Abstract
Oroantral fistula (OAF) is an epithelized, pathological communication between the maxillary antrum and oral cavity. The most common etiological factor is molar or premolar tooth extraction. The bone between maxillary sinus floor and posterior teeth is thin and occasionally the root apices of the posterior teeth reach the maxillary sinus, predisposing them to the formation of OAF. Other causes are bacterial or fungal infections, osteomyelitis, granulomatous diseases, Paget’s disease, malignancy, maxillofacial trauma and iatrogenic. Small OAFs heal spontaneously but larger fistulas, persisting more than three weeks need to be closed. In repairing the persistent OAF, the maxillary sinus must be addressed. Maxillary sinusitis may lead to the failure of closure of the OAF. The basic modus operandi is clearance of disease from the sinus and covering the defect with a suitable graft. Various local and distant flaps are used to repair the OAF. We report three cases of OAF, managed by three different techniques. We also suggest a combined approach for large OAFs, repaired in 3 layers using septal cartilage, fat, and a buccal muco-periosteal advancement flap.
Keywords: Oroantral fistula, Maxillary sinus, Sinusitis, Buccal flap, Dental extraction
Introduction
Oroantral communication is defined as an abnormal connection between the oral and maxillary sinus cavity. When this communication gets epithelized to form a pathological track is known as Oroantral fistula (OAF). The bone between the oroantral partition varies from 1–7 mm but it has been demonstrated that the bone lamella between the maxillary posterior teeth and the maxillary sinus is occasionally 0.5 mm [1, 2].
The most common cause of OAF is upper premolar or molar extraction. Other causes include trauma causing fracture of the floor of maxillary sinus, cysts and tumors, post radiation osteomyelitis, dental implantation, root canal treatment, Paget’s disease and iatrogenic. Bacteria and fungus find a route from oral cavity to maxillary sinus through OAF. However, OAF and fungal sinusitis occurring simultaneously is uncommon [3–7]. All spontaneously non-healing OAFs should be repaired at the earliest. If repair is delayed more than 3 weeks, it may lead to complications [8]. OAF can be repaired by various nonsurgical and surgical methods. Nonsurgical methods include placing material into the defect to act as a mechanical barrier. Several surgical techniques have been used for OAF closure including local rotations flaps and the distant flaps. The combinations of various local flaps to strengthen the tissue closure are also advocated [9]. Gel foam, fat and platelet rich fibrin (PRF), bone and cartilage has been used to provide extra support to the flaps especially in large OAF. In complicated OAF with bacterial or fungal maxillary sinusitis, concomitant management of OAF and sinusitis will ensure complete resolution of the infection and may prevent recurrences and complications [10]. We present three cases of OAF, managed by three different, individualized approaches according to site and size of the fistula. The cause, presentation, prevention, and management are discussed.
Cases
Case 1
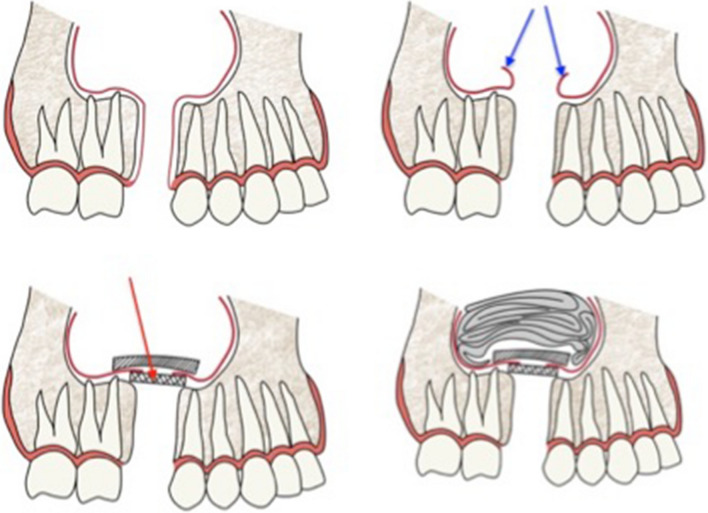
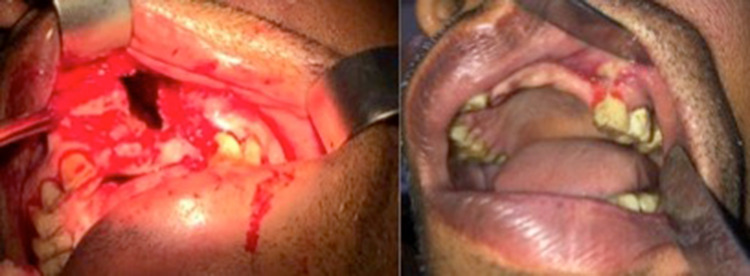
67 years male with history of left 2nd premolar and 1st molar tooth extraction three months previously, complained of regurgitation of liquids via the nose. Nasal cavities appeared normal on nasal endoscopy. An opening was identified in the region of alveolar tooth socket through which a probe could be passed up into the maxillary sinus. Computerized tomography (CT) scan showed a defect in the maxillary floor, and fluid collection with minimal mucosal hypertrophy in the maxillary sinus. Repeated antral washes through the oroantral tract were done three times till the return was clear, following which a buccal flap containing mucosa and underlying connective tissue was used to cover the defect (Fig. 1,2). The patient was followed up for 4 weeks. The wound had completely healed and the opening was closed.
Fig. 1.
Case 1- repair of OAF with a buccal flap
Fig. 2.
Diagrammatic representation of the buccal flap
Case 2
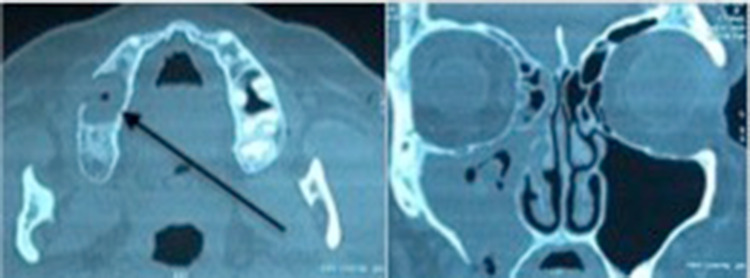
A 59 year old female patient presented with nasal regurgitation of fluids, pus discharge from right cheek area, foul smell and taste, nasal obstruction, nasal discharge, headache and postnasal drip for one and a half months. She had her second right maxillary molar extracted two months back and the opening had not closed since then. There was no pain, facial deformity, or paresthesia. Intraoral examination revealed an OAF at first molar region with purulent discharge. On CT scan, there was mucosal hypertrophy with retained secretions occupying the right maxillary sinus and a bony defect of 12.8 mm size in the floor of right maxillary antrum. Soft tissue density was identified in the ethmoids, sphenoid and frontal recess. (Fig. 3) Pseudomonas aeruginosa was identified on culture and potassium hydroxide (KOH) mount was negative. Punch biopsy from the margins of the fistulous track showed nonspecific chronic inflammatory reactions and no malignant cells or granuloma. Preoperatively, trans-alveolar antral irrigations were done thrice to clear the sinus of infection with povidone-iodine solution, 2% metronidazole and normal saline till the return was clear.
Fig. 3.
CT scan showing the defect in the alveolar process (Black arrow)
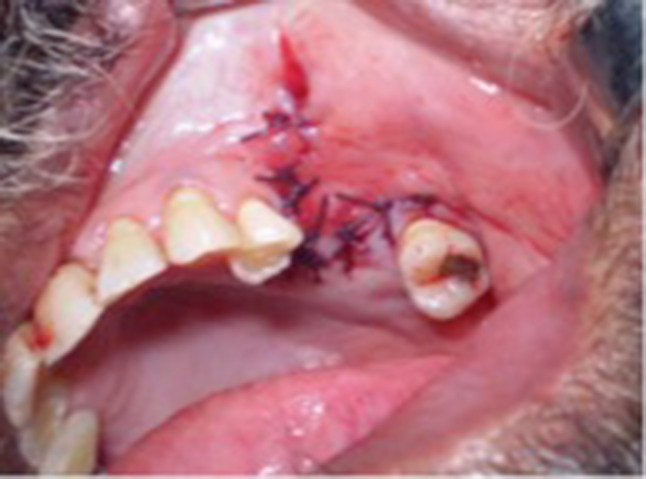
Under general anesthesia, the margins of the fistulous track were excised. Two divergent cuts were made from each end of fistula extending into the vestibule. Fungal debris was removed from the maxillary sinus. Endoscopic middle meatal antrostomy, fronto-ethmoidectomy and sphenoidotomy was done on right side. A 2 cm diameter piece of septal cartilage was harvested. The mucosa around the antral opening of OAF was elevated all around, through the sub labial opening. The cartilage was placed over the OAF opening and reflected sinus mucosa was reposed back over the cartilage graft. Maxillary sinus packed with medicated gauze. (Fig. 4) A broad based trapezoid mucoperiosteal flap was raised and buccal pad of fat harvested and stitched to the medial part of the flap as in case 1 and shown in Fig. 2. Microscopic examination of the sinus mucosa specimen revealed chronic inflammatory changes and hypertrophic stroma. At 2 weeks, the wound had healed completely.
Fig. 4.
Case 2: Diagrammatic representation of the 3 tier repair of the OAF
Case 3
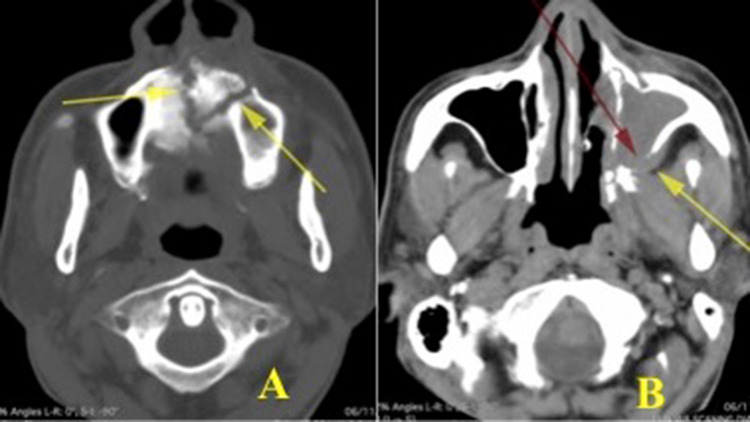
50 year-old diabetic male presented with history of left sided facial pain and swelling, regurgitation of fluids from the nose with left sided proptosis. History of tooth extraction left upper premolars was given. Examination revealed an OAF with granulation around the oral opening. Nasal endoscopy revealed necrotic granulomatous mass with loose mobile bony spicules in the middle meatus on left side and fragile inferior and middle turbinates. Tissue sent for analysis revealed invasive fungus of Rhizopus type where as the KOH mount from the oro-antral defect was negative for fungus. CT scan showed opacification of left maxillary, ethmoid and sphenoid sinus. Bony defects were noticed in the floor of right maxillary antrum along alveolar ridge and hard palate, in the roof of nasal cavity and lateral wall of sphenoid sinus and in the postero-lateral wall of maxillary sinus with extension of the soft tissue density towards infratemporal fossa. Lamina papyracea destruction with orbital invasion causing displacement of orbit was noted. There was a loose bony sequestrum at the antero- medial part of maxilla. (Fig. 5) Repeated antral wash out thrice with metronidazole and povidon-iodine 2.5% were done till the return was clear of infected material. Blood sugar was controlled in consultation with an endocrinologist. Combined endoscopic and Caldwell-luc approach was used. The debris, loose bony necrotic pieces, granulation and fungal collections were cleared. Orbital decompression along with clearance of orbital extension of disease done. There was no intracranial spread and no cerebrospinal fluid leak identified along the skull base bony dehiscence. A wide posterior based bucco-labial flap created around the OAF. Sequestrum along maxillary and nasal floor removed. The defect in posterolateral wall of maxillary sinus was explored and disease cleared. The OAF opening was covered with bucco-labial flap and stitched to the alveolar mucosa. The patient was started on anti-fungal ( Amphotericin B parenteral route followed by Voriconazole orally) along with antibiotic coverage. The general condition of the patient improved. At 2 weeks of follow-up, there was a small residual OAF which healed spontaneously and thus complete closure of the track (Fig. 6).
Fig. 5.
Axial CT scan cuts showing- A: Palatal bone erosion and formation of sequestrum. B: Defect in the postero-lateral aspect of the maxilla, erosion of pterygoid
Fig. 6.
Intraoperative defect and Post-operative healing and closure of OAF
Postoperatively, all patients were advised not to blow the nose, to avoid exploring the wound with tongue and sucking of fluids. The postoperative course was uneventful, and the fistula healed well within 2–4 weeks’ time.
Discussion
OAF is a pathological communication between the maxillary antrum and oral cavity. This communication may result from a tooth extraction or maxillofacial trauma, due to granuloma, cysts and tumors, osteomyelitis, fungal infection, post radiation or iatrogenic after a partial maxillectomy. Once a communication is established, the oral epithelium migrates to line the track. This epithelization takes place if the fistula persists for more than 48–72 h. Within the next few days, the bony defect gets organized to form a chronic fistulous track.
Predisposing factors may include excessive force applied during dental procedures, extraordinary over-pneumatized sinus, roots forming the floor of the sinus, extraordinary divergent roots, posterior maxillary pathology and preexisting bacterial or fungal maxillary sinusitis. The first premolar accounts for 5.3% of OAF, the second molar carried maximum incidence of 45%, followed by the third molars 30% and the first molars 27.2%. It is reported that about 2.2% of the first molars apices perforated the maxillary sinus floor, followed by the second molars [2]. The highest incidence is reported in the fourth and third decades of life and the lowest incidence in the second decade [11].
Depending on the location OAF can be classified as alveolo-sinusal, palatal-sinusal and vestibulo-sinusal [12]. Based on the time gap of perforation, they are described as oroantral communication (recent), oroantral fistula (lasting longer than 48 h) and chronic oroantral fistula (longstanding)[13]. Chronic OAF is lined by epithelium-ciliated columnar from antrum and squamous lining from oral mucosa.
Simultaneous OAF and fungal sinusitis are rare to occur. Cases of OAF with fungal sinusitis have been reported in literatiure [3–7]. A long-standing communication between the oral cavity and the maxillary sinus act as an access route for fungus to enter the sinus.
The classification of fungal sinusitis has evolved in the past two decades and this entity is comprised of two main type, invasive and non-invasive and five subtypes. Acute invasive fungal sinusitis, chronic invasive fungal sinusitis and chronic granulomatous invasive fungal sinusitis make up the invasive group. Non-invasive type include fungal rhinosinusitis and fungal ball (fungal mycetoma)[14]. The fungal ball, a relatively uncommon manifestation, is a tangled collection of fungal hyphae in the absence of allergic mucin. There is no fungal invasion of the sinus mucosa. It is usually caused by aspergillus fumigates but other fungi such as Pseudallescheria boydii and Alternaria can also lead to fungal ball formation [15]. A meta- analysis revealed that the common causes of odontogenic maxillary sinusitis were iatrogenic (55.97%), periodontitis (40.338%), and odontogenic cysts (6.66%)[16].
Rhizopus species is a filamentous fungus in Rhizopodaceae (Mucoraceae) family, in the order Mucorales. They are opportunistic agents of human zygomycosis (fungal infection) and sometime may prove to be fatal. It is commonly seen in diabetics and immunocompromised patients. Due to involvement of vessels, it causes decaying and extensive soft tissue destruction. Due to its aggressive nature, it is often fatal.
OAF patients, if left untreated, develop maxillary sinus disease in 50% within 48 h and 90% within two weeks [17]. Defects that are larger then 5 mm in diameter or those that present for more than 3 weeks fail to heal spontaneously and require surgical intervention by creating a barrier between oral mucosa and maxilla antral mucosa[8].
Since the Patients with OAF are most susceptible to sinus infections, therefore radiological investigations of the maxillary sinus are recommended. Computed tomography (CT) scan can assess the size of the fistula, and provide information about the health of the surrounding bone and any sinus mucosal disease [18]. A cyto-smear from the fistula opening has been advised [3] but in our case, the transoral-antral swab for potassium hydroxide (KOH) preparation was negative in case 2 and 3.
Management of established OAF can be divided into nonsurgical and surgical. Small OAF’s heal by themselves. If the fistula is less than or equal to 2 mm, noninvasive intervention as spontaneous closure by blood clot. Other methods include placing a material into the defect to act as a mechanical barrier without attempting flap closure. Synthetic graft materials, fibrin glue, xenograft, absorbable implants and acrylic splints have been used [19]. PRF has been tried in smaller fistulas [20, 21]. A well fitted soft occlusal splint has been used for hermetic seal of the opening [22]. When the opening is of moderate size between 2–6 mm, placement of gelfoam into the socket with figure of 8 suture. When the size of track is larger than 7 mm, it is advisable to repair with advancement flap.
The surgical closure of OAF is one of the more challenging problems in oral surgery. Long-term successful closure of OAF depends on the technique, the size, location of the defect, height of alveolar ridge, vestibular depth and the presence or absence of sinus disease. The nose and sinuses must be treated before the surgical closure of OAFs. The probable cause of OAF like acute or chronic sinusitis, maxillofacial trauma, granuloma, cysts and tumors, osteomyelitis, fungal infection, post radiation, perforation of Schneiderian membrane during implantation surgery, iatrogenic including partial maxillectomy and injudicious use of instruments must be looked after before repairing the track. Invasive fungal disease is managed by pre and postoperative administration of antifungal drugs like Amphotericin B, Voriconazole or posaconazole with wide surgical debridement or resection. It helps to stop the spread and eliminate the diseased process.
Several surgical techniques have been used for OAF closure, mainly local rotations (buccal and palatal flap), the distant flap (temporal muscle and tongue) and buccal pedicle fat pad graft [23]. The use of different additional materials with flap have been described by various researchers. Autogenous bone graft from the iliac crest, metal plates such as tantalum and gold and synthetic materials as hydroxyapatite, [11, 13, 24] autografts, [25] allografts (Dura mater, fascia lata), absorbable material (polyglactin, polydioxanon), [26, 27] titanium mesh with palatal flap [23] and soft tissue advancement or rotation flaps [27] have also been used.
The most widely employed flaps are: Vestibular flap, palatal flap and Buccal fat pad flap (BFP) or combination of the above. Local or free soft tissue flaps are used to close oroantral defects with or without autografts or alloplastic materials. Buccal fat pad is suitable for the closure of large posterior OAF [8, 12]. Buccal flap is best applied in the case of large fistulas located in the anterior region; the palatal flap is suitable to correct premolar defects. Problems that can be noted while harvesting BFP ranges from perforation to shrinkage of BFP [28].
A combined method to repair the OAC include a BPF covering the fistula with a layer of buccal mucosal flap over it.[29] Abdelhamid and Youssef advocate a combined palatal and buccal flap for large OAF[30]. Septal cartilage around the vomer bone piece plug and cementing has been described by Kersin and Soylu[31]. Septal cartilage grafting towards oral side as horizontal plate with buccal flap to close the OAF has been reported [32], [33]. For large fistulas, use of auricular cartilage has been recommended [34], [35]. The assessment criteria for the choice of technique are guided by: (a) size and type of defect, (b) presence or absence of sinus infection, (c) minimal donor site morbidity to the patient and (d) prosthetic considerations and experience of the operating surgeon [13]. The technique should have the added advantage for graft support and minimizing the chance of graft resorption, shrinkage or wound dehiscence [19]. Some technical points must be followed while repairing the OAF including tension free advancement of the flap, nasal irrigation, treatment of sinusitis, elimination of any diseased bone and complete excision of the fistulous tract [29]. Based on the multivariate analysis, the presence of maxillary sinusitis at the follow-up appointment was associated with a 15 times higher risk of recurrent OAF [36]. An alveoloplasty may be performed to reduce the bone height and ensure sealing of the communication with suturing of the gingival margins [18]. OAF turns chronic if the infection in the maxillary antrum is not eliminated. Regardless of the chosen technique, infection must be treated with adequate nasal drainage. This kind of therapy might require a Caldwell-luc procedure with nasal antrostomy or endoscopic sinus surgery to prevent recurrences and complications [10, 12, 37].
Our three-tier closure combined technique adds more stability and an additional support. In our case, three—tier closure was needed to provide support to the rarified alveolar bone and to maintain a good vascularization for the graft take up. Buccal fat harvested and stitched to the medial part of the buccal advancement flap in such a way that the fat fills up the fistula when the mobilized flap is stitched to the freshened margins of the fistula. Our presumption is that if the fat is left as a free graft in the fistulous track, it might slip into the maxillary sinus and if the fat is stitched to the freshened margins of the fistulous track, the buccal flap may be difficult to stitch to the margins to provide a hermetic closure.
The question arises, why OAF in our case was not healing? Whether it was due to pre-existing fungal sinusitis or fungal infection ingress from oral cavity through OAF. CT scan paranasal sinuses of our case demonstrated the diseased process in the maxillary, ethmoidal, frontal and sphenoid sinuses. It is possible that the patient was suffering from fungal sinusitis before the extraction, missed by the dental surgeon and thus prevented the healing of the wound after tooth extraction. The definite origin of the fungal infection of maxillary sinus could not be ascertained in this case.
Our technique of three-tier management with septal cartilage placement from the antral side after elevating the mucosal lining and fat stitched to the advanced buccal flap at the medial end. Septal cartilage is being used in otolaryngological practice with advantages over other materials in many reconstructive procedures. Autologous cartilage is biocompatible, non-absorbable, manipulatable, durable, non-carcinogenic, easily available, resistant to infection and cost-effective[38]. Incidence of failure is low since it does not require vascularization to integrate to the recipient site. It does not cause any visible defect at the donor site and no chances of transmission of Human immunodeficiency virus (HIV) like infections. Additionally, cartilage graft acts as a separating barrier between the sinus membrane and the oral mucosa, which helps in maintaining a successful healing. It guards the postoperative re-perforation; shrinkage of the flap and endoscopic sinus surgery helps to maintain good aeration and drainage of the sinus.
In such cases of OAF with maxillary sinusitis, surgical treatment involves accessing the maxillary sinus, it is advised that otorhinolaryngologists should undertake this procedure, since these professionals are able to deal with sequelae or complications of sinus surgery[39].
Whatsoever method is used to repair the bony defect, during surgical closure of the oroantral fistula, two basic principles must be observed. The first, that the sinus must be free from any infection and the second being that the suturing should be tension free with wide based soft tissue vascularized flap.
Conclusion
As almost all the posterior teeth extractions have the risk of OAC, the clinician must evaluate the patient thoroughly prior to extraction. OAF can lead to bacterial or fungal sinusitis if not treated early and sinusitis can lead to failure of spontaneous closure and surgical closure fistula. In non-healing OAF more than 21 days, must be investigated for sinus or comorbid conditions. The sinus must be addressed before or concomitantly with the repair of OAF procedure. Invasive fungal disease is aggressive in nature and hence early detection and prompt management is important for a good prognosis.
A combination of septal cartilage with fat and buccal advancement flap technique can be used in case of large OAF more than 10 mm in size and associated with maxillary sinus disease. It provides an additional tissue support in closure of the fistula. Proper pre and postoperative antibiotic and antifungal drugs must be administered. In cases of maxillary sinus diseases, a multidisciplinary approach with dental surgeon and nasal endoscopic surgeon should be carried out to avoid any failure. We advocate that the combined three-tier approach with fat, buccal flap and septal cartilage via Caldwell-luc approach and endoscopic sinus surgery is the best approach in large OAF and associated maxillary sinus disease. Thus assuring a complete closure of fistulous track and prevention of recurrence or failure. Since OAF with fungal maxillary sinus is rare, a differential must be kept in mind in non-healing OAF. Surgical closure of the oroantral defect, basic principles must be observed. The sinus must be free from any infection and the suturing must be tension free with wide based vascularized flap.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References:
- 1.Skoglund LA, Pedersen SS, Holst E. Surgical management of 85 perforations to the maxillary sinus. Int J Oral Surg. 1983;12(1):1–5. doi: 10.1016/s0300-9785(83)80073-8. [DOI] [PubMed] [Google Scholar]
- 2.Harrison DF. Oroantral fistula. Br J Clin Pract. 1961;15:169–174. [PubMed] [Google Scholar]
- 3.Jadhav KB, Mujib BA, Gupta N. Cytological approach for diagnosis of non-healing oroantral fistula associated with candidiasis. J Cytol. 2014;31(1):47–49. doi: 10.4103/0970-9371.130704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mane RS, Patil BC, Mohite AA. Rhinocerebral mucormycosis presenting as oroantral fistula. Clin Rhinol An Int J. 2012;5:135–137. doi: 10.5005/jp-journals-10013-1136. [DOI] [Google Scholar]
- 5.Yang SM, Park CH, Lee JH. A Case of Oroantral Fistula Complicating Fungal Sinusitis. J Rhinol. 2007;14(1):56–59. [Google Scholar]
- 6.GholinejadGhadi N, Seifi Z, Shokohi T, Aghili SR, Nikkhah M, VahediLarijani L, et al. Fulminant mucormycosis of maxillary sinuses after dental extraction in patients with uncontrolled diabetic: Two case reports. J Mycol Med. 2018;28(2):399–402. doi: 10.1016/j.mycmed.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Shams MG, Motamedi MH. Aspergilloma of the maxillary sinus complicating an oroantral fistula. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96(1):3–5. doi: 10.1016/s1079-2104(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 8.Nagaraj T, Sahu P, Saxena S, Nigam H, Biswas A. Oroantral fistula: a case report and review of literature. J Med Radiol Pathol Surg. 2018;5(4):8–10. doi: 10.15713/ins.jmrps.136. [DOI] [Google Scholar]
- 9.Awang MN. Closure of oroantral fistula. Int J Oral Maxillofac Surg. 1988;17(2):110–115. doi: 10.1016/s0901-5027(88)80162-0. [DOI] [PubMed] [Google Scholar]
- 10.Kodur S, Kiran HY, Shivakumar AM. Odontogenic fungal maxillary sinusitis: a case report of a displaced dental foreign body. Indian J Otolaryngol Head Neck Surg. 2019 doi: 10.1007/s12070-017-1167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guven O. A clinical study on oroantral fistulae. J Craniomaxillofac Surg. 1998;26(4):267–271. doi: 10.1016/s1010-5182(98)80024-3. [DOI] [PubMed] [Google Scholar]
- 12.Borgonovo AE, Berardinelli FV, Favale M, Maiorana C. Surgical options in oroantral fistula treatment. Open Dent J. 2012;6:94–98. doi: 10.2174/1874210601206010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parvini P, Obreja K, Begic A, Schwarz F, Becker J, Sader R, Salti L. Decision-making in closure of oroantral communication and fistula. Int J Implant Dent. 2019;5(1):13. doi: 10.1186/s40729-019-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakrabarti A, Denning DW, Ferguson BJ, Ponikau J, Walter Buzina B, Kita H, et al. Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope. 2019;119(9):1809–1818. doi: 10.1002/lary.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson BJ. Fungus balls of the paranasal sinuses. Otolaryngol Clin North Am. 2000;33(2):389–398. doi: 10.1016/s0030-6665(00)80013-4. [DOI] [PubMed] [Google Scholar]
- 16.Arias-Irimia O, Barona-Dorado C, Santos-Marino JA, Martínez-Rodriguez N, Martínez-González JM. Meta-analysis of the etiology of odontogenic maxillary sinusitis. Med Oral Patol Oral Cir Bucal. 2010;15(1):e70–e73. doi: 10.4317/medoral.15.e70. [DOI] [PubMed] [Google Scholar]
- 17.Dym H, Wolf JC. Oroantral communication. Oral and Maxillofac Surg Clin North Am. 2012;24(2):239–247. doi: 10.1016/j.coms.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 18.del Rey-Santamaria M, Valmaseda-Castellon E, Berini-Aytes L, Gay-Escoda C. Incidence of oral sinus communications in 389 upper third molar extraction. Med Oral Patol Oral Cir Bucal. 2006;11(4):E334–E338. [PubMed] [Google Scholar]
- 19.Sharma SP. Three-layered closure of persistent oroantral fistula using chin graft, buccal fat pad, and buccal advancement flap: a case report with review of literature. Case Rep Dent. 2019 doi: 10.1155/2019/8450749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal B, Pandey S, Roychoudhury A. New technique for closure of an oroantral fistula using platelet-rich fibrin. Br J Oral Maxillofac Surg. 2016;54(2):e31–e32. doi: 10.1016/j.bjoms.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Ghanaati S, Herrera-Vizcaino C, Al-Maawi S, Lorenz J, Miron RJ, Nelson K, et al. Fifteen years of platelet rich fibrin in Dentistry and Oromaxillofacial Surgery: How high is the level of scientific evidence? J Oral Implantol. 2018;44(6):471–492. doi: 10.1563/aaid-joi-D-17-00179. [DOI] [PubMed] [Google Scholar]
- 22.Kutuk N, Demirbas AE, Yilmaz Asan C, Bas B, Alkan A. Nonsurgical closure of oroantral communications using occlusal splints. Cumhuriyet Dent J. 2017;20(3):169. doi: 10.7126/cumudj.369104. [DOI] [Google Scholar]
- 23.de Souza LPH, SampaioDde O, de Souza Menezes BL, do Nascimento DF, Torres BC. Combined palatal flap and titanium mesh for oroantral fistula closure. Ann Maxillofac Surg. 2015;5(1):89–92. doi: 10.4103/2231-0746.161090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steiner M, Gould AR, Madion DC, Abraham MS, Loeser JG. Metal plates and foils for closure of oroantral fistulae. J Oral Maxillofac Surg. 2008;66:1551–1555. doi: 10.1016/j.joms.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 25.Er N, Tuncer HY, Karaca C, Copuroglu S. Treatment of oroantral fistulas using bony press-fit technique. J Oral Maxillofac Surg. 2013;71:659–666. doi: 10.1016/j.joms.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Visscher SH, van Minnen B, Bos RR. Closure of oroantral communications: a review of the literature. J Oral Maxillofac Surg. 2010;68:1384–1391. doi: 10.1016/j.joms.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 27.Buric N, Jovanovic G, Krasic D, Tijanic M, Buric M, Tarana S, et al. The use of absorbable polyglactin/polydioxanon implant (Ethisorb(R)) in non-surgical closure of oro-antral communication. J Craniomaxillofac Surg. 2012;40:71–77. doi: 10.1016/j.jcms.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Candamourty R, Jain MK, Sankar K, Ramesh Babu MR. Double-layered closure of oroantral fistula using buccal fat pad and buccal advancement flap. J Nat Sci Biol Med. 2012;2:203–205. doi: 10.4103/0976-9668.101930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batra H, Jindal G, Kaur S. Evaluation of different treatment modalities for closure of oro-antral communications and formulation of a rational approach. J Maxillofac Oral Surg. 2010;9(1):13–18. doi: 10.1007/s12663-010-0006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdelhamid AN, Youssef T. Large oroantral fistula repair using combined buccal and palatal flaps: a case series. Egyptian J Otolaryngol. 2018;34:48–54. doi: 10.4103/ejo.ejo_59_17. [DOI] [Google Scholar]
- 31.Kersin B, Soylu E. Closure of oroantral fistula with nasal septal cartilage, bone and bone cement: an alternative technique. Kulak Burun Bogaz Ihtis Derg. 2017;27(6):276–279. doi: 10.5606/kbbihtisas.2017.88709. [DOI] [Google Scholar]
- 32.Kansu L, Akman H, Uckan S. Closure of oroantral fistula with septal cartilage graft. Eur Arch Otolaryngol. 2010;267(11):1805–1806. doi: 10.1007/s00405-010-1340x. [DOI] [PubMed] [Google Scholar]
- 33.Saleh EA, Issa IA. Closure of large oroantral fistulas using septal cartilage. Otolaryngol Head Neck Surg. 2013;148(6):1048–1050. doi: 10.1177/0194599813482091. [DOI] [PubMed] [Google Scholar]
- 34.Ram H, Makadia H, Mehta G, Mohammad S, Singh RK, Singh N, et al. Use of auricular cartilage for closure of oroantral fistula: a prospective clinical study. J Maxillofac Oral Surg. 2016;15:293–299. doi: 10.1007/s12663-015-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isler SC, Demircan S, Cansiz E. Closure of oroantral fistula using auricular cartilage: a new method to repair an oroantral fistula. Br J Oral Maxillofac Surg. 2011;49(8):e86–e87. doi: 10.1016/j.bjoms.2011.03.262. [DOI] [PubMed] [Google Scholar]
- 36.Visscher SH, vanRoon MR, Sluiter WJ, van Minnen B, Bos RR. Retrospective study on the treatment outcome of surgical closure of oroantral communications. J Oral Maxillofac Surg. 2011;69:2956–2961. doi: 10.1016/j.joms.2011.02.102. [DOI] [PubMed] [Google Scholar]
- 37.Kamadjaja DB. The role of proper treatment of maxillary sinusitis in the healing of persistent oroantral fistula. Dent J (MajalahKedokteran Gigi) 2008;41(3):128–131. doi: 10.20473/j.djmkg.v41.i3. [DOI] [Google Scholar]
- 38.Canciz E, Gultekin A, Koltuk M, Cakarer S. Treatment of Oral fistulas. In: Motamedi MHK, editor. A textbook of advanced Oral and maxillofacial surgery. 3. InTech Open; 2016. [Google Scholar]
- 39.Meirelles RC, Neves-Pinto RM. Oroantral fistula and genian mucosal flap: a review of 25 cases. Braz J Otolaryngol. 2008;74(1):85–90. doi: 10.1016/s1808-8694(15)30756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]