Abstract
Allergic rhinitis, beginning from childhood, is a global health problem. According to the literature, allergic rhinitis has been found association with asthma and other allergic manifestations. In this study we like to find out the significance and prognostic importance of spirometry in allergic rhinitis. The study was carried out over a period of 2 years, with 63 cases and controls each. Subjects in the age of 20–55 years with allergic rhinitis and SFAR score of > / = 7 were included as a case. Participants were interviewed and sent for spirometry. Controls were recruited from the retrospective data of healthy individuals with spirometry parameters done for health checkup. These controls had an SFAR score of < 7. All the data obtained were analyzed and compared between cases and controls. The mean age of the cases and controls were 33.17 ± 10.817 and 44.41 ± 7.4, respectively. Majority of cases and controls were males (60.3% and 57.1%). A statistically significant difference in FEF25-75% among cases and controls was noted (p = 0.00), thus proving probability of developing small airway obstruction in subjects with allergic rhinitis. Subjects with allergic rhinitis have a probability of developing small airway obstruction with subclinical changes, hence necessitating the need of regular follow-up.
Keywords: Allergic rhinitis, Bronchial asthma, Pulmonary function test, Spirometry, United airway disease, Nasobronchial reflex
Introduction
Allergic rhinitis, commonly referred to as hay fever, is a global health problem beginning right from childhood. Allergic rhinitis, which begins right from childhood, affects nearly 10 to 40% of the population globally [1] and is a group of nasal and adnexal symptoms that occurs because of exposure to the allergen in a sensitized individual, which normally doesn’t cause problems in most people. An association between allergic rhinitis and bronchial asthma has been studied earlier. Many studies have reported that nearly 28–78% of patients with bronchial asthma have co-existent nasal symptoms [2].
In this study, by comparing spirometry in allergic and non-allergic subjects, we like to find out significance and prognostic importance of assessment of spirometry in allergic rhinitis. This study is aimed at adding to the existing knowledge that even if the patients are not asthmatic, but have subclinical changes in pulmonary function indices, they need to be regularly follow up.
Materials and Methods
The study was carried out in the Department of Otorhinolaryngology, in collaboration with the Department of Respiratory Medicine over a period of 2 years, from November 2018 to September 2020, after obtaining an Ethical clearance. This was a case control study with 126 subjects recruited (63 cases and controls each). Subject presenting to the Department of Otorhinolaryngology, in the age group of 20–55 years, with diagnosis of allergic rhinitis confirmed by clinical history, examination and ‘Symptoms For Allergic Rhinitis (SFAR) score’ of > / = 7 were included as cases. SFAR score was obtained by a self-explainable questionnaire given to the subject, which was prepared referring to the study by Annesi-Maesano et al. [3].Subjects with history of asthma or any lower respiratory pathology, subacute or chronic cardiorespiratory disease, chest wall disease, infectious diseases including pulmonary tuberculosis and subjects with gross nasal abnormalities, history of smoking, immunotherapy, any treatment for allergic rhinitis in the last 4 weeks were excluded. Subjects were recruited after obtaining written and informed consent. The participants were interviewed in the local language and sent to the Department of Respiratory Medicine for spirometry. All the above were performed in a single visit. Controls were recruited from the retrospective data of the age matched healthy individuals, with spirometry performed as a routine health checkup. These controls were confirmed to have an SFAR score of < 7 by telephonic interview. Other exclusion criteria were followed for controls also.
Spirometry included Forced vital capacity (FVC), Forced expiratory volume in the first second (FEV1) and Forced expiratory flow at 25–75% of vital capacity (FEF25–75%). FEV1 and FVC were considered abnormal if < 80% predicted and FEF25–75% was considered abnormal if < 65% predicted.
All the data obtained was then recorded systematically and in brief in the proforma or data collection sheet. These data were analyzed with Levene’s test for equality of variances and t-test for equality of means.
Results
In the process of the study, 63 cases and 63 controls were recruited. The important demographics are mentioned in Table 1. We noted the mean age of cases was 33.17 ± 10.817 (Mean ± SD), and that of the controls was 44.41 ± 7.4 (Mean ± SD). There were 38 and 36 male subjects with 25 and 27 female subjects in the cases and controls respectively. Thus, we noted that majority of the cases and controls were males, 60.3 and 57.1% respectively. Hence, from the demographics, it was clear that we had an identical population among cases and controls. However, since all the cases had SFAR score > / = 7, the mean SFAR score for cases was 9 ± 1.566 (Mean ± SD). Similarly, all the controls had SFAR score < 7 and hence the mean SFAR for controls was 4.4 ± 1.293 (Mean ± SD). Among the cases, 63.5% (40) subjects had perennial allergic rhinitis, while 30.5% (19) subjects had seasonal allergic rhinitis. The remaining 6% (4) subjects had features suggestive or intermittent or persistent allergic rhinitis (Table 2, Fig. 1).
Table 1.
Demographical data
| Demographics | Cases | Controls |
|---|---|---|
| Age (Mean ± SD) | 33.17 ± 10.817 | 44.41 ± 7.4 |
| Gender (M:F) | Count—38:25 Percentage—60.3:39.7 | Count—36:27 Percentage—57.1:42.9 |
| SFAR Score (Mean ± SD) | 9 ± 1.566 | 4.46 ± 1.293 |
Table 2.
Occurrence of types of allergic rhinitis
| Total | Perennial | Seasonal | Inter-Mittent | Persistent |
|---|---|---|---|---|
| 63 (100%) | 40 (63.5%) | 19 (30.5%) | 2 (3%) | 2 (3%) |
Fig. 1.

Occurence of types of allergic rhinitis
After recruiting subjects into cases based on their clinical diagnosis and SFAR scoring, spirometry was performed in each of them. While analyzing the spirometry parameters, initial step was to differentiate between obstructive and restrictive lung diseases. FEV1/FVC value of < 0.7 is suggestive of obstructive lung diseases and > / = 0.7 can be seen in restrictive lung diseases. In addition, restrictive disease will manifest with low FVC whereas obstructive disease presents with low FEF25-75%, particularly in overt airway obstruction. However, low FEV1 can be seen in both restrictive and obstructive diseases. Hence, low FEV1 and FVC is suggestive of restrictive disease or mild restrictive ventilatory defect (MRVD) while low FEV1 and FEF25-75% is suggestive of obstructive lung disease. In our study, we noticed that majority of the subjects with changes suggestive of obstructive disease also had normal or high FEV1/FVC. Hence, this could be considered as early changes or Small Airway Obstruction (SAO).
The spirometry parameters thus obtained for each of the subjects is then analyzed and the observations are shown in Table 3 and Fig. 2. It must be noted that there was statistically significant difference between FEF25-75% among cases and controls (p = 0.00). Both the cases and controls had a Mean ± SD FEF25-75% value of 75.03 ± 14.907 and 84.378 ± 14.584 respectively, with cases having a lower FEF25-75% falling at the lower borderline. Meanwhile, the other PFT parameters had similar, but clinically insignificant (p > 0.05) Mean ± SD values for cases and controls. Thus, the probability of developing respiratory compromise in subjects with allergic rhinitis, particularly Small Airway Obstruction can be noted.
Table 3.
Results of spirometry: FVC—forced vital capacity, FEV1—forced expiratory volume at 1 s, FEF 25–75%—forced expiratory flow 25–75%
| Spirometry parameters | Group | Mean | SD | p-value |
|---|---|---|---|---|
| FEV1/FVC |
Cases Controls |
0.8595 0.8732 |
0.6155 0.5288 |
0.092 |
| FVC% |
Cases Controls |
85.13 83.57 |
10.555 6.871 |
0.1645 |
| FEV1% |
Cases Controls |
84.19 84.98 |
11.206 8.280 |
0.326 |
| FEF25-75% |
Cases Controls |
75.03 84.378 |
14.907 14.584 |
0.000 |
Fig. 2.
Results of spirometry: FVC—forced vital capacity, FEV1—forced expiratory volume at 1 s, FEF 25–75%—forced expiratory flow 25–75%
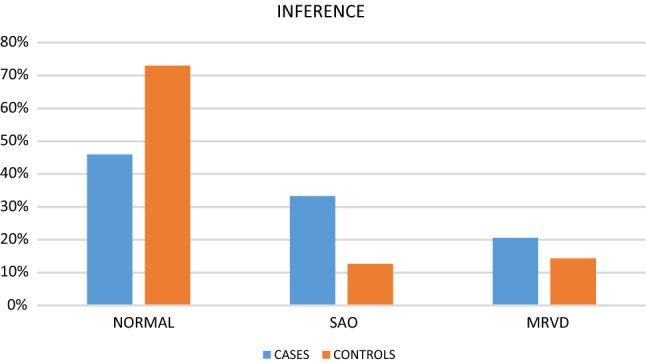
Comparing the inferences from the spirometry parameters (Table 4, Fig. 3), we noticed that 33.3% (21) cases had changes suggestive of obstructive lung disease. Meanwhile, only 12.7% (8) controls had evidence of obstructive lung disease with changes suggestive of SAO. In addition, 20.6% (13) cases and 14.3% (9) controls had evidence of mild restrictive ventilatory defect (MRVD). Hence, 53.9% (34) cases and 27% (17) controls had changes suggestive of lower respiratory disease (p = 0.1).
Table 4.
Inferences of spirometry: SAO—small airway obstruction, MRVD—mild restrictive ventilatory defect
| Inference | Group | Count | Percentage | p-value |
|---|---|---|---|---|
| Normal |
Cases Controls |
29 46 |
46.0% 73.0% |
0.1 |
| SAO |
Cases Controls |
21 8 |
33.3% 12.7% |
0.1 |
| MRVD |
Cases Controls |
13 9 |
20.6% 14.3% |
0.1 |
Fig. 3.
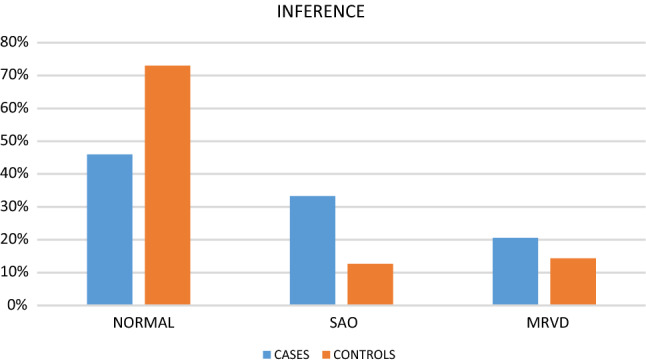
Inferences of spirometry: SAO—small airway obstruction, MRVD—mild restrictive ventilatory defect
Discussion
Allergic rhinitis claims the most common clinical condition managed in Otorhinolaryngology in-office, affecting 10–40% of the population and has been noticed to have an increasing trend [1].
Our study done was aimed at studying the co-relation of allergic rhinitis with subclinical spirometry changes of bronchial asthma. Though studies by Sluder (1919) [4] had noticed an association between allergic rhinitis and bronchial asthma in the early twentieth century itself, there have been no further studies till the late twentieth century on this topic and hence was considered to be a ‘forgotten theory’. An association between the upper and lower airways was later discussed in ‘Cellular and Neurogenic mechanism in Nose and Bronchi’—European Journal of Respiratory diseases (1983), edited by Mygind, Rasmussen and Molgaad [5]. Since then, several clinical and experimental observations suggested the possibility of united airway disease. A study by Jonathan Corren in 1998 observed that nasal symptoms were experienced by nearly 78% of patients with asthma [2]. Similarly, symptoms of bronchial asthma was experienced by nearly 38% of patients with allergic rhinitis [2]. Such a positive correlation between allergic rhinitis and spirometry is seen in our study also, with 53.9% (34) cases having features suggestive of lower respiratory disease against 27% (17) controls (p = 0.1). We noted that 33.3% (21) cases and 12.7% (8) controls had changes of airway obstruction while 20.6% (13) cases and 14.3% (9) controls had changes of restrictive airway respectively(p = 0.1).
The concept of upper and lower airway forming a unified airway, could explain this positive correlation between allergic rhinitis and spirometry noted in our study. Persson et al. [6] explained some similarities between nasal, tracheal and bronchial mucosa and their cellular mechanism. This includes the response of the mucosal end organs to various stimulus resulting in inflammation. Hence, he concluded that nasal stimulus should, therefore, increase the chance of infectious, occupational and also, allergic airway diseases [6]. Cemal Cingi et al. (2015) published a paper on nasobronchial interaction by means of literature reviews from PubMed since 1982, with database based on the terms nasal, bronchial and nasobronchial interaction [7]. Their study also explained a nasobronchial interaction due to impaired nasal filtration, aspiration of nasal contents with the inflammatory mediators and cells and nasobronchial reflex with the Trigeminal nerve as afferent and Vidian nerve as efferent [7]. Similar results were obtained by Amelia Licari et al. [8] in their study to highlight the anatomical and pathophysiological interaction between the upper and lower airways particularly in allergic rhinitis and bronchial asthma [8]. These mechanisms could be hampered in allergic rhinitis exposing the lower airway to allergens and cold air which favors bronchoconstriction as noted in the current study.
A cross sectional study to assess the spirometry parameters of subjects with allergic rhinitis by Yousser Mohammad et al. [9] can also be compared with our study. Spirometry was carried out at enrollment of subjects as well as after 2 weeks of intranasal beclomethasone therapy [9]. 60 subjects were included, 34 women and 26 men, out of which 77% subjects had persistent allergic rhinitis. 21 subjects had FEF25-75 < 65% predicted, while only 4 and 2 subjects had FEV1 and FVC < 80% predicted, respectively. All the subjects with any change in the baseline spirometry had perennial allergic rhinitis and improved with intranasal beclomethasone. They concluded that FEF25–75% may be considered an early predictor of small airway obstruction, even in the absence of any lower respiratory symptoms. Hence their study supported the ‘One airway One disease hypothesis’ [9]. Related studies had been carried out later as well. Nevine El-Helaly et al. [10] performed a study to detect the abnormalities in spirometry parameters in subjects with allergic rhinitis, and to compare these changes before and after treatment of allergic rhinitis alone, bronchial asthma alone and allergic rhinitis associated with bronchial asthma [10]. They recruited 60 subjects to the study and divided them into three different groups: Group 1 had 20 subjects with persistent allergic rhinitis only, group 2 had 20 subjects with moderate or severe bronchial asthma only and group 3 had 20 subjects with allergic rhinitis with bronchial asthma. Assessment of FVC, FEV1, peak expiratory flow (PEF) and FEF 25–75% were done on all subjects at recruitment and after treatment for 3 months with antihistamines and intranasal corticosteroids for allergic rhinitis, inhaled corticosteroids for bronchial asthma and antihistamines with both inhaled and intranasal corticosteroids for allergic rhinitis with bronchial asthma. They found that both allergic rhinitis and bronchial asthma showed a marked reduction in the FEF25–75% values than other parameters, and all the parameters of spirometry showed a significant increase on treatment in all the three groups, with lowest values noted in group 3. However, a positive correlation between FEF25–75% and FEV1% before and after treatment was found only in group 2 and 3. Thus their study provided evidence for the spirometric defects in subjects with allergic rhinitis, warranting further study later [10]. Similar results were obtained by G Ciprandi et al. [11], proposing FEF25-75% as an early marker of bronchial involvement in allergic rhinitis, particularly seasonal, with only nasal symptoms [11]. We observed a Mean ± SD FEF25-75% of 75.03 ± 14.907 and 84.378 ± 14.584 respectively, for cases and controls which points to the higher chance of developing small airway obstruction in cases (p < 0.05). However, such a statistically significant result was not observed between other spirometry parameters (p > 0.05). This difference could be due to the limited sample size and shorter duration of the current study or asthmatics with controlled manifestations.
Similarly, Tikkas R et al. [12] conducted a cross sectional case control study with 75 children to compare the spirometry parameters in non-asthmatic allergic and non-allergic children in the age group of 6–12 years and showed a statistically significant difference in the prebronchodilator spirometry parameters, i.e. FVC and FEF25–75% (p = 0.03 and 0.034 respectively) between allergic and non-allergic groups. In addition, there was a statistically significant positive increment in all the spirometry parameters i.e. FVC, FEV1, FEV1/FVC, FEF25-75% and PEF (p < 0.001) in allergy group. These conclusions were also suggestive of small airway obstruction [12].
Conclusion
From our study, we observed the possibility of a small airway obstruction (33.3% cases) with subclinical changes in FEF25–75% in subjects with allergic rhinitis. Hence, our study suggests a regular spirometry in subjects with allergic rhinitis to detect subclinical changes of small airway obstruction and obstructive lung disease. However, some limitations of study include limited sample size, lack of follow up data with or without intranasal or oral medications, use of only SFAR scoring for inclusion of subjects into allergic rhinitis and not considering the controlled asthmatics.
Acknowledgements
None.
Funding
None.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical Approval
Approval from institutional ethical committee.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, Brignardello-Petersen R, Canonica GW, Casale T, Chavannes NH, de Sousa JC. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines—2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 2.Corren J. The impact of allergic rhinitis on bronchial asthma. J Allergy Clin Immunol. 1998;101(2 SUPPL.):352–356. doi: 10.1016/S0091-6749(98)70218-0. [DOI] [PubMed] [Google Scholar]
- 3.Annesi-Maesano I, Didier A, Klossek M, Chanal I, Moreau D, Bousquet J. The score for allergic rhinitis (SFAR): A simple and valid assessment method in population studies. Allergy Eur J Allergy Clin Immunol. 2002;57(2):107–114. doi: 10.1034/j.1398-9995.2002.1o3170.x. [DOI] [PubMed] [Google Scholar]
- 4.Sluder G. Asthma as a nasal reflex. J Am Med Assoc. 1919;73(8):589. doi: 10.1001/jama.1919.02610340021006. [DOI] [Google Scholar]
- 5.Mygind N, Molgaard F, Rasmussen F. Cellular and neurogenic mechanisms in nose and bronchi. Eur J Respir Dis Suppl. 1983;128(Pt 2):383–557. [PubMed] [Google Scholar]
- 6.Persson CGA, Svensson C, Greiff L, Andersson M, Wollmer P, Alkner U, et al. Editorial: the use of the nose to study the inflammatory response of the respiratory tract. Thorax. 1992;47(12):993. doi: 10.1136/thx.47.12.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cingi C, Muluk NB, Cobanoglu B, Çatli T, Dikici O. Nasobronchial interaction. World J Clin Cases. 2015;3(6):499. doi: 10.12998/wjcc.v3.i6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Licari A, Castagnoli R, Denicolò CF, Rossini L, Marseglia A, Marseglia GL. The nose and the lung: United airway disease? Front Pediatr. 2017 doi: 10.3389/fped.2017.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammad Y, Shaaban R, Ibrahim M, Ismail M. Lung function changes in non-asthmatic allergic rhinitis patients: a case series. Prim Care Respir J [Internet] 2011;20(4):454–456. doi: 10.4104/pcrj.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-helaly N, Samy SM, Ibrahim TS, Morcos WM. Pulmonary function changes in Allergic Rhinitis with or without Bronchial Asthma. J Am Sci. 2012;8(1):110–114. [Google Scholar]
- 11.Ciprandi G, Cirillo I, Klersy C, Marseglia GL, Vizzaccaro A, Pallestrini E, Tosca M. Role of FEF25–75 as an early marker of bronchial impairment in patients with seasonal allergic rhinitis. Am J Rhinol. 2006;20(6):641–647. doi: 10.2500/ajr.2006.20.2914. [DOI] [PubMed] [Google Scholar]
- 12.Tikkas DR, Ramteke DS, Chakravarty DP, Dave DL, Srivastava DJ. A study to compare the pulmonary function test in non-asthmatic allergic and non-asthmatic non-allergic (Apparently Healthy) children-in age group 6–12 years. Pediatr Rev Int J Pediatr Res. 2018;5(1):37–42. doi: 10.17511/ijpr.2018.i01.08. [DOI] [Google Scholar]


