Introduction
Non squamous tumours of the larynx are rare. The most common non squamous neoplasms of the larynx are neuroendocrine tumours [1]. Neuroendocrine neoplasms (NEN) of larynx are classified into three types -well differentiated (typical carcinoid), moderately differentiated (atypical carcinoid) and poorly differentiated neuroendocrine carcinoma (NEC) based on the histomorphologic features [2]. Well and moderately differentiated NECs are also termed as Neuroendocrine tumour Grade 1 and 2 respectively. All these three neoplasms show different biological behaviour and prognosis thereby requiring different management [3]. We present a rare case of neuroendocrine NEC of larynx which showed a different histomorphology in small biopsy and resection specimen.
Case Report
A 43 y old gentleman presented with a history of hoarseness of voice of one month duration. On physical examination, he had a palpable left cervical level II lymph node measuring 2 × 2 cm that was firm in consistency. Systemic examination was unremarkable. A computed tomography scan showed a left false vocal cord lesion with pre and paraglottic fat involvement. Direct laryngoscopy revealed a left false vocal cord lesion without glottic extension or anterior commissure involvement.
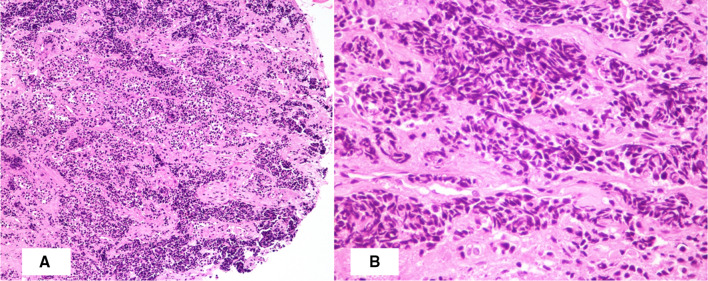
Histopathological examination of the biopsy sample obtained at laryngoscopy showed fragments of a tumour composed of small cells with high nucleocytoplasmic ratio, inconspicuous nucleoli, scant cytoplasm and coarse condensed nuclear chromatin and apoptosis. In some areas, these cells showed crush artefact (Fig. 1). Differential diagnoses considered were high grade NEC, mixed neuroendocrine-non neuroendocrine carcinoma (MiNEN) and small cell type of squamous cell carcinoma. Immunohistochemistry studies were performed. The tumour cells were positive for cytokeratin, synaptophysin and chromogranin with a high Ki67 proliferation index of 60–65%. Squamous markers such as p63 and High Molecular Weight Cytokeratin were negative. A diagnosis of poorly differentiated small cell neuroendocrine carcinoma was rendered.
Fig. 1.
Photomicrograph showing (A) tumour composed of nests and trabeculae of small cells with crush artefact (H&E X 100); (B) trabeculae of small cells with high nucleocytoplasmic ratio and hyperchromatic nuclei (H&E X 400)
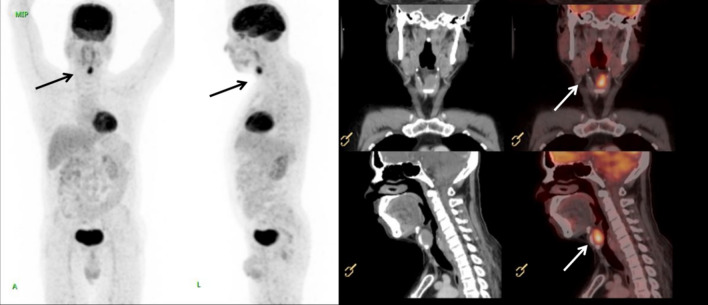
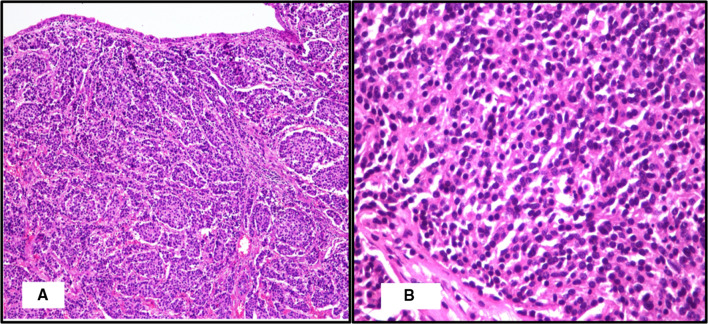
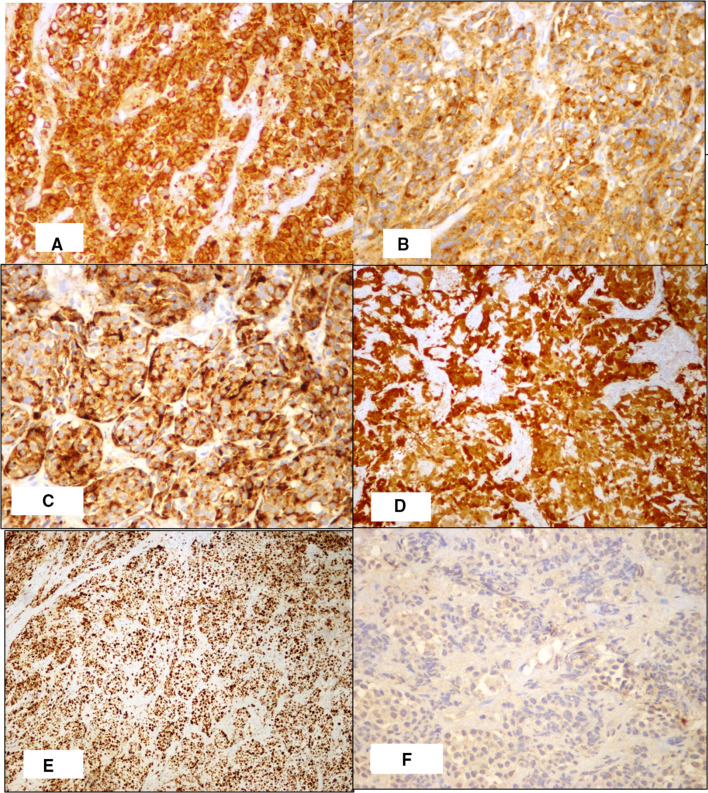
Following this biopsy report, the patient underwent Positron Emission Tomography-Computed Tomography scan which showed disease involving the left hemilarynx including left false vocal cord, anterior one third of left true vocal cord, anterior commissure and pre-epiglottic fat plane and lamina of thyroid cartilage (Fig. 2). He underwent a Supracricoid partial laryngectomy with cricohyoid-epiglottopexy [CHEP], bilateral selective neck dissection (levels 2 to 4) and a temporary tracheostomy. The specimen was received in the department of histopathology. On gross examination, a relatively well circumscribed submucosal nodule measuring 2.5 cm × 1.5 cm × 1.3 cm was seen involving the supraglottis and left false vocal cord that anteriorly infiltrated the thyroid cartilage. On microscopic examination, a partly circumscribed submucosal lesion with infiltrative borders was seen composed of organoid nests and trabeculae of monotonous cells with moderate cytoplasm, mild atypia, round to ovoid nuclei, stippled chromatin and inconspicuous nucleoli (Fig. 3). Mitotic count of 3–4 per 10 high power fields was obtained focally. The tumour infiltrated the inner lamina of thyroid cartilage. Eighty percentage of the tumor nuclei were positive for p16 immunostain and rare cells were positive for p53 (Fig. 4). Metastatic carcinoma was seen in 1 of 54 left cervical lymph nodes without extranodal extension. The histomorphology of the tumour observed in the partial laryngectomy specimen was predominantly that of moderately differentiated NEC. Given the varied spectrum of morphology of the tumour sampled in the direct laryngoscope guided biopsy and partial laryngectomy specimen with focally elevated mitotic count, high proliferation index and diffuse p16 positivity, a diagnosis of poorly differentiated small cell NEC of the larynx was made. The patient received adjuvant radiotherapy, was decannulated after completion of radiotherapy and remains alive without disease 18 months post therapy.
Fig. 2.
Computed tomography scan and Positron Emission Tomography—Computed Tomography scan showing FDG (fluorodeoxyglucose) avid soft tissue mass (marked by arrows) involving left false vocal cord, pre glottic fat plane, anterior commissure and anterior third of left true cord and invading the anterior half of left lamina of thyroid cartilage
Fig. 3.
Photomicrograph showing (A) a submucosal lesion with organoid pattern of monotonous cells (H&E X 100); (B) nests of monotonous cells with ovoid nuclei and stippled chromatin (H&E X 400)
Fig. 4.
Immunohistochemistry staining showing diffuse positivity for (A) AE1/AE3,(B) Synaptophysin, (C)Chromogranin, (D) p16 and high ki67(E) proliferation index and low (F) p53. (X 400)
Discussion
Laryngeal NENs are rare and account for less than 1% of laryngeal tumours [4]. WHO has classified head and neck NENs under three categories as well differentiated NEC or typical carcinoid tumour, moderately differentiated NEC or atypical carcinoid tumour and poorly differentiated NEC which have two subtypes namely small cell and large cell NEC [5]. In well differentiated NECs the cells exhibit mild atypia, mitotic count of less than 2 per 10 high power field, absence of necrosis and cells arranged as nests, cords and trabeculae. In moderately differentiated NEC, the tumour cells show more nuclear atypia and prominent nucleoli. Mitotic count is 2 to 10 per 10 high power fields. Small cell NECs are composed of nests, sheets and trabeculae of small cells with hyperchromatic nuclei, indistinct nucleoli, crush artefact and nuclear moulding. Large cell NECs show organoid, trabecular and palisading pattern composed of medium to large cells with abundant cytoplasm, coarse chromatin and prominent nucleoli. Necrosis is usually evident. Mitosis is more than 10 per 10 high power fields in poorly differentiated NECs. The tumour cells in all categories are by immunophenotype positive for synaptophysin, chromogranin and CD56.
Laryngeal NECs are on a morphologically continuous spectrum with an aggregate of features distinguishing the tumours [6]. There are very few studies on the molecular alterations that occur in NECs of head and neck. It is still not clear if head and neck NECs represent a diverse group of epithelial tumours showing neuroendocrine differentiation or if they are part of one disease with a range of differentiation and grades [7] as seen in our case.
Immunostaining with p16 was performed and 90% of the tumour nuclei were positive. A study done by Alos et al. observed that p16 is usually strongly and diffusely positive in high grade NECs of larynx due to Rb pathway dysregulation [8]. This positivity is not related to Human Papilloma Virus infection as seen with a subset of oropharyngeal squamous cell carcinomas. Alos et al. also observed that p53 can be overexpressed in high grade NECs suggesting a TP53 mutation. A study on 10 NECs of larynx by Halmos et al. showed no overexpression of p53 in all 10 cases [9]. In our case, p16 was strongly and diffusely positive and p53 was focally and weakly positive.
Currently there is no clear role of ki67 proliferation index in grading NENs of head and neck. Some studies have found good concordance between morphology, mitosis and ki67 labelling index whereas a few studies have showed discrepancies between the same [8]. More studies are required to assess the cut offs for ki67 index in the classification of head and neck neuroendocrine neoplasms.
Conclusion
We present this case due to its rarity and the presence of histomorphological spectrum as the tumour showed small cell morphology on biopsy and non-small cell moderately differentiated NEC like pattern on the resection specimen. It is important to grade the laryngeal NECs for prognostication and precise management. With this case we would like to emphasize the usefulness of ki67 and parameters such as p16 and p53 in assigning the tumour grade in doubtful cases. More studies are required regarding these aspects.
Acknowledgement
None
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mekala Lakshminarayanan, Email: drlmekala@gmail.com.
Ann Kurian, Email: drannkurian@gmail.com.
References
- 1.Ferlito A, Silver CE, Bradford CR, Rinaldo A. Neuroendocrine neoplasms of the larynx: an overview. Head Neck. 2009;31(12):1634–1646. doi: 10.1002/hed.21162. [DOI] [PubMed] [Google Scholar]
- 2.Rindi G, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31:1770–1786. doi: 10.1038/s41379-018-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez F, et al. How phenotype guides management of the neuroendocrine carcinomas of the larynx. J Laryngol Otol. 2018;132(7):568–574. doi: 10.1017/S0022215118000968. [DOI] [PubMed] [Google Scholar]
- 4.Hemalatha A, et al. Primary laryngeal neuroendocrine carcinoma—a rare entity with deviant clinical presentation. J Clin Diagn Res. 2014;8(9):07–08. doi: 10.7860/JCDR/2014/9766.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adel K, et al. WHO classification of head and neck tumours. 4. Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 6.Thompson DR, Bishop JA. Head and neck pathology. In: Lester DR, editor. Malignant neoplasms of the larynx, Third edn hypopharynx and trachea. Newyork: Elsevier; 2019. [Google Scholar]
- 7.Ordonez BP. Neuroendocrine carcinomas of the larynx and head and neck: challenges in classification and grading. Head Neck Pathol. 2018;12(1):1–8. doi: 10.1007/s12105-018-0894-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alos L, et al. p16 overexpression in high-grade neuroendocrine carcinomas of the head and neck: potential diagnostic pitfall with HPV-related carcinomas. Virchows Arch. 2016;469(3):277–284. doi: 10.1007/s00428-016-1982-1. [DOI] [PubMed] [Google Scholar]
- 9.Halmos, et al. Is human papillomavirus involved in laryngeal neuroendocrine carcinoma? Eur Arch Otorhinolaryngol. 2013;270:719–725. doi: 10.1007/s00405-012-2075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]