Abstract
Hemangiopericytomas (HPCs) are rare vascular tumours originating from extra capillary cells called Zimmermann’s pericytes. Only 5% of these lesions occur in the Sino nasal cavity. Sino nasal HPCs have a benign course with a high recurrence rate ranging from 9.5 to 50%. A radical surgical resection is considered the gold standard treatment either via external approach (lateral rhinotomy or Caldwell-Luc) or endoscopic approach. Three cases of Sino nasal hemangiopericytomas were treated at our institute. All these cases were operated via endoscopic approach. We are reporting their diagnostic work-up and the therapeutic management as case series. We also discuss epidemiological, clinical, morpho-pathological and radiological characteristics of this tumoral pathology. A treatment plan is also elucidated which may help to develop a long term treatment protocol for these lesions.
Keywords: Hemangiopericytoma, Endoscopic approach, Rare vascular tumor
Introduction
Hemangiopericytomas (HPCs) are rare vascular tumors arising from Zimmermann’s pericytes [1, 2]. These cells are situated surrounding all capillaries and are immature smooth muscle derived from mesenchyme. The HPC was first described in 1943 by Stout and Murray [1], may arise in any part of the body [3]; only 15%–30% are located in the head and neck region [4]. Of these, only 5% arise in the nasal cavity and paranasal sinus [5]. Other sites of predilection are the soft tissues of the scalp, face, neck, parotid gland, orbit, salivary glands and larynx. Sino nasal Hemangiopericytoma has more benign course with low risk of metastasis; however, having a recurrence rate of approximately 25% [4].
Case 1
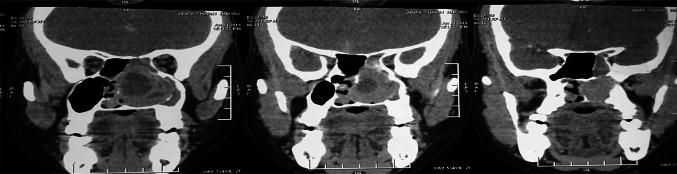
A 40-year-old female was admitted in our Department of ENT and Head-Neck Surgery in April 2019 for left chronic nasal obstruction and left nasal cavity bleeding. Endoscopic examination of nasal cavity showed a reddish vascular mass in left nasal cavity which bleed on touch. CT PNS with contrast showed ill defined enhancing soft tissue density lesion measuring 6.5 cm × 4.6 cm × 3.1 cm [AP X CC X T] involving left nasal cavity causing near total airway narrowing. It was involving left side turbinates, left ethmoid and maxillary sinuses. Marked erosion of nasal septum, bilateral cribriform plates, medial wall of left orbit, bony walls of bilateral ethmoid and left sphenoid sinuses were seen. Contralateral extension was seen involving right nasal cavity and ethmoid sinuses. Subtle intracranial extension was seen into floor of anterior cranial fossa in midline. No intra orbital extension was seen (Figs. 1, 2).
Fig. 1.
Coronal CT showing left nasal cavity mass involving left turbinates, left ethmoid and maxillary sinuses with marked erosion of nasal septum, bilateral cribriform plates, medial wall of left orbit, bony walls of bilateral ethmoid and left sphenoid sinuses. contralateral extension was seen involving right nasal cavity and ethmoid sinus
Fig. 2.
Coronal CT scan with contrast
Biopsy was taken from left nasal cavity mass in operating room under topical anaesthesia. Profuse bleeding was controlled by anterior nasal packing by conventional antibiotic soaked ribbon. On histopathological examination there were round to spindle cells arranged around multiple small capillary vessels suggestive of benign vascular tumour. On immunohistochemistry examination the specimen was positive for CD 31, CD 34, vimentin with ki-67 < 10% suggestive of hemangiopericytoma.
Before undergoing excision, MRI of brain was done for evaluating soft tissue extension. MRI showed focal abnormal signal density lesion in left nasal cavity with thick solid peripheral walls and septations. The lesion was hypointense on T2W and intermediate signal on T1W images and showed restricted diffusions on DWI & blooming on gradient images in septations and cystic component. It was causing deviation of nasal septum towards right side, laterally it was displacing medial wall of maxillary sinus and obstructing left osteomeatal complex. Superiorly it was extending into ethmoid sinus with retained secretions in bilateral frontal sinuses. There was no intraorbital and intracranial extension (Fig. 3).
Fig. 3.
Coronal MRI showing focal abnormal signal density lesion in left nasal cavity with thick solid peripheral walls and septations and was causing deviation of nasal septum towards right side, laterally it was displacing medial wall of maxillary sinus and obstructing left osteomeatal complex. superiorly it was extending into ethmoid sinus with retained secretions in bilateral frontal sinuses. there was no intraorbital and intracranial extension
It was planned to remove the tumor endoscopically under general anesthesia. Intraoperatively, a soft red, vegetative tumor, was found which bled profusely on instrumentation. Total “piece-meal” resection of tumor was done using Karl Storz microdebrider. Intraoperative blood loss was < 100 ml. Disease was cleared from all involved sinus. The histopathological examination reconfirmed hemangiopericytoma. The patient is on routine endoscopic follow-up and disease free at the time of writing this article (Fig. 4).
Fig. 4.
Pre operative, intra operative, post operative findings
Case 2
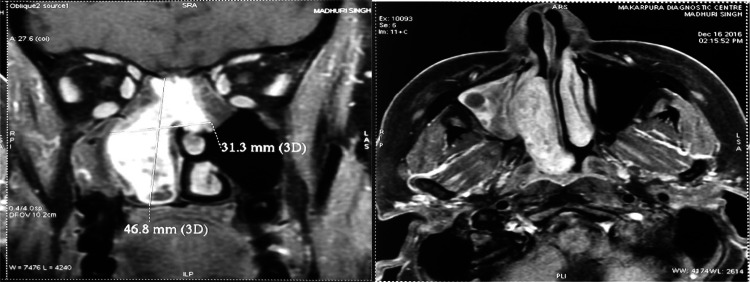
A 41-year-old female was admitted in our Department in December 2016 for right chronic nasal obstruction and bleeding [epistaxis]. A contrast CT scan showed heterogeneously enhancing dumbbell shaped lesion measuring 4.8 cm × 4.6 cm × 1.7 cm [AP X CC X T] involving right maxillary antrum extending through ostium in right nasal cavity, right middle turbinate -meatus and abutting right inferior turbinate posteriorly and just protruding in post nasal space with changes of post-obstructive sinusitis in bilateral ethmoidal and right sphenoid sinus. No intraorbital and intracranial extension was seen. Biopsy was taken from the mass following which the nose had to be packed to control bleeding. Histopathological examination revealed Hemangiopericytoma. MRI of brain was done to reveal any oblivious soft tissue/ intracranial extensions. It showed a well defined expansile heterogeneously enhancing solid mass lesion in right nasal cavity with ethmoid sinuses involvement. There was focal destruction of posterior-superior nasal septum and extension of lesion into left posterior ethmoid sinus and nasopharynx. Right middle turbinate was not visualized separately from lesion and right inferior turbinate was compressed. There was no intraorbital and intracranial extension (Fig. 5).
Fig. 5.
MRI showing well defined expansile heterogeneously enhancing solid mass lesion in right nasal cavity with ethmoid sinuses involvement and focal destruction of postero-superior nasal septum and extension of lesion into left posterior ethmoid sinus and nasopharynx. right middle turbinate was not visualized separately from lesion and right inferior turbinate was compressed without intraorbital and intracranial extension
The tumor was excised piece-meal using the endoscopic endonasal approach under general anaesthesia. Powered Instrumentation was not used and the blood loss was about 300 ml. The histopathological examination reconfirmed hemangiopericytoma.
Case 3
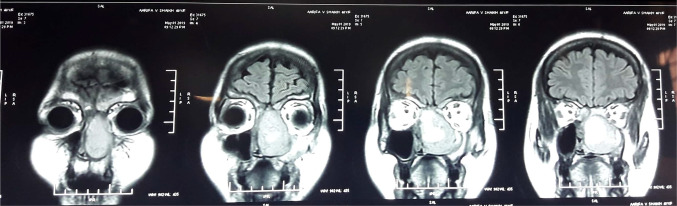
A 40-year-old male was admitted in our Department in march 2016 for left chronic nasal obstruction and left nasal cavity bleeding. CT PNS with contrast showed ill defined enhancing soft tissue density lesion measuring 4.6 cm × 5 cm × 2.2 cm [AP X CC X T] involving left nasal cavity, ipsilateral ethmoid sinus and projecting into left frontal sinus with destruction of adjacent bony nasal septum was seen. Contralateral extension was seen involving right nasal cavity and abutting right middle turbinate. Erosion of medial wall of left orbit and bony walls of left frontal sinus was seen. There was destruction of left cribriform plate with mild intracranial extension of lesion abutting left frontal lobe (Fig. 6).
Fig. 6.
CT PNS showing soft tissue density lesion of left nasal cavity, left ethmoid sinus and projecting into left frontal sinus with destruction of adjacent bony nasal septum
Biopsy taken from left nasal cavity mass suggestive of low-grade spindle cell sarcoma-hemangiopericytoma. Surgical plan was to remove the tumor using the endoscopic endonasal approach under general anaesthesia. Total tumoral excision was performed using the “piece-meal” resection method using powered instrumentation. Blood loss was < 100 ml. The histopathological examination reconfirmed hemangiopericytoma.
Disussion
The WHO classification of head and neck tumors proposed in 2005 said that Sino nasal hemangiopericytoma should be named glomangiopericytoma due to their similarity with glomus tumors [6] as there is a closer relationship of Sino nasal hemangiopericytoma to glomus tumors than to hemangiopericytoma overall [7]. However, in routine clinical practice, term “hemangiopericytoma” for all tumors with hemangiopericytoma-like histology after exclusion of other tumor entities is used [8]. Sino nasal hemangiopericytoma mainly affects middle aged patients [9], with slight female predominance [10] or without any gender predilection [11].
The etiology is still unknown [12] but theories suggest that trauma, long-term steroid use, arterial hypertension, and hormone imbalance might be predisposing risk factors for Sino nasal hemangiopericytoma [13]. On Immunochemistry Vimentin and CD34 are the only antigens detected in tumor cells of Sino nasal hemangiopericytoma [11], Other epitopes as actin, S-100 or factor XIIIa can be found in a few cases only [14]. Recently, the vessels of the Sino nasal hemangiopericytoma partially showed a positive staining for D2-40 (the so-called podoplanin antibody) [8, 15], and helped in confirming diagnosis.
The majority of Sino nasal hemangiopericytomas are located in the nasal cavity and presents with nasal bleeding and nasal obstruction [16, 17]. Local site pain occurs very rarely and is an indicative sign of local infiltration [18]. Vision impairment, headache and local swelling are less frequent symptoms [19]. During nasal examination, Sino nasal hemangiopericytoma is frequently confused and mistaken for inflammatory polpys [12]. Although histopathology can confirm the final diagnosis [19, 20], biopsy is generally not recommended (if the diagnosis is otherwise evident) as it may lead to severe bleeding [16, 21]. Therefore, radiological examination by CT and/or MRI should be performed.
A CT scan of the paranasal sinuses shows a soft-tissue mass with contrast and bone destruction [22]. However, the CT scan cannot differentiate between tumor mass and inflammatory fluid in obstructed paranasal sinuses. Hence it is recommended to get a MRI scan. On T1-weighted MRI, Sino nasal hemangiopericytomas appear as solid iso-intense masses with strong contrast enhancement; on T2-weighted imaging, the tumor mass is isointense to low intense in contrast to inflammatory fluid [23]. Conventional digital angiography is useful for identification of feeding vessel of the Sino nasal hemangiopericytoma and to plan a preoperative embolization may be helpful in reducing intraoperative blood loss [19]. A large tumor size of > 6.5 cm and the histological finding of necrosis, nuclear atypia, and a high number of mitosis are associated with a poorer prognosis [9]. However, the presence of one of these features does not predict an aggressive clinical course; it is usually the sum of several criteria for malignancy to be considered as prognostically unfavorable [1, 24]. In contrast to hemangiopericytoma located at somatic sites, Sino nasal hemangiopericytoma has a very low tendency for metastasis- 5% [4]. However, lymphatic and hematogenous metastases can occur [13]. In these rare cases regional lymph nodes as well as lungs, liver, and bone are the most common site of metastasis [10, 16]. The reason for the low-grade behavior of Sino nasal HPC is unknown. It is likely that nasal obstruction and epistaxis lead to earlier diagnosis and treatment [25].
In recent past for Sino nasal hemangiopericytoma, the treatment of choice was wide-local excision with negative margins [13, 26] usually via lateral rhinotomy [18, 20], and was even regarded as obligatory when the tumor breached the cribriform plate [27] or spread beyond the Sino nasal region [20]. An elective neck dissection is not indicated as lymphatic metastases occur rarely [28]. Recently, the tumor resection via endonasal approach became more popular in the treatment of Sino nasal hemangiopericytoma because it offers many advantages compared to the external approach like better overview for accurate assessment of the tumor insertion, the margins and the surrounding tissue, preservation of the natural physiology of the nose, the reduced risk of damaging the lacrimal structures, avoidance of outer incision with consecutive scars [16] and reduced amount of blood loss during the surgical exposure [29]. In very few cases only, an endoscopic controlled endonasal approach may be limited by certain factors like large tumor size with invasion in the pterygopalatine fossa, orbital involvement, or a highly vascular tumor [16]. However, a high vascularity of the tumor does not generally exclude an endoscopic resection provided that embolization may be successfully performed before surgery, helping to curtail intraoperative blood loss [9].
Considering an alternative treatment modality, Chemotherapy shows limited effectivity and is indicated only as palliative treatment in inoperable tumors with or without metastases [26]. Methotrexate, cyclophosphamide, vincristine and Adriamycin may show a partial remission [30].
Radiotherapy as primary therapy of hemangiopericytoma of all sites has very limited success and is associated with a recurrence rate of 87.6% during the first five years [31] compared to a recurrence rate of 47% after tumor resection as primary therapy [32]. Hence, the radiotherapy is recommended only for unresectable tumors and can also be combined with chemotherapy in these specific situatioins [20].
Recurrence rates for Sino nasal HPC vary in the literature from 9.5 to 50%. Average time to recurrence is 6 to 7 years [16] and usually associated with incomplete tumor removal. In case of recurrence also, a resection should be considered as the treatment of first choice [33], due to the good outcome after the reresection and hence evading side effects of radiation on the head and neck [16].
The prognosis of hemangiopericytoma is definitively not predictable, neither by the clinical appearance nor by the histological findings. Recurrence may occur after a prolonged disease-free interval and has even been reported 26 years after tumor resection warranting a regular, life-long surveillance of patients [11, 32]. Further, recurrence frequently precedes the development of metastases after tumor resection [28]. Based on this, we recommend approach consist of radiological evaluation followed by wide local excision of the tumor via endoscopic approach and long-term follow-up with serial endoscopic examinations and imaging studies.
Conclusion
Sino nasal hemangiopericytoma is a rare vascular tumor derived from Zimmermann’s pericyte with benign course. These tumors have a high propensity to local invasion and have an alarming recurrence rate. The diagnosis involves a complete otorhinolaryngological examination, radiological investigations and post-operative pathological examination with immunohistochemistry for confirmation. Biopsy may be avoided if diagnosis is otherwise evident like in recurrent cases, for the fear of hemorrhage. However when required biopsy can be done in OR [operating room] setup or a intraoperative Frozen Section can be employed on the day of surgical excision. The complete surgical resection is the treatment of choice, and it can be obtained endoscopically, by external approach or by combined approach. However, an endoscopic endonasal tumor resection is proved to be an excellent treatment method in our case series. Long-term follow-up of all cases is essential for optimal clinical management.
Abbreviations
- HPC
Hemangiopericytoma
- DWI
Diffusion weight image
- CT
Computed tomography
- PNS
Para nasal sinus
Compliance with ethical standards
Conflict of interest
There is no conflict of interest among authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rahulkumar Shah, Email: rahulshah.1010october@gmail.com.
Rahul Gupta, Email: doctor.rahul25@gmail.com.
Ranjan Aiyer, Email: drrgaiyer@hotmail.com.
References
- 1.Stout AP, Murray MR. Hemangiopericytoma: a vascular tumor featuring Zimmermann's pericytes. Ann Surg. 1942;116:26–33. doi: 10.1097/00000658-194207000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stout AP. Hemangiopericytoma. A study of twenty-five new cases. Cancer. 1949;2:1027–1035. doi: 10.1002/1097-0142(194911)2:6<1027::AID-CNCR2820020609>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Navarrete ML, Maeso J, Pellicer M. Hemangiopericytoma of the nasal septum. Eur Arch Oto-Rhino-Laryngol. 1990;247(6):384–386. doi: 10.1007/BF00179014. [DOI] [PubMed] [Google Scholar]
- 4.Batsakis JG, Jacobs JB, Templeton AC. Hemangioperictyoma of the nasal cavity: electron-optic study and clinical correlations. J LaryngolOtol. 1983;97:361–368. doi: 10.1017/S002221510009424X. [DOI] [PubMed] [Google Scholar]
- 5.Batsakis JG, Rice DH. The pathology of head and neck tumors: vasoformativetumors, part 9B. Head Neck Surg. 1981;3(4):326–339. doi: 10.1002/hed.2890030408. [DOI] [PubMed] [Google Scholar]
- 6.Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 7.Tse LLY, Chan JKC. Sinonasalhaemangiopericytoma like tumour: a sinonasalglomus tumour or a haemangiopericytoma? Histopathology. 2002;40(6):510–517. doi: 10.1046/j.1365-2559.2002.01396.x. [DOI] [PubMed] [Google Scholar]
- 8.Knosel T, Schulz B, Katenkamp K, Katenkamp D, Petersen I. Solitary fibrous tumor and haemangiopericytoma: what is new? Pathologe. 2010;31(2):123–128. doi: 10.1007/s00292-009-1253-x. [DOI] [PubMed] [Google Scholar]
- 9.Enzinger FM, Smith BH. Hemangiopericytoma: an analysis of 106 cases. Hum Pathol. 1976;7(1):61–82. doi: 10.1016/S0046-8177(76)80006-8. [DOI] [PubMed] [Google Scholar]
- 10.Millman B, Brett D, Vrabec DP. Sinonasalhemangiopericytoma. Ear Nose Throat J. 1994;73(9):680–687. doi: 10.1177/014556139407300912. [DOI] [PubMed] [Google Scholar]
- 11.Eichhorn JH, Dickersin GR, Bhan AT, Goodman ML. Sinonasalhemangiopericytoma. A reassessment with electron microscopy, immunohistochemistry, and long-termfollow-up. Am J SurgPathol. 1990;14(9):856–866. doi: 10.1097/00000478-199009000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Gillman G, Pavlovich JB. Sinonasalhemangiopericytoma. Otolaryngology. 2004;131(6):1012–1013. doi: 10.1016/j.otohns.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Gorenstein A, Facer GW, Weiland LH. Hemangiopericytoma of the nasal cavity. Otolaryngology. 1978;86(3):405–415. doi: 10.1177/019459987808600306. [DOI] [PubMed] [Google Scholar]
- 14.Nemes Z. Differentiation markers in hemangiopericytoma. Cancer. 1992;69(1):133–140. doi: 10.1002/1097-0142(19920101)69:1<133::AID-CNCR2820690124>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Hansen T, Katenkamp K, Katenkamp D. D2-40 staining in sinonasal-type hemangiopericytoma—further evidence of distinction from conventional hemangiopericytoma and solitary fibrous tumor. Virchows Arch. 2006;448(4):459–462. doi: 10.1007/s00428-005-0130-0. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharyya N, Shapiro NL, Metson R. Endoscopic resection of a recurrent sinonasalhemangiopericytoma. Am J Otolaryngol. 1997;18(5):341–344. doi: 10.1016/S0196-0709(97)90031-4. [DOI] [PubMed] [Google Scholar]
- 17.Fuster MAA, Sala CR, Vizcaıno VC, Hermida CD-A, Molina JV. Sinonasalhemangiopericytoma. ActaOtorrinolaringologica Espanola. 2001;52(8):699–702. [Google Scholar]
- 18.Serrano E, Coste A, Percodani J, Herve S, Brugel L. Endoscopic sinus surgery for sinonasalhaemangiopericytomas. J LaryngolOtol. 2002;116(11):951–954. doi: 10.1258/00222150260369525. [DOI] [PubMed] [Google Scholar]
- 19.Weber W, Henkes H, Metz KA, Berg-Dammer E, Kuhne D. Haemangiopericytoma of the nasal cavity. Neuroradiology. 2001;43(2):183–186. doi: 10.1007/PL00006046. [DOI] [PubMed] [Google Scholar]
- 20.Castelnuovo P, Pagella F, Delu G, Benazzo M, Cerniglia M. Endoscopic resection of nasal haemangiopericytoma. Eur Arch Oto-Rhino-Laryngol. 2003;260(5):244–247. doi: 10.1007/s00405-001-0440-z. [DOI] [PubMed] [Google Scholar]
- 21.Stout AP. Hemangiopericytoma; a study of 25 cases. Cancer. 1949;2(6):1027–1054. doi: 10.1002/1097-0142(194911)2:6<1027::AID-CNCR2820020609>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 22.Mosesson RE, Som PM. The radiographic evaluation of sinonasaltumors: an overview. OtolaryngolClin North Am. 1995;28(6):1097–1115. doi: 10.1016/S0030-6665(20)30437-0. [DOI] [PubMed] [Google Scholar]
- 23.Palacios E, Restrepo S, Mastrogiovanni L, Lorusso GD, Rojas R. Sinonasalhemangiopericytomas: clinicopathologic and imaging findings. Ear Nose Throat J. 2005;84(2):99–102. doi: 10.1177/014556130508400214. [DOI] [PubMed] [Google Scholar]
- 24.Catalano PJ, Brandwein M, Shah DK, Urken ML, Lawson W, Biller HF. Sinonasalhemangiopericytomas: a clinicopathologic and immunohistochemical study of seven cases. Head Neck. 1996;18(1):42–53. doi: 10.1002/(SICI)1097-0347(199601/02)18:1<42::AID-HED6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.Compagno J, Hyams VJ. Haemangiopericytoma-like tumour intranasal tumours: a clinicopathologic study of 23 cases. Am J ClinPathol. 1976;66:672–683. doi: 10.1093/ajcp/66.4.672. [DOI] [PubMed] [Google Scholar]
- 26.Cohen Y, Lichtig C, Robinson E. Combination chemotherapy in the treatment of metastatic hemangiopericytoma. Oncology. 1972;26(2):180–187. doi: 10.1159/000224666. [DOI] [PubMed] [Google Scholar]
- 27.Boey HP, Mitra S, Yanagisawa E. Intranasal hemangiopericytoma. Ear Nose Throat J. 1998;77(12):944–945. doi: 10.1177/014556139807701203. [DOI] [PubMed] [Google Scholar]
- 28.Reiner SA, Siegel GJ, Clark KF, Min KW. Hemangiopericytoma of the nasal cavity. Rhinology. 1990;28(2):129–136. [PubMed] [Google Scholar]
- 29.Abdel-Fattah HM, Adams GL, Wick MR. Hemangiopericytoma of the maxillary sinus and skull base. Head Neck. 1990;12(1):77–83. doi: 10.1002/hed.2880120112. [DOI] [PubMed] [Google Scholar]
- 30.Wong PP, Yagda A. Chemotherapy of malignant hemangiopericytoma. Cancer. 1978;41(4):1256–1260. doi: 10.1002/1097-0142(197804)41:4<1256::AID-CNCR2820410406>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 31.Backwinkel KD, Diddams JA. Hemangioperizytoma. Cancer. 1970;25:896–901. doi: 10.1002/1097-0142(197004)25:4<896::AID-CNCR2820250423>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 32.Sabini P, Josephson GD, Yung RT, Dolitsky JN. Hemangiopericytoma presenting as a congenital midline nasal mass. Arch Otolaryngol. 1998;124(2):202–204. doi: 10.1001/archotol.124.2.202. [DOI] [PubMed] [Google Scholar]
- 33.El-Naggar AK, Batsakis JG, Garcia GM, Luna ML, Goepfert H. Sinonasalhemangiopericytomas: a clinicopathologic and DNA content study. Arch Otolaryngol. 1992;118(2):134–137. doi: 10.1001/archotol.1992.01880020026010. [DOI] [PubMed] [Google Scholar]