Abstract
Background:
This narrative review of the literature aims to analyze the utilization of stromal vascular fraction (SVF) and decellularized extracellular matrices (dECMs) in various pathologies related to orthopedic surgery.
Methods:
A literature search was carried out in PubMed on February 15, 2022, using “Stroma Vascular Fraction and Orthopedic Surgery” and “Decellularized Extracellular Matrices and Orthopedic Surgery” as keywords. A total of 278 articles were found, of which 28 papers were selected because they seemed to be the most appropriate concerning the title of the article.
Results:
The reported results have shown that intra-articular injection of SVF seems to be a safe and efficacious method for managing knee osteoarthritis (OA). Platelet-rich plasma (PRP) and SVF are safe and effective management for intractable Achilles tendinopathy in humans, although subjects treated with SVF recover earlier. There are promising results in utilizing adipose-derived mesenchymal stromal cells in chronic lateral epicondylitis of the elbow in athletes. Ready-to-use ECM/SVF gel seems to be a good therapeutic option promoting the regeneration of the articular cartilage in subjects with injuries of the cartilage. The SVF can safely be used to treat diabetic subjects suffering from chronic foot ulcers.
Conclusion:
There are scarce high-quality data for utilizing cell-based approach in soft tissue injuries of the knee in athletes. Experimental studies indicate that SVF could be a new option to osseous regeneration. Other experimental studies support the utilization of dECMs as a scaffold for the regeneration of large osseous defects, cell-derived dECMs scaffolds to repair articular cartilage injuries, and utilization of xenogeneic acellular muscles to manage volumetric muscle loss where there is a lack of donor site.
Intra-articular injections of SVF seems to be a safe and efficacious method for managing OA of the knee joint. Platelet-rich plasma (PRP) and SVF are safe and efficacious methods for the management of intractable Achilles tendinopathy in humans, although subjects treated with SVF recover earlier.
Key Words: Bone regeneration, Cartilage regeneration, Decellularized extracellular matrices, Osteoarthritis, Stromal vascular fraction, Tendon healing
Introduction
Platelet-rich plasma (PRP), prolotherapy, and mesenchymal stem cells (MSCs) are being used more and more for the treatment of rotator cuff injury, lateral epicondylitis, Achilles tendinopathy, plantar fasciitis, OA of the knee joint, and acute muscle injuries.1
According to Gentile et al., stromal vascular fraction (SVF) cells originated from adipose stem cells (ASCs) have been used for many years in regenerative plastic surgery for autologous uses; however, poor attention has been paid to its potential allogenic function. Allogenic SVF transplants could utilize decellularized extracellular matrices (dECMs) as a donor scaffold, which are then recellularized by the patient’s ASCs, therefore developing advanced approaches for personalized utilization. In the systematic review published by these researchers on the clinical use of human allogeneic ASC transplantation, it was suggested that allogeneic transplantation of ASCs and extracellular matrix (ECM) was safe, efficacious, and without substantial complications. 2 In 2020, Blaudez et al. stated that there were future expectations about using natural ECM to decorate synthetic porous scaffolds. 3
The purpose of this narrative review of the literature is to analyze SVF and dECMs in diverse conditions related to orthopedic surgery.
Materials and Methods
The research question was: What is the current role of SVF and dECMs in pathologies related to Orthopedic Surgery? Regarding the criteria for paper selection, in this article, a literature search was performed in PubMed on February 15, 2022, using “Stroma Vascular Fraction and Orthopedic Surgery” and “Decellularized Extracellular Matrices and Orthopedic Surgery” as keywords. A total of 278 articles were found, of which 28 papers were selected because they seemed to be the most appropriate concerning the title of the article.
Results
Stromal Vascular Fraction
Osteoarthritis
One of the main global sources of disability, with a somewhat elevated prevalence in elderly people, is OA. 4 Current therapeutic modalities, such as exercise, anti-inflammatory medications, and intra-articular injections of corticosteroids, intend to alleviate the pain that subjects with OA suffer. Surgical alternatives, such as osteotomies and joint arthroplasties, are other invasive therapeutic alternatives that can be used when the abovementioned treatments fail. Substantial advances have been made to manage musculoskeletal conditions utilizing regenerative cell therapies in recent decades. Adipose-derived SVF cells holding within diverse cell types, including MSCs, are efficacious in repairing cartilaginous lesions. Intra-articular injections of PRP have been shown to act as an adjuvant to surgical treatment in subjects with knee OA. 4
In a systematic review published by Pak et al. in 2018 it was stated that in patients with OA, management with ASCs could be an alternative, given there were good information on their safety and effectiveness.5 As reported by Fotouhi et al. in 2018, MSCs treatment with intra-articular injections of PRP, SVF, and ASCs for OA had advanced well, with optimistic results. 6
In 2019, DiMatteo et al. published a systematic review demonstrating that the use of “minimally” manipulated MSCs (in the form of BMAC or SVF) was safe and provided some short-run beneficial consequences. 7 In 2019, Yokota et al. compared the clinical results of intra-articular injections with ASCs vs. SVF in individuals with OA of the knee. It was encountered that both ASCs and SVF produced clinical ameliorations, but also that ASCs gave better results SVF in terms of early pain decrease and with less adverse events. 8
In 2020, Garza et al. published a double-blinded prospective randomized controlled clinical trial (level of evidence I) to evaluate the efficacy of intra-articular MSCs for the treatment of knee OA. 9 No severe complications were observed. Intra-articular injections of SVF substantially alleviated knee pain for at least 12 months. The efficacy and safety shown in this study supported its use as a treatment option for painful OA of the knee. 9 In 2020, Tsubosaka et al. reported that the short-run clinical benefits of intra-articular SVF cell injection in knee OA were outstanding.10 In 2021, Shanmugasundaram et al. published another systematic review on the utilization of SVF injection in the treatment of OA of the knee. 11 They stated that intra-articular injection of SVFs was a secure and efficacious method for this condition.
In 2021, Filardo et al. compared three methods to exploit ACSs for OA management in a preclinical study.12 Biological samples of adipose tissue, processed by mechanical micro-fragmentation (MF), enzymatic digestion (SVF), or cell expansion (ASCs), were first characterized in vitro and then utilized in vivo in a surgically induced OA rabbit model. It was found that MF, SVF, and expanded ASCs did not produce significant local or systemic complications. Among the various techniques utilized to use the potential of adipose tissue, MF demonstrated the most encouraging results. 12
[Figure 1] shows transplantation into an osteoarthritic joint with adipose-derived SVF cells (treatment procedure).
Figure 1.
Transplantation into an osteoarthritic joint with adipose-derived stromal vascular fraction cells
Tendon Healing
In 2018, Usuelli et al. reported a randomized controlled clinical trial study, comparing the effectiveness of PRP and SVF injections for managing non-insertional Achilles tendinopathy. 13 It was found that both PRP and SVF were secure and efficacious managements for recalcitrant Achilles tendinopathy. Subjects managed with SVF had faster outcomes, indicating that such approach must be considered for those needing an earlier return to activities of daily living or sport. 13
In 2021, Khoury et al. studied the impact of ASCs injection in lateral epicondylitis of the elbow tendinopathy in tennis players. 14 The mean time to return to tennis was 3.31 months. The results of this study were encouraging and opened a new biologic therapeutic option to treat lateral epicondylitis of the elbow. 14
Articular Cartilage Defects
In 2020, Li et al. attained a novel adipose tissue-derived product, ECM/SVF gel, by simple mechanical shifting and centrifugation to extract the fat and concentrate the efficacious components. Their study tried to assess the therapeutic effectiveness of this natural biomaterial in the repair of defects of the articular cartilage. 15 Ready-to-use ECM/SVF gel seemed to be a good therapeutic option to ease the regeneration of articular cartilage. 15
Chronic Diabetes Foot Ulcers
In 2021, Carstens et al. published a phase I study to determine the security and explore the effectiveness of local injections of autologous adipose-derived SVF cells to manage non healing diabetes foot ulcers (DFUs) larger than 3 cm in diameter. No serious adverse events related to the intervention were found. The results of this study showed that SVF can be securely utilized to manage chronic DFUs, with evidence of effectiveness (wound healing). 16
Soft-tissue Sports Injuries
In a systematic review published in 2021 by Kader et al. it was concluded that there was insufficient high-quality information to utilize cell-based treatments that demonstrate ligamentous or tendinous healing, meniscal volume restoration, or post-traumatic OA amelioration/regression. 17
Bone Regeneration
In 2018Zhang et al. used SVF from human adipose tissue and human monocytes for intraoperative preparation of bone constructs. The feasibility of intraoperative preparation of SVF constructs and the superior bone regenerative capacity of SVF constructs compared with donor-matched ASCs constructs were demonstrated. 18
In 2021, Bari et al. developed a new strategy to ameliorate the osteoinductivity of SmartBone® (SB). SB scaffolds were loaded with lyosecretome, a freeze-dried formulation of MSC-secretome, containing proteins and extracellular vesicles (EVs). After two weeks, a significant enhancement of cell proliferation was encountered in SBlyo concerning SB, in which cells filled the cavities between the native trabeculae. 19
Decellularized Extracellular Matrices
The dECMs have been used in various aspects of orthopedic surgery, which are reviewed below.
Bone Regeneration
In 2017, Cunniffe et al. created porous scaffolds from decellularized growt plate (GP) ECM and assess their capacity to augment host-mediated bone regeneration following implantation in cranial defects in rats. 20 These results of this study supported the utilization of decellularized GP ECM as a scaffold for the regeneration of large bone defects. In 2018 Ghassemi et al. reported that scaffold-based bone tissue engineering, especially osteoinductive materials like demineralized bone matrix, held great promise for the future of osseous defects therapies. 21 In 2019, Freeman et al. created an “off-the-shelf” multiscale bone-ECM-derived scaffold that was mechanically stable. Once implanted in vivo, it boosted vascularization and eventually led to osseous regeneration. 22
Cartilage Regeneration
In 2016 Moradi et al. reported that an increase in sulfated glycosaminoglycans production along with upregulation of chondrogenic genes confirmed that physically treated cartilage matrix-derived scaffolds have chondrogenic potential on human dermal fibroblasts. 23
In 2017 Ghassemi et al. published that incorporating carbon nanotubes (CNTs) can substantially enhance the mechanical properties of decellularized articular cartilage while retaining its biocompatibility, suggesting CNT-incorporated decellularized articular cartilage as potential scaffolds for cartilage tissue engineering applications. 24 In 2021, Zhu et al. described the cell types utilized to secrete ECM, techniques to induce cells to secrete cartilage-like ECM, and decellularization procedures to prepare cell-derived dECM. 25
In 2021, Zhang et al. created bioinks with various concentrations of silk fibroin (SF) and dECM (SF-dECM bioinks) mixed with bone marrow MSCs (BMMSCs) for 3D bioprinting. It was demonstrated that an SF-dECM construct designed to liberate TGF-β3could promote chondrogenic differentiation of BMMSCs. In addition, it provided a good cartilage repair environment, which suggested that it was an ideal scaffold for cartilage tissue engineering. 26
In 2021, Kim et al. created polymeric nanofibrils (NFs) decorated with cartilage-derived dECM as a chondroinductive scaffold material for cartilage repair. 27 ASCs in the NF composites significantly increased the expression of chondrogenic gene markers compared with those in pellet culture.
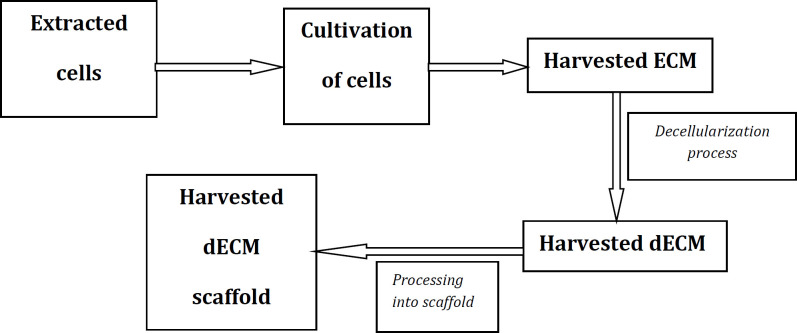
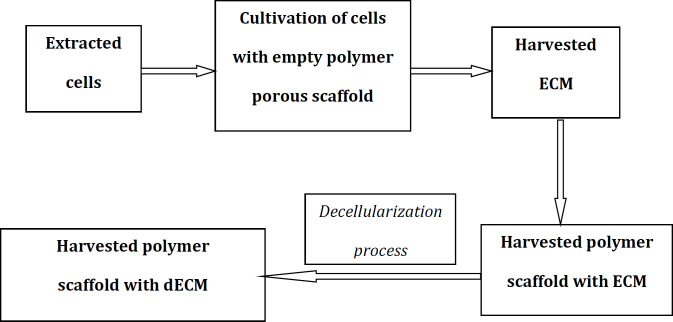
[Figure 2] shows a schematic representation of the preparation process of a dECM scaffold for articular cartilage repair. [Figure 3] shows a schematic representation of the preparation process of a composite scaffold composed of cell-derived dECM and polymer materials (a composite scaffold capable of promoting the chondrogenic differentiation of seeded MSCs).
Figure 2.
Schematic diagram of the technique for preparing a decellularized extracellular matrix (dECM) scaffold for articular cartilage repair. ECM, extracellular matrix
Figure 3.
Composite scaffold promotes the chondrogenic differentiation of seeded mesenchymal stem cells (MSCs). Schematic diagram of the technique for preparing composite scaffold composed of cell-derived decellularized extracellular matrix (dECM) and polymer materials. ECM, extracellular matrix
Muscle Regeneration
In 2018, Urciolo et al. analyzed the ability of three different decellularized skeletal muscle scaffolds to support muscle regeneration in a xenogeneic immune-competent volumetric muscle loss (VML) model.28 It was demonstrated that fibroblasts were essential for promoting effective migration and myogenesis by muscle stem cells across the scaffolds in vitro. This information supported the utilization of xenogeneic acellular muscles to manage VML diseases in the absence of donor cell implementation. 28
Discussion
It has been reported that intra-articular injection of SVF is a secure and effective method for treating subjects with OA of the knee. In humans, PRP and SVF are safe and effective for the treatment of intractable Achilles tendinopathy, albeit subjects managed with SVF recover earlier. There are promising results on the utilization of ASCs in individuals with chronic lateral epicondylitis of the elbow. Ready-to-use ECM/SVF-gel seems to be a good therapeutic option for the regeneration of articular cartilage in subjects with cartilaginous injuries. The SVF can be safely used in subjects with chronic DFUs. There is scarce information to utilize cell-based treatment in sports injuries of the soft tissues of the knee joint. Experimental studies indicate that SVF could be a new option to osseous regeneration. Other experimental studies support the utilization of decellularized GP ECM as a scaffold for the regeneration of big osseous defects, cell-derived decellularized ECM scaffolds for the repair of articular cartilage, and xenogeneic acellular muscles as a method to manage VML diseases in the absence of donor cell implementation.
The main limitations of this article are that only one search tool (PubMed) was used and that the selection of articles was made on the basis that they appeared to be the most appropriate for the title of the article. Regarding potential future implications of this review, we could say that SVF and dECMs will probably be more used in the future in clinical practice, as their current results seem promising. However, such results should be fully confirmed with well-designed studies with a high degree of scientific evidence.
Intra-articular injection of SVF seems to be a safe and efficacious method for managing OA of the knee joint. PRP and SVF seem to be safe and effective techniques for the management of intractable Achilles tendinopathy in humans, although subjects treated with SVF recover earlier.
References
- 1.Van Schaik KD, Kenneth SL. Orthobiologics: diagnosis and treatment of common tendinopathies. Semin Musculoskelet Radiol. 2021;25(6):735–44. doi: 10.1055/s-0041-1735475. [DOI] [PubMed] [Google Scholar]
- 2.Gentile P, Sterodimas A, Pizzicannella J, Dionisi L, De Fazio D, Calabrese C, et al. Systematic Review: allogenic use of stromal vascular fraction (SVF) and decellularized extracellular matrices (ECM) as advanced therapy medicinal products (ATMP) in tissue regeneration. Int J Mol Sci. 2020;21(14):4982. doi: 10.3390/ijms21144982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaudez F, Ivanovski S, Hamlet S, Vaquette C. An overview of decellularisation techniques of native tissues and tissue engineered products for bone, ligament and tendon regeneration. Methods. 2020:171:28–40. doi: 10.1016/j.ymeth.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Mehranfar S, Abdi Rad I, Mostafav E, Akbarzadeh A. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: an overview of clinical trials. Artif Cells Nanomed Biotechnol. 2019;47(1):882–90. doi: 10.1080/21691401.2019.1576710. [DOI] [PubMed] [Google Scholar]
- 5.Pak J, Lee JH, Pak N, Pak Y, Park KS, Jeon JH, et al. Cartilage regeneration in humans with adipose tissue-derived stem cells and adipose stromal vascular fraction cells: updated status. Int J Mol Sci. 2018;19(7):2146. doi: 10.3390/ijms19072146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fotouhi A, Maleki A, Dolati S, Aghebati-Maleki A, Aghebati-Maleki L. Platelet rich plasma, stromal vascular fraction and autologous conditioned serum in treatment of knee osteoarthritis. Biomed Pharmacother. 2018;104:652–60. doi: 10.1016/j.biopha.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Di Matteo B, Vandenbulcke F, Vitale ND, Iacono F, Ashmore K, Marcacci M, et al. Minimally manipulated mesenchymal stem cells for the treatment of knee osteoarthritis: a systematic review of clinical evidence. Stem Cells Int. 2019;2019:1735242. doi: 10.1155/2019/1735242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokota N, Hattori M, Ohtsuru T, Otsuji M, Lyman S, Shimomura K, et al. Comparative clinical outcomes after intra-articular injection with adipose-derived cultured stem cells or noncultured stromal vascular fraction for the treatment of knee osteoarthritis. Am J Sports Med. 2019;47(11):2577–83. doi: 10.1177/0363546519864359. [DOI] [PubMed] [Google Scholar]
- 9.Garza JR, Campbell RE, Tjoumakaris FP, Freedman KB, Miller LS, Santa Maria D, et al. Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: a double-blinded prospective randomized controlled clinical trial. Am J Sports Med. 2020;48(3):588–98. doi: 10.1177/0363546519899923. [DOI] [PubMed] [Google Scholar]
- 10.Tsubosaka M, Matsumoto T, Sobajima S, Matsushita T, Iwaguro H, Kuroda R. The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskelet Disord. 2020;21(1):207. doi: 10.1186/s12891-020-03231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanmugasundaram S, Vaish A, Chavada V, Murrell WD, Vaishya R. Assessment of safety and efficacy of intra-articular injection of stromal vascular fraction for the treatment of knee osteoarthritis-a systematic review. Int Orthop. 2021;45(3):615–25. doi: 10.1007/s00264-020-04926-x. [DOI] [PubMed] [Google Scholar]
- 12.Filardo G, Tschon M, Perdisa F, Brogini S, Cavallo C, Desando G, et al. Micro-fragmentation is a valid alternative to cell expansion and enzymatic digestion of adipose tissue for the treatment of knee osteoarthritis: a comparative preclinical study. Knee Surg Sports Traumatol Arthrosc. 2021 doi: 10.1007/s00167-020-06373-y. [DOI] [PubMed] [Google Scholar]
- 13.Usuelli FG, Grassi M, Maccario C, Vigano M, Lanfranchi L, Alfieri Montrasio U, et al. Intratendinous adipose-derived stromal vascular fraction (SVF) injection provides a safe, efficacious treatment for Achilles tendinopathy: results of a randomized controlled clinical trial at a 6-month follow-up. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):2000–10. doi: 10.1007/s00167-017-4479-9. [DOI] [PubMed] [Google Scholar]
- 14.Khoury M, Tabben M, Rolón AU, Levi L, Chamari K, PD’Hooghe P. Promising improvement of chronic lateral elbow tendinopathy by using adipose derived mesenchymal stromal cells: a pilot study. J Exp Orthop. 2021;8(1):6. doi: 10.1186/s40634-020-00320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Zhao F, Li Z, Duan X, Cheng J, Zhang J, et al. Autologous fractionated adipose tissue as a natural biomaterial and novel one-step stem cell therapy for repairing articular cartilage defects. Front Cell Dev Biol. 2020;8:694. doi: 10.3389/fcell.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carstens MH, Quintana FJ, Calderwood ST, Sevilla JP, Ríos AB, Rivera CM, et al. Treatment of chronic diabetic foot ulcers with adipose-derived stromal vascular fraction cell injections: Safety and evidence of efficacy at 1 year. Stem Cells Transl Med. 2021;10(8):1138–47. doi: 10.1002/sctm.20-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kader N, Asopa V, Baryeh K, Sochart D, Maffulli N, Kader D. Cell-based therapy in soft tissue sports injuries of the knee: a systematic review. Expert Opin Biol Ther. 2021;21(8):1035–47. doi: 10.1080/14712598.2021.1872538. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Grosfeld EC, Camargo WA, Tang H, Magri AMP, van den Beucken JJJP. Efficacy of intraoperatively prepared cell-based constructs for bone regeneration. Stem Cell Res Ther. 2018;9(1):283. doi: 10.1186/s13287-018-1026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bari E, Roato I, Perale G, Rossi F, Genova T, Mussano F, et al. Biohybrid bovine bone matrix for controlled release of mesenchymal stem/stromal cell lyosecretome: a device for bone regeneration. Int J Mol Sci. 2021;22(8):4064. doi: 10.3390/ijms22084064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunniffe GM, Díaz-Payno PJ, Ramey JS, Mahon OR, Dunne A, Thompson EM, et al. Growth plate extracellular matrix-derived scaffolds for large bone defect healing. Eur Cell Mater. 2017;33:130–42. doi: 10.22203/eCM.v033a10. [DOI] [PubMed] [Google Scholar]
- 21.Ghassemi T, Shahroodi A, Ebrahimzadeh MH, Mousavian A, Movaffagh J, Moradi A. Current concepts in scaffolding for bone tissue engineering. Arch Bone Jt Surg. 2018;6(2):90–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman FE, Browe DC, Nulty J, Von Euw S, Grayson WL, Kelly DJ. Biofabrication of multiscale bone extracellular matrix scaffolds for bone tissue engineering. Eur Cell Mater. 2019;38:168–87. doi: 10.22203/eCM.v038a12. [DOI] [PubMed] [Google Scholar]
- 23.Moradi A, Ataollahi F, Sayar K, Pramanik S, Chong PP, Khalil AA, et al. Chondrogenic potential of physically treated bovine cartilage matrix derived porous scaffolds on human dermal fibroblast cells. J Biomed Mater Res A. 2016;104(1):245–56. doi: 10.1002/jbm.a.35561. [DOI] [PubMed] [Google Scholar]
- 24.Ghassemi T, Saghatolslami N, Matin MM, Gheshlaghi R, Moradi A. CNT-decellularized cartilage hybrids for tissue engineering applications. Biomed Mater. 2017;12(6):065008. doi: 10.1088/1748-605X/aa8435. [DOI] [PubMed] [Google Scholar]
- 25.Zhu W, Cao L, Song C, Pang Z, Jiang H, Guo C. Cell-derived decellularized extracellular matrix scaffolds for articular cartilage repair. Int J Artif Organs. 2021;44(4):269–81. doi: 10.1177/0391398820953866. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Liu Y, Luo C, Zhai C, Li Z, Zhang Y, et al. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2021;118:111388. doi: 10.1016/j.msec.2020.111388. [DOI] [PubMed] [Google Scholar]
- 27.Kim HS, Mandakhbayar N, Kim HW, Leong KW, Yoo HS. Protein-reactive nanofibrils decorated with cartilage derived decellularized extracellular matrix for osteochondral defects. Biomaterials. 2021;269:120214. doi: 10.1016/j.biomaterials.2020.120214. [DOI] [PubMed] [Google Scholar]
- 28.Urciuolo A, Urbani L, Perin S, Maghsoudlou P, Scottoni F, Gjinovci A, et al. Decellularised skeletal muscles allow functional muscle regeneration by promoting host cell migration. Sci Rep. 2018;8(1):8398. doi: 10.1038/s41598-018-26371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]