Abstract
Maxillary swing approaches provide excellent exposure of the anterior, mid, and anterolateral skull base offering a wide window to approach nasopharyngeal neoplasms; however, they are also associated with complications. The present study aimed to evaluate the results of a modified total maxillary swing (TMS) approach developed to minimize postoperative complications. The modified TMS approach was used to treat five patients who had extensive juvenile nasopharyngeal angiofibromas between March and October 2019 at our tertiary care center. Surgical technique, preoperative image findings, and intra-operative findings were recorded. In the postoperative follow-up, patients were examined to rule out all possible complications associated with the procedure according to the literature. A retrospective analysis was performed to assess tumor extensions, surgical modifications, and postoperative complications. All tumors had orbital and infratemporal (lateral limit) involvement while four had intracranial involvement. No per-operative complications were reported, and postoperative clinical and endoscopic evaluation was performed at 1, 2, and 3 months. There was no evidence of complications including residue, recurrence, maxillary necrosis, ophthalmoplegia, epiphora, palatal fistula, or jaw malocclusion. Besides, minor complications such as infraorbital margin skin retraction, infraorbital serous collection, maxillo-zygomatic abscess, and unsightly scar were also not seen. Only one case presented with maxillary osteomyelitis which was resolved with mini-plate removal and antibiotics. A modified TMS approach is a prudent option to ensure complete removal of juvenile nasopharyngeal angiofibromas with negligible complications.
Keywords: Nasopharyngeal angiofibroma, Extensive, Total maxillary swing, Complications
Introduction
Juvenile nasopharyngeal angiofibromas (JNAs) are benign tumors with high vascularity seen almost exclusively in adolescent males [1, 2]. Currently presumed to arise from the sphenopalatine foramen at its superior margin, it spreads through various communications of the pterygopalatine fossa and leads to local destruction resulting in multiple morbidities [1]. Critical for all surgical approaches is adequate exposure of tumor, which depends on its extent and the neurovascular structures involved [3]. The different approaches described for limited JNAs include lateral rhinotomy, mid-facial de-gloving, and a transpalatal procedure while approaches such as maxillary swing (total, subtotal, extended total), craniofacial, infratemporal fossa type-C with or without an endoscope, or endoscopic excision alone are advocated for both limited and extensive lesions [3–10].
Introduced by Wei et al. in 1991, the maxillary swing approach provides a wide window to the nasopharynx, skull base, pterygopalatine fossa, and infratemporal fossa which is very advantageous in addressing nasopharyngeal lesions with extensions to these areas [11]. Total maxillary swing (TMS) includes the orbital floor with parts of the maxilla that are mobilized in the maxillary swing [4].
Swinging the maxilla in toto poses a risk to multiple important structures including the orbit, lacrimal duct, palate, jaw, and the maxilla itself which needs proper addressal to avoid complications. Other postoperative complications such as ophthalmoplegia, lateral rectus palsy, epiphora, palatal fistula, jaw malalignment, infraorbital margin skin retraction, etc. [3, 4] have also been reported.
The present study evaluates the results of a modified TMS approach developed by the authors to minimize postoperative complications in patients having Radowski stage III JNA [12].
Methods
During the period from March to October 2019 at our tertiary care center, five patients having extensive JNA (Radowski stage III) underwent tumor excision by a modified TMS approach. Intra-operative findings, tumor extensions, and complications were analyzed retrospectively.
Preoperative Work-up
This included clinical assessment, computed tomography (CT), angiography, contrast-enhanced magnetic resonance imaging (MRI), routine hematological investigations, vision assessment, detailed counseling, and obtaining informed consent from patients, parents, or guardians.
Surgical Technique
All patients were operated under general anesthesia. Tarsorrhaphy was performed on the ipsilateral side. The external carotid artery (ECA) was controlled above the origin of the superior thyroid artery using a transcervical approach. Modifications made to the TMS approach have been listed in Table 1 and details of the surgical steps are described next.
Table 1.
Modifications to TMS approach to minimize complications
| 1. J-shaped small palatal incision |
| 2. Limit of cheek flap elevation defined with infraorbital foramen as landmark |
| 3. Limits of orbital floor periosteal elevation defined along maxilla-ethmoidal suture and inferior orbital fissure |
| 4. Periosteal incision 5 mm below inferior orbital margin |
| 5. Zygomatic osteotomy in line with inferior orbital fissure |
| 6. Osteotomy of frontal process of maxilla in line with maxillo-ethmoidal suture |
| 7. Different incision planes of three layers during palatal osteotomy |
| 8. Vertical slitting on medial aspect of lacrimal sac |
| 9. Three-person maneuver and sequential plating during maxillary repositioning |
Palatal Flap
A J-shaped incision (Fig. 1) was made with pencil tip cautery over the mucosa of the hard palate. The palatal mucoperiosteum was elevated to about 5 mm across the midline to the contralateral side and posteriorly until the junction of the hard palate and soft palate after cauterizing the ipsilateral greater palatine artery. Mucosa behind the last molar was elevated using the same incision without a lateral extension. The flap was retracted to the opposite side with a securing suture to prevent tearing it.
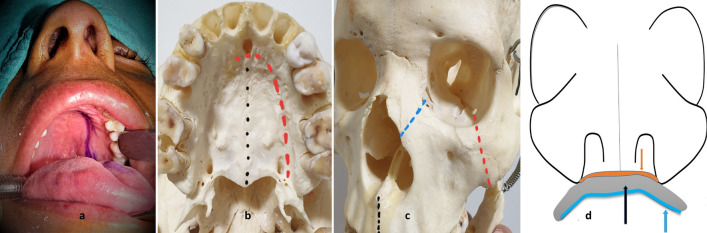
Fig. 1.
a J-shaped palatal incision mark. b Mark for midline palatal osteotomy site in black and for para-median palatal incision in red. c Osteotomy sites through zygoma in red, frontal process of the maxilla in blue and anterior midline alveolus in black. d Representation of incision lines in different planes through nasal floor mucoperiosteum in orange, palatal bone in black and palatal mucoperiosteum in blue
Exposure of Osteotomy Sites
A Weber Ferguson incision (without gingivolabial component) was made with an infra-ciliary component about 5 mm below the ciliary line. To avoid infraorbital edema, the infra-ciliary flap was elevated superficial to the orbital fat until the infraorbital margin was reached; 5 mm below this, the periosteum was incised and elevated as far as the zygoma. From the upper lip to the medial canthus, the incision was deepened to bone after transecting the periosteum. The periosteum was elevated inferiorly and laterally as far as the infraorbital foramen keeping the remaining cheek tissue attached to the maxilla (Fig. 2).
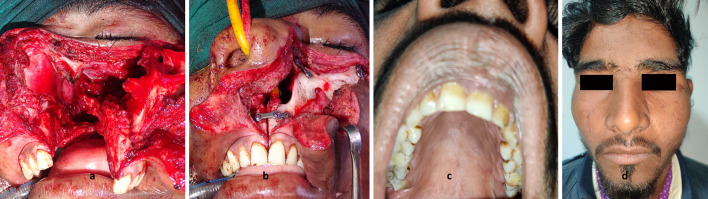
Fig. 2.
a Swung maxilla with attached cheek flap. b Maxillary alignment after plate fixation. c Well healed palate with aligned jaw. d Good facial healing with minimal scarring
Remaining infero-medially at the level of the maxillo-ethmoidal suture and infero-laterally at the level of the inferior orbital fissure, the orbital floor periosteum was elevated as far as the orbital apex while the eye globe was retracted gently. All osteotomy sites including the midline of the hard palate, zygoma, maxillary frontal process, and maxillo-ethmoidal suture were thus exposed (Fig. 1).
Osteotomies
Osteotomy at the zygoma was performed in line with the inferior orbital fissure. A curved artery was introduced through the fissure and brought under the zygoma for control and an oscillating saw was used to perform the osteotomy. Osteotomies at the frontal process of the maxilla (in line with the maxillo-ethmoidal suture), anterior alveolus (cut in the midline between the incisors), and palate (just lateral to the midline) were performed with Stryker’s sagittal oscillating saw and osteotome. The maxilla was disarticulated from the pterygoid process by placing a curved osteotome behind the last molar and gently hammering the osteotome.
Soft Tissue Addressal and Tumor Removal
The lacrimal sac was widely opened with a sharp cut at its inferior part and a vertical slit on its medial aspect. Ipsilateral nasal floor mucoperiosteum was elevated and then incised close to the inferior turbinate using scissors. The entire maxilla with attached cheek tissue was swung laterally exposing the tumor and surgical field (Fig. 2).
The tumor was separated from infratemporal fossa fat using bipolar cautery, and the maxillary artery was identified and cauterized. The tumor’s lateral, inferior, and medial attachments were separated using bipolar cautery. Finally, the superior and posterior attachments were separated by gently pulling the tumor downwards and medially. As the intra-orbital portion was removed, the eye globe was protected using a globe retractor. Gentle traction helped remove the intracranial component.
The basisphenoid, pterygoid base, and vidian canal were drilled to remove any remnants. The cavity was examined for residue using an endoscope, especially in the regions of the pterygoid base, vidian canal, sphenoid sinus, and cavernous sinus. In the case of bleeding from the cavernous region, hemostatic solution (Floseal Hemostatic Matrix, Baxter, Deerfield, Illinois, USA) was used.
Maxilla Repositioning and Closure
The maxilla was repositioned keeping the eye globe retracted to prevent entrapment. Titanium mini-plates were fixed first at mid alveolar osteotomy, then at the maxillo-zygomatic osteotomy followed by the frontal process of the maxilla (Fig. 2). We followed a “three-person maneuver” during plate and screw fixation at the alveolus, with the first person holding the maxilla in approximation to the contralateral side keeping the upper incisors at the same level, the second holding the plate and the third driving the screw in. In the first patient, holes were drilled for screw fixation before osteotomy. Due to the bone loss at the osteotomy sites, screw fixation in previously drilled holes did not give adequate jaw alignment and had to be revised during surgery in the first patient. In the rest of the cases, screw markings were made using monopolar cautery before osteotomy. Holes for screws were drilled immediately before plate fixation.
The palatal mucoperiosteum was sutured. The facial incision was closed in three layers involving the periosteal suture, inverted subcutaneous suture, and skin suture using monofilament.
Postoperative Care and Follow-up
Nasal packing and facial dressing were removed, ocular movements were assessed, lacrimal syringing was performed and lacrimal massage started on the second day after surgery. Sutures were removed on the sixth day. Nasal douching with an alkaline solution and dilute budesonide (0.5 mg of budesonide respule diluted in 250 mL of normal saline) was advised for all patients for a minimum of 4 weeks and was continued further depending on cavity status as per endoscopic examination.
At 4 weeks postoperatively, contrast-enhanced CT was performed to identify any residue. For the follow-up, patients were called for a detailed clinical and endoscopic examination every month during the initial 3 months, and every 3 months thereafter.
Results
Five adolescent males with ages ranging from 14 to 19 years and with a mean age of 16.2 years underwent excision of their JNAs by the TMS approach. One patient had undergone endoscopic excision of the tumor at another center about a year earlier while all the others were primary cases.
All patients underwent MRI and CT with angiography which revealed tumor extensions (Table 2, Fig. 3), vascularity (Table 2), and formed the basis for selection of the TMS approach for tumor excision. The ipsilateral ECA was controlled in all cases. In two patients, the ipsilateral ECA was clamped during surgery and this was relieved after tumor excision. In the remaining three, clamps were not required. Preoperative embolization was not performed in any case.
Table 2.
Patient data demonstrating tumor vascularity, blood loss and complications
| Patient | Vascularity | Significant areas involved | Blood loss (mL) | Use of topical hemostat | Postoperative complications |
|---|---|---|---|---|---|
| 1 | I/L IMA, APA | ITF, O, SS, GWS, PR, CS, ICE | 800 | No | None |
| 2 | I/L IMA, APA, ICA | ITF, O, SS, GWS, PR, CS, ICE, ICA encasement (180°) | 900 | Yes | None |
| 3 | I/L IMA, ICA | ITF, O, SS, GWS, PR, CS, ICE, ICA encasement (180°) | 700 | No | None |
| 4 | B/L IMA | ITF, O, GWS, SS, PR | 1100 | No | Osteomyelitis |
| 5 | I/L IMA, APA, ICA | ITF, O, GWS, SS, PR, CS, ICE, ICA encasement (180°) | 1300 | Yes | None |
APA, ascending pharyngeal artery; B/L, bilateral; CS, cavernous sinus; GWS, greater wing of sphenoid erosion; ICA, internal carotid artery; ICE, intracranial extension; I/L, ipsilateral; IMA, internal maxillary artery; ITF, infratemporal fossa-lateral most limit; O, orbit; PR, pterygoid root erosion; SS, sphenoid sinus
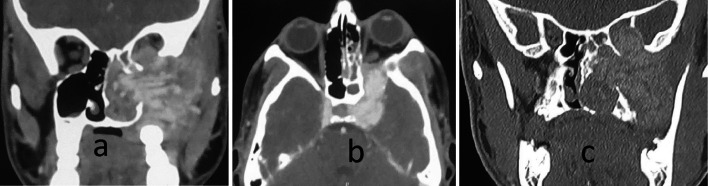
Fig. 3.
Computed tomography images showing tumor extensions to a lateral limit of infratemporal fossa; b middle cranial fossa via superior orbital fissure and internal carotid artery encasement; c orbit via inferior orbital fissure
In all cases apart from the one undergoing revision surgery, the whole maxilla was swung; however, in the revision case, the medial wall and posterior wall had already been removed in the previous surgery, therefore only the anterior wall of the maxilla, the orbital floor (maxillary portion) and hard palate could be rotated. In all cases, TMS helped in complete exposure of the infratemporal fossa, pterygopalatine fossa, orbital apex, nasopharynx, and adjoining regions of the skull base.
The mean intraoperative blood loss was 960 mL. The hemostatic solution was used in only two patients to control bleeding from the cavernous sinus. All patients required intra-operative blood transfusion. The mean operative time was 4.2 h.
One patient developed osteomyelitis of the maxilla in the alveolar region 3 weeks after surgery which mandated removal of the titanium mini-plate at the alveolus and the condition resolved with conservative management. None of our patients developed palatal fistula or malalignment of the upper incisors and none had any visual disturbance, diplopia, or complaints of epiphora post-surgery. There was no incidence of the maxillo-zygomatic abscess, infraorbital skin margin retraction, or serous collection after surgery in our patients.
In postoperative CT, no patient had any residual tumor. In the follow-up, which is continuing (minimum follow-up of 7 months completed for all patients), no patient had any symptoms, signs, or diagnostic nasal endoscopy findings indicative of recurrence.
Discussion
JNA with intracranial involvement has posed a challenge to surgeons in terms of incomplete tumor removal, recurrence, intra-operative bleeding, and neurological deficit. Involvement of the lateral-most extent of the infratemporal fossa, cavernous sinus, or pterygoid base has been reported to produce surgical difficulties resulting in incomplete tumor removal and recurrence [12, 13]. Tumor proximity to the cavernous carotid increases the risk of profuse bleeding from that region which may result in incomplete disease removal. Intra-orbital involvement (Fig. 3) of the tumor mandates an extremely cautious approach to prevent injury to the optic nerve and other orbital structures while simultaneously ensuring complete disease removal. The extensions of the tumors (Table 1) categorized them all as Radowski stage III [12].
In a primary description of the maxillary swing approach by Wei et al. [11], the orbital floor was not swung so this was a sub-total maxillary swing (SMS). The same approach was used by Roy Chowdhury et al. and Amin, while the procedure used by Mathur and Vashishth mentioned osteotomy at the inferior orbital fissure, but swinging of the orbital floor was not reported [3, 14, 15]. TMS involving swinging of the orbital floor as described by Dubey et al. was advantageous in terms of completely opening up the pterygoid base, orbit apex, pterygopalatine fossa, and the adjoining skull base [4]. Several authors have reported complications with the maxillary swing technique (Table 3). The modified technique described in this study aimed at minimizing the risk of complications.
Table 3.
Comparison of complications of maxillary swing surgery as reported in various studies
| Study | Approach | n | Complications |
|---|---|---|---|
| Dubey et al. [4] | TMS | 16b | ISO (4), IMSR (4), MZA (2), LRP (2), CO (1), DM (1) |
| Mathur and Vashishth [3] | SMS/TMSa | 5b | EP (2), PF (2), DM (1) |
| Roy Chowdhury et al. [14] | SMS | 14b | REC (4), PF (3), DM (2), BS (2) |
| Amin [15] | SMS | 4b, 3c | EP (1), PF (1), RES (1) |
| Present study | TMS | 5b | MO (1) |
n, number of cases; BS, unsightly scar, required revision; CO, complete ophthalmoplegia; DM; dental malocclusion; EP, epiphora; IMSR, infraorbital margin skin retraction; ISO, infraorbital serous collection; LRP, lateral rectus palsy; MZA, maxillo-zygomatic abscess; MO, maxillary osteomyelitis; PF, palatal fistula; REC, recurrence; RES, intracranial residue; SMS, subtotal maxillary swing (maxillary swing without orbital floor); TMS, total maxillary swing
aSwinging of orbital floor is not mentioned
bSurgery performed for juvenile nasal angiofibroma
cSurgery performed for nasopharyngeal carcinoma
Palatal Fistula
In the TMS approach described by Dubey and colleagues, an inverted U-shaped palatal incision was made and an occlusal wafer with a palatal splint was used to support the palatal mucoperiosteal flap [4, 16]. Mathur and Vashishth used a midline palatal incision while Amin and Roy Chowdhury et al. did not mention their approach to the palate [4, 14, 15]. A variable incidence of palatal fistula has been reported (Table 3).
After a palatal osteotomy, healing occurred at the palatal mucoperiosteum, palatal bone, and nasal floor mucoperiosteum. Incision lines through all three layers were kept in different parasagittal planes which complimented each other in healing (Fig. 1). The palatal mucoperiosteum was thinner medially with an increased possibility of tearing near the midline during elevation; extra caution was taken, using topical adrenaline to control bleeding from the flap, and cautery was not employed. A wafer or palatal splint was not required in any of our cases because we used a smaller J-shaped incision which healed without any complications.
Maxillary Necrosis
Maintaining the maxillofacial framework has been a concern in trans-maxillary approaches. Poor maxillary vascularity due to excessive cheek flap elevation or free reinsertion of maxillary bone has resulted in its avascular necrosis, which may ultimately lead to gross facial deformation. In 2001, Hao reported a 21.4% incidence of maxillary necrosis which required sequestrectomy [17]. Incidences of zygomatic osteomyelitis have also been reported in a study by Suárez et al. [18].
The technique described by Wei et al. and others recommended minimum flap elevation, but this limit was not defined [3, 4, 11, 14]. In this study, we used the infraorbital foramen as the lateral and inferior limit for flap elevation (Fig. 2). The alveolar mucosa was not elevated; however, in the single revision case, the alveolar mucosa was necessarily elevated for mini-plate fixation as the inferomedial maxillary wall was not present due to supposed removal in earlier surgery. The vascularity of the maxilla was preserved in all cases. In the revision case, the alveolar plate had to be removed 2 weeks after surgery as it was exposed because of a thin alveolar mucosal covering.
The TMS approach also almost completely preserves the anterior and posterior walls of the maxilla which, if carefully realigned, restores the anatomy of the maxilla and mid skull base. In all other trans-maxillary approaches such as midfacial degloving, lateral rhinotomy, and the endoscopic approach, significant removal of the anterior wall of the maxilla is required [3].
Jaw Malocclusion
Performing osteotomies leads to bone loss at each site which must be taken into consideration when the maxilla is repositioned. In the authors’ opinion, the sequential plating starting with mid-alveolar plating is important to ensure a well-aligned jawline. Maxillo-zygomatic plating as the second step and plating of the frontal process of the maxilla as the third step help to fix the last site with minimum force. This also prevents fracturing of the frontal process (which is a narrower process). While alveolar plating, screw-holes were drilled in line with prior markings after reconfirmation of proper alignment using the “three-person maneuver”. All patients had good occlusion of jaws (Fig. 2). In earlier described approaches, the dental malocclusion rate ranged between 6.25 and 20% [3, 4, 14–18], and screw holes were drilled before osteotomy.
Ophthalmoplegia
Temporary ophthalmoplegia (18.7–75%), both partial and complete, and lasting up to 6 months, has been reported as complications of swinging the orbital floor along with the maxilla and attributed to intraocular swelling [4, 19]. Dubey and Molumi [16] elevated the periosteum of the entire inferior orbital wall. We elevated the periosteum just sufficiently to expose the maxillo-ethmoid suture line medially and the inferior orbital fissure laterally, and never beyond the medial and lateral canthal ligaments, violation of which may lead to loss of ocular support. Gentle retraction of the eye globe avoided injury to it during periosteal elevation and maxillary repositioning. In the follow-up period, the ocular movements of all patients were normal.
Epiphora
After a sharp cut at the lower end of the lacrimal sac, a vertical slit was made on its medial aspect. The authors believe that this minimizes any possibility of a blockage in lacrimal flow due to postoperative adhesions or synechia. A lacrimal massage in the postoperative period complements that objective. Lacrimal flow remained patent in the postoperative period in all our patients.
The method of dealing with the lacrimal sac was not described by Amin, Mathur, and Vashishth or Roy Chowdhury et al. in their studies, though the first two groups have mentioned epiphora as a complication in their patients; however, Dubey et al. mentioned that they sectioned the sac at its lower end and there was no incidence of epiphora [3, 4, 14, 15].
Infraorbital Margin Skin Retraction and Serous Collection
An infra-ciliary incision made 5 mm from the ciliary margin and flap dissection superficial to the fat plane helped to avoid infraorbital serous collection and an unsightly scar. The maxillary periosteum was incised 5 mm inferior to the orbital rim as it helped in suturing and avoiding tears. Suturing the periosteum is an important step to prevent infraorbital margin skin retraction as also suggested by Dubey et al. [4]. These complications were not encountered in our patients.
In conclusion, in patients with extensive juvenile nasopharyngeal angiofibroma, total maxillary swing with the suggested modifications gives adequate exposure of critical areas such as the infratemporal fossa, sphenoid sinus, orbit and skull base, thus ensuring complete removal of disease. The mechanical and biological advantages of the modifications described by the authors help to minimize complications; however, the authors realize that a larger series with long-term follow-up is required to provide stronger evidence in support of the technique.
Author’s Contributions
AB: Conceptualization, Methodology, Original draft preparation. MP: Methodology, Review. MM: Methodology, Review, Editing. SV: Supervision, Review. AKT: Draft revision, study design. AS: Draft revision.
Funding
None.
Availability of Data and Material
Yes.
Compliance with Ethical Standards
Conflict of interest
The author(s) declare that they have no competing interests.
Consent to Participate
Informed consent was obtained from all individual participants/legal guardians included in the study.
Consent for Publication
Patients signed informed consent regarding publishing their data and photographs.
Ethics Approval
This retrospective review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Study has been approved by Institutional review board (Letter No. 3017396/2020/05/03/2020).
Ethical Standards
All of the procedures performed in this study were in accordance with the ethical standards of our institution and with the Helsinki Declaration of 1975, as revised in 2008. The protocol for the investigation has been approved by the Institutional Review Board, and written informed consent was obtained from each participant or each participant’s guardian.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abhishek Bhardwaj, Email: abhi04stanley@gmail.com.
Madhu Priya, Email: drpriyamadhu@gmail.com.
Manu Malhotra, Email: manumalhotrallrm@gmail.com.
Saurabh Varshney, Email: drsaurabh68@gmail.com.
Amit Kumar Tyagi, Email: ashuu.06@gmail.com.
Arpana Singh, Email: arpanaaa5687@gmail.com.
References
- 1.Stokes SM, Castle JT. Nasopharyngeal angiofibroma of the nasal cavity. Head Neck Pathol. 2010;4(3):210–213. doi: 10.1007/s12105-010-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batsakis JG. Tumors of the head and neck: clinical and pathological considerations. 2. Baltimore: Williams and Wilkins; 1979. pp. 296–300. [Google Scholar]
- 3.Mathur NN, Vashishth A. Extensive nasopharyngeal angiofibromas: the maxillary swing approach. Eur Arch Otorhinolaryngol. 2014;271(11):3035–3040. doi: 10.1007/s00405-013-2804-6. [DOI] [PubMed] [Google Scholar]
- 4.Dubey SP, Molumi CP, Apaio ML. Total maxillary swing approach to the skull base for advanced intracranial and extracranial angiofibroma. J Craniofac Surg. 2011;22:1671–1676. doi: 10.1097/SCS.0b013e31822f3c96. [DOI] [PubMed] [Google Scholar]
- 5.Kalra GS, Midya M, Bedi M. Access to the skull base—maxillary swing procedure—long term analysis. Ann Maxillofac Surg. 2018;8:86–90. doi: 10.4103/ams.ams_5_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bales C, Kotapka M, Loevner LA, Al-Rawi M, Weinstein G, Hurst R, et al. Craniofacial resection of advanced juvenile nasopharyngeal angiofibroma. Arch Otolaryngol Head Neck Surg. 2002;128:1071–1078. doi: 10.1001/archotol.128.9.1071. [DOI] [PubMed] [Google Scholar]
- 7.Cherekaev VA, Golbin DA, Kapitanov DN, Roginsky VV, Yakovlev SB, Arustamian SR. Advanced craniofacial juvenile nasopharyngeal angiofibroma. Description of surgical series, case report and review of literature. Acta Neurochir. 2011;153:499–508. doi: 10.1007/s00701-010-0922-0. [DOI] [PubMed] [Google Scholar]
- 8.Andrews JC, Fisch U, Valavanis A, Aeppli U, Makek MS. The surgical management of extensive nasopharyngeal angiofibromas with the infratemporal fossa approach. Laryngoscope. 1989;99:429–437. doi: 10.1288/00005537-198904000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Douglas R, Wormald PJ. Endoscopic surgery for juvenile nasopharyngeal angiofibroma: where are the limits. Curr Opin Otolaryngol Head Neck Surg. 2006;14:1–5. doi: 10.1097/01.moo.0000188859.91607.65. [DOI] [PubMed] [Google Scholar]
- 10.Hackman T, Synderman CH, Carrau R, Vescan A, Kassam A. Juvenile nasopharyngeal angiofibroma: the expanded endonasal approach. Am J Rhinol Allergy. 2009;23:95–99. doi: 10.2500/ajra.2009.23.3271. [DOI] [PubMed] [Google Scholar]
- 11.Wei WI, Lam KH, Sham JS. New approach to the nasopharynx: the maxillary swing approach. Head Neck. 1991;13(3):200–207. doi: 10.1002/hed.2880130306. [DOI] [PubMed] [Google Scholar]
- 12.Radkowski D, McGill T, Healy GB, Ohlms L, Jones DT. Angiofibroma. Changes in staging and treatment. Arch Otolaryngol Head Neck Surg. 1996;122(2):122–129. doi: 10.1001/archotol.1996.01890140012004. [DOI] [PubMed] [Google Scholar]
- 13.Herman P, Lot G, Chapot R, Salvan D, Huy P. Long-term follow up of juvenile nasopharyngeal angiofibromas: analysis of recurrence. Laryngoscope. 1999;109:140–147. doi: 10.1097/00005537-199901000-00027. [DOI] [PubMed] [Google Scholar]
- 14.Roy Chowdhury S, Rajkumar K, Deshmukh T. Complications of midface swing for management of juvenile nasopharyngeal angiofibroma. J Maxillofac Oral Surg. 2017;16(1):96–100. doi: 10.1007/s12663-016-0947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin AA. Maxillary swing approach for surgical resection of recurrent nasopharyngeal tumors. J Egypt Natl Canc Inst. 2007;19(3):219–223. [PubMed] [Google Scholar]
- 16.Dubey SP, Molumi CP. Critical look at the surgical approaches of nasopharyngeal angiofibroma excision and ‘‘total maxillary swing’’ as a possible alternative. Ann Otol Rhinol Laryngol. 2007;116:723–730. doi: 10.1177/000348940711601003. [DOI] [PubMed] [Google Scholar]
- 17.Hao SP. Facial translocation approach to the skull base: the viability of translocated facial bone graft. Otolaryngol Head Neck Surg. 2001;124:292–296. doi: 10.1067/mhn.2001.112308. [DOI] [PubMed] [Google Scholar]
- 18.Suárez C, Llorente JL, Muñoz C, García LA, Rodrigo JP. Facial translocation approach in the management of central skull base and infratemporal tumors. Laryngoscope. 2004;114:1047–1051. doi: 10.1097/00005537-200406000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Moreira-Gonzalez A, Pieper DR, Cambra JB, Simman R, Jackson IT. Skull base tumors: a comprehensive review of transfacial swing osteotomy approaches. Plast Reconstr Surg. 2005;115(3):711–720. doi: 10.1097/01.PRS.0000152437.71574.4F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Yes.