship of nasal polyps, infection, and inflammation. Am J Rhinol. 1999;13(5):349–356. doi: 10.2500/105065899781367537. [DOI] [PubMed] [Google Scholar]
- 19.Stoop AE, van der Heijden HA, Biewenga J, van der Baan S. Eosinophils in nasal polyps and nasal mucosa: an immunohistochemical study. J Allergy Clin Immunol. 1993;91(2):616–622. doi: 10.1016/0091-6749(93)90267-j. [DOI] [PubMed] [Google Scholar]
- 20.Huvenne W, et al. Chronic rhinosinusitis with and without nasal polyps: what is the difference? Curr Allergy Asthma Rep. 2009;9(3):213–220. doi: 10.1007/s11882-009-0031-4. [DOI] [PubMed] [Google Scholar]
- 21.Tecimer SH, Kasapoglu F, Demir UL, Ozmen OA, Coskun H, Basut O. Correlation between clinical findings and eosinophil/neutrophil ratio in patients with nasal polyps. Eur Arch Otorhinolaryngol. 2015;272(4):915–921. doi: 10.1007/s00405-014-3174-4. [DOI] [PubMed] [Google Scholar]
- 22.Rai G, Roy P, Gupta N, et al. Computed tomography score an excellent marker: differentiates eosinophilic and non-eosinophilic variants of chronic rhinosinusitis with nasal polyp. Indian J. Otolaryngol Head Neck Surg. 2019;71:1787–1792. doi: 10.1007/s12070-017-1154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derneği BBC. Assessment of patients with nasal polyposis by the neutrophil-to-lymphocyte ratio and eosinophil-to-lymphocyte ratio. Kulak Burun Bogaz Ihtis Derg. 2015;25(4):193–199. doi: 10.5606/kbbihtisas.2015.10734. [DOI] [PubMed] [Google Scholar]
- 24.Zhang ZC, Li PZ, Tang HY, Cheng L. “Correlation analysis of eosinophils in peripheral blood and polyp tissues of patients with chronicrhinosinusitis with nasal polyps. J Clin Otorhinolaryngol Head Neck Surg. 2019;33(1):14. doi: 10.13201/j.issn.1001-1781.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 25.DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017;127(3):550–555. doi: 10.1002/lary.26391. [DOI] [PMC free article] [PubMed] [Google Scholar]