Abstract
Lacrimal gland adenoid cystic carcinoma (AdCC) is associated with an aggressive clinical course and grave prognosis. A high grade transformation within adenoid cystic carcinoma of lacrimal gland is a rare condition which is even more locally aggressive with frequent neck and distant metastasis. We present a case of left lacrimal gland adenoid cystic carcinoma with high grade transformation to adenocarcinoma NOS type presenting with orbital pain and proptosis. After thorough evaluation for locoregional and distant spread of the disease, the patient underwent left orbital exenteration with orbitectomy and neck dissection with free flap reconstruction. Patient received adjuvant radiation therapy and is presently disease free for last 6 months. A multi-modality management protocol involving surgery, radiotherapy and chemotherapy has been proposed for management of lacrimal gland AdCC with high grade transformation. We report the 4th case in the literature of lacrimal gland adenoid cystic carcinoma with high grade transformation.
Keywords: Case report, Lacrimal gland carcinoma, Adenoid cystic carcinoma, Adenoid cystic carcinoma with high grade transformation, Dedifferentiated adenoid cystic carcinoma, Multi-modality management
Introduction
Lacrimal gland tumors are a kind of salivary gland tumors representing 6–12% of orbital space occupying lesions. Primary malignant epithelial tumors represent around 20–30% of all lacrimal gland tumors which are associated with significant morbidity and mortality [1–3].
Adenoid cystic carcinoma (AdCC) is the commonest malignant epithelial tumor of lacrimal gland associated with poor prognosis despite aggressive local treatment due to high rates of local recurrences and late distant metastasis [3, 4]. Classical AdCC may undergo high grade transformation (HGT) or dedifferentiation which is associated with even worse clinical outcome due to high incidence of nodal metastasis and distant metastasis [5–7].
Since first description of ‘dedifferentiated’ AdCC by Cheuk et al. in 1999 [8] around 49 cases of salivary gland AdCC with dedifferentiation/HGT have been described in literature till date—submandibular glands (13 cases), sino-nasal region (9 cases), parotid and palate (7 cases each), nasal cavity (3 cases), lacrimal gland (3 cases), tongue (2 cases), pterygopalatine, lip, pyriform and epipharynx (1 case each) [9] (Table 1). There are only 3 cases of high grade transformation in adenoid cystic carcinoma of lacrimal gland reported in literature. Here we report the 4th case of adenoid cystic carcinoma in lacrimal gland malignancy with high grade transformation (Table 2).
Table 3.
Comparative immunohistochemical features of two components
| IHC markers | Low grade area | High grade area |
|---|---|---|
| Pancytokeratin | + | + |
| EMA | + | + |
| CK7 | + | + |
| CD117 (CKIT) | Weak + | Weak + |
| p53 | Weak + | Strong + |
| Mib1 (Ki67) | Mildly increase (10%) | Strongly increase (95%) |
| p63 | Focally + | Loss |
| SMA | Loss | Loss |
| S100 | + | − |
| GDCFP15 | − | − |
| GATA3 | − | − |
| ER | − | − |
| CDX2 | − | − |
| NAPSIN-A | − | − |
| Synaptophysin | − | − |
Table 1.
Review of reported cases of adenoid cystic carcinoma with high grade transformation/dedifferentiation
| Authors | Cases |
|---|---|
| Cheuk et al. [8] | 3 |
| Moles et al. [31] | 1 |
| Terasaki et al. [4] | 1 |
| Chau et al. [32] | 1 |
| Ide et al. [33] | 1 |
| Nago et al. [34] | 6 |
| Brackrock et al. [35] | 1 |
| Sato et al. [36] | 1 |
| Seethala et al. [6] | 11 |
| Handra-Luca et al. [37] | 1 |
| Malhotra et al. [38] | 1 |
| Bonfitto et al. [39] | 7 |
| Costa et al. [40] | 1 |
| Panarelli et al. [41] | 1 |
| Boland et al. [42] | 1 |
| Argyris et al. [9] | 1 |
| Bavle et al. [43] | 1 |
| Ly et al. [44] | 1 |
| Sayar et al. [45] | 1 |
| Kusafuka et al. [46] | 1 |
| Noda et al. [47] | 1 |
| Tando et al. [48] | 1 |
| Bhardwaj et al. [49] | 1 |
| Dutta et al. [50] | 1 |
Table 2.
Clinicopathologic features of lacrimal gland AdCCs with high grade transformation case
| Case | Author | Year | Age | Sex | Tumor site | Size (cm) | Histology | |
|---|---|---|---|---|---|---|---|---|
| AdCC-LG | HG | |||||||
| 1 | Terasaki et al. [4] | 2000 | 49 | F | Orbit and middle fossa (R) | No details | Cribriform | Solid with necrosis Panarelli |
| 2 | Panarelli et al. [41] | 2011 | 52 | M | Extraconal comp. (R) | 3.2 × 3.2x2.9 | Cribriform | Myoepithelial sarcomatoid |
| 3 | Argyris et al. [9] | 2012 | 39 | F | Extraconal comp. (L) | 3 × 2.2x2 | Cribriform | Myoepithelial comedo-carcinomatous |
| 4 | Present case | 2020 | 42 | M | Extra conal and extra orbital. (L) | 5 × 4 × 1 | Solid (predominantly) + cribriform pattern | Adenocarcinoma NOS type |
Management of lacrimal gland adenoid cystic carcinoma involves a multimodality treatment. Surgery remains the main stay of the treatment with radiation therapy as adjuvant. The role of systemic therapy either in neoadjuvant or concurrent adjuvant form is still unclear.
Case Details
History
A young healthy 42 years male patient with no major medical or family history and no history of previous treatment had presented to an ophthalmologist with complaint of left eye redness and increasing swelling associated with pain. He was initially managed conservatively for around 2 months. With increasing symptoms and later proptosis, a contrast enhanced CT (CECT) scan was done which suggested a mass originating from left lacrimal gland. An external approach excision biopsy was done which suggested high grade lacrimal carcinoma, hybrid adenoid cystic and adeno carcinoma NOS type. Patient was later referred to our surgical oncology department for further management.
On clinical examination, the left eye had proptosis with scar of biopsy over left upper lid. There was exposure keratitis, chemosis with congestion and fullness in left temporal fossa region. Eye movements were partly restricted and patient had only light perception. The other eye was normal with normal vision and movements. No other significant neck node enlargement seen.
Imaging Findings
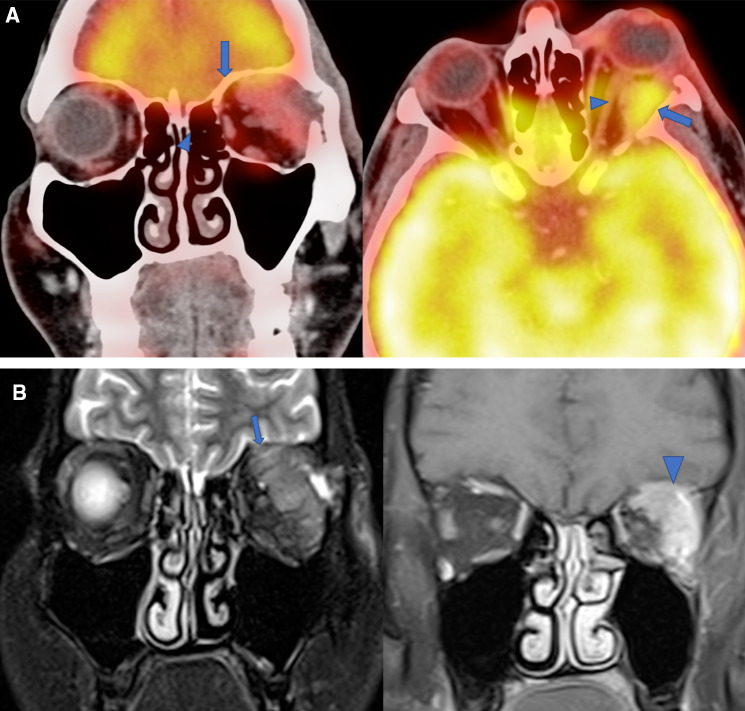
As this was a case of high grade tumor of lacrimal gland, PET-CT scan was done for locoregional extension and to evaluate nodal and distant metastasis. PET-CT showed a large metabolically active heterogeneously enhancing tumor superolateral extraconal compartment of left orbit, lacrimal gland was not seen separately. It has an intraconal and retro-orbital extension, closely adherent to superior and lateral rectus muscle as well as abutting retrobulbar portion of left optic nerve without infiltration. Reformatted bone algorithm showed thinning and erosion of roof and lateral orbital wall with lesion in close proximity to anterior cranial fossa on left side (Fig. 1a). No cervical or parotid nodes or distant metastasis seen.
Fig. 1.
Radiological images (MRI and PET CT). a Coronal and axial PECT-CT images show metabolically active left lacrimal gland tumor (large arrow) and it is abutting and minimally displacing retrobulbar portion of left optic nerve (arrowhead). b Coronal T2WFS and Post contrast T1WFS images show large heterogeneously enhancing left lacrimal gland tumor (large arrow) causing erosion of roof of orbit and adherent to dura of left anteroinferior aspect of frontal lobe (arrowhead)
Contrast enhanced MRI of orbit and brain was also done additionally to exclude any dural or intradural involvement. MRI confirmed the findings of PET-CT and additionally showed adherence of the tumor to dura of anteroinferior aspect of left frontal lobe (Fig. 1b). No intradural extension. Optic chiasma and right orbit appear normal.
Surgical Details
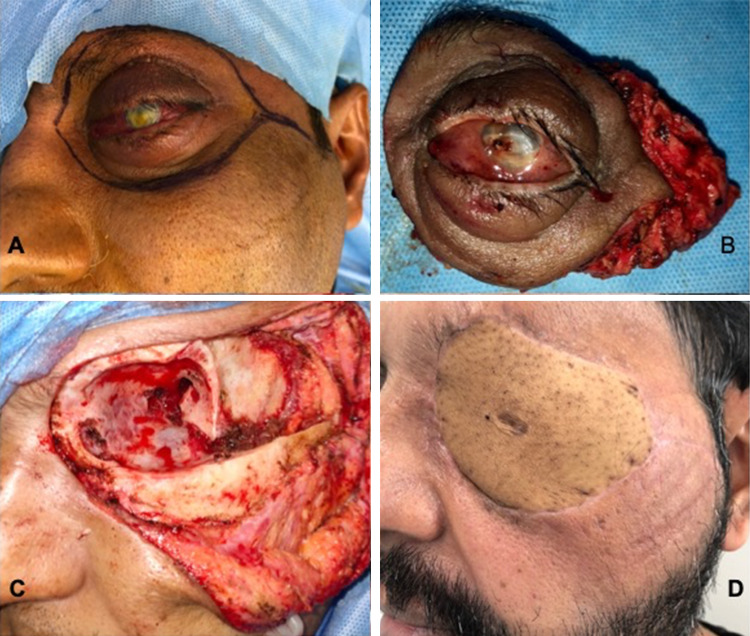
Surgery was planned after all necessary covid workup. As the tumor was high grade, locally aggressive with extra orbital involvement and abutting intra orbital contents, a radical surgical excision involving total orbital exenteration and orbitectomy was planned. The parotid region was explored for any suspicious nodes. Left selective neck dissection (Ib–III) was done.The defect was reconstructed with anterolateral thigh flap which was anastomosed with facial artery and internal jugular vein (IJV) (Fig. 2).
Fig. 2.
Clinical and surgical profile of the patient. a Clinical picture, b Surgical Specimen, c Surgical Defect, d post operative period
Final Biopsy Report
Tumor size was 5.0 × 4.0 × 1.0 cm arising from lacrimal gland involving periorbital fat, muscle (temporalis), soft tissue and periorbital fascia. Perineural invasion was present intra-tumoral without lympho-vascular invasion. The eye ball and optic nerve were close but free. Overlying skin was free. All margins and dissected nodes were free. Histologically there were two distinct tumor areas without any clear demarcation. The first predominant area is high grade component arranged in solid,trabecular and glandular component with marked nuclear enlargement (2–3 times) with desmoplastic stroma, prominent nucleoli and focal comedo necrosis seen. suggesting a high grade carcinomatous component. The second component was a classical adenoid cystic carcinoma predominantly solid area and focal cribriform pattern.
On immunohistochemistry, the conventional adenoid cystic carcinoma component was positive for cytokeratin, CD117 (C-Kit) and EMA. Immunostains for p63 highlighted myoepithelial cells in this area. High Grade carcinomatous area was diffusely positive for cytokeratin, EMA with loss of p63 in this areas. The Mib1 labelling index was approximately 95% in high grade areas while is about 10–12% in adenoid cystic component (Fig. 3; Table 3).
Fig. 3.
Histopathological and IHC findings. a H&E slide showing high and low grade area. b Mib1 index higher (95%) in high grade area as compared to 10% in low grade area
Adjuvant Therapy
Patient received adjuvant radiotherapy to primary and neck.
Discussion
Lacrimal gland tumors are rare tumors with an occurrence of less than 1 per 1 000 000 population yearly. Adenoid Cystic Carcinoma is the commonest (60%) primary epithelial malignancy of the lacrimal gland characterized by prolonged clinical course, frequent local recurrences (due to peri-neural extension) and late distant metastasis with fatal outcome [2, 3]. Lacrimal gland AdCC is very aggressive and has poor prognosis with medial overall survival of 7.6 years. Age, tumor extent, histopathologic type, perineural invasion and bone invasion affect the patient prognosis [10].
AdCC-HGT is a tumor of sixth decade (32–88 years) with a slight male predominance of 1.4:1 as against conventional AdCC which has a female predominance [6] AdCC-HGT has a more aggressive clinical course than conventional AdCC being locally aggressive with bone erosion, extra glandular involvement, higher chances of margin positivity and nodal metastasis (43–57%) [6]. A size of > 2.5 cm and solid pattern are negative prognostic factors for AdCC of lacrimal gland [2].
The present case is a male patient of 42 years with tumor size > 3 cm having extra-glandular extension (involving intra-conal structures) and bone erosion of lateral, medial and superior orbital wall (into temporal fossa, medial orbital wall and skull base erosion) without any nodal or distant metastasis.
Seethla et al. [6] gave distinctive criteria to report high grade transformation in adenoid cystic carcinoma—(a) poorly differentiated carcinoma component arranged in solid confluent nests, (b) presence of desmoplastic stroma,(c) larger pleomorphic nuclei (at least 2–3 times the size of grades I–II AdCC nuclei), (d) loss of abluminal cell layer as highlighted by p63 and (e) diffusely and strongly positive for p53. The present case fulfilled all the criteria of high grade transformation as illustrated by Seethla.
Management of lacrimal gland AdCC is controversial and involves multidisciplinary approach mainly aimed at local control and prevention of distant metastasis. Surgery is considered as the main modality of treatment for loco regional control. The extent of the surgery is largely influenced by the extent of local involvement. Traditionally orbital exenteration has been the most common surgery for lacrimal gland adenoid cystic carcinoma. Orbitectomy with bone removal may be indicated for achieving local and regional control of advanced cases of AdCC [11]. Radical surgery is indicated in patients with severe symptoms (pain, hygiene issues, severe disfigurement caused by progressive proptosis, severe exposure keratopathy), gross tumor at orbital apex, extra orbital extension into PNS and brain parenchyma irrespective of long term outcomes [11, 12]. However, Yang et al in their review pointed out that radical surgical approach did not benefit in rate of recurrence, metastasis and mortality but reduced patients quality of life because of functional disability and disfigurement [13].
Eye-sparing surgery with adjuvant radiation therapy (RT) have shown favourable local control and long-term survival outcomes in patients with orbit confined lacrimal gland AdCC (AJCC 8th T1-T2) [14, 15]. For stages T3 (AJCC 8th edition) and above eye sparing surgeries were associated with poor outcomes even with post-operative RT likely due to distant metastasis [16]. Ahmad et al. assessed the efficacy of AJCC to predict the outcomes at initial stage of diagnosis of lacrimal gland adenoid cystic carcinoma and concluded that the traditional approach of orbital exenteration with bone removal and postoperative adjuvant RT is appropriate for tumors ≥ T3 while for < T3 tumors (orbit confined) an eye sparing en bloc tumor excision followed by RT may achieve similar loco-regional control rates [12].
Hung et al. reported 5 and 10 year overall survival rates for eye sparing surgery with adjuvant radiation therapy for < T3 tumors as 81.8% and 68.2%respectively [17]. Han et al. reported a local control rate of 90% and overall survival of 90% at 89.5 months follow up period [15]. Woo et al. reported 5-year recurrence-free, disease-free and overall survival rates were 44.8%, 72.9%, and 87.4% respectively [15–17]. However, according to Yang et al. < T3 tumors of adenoid cystic carcinoma of lacrimal gland, 5 years local recurrences (83.3%), distant metastasis (35%) and mortality (20%) for eye sparing surgery followed by adjuvant radiation therapy were reported [13].
AdCC is considered as a radioresistant tumor due to its indolent growth pattern irrespective of the gland involved and requires a dose escalation for curative irradiation [3, 18]. For adjuvant radiation therapy various types including external beam photon radiation therapy—3D conformal radiation therapy (CRT) [19] or intensity modulated radiation therapy (IMRT) [20], neutron radiation therapy [21] and proton beam therapy [18] have been reported with the goal to achieve local control.[13] Adjuvant radiation therapy helps reduces the risk of recurrence (61% vs. 20%) [12]. However, ocular toxic effects of radiation therapy include dry eye, radiation retinopathy, cataract, severe corneal and conjunctival damage requiring enucleation and brain radionecrosis have to be considered [13, 15–17].
For locally advanced inoperable primary or recurrent lacrimal gland AdCC, carbon-ion radiation therapy have shown better local control with 3 year OS and LR 82.2% and 79% respectively.[22, 23] IMRT combined with raster-scanned carbon ion boost showed superior locoregional control and overall survival for advanced AdCC of head and neck [24].
Neo-adjuvant intra-arterial cytoreductive chemotherapy (IACC) have shown improved overall survival and reduced disease recurrence [25] but with substantial toxicity like myelosuppression, nausea, vomiting, fever, sepsis, renal dysfunction, ototoxicity and catheter related thrombotic/vascular compromise that limits its routine application [26]. For metastatic disease or not amenable to surgery or radiotherapy, chemotherapy has modest efficacy even in non-lacrimal AdCC [26].
Neo-adjuvant intra-venous chemotherapy with 5-Fluorouracil and cisplatin have shown cytoreduction in lacrimal gland AdCC and can be considered prior to eye sparing surgery. Although it is better tolerated, easier to deliver and associated with fewer potential serious side effects, its survival benefit is questionable [3, 13]. Concomitant platinum-based chemotherapy may be considered with radiation therapy in an attempt to enhance radio-sensitivity [26, 27].
The newer insights into molecular abnormalities underlying lacrimal gland carcinogenesis have opened up potentials for targeted therapy in extensive and metastatic lacrimal gland AdCC. Lacrimal gland AdCC have shown KRAS, NRAS, and MET gene mutations suggesting possibility of targeted therapy aimed at EGFR-RAS-RAF cascade [28].
Recent studies have shown that AdCC cells of lacrimal gland express high levels of anti-apoptosis proteins like survivin, and TNF-α. Arsenic Trioxide (As2O3) have been found to be significantly increasing the apoptosis rate in in vitro studies by inhibition of these anti-apoptotic genes [29, 30].
Conclusion
The present case report is the 4th case in literature reporting high grade transformation in lacrimal gland AdCC. High grade transformation in lacrimal gland AdCC has been associated with worse clinical outcomes owing to local aggressiveness, increased incidence of nodal and distant metastasis. Surgery for local disease and elective neck dissection with adjuvant radiation therapy is at present the most preferred treatment modality. Newer radiation therapy techniques (photon, carbon ion) significantly reduce the radiation morbidity and allow better local control enabling conservative surgical resections. Chemotherapy in the neoadjuvant settings may help reduce the tumor burden while planning for eye preserving surgery or in the adjuvant settings to enhance radio sensitivity. Understanding of molecular pathogenesis will allow development of newer targeted therapy for management of metastatic and resistant AdCC.
Acknowledgements
Dr. Nitin Trivedi, Occuloplastic Surgeon for referring the case for management.
Abbreviations
- AdCC
Adenoid cystic carcinoma
- HGT
High grade transformation
- AdCC-HGT
Adenoid cystic carcinoma with high grade transformation
- CECT
Contrast enhanced computed tomography
- RT
Radiation therapy
- 3D CRT
3 Dimensional conformal radiation therapy
- IMRT
Intensity modulated radiation therapy
- AJCC
American Joint Committee on Cancer
- IJV
Internal jugular vein
- IACC
Intra-arterial cytoreductive chemotherapy
- As2O3
Arsenic Trioxide
- TNF-α
Tumor necrosis factor
Author’s Contribution
RS—have helped with the surgical reconstruction and providing the post-operative images, JB—have helped with the surgical reconstruction and providing the post-operative images. RG—have helped in data collection and formatting.
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andreasen S, Esmaeli B, Von Holstein SL, Mikkelsen LH, Rasmussen PK, Heegaard S. An update on tumors of the lacrimal gland. Asia-Pac J Ophthalmol. 2017;6(2):159–172. doi: 10.22608/APO.201707. [DOI] [PubMed] [Google Scholar]
- 2.Von Holstein SL, Coupland SE, Briscoe D, Le Tourneau C, Heegaard S. Epithelial tumors of the lacrimal gland: a clinical, histopathological, surgical and oncological survey. Acta Ophthalmol. 2013;91(3):195–206. doi: 10.1111/j.1755-3768.2012.02402.x. [DOI] [PubMed] [Google Scholar]
- 3.Woo KI, Kim YD, Sa HS, Esmaeli B. Current treatment of lacrimal gland carcinoma. Curr Opin Ophthalmol. 2016;27(5):449–456. doi: 10.1097/ICU.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 4.Terasaki M, Tokutomi T, Maruiwa H, Sugita Y, Harada H, Shigemori M. High-grade adenoid cystic carcinoma originating from the lacrimal gland. Brain Tumor Pathol. 2000;17(3):159–163. doi: 10.1007/BF02484288. [DOI] [PubMed] [Google Scholar]
- 5.Petersson F. High-grade transformation (“Dedifferentiation”)—malignant progression of salivary gland neoplasms, including carcinoma ex pleomorphic adenoma: a review. Pathol Case Rev. 2015;20(1):27–37. [Google Scholar]
- 6.Seethala RR, Hunt JL, Baloch ZW, LiVolsi VA, Leon BE. Adenoid cystic carcinoma with high-grade transformation. Am J Surg Pathol. 2007;31(11):1683–1694. doi: 10.1097/PAS.0b013e3180dc928c. [DOI] [PubMed] [Google Scholar]
- 7.Hellquist H, Skálová A, Barnes L, Cardesa A, Thompson LDR, Triantafyllou A, et al. Cervical lymph node metastasis in high-grade transformation of head and neck adenoid cystic carcinoma: a collective international review. Adv Ther. 2016;33(3):357–368. doi: 10.1007/s12325-016-0298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheuk W, Chan JKNR. Dedifferentiation in adenoid cystic carcinoma of salivary gland: an uncommon complication associated with an accelerated clinical course. Am J Surg Pathol. 1999;23(4):465–472. doi: 10.1097/00000478-199904000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Argyris PP, Pambuccian SE, Cayci Z, Singh C, Tosios KI, Koutlas IG. Lacrimal gland adenoid cystic carcinoma with high-grade transformation to myoepithelial carcinoma: report of a case and review of literature. Head Neck Pathol. 2013;7(1):85–92. doi: 10.1007/s12105-012-0383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J, Kim HK, Kim WS, Bae TH. Extensive and aggressive growth of adenoid cystic carcinoma in the lacrimal gland. Arch Craniofacial Surg. 2020;21(2):114–118. doi: 10.7181/acfs.2019.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esmaeli B, Golio D, Kies M, DeMonte F. Surgical management of locally advanced adenoid cystic carcinoma of the lacrimal gland. Ophthal Plast Reconstr Surg. 2006;22(5):366–370. doi: 10.1097/01.iop.0000232164.00208.b4. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad SM, Esmaeli B, Williams M, Nguyen J, Fay A, Woog J, et al. American joint committee on cancer classification predicts outcome of patients with lacrimal gland adenoid cystic carcinoma. Ophthalmology. 2009;116(6):1210–1215. doi: 10.1016/j.ophtha.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Zhou C, Wang Y, Fan X, Jia R. Multimodal therapy in the management of lacrimal gland adenoid cystic carcinoma. BMC Ophthalmol. 2019;19(1):1–8. doi: 10.1186/s12886-019-1110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8. Chicago: Springer; 2017. pp. 409–414. [Google Scholar]
- 15.Han J, Kim YD, Woo KI, Sobti D. Long-term outcomes of eye-sparing surgery for adenoid cystic carcinoma of lacrimal gland. Ophthal Plast Reconstr Surg. 2018;34(1):74–78. doi: 10.1097/IOP.0000000000000877. [DOI] [PubMed] [Google Scholar]
- 16.Lin YH, Lin YH, Lin YH, Huang SM, Huang SM, Huang SM, et al. Outcomes in patients with lacrimal gland carcinoma treated with definitive radiotherapy or eye-sparing surgery followed by adjuvant radiotherapy. Radiat Oncol. 2020;15(1):1–10. doi: 10.1186/s13014-020-01601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung JY, Wei YH, Huang CH, Chen LW, Fuh CS, Liao SL. Survival outcomes of eye-sparing surgery for adenoid cystic carcinoma of lacrimal gland. Jpn J Ophthalmol. 2019;63(4):344–351. doi: 10.1007/s10384-019-00671-w. [DOI] [PubMed] [Google Scholar]
- 18.Lesueur P, Rapeaud E, De Marzi L, Goudjil F, Levy C, Galatoire O, et al. Adenoid cystic carcinoma of the lacrimal gland: high dose adjuvant proton therapy to improve patients outcomes. Front Oncol. 2020;10:1–8. doi: 10.3389/fonc.2020.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roshan V, Pathy S, Mallick S, Chander S, Sen S, Chawla B. Adjuvant radiotherapy with three-dimensional conformal radiotherapy of lacrimal gland adenoid cystic carcinoma. J Clin Diagnostic Res. 2015;9(10):XC05–XC07. doi: 10.7860/JCDR/2015/14452.6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao R, Ma D, Takiar V, Frank SJ, Fuller CD, Gunn GB, et al. Orbital carcinomas treated with adjuvant intensity-modulated radiation therapy. Head Neck. 2016;38(Suppl 1):E580–E587. doi: 10.1002/hed.24044. [DOI] [PubMed] [Google Scholar]
- 21.Gensheimer MF, Rainey D, Douglas JG, Liao JJ, Laramore GE, Jian-Amadi A, et al. Neutron radiotherapy for adenoid cystic carcinoma of the lacrimal gland. Ophthal Plast Reconstr Surg. 2013;29(4):256–260. doi: 10.1097/IOP.0b013e318295f99b. [DOI] [PubMed] [Google Scholar]
- 22.Mizoguchi N, Tsuji H, Toyama S, Kamada T, Tsujii H, Nakayama Y, et al. Carbon-ion radiotherapy for locally advanced primary or postoperative recurrent epithelial carcinoma of the lacrimal gland. Radiother Oncol. 2015;114(3):373–377. doi: 10.1016/j.radonc.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi K, Koto M, Ikawa H, Ogawa K, Kamada T. Efficacy and safety of carbon-ion radiotherapy for lacrimal gland carcinomas with extraorbital extension: a retrospective cohort study. Oncotarget. 2018;9(16):12932–12940. doi: 10.18632/oncotarget.24390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen AD, Nikoghosyan AV, Poulakis M, Höss A, Haberer T, Jäkel O, et al. Combined intensity-modulated radiotherapy plus raster-scanned carbon ion boost for advanced adenoid cystic carcinoma of the head and neck results in superior locoregional control and overall survival. Cancer. 2015;121(17):3001–3009. doi: 10.1002/cncr.29443. [DOI] [PubMed] [Google Scholar]
- 25.Tse DT, Kossler AL, Feuer WJ, Benedetto PW. Long-term outcomes of neoadjuvant intra-arterial cytoreductive chemotherapy for lacrimal gland adenoid cystic carcinoma. Ophthalmology. 2013;120(7):1313–1323. doi: 10.1016/j.ophtha.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Tourneau C, Razak ARA, Levy C, Calugaru V, Galatoire O, Dendale R, et al. Role of chemotherapy and molecularly targeted agents in the treatment of adenoid cystic carcinoma of the lacrimal gland. Br J Ophthalmol. 2011;95(11):1483–1489. doi: 10.1136/bjo.2010.192351. [DOI] [PubMed] [Google Scholar]
- 27.Meel R, Pushker N, Bakhshi S. Adjuvant chemotherapy in lacrimal gland adenoid cystic carcinoma. Pediatr Blood Cancer. 2008;53:1163–1164. doi: 10.1002/pbc.22175. [DOI] [PubMed] [Google Scholar]
- 28.Bell D, Sniegowski MC, Wani K, Prieto V, Esmaeli B, Plastic O, et al. Mutational landscape of lacrimal gland carcinomas and implications for treatment. Head Neck. 2017;38(Suppl 1):E724–E729. doi: 10.1002/hed.24078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan Y, Xing Y, Wang H. Expression of survivin in adenoid cystic carcinoma of the lacrimal gland and the effect of intervention with arsenic trioxide in vitro. Exp Ther Med. 2015;10(1):330–334. doi: 10.3892/etm.2015.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quan W, Jiang T, Zhou Y, Jiang J, Wang R, Pan X. The effect of arsenic trioxide on the expression of Tnf-α gene in adenoid cystic carcinoma Acc-2 cells. Hans J Ophthalmol. 2018;07(01):7–16. [Google Scholar]
- 31.Moles MAG. Dedifferentiation occurring in adenoid cystic carcinoma of the tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(2):177–180. doi: 10.1016/s1079-2104(99)70114-9. [DOI] [PubMed] [Google Scholar]
- 32.Chau YP, Hongyo T, Aozasa K, Chan JKC. Dedifferentiation of adenoid cystic carcinoma: report of a case implicating p53 gene mutation. Hum Pathol. 2001;32(12):1403–1407. doi: 10.1053/hupa.2001.28966. [DOI] [PubMed] [Google Scholar]
- 33.Ide F, Mishima K, Saito I. Small foci of high-grade carcinoma cells in adenoid cystic carcinoma represent an incipient phase of dedifferentiation. Histopathology. 2003;43(6):603–604. doi: 10.1111/j.1365-2559.2003.01682.x. [DOI] [PubMed] [Google Scholar]
- 34.Nagao T, Gaffey TA, Serizawa H, Sugano I, Ishida Y, Yamazaki K, et al. Dedifferentiated adenoid cystic carcinoma: a clinicopathologic study of 6 cases. Mod Pathol. 2003;16(12):1265–1272. doi: 10.1097/01.MP.0000097366.88165.08. [DOI] [PubMed] [Google Scholar]
- 35.Brackrock S, Krüll A, Röser K, Schwarz R, Riethdorf L, Alberti W. Neutron therapy, prognostic factors and dedifferentiation of adenoid cystic carcinomas(ACC) of salivary glands. Anticancer Res. 2005;25(2B):1321–1326. [PubMed] [Google Scholar]
- 36.Sato K, Ueda Y, Sakurai A, Ishikawa Y, Kaji S, Nojima T, et al. Adenoid cystic carcinoma of the maxillary sinus with gradual histologic transformation to high-grade adenocarcinoma: a comparative report with dedifferentiated carcinoma. Virchows Arch. 2006;448(2):204–208. doi: 10.1007/s00428-005-0054-8. [DOI] [PubMed] [Google Scholar]
- 37.Handra-Luca A, Planchard D, Fouret P. Docetaxel-cisplatin-radiotherapy in adenoid cystic carcinoma with high-grade transformation. Oral Oncol. 2009;45(11):e208–e209. doi: 10.1016/j.oraloncology.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Malhotra KP, Agrawal V, Pandey R. High grade transformation in adenoid cystic carcinoma of the parotid: report of a case with cytologic, histologic and immunohistochemical study. Head Neck Pathol. 2009;3(4):310–314. doi: 10.1007/s12105-009-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonfitto VL, Demasi AP, Costa AF, Bonfitto JF, Araujo VC, Altemani A. High-grade transformation of adenoid cystic carcinomas: a study of the expression of GLUT1 glucose transporter and of mitochondrial antigen. J Clin Pathol. 2010;63(7):615–619. doi: 10.1136/jcp.2010.075390. [DOI] [PubMed] [Google Scholar]
- 40.Costa AF, Altemani A, Vékony H, Bloemena E, Fresno F, Suárez C, et al. Genetic profile of adenoid cystic carcinomas (ACC) with high-grade transformation versus solid type. Cell Oncol. 2011;34(4):369–379. doi: 10.1007/s13402-011-0037-5. [DOI] [PubMed] [Google Scholar]
- 41.Panarelli JF, Zoumalan CI, Mukkamala K, Maher EA, Codrin Iacob A, Rocca DAD. Dedifferentiated adenoid cystic carcinoma of the lacrimal gland. Ophthal Plast Reconstr Surg. 2011;27(5):e119–e121. doi: 10.1097/IOP.0b013e318201cb90. [DOI] [PubMed] [Google Scholar]
- 42.Boland JM, McPhail ED, García JJ, Lewis JE, Schembri-Wismayer DJ. Detection of human papilloma virus and p16 expression in high-grade adenoid cystic carcinoma of the head and neck. Mod Pathol. 2012;25(4):529–536. doi: 10.1038/modpathol.2011.186. [DOI] [PubMed] [Google Scholar]
- 43.Bavle RM, D’Mello S, Makarla S, Hosthor SS. Dedifferentiation in adenoid cystic carcinoma. J Oral Maxillofac Pathol. 2013;17(3):474–477. doi: 10.4103/0973-029X.125225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ly CK, Cheng HM, Vermeulen T. High grade transformation in a case of adenoid cystic carcinoma associated with Epstein–Barr virus expression. Pathology. 2013;45(7):693–695. doi: 10.1097/PAT.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 45.Sayar H, Sarioǧlu S, Bakariş S, Yildirim I, Öztarakçi H. High-grade transformation of adenoid cystic carcinoma delineated with a fibrous rim: a case report. Balkan Med J. 2013;30(3):333–336. doi: 10.5152/balkanmedj.2013.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kusafuka K, Miki T, Nakajima T. Salivary adenoid cystic carcinoma with an early phase of high-grade transformation: case report with an immunohistochemical analysis. Diagn Pathol. 2013;8(1):113. doi: 10.1186/1746-1596-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noda Y, Hori Y, Kishino M, Satou S, Ogawa Y, Harada H, et al. A case of dedifferentiated adenoid cystic carcinoma in submandibular gland: morphological and immunohistochemical features. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(3):e140. doi: 10.1016/j.oooo.2014.07.165. [DOI] [Google Scholar]
- 48.Tando S, Nagao T, Kayano K, Fushiki S, Itoh K. High-gradetransformation/dedifferentiation of an adenoid cystic carcinoma of the minorsalivary gland to myoepithelial carcinoma. Pathol Int. 2018;68(2):133–138. doi: 10.1111/pin.12624. [DOI] [PubMed] [Google Scholar]
- 49.Bhardwaj M, Gupta P. Dedifferentiated adenoid cystic carcinoma ex pleomorphic adenoma of the parotid. J Cancer Res Ther. 2018;14(3):706–708. doi: 10.4103/0973-1482.179522. [DOI] [PubMed] [Google Scholar]
- 50.Dutta A, Arun P, Arun I. Adenoid cystic carcinoma with transformation to high grade carcinomatous and sarcomatoid components: a rare case report with review of literature. Head Neck Pathol. 2020;14(4):1094–1104. doi: 10.1007/s12105-019-01120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]