Abstract
We identified two novel β-defensin precursors, preproGBD-1 and preproGBD-2, in the tissues of a goat. Although the precursors were identical in 96.8% of their bases and 88.2% (60 of 68) of their amino acids, preproGBD-1 was expressed principally in the tongue and respiratory tract, whereas preproGBD-2 expression predominated throughout the intestine. These findings exemplify the phenomenon of tissue-specific expression in a family of host defense peptides that arose before the avian and mammalian lineages diverged.
Defensins are cationic, antimicrobial peptides with β-sheet structures that are stabilized by three intramolecular disulfide bonds (15). The α- and β-defensins (BDs) of vertebrates differ with respect to the pairing of their cysteine residues, but they have similar structures (31) and originated from shared ancestral genes (17). Interest in BDs has grown substantially in recent years, spurred by their potential relevance to the pathogenesis of infection in cystic fibrosis (1, 5, 23). However, our knowledge of their contribution to host defense remains fragmentary and largely circumstantial. Many BDs are expressed by epithelial cells (9, 23, 26, 27) and keratinocytes (7)—a characteristic that distinguishes them from α-defensins. Their expression in nonmyeloid cells may occur constitutively (21, 30) or in response to signals that are generated during infection, inflammation, or tissue repair (16, 20, 22, 24). This report describes two BDs of the goat and demonstrates the highly tissue-specific expression of these peptides.
Tissue and RNA preparation.
Thirty-nine necropsy tissue samples were obtained from a healthy male kid goat, by using separate sterile forceps and scissors for each. Tissues were rinsed in cold sterile saline, frozen immediately, and stored at −80°C. Total RNA was purified with the Tri-Reagent RNA isolation procedure (Molecular Research Center, Cincinnati, Ohio). One hundred microliters of cDNA was synthesized from 1 μg of each tissue RNA with an Advantage RT-for-PCR kit (Clontech, Palo Alto, Calif.) and stored frozen until used. Some limitations of this approach deserve mention. Since our samples came from a single male goat, they provided no information about the female genitourinary tract or about variations in defensin expression between animals.
Preliminary cDNA cloning.
PCR primer sets based on bovine LAP and TAP cDNA sequences were used to amplify 5 μl of goat tongue cDNA for 35 cycles, as follows: 94°C for 20 s, 55°C for 20 s, and 72°C for 1 min. P1, a sense primer (5′-CTCCTCTTCCTGGTCCTGT-3′), corresponded to nucleotides 51 to 69 of the bovine LAP cDNA sequence, and P2, an antisense primer (5′-AACTTTGAACAAAATTTATTTATTCT-3′), corresponded to the region immediately before its poly(A) tail (22). The reverse transcriptase PCR (RT-PCR) product of these primers was inserted into the TOPO TA vector (Invitrogen, Carlsbad, Calif.) and sequenced, revealing a sequence homologous to both LAP and TAP. To complete the sequence, we performed 5′ and 3′ rapid-amplification-of-cDNA-end reactions with a Marathon cDNA kit (Clontech). A template of purified goat tongue poly(A)+ RNA was used to synthesize the first-strand cDNA, which served as the template for second-strand synthesis. The Marathon cDNA adapter was ligated to the double-stranded cDNA. P3, a gene-specific primer corresponding to nucleotides 59 to 84 of goat BD-1 (GBD-1) (Fig. 1) (5′-CCTGTCTGCTGGGTCAGGATTTACTC-3′), and the Marathon adapter primer were used to get 3′-end GBD-1 cDNA. Gene-specific antisense primer P4 (5′-CGATCTGTCTAAGGGCGCAGTTTCTG-3′), complementary to nucleotides 249 to 274 of GBD-1 (Fig. 1), and the Marathon adapter primer were used to obtain the 5′ end of GBD-1 cDNA. There was a 216-bp sequence overlap between the two PCR products. The band was purified and cloned into a pCRII vector with a TA kit (Invitrogen). Sequencing was performed by the fluorescein-labeled dideoxynucleotide terminator method, and sequences were analyzed on an Applied Biosystems 373A DNA sequencer (Perkin-Elmer, Palo Alto, Calif.). Cloning of GBD-2 is described below.
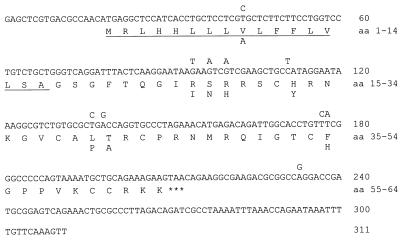
FIG. 1.
cDNA sequences of GBD-1 and GBD-2. The nucleotide and translated sequences of GBD-1 are shown in full. The nucleotide sequence of GBD-2 (311 bp) is shown above that of GBD-1 only where the sequence is different. Amino acids of GBD-2 that differ from those in GBD-1 are shown below the complete GBD-1 sequence. The putative signal sequence is underlined. aa, amino acids.
Tissue expression.
Five microliters of each goat cDNA preparation was amplified by GBD-specific primers P3 and P4. PCR was performed with an automated DNA thermal cycler for 25, 30, and 35 cycles as follows: 94°C for 20 s, 60°C for 20 s, and 72°C for 40 s. Two β-actin PCR primers (sense primer P5, 5′-CGTGGCCATCCAGGCTGTGCTGTCC-3′, and antisense primer P6, 5′-GCGATGCCAGGGTACATGGTGGTCC-3′) were designed according to the sheep β-actin cDNA sequence to assess quality and quantity of goat mRNA (expected PCR product, 530 bp). We used a master reagent mix to ensure tube-to-tube consistency in cDNA synthesis and PCR. Reaction products were visualized after electrophoresis in 1.5% agarose gels containing GelStar fluorescent stain (FMC, Rockland, Maine).
Northern blots.
Total RNA (25 μg) from each tissue was separated in a 1.0% formaldehyde agarose gel, capillary transferred to a nylon membrane (GeneScreen Plus; DuPont, Boston, Mass.), and hybridized with a 32P-labeled cDNA probe (216-bp PCR product obtained with the P3 and P4 primers). The final membrane wash prior to autoradiography was done at 65°C with 0.1% sodium dodecyl sulfate in 0.1× SSC buffer (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate).
Cloning of GBDs.
The full-length cDNA (Fig. 1) reconstructed from the overlapping 5′ and 3′ rapid-amplification-of-cDNA-end products predicts a protein, GBD-1, with 64 amino acid residues, a calculated mass of 7,258 Da, and a pI of 11.99. GBD-1 contains a typical signal peptide and a propeptide whose C-terminal domain includes six cysteines with a typical BD motif. GBD-1 cDNA is 95.8% identical to ovine BD (sheep BD-1), 88.4% identical to bovine LAP, and 71% identical to porcine lingual pBD-1.
To look for additional GBDs, we subcloned and sequenced a PCR product obtained by amplifying jejunum cDNA with P7 (5′-GAGCTCGTGACGCCAACATGAGG-3′), a sequence complementary to nucleotides 1 to 23 of GBD-1, and the antisense primer P2. This identified a second GBD, GBD-2, whose 311-nucleotide cDNA sequence was identical to GBD-1 in 301 (96.8%) of its bases (Fig. 1). The nucleotide differences between the isoforms would impart eight amino acid differences, one in the signal peptide and seven in the propeptide (Fig. 1).
Tissue expression.
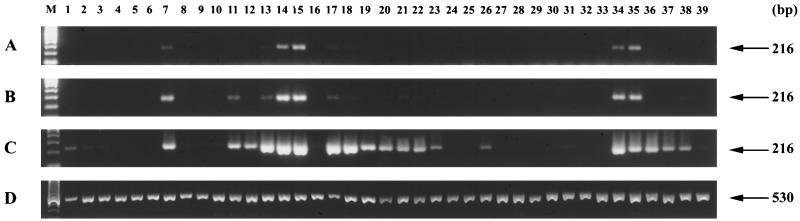
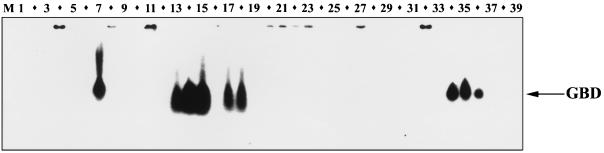
We examined the expression of GBD-1 and -2 in different goat tissues by RT-PCR, with primers that amplified both peptides. After 25 cycles, GBD transcripts were prominent only in the tongue, stomach (omasum and abomasum), respiratory tract (turbinates and trachea), and small intestine (jejunum and ileum). After 35 cycles, the message was readily detected in other respiratory and gastrointestinal tract tissues but was still not evident in the bone marrow, spleen, liver, lymph nodes, thymus, or male reproductive tract (Fig. 2). The female reproductive tract was not examined. By Northern blotting (Fig. 3), GBD transcripts were detected in the tongue, stomach, small intestine, nasal turbinates, trachea, and bronchi.
FIG. 2.
Tissue expression of GBDs. Expression in various tissues was examined by RT-PCR. GBD expression after 25, 30, and 35 PCR cycles is shown, and β-actin expression after 35 cycles is shown. The primers (GBD, P3 and P4; β-actin, P5 and P6) are described in the text. Lanes: M, molecular size markers; 1, bone marrow; 2, mandibular node; 3, mediastinal node; 4, spleen; 5, thymus; 6, pericardium; 7, tongue; 8, mandibular salivary gland; 9, parotid salivary gland; 10, sublingual salivary gland; 11, tonsils; 12, esophagus; 13, reticulum stomach; 14, omasum stomach; 15, abomasum stomach; 16, duodenum; 17, jejunum; 18, ileum; 19, proximal colon; 20, medial colon; 21, distal colon; 22, rectum; 23, anal area; 24, pancreas; 25, liver; 26, gallbladder; 27, renal medulla; 28, renal cortex; 29, bladder-ureter; 30, penis; 31, testis; 32, prostate; 33, epididymis; 34, nasal turbinate; 35, trachea; 36, bronchus; 37, lung; 38, eye; 39, ileocecal node.
FIG. 3.
Northern blot analysis. Total RNA prepared from 39 different goat tissues was probed with the 216-bp PCR product amplified by P3 and P4. The 32P-labeled probe detected both GBD-1 and GBD-2. See the legend to Fig. 2 for identification of lanes.
To distinguish GBD-1 from GBD-2, we subcloned and sequenced the RT-PCR products of P3 and P4 (Fig. 2) from 13 lingual clones, 18 respiratory tract clones (10 from the trachea and 4 each from the bronchi and lung), and 20 digestive tract clones (four each from the abomasum, jejunum, ileum, medial colon, and rectum). All 31 tongue and respiratory tract clones corresponded to GBD-1, and all 20 gastrointestinal tract clones corresponded to GBD-2.
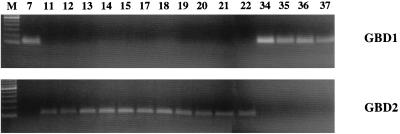
To confirm the differential expression of GBD-1 and GBD-2 in GBD-positive goat tissues by RT-PCR, we designed two defensin-specific primer sets. P8, a sense primer (5′-ACTCAAGGAATAAGAAGTCG-3′) corresponding to nucleotides 81 to 100, and P9, an antisense primer (5′-CATTTTACTGGGGGCCCGAA-3′) complementary to nucleotides 177 to 196 of the GBD-1 cDNA sequence, were used to obtain a 116-bp GBD-1 PCR product. For GBD-2, a sense primer, P10 (5′-ACTCAAGGAATAATAAATCA-3′), corresponding to nucleotides 81 to 100, and an antisense primer, P11 (5′-CATTTTACTGGGGGCCCGTG-3′), complementary to nucleotides 177 to 196 of the GBD-2 cDNA sequence, were used.
The findings, shown in Fig. 4, confirm the results of our sequencing studies. Minimal expression of GBD-1 and -2 took place in bone marrow, liver, kidney, thymus, spleen, or lymph nodes. GBD-1 expression predominated in the tongue, trachea, bronchi, and lung. GBD-2 expression prevailed throughout the intestinal tract: in the stomach, jejunum, ileum, colon, and rectum.
FIG. 4.
Differential expression of GBD-1 and GBD-2. Differential expression in selected GBD-expressing goat tissues was examined by RT-PCR. The specific primers for GBD-1 were P8 and P9 at an annealing temperature of 58°C. The specific primers for GBD-2 were P10 and P11 at an annealing temperature of 52°C. Additional details are provided in the text. See the legend to Fig. 2 for identification of lanes.
Since the GBD-1 and -2 genes undoubtedly arose via duplication of an ancestral gene also common to sheep BD-1 (see Fig. 6), their divergent patterns of expression must reflect different tissue-specific regulatory elements. The benefit of selective expression of GBD-1 and -2 is not clear. Although the peptides have almost identical primary structures, their antimicrobial properties have not been tested. Perhaps GBD-1 and -2 differ significantly in activity against airborne and food-borne organisms or in their resistance to other factors (e.g., proteases) that are differentially expressed in the respiratory and digestive tracts. Peptide level studies that examine such possibilities would be of interest.
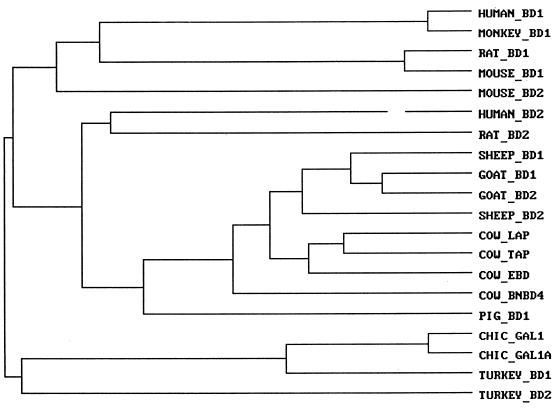
FIG. 6.
Dendrogram. The prepropeptide sequences shown in Fig. 5 were analyzed by the Clustal program (IntelliGenetics). CHIC, chicken.
Figure 5 shows the sequences of GBD-1 and -2 and of BDs found in sheep (11, 12), cattle (4, 5, 22, 25), pigs (29), humans (2, 7), rhesus monkeys (14), rats (13, 19), mice (11), chickens (3, 8), and turkeys (3). Figure 6 presents a dendrogram obtained with the Clustal program (IntelliGenetics) by using the amino acid sequences shown in Fig. 5. As expected, chicken and turkey BDs are most closely related to each other. The early branching of the avian and mammalian BDs indicates that both arose from a gene that existed before these lineages diverged. The similarity of the human and rhesus monkey BD-1 sequences and those of the rat and mouse implies their relatively recent divergence from a shared precursor. Since sheep, goats, and cattle are all ruminants, it is not surprising that all of their BD peptides cluster.
FIG. 5.
Sequences of BDs. References for these peptides are as follows: human BD-1 (2), rhesus monkey BD-1 (14), rat BD-1 (19), mouse BD-1 (11), mouse BD-2 (18), human BD-2 (7), rat BD-2 (13), sheep BD-1 (9, 12), sheep BD-2 (9, 11), cow LAP (22), cow TAP (4), cow EBD (25), cow BNBD4 (28), pig BD-1 (30), chicken (CHIC) GAL-1 (3), chicken GAL-1A (unpublished data); turkey BD-1 (3), turkey BD-2 (3).
Nucleotide sequence accession numbers.
The sequences of GBD-1 and GBD-2 were deposited in the EMBL database (accession numbers: Y17679, GBD-1, and AJ009877, GBD-2).
Acknowledgments
This work was supported by grants from the NIH, AI 22839 and AI 40248, and by a Fogarty Award, TW00355.
We thank Gwen Laird and Jean Laufer for their expert technical assistance.
REFERENCES
- 1.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson J M. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Investig. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bensch K W, Raida M, Magert H J, Schulz-Knappe P, Forssmann W G. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 3.Brockus C W, Jackwood M W, Harmon B G. Characterization of beta-defensin prepropeptide mRNA from chicken and turkey bone marrow. Anim Genet. 1998;29:283–289. doi: 10.1046/j.1365-2052.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 4.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy W L, Bevins C L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallagher D S, Jr, Ryan A M, Diamond G, Bevins C T, Womack J E. Somatic cell mapping of beta-defensin genes to cattle syntenic group U25 and fluorescence in situ localization to chromosome 27. Mamm Genome. 1995;6:554–556. doi: 10.1007/BF00356177. [DOI] [PubMed] [Google Scholar]
- 6.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 7.Harder J, Bartels J, Christophers E, Schroder J M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 8.Harwig S S, Swiderek K M, Kokryakov V N, Tan L, Lee T D, Panyutich E A, Aleshina G M, Shamova O V, Lehrer R I. Gallinacins: cysteine-rich antimicrobial peptides of chicken leukocytes. FEBS Lett. 1994;342:281–285. doi: 10.1016/0014-5793(94)80517-2. [DOI] [PubMed] [Google Scholar]
- 9.Huttner K M, Bevins C L. Antimicrobial peptides as mediators of epithelial host defense. Pediatr Res. 1999;45:785–794. doi: 10.1203/00006450-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Huttner K M, Brezinski-Caliguri D J, Mahoney M M, Diamond G. Antimicrobial peptide expression is developmentally regulated in the ovine gastrointestinal tract. J Nutr. 1998;128:297S–299S. doi: 10.1093/jn/128.2.297S. [DOI] [PubMed] [Google Scholar]
- 11.Huttner K M, Kozak C A, Bevins C L. The mouse genome encodes a single homolog of the antimicrobial peptide human beta-defensin 1. FEBS Lett. 1997;413:45–49. doi: 10.1016/s0014-5793(97)00875-2. [DOI] [PubMed] [Google Scholar]
- 12.Huttner K M, Lambeth M R, Burkin H R, Burkin D J, Broad T E. Localization and genomic organization of sheep antimicrobial peptide genes. Gene. 1998;206:85–91. doi: 10.1016/s0378-1119(97)00569-6. [DOI] [PubMed] [Google Scholar]
- 13.Jia, H. P., G. S. Wang, K. Wiles, B. F. Tack, and P. B. McCray, Jr. 1998. Rattus norvegicus beta defensin-2 mRNA, complete cds. GenBank locus AF068861, accession no. AF0688612. [Online.] http://www.ncbi.nlm.nih.gov.
- 14.Kwok, J., G. Hurlock, X. Wu, C. Penland, and J. J. Wine. 14 July 1997, posting date. Macaca mulatta beta defensin-1 homolog (rhBD-1) mRNA. GenBank locus AF014016, accession no. AF014016. [Online.] http://www.ncbi.nlm.nih.gov.
- 15.Lehrer R I, Ganz T. Endogenous vertebrate antibiotics: defensins, protegrins, and other cysteine-rich antimicrobial peptides. Ann N Y Acad Sci. 1996;797:228–239. doi: 10.1111/j.1749-6632.1996.tb52963.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Wang L, Jia H P, Zhao C, Heng H H Q, Schutte B C, McCray P B, Jr, Ganz T. Structure and mapping of the human beta-defensin HBD-2 gene and its expression at sites of inflammation. Gene. 1998;222:237–244. doi: 10.1016/s0378-1119(98)00480-6. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Zhao C, Heng H H, Ganz T. The human beta-defensin-1 and alpha-defensins are encoded by adjacent genes: two peptide families with differing disulfide topology share a common ancestry. Genomics. 1997;43:316–320. doi: 10.1006/geno.1997.4801. [DOI] [PubMed] [Google Scholar]
- 18.Morrison G M, Davidson D J, Dorin J R A. A novel mouse beta defensin, Defb2, which is upregulated in the airways by lipopolysaccharide. FEBS Lett. 1999;442:112–116. doi: 10.1016/s0014-5793(98)01630-5. [DOI] [PubMed] [Google Scholar]
- 19.Page, R. A., and A. N. Malik. 21 September 1998, posting date. Rattus norvegicus beta defensin-1 (BD-1) mRNA, complete cds. GenBank locus AF093536, accession no. AF093536. [Online.] http://www.ncbi.nlm.nih.gov.
- 20.Russell J P, Diamond G, Tarver A P, Scanlin T F, Bevins C L. Coordinate induction of two antibiotic genes in tracheal epithelial cells exposed to the inflammatory mediators lipopolysaccharide and tumor necrosis factor alpha. Infect Immun. 1996;64:1565–1568. doi: 10.1128/iai.64.5.1565-1568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnapp D, Reid C J, Harris A. Localization of expression of human beta defensin-1 in the pancreas and kidney. J Pathol. 1998;186:99–103. doi: 10.1002/(SICI)1096-9896(199809)186:1<99::AID-PATH133>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Schonwetter B S, Stolzenberg E D, Zasloff M A. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 23.Singh P K, Jia H P, Wiles K, Hesselberth J, Liu L, Conway B A, Greenberg E P, Valore E V, Welsh M J, Ganz T, Tack B F, McCray P B., Jr Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stolzenberg E D, Anderson G M, Ackermann M R, Whitlock R H, Zasloff M. Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci USA. 1997;94:8686–8690. doi: 10.1073/pnas.94.16.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarver A P, Clark D P, Diamond G, Russell J P, Erdjument- Bromage H, Tempst P, Cohen K S, Jones D E, Sweeney R W, Wines M, Hwang S, Bevins C L. Enteric beta-defensin: molecular cloning and characterization of a gene with inducible intestinal epithelial cell expression associated with Cryptosporidium parvum infection. Infect Immun. 1998;66:1045–1056. doi: 10.1128/iai.66.3.1045-1056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valore E V, Park C H, Quayle A J, Wiles K R, McCray P B, Jr, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Investig. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinberg A, Krisanaprakornkit S, Dale B A. Epithelial antimicrobial peptides: review and significance for oral applications. Crit Rev Oral Biol Med. 1998;9:399–414. doi: 10.1177/10454411980090040201. [DOI] [PubMed] [Google Scholar]
- 28.Yount, N. Y., J. Yuan, A. P. Tarver, G. Diamond, J. N. Levy, P. A. Mcguire, C. Mccullough, J. S. Cullor, C. L. Bevins, and M. E. Selsted. 17 July 1997, revision date. Bos taurus neutrophil beta-defensin 4 (BNBD4). GenBank locus AF008307, accession no. AF008307U60447U60446. [Online.] http: //www.ncbi.nlm.nih.gov.
- 29.Zhang G, Wu H, Shi J, Ganz T, Ross C R, Blecha F. Molecular cloning and tissue expression of porcine beta-defensin-1. FEBS Lett. 1998;424:37–40. doi: 10.1016/s0014-5793(98)00134-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhao C, Wang I, Lehrer R I. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann G R, Legault P, Selsted M E, Pardi A. Solution structure of bovine neutrophil beta-defensin-12: the peptide fold of the beta-defensins is identical to that of the classical defensins. Biochemistry. 1995;34:13663–13671. doi: 10.1021/bi00041a048. [DOI] [PubMed] [Google Scholar]