Abstract
Immune responses against enterotoxigenic Escherichia coli (ETEC) were examined in Bangladeshi adults with naturally acquired disease and compared to responses in age-matched Bangladeshi volunteers who had been orally immunized with a vaccine consisting of inactivated ETEC bacteria expressing different colonization factor antigens (CFs) and the B subunit of cholera toxin. B-cell responses in duodenal biopsy samples, feces, intestinal washings, and blood were determined. Because most of the patients included in the study were infected with ETEC expressing CS5, immune responses to this CF were studied most extensively. Vaccinees and patients had comparable B-cell responses against this antigen in the duodenum: the median numbers of antibody-secreting cells (ASC) were 3,300 immunoglobulin A (IgA) ASC/107 mononuclear cells (MNC) in the patient group (n = 8) and 1,200 IgA ASC/107 MNC in the vaccinees (n = 13) (not a significant difference). Similarly, no statistically significant differences were seen in the levels of duodenal B cells directed against enterotoxin among vaccinees and patients. A comparison of the capacities of the various methods used to assess mucosal immune responses revealed a correlation between numbers of circulating B cells and antibody levels in saponin extracts of duodenal biopsy samples (r = 0.58; n = 13; P = 0.04) after vaccination. However, no correlation was seen between blood IgA ASC and duodenal IgA ASC after two doses of vaccine. Still, a correlation between numbers of CF-specific B cells in blood sampled from patients early during infection and numbers of duodenal B cells collected 1 week later was apparent (r = 0.70; n = 10; P = 0.03).
Enterotoxigenic Escherichia coli (ETEC) is one of the most common causes of childhood diarrhea in developing countries, in addition to being the principal etiologic agent of traveler's diarrhea (5). These noninvasive bacteria cause disease by secreting one or two enterotoxins, namely the cholera toxin (CT)-like heat-labile enterotoxin (LT) and the nonimmunogenic heat-stable enterotoxin (ST), both of which interact with the intestine to yield the electrolyte-rich, watery diarrhea characteristic of the illness. A prerequisite for the targeting of the toxins to the intestinal mucosa is the close adherence of the bacteria to the intestinal wall, which is mediated by fimbriae named colonization factor antigens (CFAs) (11). To date, 20 different colonization factors (CFs) have been described, the most prevalent ones being CFA/I, CFA/II, and CFA/IV. CFA/II is composed of three separate antigens named coli surface antigen 1 (CS1), CS2, and CS3; similarly, CFA/IV is composed of the three antigens CS4, CS5, and CS6.
The age-associated decrease in the incidence of ETEC infections in the developing world has been suggested to be the result of the development of protective immunity (5). Cravioto et al. (7) monitored a cohort of Mexican infants prospectively and found that the infants were protected from reinfection by ETEC strains expressing a specific CFA if they had previously been infected by a strain expressing the same CFA. Neither the O antigens nor LT was shown to be protective in the Mexican study. It has also been shown that American volunteers who were experimentally infected with ETEC were protected against challenge with homologous ETEC strains (16). However, the same volunteers were not protected upon challenge with an ETEC strain heterologous in all traits except for the expression of LT (16). Nevertheless, both a large field trial in Bangladesh (6) and a study of Finnish travelers to Morocco (22) demonstrated that an oral whole-cell cholera vaccine containing the B subunit of CT (CTB) gave rise to protection against diarrhea caused by LT-expressing E. coli.
The elaboration of an ETEC vaccine has been deemed to be of high priority because ETEC infections lead to high mortality and morbidity levels among children in developing countries. An inactivated ETEC vaccine consisting of a combination of CTB and formalin-inactivated ETEC bacteria expressing the most-prevalent CFAs has been tested on Swedish (1, 2, 13, 33, 34) as well as Egyptian volunteers (26, 27). The vaccine is given orally because elicitation of mucosal immune responses in the intestine has been considered to be of prime importance for protection. To assess the immunogenicity of this vaccine and other vaccine candidates, we deemed it important to study the immune responses that develop after infection in a population in which ETEC is endemic, which is the major target for vaccination. In a previous study, mucosal immune responses after ETEC disease were analyzed in intestinal lavages collected from Bangladeshi convalescents (28). No clear relationship between immunoglobulin A (IgA) responses in intestinal lavage fluid and IgA levels in easily accessible specimens, such as saliva, serum, and breast milk, was found in that study (28). The aims of this study were to compare various methods of monitoring intestinal immune responses to ETEC and to compare the immune response induced by the oral inactivated-ETEC vaccine with that seen after natural infection with CFA-positive ETEC.
MATERIALS AND METHODS
Vaccinees.
Twenty-seven volunteers, 25 men and 2 women, were enrolled in the study, which was approved by the Ethical Review Committee of the International Centre for Diarrhoeal Disease Research in Bangladesh (ICCDR,B). Prior to inclusion in the study, the medical histories of participants were taken and sick persons, as well as those who had experienced diarrhea during the 6 months preceding the study, were excluded. The volunteers were of low socioeconomic status and were between the ages of 17 and 49 years (median age, 22 years). They gave informed consent to participate in the study and were divided into two groups, one in which intestinal lavages (n = 14) were performed and one from which duodenal punch biopsy samples (n = 13) were taken. Blood samples (15 ml of venous blood) and stool specimens were collected from all volunteers. Sampling occurred prior to vaccination (day 0) and 1 week after the second dose of the ETEC vaccine (day 21).
The ETEC vaccine (lot E001) was produced by SBL Vaccin AB, Stockholm, Sweden. Each dose of vaccine consisted of 1 mg of recombinant CTB (25) and 2 × 1010 bacteria of each of five different strains expressing CFA/I and the different subcomponents of CFA/II and CFA/IV (CS1 through CS6), amounting to a total of 1011 formalin-inactivated ETEC bacteria. The volunteers were given two oral doses of vaccine 2 weeks apart. One vial of vaccine was added to 150 ml of tap water mixed with two sachets of sodium bicarbonate-tartaric acid (Samarin; Cederroth AB, Upplands Väsby, Sweden) to protect the CTB component from the low pH of the stomach. Vaccinees fasted overnight prior to immunization and were not allowed to eat for 1 h after vaccination.
Patients.
Twenty adult patients hospitalized because of ETEC diarrhea at the Clinical Research and Service Centre of the ICDDR,B were enrolled in this study after giving informed consent. All the patients were male and were between the ages of 17 and 50 years (median age, 27.5 years). We failed to enroll female patients in the study because the women found it nearly impossible to come for follow-up sampling due to family obligations. Prior to inclusion in the study, the patients had suffered from diarrhea for 3 to 72 h (median duration, 8 h). They were hospitalized for 1 to 7 days (median, 3 days) and suffered from watery diarrhea and vomiting but had normal body temperatures. The patients exhibited varying degrees of dehydration as assessed by the Denvar scale (35): severe (40% of the cases), moderate (40%), and mild (20%). Only patients infected with CFA-expressing ETEC were recruited. Three of 7 (43%) of the patients infected with ETEC strains producing ST only and 5 of 13 (38%) of those infected with ETEC strains producing both LT and ST experienced severe dehydration. All patients were given oral rehydration therapy, supplemented by intravenous-fluid therapy when deemed necessary. More than half of the patients received antibiotics, usually erythromycin. Three of the patients had coinfections with Giardia lamblia and received metronidazole treatment.
Clinical specimens were collected from the patients as follows: stool was collected on the day of hospitalization (day 0) and on day 9, whereas blood samples and duodenal biopsy samples were obtained 3 days after the onset of diarrhea (day 3) and 1 week later (day 9), after the identification of CFA-positive ETEC strains in the patients' fecal samples.
Bacteriological analysis.
Fecal specimens isolated from patients at the time of arrival at the hospital were cultured on MacConkey agar plates for the detection of lactose-fermenting colonies (E. coli or yersiniae). Non-lactose-fermenting colonies were further cultured on Brucella agar to exclude the presence of Campylobacter spp., and on taurocholate-tellurite-gelatin agar and Salmonella-Shigella agar to ensure that neither Vibrio cholerae nor Salmonella species nor Shigella species were present. In some cases, biochemical and serological tests were performed on non-lactose-fermenting colonies to exclude the presence of diarrheagenic bacteria other than ETEC. ETEC was grown overnight on CFA agar with or without bile salts for optimal expression of CFAs (19). Bacterial colonies were assayed for CFA expression by slide agglutination using monoclonal antibodies specific for CFA/I, CS1, CS2, CS3, or CS5 (17). Dot blots using the same panel of monoclonal antibodies were later carried out to confirm the results obtained in slide agglutination assays as well as for the detection of CS4 and CS6; the latter two antigens cannot be detected by slide agglutination (12). CFA-positive E. coli strains were assayed for the presence of enterotoxins by using a ganglioside GM1-enzyme-linked immunosorbent assay (ELISA) for the detection of LT (29) and an inhibition-GM1-ELISA for the detection of ST (31).
Duodenal biopsies.
Duodenal punch biopsy samples were taken from vaccinees and patients by a standard endoscopic procedure using a local anesthetic and biopsy forceps. In general, 15 to 17 punch biopsy samples, 1 to 2 mm in diameter, were obtained from different parts of the duodenum of each subject.
(i) Saponin extracts.
Two duodenal punch biopsy samples were incubated at 4°C overnight in phosphate-buffered saline (PBS) containing 2% (wt/vol) saponin (Sigma Chemical Co., St. Louis, Mo.) in order to extract immunoglobulins from the tissue specimens. The extracts were then centrifuged at a high speed, and the supernatant was stored at −70°C until it was assayed for protein contents by the Bio-Rad Protein Assay (Bio-Rad Laboratories AB, Hercules, Calif.) as well as for antibodies by ELISA.
(ii) Intestinal lymphocytes.
Twelve to 16 duodenal punch biopsy specimens were chopped into very small pieces with a surgical blade while they were incubated on ice. The homogenized tissue was incubated in a Bacillus thermoproteolyticus thermolysin (Boehringer, Mannheim, Germany) solution (0.5 mg ml−1) at 4°C for 30 min and was subsequently filtered through a large-diameter nylon mesh (0.150 by 0.08 mm) (J. H. Tidbeck AB, Haninge, Sweden). Nondigested tissue was treated with collagenase/dispase (Boehringer), diluted to 1 mg ml−1 in Iscove's medium (Gibco, Paisley, United Kingdom), and again passed through a large-diameter mesh as described above. Any remaining tissue which did not pass through the filter was discarded. The filtered cells were pooled and treated with 2.5 mg of DNAse type IV (Sigma) ml−1 diluted in Iscove's medium at 37°C for 20 min. Next, the cells were passed through a small-diameter polyester mesh (0.045 by 0.038 mm), spun down, and counted in a Bürker chamber. The proportion of dead cells was determined by trypan blue (Merck, Darmstadt, Germany) staining and never accounted for more than 10% of all cells. Lastly, the cells were diluted to the appropriate concentrations in Iscove's medium supplemented with 10% fetal calf serum, 2 mM glutamine, and 100 μg of gentamicin (Essex Läkemedel AB, Stockholm, Sweden) ml−1 and were added to enzyme-linked immunospot (ELISPOT) plates for the determination of the numbers of antibody-secreting cells (ASC).
Intestinal lavage.
Volunteers, who had fasted overnight, drank 250 ml of lavage solution (6.5 g of NaCl, 1.8 g of NaHCO3 and 0.75 g of KCl per liter of water) every 10 min until a clear, watery stool appeared. The mean volume of lavage solution ingested by the volunteers was 4.9 liters. Two 250-ml samples of “clear” intestinal lavage fluid were collected from each volunteer, and the specimens were filtered through cheesecloth. Next, a 50-ml aliquot of each of the two samples was treated by the addition of soybean trypsin inhibitor (STI) (final concentration, 100 μg ml−1) (Sigma) and EDTA (final concentration, 0.05 M) (Merck) and kept on ice. The two specimens were pooled and then centrifuged at 1,000 × g for 15 min, and the pellet was discarded. The supernatant was next treated by the addition of phenylmethylsulfonyl fluoride (PMSF; final concentration, 2 mM) (Sigma) and left to stand at room temperature for 15 min. Lastly, bovine serum albumin (Sigma) and sodium azide were added to final concentrations of 1 mg ml−1 and 0.02% (wt/vol), respectively. The gut lavage fluid was freeze-dried and reconstituted to one-fifth of the original volume before analysis of antibody contents by ELISA (2).
Stool specimens.
Stool specimens were immediately stored at −70°C upon collection. Antibodies were extracted by the following procedure. Four grams of thawed stool was mixed with 16 ml of a solution containing STI (100 mg ml−1) (Sigma), EDTA (0.05 M) (Merck), and PMSF (10 mM) (Sigma), dissolved in PBS (pH 7.2) supplemented with 0.05% Tween 20 (PBS-Tween). The mixture was left to stand on the bench at room temperature for 15 min with intermittent shaking. Next, the mixture was filtered through cheesecloth to remove any solid particles and centrifuged at 20,000 × g for 30 min. Bovine serum albumin (final concentration, 0.1% [wt/vol]) and sodium azide (final concentration, 0.02%) were added to the supernatant after the pellet was discarded. Aliquots of the supernatant were stored at −70°C until they were assayed by ELISA for total and specific IgA antibody contents.
Blood samples.
Venous blood (15 ml) was collected from volunteers prior to vaccination (day 0) and 7 days after the second immunization (day 21). Similarly, venous blood was collected from patients on day 3 after arrival at the hospital and approximately 1 week later, on day 9. Mononuclear cells (MNC) were isolated from the blood samples by gradient centrifugation on Ficoll-Isopaque (Pharmacia, Uppsala, Sweden).
ASC.
The ELISPOT method was used to quantitate numbers of ASC (8). Nitrocellulose-bottomed 96-well plates (Millititer HA; Millipore Corp., Bedford, Mass.) were coated with either 10 μg of purified CFA/I ml−1, 20 μg of CFA/II (CS1 plus CS3) ml−1, 10 μg of CFA/IV (CS5) ml−1, or 3 nmol of GM1 ganglioside (Sigma) ml−1 at 4°C overnight. The GM1-coated wells were washed in PBS, whereupon 2.5 μg of CT (List Biological Laboratories, Inc., Campbell, Calif.) ml−1 was added and the wells were reincubated at 37°C for 2 h. Sites with nonbound antigen were blocked by the addition of 1% bovine serum albumin-PBS. Intestinally derived MNC suspensions were seeded at 5 × 104 cells per CFA-coated well and 104 cells per GM1-CTB-coated well; 10-fold higher numbers were used when peripheral blood MNC were assayed. Next, the plates containing the cells were incubated at 37°C for 3 h in a 7.5% CO2 thermostat. Finally, horseradish peroxidase-conjugated antibodies specific for human IgA (diluted 1:250 in PBS-Tween), IgM, or IgG (diluted 1:500 for the latter two isotypes) (Southern Biotechnology Associates, Birmingham, Ala.) were added to the wells, which were incubated at 4°C overnight. Spots were developed by the addition of the chromogen 3-amino-9-ethylcarbazole (Sigma) and enumerated under low magnification with the aid of a stereomicroscope. Duplicate assays were performed for each antigen and isotype.
Determination of levels of specific antibodies and total IgA.
Total IgA contents were determined for all fecal-extract and intestinal-lavage-fluid specimens as previously described (2). For the assessment of specific IgA titers, flat-bottom ELISA plates (NUNC, Roskilde, Denmark) were coated with purified CFA/I, CFA/II (CS1 plus CS3 or CS2), or CFA/IV (CS5) diluted in PBS to a concentration of 1 μg ml−1. Samples were threefold serially diluted and tested in duplicate. A GM1-ELISA method was used for the determination of CT-specific antibodies (30). Antibody titers were expressed as units per microgram and were obtained by calculating the specific titer (in units per milliliter), i.e., the reciprocal interpolated dilution giving rise to an absorbance of 0.2 above the background absorbance at 450 nm, dividing it by the total IgA contents (in micrograms per milliliter), and multiplying by 10. A vaccinee with at least a twofold increase in the titer of specific IgA per total IgA content was considered a responder. A similar computation was performed when specific antibody titers in saponin extracts were determined, except that specific IgA levels in these samples were related to total protein contents instead of total IgA levels. The reason for this was that the protein assay was much quicker and the quantity of protein extract needed for protein determination was much smaller than that required for IgA analysis.
Statistics.
For comparisons of matched data such as preimmune and postimmune samples from vaccinees or late and early samples from patients, the paired nonparametric Wilcoxon signed rank test was used (InStat program; Graph Pad Software Inc., San Diego, Calif.). For nonpaired groups, such as vaccinees and patients, the Mann-Whitney test was used (Graph Pad). Correlations between various immune parameters were carried out by using the Spearman rank correlation coefficient (Graph Pad).
RESULTS
Comparison of patients and vaccinees.
In this study, determination of ETEC-specific duodenal B cells was used as the reference method for evaluating intestinal immunity to ETEC. The study had two main objectives: to compare the immune responses elicited by ETEC vaccination and natural disease and to evaluate to what extent immune responses obtained in easily accessible clinical specimens reflect B-cell responses in duodenal biopsy specimens.
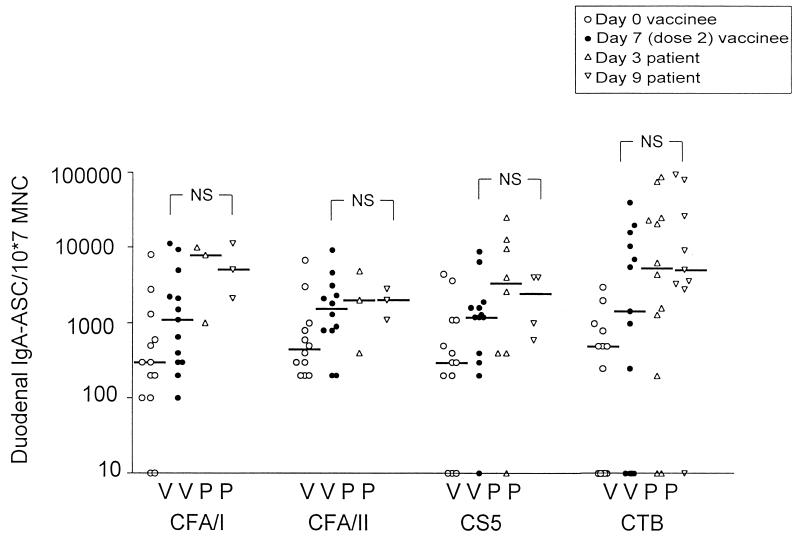
The numbers of IgA ASC directed against most of the CFAs included in the vaccine, i.e., CFA/I, CFA/II (CS1 plus CS3), and CFA/IV (CS5), were determined in the duodena of 13 vaccinees and related to the total number of intestinal MNC (Fig. 1). Although the adult volunteers enrolled in the study had already had very high numbers of ASC against all the different antigens studied before vaccination, significant and near-significant IgA ASC responses were seen against all antigens 1 week after two oral doses of ETEC vaccine were given. Thus, the median fold increase was 3.7 for CFA/I (P = 0.06), 3.6 for CFA/II (P = 0.05), and 4.0 for CS5 (P = 0.06) (Fig. 1). The responder frequency, defined as the proportion of vaccinees with a twofold or greater increase in IgA ASC numbers after immunization, was 6 of 13 for CFA/I, 7 of 12 for CFA/II, and 8 of 13 for CS5. All the vaccinees who responded significantly with IgA ASC against CFA/I also responded against CFA/II; however, only five of the eight vaccinees who responded to CS5 responded to CFA/I.
FIG. 1.
Duodenal IgA ASC responses against CFAs and CTB in adult Bangladeshi vaccinees and patients. Open circles, individual preimmune values for volunteers; solid circles, individual values seen 7 days after two oral doses of the ETEC vaccine, i.e., on day 21; triangles, individual values for patients on day 3 after hospitalization; inverted triangles, individual values for patients on day 9; horizontal bars, median ASC values. No statistically significant differences (NS, P ≥ 0.05) were seen between vaccinees and patients monitored either on day 3 or on day 9.
Duodenal biopsies for the isolation of intestinal lymphocytes were performed on ETEC patients at an early stage of infection, i.e., on day 3 after hospitalization, when infection with CFA-positive ETEC had been diagnosed, and 1 week later. Studies with North American volunteers have shown that the mean incubation time of ETEC diarrhea is 2 days (range, 1 to 5 days) after challenge with virulent ETEC bacteria (16). The patients in our study usually had experienced symptoms for less than 1 day upon admission to the hospital, suggesting that, on average, they had been infected with ETEC for 3 days before enrollment in the study. Thus, the biopsy specimens collected on day 3 corresponded to specimens collected on day 6 after the onset of infection.
The number of duodenal B cells that secreted antibodies against the CFA of the ETEC strain infecting the respective patient was determined for 14 patients at an early stage of infection (approximately day 6) and for 10 of these patients at a later stage (approximately day 12) after the onset of infection (Fig. 1). Most of the patients, 8 of 14, were infected with CS5-expressing strains, and of the remaining patients, 3 were infected with CFA/I-producing strains and 3 with CFA/II-expressing strains. The median IgA ASC levels were higher on day 3 (day 6 after the onset of infection) than on day 9 (day 12 after the onset of infection) for most of the CFA-specific duodenal B-cell responses, but this difference was not statistically significant. In the comparison of duodenal B-cell responses to CFAs induced by vaccination and infection, it was found that the median duodenal IgA ASC levels against CS5, CFA/II, and CTB were comparable for patients and vaccinees; responses against CFA/I were somewhat higher in the patients, but this difference was not statistically significant (Fig. 1).
The proportion of patients who responded significantly with CFA-specific duodenal B cells could not be determined by the definition used for vaccinees, i.e., a twofold increase in specific B-cell numbers, since preinfection values were not available. Instead, a patient was defined as a responder if he had ASC numbers exceeding the 75th percentile (upper quartile) of the preimmune levels recorded for vaccinees for that particular CFA. By this definition, 93% (13 of 14) of the patients responded significantly against the CFAs of their own ETEC strains on either day 3 or day 9 after admission. The nonresponder was sampled only on day 3.
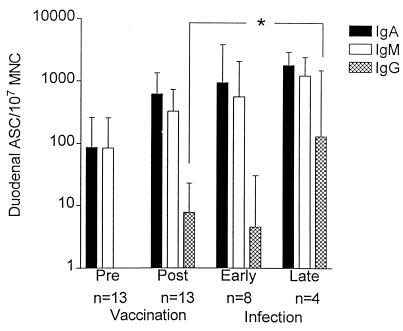
We also studied the duodenal CS5-specific ASC responses of the IgG and IgM isotypes in 13 vaccinees and 8 patients. CS5 was chosen because it was the most common CFA expressed by the ETEC strains infecting our group of patients. The dominant isotypes in the patients, as well as in the vaccinees, were IgA and IgM (Fig. 2). Even though preimmune ASC levels were high in most of the volunteers, vaccination gave rise to a near-significant CS5-specific duodenal IgA ASC response (P = 0.06), with a responder frequency of 8 of 13; a statistically significant increase in IgG ASC (P = 0.004), with 6 of 11 vaccinees responding significantly; but no significant IgM ASC responses. The magnitudes of the duodenal IgA ASC and IgM ASC responses induced by natural infection to CS5 did not differ significantly from those seen after vaccination (Fig. 2). In contrast, the levels of IgG ASC against CS5 were significantly elevated among patients at a late stage of infection compared to vaccinees (P = 0.01) (Fig. 2).
FIG. 2.
CS5-specific duodenal ASC responses of the IgA, IgM, and IgG isotypes monitored in vaccinees and patients. Bars, geometric mean values; error bars, 1 SEM.
The intestinal B-cell responses against CTB were also determined for patients and vaccinees. In 8 of 13 vaccinees, the levels of enterotoxin-specific IgA-producing B cells increased significantly after vaccination (P = 0.02) (Fig. 1). The numbers of duodenal CTB-specific ASC were assessed for patients infected with LT-expressing strains (12 patients altogether). A majority of these patients, 83% (10 of 12), responded significantly with IgA ASC against CTB, i.e., they had levels of IgA ASC against enterotoxin that exceeded the 75th percentile of the preimmune levels against enterotoxin in nonimmunized, noninfected volunteers. There was no statistically significant difference between patients and vaccinees with regard to levels of duodenal IgA ASC specific for CTB (Fig. 1).
Blood ASC.
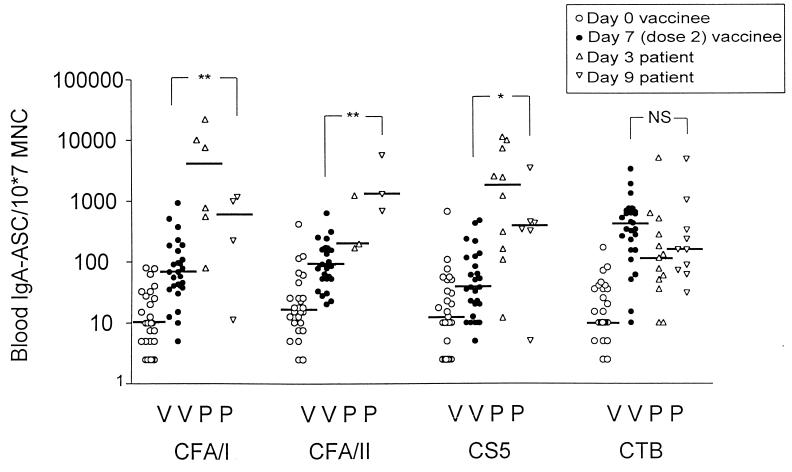
A frequently used test for assessing mucosally derived immune responses to bacterial pathogens is the determination of ASC circulating in peripheral blood. In the present study, blood ASC responses of the IgA isotype were recorded for 19 patients infected with ETEC strains expressing CFA/I (n = 6), CFA/II (n = 3), or CS5 (n = 10) and for 27 vaccinees. Rather high preimmune levels of CFA-specific blood IgA ASC, i.e., medians on the order of 10 IgA ASC/107 MNC, were seen in the vaccinees (Fig. 3). Two doses of vaccine gave rise to significant CFA-specific IgA ASC in a majority of volunteers: the median values were 68 IgA ASC/107 MNC for CFA/I, 88 IgA ASC/107 MNC for CFA/II, and 38 IgA ASC/107 MNC for CS5. Compared to the preimmune levels, these responses were all statistically significant (P = 0.02 to 0.0001) (Fig. 3). The vaccinees also had significant, albeit lower, numbers of ASC that produced IgG antibodies against the CFAs (median levels, 15 IgG ASC/107 MNC for CFA/I as well as for CS5, and 22 IgG ASC/107 MNC for CFA/II). These responses were also statistically significant (P = 0.01 to 0.004). No statistically significant IgM ASC responses were seen in the blood on day 21, i.e., 1 week after the second dose of vaccine.
FIG. 3.
Blood IgA ASC responses against CFAs and CTB in adult Bangladeshi vaccinees and patients. Open circles, individual preimmune values for volunteers; solid circles, individual postimmunization values seen after two oral doses of the ETEC vaccine; triangles, individual values for patients on day 3 after hospitalization; inverted triangles, individual values for patients on day 9; horizontal bars, median ASC values. NS, P ≥ 0.05; ∗, P < 0.05 to 0.01; ∗∗, P < 0.01 to 0.001.
Natural infection elicited high IgA ASC responses to CFAs in peripheral blood (Fig. 3). Infection-induced responses tended to peak on day 3 after admission to hospital, i.e., around day 6 after the onset of infection, rather than on day 9 (approximately day 12 after the onset of infection). The median number of IgA ASC/107 MNC directed against CFA/I was 4,100; corresponding values were 1,300 for CFA/II and 1,800 for CS5. In contrast to the duodenal IgA ASC responses, the blood IgA ASC responses against CFAs were clearly higher in the patient group than in the vaccinated group (P = 0.03 to 0.0006). However, the samples were collected at rather different times, i.e., on day 21 for vaccinees and on day 9 for patients.
At variance with the CFA-specific IgA ASC response in duodenal biopsy specimens, the levels of IgA ASC in the blood directed against CTB were higher in the vaccinated group than among the patients (Fig. 3). However, this difference was not statistically significant.
We also compared the blood ASC responses of the IgA, IgM, and IgG isotypes to CS5 after vaccination and during natural infection. In the blood, CS5-specific ASC of the IgA isotype were predominant; ASC of the IgG and IgM isotypes were of similar but lower magnitudes in vaccinees and patients alike. On day 3 after the onset of diarrhea, the 10 patients infected with CS5-expressing ETEC had the following geometric mean levels of ASC against CS5 in peripheral blood: 900 IgA ASC/107 MNC (geometric mean ± 1 standard error of the mean [SEM], 440 to 1,840), 81 IgM ASC/107 MNC (35 to 190), and 27 IgG ASC/107 MNC (8 to 92). Corresponding values for the six patients who were also assayed on day 9 were 262 IgA ASC/107 MNC (110 to 630), 12 IgM ASC/107 MNC (5 to 28), and 21 IgG ASC/107 MNC (9 to 50).
Comparison of various methods for assessment of mucosal immune responses in vaccinees.
An important goal was to evaluate whether ETEC-specific IgA ASC in the blood or antibody levels in fecal extracts, duodenal-tissue extracts, or intestinal lavage fluid reflected the numbers of ETEC-specific B cells determined in the duodenum. For this purpose, vaccine-induced immune responses against CFA/I and CTB determined by the above-mentioned methods were compared, both with respect to the magnitude of the immune response and with respect to the frequency of responders, i.e., the percentage of vaccinees with at least a twofold increase in antibody titers or in the number of ETEC-specific B cells after immunization (Table 1).
TABLE 1.
Comparison of various methods for monitoring immune responses to the ETEC vaccine in adult Bangladeshi volunteers
| Immune parameter | CFA/I
|
CTB
|
||||
|---|---|---|---|---|---|---|
| Responder frequencya | Fold increaseb | Statistical significancec | Responder frequency | Fold increase | Statistical significance | |
| Gut ASC | 6/13 (46) | 3.7 | P = 0.006 | 8/13 (62) | 10 | P = 0.02 |
| Blood ASC | 22/27 (81) | 9.0 | P = 0.0001 | 25/27 (93) | 299 | P < 0.0001 |
| Extracted Ab | 9/13 (69) | 2.2 | P = 0.001 | 12/13 (92) | 6.2 | P < 0.01 |
| Lavage Ab | 8/14 (57) | 1.8 | NS | 13/14 (93) | 4.6 | P = 0.0001 |
| Fecal Ab | 10/18 (56) | 2.3 | NS | 14/18 (78) | 7.0 | P = 0.0005 |
Expressed as the number of responders/total number tested (percent responders). A vaccinee with at least a twofold increase in ASC numbers or antibody (Ab) titer was considered a responder.
The median postimmunization value (after two oral doses of vaccine) divided by the median preimmunization value equals the fold increase of the respective immune parameter.
The statistical significance of the fold increase induced by vaccination was determined by the Wilcoxon signed rank test. NS, not significant.
ETEC-specific IgA levels in saponin extracts of duodenal biopsy specimens were determined for vaccinees prior to and following oral ETEC immunization. The median IgA levels increased from 1.5 to 3.4 U μg−1 for CFA/I and from 2.8 to 18 U μg−1 for CTB in the duodenal extracts, which was statistically significant in both cases (P < 0.01). In intestinal-lavage fluid, the median preimmune and postimmune IgA titers against CFA/I were 1.5 and 2.8 U μg−1, respectively, while the median IgA titers against CTB increased from 3.0 U μg−1 prior to immunization to 12 U μg−1 after vaccination (Table 1). The titers of fecal antibody against CFA/I increased from 1.5 to 4.4 U μg−1 after vaccination, and the median CTB-specific IgA level in the stool was 6.4 U μg−1 before immunization and 35 U μg−1 after immunization (Table 1). Irrespective of the method used to estimate intestinal immunity, immune responses of very similar magnitudes were noted for the ETEC vaccinees (Table 1). Hence, the increase after immunization was two- to threefold for CFA/I-specific responses and around sevenfold for enterotoxin-specific responses; the magnitudes of the blood ASC responses to both antigens were higher.
Correlations between different immune responses.
In order to elucidate whether the methods listed in Table 1 actually measured the same responses, correlation analyses were carried out. A correlation was seen for vaccinees between the CTB-specific IgA responses in fecal samples and lavage fluid (r = 0.66; P = 0.01; n = 13). We could not determine whether there was a correlation between titers of antibody in lavage fluid and numbers of duodenal ASC because these assays were not performed on the same individuals. Nevertheless, there was a correlation for patients on day 3 (but not on day 9) between the levels of CFA-specific IgA in the stool and the numbers of duodenal IgA ASC (r = 0.68; n = 14; P = 0.007). No statistically significant correlation was seen between titers of antibody in duodenal protein extracts and numbers of duodenal ASC for either CFA/I or CTB (n = 13), even though the samples were collected from the same part of the intestine and at the same time. However, a correlation was found between the titers of IgA against CTB determined in saponin extracts and the numbers of blood IgA ASC directed against CTB in vaccinees (r = 0.58; n = 13; P = 0.04). No correlation was found between CTB-specific or CFA-specific blood IgA ASC and duodenal IgA ASC collected on the same day from vaccinees. However, a correlation was seen between CFA-specific IgA ASC isolated from the blood on day 3 (i.e., 6 days after the onset of infection) and duodenal IgA ASC collected on day 9 from infected individuals (r = 0.70; n = 10; P = 0.03).
Immune responses to ETEC antigens elicited by natural infection versus vaccination.
IgA responses induced by natural ETEC disease and vaccination were compared (Table 2). Although naturally acquired ETEC infection tended to elicit higher IgA responses to both CFA/I and CTB, these differences were statistically significant only with respect to CFA/I-specific IgA titers in fecal extracts and numbers of blood ASC directed against both antigens.
TABLE 2.
Comparison of immune responses to ETEC induced by natural disease and vaccination of adult Bangladeshi subjects
| Immune parameter | Mediana
(25th–75th percentile) (n) for:
|
Fold
increaseb
|
Statistical
significancec
|
||||
|---|---|---|---|---|---|---|---|
| Patients
|
Vacinees, day 21 | Day 3 | Day 9 | Day 3 | Day 9 | ||
| Day 3 | Day 9 | ||||||
| CFA-specific | |||||||
| Gut ASCd | 3,300 (400–11,000) (n = 8) | 2,500 (600–4,000)e (n = 4) | 1,200 (400–1,600) (n = 13) | 2.8 | 2.1 | NS | NS |
| Blood ASCd | 1,800 (230–4,800) (n = 10) | 380 (320–430) (n = 6) | 38 (16–99) (n = 27) | 48 | 10 | P = 0.0006 | P = 0.028 |
| Fecal Abf | 0.92 (0.28–2.5) (n = 6) | 18 (8–160) (n = 5) | 3.4 (1.0–6.6) (n = 18) | (3.4) | 5.2 | NS | P = 0.005 |
| CTB-specific | |||||||
| Gut ASC | 5,400 (750–24,000) (n = 12) | 5,100 (3,300–9,200) (n = 9) | 1,400 (0–7,000) (n = 13) | 3.7 | 3.5 | NS | NS |
| Blood ASC | 110 (50–180) (n = 13) | 150 (80–280) (n = 10) | 420 (190–660) (n = 27) | (3.7) | (2.8) | P = 0.028 | NS |
| Fecal Ab | 7.2 (2.5–20) (n = 8) | 33 (9–600) (n = 8) | 35 (11–66) (n = 18) | (5) | 1 | NS | NS |
Values are numbers of ASC or titers of antibody (Ab).
How many times higher the immune response was in patients than in vaccinees. Parentheses indicate that the immune response was higher in vaccinees.
Calculated by the Mann-Whitney test. A P value of <0.05 was considered significant. NS, not significant.
CFA/I.
The 25th to 75th percentile could not be calculated due to the paucity of patients; instead, the lowest and highest values are given.
CS5.
DISCUSSION
As yet, no study of intestinal immune responses in ETEC patients based on the determination of ASC responses in the duodenum has been reported. Our assumption that intestinal ASC may reflect protective immunity against ETEC is based on studies with animals. Thus, in a rabbit model, Evans and coworkers (9) found a highly significant inverse correlation between numbers of lamina propria cells producing IgA antibodies against CFA and the diarrheal response to challenge with an ETEC strain expressing the same CFA. Another study showed that when hybridomas derived from Peyer's patch lymphocytes which produced monoclonal IgA directed against bacterial antigen were implanted into mice, IgA was secreted into the gut lumen and the mice were protected against cholera (4), a disease that closely resembles ETEC disease. Furthermore, animal experiments have revealed that antisera against enterotoxin and lipopolysaccharide deposited locally in the intestine conferred synergistic protective immunity against challenge with virulent ETEC bacteria (3).
In this study we show that an oral ETEC vaccine and clinical ETEC infection induced ETEC-specific B-cell responses of comparable magnitudes in the intestine. Furthermore, the same isotype pattern, i.e., predominantly IgA ASC and IgM ASC, developed in the duodena of vaccinees and patients against ETEC CFs. In contrast, natural infection gave rise to 40-fold-higher levels of CFA-specific IgA ASC in blood than vaccination. However, it is possible that the latter difference may be an overestimation, since we measured blood ASC responses only after two doses of vaccine, and recent evaluations of the same vaccine with Egyptian adults have revealed that circulating CFA-specific IgA ASC responses peak after one oral dose of vaccine and decrease considerably after two doses, both with respect to responder frequency and with respect to the magnitude of the response (26). In our study, we could show that blood ASC specific for CFAs were predominantly of the IgA isotype in vaccinees and patients alike; ASC of the IgM and IgG isotypes were much less frequent. Regarding the B-cell responses to enterotoxin, the duodenal ASC levels were of the same magnitude in patients and vaccinees, but the blood ASC levels were higher in the vaccinated subjects.
It is not known whether ETEC colonize the whole small intestine or only parts of it. Levine et al. (15) have reported that ETEC could be cultured from jejunal fluid taken from North American volunteers experimentally infected with ETEC. In some instances, we were able to culture ETEC from the duodenal punch biopsy specimens of the infected patients. However, it is not clear whether the duodenum is the optimal part of the gut for studies of ETEC-specific intestinal immunity in humans. The parts of the small intestine exposed to the highest ETEC load are probably the sites where the largest numbers of ETEC-specific B cells will be found (21). Thus, there is a risk that we have underestimated the intestinal ASC responses in patients if the duodenum proves not to be the preferential colonization site of ETEC. This argument may be even more relevant to the immune responses determined for vaccinees, since Ogra and Karzon (21) showed that the secretory IgA responses to poliovirus of children immunized in the colon with an inactivated polio vaccine were entirely limited to the immunized intestinal segment.
An important question is whether the ETEC vaccine elicits immune responses of similar magnitudes in individuals who live in areas of ETEC endemicity and in those who live in areas where ETEC is not endemic. A comparison was made with the results of two Swedish studies in which volunteers received several doses of an oral cholera vaccine, consisting of the same amount of CTB as is present in the ETEC vaccine (23). Whereas the magnitudes of the vaccine-induced duodenal B-cell responses seemed to be comparable in populations in areas of ETEC endemicity and elsewhere, the responder frequency appeared to differ somewhat between the two populations. We found that 62% of the Bangladeshi vaccinees had significant duodenal B-cell responses to CTB, which may be compared to 80% among the Swedes (18, 23). One possible explanation for the relatively modest frequency of responders among the Bangladeshi vaccinees is that the high levels of ETEC-specific ASC observed prior to immunization may have neutralized vaccine antigens in the gut lumen, thus partly impeding the vaccine from reaching the gut-associated lymphoid tissue and further boosting immune responses (14, 32). Another explanation for the lower responder frequency in the population in the area of endemicity could be that the time of sampling was suboptimal. Thus, it is possible that maximal ETEC vaccine-induced responses are seen after one oral dose of vaccine in a population in an area of ETEC endemicity, as recently shown in an adult Egyptian population (26), but that two doses are required in a population in an area where ETEC is not endemic.
Although the determination of numbers of intestinal ASC is probably the most relevant assay of ETEC immunity, it is an invasive and time-consuming test which cannot be performed routinely, especially not on children. Therefore, it is desirable to find alternative methods based on more easily accessible specimens that reflect intestinal immunity. In this study we could show that antibody responses determined in stool extracts as well as intestinal-lavage fluid correlated with B-cell responses in duodenal biopsy specimens. In addition, a relationship was found between levels of circulating B cells and antibody titers in saponin extracts of duodenal biopsy specimens, but not with B cells isolated from the duodenum on the same day. However, a correlation did exist between levels of circulating B cells early during infection and B cells isolated from intestinal biopsy specimens 1 week later. These differences in kinetics may reflect the fact that B cells leave the intestinal mucosa and enter the circulation at the early stage of infection and later return to the site of induction, i.e., the intestinal mucosa. It is clear however, that B cells in the blood cannot be said to be proxy measures of intestinal B-cell responses. The finding that there was a much larger increase in blood ASC responses to ETEC antigens than in duodenal B-cell responses after two doses of vaccine underscores this point.
There is controversy as to how representative fecal antibodies are of intestinal immune responses. Ferguson et al. have suggested that measurement of fecal antibodies should be abandoned (10). These authors reported that fecal antibody measurements and intestinal-lavage antibody determinations yielded similar frequencies of responders among patients with active inflammatory bowel disease but that fecal antibody measurements could not be used to study specific antibodies in patients suffering from milder intestinal disorders. However, we could show a correlation between levels of fecal IgA against CFAs and the number of B cells specific for CFAs in the duodena of patients. In addition, it was recently demonstrated that a significant correlation existed between anti-CFA IgA titers in fecal extracts and intestinal-lavage fluid in Swedish volunteers who were given two doses of the ETEC vaccine (1). Feces may also be utilized for determining enterotoxin-specific IgA levels after vaccination, since we observed a correlation between CTB-specific IgA titers in lavage fluid and fecal extracts in the present study. The poor sensitivity of the fecal antibody assay, however, makes it less useful for assaying intestinal antibodies against CFAs induced by vaccination, at least in populations in areas of endemicity. To conclude, the determination of fecal antibody titers may be of value in monitoring intestinal immune responses in selected groups, most likely those with strong immune responses. We therefore propose that intestinal lavage should continue to be used for the evaluation of immunity to ETEC in settings where ETEC is endemic. It is a noninvasive method that reflects local immune responses. Whether alternative methods could be used for studies of ETEC immunity among infants remains to be evaluated.
A key question, which has not yet been resolved, is which antibody specificities, isotypes, and titers confer protective immunity against ETEC. Epidemiological studies in Mexico (7), studies with American volunteers (16), and animal studies suggest that immunity against bacteria is more important than immunity against toxins for prolonged protection (4, 9). It has been estimated that about one-third of ETEC strains express only the LT (11), which, due to its small size (19 amino acids), does not elicit adaptive immune responses. No one has yet been able to construct a conjugate protein containing ST with conserved immunogenicity and ablated toxicity. Thus, immunity against the bacteria is especially important for these strains. Notwithstanding, serotype-specific immunity does not appear to be very important in ETEC disease (7). A study of piglets infected with porcine ETEC strains revealed that the animals could be protected against disease caused by virulent ETEC by the enteric administration of egg yolk IgG directed against homologous CFs (36). Absorption of the antibody solution with fimbriae reduced its protective efficacy despite the presence of weakly agglutinating antibodies to O antigens. Interference with colonization appeared to be the main protective mechanism of this antibody preparation, since healthy piglets lacked ETEC organisms adherent to ileal sections, in contrast to sick piglets, as evidenced by scanning electron microscopy (36). In summary, anti-CF immunity is probably the cornerstone of long-lasting immunity to ETEC. More than 20 different CFs have been described in human ETEC strains, which may seem to preclude the production of a vaccine covering all fimbrial varieties. The vaccine presented in this study contains the most-prevalent CFs, which have been estimated to be present on 50 to 85% of clinical ETEC isolates (11). Fortunately, there is some antigenic homology between some of these antigens (11, 20), and natural infection has been proven to boost immunity against partly heterologous CFs (24). In the future, we would like to determine if immune responses against homologous and heterologous CFs as well as other ETEC antigens give rise to protective immunity. Hopefully, such studies may be conducted among children residing in areas of ETEC endemicity, which is the target group for future immunization against ETEC disease.
ACKNOWLEDGMENTS
The Swedish Agency for Research Collaboration with Developing Countries (SAREC), the World Health Organization, The Göteborg Medical Society, and The Swedish Medical Research Council supported this work (grant 16X09084).
REFERENCES
- 1.Ahrén C, Jertborn M, Svennerholm A-M. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia colivaccine and associated immunoglobulin A responses in blood. Infect Immun. 1998;66:3311–3316. doi: 10.1128/iai.66.7.3311-3316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahrén C, Wennerås C, Holmgren J, Svennerholm A-M. Intestinal antibody response after oral immunization with a prototype cholera B subunit-colonization factor antigen Escherichia colivaccine. Vaccine. 1993;11:929–934. doi: 10.1016/0264-410x(93)90380-g. [DOI] [PubMed] [Google Scholar]
- 3.Ahrén C M, Svennerholm A-M. Synergistic protective effect of antibodies against Escherichia colienterotoxin and colonization factor antigens. Infect Immun. 1982;38:74–79. doi: 10.1128/iai.38.1.74-79.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apter F M, Michetti P, Winner III L S, Mack J A, Mekalanos J J, Neutra M R. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio choleraeand cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993;61:5279–5285. doi: 10.1128/iai.61.12.5279-5285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black R E. Epidemiology of diarrhoeal disease: implications for control by vaccines. Vaccine. 1993;11:100–106. doi: 10.1016/0264-410x(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 6.Clemens J D, Sack D A, Harris J R, Chakraborty J, Neogy P K, Stanton B, Huda N, Khan M U, Kay B A, Khan M R, Ansaruzzaman M, Yunus M, Rao M R, Svennerholm A-M, Holmgren J. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale trial. J Infect Dis. 1988;158:372–377. doi: 10.1093/infdis/158.2.372. [DOI] [PubMed] [Google Scholar]
- 7.Cravioto A, Reyes R E, Trujillo F, Uribe F, Navarro A, de la Roca J M, Hernández J M, Pérez G, Vázquez V. Risk of diarrhea during the first year of life associated with initial and subsequent colonization by specific enteropathogens. Am J Epidemiol. 1990;131:886–904. doi: 10.1093/oxfordjournals.aje.a115579. [DOI] [PubMed] [Google Scholar]
- 8.Czerkinsky C, Nilsson L-Å, Nygren H, Ouchterlony Ö, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;115:31–37. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 9.Evans D G, de la Cabada F J, Evans D J., Jr Correlation between intestinal immune response to colonization factor antigen/I and acquired resistance to enterotoxigenic Escherichia colidiarrhea in an adult rabbit model. Eur J Clin Microbiol. 1982;1:178–185. doi: 10.1007/BF02019620. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson A, Humphreys K A, Croft N M. Technical report: results of immunological tests on faecal extracts are likely to be extremely misleading. Clin Exp Immunol. 1995;99:70–75. doi: 10.1111/j.1365-2249.1995.tb03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaastra W, Svennerholm A-M. Colonization factors of human enterotoxigenic Escherichia coli(ETEC) Trends Microbiol. 1996;4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 12.Helander A, Grewal H M S, Gaastra W, Svennerholm A-M. Detection and characterization of the coli surface antigen 6 of enterotoxigenic Escherichia coliby using monoclonal antibodies. J Clin Microbiol. 1997;35:867–872. doi: 10.1128/jcm.35.4.867-872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jertborn M, Ahrén C, Holmgren J, Svennerholm A-M. Safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia colivaccine. Vaccine. 1998;16:255–260. doi: 10.1016/s0264-410x(97)00169-2. [DOI] [PubMed] [Google Scholar]
- 14.Kantele A, Kantele J M, Arvilommi H, Mäkelä P H. Active immunity is seen as a reduction in the cell response to oral live vaccine. Vaccine. 1991;9:428–431. doi: 10.1016/0264-410x(91)90130-x. [DOI] [PubMed] [Google Scholar]
- 15.Levine M M, Black R E, Clements M L, Young C R, Cheney C P, Schad P, Collins H, Boedeker E C. Prevention of enterotoxigenic Escherichia coli diarrheal infection in man by vaccines that stimulate anti-adhesion (anti-pili) immunity. In: Boedeker E C, editor. Attachment of organisms to the gut mucosa. II. Boca Raton, Fla: CRC Press, Inc.; 1984. pp. 223–244. [Google Scholar]
- 16.Levine M M, Nalin D R, Hoover D L, Bergquist E J, Hornick R B, Young C R. Immunity to enterotoxigenic Escherichia coli. Infect Immun. 1979;23:729–736. doi: 10.1128/iai.23.3.729-736.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Vidal Y, Svennerholm A-M. Monoclonal antibodies against different subcomponents of colonization factor antigen II of enterotoxigenic Escherichia coli. J Clin Microbiol. 1990;28:1906–1912. doi: 10.1128/jcm.28.9.1906-1912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattsson A, Lönroth H, Quiding-Järbrink M, Svennerholm A-M. Induction of B cell responses in the stomach of Helicobacter pylori-infected subjects after oral cholera vaccination. J Clin Investig. 1998;102:51–56. doi: 10.1172/JCI22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McConnell M M, Chart H, Field A M, Hibberd M, Rowe B. Characterization of a putative colonization factor (PCFO166) of enterotoxigenic Escherichia coliof serogroup O166. J Gen Microbiol. 1989;135:1135–1144. doi: 10.1099/00221287-135-5-1135. [DOI] [PubMed] [Google Scholar]
- 20.McConnell M M, Chart H, Rowe B. Antigenic homology within human enterotoxigenic Escherichia colifimbrial colonization factor antigens: CFA/I, coli-surface-associated antigens (CS)1, CS2, CS4 and CS17. FEMS Microbiol Lett. 1989;61:105–108. doi: 10.1016/0378-1097(89)90179-1. [DOI] [PubMed] [Google Scholar]
- 21.Ogra P L, Karzon D T. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with polio vaccine. J Immunol. 1969;102:1423–1430. [PubMed] [Google Scholar]
- 22.Peltola H, Siitonen A, Kyrönseppä H, Simula I, Mattila L, Oksanen P, Kataja M J, Cadoz M. Prevention of travellers' diarrhoea by oral B-subunit/whole-cell cholera vaccine. Lancet. 1991;338:1285–1289. doi: 10.1016/0140-6736(91)92590-x. [DOI] [PubMed] [Google Scholar]
- 23.Quiding M, Nordström I, Kilander A, Andersson G, Hanson L Å, Holmgren J, Czerkinsky C. Intestinal immune responses in humans: oral cholera vaccination induces strong intestinal antibody responses and interferon-γ production and evokes local immunological memory. J Clin Investig. 1991;88:143–148. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudin A, Wiklund G, Wennerås C, Qadri F. Infection with colonization factor antigen I-expressing enterotoxigenic Escherichia coliboosts antibody responses against heterologous colonization factors in primed subjects. Epidemiol Infect. 1997;119:391–393. doi: 10.1017/s0950268897008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez J, Holmgren J. Recombinant system for overexpression of cholera toxin B subunit in Vibrio choleraeas a basis for vaccine development. Proc Natl Acad Sci USA. 1989;86:481–485. doi: 10.1073/pnas.86.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savarino S J, Brown F M, Hall E, Bassily S, Youssef F, Wierzba T, Peruski L, El-Masry N A, Safwat M, Rao M, Jertborn M, Svennerholm A-M, Lee Y J, Clemens J D. Safety and immunogenicity of an oral, killed enterotoxigenic Escherichia coli-cholera toxin B subunit vaccine in Egyptian adults. J Infect Dis. 1998;177:796–799. doi: 10.1086/517812. [DOI] [PubMed] [Google Scholar]
- 27.Savarino S J, Hall E R, Bassily S, Brown F M, Youssef F, Wierzba T F, Peruski L, El-Masry N A, Safwat M, Rao M, El Mohamady H, Abu-Elyazeed R, Naficy A, Svennerholm A-M, Jertborn M, Lee Y J, Clemens J D. Oral, inactivated, whole cell enterotoxigenic Escherichia coliplus cholera toxin B subunit vaccine: results of the initial evaluation in children. J Infect Dis. 1999;179:107–114. doi: 10.1086/314543. [DOI] [PubMed] [Google Scholar]
- 28.Stoll B J, Svennerholm A-M, Gothefors L, Barua D, Huda S, Holmgren J. Local and systemic antibody responses to naturally acquired enterotoxigenic Escherichia colidiarrhea in an endemic area. J Infect Dis. 1986;153:527–534. doi: 10.1093/infdis/153.3.527. [DOI] [PubMed] [Google Scholar]
- 29.Svennerholm A-M, Holmgren J. Identification of Escherichia coliheat-labile enterotoxin by means of a ganglioside immunosorbent assay (GM1-ELISA) procedure. Curr Microbiol. 1978;1:19–23. [Google Scholar]
- 30.Svennerholm A-M, Holmgren J, Black R, Levine M, Merson M. Serologic differentiation between antitoxin responses to infection with Vibrio cholerae and enterotoxin-producing Escherichia coli. J Infect Dis. 1983;147:514–522. doi: 10.1093/infdis/147.3.514. [DOI] [PubMed] [Google Scholar]
- 31.Svennerholm A-M, Wikström M, Lindblad M, Holmgren J. Monoclonal antibodies against Escherichia coliheat-stable toxin (STa) and their use in a diagnostic ST ganglioside GM1-enzyme-linked immunosorbent assay. J Clin Microbiol. 1986;24:585–590. doi: 10.1128/jcm.24.4.585-590.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker W A, Isselbacher K J. Intestinal uptake of macromolecules: effect of oral immunization. Science. 1972;177:608–610. doi: 10.1126/science.177.4049.608. [DOI] [PubMed] [Google Scholar]
- 33.Wennerås C, Svennerholm A-M, Czerkinsky C. Vaccine-specific T cells in human peripheral blood after oral immunization with an inactivated enterotoxigenic Escherichia colivaccine. Infect Immun. 1994;62:874–879. doi: 10.1128/iai.62.3.874-879.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wennerås C, Svennerholm A-M, Åhrén C, Czerkinsky C. Antibody-secreting cells in human peripheral blood after oral immunization with an inactivated enterotoxigenic Escherichia colivaccine. Infect Immun. 1992;60:2605–2611. doi: 10.1128/iai.60.7.2605-2611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Manual for the treatment of diarrhoea: for use by physicians and other senior health workers. CDD/SER/80.2 Rev 2. Geneva, Switzerland: World Health Organization; 1990. [Google Scholar]
- 36.Yokoyama H, Peralta R C, Diaz R, Sendo S, Ikemori Y, Kodama Y. Passive protective effect of chicken egg yolk immunoglobulins against experimental enterotoxigenic Escherichia coliinfection in neonatal piglets. Infect Immun. 1992;60:998–1007. doi: 10.1128/iai.60.3.998-1007.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]