Abstract
Schwannoma in cervical region arising from Sympathetic chain is a very rare entity. Ancient schwannoma is the term used to describe a schwannoma that has undergone changes viz loss of antoni Type A cells, perivascular hyalinization, calcification, cystic necrosis, hemorrhage and presence of degenerative nuclear changes. Ackerman and Taylor (Cancer 4:669–691, 1951) introduced the term “ancient schwannoma”. Badawi (Eur J Surg Oncol 28(1):88–90, 2002) in 2002 reported the first case of ancient schwannoma of the sympathetic chain. Surgery is the choice of treatment. In most of the cases Horner’s syndrome may or may not be the presenting symptom but may appear postoperatively. First bite syndrome is another complication of surgery. These must be kept in mind and proper counseling should be done preoperatively. Fine needle aspiration cytology (FNAC) may be misleading. To diagnose, radiological investigations like CT/MRI are helpful. They may mimic carotid body tumor, vagal schwannoma, necrotic lymph node and sarcoma.
Keywords: Schwannoma, Ancient, Carotid body, Cervical sympathetic chain, Horner’s syndrome
Introduction
A schwannoma is a uncommon benign perineural tumor of neuroectodermal derivation composed of schwann cells of the neural sheath of motor and sensory peripheral nerves. They rarely turn malignant. The etiology is still unknown. It may develop at any age and there is no gender predilection. 25–40% of schwannomas occur in head and neck region.
Verocay in 1910 first described a group of neurogenic tumors and referred them as Neurinomas. Stout [3] in 1935 described their origin from the Schwann cell termed them as schwannomas. In the head and neck region, neural tumors are derived from cells taking origin from the neural crest cell. Schwannomas can arise from any nerve covered with Schwann cell sheath. Therefore all cranial nerves except optic and olfactory nerves or sympathetic nerve or trunk can develop Schwannomas. Vagus nerve is the commonest nerve to be involved in the neck. The histogenesis of these tumors is not known. They are slow growing, circumscribed, presenting submucosally, may or may not be painful. The nerve of origin is not demonstrable in 50% cases. These tumors do not cause any damage to the nerve of origin as the nerve is splayed around the tumor in its capsule. The lateral cervical region and the mouth are the most common sites. Ancient schwannoma is the term used to describe a schwannoma that has undergone changes viz loss of antoni Type A cells, perivascular hyalinization, calcification, cystic necrosis, hemorrhage and presence of degenerative nuclear changes. It usually appears as slow growing, solitary and asymptomatic mass. Ackerman and Taylor [1] introduced the term “ancient schwannoma”. Badawi [2] in 2002 reported the first case of ancient schwannoma of the sympathetic chain. Horner’s syndrome may or may not be the presenting symptom in most of the cases but may appear postoperatively in most of the cases. First bite syndrome is another complication of surgery. These must be kept in mind and proper counseling should be done preoperatively.
Case Report
26 years old female complained of slow growing swelling in the upper neck just below the angle of mandible on left side. No difficulty in breathing, swallowing, pain or discomfort. On examination, approximately 4*3 cm mass, palpable below the angle of mandible on left side (Fig. 1). The mass was soft, mobile, non pulsatile, nontender and no changes observed over the skin. Oropharygo-laryngoscopic examination did not reveal any abnormality. Fine needle aspiration biopsy reported it a cystic mass. CT scan with contrast was done. It revealed a well-defined heterogeneously enhancing mass lesion 42*25 mm at the bifurcation of left carotid artery with splaying of left internal and external carotid vessels (Lyre sign). It was lying corresponding to hyoid bone and was indenting upon the left internal jugular vein displacing it antero-laterally (Fig. 2a, b). After routine investigations the patient was operated upon. Left horizontal incision in the crease and tumor was exposed. The vagus nerve was pushed laterally over the mass. The mass was removed by intracapsular dissection (Fig. 3). The cut section of the mass showed cystic changes with hemorrhages (Fig. 4).
Fig. 1.

Mass below the angle of mandible
Fig. 2.
CT scan with contrast showing displacement of vessels
Fig. 3.

Peroperative picture showing the mass
Fig. 4.
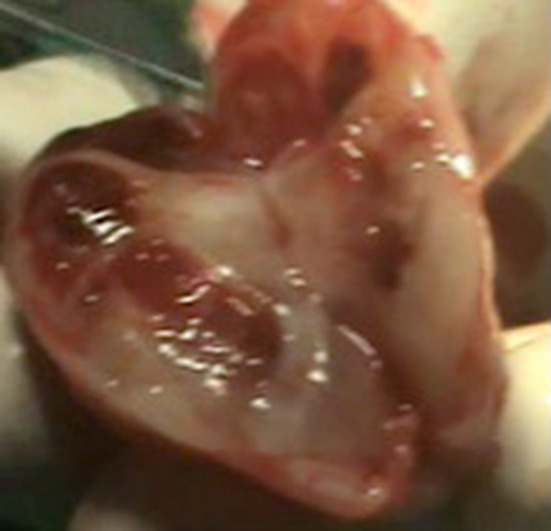
Cut section of the mass showing hemorrhages and cystic changes
Section studied shows a partly encapsulated tumor showing spindle cells in hyper cellular and hypo cellular areas. Individual cells have ill-defined eosinophilic cytoplasm with some cells showing markedly pleomorphic nuclei. Extensive degenerative changes comprising of areas of hyalinization and congested blood cells. No necrosis seen. Mitoses 0–1/10 HPF (Fig. 5). In view of extensive degenerative changes comprising of areas of hyalinization and congested blood cells: the diagnosis of ancient variant of schwannoma is favoured. Immunohistochemistry studies-the spindle cell express Vimentin and S100. Ki67 index is up to 5% (Fig. 6a, b). Two days postoperatively the patient complained of headache and retro-orbital pain on left side. Ophthalmologist consultation revealed it to be Horner’s syndrome (miosis, ptosis, anhidrosis and enophthalmos) (Fig. 7). The patient was put on analgesics.
Fig. 5.

Microscopic Picture-Partly encapsulated tumor showing spindle cells in hypercellular and hypocellular areas. Individual cells have ill-defined eosinophilic cytoplasm
Fig. 6.
Immunohistochemistry
Fig. 7.

Horner’s Syndrome
Discussion
Schwannomas, soft tissue neurogenic tumors, are known to occur in almost any anatomic location of the body, including the head and neck region, mediastinum, retro peritoneum, trunk, pelvis, perineum and upper and lower extremities. In the neck, it may arise from the last four cranial nerves and Vagus Nerve is the commonest site of origin of these tumors [4].
Ackerman and Taylor introduced the term “ancient schwannoma” in 1951. They described 10 cases out of 40 schwannomas of thorax with hyalinization suggestive of degenerative changes. Ancient schwannoma is a degenerative neurilemmoma or schwannoma, characterized by distinct degenerative morphological changes in the tumor, which may include cystic necrosis, stromal edema, xanthomatous change, fibrosis, perivascular hyalinization, calcification, degenerative nuclei with pleomorphism, lobulation and hyperchromacia. These changes are attributed to the growth and the ageing. Over a long period of growth, there is vascular insufficiency, which ultimately leads to areas of degeneration. Inspite of all the changes in ancient schwannoma, they behave similar to their original counterpart. The schwannomas arising from sympathetic chain in the neck are very rare. A review of literature disclosed fewer than 45 cases of schwannoma of sympathetic chain and only nine cases of “ancient schwannoma” of sympathetic chain (excluding this case). Bowles et al. [5] described three cases and added one of their own [2, 5, 6, 9]. Further exploration of literature lead us to add five more cases [7, 8, 10–12]. Table 1. They may present as neck mass. Some time it may present with Horner’s syndrome or pulsatile mass mimicking carotid body tumor with radiological evidence of Lyre sign [13]. Ancient schwannomas of the neck region are usually misdiagnosed and preoperative investigations are usually fruitless [14]. These tumors are either sessile or pedunculated. When sessile, they do not cause much of the symptoms. Pain or discomfort, dyspnea, hoarseness and sensation of pressure or foreign body are the main symptoms. Fine needle aspiration biopsy is usually misleading due to degeneration and cyst formation. As in our case the Fine needle aspiration showed cystic lesion. Ultra sound scan, C.T. Scan/M.R.I. with contrast have revolutionized the diagnosis of these tumors, preoperative planning and prevent per operative disaster.
Table 1.
Ancient schwannoma cervical sympathetic chain- literature
| Author | Patient | Signs/symptoms | Investigations | Initial diagnosis | Management | Post-operative complication |
|---|---|---|---|---|---|---|
| Badawi et al. [2] | 23 F | Asymptomatic Nonpulsatile neck mass- ant neck triangle Left side | FNAC, TFT, Thyroid scan | Thyroid mass | External surgical approach | Horner’s |
| Athar et al. [6] | 41 F | Non tender mass 20 years Right upper neck | FNAC, CT scan | Parapharyngeal mass | External surgical approach | Horner’s |
| Iacconi et al. [7] | 31 M | Asympatomatic Right upper lateral neck mass | US scan, CT scan, MRI, Digital substraction angiography | Lymphadinopathy, Neural tumour | Right submandibular incision, External approach | Horner’s |
| Kim et al. [8] | 58 F | Huge left lateral mass, dysphagia, horner’s Syndrome | FNAC, CT scan, MRI | Schwannoma-CSC/vagus, malignant peripheral nerve tumour, sarcoma | Ext cervical route surgery | More prominent Horner’s |
| Bihani et al. [9] | 50 F | Non pulsatile, non tender, anterior to sternomastoid, 2 years | FNAC, CT, MRI | Carotid body tumour | Surgical excision, Ext approach | Postop Horner’s |
| Lohiya et al. [10] | 40 F | Painless swelling left side neck | MRI, US Scan | Schwannoma/neurofibroma | External Surgical excision | Horner’s |
| Bowles et al. [5] | 36 F | Painless left sided neck lump- 1 month | US scan, FNAC, MRI | Schwannoma | External surgical approach | Nil |
| Baker et al. [11] | 36 F | Slow growing neck mass, Painful -8 month with 2 months dysphagia | CT scan, MRI, | Vagal/Sympathetic schwannoma | Ext Surgical excision | Horner’s |
| Lim et al. [12] | 73 M | Progressive neck mass, dysphagia,dysphonia, transmitted pulsations | CT scan with contrast | Carotid body tumour | External surgical excision | Horner’s |
| Present case | 26F | Painless mass left side below angle of mandible | FNAC, CT scan with contrast | Carotid body tumour Tubercular lymph node | External cervical route | Horner’s |
Neural tumors are derived from cells taking origin from the neural crest cell. They can be divided into two main groups, the nerve sheath tumors (Schwann cell) and the tumors arising from the sympathoblast. The Schwann cell is the parent cell of the common clinical tumors, the schwannoma and the neurofibroma. Some nerve sheath tumors may contain histologically clear components of both neurofibroma and schwannoma within the same specimen. This explains the confusion in making a correct diagnosis [15]. Schwannomas were wrongly termed as neuromas or neurilemmomas, in the past. So, the term schwannoma should be used. Neurolemmoma can be used but is misleading [16]. The typical features of compact twisted bundles of spindle cells with elongated nuclei, in palisading pattern and alternating areas of acellular fine cytoplasmic fibrils and eosinophilic masses called verocay bodies (Antoni A) and irregularly arranged masses of elongated cells and fibres with areas of cystic degeneration and edema (Antoni Type B). The classic features with verocay bodies are present in one-third of cases [15]. Schwannoma can arise from any nerve covered with Schwann cell sheath. Therefore all cranial nerves except optic and olfactory nerves or sympathetic nerve or trunk or spinal nerve roots can develop schwannomas. Unlike neurofibromas, these tumors do not engulf the nerve of origin. The fibres are splayed out over the outer aspect of the capsule rather than incorporating into the tumor mass. That is why the function of the nerve of origin is not impaired. Only in 50% of cases, a direct relationship with the nerve is established. These tumors are usually solitary, solid, may appear pink, yellow or pearly grey, slow growing and circumscribed. These tumors are initially asymptomatic. As these tumors enlarge in size, they produce the symptoms of pressure and compression according to the size. On microscopy, ancient schwannomas show areas of cellularity intermixed with myxoid matrix like other conventional schwannomas. The cellular regions however tend to be sclerosed or fibrotic and with time may undergo degenerative changes leading to hematomas and cysts. Nuclear palisades seen in classic schwannoma are absent. The absence of mitosis and preservation of cohesive clusters of spindle cells differentiate these tumors from malignant one. But at the same time, the histopathological features of degeneration and nuclear atypia in ancient schwannoma may easily lead to the diagnosis of malignant mesenchymal neoplasm [17]. Positivity with S100 in Immunohistochemistry is a feature of neural origin [18].
Horner’s syndrome (miosis, ptosis, anhidrosis and enophthalmos) and First bite syndrome (acute pain in parotid region/mouth on first bite of each meal) are the common complications of surgical treatment of Schwannoma arising from cervical sympathetic chain. Out of all nine cases of ancient schwannoma reported in English literature, eight had Horner’s syndrome postoperatively. Our present case too had Horner’s syndrome postoperatively. Laccourreye et al. [19], reviewed the literature and found 48 of 106 patients had developed first bite syndrome after upper neck surgery involving superior sympathetic ganglion. Casserly et al. [20] reported a case of cervical sympathetic schwannoma who later developed first bite syndrome. There are many reports of cervical sympathetic schwannomas with postoperative first bite syndrome after surgery but none in ancient schwannoma from cervical sympathetic chain. First bite syndrome is due to the damage to the sympathetic supply to parotid gland. Malignant transformation has been reported in ancient schwannoma [21].
The differential diagnosis includes carotid body tumor, necrotizing Lymph node, branchial cleft cyst, lymphangioma and soft tissue sarcomas. Carotid body tumors are the most common tumors at carotid bifurcation. Widening of bifurcation and displacement of vessels at that point- Lyre Sign, may be produced by schwannoma of cervical sympathetic chain [20].
Elective treatment is by surgery, as the tumor is radio-resistant. The external surgical approach is favoured in most patients for excision of benign tumors. Complete excision of the tumor is mandatory in order to prevent recurrence. These tumors can usually be enucleated without fear of recurrence and preservation of functional integrity. Recurrence is very rare, even if a portion of the capsule is left behind [22].
Conclusion
Schwannoma in cervical region arising from Sympathetic chain is a very rare entity. They arise from the schwann cells of the nerve sheaths. They may mimic Vagal tumours or carotid body tumours. Cystic changes occur in long standing cases to be termed as Ancient schwannoma. Fine needle aspiration is of little significance. CT/MRI with contrast helps in diagnosis. Surgery is the treatment of choice. Recurrence is rare. Horner’s syndrome and first bite syndrome must be kept in mind preoperative and postoperative as well.
Funding
No funding source.
Compliance with Ethical Standards
Conflict of interest
There is no conflict of interest among the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Ethical Approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ackerman LV, Taylor FH. Neurogenous tumors within the thorax: a clinicopathological evaluation of forty-eight cases. Cancer. 1951;4:669–691. doi: 10.1002/1097-0142(195107)4:4<669::AID-CNCR2820040405>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 2.Badawi RA, Scott-Coombs D. Ancient schwannoma masquerading as thyroid mass. Eur J Surg Oncol. 2002;28(1):88–90. doi: 10.1053/ejso.2001.1159. [DOI] [PubMed] [Google Scholar]
- 3.Stout AP. Peripheral manifestations of the specific nerve sheath tumor(neurilemmoma) Am J Cancer. 1935;24:751. doi: 10.1158/ajc.1935.751. [DOI] [Google Scholar]
- 4.Verma R, Sardana NK (2005) Schwannoma of vallecula. Ind J Otol HNS. Special Issue 151-3
- 5.Bowles PF, Cheong RCT, Cartwright S, Pelser A. Ancient schwannoma of the cervical sympathetic chain. Clin Case Rep. 2017;5(7):1077–1080. doi: 10.1002/ccr3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Athar PPS, Norhan NA, Rahman MSM. Ancient schwannoma of cervical sympathetic chain: a case report. Malays J Med Sci. 2007;14(1):75–78. [PMC free article] [PubMed] [Google Scholar]
- 7.Iacconi P, Faggioni M, De Bartolomeis C, Iacconi C, Caldarelli C. Cervical sympathetic chain schwannoma: a case report. Acta Otorhinolaryngol Ital. 2012;32(2):133–136. [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SW, Sah DJ, Kwak SG, Hwang JY. Giant ancient schwannoma of lateral neck presenting with preoperative Horner’s Syndrome. Korean J Otolaryngol-Head Neck Surg. 2012;55(11):728–731. doi: 10.3342/kjori-hns.2012.55.11.728. [DOI] [Google Scholar]
- 9.Bihani A, Hardikar P, Dokhe Y, Dabholkar J. Ancient Schwannoma of cervical sympathetic chain masquerading as carotid body tumour. Int Surg J (S.I.) 2016;2(2):274–277. doi: 10.5455/2349-2902.isj20150530. [DOI] [Google Scholar]
- 10.Lohiya TA, Shah KD, Patil AV, Bradoo RA. Ancient schwannoma of cervical sympathetic chaina rare entity. Odisha J Otolaryngol HNS. 2015;9(II):37–39. [Google Scholar]
- 11.Baker AT, Homewood TJ, Baker TR. Cervical sympathetic chain schwannoma Masquerading as a Vagus nerve schwannoma complicated by postoperative Horner’s syndrome and facial pain: a case report. Int J Surg Case Rep. 2018;49:4–7. doi: 10.1016/j.ijscr.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim CC, Chong HS, Yong DJ, Foong SK, Narayanan P (2018) Ancient schwannoma of cervical sympathetic chain masquerading as carotid body tumour. Med J Malaysia 73(2). pdfs.sementicscholsr.org [PubMed]
- 13.Ozluzedik S, Ozcan M, Unal T, Unal A, Tezer MS, Seckin S (2007) Cervical sympathetic chain schwannoma: two different clinical presentations. Tumori 93:305–307 (citeseerx.ist.psu.edu/viewdoc/download? doi = 10.1.1.651.3611&rep = rep1&type = pdf) [DOI] [PubMed]
- 14.Bindra R, Gupta S, Gupta N, Asotra S, Sharma A. Ancient scwannoma of the neck mimicking soft tissue sarcoma. J Cancer Res Ther. 2010;6:234–235. doi: 10.4103/0973-1482.65234. [DOI] [PubMed] [Google Scholar]
- 15.Colreavy MP, Lacy PD, Hughes J, et al. Head and neck schwannomas—a ten-year review. J Laryngol Otal. 2000;114:119–124. doi: 10.1258/0022215001905058. [DOI] [PubMed] [Google Scholar]
- 16.Stell PM, Maran AGD (1994) Peripheral nerve tumours. Head and neck surgery. Ed Maran AGD, Wilson JA; third Ed: pp 79–80. Butterworth-Heinemann
- 17.Goldblum JR, Flope AL, Weiss SW. Benign tumours of peripheral nerves. In: Enzinger FM, Weiss SW, editors. Soft tissue tumours. 6. Philadelphia: Elsevier; 2014. pp. 823–824. [Google Scholar]
- 18.Sai M, Ratnagiri R. Retroperitoneal ancient schwannoma: two cases and review of literature. J Can Res Ther. 2014;10(2):368–370. doi: 10.4103/0973-1482.136660. [DOI] [PubMed] [Google Scholar]
- 19.Laccourreye O, Werner A, Garcia D, Malinvaud D, Tran Ba Huy P, Bonfil P. Review; first bite syndrome. Euro Ann Otolaryngol HN Dis. 2013;130:269–273. doi: 10.1016/j.anorl.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Casserly P, Kiely P, Fenton JE. Cervical sympathetic chain schwannoma masquerading as carotid body tumour with a postoperative complication of first bite syndrome. Eur Arch Otolaryngol. 2009;266(10):1659–1662. doi: 10.1007/s00405-008-0902-7. [DOI] [PubMed] [Google Scholar]
- 21.Mikami Y, Hidaka T, Akisada T, Takemoto T, Irei I, Manabe T. Malignant peripheral nerve sheath tumours arising in benign ancient schwannoma: a case report with an immunohistochemical study. Pathol Int. 2000;50:156–161. doi: 10.1046/j.1440-1827.2000.01019.x. [DOI] [PubMed] [Google Scholar]
- 22.Krag LV, Soule EH, Masson JK. Benign and malignant neurilemmoma of head and neck. Surg Gynecol Obstr. 1960;11:211. [PubMed] [Google Scholar]




