Abstract
A low-grade fibromyxoid sarcoma (LGFMS) is an extremely rare tumor first described by Evans in 1987. LGFMS is a type of soft tissue sarcoma characterized by a deceptively benign histological appearance but completely malignant behavior. LGFMS is usually seen in the deep soft tissues of the extremities and trunk. We have examined many reviews, case reports and case series previously published in PubMed and Google Scholar. To date, only five cases have been reported in the maxilla. LGFMS generally affects young adults, but it can also be seen in children and older adults. A radical surgical approach is the most recommended treatment option. LGFMS has a very low mitotic activity; therefore, it is considered that neither chemotherapy nor radiotherapy has a significant effect on long-term LGFMS prognosis. However, to date, there has been no study suggesting any protocol for the follow-up of patients with LGFMS. In this report, we present a case with LGFMS located in the maxillary sinus, in which despite radiotherapy following extensive surgical excision, the tumor recurred in a short period of three months and reached its former size.
Keywords: Low-grade fibromyxoid sarcoma, LGFMS, Sarcoma, Maxillary sinus, Paranasal sinus sarcoma
Introduction
Low-grade fibromyxoid sarcoma (LGFMS) is a variant of fibrosarcoma that usually occurs in the deep soft tissues of the extremities in young or middle-aged adults [1]. Primary sarcomas of the head and neck region are very rare and constitute approximately 1% of malignancies in this region [2]. Although LGFMS acts like a benign tumor with a slow growth pattern, it has very high potential for local recurrence and distant metastasis [3]. The incidence of LGFMS is very similar between men and women, and it is a tumor that can be seen at all ages [4, 5]. LGFMS is primarily seen in the deep soft tissues of the extremities and trunk, and a small number of cases have been reported in the head and neck region [3, 4]. It is genetically characterized by translocation on chromosomes 7 and 16 [6]. Immunohistochemically, most cells of this sarcoma are strongly positive for vimentin but are generally negative for α-actin, desmin, S-100 protein, cytokeratin, CD34, and CD56 [7]. LGFMS typically appears as a well-circumscribed, encapsulated mass without necrosis or bleeding foci [8]. We aimed to obtain a more accurate idea of LGFMS by reviewing the literature and examining relapse, recurrence, gender and affected areas in cases reported to date. As a result of this detailed literature review, we summarized the data obtained in a table, which constitutes the most comprehensive list of LGFMS cases with the head and neck localization in the literature (Table 1).
Table 1.
List of all case reports obtained after a comprehensive review of the literature (PubMed and Google Scholar)
| Case | Age | Sex | Location | Outcome (If Known) | References |
|---|---|---|---|---|---|
| 1 | 26 | M | NECK | Multiple recurrent, alive at 44 years | Evans [9] |
| 2 | 3y 10 m | M | JAW | Local recurrence after 3 months; local recurrence and concomitant lung metastasis after 15 years with surgery and radiation; NED 2.5 years later | Papadimitriou et al. [10] |
| 3 | 36 | M | NECK | NED at 55 mo | Lane et al. [11] |
| 4 | N/A | N/A | HEAD/NECK | N/A | Folpe et al. [12] |
| 5 | N/A | N/A | HEAD/NECK | N/A | Folpe et al. [12] |
| 6 | N/A | N/A | HEAD/NECK | N/A | Folpe et al. [12] |
| 7 | N/A | N/A | HEAD/NECK | N/A | Folpe et al. [12] |
| 8 | 41 | M | LEFT SUPRACLAVICULAR REGION | N/A | Zamenik and Michal [13] |
| 9 | 57 | F | CHEEK | N/A | Botev et al. [14] |
| 10 | 22 | M | NECK | NED at 38 months | Guillou et al. [15] |
| 11 | 40 | F | SCM | NED at 12 months | Marglani et al. [16] |
| 12 | 57 | M | THYROID | NED at 13 months | Merchant [17] |
| 13 | 1Y 10 M | M | CHEEK | NED after 6 months | Tang et al. [18] |
| 14 | 57 | M | NECK | N/A | Viswanathan et al. [19] |
| 15 | 27 | M | JAW | NED after 22 months | Rekhi et al. [20] |
| 16 | 31 | M | FACE | N/A | Rekhi et al. [20] |
| 17 | 69 | M | NECK | 2 recurrences after 10 years | Rekhi et al. [20] |
| 18 | 26 | M | NECK | Multiple recurrences treated with excision, NED at 44 years | Evans [3] |
| 19 | 9 | M | NECK | Multiple recurrences, followed by radiation after last; lung and chest wall metastases at 25 years treated with multiple resections; died of metastatic tumor at 42 years | Evans [3] |
| 20 | 84 | F | RIGHT FOREHEAD | After resection with a 1-cm skin margin, recurrence occurred at 15 months postoperatively. Additional wide excision was subsequently performed with a 2-cm skin margin. Recurrence and metastasis have not been observed for 1 year after the second excision | Abe et al. [7] |
| 21 | 53 | F | NECK | NED after 91 months | Maretty-Nielsen et al. [21] |
| 22 | 35 | M | NECK | NED after 63 months | Maretty-Nielsen et al. [21] |
| 23 | N/A | N/A | THYROİD | N/A | Dong and Zhang [22] |
| 24 | 14 | M | CHEEK | NED after 12 months | He et al. [23] |
| 25 | 41 | M | NECK |
LUNG MET 48 MONTH 271 MONTHS DİED OF DİSEASE |
Prieto-Granada et al. [24] |
| 26 | 40 | M | HARD PALATE | NED after 4 months | Soma et al. [25] |
| 27 | 63 | F | MASSETER MUSCLE | NED after 12 months | Lee et al. [26] |
| 28 | 16 | M | MAXILLA | NED after 6 months | Spalthoff et al. [27] |
| 39 | 6 | M | PESTERİOR CERVİCAL SPİNE | One patient was disease-free at 12 months post-excision. The other patient was alive 10 months after surgery with multiple sub-centimeter pulmonary nodules too small to biopsy and enlarged posttracheal and jugular chain lymph nodes (up to 1.4 cm) suggesting disseminated disease | Cowan et al. [4] |
| 30 | 43 | F | FACİAL SKİN | One patient was disease-free at 12 months post-excision. The other patient was alive 10 months after surgery with multiple sub-centimeter pulmonary nodules too small to biopsy and enlarged posttracheal and jugular chain lymph nodes (up to 1.4 cm) suggesting disseminated disease | Cowan et al. [4] |
| 31 | 45 | M | MANDİBLE | One patient was disease-free at 12 months post-excision. The other patient was alive 10 months after surgery with multiple sub-centimeter pulmonary nodules too small to biopsy and enlarged posttracheal and jugular chain lymph nodes (up to 1.4 cm) suggesting disseminated disease | Cowan et al. [4] |
| 32 | 73 | M | LARYNX | One patient was disease-free at 12 months post-excision. The other patient was alive 10 months after surgery with multiple sub-centimeter pulmonary nodules too small to biopsy and enlarged posttracheal and jugular chain lymph nodes (up to 1.4 cm) suggesting disseminated disease | Cowan et al. [4] |
| 33 | 35 | M | MANDİBLE | N/A | Chaudhuri et al. [28] |
| 34 | 61 | M | MANDİBLE | NED after 24 months | Kargahi et al. [29] |
| 35 | 2 M | M | NECK | NED after 4 months | Zakiyah [30] |
| 36 | 40 | F | POSTERIOR NASAL CAVITY | NED after 60 months | Sohn et al. [31] |
| 37 | N/A | N/A | TONGUE | N/A | Pellini et al. [32] |
| 38 | 18 | F | BUCCAL MUCOSA | NED after 18 months | Kanato et al. [33] |
| 39 | 44 | F | EXTERNAL AUDITORY CANAL | NED after 18 months | Kumari et al. [34] |
| 40 | 57 | F | PARAPHARYNGEAL SPACE | NED after 24 months | Toro et al. [35] |
| 41 | N/A | N/A | MAXİLLARY SINUS | NED after 148 months | Koucky et al. [36] |
| 42 | N/A | N/A | MAXİLLARY SINUS | NED after 148 months | Koucky et al. [36] |
| 43 | N/A | N/A | NASAL CAVİTY | NED after 148 months | Koucky et al. [36] |
| 44 | 45 | M | MAXILLA | NED after 12 months | Flores et al. [1] |
| 45 | 78 | M | NECK | NED after 12 months | Park et al. [8] |
M male, F female, N/A information was not available, NED no evidence of disease
Case
A 56-year-old male patient presented to our clinic with a painless swelling growing slowly in the right malar region. On his physical examination, there was a swelling extending to the right upper hard palate. The endoscopic examination revealed a soft tissue mass filling the middle meatus in the right nasal passage. The nasopharynx was natural, and all routine blood tests were normal. Contrast-enhanced paranasal sinus, neck and thorax tomographies were taken. Diagnostic imaging revealed a contrast-enhancing soft tissue mass in the right maxillary sinus, which had eroded the bone structures of the sinus. The mass extended into the right nasal passage and filled the middle meatus (Figs. 1, 2). However, no signs of invasion or metastasis were detected. A punch biopsy was performed from the part of the mass extending to the nasal passage under local anesthesia. Histologically, the mass was characterized by epithelioid cells with a fibrotic basis. In the immunohistochemical examination, vimentin and MUC4 were positive while S100, CD34, desmin, actin, keratin, and epithelial membrane antigens (AE1/AE3, LMWK, and HMWK) were negative. Thus, we planned the excision of the entire tumor, including the surrounding soft tissue, under general anesthesia. We made a Weber-Ferguson incision to reach the tumor (Fig. 3). After flap elevation, it was observed that the maxillary anterior wall bone structure was completely lost. The tumor was seen to have expanded into surrounding tissues (Fig. 4). The tumor was macroscopically removed en-block (Figs. 5, 6). The pathological examination revealed tumor cells growing in nests surrounded by partially spindle-like cells and partially myxoid stroma. Some atypical mitotic figures were also seen (Figs. 7, 8). Immunohistochemically, vimentin was positive in tumor cells, but actin, CD31, CD34, S100, and desmin were negative. The Ki-67 proliferation index was 10–20%. The fluorescent in situ hybridization test was positive for FUS gene rearrangement. Thus, the patient was diagnosed with LGFMS. Radiotherapy was initiated from the third week after surgery. At the end of the postoperative third month, the follow-up computed tomography showed that the tumor had filled the maxillary sinus again and reached its initial size (Figs. 9, 10).
Fig. 1.
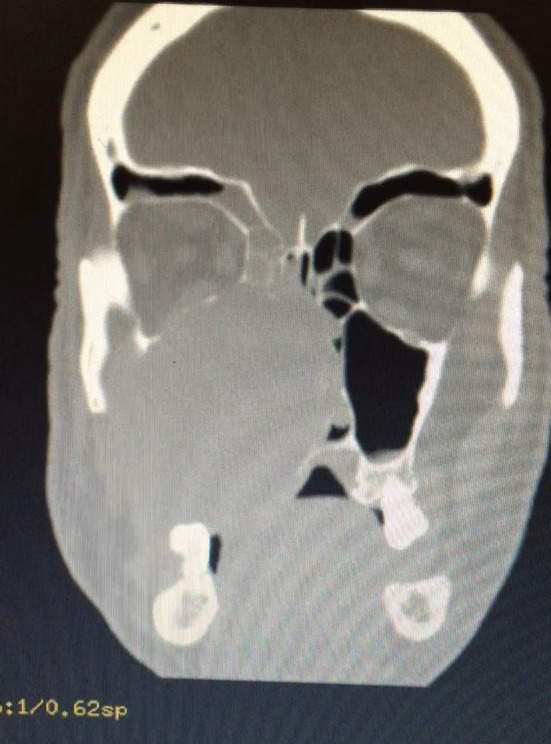
Preoperative computed tomography image in the coronal plane
Fig. 2.
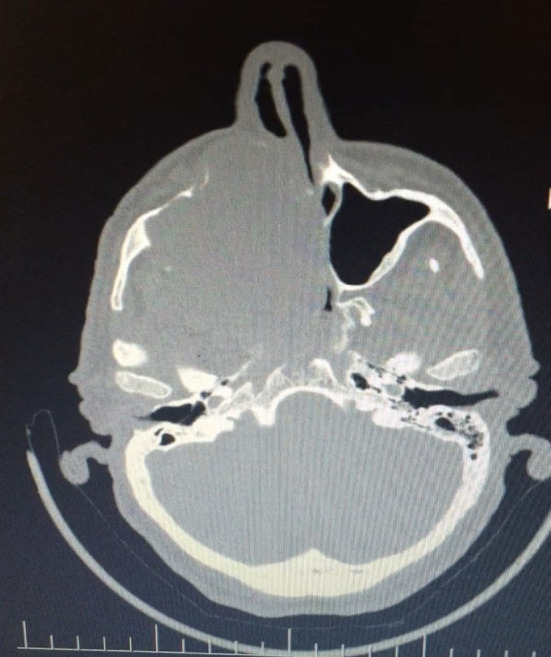
Preoperative computed tomography image in the axial plane
Fig. 3.
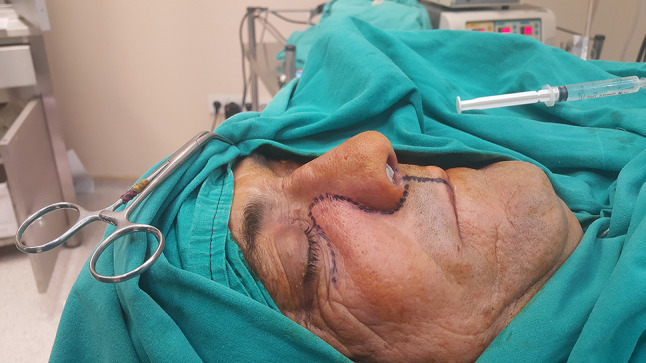
Weber-Ferguson incision
Fig. 4.
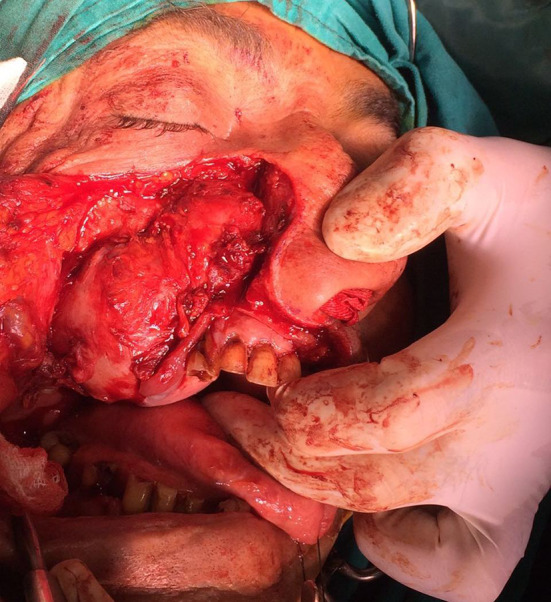
Appearance of the mass after flap elevation revealing the defect on the anterior wall of the maxilla
Fig. 5.
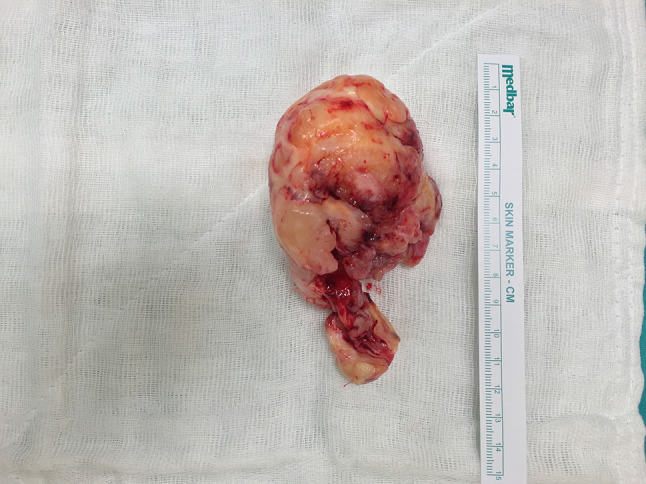
Macroscopic image of the mass that was removed en-block together with the part extending into the nasal passage
Fig. 6.
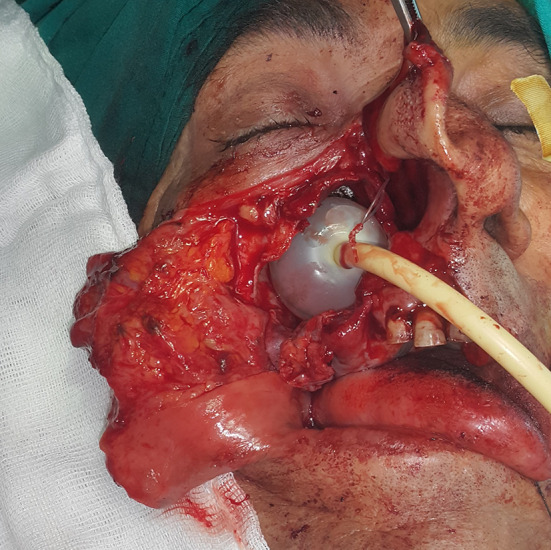
A Foley catheter inserted in the sinus cavity and inflated to 15 cc sf after the removal of the mass in order to provide support for the eroded maxillary sinus bone structure during healing and prevent cosmetic deformity
Fig. 7.

Low cell rate and dense vascular network tumor morphology in diffuse myxoid focal collagenized stroma (H & E × 100)
Fig. 8.
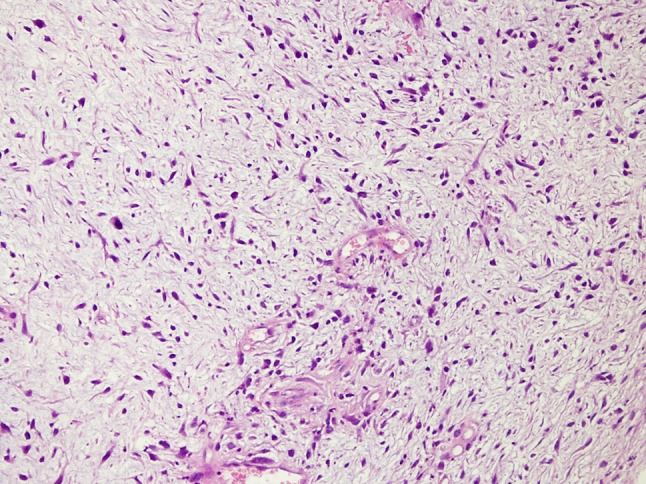
Spindle cells with mild pleomorphic nuclei in fibromyxoid stroma (H & E × 200). The pathology revealed tumor-cells growing in nests surrounded by partially spindle-like cells and partially myxoid stroma. Some cells were eosinophilic and others showed clear cell-like cytoplasm. Many mitoses were observed with some atypical mitotic figures
Fig. 9.
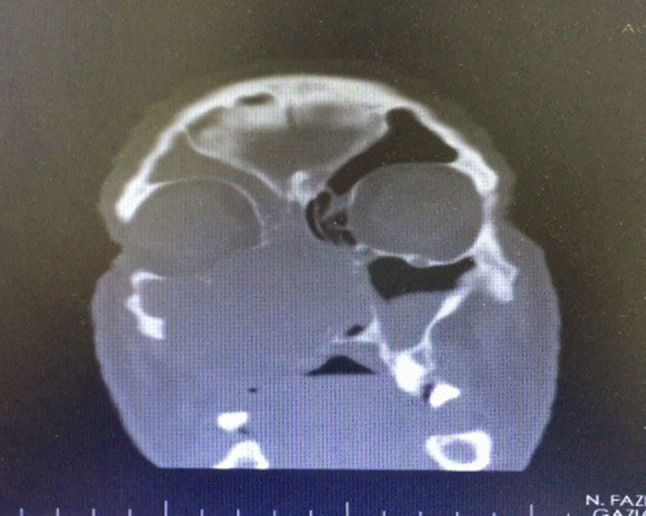
Postoperative third-month follow-up computed tomography image in the coronal plane
Fig. 10.
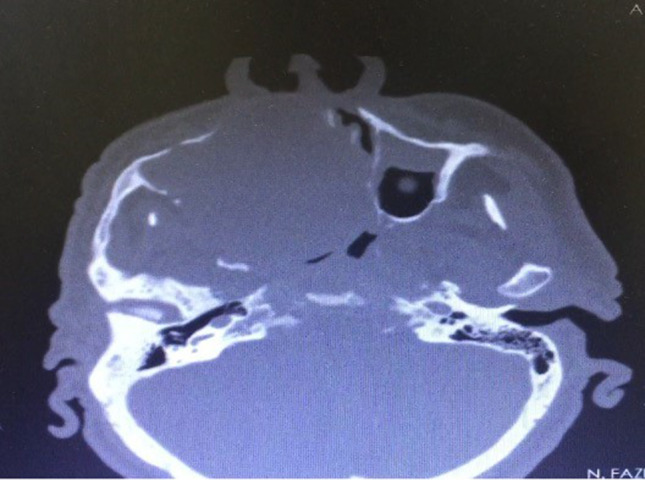
Postoperative third-month follow-up computed tomography image in the axial plane
Discussion
LGFMS is a benign tumor rarely seen in the head and neck region. Despite acting like a benign tumor with a slow course, it can exhibit malignant behaviors such as distant metastasis and local recurrence [8]. In the head and neck region, the maxillary sinus localization of LGFMS is even rarer, and our literature review revealed only five reported cases in total [1, 9–11]. Although there are many case reports or series about LGFMS in the literature, LGFMS continues to be a challenging and complex clinical entity for surgeons. Since it was first described by Evans in 1987, confusions remain concerning the diagnosis and treatment of LGFMS [12]. Based on our own clinical experience, we can state that unfortunately, it is very difficult to diagnose these patients in centers that are not equipped with molecular, genetic and immunohistochemical laboratories. Even in most experienced clinics, fine needle aspiration biopsy (FNAB), which is the first procedure used for a pathological evaluation in head and neck masses, is insufficient in diagnosing this tumor [13]. In a neck mass that cannot be diagnosed with FNAB, if there is no necrosis or hemorrhage in the mass due to radiological invasion, metastasis or high mitosis, it naturally becomes difficult to make a radical surgery decision. Even if a radical surgery decision is taken, this does not necessarily improve the condition of the patient or the course of the disease. Nevertheless, the radical surgical approach maintains its exaggerated popularity as a treatment method recommended by many surgeons and considered to be the most beneficial for the patient [1, 2, 10, 13–15]. The surgical treatment of LGFMS, which also occurs in the extremities in addition the head and neck region, does not affect the comfort of the patient as dramatically as the large resection of a tumor in the maxillary sinus. Unfortunately, the large surgical excision of LGFMS in the head and neck region, especially in the maxilla, which has been previously described in a few cases in the literature, creates hesitations in the surgical approach due to the possibility of resulting in serious cosmetic deformities [11, 13]. Cosmetic concerns clearly should not change the treatment algorithm of this malignant tumor; however, the literature contains controversial discussions concerning the benefits of radical surgery, with patients often experiencing frequent and short-term recurrences and distant metastases and requiring multiple operations [3, 4, 16, 17]. In the first and longest-term follow-up study, LGFMS was detected in the head and neck region in two patients, one aged 26 and the other nine years, requiring nine and seven operations, respectively after the first excision of LGFMS [3]. Distant metastasis developed in the nine-year-old patient despite radiotherapy application after the last recurrent tumor excision, and the 42-year-old patient died due to distant metastasis after repeated metastasis operations (six operations).
According to Ewans, the mean recurrence time of LGFMS is 3.5 years, while the average duration of distant metastasis is five years. However, such long-term follow-ups are very limited in the literature. In this respect, the literature can be considered insufficient in terms of revealing the true recurrence, distant metastasis capacity and progression rate of the disease. On the other hand, recurrence after surgery is not the only problem caused by the genetic and histological features of LGFMS. Another factor that makes treatment difficult is the low mitosis rate, which is one of the most characteristic features of the tumor [8, 18]. The low mitotic rate and fibrotic background also limit the effectiveness of radiotherapy and chemotherapy, which are highly effective in malignant tumors [19, 20]. This situation leads to the inability to prevent recurrence associated with residual tumor at microscopic level after surgery. As we mentioned in the case we presented, although we were sure of the surgical margins and started radiotherapy immediately after surgery, our patients relapsing in a very short time presented serious evidence for that it is a difficult disease to treat.
Lastly, during the literature review on LGFMS conducted as part of this study, we found 36 cases for which gender and age information was available, and they had a wide age range from two months to 84 years, with the mean value being 38.6 years, which is consistent with previous reviews [4, 15]. However, based on the gender data of 36 patients, among the patients with head and neck involvement, LGFMS was 2.6 times more common in males. In the literature, equal or similar prevalence is generally reported for both genders [4, 5, 15].
In conclusion, LGFMS is a soft tissue tumor that should be diagnosed genetically and immunohistochemically. The benefit of FNAB in diagnosis is very limited. The best option for its treatment is radical surgical excision, although it does not provide a desired satisfaction level in today’s conditions. Although the effects of radiotherapy and chemotherapy remain controversial in the literature, we know that radiotherapy was not beneficial for our patient. We consider that all scientific discussions concerning this issue should continue, and further diagnosis and treatment studies should be conducted on LGFMS.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flores RM, Rojas BV, Gómez VM, Burgos C. Low-grade fibromyxoid sarcoma of the maxilla. Report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;129(1):e33. doi: 10.1016/j.oooo.2019.06.092. [DOI] [Google Scholar]
- 2.Tajudeen BA, Fuller J, Lai C, Grogan T, Elashoff D, Abemayor E, et al. Head and neck sarcomas: the UCLA experience. Am J Otolaryngol. 2014;35(4):476–481. doi: 10.1016/j.amjoto.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans HL. Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol. 2011;35(10):1450–1462. doi: 10.1097/PAS.0b013e31822b3687. [DOI] [PubMed] [Google Scholar]
- 4.Cowan ML, Thompson LD, Leon ME, Bishop JA. Low-grade fibromyxoid sarcoma of the head and neck: a clinicopathologic series and review of the literature. Head Neck Pathol. 2016;10(2):161–166. doi: 10.1007/s12105-015-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logan SJ, Perricone A, Farris AB, Edgar M. Low-grade fibromyxoid sarcoma: a potentially useful histologic finding. Histopathology. 2020;77(2):329–331. doi: 10.1111/his.14142. [DOI] [PubMed] [Google Scholar]
- 6.Rose B, Tamvakopoulos GS, Dulay K, Pollock R, Skinner J, Briggs T, et al. The clinical significance of the FUS-CREB3L2 translocation in low-grade fibromyxoid sarcoma. J Orthop Surg Res. 2011;6(1):15. doi: 10.1186/1749-799X-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abe Y, Hashimoto I, Nakanishi H. Recurring facial low-grade fibromyxoid sarcoma in an elderly patient: a case report. J Med Invest. 2012;59(3–4):266–269. doi: 10.2152/jmi.59.266. [DOI] [PubMed] [Google Scholar]
- 8.Park JM, Lim HR, Kim JH, Lee DH. Giant low-grade fibromyxoid sarcoma in the neck. Korean J Otorhinolaryngol Head Neck Surg. 2020;63(9):432–435. doi: 10.3342/kjorl-hns.2019.00857. [DOI] [Google Scholar]
- 9.Evans HL. Low-grade fibromyxoid sarcoma. A report of 12 cases. Am J Surg Pathol. 1993;17(6):595–600. doi: 10.1097/00000478-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Papadimitriou JC, Ord RA, Drachenberg CB. Head and neck fibromyxoid sarcoma: clinicopathological correlation with emphasis on peculiar ultrastructural features related to collagen processing. Ultrastruct Pathol. 1997;21(1):81–87. doi: 10.3109/01913129709023250. [DOI] [PubMed] [Google Scholar]
- 11.Lane KL, Shannon RJ, Weiss SW. Hyalinizing spindle cell tumor with giant rosettes: a distinctive tumor closely resembling low-grade fibromyxoid sarcoma. Am J Surg Pathol. 1997;21(12):1481–1488. doi: 10.1097/00000478-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Folpe AL, Lane KL, Paull G, Weiss SW. Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor with giant rosettes: a clinicopathologic study of 73 cases supporting their identity and assessing the impact of high-grade areas. Am J Surg Pathol. 2000;24(10):1353–1360. doi: 10.1097/00000478-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Zámečník M, Michal M. Low-grade fibromyxoid sarcoma: a report of eight cases with histologic, immunohistochemical, and ultrastructural study. Ann Diagn Pathol. 2000;4(4):207–217. doi: 10.1053/adpa.2000.8122. [DOI] [PubMed] [Google Scholar]
- 14.Botev B, Casale M, Vincenzi B, D’ascanio L, Santini D, Esposito V, et al. A giant sarcoma of the parotid gland: a case report and review of the literature. In Vivo. 2006;20(6B):907–910. [PubMed] [Google Scholar]
- 15.Guillou L, Benhattar J, Gengler C, Gallagher G, Ranchere-Vince D, Collin F, et al. Translocation-positive low-grade fibromyxoid sarcoma: clinicopathologic and molecular analysis of a series expanding the morphologic spectrum and suggesting potential relationship to sclerosing epithelioid fibrosarcoma: a study from the French Sarcoma Group. Am J Surg Pathol. 2007;31(9):1387–1402. doi: 10.1097/PAS.0b013e3180321959. [DOI] [PubMed] [Google Scholar]
- 16.Marglani O, Commons S, Lamothe A. Radiation-induced low-grade fibromyxoid sarcoma of the sternocleidomastoid muscle. J Otolaryngol. 2007;36(5):E73–E75. [PubMed] [Google Scholar]
- 17.Merchant SH. Low grade fibromyxoid sarcoma: report of a case with epithelioid cell morphology, masquerading as a papillary thyroid carcinoma. Acta Cytol. 2009;53(6):689–692. doi: 10.1159/000325411. [DOI] [PubMed] [Google Scholar]
- 18.Tang Z, Zhou ZH, Lv CT, Qin LY, Wang Y, Tian G, et al. Low-grade fibromyxoid sarcoma: clinical study and case report. J Oral Maxillofac Surg. 2010;68(4):873–884. doi: 10.1016/j.joms.2009.04.136. [DOI] [PubMed] [Google Scholar]
- 19.Viswanathan S, Rahman K, Pallavi S, Sachin J, Patil A, Chaturvedi P, et al. Sarcomatoid (spindle cell) carcinoma of the head and neck mucosal region: a clinicopathologic review of 103 cases from a tertiary referral cancer centre. Head Neck Pathol. 2010;4(4):265–275. doi: 10.1007/s12105-010-0204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rekhi B, Deshmukh M, Jambhekar NA. Low-grade fibromyxoid sarcoma: a clinicopathologic study of 18 cases, including histopathologic relationship with sclerosing epithelioid fibrosarcoma in a subset of cases. Ann Diagn Pathol. 2011;15(5):303–311. doi: 10.1016/j.anndiagpath.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Maretty-Nielsen K, Baerentzen S, Keller J, Dyrop HB, Safwat A. Low-grade fibromyxoid sarcoma: incidence, treatment strategy of metastases, and clinical significance of the FUS gene. Sarcoma. 2013;2013:256280. doi: 10.1155/2013/256280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong W, Zhang H. Low-grade fibromyxoid sarcoma of the thyroid: a case report. Ann Acad Med Singap. 2013;42(1):55–56. doi: 10.47102/annals-acadmedsg.V42N1p55. [DOI] [PubMed] [Google Scholar]
- 23.He KF, Jia J, Zhao YF. Low-grade fibromyxoid sarcoma with cystic appearance and osseous metaplasia in the cheek: a case report and review of the literature. J Oral Maxillofac Surg. 2013;71(6):1143–1150. doi: 10.1016/j.joms.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Prieto-Granada C, Zhang L, Chen HW, Sung YS, Agaram NP, Jungbluth AA, et al. A genetic dichotomy between pure sclerosing epithelioid fibrosarcoma (SEF) and hybrid SEF/low-grade fibromyxoid sarcoma: a pathologic and molecular study of 18 cases. Genes Chromosomes Cancer. 2015;54(1):28–38. doi: 10.1002/gcc.22215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soma S, Bhat S, Shetty SK. Low grade fibromyxoid sarcoma of the palate: a case report. J Clin Diagn Res. 2015;9(10):XD01–XD2. doi: 10.7860/JCDR/2015/14670.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee EJ, Hwang HJ, Byeon HK, Park HS, Choi HS. A low grade fibromyxoid sarcoma originating from the masseter muscle: a case report. J Med Case Rep. 2015;9(1):176. doi: 10.1186/s13256-015-0658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spalthoff S, Bredt M, Gellrich NC, Jehn P. A rare pathology: low-grade fibromyxoid sarcoma of the maxilla. J Oral Maxillofac Surg. 2016;74(1):219e1-10. doi: 10.1016/j.joms.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhuri K, Kasimsetty CR, Lingappa A, Gujjar PV. Low-grade fibromyxoid sarcoma involving the mandible: a diagnostic dilemma. J Oral Maxillofac Pathol. 2016;20(2):334. doi: 10.4103/0973-029X.185914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kargahi N, Arjang E, Shahnaseri S, Keshani FJME. Low-grade Myxofibrosarcoma in the mandible: a rare case report. Middle East J Cancer. 2017;8(2):113–116. [Google Scholar]
- 30.Zakiyah R (2017) An infantile low grade fibromyxosarcoma of the neck: a rare case. J Islamic Med Res 1(2)
- 31.Sohn JH, Lee K, Cho KR. Low-grade fibromyxoid sarcoma arising in posterior nasal cavity: case report and review of the literature. Korean J Otorhinolaryngol Head Neck Surg. 2018;61(11):624–629. doi: 10.3342/kjorl-hns.2018.00437. [DOI] [Google Scholar]
- 32.Pellini R, De Virgilio A, Petruzzi G, Pichi B, Mercante G, Malvezzi L, et al. Low-grade fibromyxoid sarcoma of the tongue: a rare nosological entity. Otorinolaringologia. 2019;69(3):188–191. doi: 10.23736/S0392-6621.18.02205-1. [DOI] [Google Scholar]
- 33.Kanato T, Kalyani S, Lailyang T, Santosh D, Rebecca T, Charai H. Low grade fibromyxoid sarcoma in oral cavity: a rare case report. Indian J Otolaryngol Head Neck Surg. 2019;71(Suppl 1):25–26. doi: 10.1007/s12070-015-0946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumari K, Thota R, Chaudhary HL, Sharma MC, Thakar A, Singh G. Low-grade fibromyxoid sarcoma of the external auditory canal: a rare pathology and unusual location. Head Neck Pathol. 2020;14(1):276–282. doi: 10.1007/s12105-019-01030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toro C, Costa P, Vecchio GM, Magro G. Low-grade fibromyxoid sarcoma of the parapharyngeal space: a case report and review of the literature. Oral Maxillofac Surg Cases. 2020;2020:100152. doi: 10.1016/j.omsc.2020.100152. [DOI] [Google Scholar]
- 36.Koucky V, Kalfert D, Kodetova Novakova D, Plzak J (2020) Low-grade fibromyxoid sarcoma of the maxillary sinus. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub [DOI] [PubMed]
- 37.Evans HLJ. Low-grade fibromyxoid sarcoma: a report of two metastasizing neoplasms having a deceptively benign appearance. Am j clin pathol. 1987;88(5):615–619. doi: 10.1093/ajcp/88.5.615. [DOI] [PubMed] [Google Scholar]
- 38.Domanski H, Mertens F, Panagopoulos I, Åkerman MJC. Low-grade fibromyxoid sarcoma is difficult to diagnose by fine needle aspiration cytology: a cytomorphological study of eight cases. Cytopathology. 2009;20(5):304–314. doi: 10.1111/j.1365-2303.2008.00587.x. [DOI] [PubMed] [Google Scholar]
- 39.Mustafa S, VandenBussche CJ, Ali SZ, Siddiqui MT, Wakely PE., Jr Cytomorphologic findings of low-grade fibromyxoid sarcoma. J Am Soc Cytopathol. 2020;9(3):191–201. doi: 10.1016/j.jasc.2020.01.006. [DOI] [PubMed] [Google Scholar]


