Abstract
Ethmoidal infundibulum (EI) is an important part of the osteomeatal complex, which provides the main pathway for the maxillary sinus drainage. This study aimed to compare the length, width, and angulation of EI in patients with/without maxillary sinusitis using computed tomography (CT). This cross-sectional study evaluated 818 spiral CT scans of patients with/without maxillary sinusitis (n = 409 maxillary sinuses in each group) classified according to the clinical symptoms and the Lund-Mackay score for mucosal thickening. The degree of mucosal thickening (on axial and coronal sections), anatomical variations namely concha bullosa (CB), ethmoidal bulla (EB), and Haller cells (HCs), the form of EI (on coronal sections), the length, width and angulation of EI, and degree of nasal septal deviation (on coronal sections) were all evaluated. Data were analyzed by parametric and non-parametric tests (alpha = 0.05). The frequency of EB (P < 0.001), EI form (P < 0.001) and HC (P = 0.011), and the length and width of EI (P < 0.001) were significantly different in patients with and without maxillary sinusitis. The length and width of EI were significantly correlated with the degree of mucosal thickening (P < 0.01). The frequency of CB (P = 0.002), EB (P < 0.001), and HC (P = 0.002), and the EI form (P < 0.001) were significantly different in groups with different degrees of mucosal thickening. EI was wider and shorter in patients with maxillary sinusitis. By an increase in mucosal thickness, the length of EI decreased while its width increased. Also, the frequency of EB and HC, and the EI form were significantly different in the two groups.
Keywords: Anatomical variations, Computed tomography, Ethmoid bone, Maxillary sinusitis
Introduction
Chronic sinusitis is a common inflammatory condition characterized by chronic inflammation of the sinus lining due to various reasons. It is often associated with nasal involvement. Thus, the term rhinosinusitis is often used to describe inflammation of the lining mucosa of the nasal cavity and paranasal sinuses [1].
The maxillary sinus is the first sinus that develops, and is more commonly affected by sinusitis than other paranasal sinuses [2, 3]. The secretions of the maxillary sinus are drained into the middle meatus through the osteomeatal complex (OMC) [4]. Obstruction of this narrow path is responsible for development of chronic sinusitis [5]. Several anatomical variations can affect the function of OMC, and play a role in its obstruction.
Computed tomography (CT) is currently the diagnostic modality of choice for assessment of the OMC due to its ability in optimal visualization of the hard and soft tissues. CT is used as a diagnostic tool for detection of anatomical variations and pathological mucosal anomalies and also for preoperative assessment of the OMC to serve as a guide map for surgeons [6].
The Lund-Mackay score is a simple scoring system for grading of the severity of chronic sinusitis according to the CT findings [7]. In this system, the maxillary, frontal, sphenoid, and anterior and posterior ethmoid sinuses are scored 0 if clear, scored 1 in case of partial opacification, and scored 2 in case of complete opacification. The OMC is scored 0 if open and scored 2 if obstructed. The total score can range from 0 to 24 (0–12 at each side) [8].
Concha bullosa (CB), Haller cells (HCs), ethmoidal bulla (EB), nasal septal deviation (NSD), and different forms of ethmoidal infundibulum (EI) are among the anatomical variations of this region, which can affect the OMC and EI, and impair the process of drainage. This issue has been the topic of many investigations, and controversial results have been reported regarding the association of anatomical variations and sinusitis [9–13].
EI is an important part of the OMC, and its dimensions i.e. its length and width can affect the maxillary sinus drainage. Limited studies with controversial results are available in this respect. For instance, de Carvalho et al. [14] and Paşaoğlu et al. [10] found no correlation between the length of EI and sinus drainage while Thorp et al. [15] reported shorter length of EI in patients with sinusitis. On the other hand, therapeutic interventions for sinusitis target the narrowing of this area, and aim to widen the drainage path. However, some other studies have reported increased width of EI in patients with sinusitis [10, 15]. Aside from the controversial results on this topic, it is noteworthy that variations of the uncinate process (UP) such as its lateral or medial deviation affect the dimensions of EI and subsequently the quality of drainage [16, 17]. However, this issue has not been addressed in previous studies.
Considering all the above and the existing controversies in the literature, the relationship of anatomical variations and dimensions of EI with chronic sinusitis has not been well elucidated. On the other hand, search of the literature by the authors yielded no study with the abovementioned conditions on the correlation of EI dimensions and chronic sinusitis. Thus, this study was designed to assess the relationship of EI with chronic sinusitis of the maxillary sinuses by eliminating the effect of some anatomical variations such as the medial or lateral deviation of UP, uncinate bulla, curved UP, paradoxical middle concha and bifid turbinate, and taking into account limited anatomical variations such as CB, EB, HC, NSD, and EI form (determined based on the superior attachments of the UP). Also, the Lund-Mackay score was considered as an indicator of sinusitis to compare the width, length and angulation of EI in patients with and without maxillary sinusitis.
Methods
This cross-sectional study evaluated 818 spiral CT scans of patients in two groups with and without maxillary sinusitis (n = 409 maxillary sinuses in each group) during 2015–2019. The study was conducted at the Oral and Maxillofacial Radiology Department of School of Dentistry, Guilan University of Medical Sciences, and approved by the ethics committee of this university (approval ID: IR.GUMS.REC.1398.479). Sample size was calculated to be 409 sinuses in each group according to a study by de Carvalho et al. [14] assuming alpha = 0.05, 95% confidence interval, and 80% study power.
Patients with a history of chronic sinusitis and symptoms such as nasal congestion, nasal discharge, decreased sense of smell, and mid-facial pain who acquired a Lund-Mackay score of ≥ 6 at each side according to their CT scan [18] with definite involvement of the maxillary sinus were assigned to the maxillary sinusitis group. If the acquired score in one side was < 6, the respective side would be excluded from the study.
Individuals who obtained CT scans for other purposes, such as evaluation of the lacrimal duct, and did not have the symptoms of sinusitis or had isolated involvement of paranasal sinuses other than the maxillary sinus on their CT scans (Lund-Mackay score < 3) [18] were assigned to the healthy control group without maxillary sinusitis. All patients were over 18 years of age, and had high-quality CT scans in coronal and axial sections. The exclusion criteria were (i) images showing anatomical variations of the UP such as uncinate bulla, its medial or lateral deviation, or curved UP, (ii) variations of the middle turbinate including paradoxical middle turbinate and bifid turbinate, (iii) history of sinus surgery, malignancy, paranasal masses, or trauma, (iv) sinusitis with odontogenic origin, and (v) poor-quality or low-resolution images.
The spiral CT scans had been obtained by Philips (PA, USA), Siemens (PA, USA), or General Electric (USA) 8- and 16-slice CT scanners with the exposure settings of 110 or 120 kVp, and 100 or 147 mAs, with 1–3 mm slice thickness. The CT images in the coronal section were evaluated in PACS Viewer in terms of NSD, OMC (length, width and angulation of EI), presence/absence of extensive and bulbous-type CB in the middle turbinate, HC, EB, and form of EI as anatomical variations of this region.
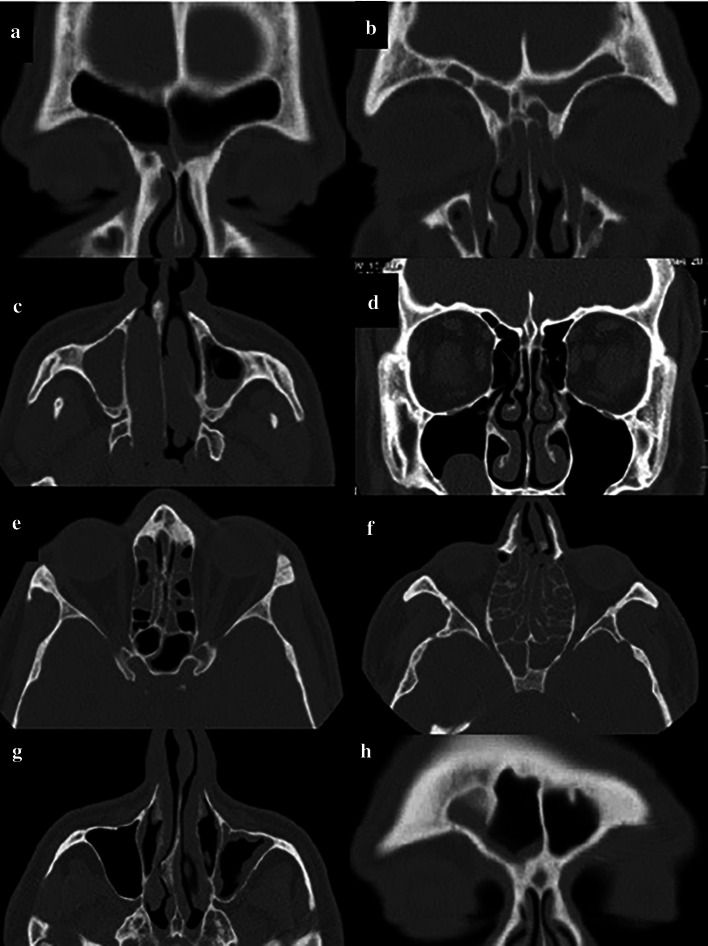
According to the Lund-Mackay scoring system and using the coronal and axial sections, each sinus (frontal, anterior ethmoid, posterior ethmoid, maxillary, and sphenoid) was allocated a score from 0 to 2 (0: no abnormality, 1: partial opacification, 2: complete opacification). Also, the OMC was allocated a score of 0 (not obstructed) or 2 (obstructed). According to this scoring system, each side with a score ≥ 6 with maxillary sinus involvement was assigned to the maxillary sinusitis group while the sides with a score < 3 and absence of maxillary sinusitis were assigned to the healthy control group without maxillary sinusitis (Fig. 1).
Fig. 1.
Scoring of CT scans according to the Lund-Mackay scoring system. a Coronal section of the right and left frontal sinus with score 1; b Coronal section of the right and left frontal sinus with score 2; c Axial section of the right maxillary sinus with score 2 and left maxillary sinus with score 1, d Coronal section of the left OMC with score 2 and right OMC with score 0; e Axial section of the anterior and posterior ethmoid and sphenoid sinuses of each side with score 1; f Axial section of the anterior and posterior ethmoid and sphenoid sinuses of each side with score 2; g Axial section of the maxillary sinus of the right side with score 0 and left side with score 1; h Coronal section of the frontal sinus with score 1 in the right side and score 0 in the left side
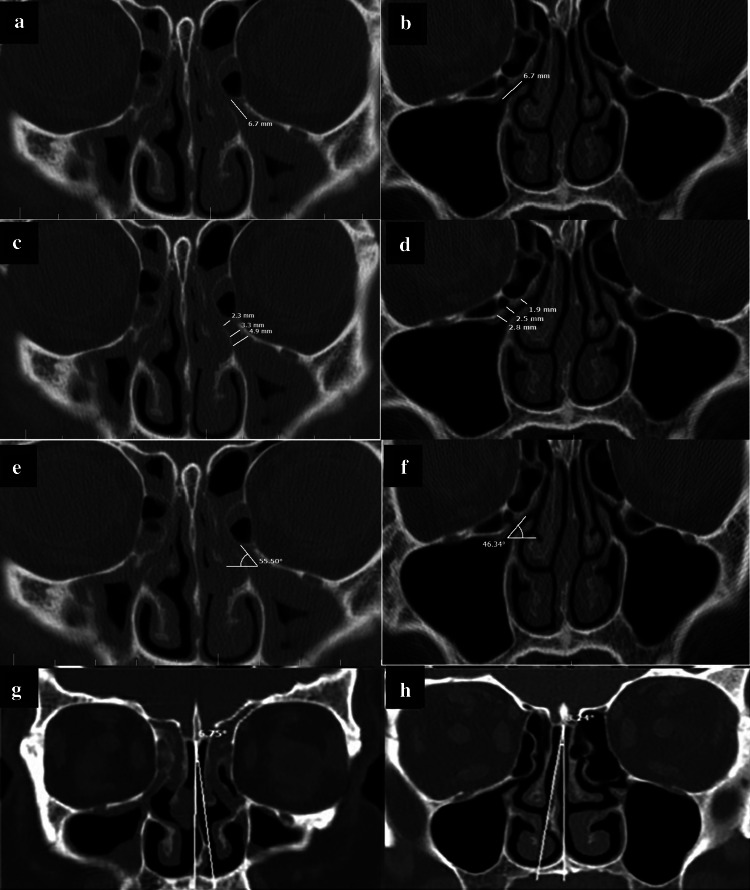
To measure the width, length and angulation of EI and NSD, the ruler and angle features of the PACS software were used on coronal sections (Fig. 2a–h).
Fig. 2.
Measuring the length, width and angle of EI and NSD in the two groups with/without maxillary sinusitis; a, b Measuring the length; c, d Measuring the width; e, f Measuring the angle; g, h Measuring the degree of NSD
Length of EI
To measure the length of EI, the distance between the initiation of ostium and the free end of UP was measured and reported in millimeters (Fig. 2a–b).
Width of EI
To measure the width of EI, three areas were selected along its path (initiation, center, and end), and the distance between the UP and the medial or inferomedial wall of the orbit (or HC if present) was measured in millimeters. The mean of the three values was calculated to determine the final mean width of the EI (Fig. 2c, d).
Angle of EI
The angle between the line passing through the center of EI and the horizontal line (which was parallel to the line tangent to the most inferior point of the maxillary sinus floor) was considered as the angle of EI (Fig. 2e, f).
NSD
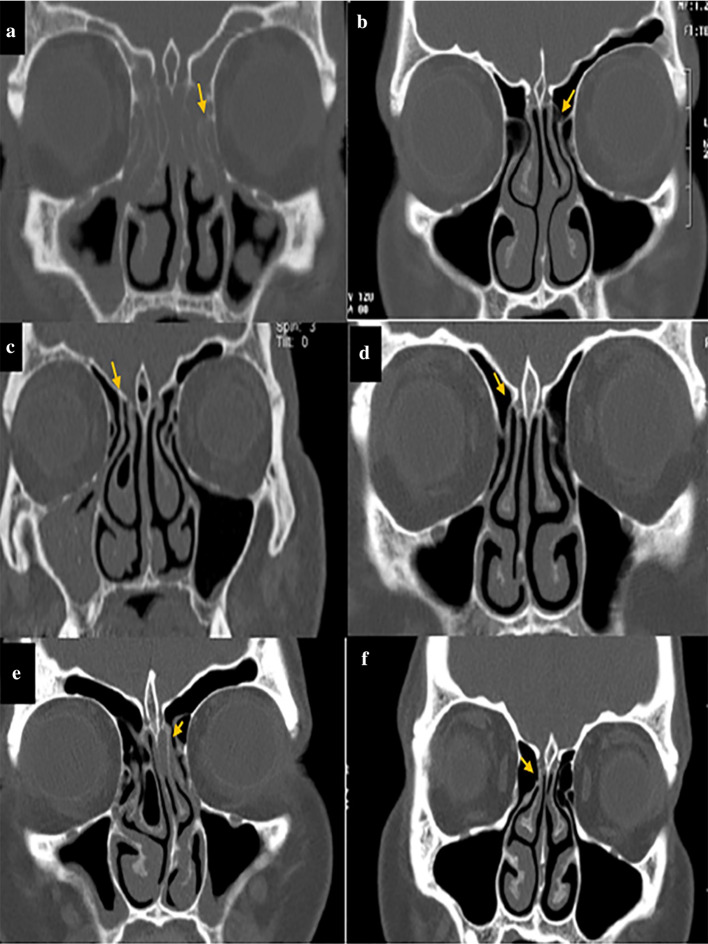
The angle formed between the line passing through the crista galli to the anterior nasal spine and the most prominent point of the deviated septum was considered as the angle of NSD (Fig. 2g, h). Also, according to a study by Orlandi et al. [19] who reported 10° septal deviation as the cutoff point, the obtained NSD values were categorized into two groups of NSD < 10°, and NSD ≥ 10°, and the two groups were compared in this respect. Moreover, the form of EI was divided into three types based on the attachment of the UP to lamina papyracea (type A), cribriform plate (base of skull) (type B), and middle concha (type C) as shown in Fig. 3. All CT scans were evaluated by a senior post-graduate student of oral and maxillofacial radiology under the supervision of an oral and maxillofacial radiologist.
Fig. 3.
Different forms of EI in patients with/without maxillary sinusitis; a, b Attachment to lamina papyracea (arrow); c, d: Attachment to the cribriform plate (base of skull) (arrow), e, f Attachment to middle concha (arrow)
Statistical analysis
Data were analyzed using SPSS version 22 (SPSS Inc., IL, USA). The Mann–Whitney test, independent t-test, and Chi-square test were used to compare the length, width and angle of EI, NSD, form of EI, and anatomical variations between the two groups with/without maxillary sinusitis. Comparison of length, width and angle of EI, NSD, EI form, and anatomical variations based on the degree of mucosal thickening of the maxillary sinus in the two groups with/without maxillary sinusitis was performed using the Kruskal–Wallis test followed by pairwise comparisons with the Mann–Whitney test with Bonferroni adjustment, as well as one-way ANOVA, Welch’s ANOVA, Chi-square test, and Fisher’s exact test. P < 0.05 was considered statistically significant.
Results
A total of 818 CT scans of patients with/without maxillary sinusitis were evaluated. The mean age of participants was 40.04 ± 15.10 years (range 18–86 years). There were 177 (43.3%) females and 232 (56.7%) males in the maxillary sinusitis group, and 247 (60.4%) females and 162 (39.6%) males in the healthy control group.
Table 1 presents the frequency of CB, EB, EI forms, HC, and NSD in the two groups with/without maxillary sinusitis, separately in males and females. Of anatomical variations, the frequency of EB and HC, and the EI forms were significantly different in the two groups with/without maxillary sinusitis. Also, the frequency of EB and EI forms in females, and the frequency of CB, EB, HC, and EI forms in males were significantly different in the two groups with/without maxillary sinusitis.
Table 1.
Frequency of CB, EB, EI forms, HC, and NSD in males and females of the two groups with/without maxillary sinusitis (n = 409 maxillary sinuses in each group)
| Goups | CB | EB | EI form | HC | NSD | NSD side | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present | Absent | Present | Absent | A | B | C | NA | Present | Absent | < 10 | ≥ 10 | Towards the involved sinus |
Opposite the involved sinus |
|
|
Normal N = 409 |
143 (52.2) |
266 (48.9) |
390 (55.9) |
19 (15.8) |
309 (54.9) |
21 (87.5) |
42 (73.7) |
37 (21.3) |
125 (57.3) |
284 (47.3) |
275 (50.2) |
134 (49.6) |
204 (50.2) |
205 (49.8) |
|
Sinusities N = 409 |
131 (47.8) |
278 (51.1) |
308 (44.1) |
101 (84.2) |
254 (45.1) |
3 (12.5) |
15 (26.3) |
137 (78.7) |
93 (42.7) |
316 (52.7) |
273 (49.8) |
136 (50.4) |
202 (49.8) |
207 (50.2) |
| P value | 0.374* | < 0.001* | < 0.001* | 0.011* | 0.882* | 0.889* | ||||||||
| Female | ||||||||||||||
|
Normal N = 247 |
78 (55.3) |
169 (59.7) |
236 (63.8) |
11 (20.4) |
183 (62.0) |
16 (100.0) |
25 (71.4) |
23 (29.5) |
64 (58.7) |
183 (58.7) |
173 (58.8) |
74 (56.9) |
122 (58.9) |
125 (57.6) |
|
Sinusities N = 162 |
63 (44.7) |
114 (40.3) |
134 (36.2) |
43 (79.6) |
112 (38.0) |
0 |
10 (28.6) |
55 (7.05) |
45 (41.3) |
132 (41.9) |
121 (41.2) |
56 (43.1) |
85 (41.1) |
92 (42.4) |
| P value | 0.387* | < 0.001* | < 0.001 | 0.910* | 0.712* | 0.781* | ||||||||
| Male | ||||||||||||||
|
Normal N = 162 |
65 (48.9) |
97 (37.2) |
154 (47.0) |
8 (12.1) |
126 (47.0) |
5 (62.5) |
17 (77.3) |
14 (14.6) |
61 (56.0) |
101 (35.4) |
102 (40.2) |
60 (42.9) |
82 (41.2) |
80 (41.0) |
|
Sinusities N = 232 |
68 (51.1) |
164 (62.8) |
174 (53.0) |
58 (87.9) |
142 (53.0) |
3 (37.5) |
5 (22.7) |
82 (85.4) |
48 (44.0) |
184 (64.6) |
152 (59.8) |
80 (57.1) |
117 (58.8) |
115 (59.0) |
| P value | 0.026* | < 0.001* | < 0.001* | < 0.001* | 0.602* | 0.971* | ||||||||
Significant level is P < 0.05
*Chi square test, **Fisher’s Exact test
Data regarding the degree of NSD had non-normal distribution. Thus, the Mann–Whitney test was applied, which showed no significant difference in this respect between the two groups (P = 0.537) in males (P = 0.235) or in females (P = 0.824).
Table 2 presents the length, width and angulation of EI in males and females in the two groups with/without maxillary sinusitis. Patients with maxillary sinusitis had significantly shorter and wider EI. Similar results were obtained in males and females.
Table 2.
Length, width and angulation of EI in the two groups with/without maxillary sinusitis separately in males and females
| Groups | Length (mm) Median interquartile range |
Width (mm) Median interquartile range |
Mean angle (degreses) |
|---|---|---|---|
|
Normal Sinusities |
6.30 (5.40–7.20) 5.50 (4.75–6.50) |
2.06 (1.83–2.40) 3.00 (2.44–3.54) |
48.17 ± 8.87 47.44 ± 7.85 |
| P value | < 0.001* | < 0.001* | 0.210** |
| Female | |||
|
Normal Sinusities |
6.30 (5.50–7.30) 5.50 (4.75–6.40) |
2.06 (1.83–2.43) 2.86 (2.36–3.46) |
47.71 ± 8.66 46.61 ± 7.47 |
| P value | < 0.001* | < 0.001* | 0.174** |
| Male | |||
|
Normal Sinusities |
6.20 (5.40–7.20) 5.50 (4.72–6.57) |
2.13 (1.80–2.40) 3.06 (2.46–3.70) |
48.88 ± 9.16 48.07 ± 8.08 |
| P value | < 0.001* | < 0.001* | < 0.365** |
The length and width values are the median values (first quartile-third quartile)
Significant level is P < 0.05
*Mann Whitney test, **Independent t-test
Table 3 presents the length, width, and angulation of EI based on the maxillary sinus mucosal thickness (Lund-Mackay score). The length and width of EI were significantly different in the two groups. The EI became shorter and wider as the mucosal thickness increased (higher Lund-Mackay score). Similar results were obtained in males and females.
Table 3.
Mean length, width, and angulation of EI based on the maxillary sinus mucosal grading (Lund-Mackay score)
| Groups | Length (mm) Median interquartile range |
Width (mm) Median interquartile range |
Mean angle (degree) |
|---|---|---|---|
|
Normal N = 409 |
6.30 (5.40–7.20)a# | 2.06 (1.83–2.40)a | 48.17 ± 8.87 |
|
Partial opacification N = 48 |
5.60 (4.80–6.55)b | 2.93 (2.40–3.46)b | 47.27 ± 7.77 |
|
Complete opacification N = 48 |
4.85 (4.20–5.57)c | 3.60 (2.76–4.25)c | 48.71 ± 8.39 |
| P value | < 0.001* | < 0.001* | 0.236** |
| Female | |||
|
Normal N = 247 |
6.30 (5.50–7.30)a | 2.06 (1.83–2.43)a | 47.71 ± 8.66 |
|
Partial opacification N = 161 |
5.60 (4.95–6.45)b | 2.86 (2.36–3.40)b | 46.58 ± 7.56 |
|
Complete opacification N = 16 |
4.60 (4.22–5.45)c | 3.53 (2.22–4.26)b | 46.92 ± 6.81 |
| P value | < 0.001* | < 0.001* | 0.392** |
| Male | |||
|
Normal N = 162 |
6.20 (5.40–7.20)a | 2.13 (1.80–2.40)a | 48.88 ± 9.16 |
|
Partial opacification N = 200 |
5.60 (4.80–6.70)a | 3.00 (2.43–3.65)b | 47.83 ± 7.91 |
|
Complete opacification N = 32 |
4.90 (4.12–5.67)c | 3.60 (2.98–4.14)c | 49.60 ± 9.05 |
| P value | < 0.001* | < 0.001* | 0.368** |
The length and width values are the median values (first quartile-third quartile)
Significant level is P < 0.05
*#Different lowercase letters (a, b, c) in the same column indicate significant differences (P < 0.05), and similar lowercase letters indicate no significant difference (P > 0.05) among groups (Bonferroni adjustment)
Kruskal Wallis test, **Welch’s ANOVA, ***One Way ANOVA
Table 4 compares the frequency of anatomical variations based on the maxillary sinus mucosal thickness. Significant differences were noted in the frequency of CB, EB, EI forms, and HC between different grades of maxillary mucosal thickness. Similar results were obtained in males and females. However, in females, the frequency of CB, EB and EI forms was significantly different among different mucosal thicknesses.
Table 4.
Frequency of anatomical variations based on the maxillary sinus mucosal grading (n = 409 maxillary sinuses in each group)
| Groups | CB | EB | EI form | HC | NSD | NSD side | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present | Absent | Present | Absent | A | B | C | N.A | Present | Absent | < 10 | ≥ 10 | Towards the involved sinus | Opposite to involved sinus | |
| Normal |
143 (35.0) |
266 (65.0) |
390 (95.4) |
19 (4.6) |
309 (75.6) |
21 (5.1) |
42 (10.3) |
37 (9.0) |
125 (30.6) |
284 (69.4) |
275 (67.2) |
134 (32.8) |
204 (49.9) |
205 (50.1) |
| Partial opacification |
126 (34.9) |
235 (65.1) |
285 (78.9) |
76 (21.1) |
241 (66.8) |
3 (0.8) |
14 (3.9) |
103 (28.5) |
89 (24.7) |
272 (75.3) |
238 (65.9) |
123 (34.1) |
176 (48.8) |
185 |
| (51.2) | ||||||||||||||
| Total opacification |
5 (10.4) |
43 (89.6) |
23 (47.9) |
25 (52.1) |
13 (27.1) |
0 |
1 (2.1) |
34 (70.8) |
4 (8.3) |
44 (91.7) |
35 (72.9) |
13 (27.1) |
26 (54.2) |
22 (45.8) |
| P value | 0.002* | < 0.001* | < 0.001* | 0.002* | 0.619* | 0.773* | ||||||||
| Female | ||||||||||||||
| Normal |
78 (31.6) |
169 (68.4) |
236 (95.5) |
11 (4.5) |
183 (74.1) |
16 (6.5) |
25 (10.1) |
23 (9.3) |
64 (25.9) |
183 (74.1) |
173 (70.0) |
74 (30.0) |
122 (49.4) |
125 (50.6) |
| Partial opacification |
62 (38.5) |
99 (61.5) |
124 (77.0) |
37 (23.0) |
109 (67.7) |
0 |
10 (6.2) |
42 (26.1) |
42 (26.1) |
119 (73.9) |
107 (66.5) |
54 (33.5) |
76 (47.2) |
85 (52.8) |
| Total opacification |
1 (6.3) |
15 (93.8) |
10 (62.5) |
6 (37.5) |
3 (18.8) |
0 | 0 |
13 (81.3) |
3 (18.8) |
13 (81.3) |
14 (87.5) |
2 (12.5) |
9 (56.3) |
7 (43.8) |
| P value | 0.023* | < 0.001* | < 0.001** | 0.809* | 0.205* | 0.758* | ||||||||
| Male | ||||||||||||||
| Normal |
65 (40.1) |
97 (59.9) |
154 (95.1) |
8 (4.9) |
126 (77.8) |
5 (3.1) |
17 (10.5) |
14 (8.6) |
61 (37.7) |
101 (62.3) |
102 (63.0) |
60 (37.0) |
82 (50.6) |
80 (49.4) |
| Partial opacification |
64 (32.0) |
136 (68.0) |
161 (80.5) |
39 (19.5) |
132 (66.0) |
3 (1.5) |
4 (2.0) |
61 (30.5) |
47 (23.5) |
153 (76.5) |
131 (65.5) |
69 (34.5) |
100 (50.0) |
100 (50.0) |
| Total opacification |
4 (12.5) |
28 (87.5) |
13 (40.6) |
19 (59.4) |
10 (31.3) |
0 |
1 (3.1) |
21 (65.5) |
1 (3.1) |
31 (96.9) |
21 (65.6) |
11 (34.4) |
17 (53.1) |
15 (46.9) |
| P value | 0.008* | < 0.001* | < 0.001** | < 0.001* | 0.873* | 0.947* | ||||||||
Significant level is P < 0.05
*Chi square test, **Fisher’s Exact test
The degree of NSD was not significantly different in different mucosal thicknesses (median value of 8.23° in the normal group, 8.12° in partial opacification group, and 7.52° in complete opacification group; P = 0.440). The difference in degree of NSD based on the mucosal thickness was not significant in males (P = 0.493) or females (P = 0.168).
Discussion
This study compared the length, width, and angulation of EI in patients with/without maxillary sinusitis using spiral CT. The results showed that the frequency of CB was not significantly different between the two groups; this finding was in line with some [8, 10, 13, 20–22] and in contrast to some other [23–25] studies. The degree of NSD, its direction of deviation, and its different classes were compared between the two groups according to previous studies [19, 20, 26]. However, no significant difference was noted between the two groups in this respect, which was similar to some [8, 10, 13, 20, 22, 24, 27] and different from some other studies [21, 23]. Yasan et al. [28] assessed the correlation of NSD with chronic sinusitis after eliminating the effect of other anatomical variations. They only observed such a correlation in severe cases of NSD (NSD > 21°). Mohebbi et al. [29] did not report such a correlation, even for cases with NSD > 20°. In our study, all cases had NSD < 21°.
Direction of deviation was not significantly different between the two groups with/without maxillary sinusitis. Karki et al. [24] found no correlation between deviated septum and maxillary sinusitis of the same side. However, Fadda et al. [25] found a correlation between NSD in the left side and maxillary sinusitis of the same side. Kucybała et al. [21] reported higher prevalence of bilateral maxillary sinusitis in NSD patients. In comparison of different subgroups of NSD between patients with/without maxillary sinusitis, no significant difference was noted between the two groups of NSD < 10° and NSD ≥ 10°.
Absence of a significant difference in CB and NSD between the two groups with/without maxillary sinusitis can be due to the fact that a nasal air channel remained between the medial surface of the concha and the adjacent nasal septum surface in the majority of patients.
Regarding the correlation of HC and maxillary sinusitis, some previous studies reported a significant correlation between HC and maxillary sinusitis [11, 12] while some others did not [10, 22]. Also, a previous study reported a relationship between the presence of EB and sinusitis [30] while some others did not report such a correlation [8, 9]. Paşaoğlu et al. [10] reported the association of EB and maxillary sinusitis. In our study, the two groups were significantly different regarding the presence of EB and HC. The EB and HC were significantly more prevalent in the healthy control group; thus, they cannot serve as risk factors for maxillary sinusitis.
Regarding the form of EI, both group had a higher frequency of form A (attachment of the UP to lamina papyracea); however, it had a higher frequency in the healthy control group. Non-attachment of the UP ranked second in terms of frequency in the maxillary sinusitis group. This finding was somehow similar to that of Srivastava et al. [31] who reported that form A followed by no-attachment were the most common forms. The two groups with/without maxillary sinusitis were significantly different regarding the form of EI; while, some studies did not find such a difference [10, 32]. Variations in the results of studies can be due to different definitions of anatomical variations or sinusitis. For instance, some studies considered > 3 mm mucosal thickness as sinusitis [20, 21] while some others considered any degree of thickening on radiographs as sinusitis [27]. In our study, patients with clinical signs and symptoms, Lund-Mackay score of ≥ 6, and apparent maxillary sinus involvement on CT scans were assigned to the maxillary sinusitis group. Although the majority of studies reviewed here are recent, difference between our findings and some older studies may be attributed to the technique of imaging as well, because in the past, patients’ head had an extended position in imaging for the coronal view, and the magnitude of head extension was an influential factor on the precise angle of coronal CT scans. Also, in some cases, modified coronal plane would form 50 to 75° angle with the infraorbitomeatal (Frankfurt) plane [33]. Thus, it may be stated that the obtained coronal images were angulated, and were different from the actual coronal view. The slice thickness is another important parameter in this respect, because measurements made on thick slices are not accurate, and anatomical variations cannot be well detected on thick slices. On the other hand, the current advances in window width and window level adjustments have greatly enhanced the visualization of images, and observations are now less dependent on personal experience. However, as mentioned earlier, these parameters are important when comparing the results with those of older studies.
Studies on the length and width of EI are limited. Thorp et al. reported shorter EI in patients with sinusitis [15]. Akay et al. [13] reported shorter EI in patients with partial opacification of the maxillary sinus. de Carvalho et al. [14] reported longer EI in patients with antral pseudocyst and shorter EI in patients with complete opacification of the maxillary sinus. However, they concluded that EI length is not a determining factor for optimal drainage of the maxillary sinus because a previous obstruction may be present in the OMC. Alternatively, presence of antral pseudocyst may be due to defects in the structure of caliciform cells, causing obstruction and accumulation of secretions [14]. Paşaoğlu et al. [10] reported results similar to de Carvalho et al. [14] and found no significant correlation between EI length and maxillary sinusitis. In our study, EI length was significantly shorter in patients with maxillary sinusitis; this finding was similar to that of Thorp et al. [15] and somehow similar to that of Akay et al. [13]. Shorter length of EI in patients with maxillary sinusitis, and longer EI in the healthy control group can be attributed to the ciliary function. In those with longer EI, the cilia can still perform their function in part of the EI path, and contribute to the process of drainage. However, in those with shorter EI, inflammation of the mucosa, in case of occurrence, can lead to complete obstruction of the drainage path in a shorter time.
Regarding the EI width, Thorp et al. [15] measured the EI width only at the orifice of EI while Paşaoğlu et al. [10] measured its width only at the narrowest part of the EI path. Both studies reported wider EI in patients with sinusitis. Generato Sogono et al. [20] reported that size of EI, measured at the initial part, was greater in patients with maxillary sinusitis, and each 1 mm increase in its size increased the odds of maxillary sinusitis by 1.4 times. On the other hand, some studies did not find a correlation between the size of ostium and maxillary sinusitis [11, 34]. In this study, we measured three width values along the path of EI and calculated the mean value for higher accuracy. We found that patients with maxillary sinusitis had a wider EI, which was in line with the results of Thorp et al. [15] and Paşaoğlu et al. [10] and somehow similar to the findings of Generato Sogono et al. [20]. The mass effect of inflammatory secretions and decreased ciliary activity may be responsible for the widening of EI [10]. Differences in the results of studies may be due to variations in the inclusion criteria, age of patients, measurement techniques, and racial differences [13].
No previous study has evaluated the angulation of EI. However, Thorp et al. [15] measured the angle between the lateral border of the UP and the lateral nasal wall. Pruna [35] measured the mediolateral angle of the UP from its superior free end to the inferior turbinate, and Khojastepour et al. [11] measured the angle between the UP and the horizontal line passing through its superior end. They found no significant difference in this parameter; however, medial or lateral deviation of the UP affected this angle [16, 17]. Thus, in the current study, we eliminated these parameters and analyzed the angle of EI path. Although this angle was smaller in maxillary sinusitis group, the difference between the two groups was not significant. There is no previous study on this topic to compare our results with.
Regarding the dimensions of the drainage system in males and females, de Carvalho et al. [14] reported longer EI in the right side in males and Akay et al. [13] found no significant difference between males and females. None of them assessed the effect of sinusitis in this respect.
Comparison of the EI length, width and angulation based on mucosal thickening score according to the Lund-Mackay scoring system revealed significant differences among the groups in EI length and width, but not angulation. By an increase in mucosal thickening, EI became wider and shorter. Paşaoğlu et al. [10] found no correlation between the grade of sinusitis and EI length, but EI width was greater in patients with partial and complete opacification of the maxillary sinus, which was in line with our results. Anatomical variations, except for NSD, had a significant correlation with the Lund-Mackay score.
Regarding the relationship of CB with grade of mucosal thickening of the maxillary sinuses, some studies found no association [8, 10] while Caughey et al. [36] reported larger CB in patients who acquired higher Lund-Mackay scores. In our study, CB was more frequent in normal individuals and those with partial opacification, compared with complete opacification. However, a large percentage of normal individuals and those with partial/complete opacification did not have CB; thus, despite the significant difference in the frequency of CB, it cannot play a role in development of sinusitis.
Paşaoğlu et al. [10] reported EB as an independent risk factor for development of maxillary sinusitis such that risk of sinusitis in patients with EB was 1.48 times higher than that in individuals without it. In our study, EB was more prevalent in normal individuals and those with partial opacification. Although this difference was significant, EB cannot play a role in development of sinusitis since a high percentage of normal individuals had EB.
Regarding the presence of HC in patients with different thicknesses of the maxillary sinus mucosa, Paşaoğlu et al. [10] found no significant correlation while Caughey et al. [36] reported larger anteroposterior, superior-inferior and mediolateral dimensions of HC in patients with Lund-Mackay score > 0 for the maxillary sinus. The difference in this respect was also significant in our study; however, since HC was present in a high percentage of normal individuals and those with partial/complete opacification, presence of HC does not increase the risk of mucosal thickening.
The current study found a significant association between the form of EI and mucosal thickening. However, Paşaoğlu et al. [10] found no such a correlation. We did not find a correlation between NSD and degree of mucosal thickening, which was in agreement with the results of Kaygusuz et al. [8]. However, Paşaoğlu et al. [10] reported significantly higher prevalence of partial opacification in patients with NSD.
Considering all the above, it seems that individual assessment plays a fundamental role in the occurrence, prognosis, and treatment of sinusitis. For instance, in contrast to the general opinion, narrowing of the drainage path does not necessarily cause sinusitis because the mass effect of inflammation and mucosal thickening may compensate the narrowing and slightly widen the drainage path. In this study, wider EI was noted in the sinusitis group. On the other hand, recurrence has been reported following surgical widening of the area and uncinectomy. Thus, role of other parameters, such as genetic susceptibility, systemic diseases, biological factors, function of cilia, and environmental and occupational factors should also be taken into account. Regarding the relationship of anatomical variations with sinusitis, the role of their size, their vicinity to the drainage path, and evidence of inflammation in anatomical variations such as HC and EB should not be overlooked. On the other hand, type of microorganisms in chronic sinusitis is reportedly different in young and old patients. Also, variations in the bacterial flora have been reported in the first and next surgical procedures [37], which should be considered in pharmaceutical therapy and may be responsible for treatment failure. Microbial culture from the area can help in precise determination of the causative microorganisms, especially in treatment-resistant cases. Future prospective studies are required to analyze the role of each parameter in development of sinusitis.
Conclusion
Patients with maxillary sinusitis had wider and shorter EI and by an increase in mucosal thickening, the EI length decreased while its width increased. Some anatomical variations had significantly different frequency in patients with/without maxillary sinusitis such as EB, EI form, and HC. Except for NSD, other anatomical variations were significantly correlated with the degree of mucosal thickening.
Acknowledgements
This study approved by Vice Chancellery for Research and Technology and Dental Sciences Research Center of Guilan University of medical sciences, ID: IR.GUMS.REC.1398.479.
Compliance with ethical standards
Conflict of interest
There is no conflict of intrest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zahra Yousefi, Email: zahrayousefi71@yahoo.com.
Zahra Dalili Kajan, Email: zahradalili@yahoo.com.
Mir Mohammad Jalali, Email: mmjalali@gmail.com.
Negar Khosravifard, Email: ngkhosravi@yahoo.com.
Elahe Rafiei, Email: rafiee.elahe@yahoo.com.
References
- 1.Philpott C. Rhinosinusitis: definition, classification and diagnosis. In: Watkinson JC, Clarke RW, editors. Scott-Brown's Otorhinolaryngology and Head and Neck Surgery. Florida: CRC Press; 2018. [Google Scholar]
- 2.Tetradis S. Paranasal sinus disease. In: Mallya SM, Lam EW, editors. White and Pharoah’s oral radiology principles and interpretation. 8. Amsterdam: Elsevier; 2019. [Google Scholar]
- 3.Teul I, Zbislawski W, Baran S, Czerwinski F, Lorkowski J. Quality of life of patients with diseases of sinuses. J Physiol Pharmacol. 2007;58(5):691–697. [PubMed] [Google Scholar]
- 4.Som PM, Curtin HD. Head and Neck Imaging. Amsterdam: Elsevier; 2011. [Google Scholar]
- 5.Bandyopadhyay R, Biswas R, Bhattacherjee S, Pandit N, Ghosh S. Osteomeatal complex: a study of its anatomical variation among patients attending North Bengal medical college and hospital. Indian J Otolaryngol Head Neck Surg. 2015;67(3):281–286. doi: 10.1007/s12070-015-0874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alsowey AM, Abdulmonaem G, Elsammak A, Fouad Y. Diagnostic performance of multidetector computed tomography (MDCT) in diagnosis of sinus variations. Pol J Radiol. 2017;82:713–725. doi: 10.12659/PJR.903684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3):35–40. doi: 10.1016/S0194-5998(97)70005-6. [DOI] [PubMed] [Google Scholar]
- 8.Kaygusuz A, Haksever M, Akduman D, Aslan S, Sayar Z. Sinonasal anatomical variations: their relationship with chronic rhinosinusitis and effect on the severity of disease—a computerized tomography assisted anatomical and clinical study. Indian J Otolaryngol Head Neck Surg. 2014;66(3):260–266. doi: 10.1007/s12070-013-0678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaya M, Çankal F, Gumusok M, Apaydin N, Tekdemir I. Role of anatomic variations of paranasal sinuses on the prevalence of sinusitis: computed tomography findings of 350 patients. Niger J Clin Pract. 2017;20(11):1481–1488. doi: 10.4103/njcp.njcp_199_16. [DOI] [PubMed] [Google Scholar]
- 10.Paşaoğlu L, Toprak U, Üstüner E, Temel E, Özer H. Are variations of paranasal sinuses and infundibular trace length responsible for development of maxillary sinusitis. OMICS J Radiol. 2017 doi: 10.4172/2167-7964.1000270. [DOI] [Google Scholar]
- 11.Khojastepour L, Haghnegahdar A, Khosravifard N. Role of sinonasal anatomic variations in the development of maxillary sinusitis: a cone beam CT analysis. Open Dent J. 2017;11:367–374. doi: 10.2174/1874210601711010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamdi P, Nimma V, Ramchandani A, Ramaswami E, Gogri A, Umarji H. Evaluation of haller cell on CBCT and its association with maxillary sinus pathologies. J Indian Acad Oral Med Radiol. 2018;30(1):41–45. doi: 10.4103/jiaomr.jiaomr_22_18. [DOI] [Google Scholar]
- 13.Akay G, Yaman D, Karadağ Ö, Güngör K. Evaluation on the relationship of dimensions of maxillary sinus drainage system with the anatomical variations and sinusopathies: cone-beam computed tomography findings. Med Prince Pract. 2019 doi: 10.1159/000504963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Carvalho ABG, Costa ALF, Fuziy A, de Assis ACS, Veloso JRC, Junior LRCM, et al. Investigation on the relationship of dimensions of the maxillary sinus drainage system with the presence of sinusopathies: a cone beam computed tomography study. Arch Oral Biol. 2018;94:78–83. doi: 10.1016/j.archoralbio.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Thorp M, Roche P, Nilssen E, Mortimore S. Complicated acute sinusitis and the computed tomography anatomy of the ostiomeatal unit in childhood. Int J Pediatr Otorhinolaryngol. 1999;49(3):189–195. doi: 10.1016/S0165-5876(99)00200-1. [DOI] [PubMed] [Google Scholar]
- 16.Wardani RS, Wardhana A, Mangunkusumo E, Wulani V, Senior BA. Radiological anatomy analysis of uncinate process, concha bullosa, and deviated septum in chronic rhinosinusitis. Oto Rhino Laryngol Indones. 2017;47(1):16–24. doi: 10.32637/orli.v47i1.191. [DOI] [Google Scholar]
- 17.Khojastepour L, Mirhadi S, Mesbahi SA. Anatomical variations of ostiomeatal complex in CBCT of patients seeking rhinoplasty. J Dent (Shiraz) 2015;16(1):42–48. [PMC free article] [PubMed] [Google Scholar]
- 18.Ashraf N, Bhattacharyya N. Determination of the “incidental” Lund score for the staging of chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2001;125(5):483–486. doi: 10.1067/mhn.2001.119324. [DOI] [PubMed] [Google Scholar]
- 19.Orlandi RR. A systematic analysis of septal deviation associated with rhinosinusitis. Laryngoscope. 2010;120(8):1687–1695. doi: 10.1002/lary.20992. [DOI] [PubMed] [Google Scholar]
- 20.Generato Sogono P, Gozun Songco C. Prevalence of nasal septal deviation, concha bullosa, and infundibular size and their association with maxillary sinusitis by computed tomography in filipinos with paranasal sinus disease. J Otorhinolaryngol Facial Plast Surg. 2019;5(1):1–6. [Google Scholar]
- 21.Kucybała I, Janik KA, Ciuk S, Storman D, Urbanik A. Nasal septal deviation and concha bullosa–do they have an impact on maxillary sinus volumes and prevalence of maxillary sinusitis? Pol J Radiol. 2017;82:126–133. doi: 10.12659/PJR.900634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demirel O, Ozlem Ucok C, Toraman Alkurt M. Evaluation of osteomeatal complex anomalies and maxillary sinus diseases using cone beam computed tomography. Curr Med Imaging. 2017;13(4):397–405. [Google Scholar]
- 23.Mendiratta V, Baisakhiya N, Singh D, Datta G, Mittal A, Mendiratta P. Sinonasal anatomical variants: CT and endoscopy study and its correlation with extent of disease. Indian J Otolaryngol Head Neck Surg. 2016;68(3):352–358. doi: 10.1007/s12070-015-0920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karki S, Pokharel M, Suwal S, Poudel R. Prevalence of anatomical variations of the sinonasal region and their relationship with chronic rhinosinusitis. Kathmandu Univ Med J. 2016;56(4):342–346. [PubMed] [Google Scholar]
- 25.Fadda G, Rosso S, Aversa S, Petrelli A, Ondolo C, Succo G. Multiparametric statistical correlations between paranasal sinus anatomic variations and chronic rhinosinusitis. Acta Otorhinolaryngol Ital. 2012;32(4):244–251. [PMC free article] [PubMed] [Google Scholar]
- 26.Karatas D, Koç A, Yüksel F, Dogan M, Bayram A, Cihan MC. The effect of nasal septal deviation on frontal and maxillary sinus volumes and development of sinusitis. J Craniofac Surg. 2015;26(5):1508–1512. doi: 10.1097/SCS.0000000000001809. [DOI] [PubMed] [Google Scholar]
- 27.Smith KD, Edwards PC, Saini TS, Norton NS. The prevalence of concha bullosa and nasal septal deviation and their relationship to maxillary sinusitis by volumetric tomography. Int J Dent. 2010 doi: 10.1155/2010/404982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasan H, Doĝru H, Baykal B, Döner F, Tüz M. What is the relationship between chronic sinus disease and isolated nasal septal deviation? Otolaryngol Head Neck Surg. 2005;133(2):190–193. doi: 10.1016/j.otohns.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Mohebbi A, Ahmadi A, Etemadi M, Safdarian M, Ghourchian S. An epidemiologic study of factors associated with nasal septum deviation by computed tomography scan: a cross sectional study. BMC Ear Nose Throat Disord. 2012;12(1):15. doi: 10.1186/1472-6815-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dasar U, Gokce E. Evaluation of variations in sinonasal region with computed tomography. World J Radiol. 2016;8(1):98–108. doi: 10.4329/wjr.v8.i1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava M, Tyagi S. Role of anatomic variations of uncinate process in frontal sinusitis. Indian J Otolaryngol Head Neck Surg. 2016;68(4):441–444. doi: 10.1007/s12070-015-0932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuli IP, Sengupta S, Munjal S, Kesari SP, Chakraborty S. Anatomical variations of uncinate process observed in chronic sinusitis. Indian J Otolaryngol Head Neck Surg. 2013;65(2):157–161. doi: 10.1007/s12070-012-0612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Som PM. CT of the paranasal sinuses. Neuroradiology. 1985;27(3):189–201. doi: 10.1007/BF00344487. [DOI] [PubMed] [Google Scholar]
- 34.Mathew R, Omami G, Hand A, Fellows D, Lurie A. Cone beam CT analysis of Haller cells: prevalence and clinical significance. Dentomaxillofac Radiol. 2013 doi: 10.1259/dmfr.20130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pruna X. Morpho-functional evaluation of ostiomeatal complex in chronic sinusitis by coronal CT. Eur Radiol. 2003;13(6):1461–1468. doi: 10.1007/s00330-002-1644-3. [DOI] [PubMed] [Google Scholar]
- 36.Caughey RJ, Jameson MJ, Gross CW, Han JK. Anatomic risk factors for sinus disease: fact or fiction? Am J Rhinol. 2005;19(4):334–339. doi: 10.1177/194589240501900402. [DOI] [PubMed] [Google Scholar]
- 37.Leszczyńska J, Stryjewska-Makuch G, Ścierski W, Lisowska G. Bacterial flora of the nose and paranasal sinuses among patients over 65 years old with chronic rhinosinusitis who underwent endoscopic sinus surgery. Clin Interv Aging. 2020;15:207–215. doi: 10.2147/CIA.S215917. [DOI] [PMC free article] [PubMed] [Google Scholar]