Abstract
Background
The symptoms and signs of schizophrenia have been linked to high levels of dopamine in specific areas of the brain (limbic system). Antipsychotic drugs block the transmission of dopamine in the brain and reduce the acute symptoms of the disorder. An original version of the current review, published in 2012, examined whether antipsychotic drugs are also effective for relapse prevention. This is the updated version of the aforesaid review.
Objectives
To review the effects of maintaining antipsychotic drugs for people with schizophrenia compared to withdrawing these agents.
Search methods
We searched the Cochrane Schizophrenia Group's Study‐Based Register of Trials including the registries of clinical trials (12 November 2008, 10 October 2017, 3 July 2018, 11 September 2019).
Selection criteria
We included all randomised trials comparing maintenance treatment with antipsychotic drugs and placebo for people with schizophrenia or schizophrenia‐like psychoses.
Data collection and analysis
We extracted data independently. For dichotomous data we calculated risk ratios (RR) and their 95% confidence intervals (CIs) on an intention‐to‐treat basis based on a random‐effects model. For continuous data, we calculated mean differences (MD) or standardised mean differences (SMD), again based on a random‐effects model.
Main results
The review currently includes 75 randomised controlled trials (RCTs) involving 9145 participants comparing antipsychotic medication with placebo. The trials were published from 1959 to 2017 and their size ranged between 14 and 420 participants. In many studies the methods of randomisation, allocation and blinding were poorly reported. However, restricting the analysis to studies at low risk of bias gave similar results. Although this and other potential sources of bias limited the overall quality, the efficacy of antipsychotic drugs for maintenance treatment in schizophrenia was clear. Antipsychotic drugs were more effective than placebo in preventing relapse at seven to 12 months (primary outcome; drug 24% versus placebo 61%, 30 RCTs, n = 4249, RR 0.38, 95% CI 0.32 to 0.45, number needed to treat for an additional beneficial outcome (NNTB) 3, 95% CI 2 to 3; high‐certainty evidence).
Hospitalisation was also reduced, however, the baseline risk was lower (drug 7% versus placebo 18%, 21 RCTs, n = 3558, RR 0.43, 95% CI 0.32 to 0.57, NNTB 8, 95% CI 6 to 14; high‐certainty evidence). More participants in the placebo group than in the antipsychotic drug group left the studies early due to any reason (at seven to 12 months: drug 36% versus placebo 62%, 24 RCTs, n = 3951, RR 0.56, 95% CI 0.48 to 0.65, NNTB 4, 95% CI 3 to 5; high‐certainty evidence) and due to inefficacy of treatment (at seven to 12 months: drug 18% versus placebo 46%, 24 RCTs, n = 3951, RR 0.37, 95% CI 0.31 to 0.44, NNTB 3, 95% CI 3 to 4).
Quality of life might be better in drug‐treated participants (7 RCTs, n = 1573 SMD ‐0.32, 95% CI to ‐0.57 to ‐0.07; low‐certainty evidence); probably the same for social functioning (15 RCTs, n = 3588, SMD ‐0.43, 95% CI ‐0.53 to ‐0.34; moderate‐certainty evidence).
Underpowered data revealed no evidence of a difference between groups for the outcome ‘Death due to suicide’ (drug 0.04% versus placebo 0.1%, 19 RCTs, n = 4634, RR 0.60, 95% CI 0.12 to 2.97,low‐certainty evidence) and for the number of participants in employment (at 9 to 15 months, drug 39% versus placebo 34%, 3 RCTs, n = 593, RR 1.08, 95% CI 0.82 to 1.41, low certainty evidence).
Antipsychotic drugs (as a group and irrespective of duration) were associated with more participants experiencing movement disorders (e.g. at least one movement disorder: drug 14% versus placebo 8%, 29 RCTs, n = 5276, RR 1.52, 95% CI 1.25 to 1.85, number needed to treat for an additional harmful outcome (NNTH) 20, 95% CI 14 to 50), sedation (drug 8% versus placebo 5%, 18 RCTs, n = 4078, RR 1.52, 95% CI 1.24 to 1.86, NNTH 50, 95% CI not significant), and weight gain (drug 9% versus placebo 6%, 19 RCTs, n = 4767, RR 1.69, 95% CI 1.21 to 2.35, NNTH 25, 95% CI 20 to 50).
Authors' conclusions
For people with schizophrenia, the evidence suggests that maintenance on antipsychotic drugs prevents relapse to a much greater extent than placebo for approximately up to two years of follow‐up. This effect must be weighed against the adverse effects of antipsychotic drugs. Future studies should better clarify the long‐term morbidity and mortality associated with these drugs.
Plain language summary
Maintenance treatment with antipsychotic drugs for schizophrenia
Antipsychotic drugs are the mainstay of treatment of schizophrenia, not only in the event of acute episodes, but also in the long‐term perspective. While people might want to stop their treatment at some stage, recurrences of psychotic symptoms are known to occur after treatment discontinuation. Relapses can lead to risk of harm, loss of autonomy and substantial distress for individuals and their families.
The current report presents the update version of a systematic review previously published in 2012, and is based on 75 randomised controlled trials (RCTs) published over a long period since the 1950s and including more than 9000 participants. The effects of all antipsychotic drugs are here compared to those of placebo ‐ namely drug discontinuation ‐ for maintenance treatment, that is prevention of relapses. The aim is to explore the benefits and risks of each of the two options.
The results of this review show very consistently that antipsychotic drugs effectively reduce relapses and need for hospitalisation. Indeed, in case of treatment discontinuation, the risk of relapse at one year is almost three times higher. Antipsychotic drugs appear to have a positive effect on the ability to engage in activities and relationships, and on the possibility to fulfil remission from symptoms, although less evidence is available in this regard. Though based again on a lower number of reports, people continuing their treatment tend to experience higher satisfaction with their life, which confirms the negative consequences on well‐being of being at higher risk for recurrence. Conversely, antipsychotic drugs are, as a group, associated with a number of side effects such as movement disorders, weight gain and sedation. However, this review allows more understanding of the fact that stopping treatment is far more harmful than thoughtfully maintaining it.
Unfortunately, studies included in this review do generally last up to one year, and this makes difficult to clarify the longer‐term effect of these drugs. It is however true that the longer the study the more likely that other factors ‐ e.g. environmental – may accumulate and complicate the interpretation of results. Most of all, this review supports the advantages of antipsychotic drugs among many different types of participants. The best strategy would be therefore to continue treatment with antipsychotics, eventually discussing and adapting it if any adverse effect occurs.
Summary of findings
Summary of findings 1. Maintenance treatment with antipsychotic drugs versus placebo/no treatment for schizophrenia.
| Maintenance treatment with antipsychotic drugs versus placebo/no treatment for schizophrenia | ||||||
| Patient or population: schizophrenia Setting: inpatients and outpatients Intervention: maintenance treatment with antipsychotic drugs Comparison: placebo/no treatment | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Maintenance treatment with antipsychotic drugs versus placebo/no treatment | |||||
|
Relapse: 7 to 12 months Follow‐up: 7‐12 months |
606 per 1.000 | 230 per 1.000 (194 to 273) | RR 0.38 (0.32 to 0.45) | 4249 (30 RCTs) | ⊕⊕⊕⊕ HIGH1 2 3 4 | |
|
Leaving the study early: due to any reason (acceptability of treatment) Follow‐up: 1‐24 months |
541 per 1.000 | 292 per 1.000 (265 to 330) | RR 0.54 (0.49 to 0.61) | 7001 (56 RCTs) | ⊕⊕⊕⊕ HIGH5 6 | |
|
Service use: number of participants hospitalised Follow‐up: 1‐36 months |
177 per 1.000 | 76 per 1.000 (57 to 101) | RR 0.43 (0.32 to 0.57) | 3558 (21 RCTs) | ⊕⊕⊕⊕ HIGH6 7 | |
|
Death: due to suicide Follow‐up: 1‐15 months |
1 per 1.000 | 1 per 1.000 (0 to 4) | RR 0.60 (0.12 to 2.97) | 4634 (19 RCTs) | ⊕⊕⊝⊝ LOW6 8 | |
|
Quality of life (various scales; low score=better) Follow‐up: 3‐18 months |
The mean quality of life in the intervention group was 0.32 standard deviations lower (from 0.57 to 0.07 standard deviations lower), with lower scores reflecting a better condition. | ‐ | 1573 (7 RCTs) | ⊕⊕⊝⊝ LOW5 6 9 10 11 | SMD ‐0.32 (‐0.57 to ‐0.07) | |
|
Number of participants in employment Follow‐up: 9‐15 months |
344 per 1.000 | 372 per 1.000 (282 to 486) | RR 1.08 (0.82 to 1.41) | 593 (3 RCTs) | ⊕⊕⊝⊝ LOW6 12 13 | |
|
Social functioning (various scales; low score=better) Follow‐up: 1‐15 months |
The mean social functioning in the intervention group was 0.43 standard deviations lower (from 0.53 to 0.34 standard deviations lower), with lower scores reflecting a better condition. | ‐ | 3588 (15 RCTs) | ⊕⊕⊕⊝ MODERATE6 14 15 | SMD ‐0.43 (‐0.53 to ‐0.34) | |
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Publication bias: rated 'undetected' ‐ although the funnel plot was asymmetrical, the trim and fill test did not change the point estimate and the point estimate was also similar when only large studies were included (Analysis 3.5).
2 Risk of bias: rated 'no' ‐ many studies did not report the methods for sequence generation and/or allocation concealment. However, in subgroup analysis (Analysis 2.8) studies reporting high standards of methods showed a similar effect size as compared to studies with unclear methods. Also, in a sensitivity analysis excluding studies with unclear methods (Analysis 3.10 and Analysis 3.11), the effect sizes did not change substantially. Early terminated studies were not judged to contribute substantial weight to this outcome.
3 Inconsistency: rated 'no' ‐ the P value for heterogeneity was statistically significant and the I2 higher than 50%. However, results of individual studies differed rather in magnitude of effect (which could be partly explained by subgroup analyses) rather than in direction of effect. Therefore, this inconsistency does not challenge the overall results.
4 No indirectness was found in terms of study population nor of interventions. In terms of outcome, we followed the original authors definitions of relapse. These definitions used different criteria, but all addressed symptomatic deterioration related to relapse. Therefore, this was not judged to lead to indirectness.
5 Inconsistency: rated 'no' ‐ the P value for heterogeneity was statistically significant and the I‐square higher than 50%. However, results of individual studies differed rather in magnitude of effect than in direction of effect, which was the same in almost all the studies. Therefore, this inconsistency does not challenge the overall results.
6 Publication bias: it is unlikely that a study was unpublished because of unfavourable data in a secondary outcome. As a possible publication bias had no effect on the results for the primary outcome (relapse at 7 to 12 months), we deem that there was no relevant publication bias for this secondary outcome.
7 Indirectness: hospitalisation due to relapse was our primary interest, but in some studies reasons for hospitalisation were unclearly reported. Overall, we do not deem that this uncertainty was an important source of indirectness.
8 Imprecision: rated 'very serious' ‐ only few studies with few events contributed data to this outcome. The CI was wide, ranging from substantial harm to substantial benefit.
9 Risk of bias: rated 'serious' ‐ five out of seven studies were terminated early after interim analyses, possibly leading to overestimation of effect.
10 Indirectness: some rating scales used in the studies have been criticised for eventually not measuring what people understand by quality of life. However, it was decided not to further lower the quality of evidence for this outcome after downgrading for other factors, despite some uncertainty.
11 Imprecise data ‐ only a few studies provided data for this outcome and the confidence interval was large.
12 Indirectness: rated 'serious' ‐ the only three studies included mixed groups of employed and non‐employed participants at baseline, and it is unclear whether employment was supported or competitive employment.
13 Imprecision: rated 'serious' ‐ only three studies contributed to this event which depends on various factors (e.g. the existence of supported employment, rural versus service economy etc).
14 Risk of bias: rated 'serious' ‐ eleven out of fifteen studies were terminated early after interim analyses, possibly leading to overestimation of effects.
15 Indirectness: rated 'no' ‐ different rating scales were used in the studies, but this was not judged to challenge the results.
Background
Description of the condition
Schizophrenia is often a chronic and disabling psychiatric disorder. It afflicts approximately 1% of the population worldwide with few gender differences (McGrath 2008). Its typical manifestations are 'positive' symptoms such as fixed, false beliefs (delusions) and perceptions without cause (hallucinations); 'negative' symptoms such as apathy and lack of drive, disorganisation of behaviour and thought; and catatonic symptoms such as mannerisms and bizarre posturing (Carpenter 1994). The degree of suffering and disability is considerable with 80% to 90% of people not employed (Marvaha 2004) and up to 10% dying (Tsuang 1978).
Description of the intervention
Antipsychotic drugs are the mainstay of treatment for schizophrenia. They can be classified according to their biochemical structure (e.g. butyrophenones, phenothiazines, thioxanthenes, etc.), the doses necessary for an antipsychotic effect (high‐potency versus low‐potency antipsychotic drugs), and their risk of producing movement disorders ('atypical' versus 'typical' antipsychotic drugs). What they all have in common is that they block, to a greater or lesser extent, the transmission of dopamine in the brain. Currently there is not a single antipsychotic drug available that is not a dopamine receptor antagonist and the hypothesis that dopamine plays a role in the causation of schizophrenia has been partly derived from the mechanism of action of antipsychotic drugs (Berger 2003). Furthermore, there is no firm evidence that ‐ except for clozapine and possibly some other second‐generation antipsychotic drugs (Kane 1988; Leucht 2009; Leucht 2009a; Leucht 2013; Wahlbeck 1999) ‐ any of these agents is more effective than another (Klein 1969). Early (non‐systematic) reviews (Baldessarini 1985; Davis 1975) have shown that keeping people with schizophrenia on antipsychotic drugs after successful treatment of the acute episode substantially lowers relapse risk, for example, from 53.2% to 15.6% within a period of approximately 9.7 months (Gilbert 1995). Conversely, the side‐effect burden can be considerable, as antipsychotic drugs produce movement disorders, sedation, weight gain and are even related with sudden death. Therefore, clinicians and those with schizophrenia often face a trade‐off between protection against further psychotic episodes and adverse effects.
How the intervention might work
The theory is that schizophrenia is a chronic disorder caused by hyperdopaminergic states in the limbic system (Berger 2003). All antipsychotic drugs block dopamine receptors. Continuous treatment with antipsychotic drugs may be necessary to keep the dopaminergic tone low and to avoid psychotic relapses.
Why it is important to do this review
Although previous reviews had shown that maintenance treatment with antipsychotic drugs reduces relapse rates (Baldessarini 1985; Davis 1975; Gilbert 1995), they did not meet modern systematic review criteria and addressed only one outcome (relapse). The present review is an update of the previous Cochrane Review of Maintenance treatment with antipsychotic drugs for schizophrenia (Leucht 2012b). This update is important, because a lot of evidence has emerged since 2012.
Objectives
To review the effects of maintaining antipsychotic drug treatment for people with schizophrenia compared with withdrawing these agents.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials (RCTs). We excluded quasi‐randomised trials, such as those where allocation is undertaken on surname. If a trial was described as double‐blind, but it was implied it had been randomised, we included it, but excluded such trials in a sensitivity analysis. Randomised cross‐over studies were eligible but only data up to the point of first cross‐over were used because of the instability of the problem behaviours and the likely carry‐over effects of the treatments (Elbourne 2002).
Types of participants
We included people with schizophrenia and schizophrenia‐like psychoses (schizophreniform and schizoaffective disorders) who had stabilised on antipsychotic medications. There is no clear evidence that the schizophrenia‐like psychoses are caused by fundamentally different disease processes or require different treatment approaches (Carpenter 1994).
Types of interventions
Antipsychotic drugs: any dose or mode of administration (oral or by injection). There is no evidence for large differences in the efficacy of the available antipsychotic drugs (e.g. Davis 1989; Duggan 2005; Leucht 2009; Srisurapanont 2004). All currently available antipsychotic drugs have in common that they act via the blockade of dopamine and their classification according to their chemical properties (e.g. butyrophenones, thioxanthenes or phenothiazines) does not have an important clinical impact. Other classifications into 'low‐ versus high‐potency' or 'typical versus atypical' are continuums, at best (Leucht 2009). We therefore decided to include all antipsychotic drugs that are currently on the market in at least one country.
Active or inactive placebo, or no treatment.
Types of outcome measures
The outcomes were analysed for different lengths of follow‐up: up to three months, four to six months, seven months to one year and more than one year.
Primary outcomes
Relapse at one year (seven to 12 months) as defined by the original studies or by a deterioration in mental state requiring further treatment. Overall relapse and relapse at other time points were considered as secondary outcomes.
Secondary outcomes
The following outcomes were added to the list for this update: number of participants in symptomatic remission, number of participants in sustained remission, number of participants in recovery, social functioning.
1. Relapse
1.1 Across the pre‐specified time periods (please see above). 1.2 Independent of duration
2. Leaving the study early
2.1 Due to any reason (acceptability of treatment) 2.2 Due to adverse events (overall tolerability) 2.3 Due to inefficacy
3. Global state
3.1 Improved (at least minimally) 3.2 In symptomatic remission 3.3 In sustained remission 3.4 In recovery
4. Service use
4.1 Number hospitalised 4.2 Number discharged
5. Death
5.1 Due to any reason 5.2 Due to natural causes 5.3 Due to suicide
6. Suicidal behaviour
6.1 Number with suicide attempts 6.2 Number with suicide ideation
7. Violent/aggressive behaviour
8. Adverse effects
8.1 General: at least one adverse event 8.2 Specific: movement disorders 8.2.1 At least one movement disorder 8.2.2 Akathisia 8.2.3 Akinesia 8.2.4 Dyskinesia 8.2.5 Dystonia 8.2.6 Rigor 8.2.7 Tremor 8.2.8 Use of antiparkinson medication 8.3 Specific: sedation 8.4 Specific: weight gain
9. Satisfaction with care (any published rating scale)
9.1 Participants satisfied 9.2 Carers satisfied
10. Quality of life (any published rating scale)
11. Functioning
11.1 Number in employment 11.2 Social functioning (any published rating scale)
'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2008) and used GRADEPRO to import data from Review Manager to create a 'Summary of findings' table. This table provides outcome‐specific information concerning the overall certainty of evidence from each included study in the comparison, the magnitude of effect of the interventions examined and the sum of available data on all outcomes that we rated as important to patient care and decision making. We anticipated including the following long‐term main outcomes in a 'Summary of findings' table:
relapse: seven to 12 months;
leaving the study early: due to any reason (acceptability of treatment);
service use: number hospitalised;
death: due to suicide;
quality of life (any published rating scale);
functioning: number in employment;
functioning: social functioning (any published rating scale).
Search methods for identification of studies
No language restriction was applied within the limitations of the search tools.
Electronic searches
Cochrane Schizophrenia Group’s Study‐Based Register of Trials
On 10 October 2017, the Information Specialist searched the register using the following search strategy which has been developed based on literature review and consulting with the authors of the review:
((*Cessation* OR *Discontinu* OR *Halt* OR *Maintain* OR *Maintenance* OR *Recur* OR *Rehospitali* OR *Re‐Hospitali* OR *Relaps* OR *Stop* OR *Withdr*) in Title OR Abstract Fields of REFERENCE OR (Maintenance Treatment*) in Intervention Field of STUDY) AND ((*Amisulpride* OR *Aripiprazole* OR *Asenapine* OR *Benperidol* OR *Brexpiprazole* OR *Cariprazine* OR *Chlorpromazine* OR *Clopenthixol* OR *Clozapine* OR *Flupenthixol* OR *Fluphenazine* OR *Fluspirilene* OR *Haloperidol* OR *Iloperidone* OR *Levomepromazine* OR *Methotrimeprazine* OR *Loxapine* OR *Lurasidone* OR *Molindone* OR *Olanzapine* OR *Paliperidone* OR *Penfluridol* OR *Perazine* OR *Perphenazine* OR *Pimozide* OR *Quetiapine* OR *Risperidone* OR *Sertindole* OR *Sulpiride* OR *Thioridazine* OR *Thiothixene* OR *Trifluoperazine* OR *Ziprasidone* OR *Zotepine* OR *Zuclopenthixol*) in Intervention Field of STUDY)
In such study‐based register, searching the major concept retrieves all the synonyms and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics (Shokraneh 2017).
This register is compiled by systematic searches of major resources (AMED, BIOSIS, CINAHL, ClinicalTrials.Gov, Embase, MEDLINE, PsycINFO, PubMed, WHO ICTRP) and their monthly updates, ProQuest Dissertations and Theses A&I and its quarterly update, Chinese databases (CBM, CNKI, and Wanfang) and their annual updates, handsearches, grey literature, and conference proceedings (see Group’s Module). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
This search was conducted for a broader project and includes studies comparing antipsychotic drugs for relapse prevention (head‐to‐head studies).
On 3 July 2018 first and then on 11 September 2019, a further updated search of the register was performed. The following search strategy, which was also developed consulting with the authors of the review, was used in both cases:
(*{AP}* in Intervention Field of Study) AND ((*Cessation* OR *Discontinu* OR *Halt* OR *Maintain* OR *Maintenance* OR *Recur* OR *Rehospitali* OR *Re‐Hospitali* OR *Relaps* OR *Stop* OR *Withdr*) in Title OR Abstract Fields of REFERENCE OR (Maintenance Treatment*) in Intervention Field of STUDY); {AP} refers to all antipsychotic drugs in the register.
For previous searches, please see Appendix 1.
Searching other resources
1. Reference searching
We inspected the references of all included studies and of previous reviews (e.g. Davis 1975; Gilbert 1995) for more trials. The targeted update version of this review performed in 2016 was also inspected (New Reference).
2. Personal contact
We contacted the first author of each included study for missing information and for the existence of further studies.
3. Drug companies
We contacted the manufacturers of antipsychotic drugs and asked them about further relevant studies and for missing information on identified studies.
Data collection and analysis
Selection of studies
For the 2019 search, two review authors (JS, AC) identified and independently inspected citations. For the 2018 search, identified citations were independently inspected by two review authors (AC, JL). For the 2017 search, identified citations were independently inspected by two review authors (among JS, AC and JL). For the original search, two review authors (SL, KK) identified and independently inspected citations. We identified potentially relevant reports and ordered full‐text papers for reassessment. Where disagreements arose we asked a third member of the team for help, and if it was impossible to decide, the full papers were ordered for assessment. This process was repeated for the full papers. If it was impossible to resolve disagreements these studies were added to those awaiting classification and we contacted the authors of the papers for clarification.
Data extraction and management
1. Extraction
For this update, three review authors (AC, JL, JS) independently extracted data from included studies. For the original review, three review authors (SL, MT, KK) independently extracted data from the included studies. Any disagreement was discussed with another member of the review team, decisions documented and, if necessary, we contacted authors of studies for clarification. The studies included in the original review were closely inspected in order to collect data on the outcomes that were added to the list within the updating process, and to look for potentially new information from eventual recent secondary publications.
2. Management
For the original review, we extracted data onto standard simple forms. For the review update, we extracted data using electronic forms in Microsoft Access.
3. Scale‐derived data
3.1 Valid measures
We included continuous data from rating scales only if: (a) the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000); (b) the measuring instrument was not written or modified by one of the trialists.
3.2 Endpoint versus change data
Since there is no principal statistical reason why endpoint and change data should measure different effects (Higgins 2011, we decided primarily to use scale change data. If change data were not available we used endpoint data. Endpoint and change data were presented in separate subgroups, then pooled in the final analysis.
4. Common measure
To facilitate comparison between trials, we intended to convert variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
5. Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for maintenance treatment.
Assessment of risk of bias in included studies
Three review authors (AC, JL, JS) for this update and three review authors (SL, MT, KK) for the original review worked independently by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess trial quality. This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article, such as sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, other potential sources of bias (i.e. fraud, premature interruption of the studies, baseline clinical imbalances among study groups).
Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain additional information.
We noted the level of risk of bias in the text of the review, the 'Risk of bias' tables and in the Table 1.
Measures of treatment effect
1. Dichotomous data
The review focused on binary data, which are easier to interpret and can be more intuitively understood. For binary outcomes we calculated a standard estimation of the random‐effects (Der‐Simonian 1986) risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios (ORs) and that ORs tend to be interpreted as RR by clinicians (Deeks 2000). This mis‐interpretation then leads to an overestimate of the impression of the effect. For statistically significant results we calculated the number needed to treat for an additional beneficial outcome/number needed to treat for an additional harmful outcome statistic (NNTB/NNTH), and its 95% CI as the inverse of the risk difference (RD).
Where possible, efforts were made to convert outcome measures to dichotomous data. This could be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It was generally assumed that if there had been a 20% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS; Kay 1986), this could be considered as a minimally significant response (Leucht 2005a; Leucht 2005b). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2. Continuous data
2.1 Summary statistic
For continuous outcomes we estimated a mean difference (MD) between groups. MDs were based on the random‐effects model as this takes into account any differences between studies even if there is no statistically significant heterogeneity. In the case of where scales were judged of such similarity to allow pooling, we calculated the standardised mean difference (SMD) and, whenever possible, transformed the effect back to the units of one or more of the specific instruments.
All the numbers were entered in a way that a decrease in score should indicate improvement (for change data), and a lower score a better outcome (for endpoint data), in order to provide a similarity to the Positive and Negative Syndrome Scale (PANSS, Kay 1986), and make the numbers comparable and easy to interpret. When a rating scale construct provided for a higher score to indicate a better outcome, a minus (‐) was added before the numbers.
2.3 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to all data before inclusion:
(a) data from studies of at least 200 participants were entered in the analysis irrespective of the following rules, because skewed data pose less of a problem in large studies;
(b) change data: when continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to determine whether data are skewed or not. We entered the study, because change data tend to be less skewed and because excluding studies would also lead to bias, because not all the available information was used;
(c) endpoint data: when a scale starts from the finite number zero, we subtracted the lowest possible value from the mean, and divided this by the standard deviation. If this value was lower than 1, it strongly suggested a skew and the study was excluded. If this ratio was higher than 1 but below 2, there is suggestion of skew. We entered the study and tested whether its inclusion or exclusion substantially changed the results. If the ratio was larger than 2 the study was included, because skew is less likely (Altman 1996; Higgins 2011).
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. First, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, CIs unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
Where clustering is not accounted for in primary studies, we presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering had been incorporated into the analysis of primary studies, we present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICCs [design effect = 1 + (m ‐ 1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in schizophrenia, randomised cross‐over studies were eligible but only data up to the point of first cross‐over.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, especially two appropriate dose groups of an antipsychotic drug, the different dose arms were pooled and considered to be one. Where the additional treatment arms were not relevant, we did not reproduce these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss to follow‐up, data must lose credibility (Xia 2009). The loss to follow‐up in randomised schizophrenia trials is often considerable calling the validity of the results into question. Nevertheless, it is unclear which degree of attrition leads to a high degree of bias. We did not exclude trials from outcomes on the basis of the percentage of participants completing them. However, we used the 'Risk of bias' tool described above to indicate potential bias when more than 25% of the participants left the studies prematurely, when the reasons for attrition differed between the intervention and the control group and when no appropriate imputation strategies were applied.
2. Dichotomous data
We presented data on a 'once‐randomised‐always‐analyse' basis, assuming an intention‐to‐treat (ITT) analysis. If the authors applied such a strategy, we used their results. If the original authors presented only the results of the per‐protocol or completer population, we assumed that those participants lost to follow‐up would have had the same percentage of events as those who remained in the study.
3. Continuous data
3.1 General
ITT was used when available. We anticipated that in some studies, in order to perform an ITT analysis, the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leon 2006). Therefore, where LOCF data have been used in the analysis, they are indicated in the review.
3.2 Missing standard deviations
Where there are missing measures of variance for continuous data but an exact standard error and CI are available for group means, either 'P' value or 't' value are available for differences in mean, we calculated the standard deviation value according to method described in Section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If standard deviations were not reported and could not be calculated from available data, we asked authors to supply the data. In the absence of data from authors, we used the mean standard deviation from other studies.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations that we had not predicted would arise and, where found, discussed such participant groups or situations.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods, which we had not predicted, would arise and discussed any such methodological outliers.
3. Statistical heterogeneity
3.1. Visual inspection
We inspected graphs visually to investigate the possibility of statistical heterogeneity.
3.2. Employing the I² statistic
We investigated heterogeneity between studies by considering the I² statistic alongside the Chi² P value. The I² statistic provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I² statistic depends on both the magnitude and direction of effects and the strength of evidence for heterogeneity (e.g. P value from the Chi² test, or 95% CIs for the I² statistic). An I² statistic estimate equal or greater than 50% accompanied by a statistically significant Chi² statistic would be interpreted as evidence of substantial levels of heterogeneity (Higgins 2011). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots were possible, we sought statistical advice in their interpretation.
Data synthesis
We employed a random‐effects model for analyses (Der‐Simonian 1986). We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This does seem true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. Therefore, the random‐effects model is usually more conservative in terms of statistical significance, although as a disadvantage it puts added weight onto smaller studies, which can either inflate or deflate the effect size. We examined in a secondary analysis whether using a fixed‐effect model markedly changed the results of the primary outcome.
Subgroup analysis and investigation of heterogeneity
Reasons for heterogeneity in the primary outcome were explored by the following subgroup analyses and restricted‐maximum‐likelihood‐random‐effect meta‐regressions, the latter performed using meta v4.9‐9 (Schwarzer 2007) in R statistical language v3.6.2 (R Core Team 2018). The R code used for meta‐regressions is reported in Appendix 2. Post‐hoc analyses are marked with an asterisk.
Subgroup analyses addressed people with only one episode of schizophrenia and people in remission at baseline, who may both have a better prognosis. We examined people who had been stable for different durations before study entry (at least three, six, nine, 12 and more than 12 months) to find out whether after long‐term stability antipsychotic drugs are no longer necessary. Abrupt versus gradual withdrawal of the pre‐study antipsychotic drug, defined as a minimum taper period of three weeks or depot treatment before the study following Viguera 1997*, was examined because abrupt withdrawal may lead to rebound psychoses. Other subgroup analyses addressed: single antipsychotic drugs*, depot versus oral medication* (depot drugs are thought to be superior due to better compliance), first‐ versus second‐generation antipsychotic drugs* (to address the debate whether the more expensive second‐generation drugs are more efficacious), unblinded versus blinded trials* and studies with appropriate and unclear allocation concealment methods*.
Duration of stability before study entry and duration of taper in the placebo group were also examined by meta‐regressions. Other meta‐regressions addressed severity of illness at baseline, mean dose in chlorpromazine equivalents and study duration. Meta‐regressions were performed only if at least 10 studies per comparison were available (Higgins 2011). For the dose conversion to chlorpromazine equivalents, doses were transferred following the conversion factors provided by available publications (Davis 1974, Gardner 2010, Gopal 2010). Regarding long‐acting injectable drugs, the mean daily dose was obtained by dividing the given dose by the injection interval, and then transferred to chlorpromazine equivalents.
Sensitivity analysis
All sensitivity analyses were made only for the primary outcome. Some of them were performed post‐hoc, due to the fact that reviewers of the original Lancet publication (Leucht 2012a) asked for them.
1. Implication of randomisation
We excluded studies in a sensitivity analysis if they were described in some way as to imply randomisation. If there was no substantive difference when the implied randomised studies were excluded or added to those with better description of randomisation, then all data were employed from these studies.
2. Implication of non double‐blind trials
We excluded trials in a sensitivity analysis if they were not double‐blinded. If there was no substantive difference when the non double‐blind studies were excluded or added to the double‐blind studies, then all data were employed from these studies.
3. Fixed‐effect model
A sensitivity analysis was performed employing a fixed‐effect model for the analysis of data for all the relevant studies, in order to examine whether applying a different approach markedly changed the results of the primary outcome or not.
4. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings when we used our assumption compared with completer data only. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
5. Inclusion of large studies only
We included trials in a sensitivity analysis only if at least 200 participants were enrolled.
6. Exclusion of studies that used clinical criteria to diagnose the participants
We excluded trials in a sensitivity analysis if either enrolled participants were diagnosed with schizophrenia only on a clinical basis, or no mention to the use of specific operational diagnostic criteria was made.
7. Inclusion of only those participants who had been in the trials without a relapse for specific time intervals
Secondary analyses were performed entering only data of those participants who had not relapsed for various durations after study start (three months, six months, nine months). Relapse risks resulted therefore from the number of relapse events from the beginning at the time interval till the end of a study, divided by the patients at risk of relapse, who had not relapsed before.
8. Exclusion of studies with unclear randomisation/allocation concealment methods
We excluded trials in a sensitivity analysis if a detailed description of the randomisation method used was not provided. In another sensitivity analysis, trials were excluded whether the method of allocation concealment was judged to be inadequately clarified
Summary of findings and assessment of the certainty of the evidence
Results
Description of studies
For substantive description of studies please see Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The original search in the CSG register yielded 1163 reports and two previous reviews contained 66 (Gilbert 1995) and 24 studies (Davis 1975). The update search in 2011 identified another 669 reports. Overall, 185 studies were closely inspected. We included 116 publications on 65 studies and we excluded 69 publications on 49 studies. See Study flow diagram (Figure 1).
1.
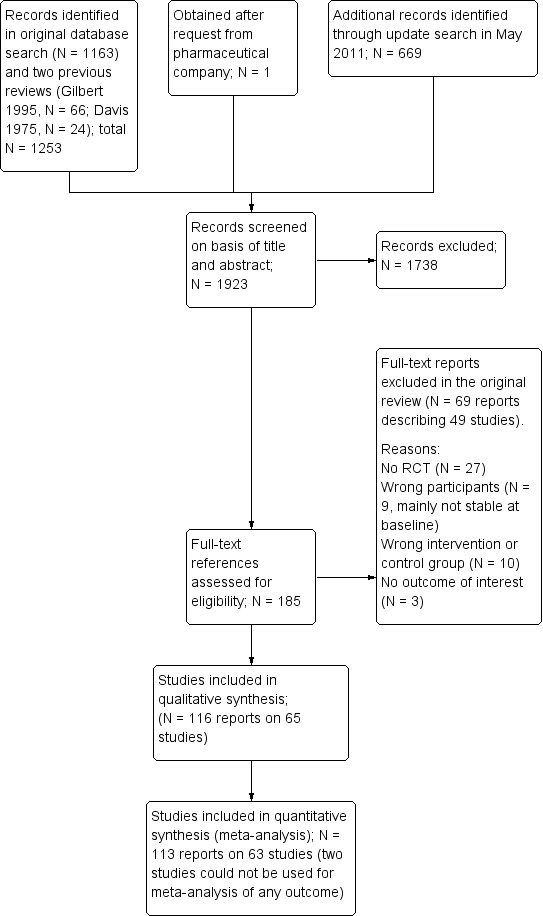
Study flow diagram (results of the original search)
For the review update in 2018: 3 reports describing the 2 studies originally excluded from quantitative synthesis were moved to excluded studies (no usable data for outcomes of interest); 3 reports on 1 study, originally excluded (short duration of follow‐up), were moved to included studies; one report originally included as independent study was moved as secondary publication of another included study.
The update search performed in October 2017 yielded 3562 reports; the update search performed in July 2018 yielded 295 reports; the update search performed in September 2019 yielded 137 reports. An additional five reports were identified through other sources (handsearch in our research group's database of schizophrenia trials). Overall, 198 reports were closely inspected within the update process. We included 80 publications on 12 studies, and we excluded 35 studies (81 publications); five reports are still awaiting classification after contacting the corresponding authors, and three reports on three ongoing and potentially relevant trials were also identified. Twenty‐nine reports were moved as secondary publications of already included/excluded studies. See Study flow diagram (Figure 2).
2.
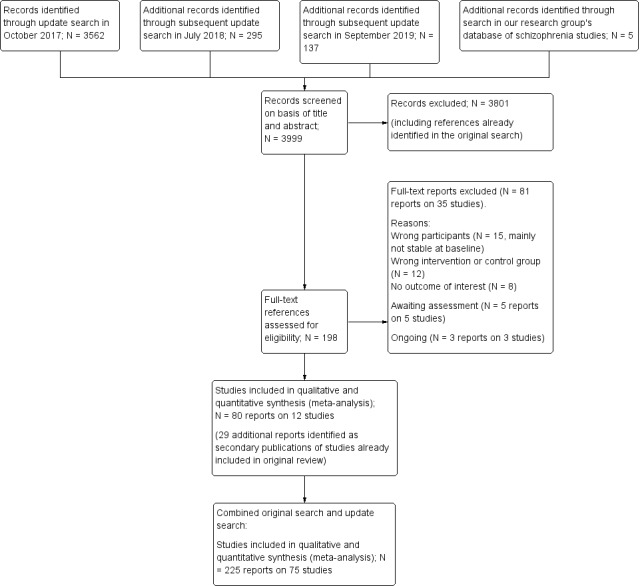
Study flow diagram (results of the 2017/2018/2019 update search and combined results of the original search and the update search)
For this update, one study (originally referenced as 'Pfizer 2000', previously included as an independent study obtained from a pharmaceutical company) was found to be an unpublished report of another included study (Ziprasidone 2002), and was therefore moved into this study; the references reported a slightly different sample size, as one recruiting centre was removed due to protocol deviation, but the reported study ID was the same (128‐303). One study (Olanzapine 1999) was previously excluded due to short duration of follow‐up (one to three days), but was then moved to included studies (a sensitivity analysis of the outcome relapse excluding this study was performed and found no different results); two studies (Gitlin 1988 and Hirsch 1996) were previously included in the qualitative synthesis but not in the meta‐analysis, due to the absence of usable outcome data. For this update, they were moved to excluded studies (see Figure 1).
Overall, 225 publications on 75 studies were included and 150 publications on 85 studies were excluded. See Study flow diagram (Figure 2).
Included studies
Seventy‐five studies (9145 participants) met the inclusion criteria.
1. Length of trials
Of the included studies, 17 had a duration up to three months. Twenty‐six studies lasted up to six months and 25 up to 12 months. Seven studies lasted more than 12 months. The longest study had a duration of three years.
2. Participants
In 33 of the 65 studies, participants were diagnosed according to clinical diagnoses (i.e. specific diagnostic criteria were not mentioned). The others used a variety of tools, combinations of tools and versions of those tools.
| Number of studies | Diagnostic tool | Version | + additional tool |
| 4 | Diagnostic and Statistical Manual | II | |
| 5 | III | ||
| 2 | III‐R | ||
| 9 | IV | ||
| 10 | IV‐TR | ||
| 1 | IV Axis I Disorders (Structured Clinical Interview) | ||
| 3 | Present State Examination (PSE) | ||
| 3 | Research Diagnostic Criteria (RDC) | ||
| 1 | + Schedule for Affective Disorder | ||
| 1 | + PSE + Feighner's criteria | ||
| 1 | Feighner's criteria | ||
| 1 | International Statistical Classification of Diseases and Related Health Problems (ICD‐9) | + RDC | |
| 1 | + PSE | ||
The average age of participants was around 45 years old, and the mean duration of illness well over two decades (26.2 years). In 13 studies, participants were in remission at baseline.
3. Setting
Twenty‐nine studies were conducted in hospitals (at least at the start of the trial) and 34 studies in outpatients. Seven studies included both inpatients and outpatients. Several important and [mostly] quite recent studies did not report on setting (Asenapine 2011, Lurasidone 2016, Paliperidone depot1M 2010, Penfluridol 1987, Quetiapine 2007).
4. Study size
The average number of participants was 122 (median 67). Chlorpromazine 1968 was the largest study with 420 participants, while Chlorpromazine 1975 was the smallest, randomising only 14 people. Thirty‐four studies had fewer than 50 participants and 19 randomised more than 200. The oldest trials were some of the largest but, in recent years the size did seem to be increasing (Figure 3).
3.

Size of trial over time
5. Interventions
Seventy‐three studies compared maintenance treatment with antipsychotic drugs and inactive placebo; two open randomised controlled trials (RCTs) compared antipsychotic drugs with no treatment. No data on active placebo as a comparator were available. In most studies flexible doses of antipsychotic drugs were employed although some trials did use fixed doses (see table below). The older trials did have ranges which could have included using doses that would be considered very high now. For example, the doses in Pimozide 1971 and Trifluoperazine 1969 were very high (pimozide 40 mg/day and trifluoperazine 80 mg/day, respectively) and in Various drugs 1982 (chlorpromazine 75 mg/day, haloperidol 3 mg/day) they were very low. However, in most cases most participants would have been given doses of drugs well within the usual ranges employed in current day‐to‐day practice.
| Flexible doses | Fixed doses | ||
| Drug | Dose range | Drug | Fixed doses |
| aripiprazole | 10 mg/day to 30 mg/day | aripiprazole long‐acting | 300 mg or 400 mg four‐weekly |
| brexpiprazole | 1 mg/day to 4 mg/day | haloperidol decanoate | 60 mg four‐weekly |
| cariprazine | 3 mg/day to 9 mg/day | olanzapine | 10 mg/day, 15 mg/day or 20 mg/day |
| chlorpromazine (equivalent) | 50 mg/day to 1000 mg/day | paliperidone depot | 25 mg, 50 mg,100 mg or 150 mg four‐weekly or 175 mg, 263 mg, 350 mg or 525 mg twelve‐weekly |
| flupenthixol depot | 20 mg to 40 mg three‐weekly | zotepine | 300 mg/day |
| fluphenazine decanoate | 1.25 mg to 5 mg twice‐weekly | ||
| fluphenazine depot | 12.5 mg to 25 mg three‐weekly or 25 mg to 50 mg four‐weekly | ||
| iloperidone | 8 mg/day to 24 mg/day | ||
| paliperidone | 3 to mg/day 15 mg/day | ||
| penfluridol | 10 mg/week to 160 mg/week | ||
| perphenazine | 8 mg/day to 24 mg/day | ||
| pimozide | 2 mg/day to 40 mg/day | ||
| prochlorpromazine | 15 mg/day to 150 mg/day | ||
| promazine | 200 mg/day to 400 mg/day | ||
| quetiapine | 500 mg/day to 800 mg/day | ||
| thioridazine | 75 mg/day to 1000 mg/day | ||
| trifluoperazine | 5 mg/day to 50 mg/day | ||
| ziprasidone. | 40 mg/day to 160 mg/day | ||
In a number of studies various antipsychotic drugs could be administered.
6. Sponsor
Most studies had either a neutral sponsor or sponsorship was not indicated. Twenty‐five studies were industry sponsored (Aripiprazole 2003; Aripiprazole 2017; Aripiprazole depot 2012; Asenapine 2011; Brexpiprazole 2017; Cariprazine 2016; Fluphenazine depot 1992; Iloperidone 2016; Lurasidone 2016; Olanzapine 2003; Paliperidone 2007; Paliperidone 2014; Paliperidone depot1M 2010; Paliperidone depot1M 2015; Paliperidone depot3M 2015; Penfluridol 1970; Penfluridol 1974b; Quetiapine 2007; Quetiapine 2009a; Quetiapine 2009b; Quetiapine 2010; Various drugs 1971; Various drugs 1989; Ziprasidone 2002; Zotepine 2000). Frequently medication was provided by the manufacturers of the antipsychotic drugs, but we did not record such studies as primarily 'industry sponsored'.
7. Outcomes
7.1 Relapse
The main relapse criteria in 25 studies was clinical judgement. However, in 24 studies various rating‐scale‐based definitions of relapse were used, in another 16 studies we took relapse as 'need of medication', in four 'admission to hospital', in two 'dropout due to worsening of symptoms', and, finally, in four the criteria used for 'relapse' was not indicated.
7.2 Leaving the study early
The number of participants leaving the study early was recorded by category ('any reason', 'adverse events' and 'lack of efficacy'). In the more recent trials, efficacy‐related adverse events (e.g. exacerbation of psychosis) are often grouped with tolerability‐related adverse events as "Leaving the study early due to adverse events". Where detailed data on leaving early were available, data on 'exacerbation of psychosis' were not entered as 'adverse events'.
7.3 Service use
Service use was described as the number of people re‐hospitalised and the numbers discharged during the trial. When reasons for hospitalisation were provided, we decided to enter data relative to people rehospitalised due to relapse or exacerbation of psychosis.
7.4 Scales
Scales that provided usable data are described below. We had, however, a priori, decided in the protocol to focus on dichotomous outcomes apart from quality of life and social functioning (see Measures of treatment effect). However, a few authors used rating scales to examine extrapyramidal adverse effects and defined cut‐offs to decide whether participants had a particular side effect or not. We used these data and explain below which cut‐offs were used.
7.4.1 Adverse effects scales
7.4.1.1 Abnormal Involuntary Movement Scale (AIMS) (Guy 1976) This scale has been used to assess tardive dyskinesia, a long‐term, drug‐induced movement disorder and short‐term movement disorders such as tremor. A low score indicates low levels of abnormal involuntary movements. In Fluphenazine depot 1982, all participants with any positive AIMS score were considered to have tardive dyskinesia. In Olanzapine 2003, the cut‐off was 3 or more on any item, or 2 or more on any two of the items. In Fluphenazine 1980 the cut‐off was any item rated 2. In Quetiapine 2010, the cut‐off was 2 or more on the global severity item.
7.4.1.2 Barnes Akathisia Scale (BAS) (Barnes 1989) The scale comprises items rating the observable, restless movements that characterise akathisia, a subjective awareness of restlessness, and any distress associated with the condition. These items are rated from 0 (normal) to 3 (severe). In addition, there is an item for rating global severity (from 0 (absent) to 5 (severe)). A low score indicates low levels of akathisia. In Olanzapine 2003 all participants with a BAS score of 2 or more were considered to have akathisia. In Quetiapine 2010 the cut‐off was 2 or more on the global severity item.
7.4.1.3 Simpson‐Angus Scale (SAS) (Simpson 1970) The 10‐item scale, with a scoring system of 0 to 4 for each item, measures drug‐induced parkinsonism, a short‐term drug‐induced movement disorder. A low score indicates low levels of parkinsonism. In Olanzapine 2003 all participants with a SAS score of 4 or more were considered to have parkinsonism. In Quetiapine 2010 the cut‐off was 1 or more on the mean SAS score.
7.4.2 Satisfaction with care scales
7.4.2.1 Participant Satisfaction with Medication Questionnaire ‐ Modified (PSMQ‐M) (Kalali 1999)
This self‐administered instrument consists of a 4‐part list of items rated according to 6‐point Likert scales, and measures the patient's satisfaction with current medication (ranging from "extremely satisfied" to "extremely unsatisfied") and the side effects burden (ranging from "no side effects" to "much more side effects"), with respect to previous antipsychotic medications. At the end, the patient is asked to state his preference for "current" versus "previous" medication. This instrument was applied in Aripiprazole depot 2012. The proportion of participants defined by the study authors as "at least very satisfied" was taken into the analysis for the present review.
7.4.2.2 Medication Satisfaction Questionnaire (MSQ) (Vernon 2010)
The instrument consists of a single question, read aloud by the clinician to the patient ("Overall, how satisfied are you with your current antipsychotic medication"). The answer has to be given according to a 7‐point Likert scale, ranging from 1 ("extremely dissatisfied") to 7 ("extremely satisfied). A 1‐point change over time may be considered as clinically meaningful. The proportion of participants defined as "satisfied with medication" according to this instrument was entered into the analysis for the current review, This scale was applied in Paliperidone depot1M 2015.
7.4.3 Quality of life scales
7.4.3.1 Heinrichs‐Carpenter Quality of Life Scale (QLS) (Carpenter 1994) This semi‐structured interview is administered and rated by trained clinicians. It contains 21 items rated on a 7‐point scale based on the interviewer's judgement of patient functioning. A total quality‐of‐life score and four subscale scores are calculated, with higher scores indicating less impairment. This scale was applied in Olanzapine 2003.
7.4.3.2 Symptom Questionnaire of Kellner and Sheffield (SQKS) (Kellner 1973)
The 30‐item self‐completion questionnaire measures subjective well‐being. A total score and four subscale scores are obtainable from the questionnaire. This instrument was applied in Various drugs 1981b.
7.4.3.3 Self‐report Quality of Life Scale (SQLS) (Wilkinson 2000) The scale is a self‐administered rating scale that includes 33 items concerning the patient's symptoms and well‐being over the preceding seven days, on a scale from 0 (never) to 4 (always). Total scores range from 0 to 100, with low scores representing a better outcome. Results based on this rating scale were found in Paliperidone 2007 and Paliperidone depot1M 2010.
7.4.3.4 Schizophrenia Quality of Life (S‐QoL) (Auquier 2003, Boyer 2010)
The scale is a self‐administered questionnaire to assess health‐related quality of life among people with schizophrenia, defined as the discrepancy between expectation and current life experience. The original version is composed of 41 items, and a shortened 18‐item version has been validated, with high degree of comparability with the original one. It is a multidimensional instrument with high reliability, validity and sensitivity to change. It evaluates 8 dimensions (psychological well‐being, self‐esteem, family relationships, relationships with friends, resilience, physical well‐being, autonomy and sentimental life). Each of the items is accompanied by a 5‐point Likert scale (1 = less than expected; 5 = more than expected, with the negatively worded item scores reversed). The score of each of the eight dimensions can be obtained by computing the mean of each item score within the dimension; by summing up every dimension score a total score is obtained. Quetiapine 2009a and Quetiapine 2009b applied this instrument.
7.4.3.5 EuroQol 5 Dimension ‐ Visual Analog Scale (EQ‐5D VAS) (EuroQol 1990)
The EQ‐5D is a self‐administered standardised measure of health status, applicable to a wide range of health conditions, and it is used to evaluate health care from a clinical and economic point of view, as well as in population health surveys. It consists of two parts: the EQ‐5D descriptive system and the EQ 20‐cm visual analogue scale (VAS). The first parts consists of one question in each of five dimensions (mobility, self‐care, pain, usual activities, and anxiety) with five possible response levels per question (level 1= no problem; level 5= extreme problems). The 20‐cm VAS has endpoints labelled "best imaginable health state" (anchored at 100) and "worst imaginable health state" (anchored at 0). Respondents are asked to indicate how they rate their own health by drawing a line from an anchor box to that point on the EQ‐VAS, which best represents their own health on a specified time period (usually that day). This instrument was used in Lurasidone 2016.
7.4.4 Social functioning scales
7.4.4.1 Personal and Social Performance (PSP) (Morosini 2000)
The scale is a validated clinician‐reported instrument that has been widely used in clinical trials to assess personal and social functioning of patients with psychiatric disorders. It is based on four distinct domains: (a) socially useful activities, (b) personal and social relationships, (c) self‐care, (d) disturbing and aggressive behaviour. Each PSP domain is assessed on a 6‐point severity scale ranging from "absent" to "very severe" difficulties in the specified area. After each domain is scored, raters determine one total score by selecting a 10‐point range within a 100‐point scale based on the domain scores following PSP scoring guidelines. The higher the score, the better the functioning. A variation of eight points over time should be classified as clinically significant. This scale was applied in the studies Aripiprazole depot 2012, Brexpiprazole 2017, Cariprazine 2016, Paliperidone 2007, Paliperidone 2014, Paliperidone depot1M 2010, Paliperidone depot1M 2015, Paliperidone depot3M 2015, Quetiapine 2009a and Quetiapine 2009b.
7.4.4.2 Global Assessment of Functioning (GAF) (American Psychiatric Association 1987)
The scale is a numeric scale (0 to 100 points) used by clinicians to subjectively rate the severity of mental illnesses in terms of their impact on day‐to‐day life. It is a brief and easy‐to‐administer scale, although based on a single global impression. It is broken into 10 sections, so that the higher the score, the better the functioning of the patients. Results derived from this scale were found in Ziprasidone 2002.
7.4.4.3 Sheehan Disability Scale (SDS) (Sheenan 1983)
The SDS is a brief, 5‐item self‐administered tool that assesses functional impairment in three areas: work/school, social life and family life. The patient has to rate the extent to which each area is affected by his/her symptoms. Total score is obtained by summing the single dimension score into one measure, and ranges from 0 (unimpaired) to 30 (highly impaired). No cut‐off has been recommended, but a score of 5 or more on any of the three areas could be classified as significant functional impairment. This scale was applied in Iloperidone 2016.
7.4.4.4 Specific Level of Functioning (SLOF) (Schneider 1983)
The scale is a 43‐item multidimensional behavioural survey assessing schizophrenia patients' current functioning and observable behaviour, and it is focused on the person's skills rather than deficits. It can be administered to the patient him/herself or to his/her caregiver. It comprises six subscales: (a) physical functioning, (b) personal care skills, (c) interpersonal relationships, (d) social acceptability, (e) activities of community living and (f) work skills. Each question is rated on a 5‐point Likert scale. Total scores range from 43 to 215, with higher scores representing better overall functioning. This instrument was applied in Lurasidone 2016.
7.4.4.5 Global Assessment Scale (GAS) (Endicott 1976) and Children Global Assessment Scale (CGAS) (Shaffer 1983)
The GAS is a rating scale used to evaluate the overall functioning of a person seeking mental health services, during a specified time period, independently of specific mental health diagnoses. It has proven to have good reliability and high sensitivity to change over time. The scale values range from 1 to 100, with higher scores representing better functioning and scores above 70 indicating good functioning. The CGAS is and adaptation of the GAS, designed to reflect the lower level of functioning of children and adolescents with respect to adults. Fluphenazine depot 1981 reported data from GAS, while the adaptation for children was used in Aripiprazole 2017.
7.5 Other adverse effects
Other adverse events such as death, suicide, suicide attempts, suicidal ideation, violent/aggressive behaviour, at least one adverse event, at least one movement disorder, akathisia, akinesia, dystonia rigor, tremor, use of antiparkinson medication, tardive dyskinesia, sedation and weight gain were reported in a dichotomous manner in terms of the number of participants with a given side effect.
7.6 Global state: number of participants improved (at least minimally)
The number of people who improved at the end of the studies was assessed by the Clinical Global Impression (CGI) scale (Guy 1976) or similar rating systems. The CGI compares the conditions of the person standardised against other people with the same diagnosis. A 7‐point scoring system is used with low scores showing decreased severity, overall improvement, or both. The outcome was defined as the number of participants 'at least minimally improved' (CGI score of 3 or less). When other scales were used in the original studies (e.g. PANSS, BPRS), data based on the '20% reduction of score' cut‐off were used. If data based on these thresholds were not available, we used the numbers presented by the original authors (study‐defined improvement), when available.
7.7 Global state: number of participants in symptomatic remission
The number of participants in symptomatic remission was defined by either 'mild or better' at the Clinical Global Impression (CGI) or similar rating systems, or using the operational criteria for remission in schizophrenia proposed by Andreasen et al (Andreasen 2005), without employing any duration threshold. In this case, a score of 'mild or less' at all eight core symptoms (delusions, hallucinatory behaviour and unusual thought content for the positive dimension, conceptual disorganisation and mannerism/disorders of posture for the disorganisation dimension, blunted affect, social withdrawal and lack of spontaneity/flow of conversation for the negative dimension) constitutes the symptom severity level of remission. If data based on these criteria were not available, other definitions of remission used by the original Authors ‐ with no mention to its duration ‐ were accepted. It should be noted that we defined this outcome as cross‐sectional and representative of the clinical severity level of patients, independent on the fact that the patients were achieving or maintaining it.
7.8 Global state: number of participants in sustained remission
This outcome was defined as either the number of participants achieving and maintaining the aforementioned symptom severity level (symptomatic remission) for a minimum period of six months, as proposed by Andreasen 2005, or the number already in remission at baseline and maintaining the same severity level for the whole duration of the study (if lasting at least six months).
7.9 Global state: number of participants in recovery
At present, more research is needed in order to achieve consensus regarding operational criteria for recovery (Andreasen 2005). Therefore, every definition of recovery provided by the original studies, including symptom severity, social‐occupational functioning and data on subjective recovery, was accepted.
7.10 Number of participants in employment
This outcome was described as the number of participants being employed at the end of the trials.
Excluded studies
We excluded 85 studies. Twenty‐six studies were excluded because they were not (appropriately) randomised. Twenty‐four studies were excluded because they did not examine suitable participants (e.g. participants had not been stabilised on antipsychotic drugs before study start. Twenty‐two studies were excluded because the interventions were not appropriate for this review ‐ most, for example, did not use a placebo control group. Thirteen studies were excluded because they did not report any usable or relevant outcomes.
Risk of bias in included studies
For graphical representations of our judgements of risk of bias please refer to Figure 4 and Figure 5. Full details of judgements are seen in the 'Risk of bias' tables.
4.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
5.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In 22 studies, random sequence generation was adequate. In the remaining 53 studies this was unclear. Among these, 50 studies were described as "randomised", but 39 of these did not provide any further details about random sequence generation. Eleven studies gave further information about randomisation, but these details were rather superficial and we still had to rate them as 'unclear'. Three further studies (Haloperidol 1973; Penfluridol 1987; Various drugs 1989) did not provide any information about sequence generation, but they were double‐blind and we assumed they were also randomised.
In 26 studies, allocation concealment was rated as adequate. For example, some studies reported that the only people with access to the identity of patients were the hospital pharmacist (e.g. Chlorpromazine 1976; Trifluoperazine 1972), a research assistant (e.g. Fluphenazine depot 1973), a psychiatrist without contact to participants (Various drugs 1962b) or a unit secretary (Various drugs 1971). Aripiprazole depot 2012, Brexpiprazole 2017, Iloperidone 2016Lurasidone 2016, Olanzapine 2003, Paliperidone 2007, Paliperidone 2014, Paliperidone depot1M 2010, Paliperidone depot1M 2015 and Paliperidone depot3M 2015 used an interactive voice‐response system for allocation concealment. One study (Ziprasidone 2002) used treatment cards numbered for each participant and investigators and pharmacists allocated numbers to people. Quetiapine 2010 reported that AstraZeneca prepared individually‐numbered study drugs and packed them according to the randomisation sequence. Two studies mentioned that codes were not broken until the time of the analysis and that the code was unknown to the investigators (Haloperidol depot 1982, Zotepine 2000).
The remaining 49 studies ‐ often undertaken well beyond the period when the need for good reporting was widely recognised (CONSORT) ‐ did not provide any details on allocation concealment. Therefore, it was unclear for most of the studies whether adequate allocation concealment methods were used.
Blinding
Concerning bias related to blinding of participants and personnel, we rated seven studies to have a low risk of bias. In them it was either tested that blinding had worked (Chlorpromazine 1962; Fluphenazine depot 1973; Perphenazine 1963; Various drugs 1971), or the authors had applied specific measures to improve blinding (e.g. prophylactic antiparkinson medication to avoid unmasking by side effects, Fluphenazine 1979; medication was administered by a person distinct from other study personnel, Paliperidone depot1M 2015 and Paliperidone depot3M 2015).
Six studies were rated with a high risk of bias. Various drugs 2011 was an open study, without providing any further information. In Various drugs 1964a, the placebo group received medication only every other day and blinding was not fully maintained. Various drugs 1968 and Various drugs 1981a reported that nurses had made correct guesses as to who was on drug and who was on placebo. In Fluphenazine depot 1982 evaluating psychiatrists and participants were unaware of the contents of the injections, while treating psychiatrists seemed to be aware of it. Various drugs 1993 was an open trial with rating scales being additionally rated by a second blind assessor.
In the other 62 studies, we rated the risk of bias as unclear. All these studies were described as double‐blind, with no further relevant information.
Concerning blinding of outcome assessment, all studies were rated as 'low risk of bias' concerning what we designated as 'more' objective outcomes, because we considered blinding to be less important for these.
As to subjective outcomes, we rated seven studies to have a low risk of bias. In them it was either tested that blinding had worked (Chlorpromazine 1962; Fluphenazine depot 1973; Perphenazine 1963; Various drugs 1971) or the authors had applied specific measures to improve blinding (e.g. prophylactic antiparkinson medication to avoid unmasking by side effects, Fluphenazine 1979; medication was administered by a person distinct from other study personnel, Paliperidone depot1M 2015 and Paliperidone depot3M 2015).
Four studies were rated with a high risk of bias for subjective outcomes. Various drugs 2011 was an open study, without providing any further information. In Various drugs 1964a, the placebo group received medication only every other day and blinding was not fully maintained. Various drugs 1968 and Various drugs 1981a reported that nurses had made correct guesses as to who was on drug and who was on placebo.
In the other 64 studies, we rated the risk of bias for subjective outcomes as 'unclear'. With the exception of Various drugs 1993 (an open trial with rating scales being additionally rated by a second blind assessor), all these 64 studies were described as double‐blind. But as antipsychotic drugs have adverse effects we considered that we should make a conservative judgment about the success of blinding. Many of these reports did not provide any details about how double‐blind conditions were maintained. It was usually just stated that the studies were "double‐blind" or it was simply indicated that "identical capsules" were used. Some studies using depot antipsychotic drugs reported that sesame oil injections were used in the placebo groups (e.g. Fluphenazine depot 1968 and Various drugs 1984a).
Incomplete outcome data
The number of participants leaving the studies early was frequently high leading to a judgement of high risk of bias in 30 included studies. The most frequent reason for leaving the studies early was 'relapse', because many studies had predefined in their protocols that once participants had relapsed they had to discontinue the trial. This had two consequences: the primary outcome relapse was frequently not affected by attrition, because most participants reached this end point. However, there was a risk of bias for other outcomes (e.g. adverse effects), because the reasons for leaving the studies early differed between participants on placebo (mainly relapse/inefficacy) and participants on antipsychotic drugs (other reasons).
Only 19 studies used survival analyses to examine relapse rates, while most others simply counted the numbers of participants who relapsed. We, therefore, had to restrict this review to analysis of relapse rates rather than more sensitive parameters such as 'time to relapse'.
Selective reporting
We judged 63 studies to be free of selective reporting. However, many did not (sufficiently) report on predefined outcomes.
| Studies with insufficient reporting of pre‐defined outcomes |
| Aripiprazole 2003, Haloperidol 1991, Iloperidone 2016, Lurasidone 2016, Olanzapine 2003, Penfluridol 1970, Penfluridol 1974c, Quetiapine 2007, Quetiapine 2009a, Quetiapine 2009b, Various drugs 1962a, Zotepine 2000, |
Other potential sources of bias
We judged 44 studies to be free of other potential sources of bias ‐ as far as we could detect. However, for six trials this was unclear (please see table below). Fourteen studies were terminated prematurely after pre‐planned interim analyses. One study had baseline imbalances in terms of the mean number of previous hospitalisations and mean age and duration of illness (Quetiapine 2007). This trial was also terminated prematurely. For Fluphenazine depot 1992, there were imbalances of gender and baseline medication. In five studies participants who relapsed were discontinued and their code was broken, which can be a threat for blinding (Chlorpromazine 1962; Trifluoperazine 1972; Various drugs 1960; Various drugs 1968; Various drugs 1986a) as can be administration of additional antipsychotic drugs in case of deterioration (Fluphenazine depot 1968). In Various drugs 1962b, three out of 19 participants in the placebo group continued to receive active medication (also terminated prematurely).
| Unclear if free from 'other biases' |
| Fluphenazine 1982, Lurasidone 2016, Olanzapine 1999, Various drugs 1964a, Various drugs 1989, Various drugs 1986b |
| Terminated prematurely after pre‐planned interim analyses |
| Aripiprazole 2017, Aripiprazole depot 2012, Brexpiprazole 2017,Cariprazine 2016, Iloperidone 2016, Olanzapine 2003, Paliperidone 2007, Paliperidone 2014, Paliperidone depot1M 2010, Paliperidone depot3M 2015, Quetiapine 2007, Quetiapine 2009a, Quetiapine 2009b, Various drugs 2011 |
Effects of interventions
See: Table 1
1. Comparison 1. Maintenance treatment with antipsychotic drugs versus placebo/no treatment
1.1 Relapse
Antipsychotic medication was clearly more effective than placebo in preventing relapse in studies lasting up to three months (percentage of participants relapsed drug 12% versus placebo 35%, 44 randomised controlled trials (RCTs), n = 6362, risk ratio (RR) 0.34, 95% confidence intervals (CI) 0.28 to 0.40, number needed to treat for an additional beneficial outcome (NNTB) 4, 95% CI 3 to 5, Analysis 1.1); four to six months (drug 18% versus placebo 49%, 49 RCTs, n = 7599, RR 0.36, 95% CI 0.31 to 0.42, NNTB 3, 95% CI 3 to 4, Analysis 1.1); seven to 12 months (primary outcome: drug 24% versus placebo 61%, 30 RCTs, n = 4249, RR 0.38, 95% CI 0.32 to 0.45, NNTB 3, 95% CI 2 to 3; high‐certainty evidence, Analysis 1.1); more than 12 months (drug 31% versus placebo 68%, 10 RCTs, n = 1786, RR 0.46, 95% CI 0.33 to 0.64, NNTB 3, CI 2 to 4, Analysis 1.1), and all studies combined (drug 22% versus placebo 58%, 71 RCTs, n = 8666, RR 0.35, 95% CI 0.30 to 0.40, NNTB 3, 95% CI 2 to 3, Analysis 1.2). There was considerable heterogeneity of study results up to three months (P <.0001, I2=50%), four to six months (P < 0.00001, I2 = 68%), seven to 12 months (P < 0.00001, I2 = 71%), more than 12 months (P < 0.00001, I2 = 90%); and all studies combined (P < 0.00001, I2 = 78%). However, in all studies the relapse rates were lower in the antipsychotic drug group than in the placebo group. Therefore, the heterogeneity expressed a difference in the magnitude of the superiority rather than in the direction of the effect. Subgroup analyses and meta‐regressions showed that the heterogeneity may be in part explained by study duration and differences between oral and depot medication (see sections 2.5 and 2.10 below).
1.1. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 1: Relapse: 1. Within pre‐specified time periods
1.2. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 2: Relapse: 2. Independent of duration
The funnel plot of the primary outcome 'relapse at 12 months' was asymmetrical (see Figure 6), and this was corroborated by Egger's regression test (intercept ‐1.39, t value 2.46, degrees of freedom (df) 28, P = 0.02042, Egger 1997) and a contour‐enhanced funnel‐plot (Peters 2008, the plot can be received from the authors upon request). However, when adjusted by Duval's trim and fill method (Duval 2000) the RR did not change substantially (RR 0.45, 95% CI 0.38 to 0.54), neither did it when only large studies (defined as > 200 participants) were included (10 RCTs, n = 2950, RR 0.37, 95% CI 0.31 to 0.45, Analysis 3.5).
6.
Funnel plot of comparison: 1 Maintenance treatment with antipsychotic drugs versus placebo/no treatment, outcome: Relapse
3.5. Analysis.

Comparison 3: Sensitivity analysis (relapse at 12 months), Outcome 5: Inclusion of only large studies (> 200 participants)
1.2 Leaving the study early
1.2.1 Due to any reason (acceptability of treatment)
Studies lasting up to three months (drug 9% versus placebo 29%, 11 RCTs, n = 517, RR 0.34, 95% CI 0.17 to 0.67); between four to six months (drug 22% versus placebo 44%, 18 RCTs, n = 1792, RR 0.49, 95% CI 0.37 to 0.65, NNTB 6, 95% CI 4 to 10); between seven to 12 months (drug 36% versus placebo 62%, 24 RCTs, n = 3951, RR 0.56, 95% CI 0.48 to 0.65, NNTB 4, 95% CI 3 to 5), high‐certainty evidence; and more than 12 months (drug 35% versus placebo 53%, 5 RCTs, n = 741, RR 0.64, 95% CI 0.51 to 0.82) showed a clear difference in favour of antipsychotic medication. Overall, there was a clear difference ‐ to conventional levels of statistical significance ‐ in favour of antipsychotic medication (drug 30% versus placebo 54%, 56 RCTs, n = 7001, RR 0.54, 95% CI 0.49 to 0.61, NNTB 4, 95% CI 4 to 6, Analysis 1.3). There was considerable heterogeneity within the group of studies lasting up to six months (P =.005, I2 = 54%), in studies lasting up to12 months (P < 0.00001, I2= 80%) and in all studies combined (P < 0.00001, I2 = 69%), but again this reflected heterogeneity in the degree of superiority rather than in the direction of the effect.
1.3. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 3: Leaving the study early: 1. Due to any reason (acceptability of treatment)
1.2.2 Due to adverse events (overall tolerability)
There was not a clear difference in studies lasting up to three months (drug 1% versus placebo 0%, 10 RCTs, n = 371, RR 2.84, 95% CI 0.12 to 65.34); four to six months (drug 4% versus placebo 4%, 15 RCTs, n = 1852, RR 1.20, 95% 95% CI 0.63 to 2,28); and seven to 12 months (drug 5% versus placebo 4%, 23 RCTs, n = 3870, RR 1.16, 95% CI 0.69 to 1.97), while the difference was statistically significant in studies lasting more than 12 months (drug 4% versus placebo 1%, 5 RCTs, n = 534, RR 5.70, 95% CI 1.28 to 25.33, NNTB 50, 95% CI 17 to 50). Overall, there was not a clear difference between groups (drug 5% versus placebo 3%, 53 RCTs, n = 6627, RR 1.27, 95% CI 0.85 to 1.89, Analysis 1.4). There was some heterogeneity in the group of studies lasting seven to 12 months (P = 0.009, I2 = 48%) and overall (P = 0.007, I2 = 43%). A possible explanation is that in particular, in recent trials not only tolerability‐related adverse events, but also efficacy‐related adverse events (e.g. exacerbation of psychosis) were summarised as "leaving the study due to adverse events". This may explain the clearest outlier (Olanzapine 2003), where all leaving due to adverse events were efficacy‐related. Removing this study and Ziprasidone 2002 (where details about dropout due to adverse events were not presented), results in the data becoming homogeneous. Statistically significantly more patients in the antipsychotic group left early for adverse events at 12 months (RR 1.56, 95% CI 1.04 to 2.34; heterogeneity test: P = 0.4, I² = 5%), and overall (RR 1.51, 95% CI 1.07 to 2.13; heterogeneity test: P = 0.23, I² = 15%).
1.4. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 4: Leaving the study early: 2. Due to adverse events (overall tolerability)
1.2.3 Due to inefficacy
Studies lasting up to three months (drug 5% versus placebo 27%, 11 RCTs, n = 421, RR 0.21, 95% CI 0.07 to 0.64), four to six months (drug 14% versus placebo 36%, 16 RCTs, n = 1661, RR 0.41, 95% CI 0.31 to 0.54, NNTB 5, 95% CI 3 to 9), seven to 12 months (drug 18% versus placebo 46%, 24 RCTs, n = 3951, RR 0.37, 95% CI 0.31 to 0.44, NNTB 3, 95% CI 3 to 4), and more than 12 months (drug 11% versus placebo 25%, 4 RCTs, n = 504, RR 0.43, 95% CI 0.29 to 0.64) showed a clear difference in favour of antipsychotic medication. Overall, there was a statistically significant difference in favour of antipsychotic medication (drug 16% versus placebo 40%, 55 RCTs, n = 6537, RR 0.38, 95% CI 0.32 to 0.43, NNTB 4, 95% CI 3 to 5, Analysis 1.5). The results at three months (P = 0.05, I2 = 50%), at seven to 12 months (P < 0.0001, I2 = 64%) and pooling all studies (P < 0.0001, I2= 50%) were heterogeneous, but, with the exception of Penfluridol 1987 and Various drugs 1964b, all studies showed at least a trend in favour of antipsychotic drugs. Thus, again, we feel the heterogeneity reflected differences in the degree of superiority rather than in the direction of the effect. Re‐inspection of Penfluridol 1987 and Various drugs 1964b did not reveal clear reasons why these studies showed a slight trend in favour of placebo.
1.5. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 5: Leaving the study early: 3. Due to inefficacy
1.3 Global state
1.3.1 Number of participants improved (at least minimally)
Studies in the up to three months category (drug 46% versus placebo 6%, 3 RCTs, n 0 = 119, RR 4.76, 95% CI 1.65 to 13.68, NNTB 3, 95% CI 2 to 6), and studies in the four to six months category (drug 30% versus placebo 11%, 8 RCTs, n=1037, RR 2.33, 95% CI 1.69 to 3.21, NNTB 4, 95% CI 2 to 8) showed a clear significant difference in favour of antipsychotic medication. The impression was the same in the seven to 12 months category (drug 24% versus placebo 15%, 4 RCTs, n = 388, RR 1.67, 95% CI 0.89 to 3.13), and in studies lasting more than 12 months (drug 23% versus placebo 17%, 1 RCT, n = 334, RR 1.36, 95% CI 0.88 to 2.09), but the difference did not reach conventional levels of statistical significance. When all studies were combined drugs were again significantly superior to switching to placebo (drug 29% versus placebo 13%, 16 RCTs, n = 1878, RR 2.12, 95% CI 1.58 to 2.85, NNTB 5, CI 3 to 8, Analysis 1.6). No significant heterogeneity was found.
1.6. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 6: Global state: number of participants improved (at least minimally)
1.3.2 Number of participants in symptomatic remission
This outcome was added to the list of outcomes for this update. One small study in the four to six months category (drug 70% versus placebo 40%, 1 RCT, n = 40, RR 1.75, 95% CI 0.79 to 3.87, NNTB 3, 95% CI 2 to 20) and studies in the seven to 12 months category (drug 52% versus placebo 31%, 5 RCTs, n = 807, RR 1.70,95% CI 1.11 to 2.59, NNTB 5, 95% CI 3 to 14) showed a significant difference in favour of antipsychotic medication. Again, the impression was the same in one small study in the up to three months category (drug 50% versus placebo 20%, 1 RCT, n = 20, RR 2.50, 95% CI 0.63 to 10.00), but the difference was not statistically significant. When all studies were combined drugs were clearly superior to placebo (drug 53% versus placebo 31%, 7 RCTs, n = 867, RR 1.73, 95% CI 1.20 to 2.48, NNTB 5, 95% CI 3 to 10, Analysis 1.7). There were heterogeneous results at 12 months (P < 0.0001, I2 = 82%) and overall (P = 0.0007, I2 = 74%), but by removing the only study that clearly was outlying (Aripiprazole 2017) all others showed concurred on the direction of effect in favour of antipsychotic drugs, and heterogeneity expressed differences in the degree of superiority rather than in the direction of effect (all the studies combined: P = 0.16, I2 = 37%). This may be explained by the different criteria used to define symptomatic remission among the studies.
1.7. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 7: Global state: number of participants in symptomatic remission
1.3.3 Number of participants in sustained remission
This outcome was added to the list of outcomes for the current update. Studies in the seven to 12 months category (drug 27% versus placebo 16%, 6 RCTs, n = 1443, RR 1.83, 95% CI 1.49 to 2.25, NNTB 7, 95% CI 5 to 12), studies in the more than 12 months category (drug 76% versus placebo 61%, 2 RCTs, n = 364, RR 1.29, 95% CI 1.13 to 1.47, NNTB 6, 95% CI 4 to 10), and all the studies combined (drug 36% versus placebo 26%, 8 RCTs, n = 1807, RR 1.67, 95% CI 1.28 to 2.19, NNTB 7, 95% CI 5 to 10, Analysis 1.8) showed a clear and statistically significant difference in favour of antipsychotic medication. The results pooling all the studies were slightly heterogeneous (P =.03, I2= 55%), but the direction of effect was the same among all the studies, reflecting at least a trend in favour of antipsychotic drugs.
1.8. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 8: Global state: number of participants in sustained remission
1.3.4. Number of participants in recovery
No data were available for this outcome.
1.4. Service use
1.4.1 Number of participants hospitalised
Studies lasting seven to 12 months (drug 4% versus placebo 13%, 11 RCTs, n = 2119, RR 0.36, 95% CI 0.23 to 0.56, NNTB 10, 95% CI 6 to 20), and more than 12 months (drug 17% versus placebo 31%, 4 RCTs, n = 965, RR 0.55, 95% CI 0.44 to 0.69, NNTB 7, 95% CI 3 to 10) showed a statistically and clinically significant difference in favour of antipsychotic medication. There was no clear difference for studies lasting up to three months (drug 4% versus placebo 7%, 2 RCTs, n = 55, RR 0.42, 95% CI 0.04 to 4.06), but these short‐term data are only based on two small studies. Again, the difference was not statistically significant in four studies lasting four to six months (drug 3% versus placebo 11%, 4 RCTs, n = 419, RR 0.19, 95% CI 0.03 to 1.32). Overall, there was a clear difference in favour of antipsychotic medication (drug 7% versus placebo 18%, 21 RCTs, n = 3558, RR 0.43, 95% CI 0.32 to 0.57, NNTB 8, 95% CI 6 to 14,high‐certainty evidence; Analysis 1.9). There was some heterogeneity for studies lasting four to six months (P =.04, I2 = 63%), but all studies showed favoured antipsychotic drugs.
1.9. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 9: Service use: number of participants hospitalised
1.4.2 Number of participants discharged
Three studies in inpatients reported on the number of participants who could be discharged. There was no significant difference between groups (drug 5% versus placebo 1%, 3 RCTs, n = 404, RR 2.76, 95% CI 0.69 to 11.06, Analysis 1.10). All the three studies lasted four to six months.
1.10. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 10: Service use: number of participants discharged
1.5 Death
1.5.1 Any
In tota,l there were nine deaths in the drug group and eight deaths in the placebo group. There was no significant difference between groups in studies lasting up to three months (drug 0% versus placebo 0%, 3 RCTs, n = 415, RR not estimable), between four to six months (drug 0.9% versus placebo 0.2%, 6 RCTs, n = 1159, RR 2.30, 95% CI 0.59 to 8.98), seven to 12 months (drug 0.1% versus placebo 0.5%, 15 RCTs, n = 3273, RR 0.35, 95% CI 0.11 to 1.12), in one study lasting more than 12 months (drug 1.2% versus placebo 0%, 1 RCT, n = 334, RR 5.18, 95% CI 0.25 to 107.12), and in all studies combined (drug 0.3% versus placebo 0.3%, 25 RCTs, n = 5181, RR 0.90, 95% CI 0.39 to 2.11, Analysis 1.11).
1.11. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 11: Death: due to any reason
1.5.2 Due to natural causes
Studies lasting up to three months (drug 0% versus placebo 0%, 2 RCTs, n = 379, RR not estimable), four to six months (drug 0.9% versus placebo 0.2%, 6 RCTs, n = 1159, RR 2.30, 95% CI 0.59 to 8.98), seven to 12 months (drug 0.1% versus placebo 0.1%, 16 RCTs, n = 3354, RR 0.53, 95% CI 0.11 to 2.58), in one study lasting more than 12 months (drug 0.6% versus placebo 0%, 1 RCT, n = 334, RR 3.11, 95% CI 0.13 to 75.78), and in all studies combined (drug 0.3% versus placebo 0.1%, 25 RCTs, n=5226, RR 1.35, 95% CI 0.50 to 3.6, Analysis 1.12) did not reveal a significant difference between groups.
1.12. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 12: Death: due to natural causes
1.6. Suicidal behaviour
1.6.1 Death due to suicide
Studies up to three months (drug 0% versus placebo 0%, 3 RCTs, n = 415, RR not estimable), four to six months (drug 0% versus placebo 0%, 3 RCTs, n = 1033, RR not estimable), seven to 12 months (drug 0% versus placebo 0.2%, 12 RCTs, n = 2852, RR 0.35, 95% CI 0.06 to 2.21), one study lasting more than 12 months (drug 0.6% versus placebo 0%, 1 RCT, n = 334, RR 3.11, 95% CI 0.13 to 75.78), and all studies combined irrespective of their duration (drug 0.04% versus placebo 0.1%, 19 RCTs, n = 4634, RR 0.60, 95% CI 0.12 to 2.97,low‐certainty evidence; Analysis 1.13) did not show clear differences.
1.13. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 13: Death: due to suicide
1.6.2 Number of participants with suicide attempts
There was also no clear difference in terms of suicide attempts in two studies lasting four to six months (drug 0.3% versus placebo 0%, 3 RCTs, n = 776, RR 3.00, 95% CI 0.13 to 71.51), and seven to 12 months (drug 0.2% versus placebo 0.5%, 9 RCTs, n = 2347, RR 0.48, 95% CI 0.13 to 1.69). Also, when all studies were combined irrespective or their duration, there was no difference between groups (drug 0.2% versus placebo 0.3%, 12 RCTs, n = 3123, RR 0.61, 95% CI 0.19 to 1.99, Analysis 1.14).
1.14. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 14: Number with suicide attempts
1.6.3 Number of participants with suicide ideation
There was no significant difference in the number of participants with suicidal ideation in one study in the up to three months category (drug 0% versus placebo 6%, 1 RCT, n = 49, RR 0.17, 95% CI 0.01 to 3.88), in one study in the four to six months category (drug 0% versus placebo 0%, 1 RCT, n = 386, RR not estimable), in studies in the seven to 12 months category (drug 0.9% versus placebo 2%, 10 RCTs, n = 2486, RR 0.52, 95% CI 0.24 to 1.09), in one study lasting more than 12 months (drug 3% versus placebo 2.4%, 1 RCT, n = 334, RR 1.30, 95% CI 0.35 to 4.74), and in all studies combined irrespective of duration (drug 1% versus placebo 1.8%, 13 RCTs, n = 3255, RR 0.61, 95% CI 0.99 to 1.16, Analysis 1.15).
1.15. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 15: Number with suicide ideation
1.7 Violent/aggressive behaviour
There were data in one small study in the up to three months category (drug 0% versus placebo 8%, 1 RCT, n = 26, RR 0.33, 95% CI 0.01 to 7.50), two studies in the four to six months category (drug 4% versus placebo 9%, 2 RCTs, n = 350, RR 0.46, 95% CI 0.2 to 1.08), and one study lasting more than 12 months (drug 0% versus placebo 1%, 1 RCT, n = 334, RR 0.21, 95% CI 0.01 to 4.28). We found no clear difference in the number of participants with aggressive behaviour. However, in studies lasting seven to 12 months (drug 1% versus placebo 5%, 8 RCTs, n = 2146, RR 0.35, 95% CI 0.19 to 0.66, NNTB 50, 95% CI 20 to 100), and in all studies combined irrespective of their duration (drug 1% versus placebo 5%, 12 RCTs, n=2856, RR 0.37, 95% CI 0.24 to 0.59, Analysis 1.16) fewer participants in the drug group than in the placebo group were violent/aggressive.
1.16. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 16: Violent/aggressive behaviour
1.8 Adverse effects
1.8.1 At least one adverse effect
One study in the up to three months category (drug 36% versus placebo 69%, 1 RCT, n = 49, RR 0.53, 95% CI 0.30 to 0.93, NNTB 3, 95% CI 2 to 25) showed a clear difference between groups. Four studies in the four to six months category (drug 49% versus placebo 52%, 4 RCTs, n = 1079, RR 0.98, 95% CI 0.85 to 1.12) studies lasting seven to 12 months (drug 42% versus placebo 34%, 12 RCTs, n = 2890, RR 1.15, 95% CI 0.99 to 1.33), and all studies combined irrespective of their duration (drug 44% versus placebo 38%, 18 RCTs, n = 4352, RR 1.10, 95% CI 0.98 to 1.25, Analysis 1.17) did not indicate a clear difference between groups. This was statistically significant, however, in one study lasting more than 12 months (drug 39% versus placebo 22%, 1 RCT, n = 334, RR 1.75, 95% CI 1.24 to 2.45). The results at 12 months (P = 0.004, I2 = 60%) and overall (P < 0.0001, I2 = 66%) were heterogeneous. Similarly to the outcome 'leaving the study early due to adverse events' (see Section 1.2.2 above) it should be noted that in particular in recent trials efficacy‐related events can also be adverse events that may in part explain the heterogeneity. Haloperidol 1973 even showed clearly more adverse events in the placebo group. The authors discussed this finding as withdrawal effects after abrupt stopping of medication. However, excluding this outlying study did not change the results (all studies pooled: RR 1.13, 95% CI 1.00 to 1.27; heterogeneity test: P = 0.0002, I² = 64%).
1.17. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 17: Adverse effects: at least one adverse event
1.8.2 Movement disorders
1.8.2.1 At least one movement disorder
Studies lasting up to three months (drug 29% versus placebo 10%, 4 RCTs, n = 158, RR 2.42, 95% CI 0.70 to 8.33) did not reveal any difference between groups. However, studies lasting four to six months (drug 18% versus placebo 11%, 8 RCTs, n = 1658, RR 1.45, 95% CI 1.06 to 1.99), seven to 12 months (drug 12% versus placebo 6%, 16 RCTs, n = 3126, RR 1.55, 95% CI 1.17 to 2.05), and all studies combined irrespective of their duration (drug 14% versus placebo 8%, 29 RCTs, n =5276, RR 1.52, 95% CI 1.25 to 1.85, number needed to treat for an additional harmful outcome (NNTH) 20, 95% CI 14 to 50, Analysis 1.18) showed a clear and statistically significant difference in favour of placebo. In one study lasting more than 12 months the difference between groups did not reach conventio nal levels of statistical significance (drug 9% versus placebo 7%, 1 RCT, n = 334, RR 1.21, 95% CI 0.58 to 2.54).
1.18. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 18: Adverse effects: movement disorders: at least one movement disorder
1.8.2.2 Akathisia
Studies in the up to three‐month category (drug 14% versus placebo 4%, 2 RCTs, n = 69, RR 2.68, 95% CI 0.49 to 14.82), in the four to six months category (drug 9% versus placebo 2%, 6 RCTs, n = 1191, RR 2.14, 95% CI 0.50 to 9.11), in the seven to 12 months category (drug 5% versus placebo 3%, 12 RCTs, n = 2620, RR 1.07, 95% CI 0.71 to 1.61), one study in the more than 12 months category (drug 3% versus placebo 2%, 1 RCT, n = 334, RR 1.73, 95% CI 0.42 to 7.11), and all studies combined irrespective of their duration (drug 6% versus placebo 3%, 21 RCTs, n = 4214, RR 1.49, 95% CI 0.93 to 2.38, Analysis 1.19) did not show clear differences. Results at six months (P = 0.009, I2 = 67%) were heterogeneous due to one outlying trial (Various drugs 1975) in which more participants in the placebo group had akathisia. Re‐inspection of this study did not reveal an obvious explanation. Removing this study reduced heterogeneity and then statistically significantly more participants in the drug group suffered from this adverse effect (RR 4.07, 95% CI 1.46 to 11.33; heterogeneity test: P = 0.24, I2 = 27%).
1.19. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 19: Adverse effects: movement disorders: akathisia
1.8.2.3 Akinesia
There was no clear difference in the one small study in the up to three months category (drug 6% versus placebo 6%, 1 RCT, n = 49, RR 0.97, 95% CI 0.09 to 9.92), nor in two studies lasting between seven and 12 months (drug 0% versus placebo 0,01%, 2 RCTs, n = 348, RR 0.16, 95% CI 0.01 to 3.98), nor in all the three studies combined (drug 1% versus placebo 1%, 3 RCTs, n = 397, RR 0.52, 95% CI 0.08 to 3.42, Analysis 1.20).
1.20. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 20: Adverse effects: movement disorders: akinesia
1.8.2.4 Dyskinesia
Three studies in the four to six months category (drug 2% versus placebo 13%, 3 RCTs, n = 418, RR 0.31, 95% CI 0.11 to 0.84), and all studies combined (drug 1% versus placebo 4%, 18 RCTs, n = 3200, RR 0.55, 95% CI 0.33 to 0.91, Analysis 1.21) showed a clear difference in favour of antipsychotic medication. There was no difference in one study in the up to three months category (drug 3% versus placebo 0%, 1 RCT, n = 49, RR 1.50, 95% CI 0.06 to 34.91), in studies in the seven to 12 months category (drug 2% versus placebo 2%, 13 RCTs, n = 2399, RR 0.69, 95% CI 0.37 to 1.27), and in one study lasting more than 12 months (drug 1% versus placebo 2%, 1 RCT, n = 334, RR 0.35, 95% CI 0.04 to 3.29).
1.21. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 21: Adverse effects: movement disorders: dyskinesia
1.8.2.5 Dystonia
For dystonia there was no clear difference in the one study in the up to three months category (drug 6% versus placebo 0%, 1 RCT, n = 49, RR 2.50, 95% CI 0.13 to 49.22), two studies in the four to six months category (drug 16% versus placebo 9%, 2 RCTs, n = 382, RR 1.75, 95% CI 0.94 to 3.29), studies in the seven to 12 months category (drug 1% versus placebo 1%, 9 RCTs, n = 2002, RR 1.63, 95% CI 0.65 to 4.09), and one study in the more than 12 months category (drug 0% versus placebo 1%, 1 RCT, n = 334, RR 0.21, 95% CI 0.01 to 4.28). When all studies were pooled irrespective of their duration, there was a suggestion of superiority of placebo but this did not quite reach conventional levels of statistical significance (drug 4% versus placebo 2%, 13 RCTs, n = 2767, RR 1.63, 95% CI 0.99 to 2.7, Analysis 1.22).
1.22. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 22: Adverse effects: movement disorders: dystonia
1.8.2.6 Rigor
There was never any clear difference between groups in terms of rigor. Two studies in the up to three months category (drug 19% versus placebo 15%, 2 RCTs, n = 69, RR 1.2, 95% CI 0.22 to 6.62), three studies in the four to six months category (drug 17% versus placebo 8%, 3 RCTs, n = 160, RR 1.98, 95% CI 0.67 to 5.85), four studies in the seven to 12 months category (drug 1% versus placebo 0%, 4 RCTs, n = 693, RR 1.80, 95% CI 0.29‐2.79), and all studies combined (drug 6% versus placebo 2%, 9 RCTs, n = 922, RR 1.39, 95% CI 0.70 to 2.79, Analysis 1.23) did not suggest a clear difference between groups.
1.23. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 23: Adverse effects: movement disorders: rigor
1.8.2.7 Tremor
Two studies in the up to three months category (drug 23% versus placebo 15%, 2 RCTs, n=69, RR 1.20, 95% CI 0.46 to 3.16), three studies in the four to six months category (drug 8% versus placebo 10%, 3 RCTs, n = 160, RR 0.92, 95% CI 0.33 to 2.61), one study in the more than 12 months category (drug 1% versus placebo 2%, 1 RCT, n = 334, RR 0.52, 95% CI 0.10 to 2.79), and all studies combined (drug 5% versus placebo 3%, 18 RCTs, n = 3353, RR 1.37, 95% CI 0.95 to 1.98, Analysis 1.24) did not reveal clear differences in terms of tremor. Only the 12 studies in the seven to 12 months category showed a clear superiority for placebo (drug 5% versus placebo 2%, 12 RCTs, n = 2790, RR 1.62, 95% CI 1.04 to 2.54).
1.24. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 24: Adverse effects: movement disorders: tremor
1.8.2.8 Use of antiparkinson medication
No clear differences were found in the four to six months category (drug 22% versus placebo 13%, 3 RCTs, n = 841, RR 1.53, 95% CI 0.90 to 2.61) and in one study in the more than 12 months category (drug 19% versus placebo 19%, 1 RCT, n = 334, RR 1.00, 95% CI 0.64 to 1.57). In the seven to 12 months category (drug 23% versus placebo 17%, 9 RCTs, n = 1733, RR 1.37, 95% CI 1.06 to 1.78, NNTH 11, 95% CI 7 to 33) and overall, there was a clear difference in favour of placebo (drug 22% versus placebo 16%, 13 RCTs, n = 2908, RR 1.35, 95% CI 1.10 to 1.65, NNTH 13, 95% CI 8 to 33, Analysis 1.25).
1.25. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 25: Adverse effects: movement disorders: use of antiparkinson medication
1.8.3 Sedation
One small study in the up to three months category (drug 0% versus placebo 20%, 1 RCT, n = 20, RR 0.20, 95% CI 0.01 to 3.70), showed no clear difference. This also applied to studies lasting between four to six months (drug 6% versus placebo 3%, 7 RCTs, n = 1880, RR 1.37, 95% CI 0.89 to 2.12), and one study lasting more than 12 months (drug 1% versus placebo 1%, 1 RCT, n = 334, RR 1.04, 95% CI 0.15 to 7.27). Studies lasting between seven and 12 months (drug 11% versus placebo 7%, 9 RCTs, n = 1844, RR 1.78, 95% CI 1.25 to 2.53), and all studies combined (drug 8% versus placebo 5%, 18 RCTs, n = 4078, RR 1.52, 95% CI 1.24 to 1.86, NNTH 50, 95% CI not significant, Analysis 1.26) showed a clear difference in favour of placebo.
1.26. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 26: Adverse effects: sedation
1.8.4 Weight gain
Four studies in the four to six months category showed no clear difference (drug 5% versus placebo 3%, 4 RCTs, n = 1039, RR 1.49, 95% CI 0.81 to 2.73). However, studies lasting seven to 12 months (drug 10% versus placebo 7%, 14 RCTs, n = 3394, RR 1.80, 95% CI 1.17 to 2.77, NNTH 25, 5% CI 17 to 50), one study lasting more than 12 months (drug 13% versus placebo 6%, 1 RCT, n = 334, RR 2.18, 95% CI 1.06 to 4.48), and all studies combined (drug 9% versus placebo 6%, 19 RCTs, n = 4767, RR 1.69, 95% CI 1.21 to 2.35, NNTH 25, 95% CI 20 to 50, Analysis 1.27) did suggest a clear difference in favour of placebo. There was some heterogeneity in the 12 months results (P=0.006, I2=56%) and pooling all the studies (P=0.02, I2=45%). Removing the clearest outliers (Cariprazine 2016, Lurasidone 2016, Iloperidone 2016), which showed greater weight gain in the placebo group, reduced heterogeneity, but only to some degree and, overall, antipsychotic drugs did seem to cause more weight gain (all studies combined: RR 2.01, 95% CI 1.43 to 2.81, heterogeneity test: P = 0.12, I2 = 30%) The heterogeneity expressed differences more in the degree of weight gain rather than in the direction of effect. This may be partially explained by the use of different criteria to define and report weight gain among the original studies.
1.27. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 27: Adverse effects: weight gain
1.9 Satisfaction with care (any published rating scale)
1.9.1 Number of participants satisfied
No data on this outcome were available in the original review. In this update, drug was clearly superior to placebo in one study in the seven to 12 months category (drug 74% versus placebo 63%, 1 RCT, n = 403, RR 1.19, 95% CI 1.02 to 1.38), and in one study lasting more than 12 months (drug 84% versus placebo 69%, 1 RCT, n = 334, RR 1.22, 95% CI 1.08 to 1.38). When the two studies were combined, the difference remained statistically (drug 78% versus placebo 66%, 2 RCTs, n = 737, RR 1.21, 95% CI 1.10 to 1.33; Analysis 1.28). No heterogeneity was identified (P=0.75, I2 = 0%).
1.28. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 28: Participant´s satisfaction with care
1.9.2 Number of carers satisfied
No data were available for this outcome.
1.10 Quality of life (any published rating scale)
Studies were divided in subgroups according to time points and rating scales used; endpoint and change data were presented in separate subgroups. Seven studies provided data on this outcome (two studies lasting up to 3 months, four studies in the seven to 12 months category and one study lasting more than 12 months). Across different subgroups, the superiority of drugs was not always clear, with effect sizes ranging from minimum mean difference (MD) ‐2.00 (2 RCTs, 379 participants, 95% CI ‐5.80 to 1.80) to maximum MD ‐ 11.36 (1 RCT, 304 participants, 95% CI ‐14.67 to ‐8.05) (Analysis 1.29). As an additional analysis, all the studies (across different time points and scales) were combined and a standardised mean difference (SMD) was calculated: antipsychotic drugs were found clearly and statistically significantly superior to placebo (7 RCTs, n = 1573, SMD ‐0.32 95% CI ‐0.57 to ‐0.07, Analysis 1.30). Tentative back‐calculation to the Schizophrenia Quality of Life Scale (used in Paliperidone 2007 and Paliperidone depot1M 2010) yielded a MD of 4.4 points. When pooling all the studies, there was significant heterogeneity (P =.01, I2 = 64%), which may be in part due to the use of different scales (see Discussion, 2.9 below), but the direction of effect was the same in all studies.
1.29. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 29: Quality of life (various scales, different timepoints)
1.30. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 30: Quality of life (across all scales and timepoints)
1.11 Number of participants in employment
There was no clear difference in terms of number of people employed in two studies in the seven to 12 months category (drug 48% versus placebo 50%, 2 RCTs, n = 259, RR 0.96, 95% CI 0.75 to 1.23), nor in the one study lasting more than 12 months (drug 31% versus placebo 22%, 1 RCT, n = 334, RR 1.39, 95% CI 0.97 to 2.00), nor in all the studies combined (drug 39% versus placebo 34%, 3 RCTs, n = 593, RR 1.08, 95% CI 0.82 to 1.41, Analysis 1.31).
1.31. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 31: Number of participants in employment
1.12 Social functioning (any published rating scale)
This outcome was added to the list of outcomes for the present update. Studies were divided in subgroups according to time points and rating scales used. Endpoint and change data were presented separately in subgroups. Antipsychotic medication was clearly rated as superior to placebo in terms of participants´ social functioning in studies in the up to three months category (3 RCTs, n = 499, MD ‐4.32, 95% CI ‐6.69 to ‐1.94), in the four to six months category (1 RCT, n = 270, MD ‐2.00, 95% CI ‐3.60 to ‐0.40), in studies lasting seven to 12 months (10 RCTs, n = 2490, MD ‐4.89 95% CI ‐6.00 to ‐3.79), and in the one study lasting more than 12 months (1 RCT, n = 334, MD ‐3.60 CI ‐6.76 to ‐0.44) (Analysis 1.32). As an additional analysis, all studies were combined irrespective of their duration and the scale used, and a standardised mean difference calculated. This analysis also showed superiority of active drugs to placebo (15 RCTs, n=3588, SMD ‐0.43 CI ‐0.53 to ‐0.34,moderate‐certainty evidence; Analysis 1.33). Tentative back‐calculation to the Personal and Social Performance schedule (used in 10 out of 15 studies) yielded an MD of 5.2 points. There was heterogeneity when pooling all the studies (P=.03, I2 = 45%) which may be partially explained by the use of different scales (see Discussion 2.11 below), but all the studies showed a result tending to favour antipsychotic drugs.
1.32. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 32: Social Functioning (various scales, different timepoints)
1.33. Analysis.

Comparison 1: Maintenance treatment with antipsychotic drugs versus placebo/no treatment, Outcome 33: Social Functioning (across all scales and timepoints)
2. Subgroup analyses (relapse at 12 months)
All subgroup analyses were conducted only on the primary outcome 'relapse at sevevn to 12 months'.
2.1 Participants with a first episode of psychosis
There was no clear difference between studies that included only people with a first episode (drug 26% versus placebo 61%, 8 RCTs, n = 528, RR 0.47, 95% CI 0.38 to 0.58) and studies in people who had already experienced several episodes (drug 23% versus placebo 59%, 24 RCTs, n = 3585, RR 0.38, 95% CI 0.31 to 0.46); (test for subgroup differences: Chi2 =2.12, df = 1 (P = 0.15), I2 = 52.8%, Analysis 2.1).
2.1. Analysis.

Comparison 2: Subgroup analysis (relapse at 12 months), Outcome 1: Subgroup analysis: participants with a first episode
2.2 Participants in remission at baseline
There was also no difference between the results of studies that included only participants who were in remission at baseline (drug 27% versus placebo 52%, 10 RCTs, n = 1050, RR 0.44, 95% CI 0.33 to 0.60) and the rest of the studies (drug 22% versus placebo 62%, 19 RCTs, n = 3063, RR 0.36, 95% CI 0.30 to 0.44); (test for subgroup differences: Chi2 = 1.25, df =1 (P = 0.26), I2 = 20%, Analysis 2.2).
2.2. Analysis.

Comparison 2: Subgroup analysis (relapse at 12 months), Outcome 2: Subgroup analysis: participants in remission at baseline
2.3 Participants who had been stable for various periods before entering the trials
Five studies included only participants who were stable for at least one month. Antipsychotic drugs significantly reduced relapse rates compared to placebo (drug 22% versus placebo 65%, 6 RCTs, n = 574, RR 0.32, 95% CI 0.20 to 0.50). The same pattern was found for studies with participants stable at least three months (drug 1% versus placebo 54%, 10 RCTS, n = 2250, RR 0.34, 95% CI 0.26 to 0.43), stable at least 12 months (drug 21% versus placebo 60%, 5 RCTs, n = 326, RR 0.31, 95% CI 0.17 to 0.57), and at least three to six years (drug 22% versus placebo 63%, 2 RCTs, n = 54, RR 0.38, 95% CI 0.18 to 0.78). One small study included participants who were stable for at least six months and the difference between drug and placebo was not statistically significant (drug 10% versus placebo 30%, 1 RCT, n = 20, RR 0.33, 95% CI 0.04 to 2.69). Overall, there was no clear difference between the different durations of pre‐trial stability (test for subgroup differences: Chi² = 0.21, df = 4 (P =1.00), I² = 0%, Analysis 2.3).
2.3. Analysis.

Comparison 2: Subgroup analysis (relapse at 12 months), Outcome 3: Subgroup analysis: various durations of stability before entering the study
2.4 Abrupt withdrawal versus tapering
There was no clear difference between studies in which antipsychotics were abruptly withdrawn (drug 27% versus placebo 62%, 18 RCTs, n = 2348, RR 0.43, 95% CI 0.35 to 0.53) or slowly tapered (drug 18% versus placebo 56%, 11 RCTs, n = 1765, RR 0.33, 95% CI 0.24 to 0.44); (test for subgroup differences: Chi2 = 2.37, df = 1 (P = 0.12), I2 = 57.8%, Analysis 2.4).
2.4. Analysis.

Comparison 2: Subgroup analysis (relapse at 12 months), Outcome 4: Subgroup analysis: abrupt withdrawal versus tapering
2.5 to 2.6 Single antipsychotic drugs and depot versus oral medication
The test for subgroups differences between single antipsychotics was not statistically significant( Chi2 =15.08, df =9 (P = 0.09), I² = 40.3%, Analysis 2.5). When the subgroup of studies using depot antipsychotics (drug 17% versus placebo 55%, 10 RCTs, n = 1705, RR 0.30, 95% CI 0.23 to 0.39) was compared with the subgroup of studies using oral antipsychotics (drug 29% versus placebo 63%, 16 RCTs, n = 2187, RR 0.46, 95% CI 0.38 to 0.55) a clear, statistically significant superiority of the depot formulations emerged (test for subgroup differences: Chi2 =6.87, df =1 (P =0.009), I2 =85.4%, Analysis 2.6).
2.5. Analysis.

Comparison 2: Subgroup analysis (relapse at 12 months), Outcome 5: Subgroup analysis: single antipsychotic drugs
2.6. Analysis.

Comparison 2: Subgroup analysis (relapse at 12 months), Outcome 6: Subgroup analysis: depot versus oral drugs
2.7 First‐ versus second‐generation antipsychotic drugs
There was no clear difference in reduction of relapse risk between first‐generation antipsychotics (drug 24% versus placebo 62%, 18 RCTs, n=1430, RR 0.35, 95% CI 0.25 to 0.48) and second‐generation antipsychotics (drug 23% versus placebo 58%, 11 RCTs, n = 2683, RR 0.39, 95% CI 0.32 to 0.48)(test for subgroup differences: Chi2 = 0.36, df = 1 (P = 0.55), I2 = 0%, Analysis 2.7).
2.7. Analysis.

Comparison 2: Subgroup analysis (relapse at 12 months), Outcome 7: Subgroup analysis: first‐ versus second‐generation antipsychotic drugs
2.8 Appropriate versus unclear allocation concealment
The degree of relapse reduction by antipsychotics was not different in studies that used appropriate allocation concealment (drug 22% versus placebo 59%, 13 RCTs, n = 2708, RR 0.37, 95% CI 0.30 to 0.45) and studies in which this was unclear (drug 25% versus placebo 61%, 16 RCTs, n = 1405, RR 0.41, 95% CI 0.30 to 0.54); (test for subgroup differences: Chi2 = 0.29, df = 1 (P = 0.59), I2 = 0%, Analysis 2.8).
2.8. Analysis.

Comparison 2: Subgroup analysis (relapse at 12 months), Outcome 8: Subgroup analysis: appropriate versus unclear allocation concealment
2.9 Blinded versus open trials
The relapse risk reduction by antipsychotics was slightly larger in two open trials (drug 17% versus placebo 65%, 2 RCTs, n = 257, RR 0.26, 95% CI 0.17 to 0.39) than in the double‐blind studies (drug 24% versus placebo 59%, 27 RCTs, n = 3856, RR 0.40, 95% CI 0.33 to 0.48); (test for subgroup differences: Chi2 = 3.57, df = 1 (P = 0.06), I2 = 72%, Analysis 2.9).
2.9. Analysis.

Comparison 2: Subgroup analysis (relapse at 12 months), Outcome 9: Subgroup analysis: blinded versus open trials
2.10 Meta‐regressions
All meta‐regressions were conducted only on the primary outcome 'relapse at seven to 12 months', except for the meta‐regression on study duration, which was performed on all the studies reporting data on relapse (using the longest time point available).
2.10.1 Severity of illness at baseline (relapse at 12 months)
The studies used many different scales (e.g. Clinical Global Impression scale (CGI), Brief Psychiatric Rating Scale (BPRS), Positive and Negative Syndrome Scale (PANSS)) to assess participants' severity at baseline. Therefore, a meta‐regression based on a scale‐defined severity of the illness was impossible. The subgroup analysis comparing participants in remission at baseline with the rest of the studies did not yield a significant difference (see Section 2.2 above).
2.10.2 Duration the participants were stable before the start of the study (relapse at 12 months)
There was no clear effect on the difference in relapse risk at seven to 12 months based on the duration the participants had been stable before they entered the studies (slope 0.0002, CI ‐0.0029 to 0.0033, P = 0.904, see Figure 7).
7.
Meta‐regression on duration of clinical stability before study start (relapse at 12 months)
The size of the bubbles is proportional to the inverse variance of the treatment effect.
2.10.3 Duration of taper in the placebo group (relapse at 12 months)
There was also no clear effect on the difference in relapse risk at seven to 12 months based on how rapidly the medication was withdrawn from the placebo group (slope ‐0.0005, CI ‐0.0120 to 0.0110, P =0.934, see Figure 8).
8.
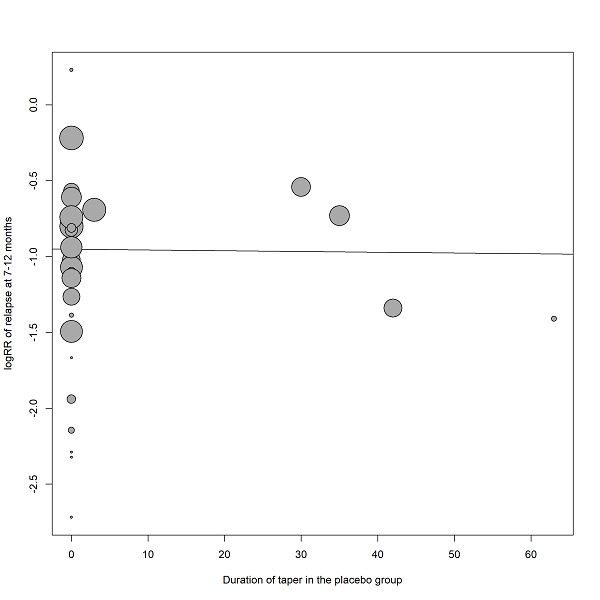
Meta‐regression on duration of taper in the placebo group (relapse at 12 months)
The size of the bubbles is proportional to the inverse variance of treatment effect.
2.10.4 Mean dose in chlorpromazine equivalents (relapse at 12 months)
When the mean dose in chlorpromazine equivalents used in the antipsychotic drug groups was taken into the meta‐regression, yet again there was no clear statistically significant effect on the difference in relapse risk at seven to 12 months (slope 0.0002, CI ‐0.0007 to 0.0011, P = 0.703, see Figure 9).
9.
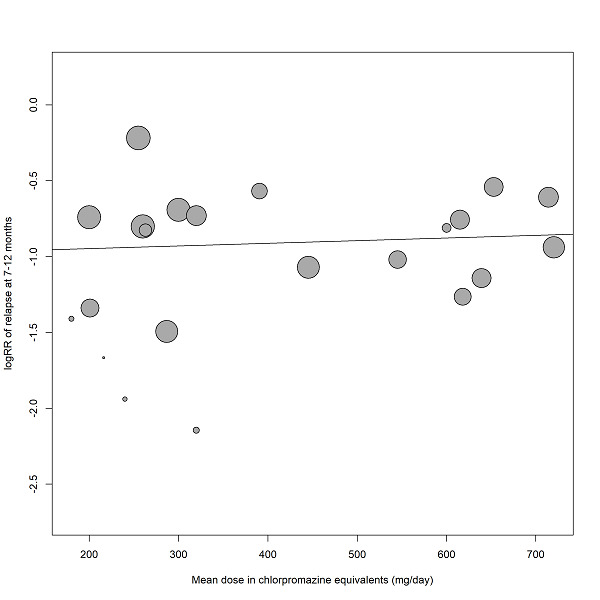
Meta‐regression on mean dose in chlorpromazine equivalents (relapse at 12 months)
The size of the bubbles is proportional to the inverse variance of treatment effect.
2.10.5 Study duration (relapse, all studies included)
There was, however, a clear statistically significant association in study duration with the difference relapse risk between antipsychotic drugs and placebo. The superiority of antipsychotic drugs was smaller in longer trials than in shorter studies (slope =0.0065 95% CI 0.0026 to 0.0104, P =0.001, see Figure 10).
10.
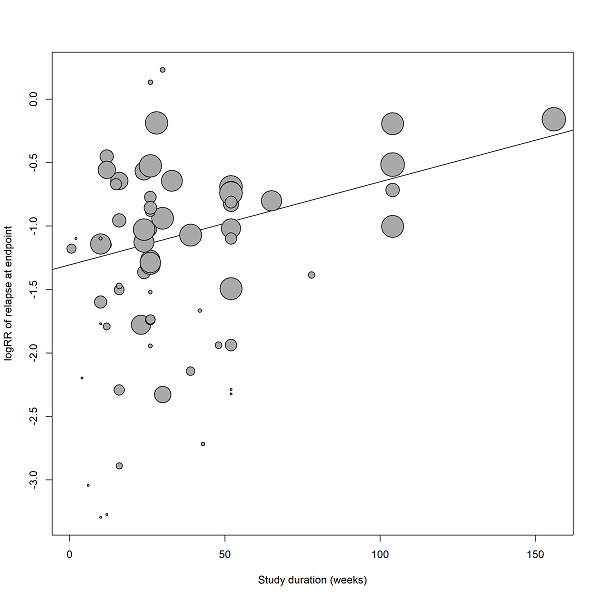
Meta‐regression on study duration (relapse, all studies included).
The size of the bubbles is proportional to the inverse variance of treatment effect.
3. Sensitivity analyses (relapse at 12 months)
All sensitivity analyses were conducted only for the primary outcome 'relapse at seven to 12 months'.
3.1 Exclusion of studies for which randomisation was implied because they were double‐blind
There was one study (Various drugs 1989) for the primary outcome relapse seven to 12 months that was not explicitly described as randomised, although randomisation was likely because it was double blind. Excluding this study did not change the overall results (drug 23% versus placebo 59%, 28 RCTs, n=4098, RR 0.39, 95% CI 0.33 to 0.46, NNTB 3, 95% CI 2 to 3, Analysis 3.1).
3.1. Analysis.

Comparison 3: Sensitivity analysis (relapse at 12 months), Outcome 1: Exclusion of studies that were not explicitly described as randomised
3.2 Exclusion of randomised, open studies
There were two randomised, open studies (Various drugs 1993; Various drugs 2011). Excluding these studies did not change the overall results (drug 24% versus placebo 59%, 27 RCTs, n = 3856, RR 0.40, 95% CI 0.33 to 0.48, NNTB 3, 95% CI 2 to 3, Analysis 3.2).
3.2. Analysis.

Comparison 3: Sensitivity analysis (relapse at 12 months), Outcome 2: Exclusion of non‐double‐blind studies
3.3 Fixed‐effect model
When a fixed‐effect model was applied, antipsychotic medication remained significantly more effective than placebo in preventing relapse (drug 23% versus placebo 59%, 29 RCTs, n = 4113, RR 0.38, 95% CI 0.35 to 0.41, NNTB 3, 95% CI 3 to 3, Analysis 3.3).
3.3. Analysis.

Comparison 3: Sensitivity analysis (relapse at 12 months), Outcome 3: Fixed‐effects model
3.4 Original authors' assumptions on attrition
There was no important difference if the original data of the authors' rather than our assumption on participants who had discontinued the studies was applied (drug 23% versus placebo 59%, 29 RCTs, n = 4113, RR 0.39, 95% CI 0.32 to 0.46, NNTB 3, 95% CI 2 to 3, Analysis 3.4).
3.4. Analysis.

Comparison 3: Sensitivity analysis (relapse at 12 months), Outcome 4: Original authors' assumptions on dropouts
3.5 Inclusion of large studies only (>200 participants)
Including only large studies did not markedly change the effect size (see publication bias above, Analysis 3.5).
3.6 Exclusion of studies that used clinical criteria to diagnose the participants
Excluding studies that did not use standardised diagnostic criteria did not change the overall results (drug 25% versus placebo 60%, 22 RCTs, n = 4054, RR 0.41, 95% CI 0.34 to 0.48, NNTB 3, 95% CI 2 to 3, Analysis 3.6).
3.6. Analysis.

Comparison 3: Sensitivity analysis (relapse at 12 months), Outcome 6: Exclusion of studies with clinical diagnosis
3.7 to 3.9 Inclusion of only those participants who had been in the trials without a relapse for three, six and nine months
Even when only participants who had not relapsed for three (drug 13% versus placebo 43%, 29 RCTs, n = 4622, RR 0.32, 95% CI 0.24 to 0.42, NNTB 5, 95% CI 3 to 10, Analysis 3.7), six (drug 11% versus placebo 39%, 20 RCTs, n = 2549, RR 0.30, 95% CI 0.20 to 0.45, NNTB 3, 95% CI 2 to 3, Analysis 3.8), or nine months (drug 9% versus placebo 32%; 15 RCTs, n = 1806, RR 0.32, 95% CI 0.19 to 0.52, NNTB 3, 95% CI 2 to 3, Analysis 3.9), after study start were included in the analysis, antipsychotic drugs were still clearly more effective than placebo. In the review update, data concerning this analysis were extracted from the start, due to a systematic error in the imputation of non‐relapsed patients in the original review, but the original findings were comparable to the current ones (Leucht 2012a, Leucht 2012b).
3.7. Analysis.

Comparison 3: Sensitivity analysis (relapse at 12 months), Outcome 7: Three months stable
3.8. Analysis.

Comparison 3: Sensitivity analysis (relapse at 12 months), Outcome 8: Six months stable
3.9. Analysis.

Comparison 3: Sensitivity analysis (relapse at 12 months), Outcome 9: Nine months stable
3.10 Exclusion of studies with unclear randomisation methods
Excluding studies with unclear randomisation methods did not markedly change the overall results (drug 22% versus placebo 59%, 11 RCTs, n = 2644, RR 0.36, 95% CI 0.29 to 0.43, Analysis 3.10).
3.10. Analysis.

Comparison 3: Sensitivity analysis (relapse at 12 months), Outcome 10: Exclusion of studies with unclear randomisation method
3.11 Exclusion of studies with unclear allocation concealment methods
Excluding studies with unclear allocation concealment methods did not markedly change the overall results (drug 22% versus placebo 59%, 13 RCTs, n = 2708, RR 0.37, 95% CI 0.30 to 0.45, Analysis 3.11).
3.11. Analysis.

Comparison 3: Sensitivity analysis (relapse at 12 months), Outcome 11: Exclusion of studies with unclear allocation concealment method
'Summary of findings' table
The results of seven a priori chosen outcomes ‐ relapse (seven to 12 months), leaving the study early due to any reason, service use (number of patients hospitalised), death due to suicide, quality of life, number of patients in employment and social functioning ‐ were considered more closely in a 'Summary of findings' table (see Table 1). Based on this tool, we considered the results for the outcomes relapse, leaving the studies early due to any reason and rehospitalisation to be high, for social functioning to be moderate, for suicide to be poor, and for quality of life and employment to be very poor. This is consistent with the judgements emerging from the original review data. The judgements derived from this instrument were used for the discussion section of the review (see Summary of main results).
Discussion
Summary of main results
1. General
This review currently includes 75 studies involving 9145 participants that compared antipsychotic maintenance treatment with placebo. The included studies were published over a long period (from 1959 to 2017) and in different settings (e.g. inpatients and outpatients) and different countries. Despite this variety, the results consistently demonstrated a superiority of antipsychotic drugs in the primary outcome relapse at seven to 12 months. This superiority remained robust in a number of sensitivity analyses. However, many included studies were relatively small; 47 randomised fewer than 100 people and 34 fewer than 50 people. Many trials were of short duration, only four studies lasted two years and only one study had a duration of three years. Thus, nothing is known from trials about the effects of antipsychotic drugs compared with placebo after three years. Furthermore, while almost all studies reported on relapse and leaving the study early, all other outcomes were much more sparsely recorded (e.g. adverse effects, quality of life, employment status, subjective outcomes such as participants' satisfaction with care). As it is unfortunately typical for randomised trials in schizophrenia, the methods of randomisation, allocation concealment and blinding were frequently not reported. However, as those studies that reported appropriate allocation methods yielded similar results, this potential source of bias should not challenge the overall findings.
All the results emerging from the review update are in line with those of the original review (Leucht 2012a, Leucht 2012b). For the current review update, some additional outcomes were investigated: antipsychotic drugs were found to be significantly superior to placebo in terms of promoting clinical remission status and better social functioning. No data were available on the efficacy of these drugs in terms of promoting recovery.
2. Treatment effects
2.1 Relapse
The results demonstrate that antipsychotic drugs reduce relapse rates more effectively than placebo. This effect was apparent as early as three months after discontinuation of antipsychotic drugs and remained significant in studies between 13 and 36 months. However, studies lasting longer than 12 months were scarce. This is even more important since the meta‐regression on study duration showed that the difference in patients with a relapse between drug and placebo gets smaller over time (Figure 7). There were frequent instances of significant heterogeneity, which may be due to differences in drugs, participants (e.g. degree of severity at baseline), or definitions of relapse. Nevertheless, almost all individual studies favoured antipsychotic drugs and therefore the heterogeneity reflected differences in the degree of superiority rather than differences in the direction of the effect. We continued to feel justified that this finding has a high degree of certainty.
2.2 Leaving the study early
Clearly fewer in the drug group than in the placebo group left the studies early because of 'any reason' or due to inefficacy of treatment. Leaving a study because of 'any reason' is often considered to be a measure of acceptability of treatment. We would be hesitant to apply this interpretation here because relapses were the most frequent reason for leaving the studies early and in many studies it was predefined by the protocol that participants had to discontinue once they had relapsed. Therefore, it was not really the participants' choice ('acceptability') to remain in a trial or not, and leaving the study early reflected efficacy rather than tolerability.
That more in the placebo group left the studies early due to 'inefficacy of treatment' supports the relapse‐preventing effect of antipsychotics.
There was no difference in the number of participants leaving the studies early 'due to adverse events'. It should be noted that events such as 'worsening of psychosis' are, by definition, also recorded as adverse events ‐ especially in more recent trials. In part, this may explain the significant heterogeneity of results. Moreover, this mix of tolerability‐ and efficacy‐related adverse events shows that 'leaving the studies early due to adverse events' is not an ideal measure of overall tolerability.
2.3 Global state
In the current update, the effect of antipsychotic drugs on the participants' clinical picture, when compared to placebo, was addressed in various ways.
When investigating the number of participants at least minimally improved at follow‐up (Clinical Global Impression ‐ Improvement score, or similar rating instruments), the results showed that antipsychotic drugs improved participants' global state more than placebo. But these findings also show that many participants were 'stable', but not in remission at study start. If they had all been in remission, further improvements would not have been possible. This demonstrates the importance of our subgroup analysis on people in remission at baseline.
The number of participants in symptomatic remission was addressed as an outcome for this update, along with the number of participants in sustained remission and in recovery. Symptomatic remission was defined following available criteria (e.g. Andreasen 2005 without time criterion) or considering being "at least mildly ill" (Clinical Global Impression ‐ Severity score or similar rating instruments) as a threshold. It could represent a cross‐sectional vision of the patients' severity of illness at various time points. Our results showed that antipsychotic drugs were significantly superior to placebo, even though only seven studies provided data on this outcome. All single studies showed at least a trend in favour of antipsychotic drugs, so that heterogeneity reflected differences in the degree of superiority rather than in the direction of the effect. Heterogeneity could also be explained by differences in the degree of severity at baseline, so that an unclear proportion of patients either achieved or maintained the remission level across the studies.
Eight studies lasting more than six months showed a significant superiority of antipsychotic drugs for sustained remission, which was defined as stabilised remission status for at least six months. Only a slight heterogeneity was found when pooling all the study results. Both symptomatic and sustained remission are important outcomes for schizophrenia patients, with the latter one being more linked to the possibility to fulfil a role in society and to achieve functional remission, but not representing it.
Among the included studies, no data were actually found on recovery, which can be multidimensionally conceptualised as comprising both objective (symptom severity and level of functioning) and subjective elements, such as quality of life and satisfaction with care (Vita 2018). We suggest that future long‐term studies should report at least sustained remission, or report data on both remission definitions separately: two studies included in the present review reported them separately (Aripiprazole 2017; Quetiapine 2007). Functional outcome is also a priority target for therapeutic interventions in schizophrenia; future trials should therefore focus on clinical remission, as well as social functioning and recovery data.
2.4 Service use
Fewer participants in the drug group had to be re‐hospitalised when compared with those allocated to placebo. Again, there was moderate heterogeneity, but all individual studies favoured to some degree the antipsychotic drugs. This finding is important, because in many industrialised countries hospitalisation contributes considerably to the direct cost of schizophrenia. Only 17 studies provided data on this outcome. Although it should be noted that only 34 trials were conducted in outpatients (in inpatients rehospitalisation cannot be an outcome), and although it depends on the setting how easily patients are admitted, this relatively hard and easy‐to‐measure outcome should be recorded in all future trials.
Many older trials were conducted in inpatient settings. Under these circumstances it was of interest to analyse whether the participants could be stabilised to such an extent that they could be discharged at the end of the trial. There was no clear difference between drug and placebo; however, only three trials contributed to this outcome and results are inconclusive.
2.5 Death and suicidal behaviour
There was no clear difference in the number of participants dying for any reason, natural causes or suicide. There was also no difference in the number of suicide attempts and suicidal ideation; however, in most studies the outcome death was not clearly reported. This is problematic, because there is some epidemiological evidence that long‐term treatment with antipsychotic drugs may increase mortality (Ray 2009; Weinmann 2009). Conversely, it is hoped that maintenance treatment with antipsychotic drugs might reduce suicides and another epidemiological study showed that treatment with antipsychotic drugs was associated with reduced mortality (Tiihonen 2009). We feel that future long‐term studies should consistently report this hard and important outcome.
2.6 Violent/aggressive behaviour
Fewer participants in the antipsychotic drug group had aggressive episodes. Although this finding is based on only 12 trials, it is an argument in favour of the use of antipsychotic drugs for maintenance treatment. Although the overall incidence is low, violence seems to be more frequent among people with schizophrenia compared to the general population contributing to the stigma of the disorder (Walsh 2002).
2.7 Adverse effects
Adverse effects were often poorly and incompletely reported. Nevertheless, antipsychotic drugs produced more movement disorders in terms of at least one movement disorder, akathisia (after removing an outlier) and use of antiparkinson medication. They also produced more sedation and weight gain. We highlight that we combined all antipsychotic drugs in the analysis, but antipsychotic drugs differ largely in their risk for these adverse events. For example, high‐potency conventional antipsychotic drugs, such as haloperidol, produce many movement disorders while many newer, so‐called second‐generation antipsychotic drugs, such as olanzapine, are associated with significant weight gain (Leucht 2009). Therefore, our tolerability findings are not generalisable to all compounds. Dyskinesia was the only outcome that occurred more frequently in the placebo group. At first glance this finding is peculiar. We speculate that these dyskinesias frequently were withdrawal dyskinesia after abrupt stopping of antipsychotic drugs rather than tardive dyskinesia. However, it was usually not clearly reported when this adverse event occurred. This is another example for a need of better side‐effect reporting in randomised schizophrenia trials (Papanikolaou 2004; Pope 2010).
2.8 Satisfaction with care
No data on either participant's or carer's satisfaction with care were available in the original review. However, in this update, two studies provided data on participant's satisfaction with care. Results were inconclusive due to the small number of participants included in the analysis; however, a significant superiority of drug to placebo was shown. We suggest that future trials should focus on this important outcome, in order to have the possibility to build reliable conclusions on this point.
2.9 Quality of life
Seven studies reported this outcome, which was evaluated using different rating instruments: two studies (Paliperidone 2007, Paliperidone depot1M 2010) used the Self‐report Quality of Life Scale (SQLS), two studies (Quetiapine 2009a, Quetiapine 2009b) used the Schizophrenia Quality of Life (S‐QoL); considering the other three studies, each one applied one different rating scale: Olanzapine 2003 used the Heinrichs Carpenter Quality of Life Scale (QLS), Lurasidone 2016 applied the EuroQOL Visual Analog Scale (EQ5D‐VAS) and Various drugs 1981b used the Symptom Questionnaire of Kellner and Sheffield. Four studies showed better quality of life in the antipsychotic drug groups and three studies showed no significant difference; when all the studies were combined, the superiority of active medication was statistically significant. Due to the small number of trials this finding is not robust and more evidence is needed. Furthermore, the seven trials applied different rating scales, providing heterogeneous conclusions in terms of statistical significance. However, the direction of effect was always consistent across the trials, tending to favour antipsychotic drugs. The relevance of the actual finding is, however, high, because we had assumed that due to their side effects antipsychotic drugs could worsen quality of life. If confirmed by further trials, improved quality of life would be another strong argument for maintenance treatment with antipsychotic drugs. As a limitation, it needs to be considered that patients with a relapse were typically included in this outcome. For evaluating the quality of life of patients while taking maintenance treatment (and experiencing side effects), the quality‐of‐life‐ratings before recurrence of psychotic symptoms would be of additional interest. However, these data were not typically 'not available'. A targeted update review performed by another team in 2016 (New Reference), found that maintenance treatment may make little or no difference to quality of life (standardised mean difference (SMD) ‐0.42 95% confidence interval (CI) ‐0.96 to 0.13, 4 RCTs, 804 participants). In that review, four studies (all included in the present review) were included (Lurasidone 2016, Olanzapine 2003, Paliperidone 2007 and Various drugs 1981b). Quality of life was measured using four different rating scales in the four studies and back‐estimated to SQLS. When inspecting the studies mentioned above, it was noted that in the targeted update the Lurasidone 2016 results were entered in the wrong direction of effect, with higher scores indicating improvement (the opposite as in the other studies). The analysis based on the four studies was performed again using the statistical method applied by the targeted update team, considering this mistake (SMD ‐0.46 95% CI ‐0.93 to 0.00, P = 0.05).
2.10 Number of participants in employment
Only three studies addressed this outcome and did not find a significant difference. This finding is inconclusive and highlights the limitations of the current evidence. It is clear that antipsychotic drugs suppress symptoms of schizophrenia, but whether this also leads to better functional outcomes is unclear. A review suggested that 80% to 90% of people with schizophrenia are not employed (Marvaha 2004). In this review update we therefore investigated the effects of antipsychotic drugs on social functioning (see next paragraph).
2.11 Social functioning
Fifteen studies reported this outcome. The studies applied different rating scales: 10 studies used Performance and Social Participation schedule (Aripiprazole depot 2012, Brexpiprazole 2017, Cariprazine 2016, Paliperidone 2007, Paliperidone 2014, Paliperidone depot1M 2010, Paliperidone depot1M 2015, Paliperidone depot3M 2015, Quetiapine 2009a; Quetiapine 2009b), while in the other five studies, different rating scales were applied in one study each: the Global Assessment of Functioning (Ziprasidone 2002), the Global Assessment Scale (Fluphenazine depot 1981) and its Children version (Aripiprazole 2017), the Specific Levels of Functioning (Lurasidone 2016) and the Sheehan Disability Schedule (Iloperidone 2016). For that reason, effects were analysed separately using MDs. At all time points and with all the scales used, the direction of effect was in favour of antipsychotic treatment, mostly (in 13 out of 15 studies) statistically significant. As an additional analysis, all studies were combined across different rating scales and time points using SMD. This analysis also showed statistically significant superiority of active medication. Analysing the studies using different statistical methods would probably not have changed the conclusions, since all the studies revealed at least a trend in favour of active drugs. As social functioning is regarded as one of the areas which should be taken into account for recovery beyond clinical remission, this is an important finding for clinical practice. It has been argued that functional remission is a more important criterion for recovery than being symptom free in order to be able to fulfil private and professional roles and to achieve social integration (Burns 2007, Vita 2018). Future trials should continue to consider social functioning as one of the outcomes, and, if our finding is confirmed, improved social functioning is another argument in favour of maintenance treatment with antipsychotic drugs in schizophrenia patients. As in the assessment of quality of life, it needs to be considered as a limitation that patients with a relapse were typically included in this outcome. For evaluating the social functioning of patients while taking maintenance treatment, the social‐functioning ratings before recurrence of psychotic symptoms would be of additional interest. However, tthese data were not typically not available.
3. Publication bias
The funnel plot was clearly asymmetrical suggesting the possibility of a publication bias. However, other reasons than unpublished studies can make funnel plots asymmetrical. For example, small studies are often conducted in single centres with very motivated investigators who make sure that drugs are compliantly taken. This may be more difficult in large, multi‐centre studies. To examine the impact of potentially undetected small studies we undertook a sensitivity analysis in which we only included larger studies (which we defined by a sample size of at least 200). In this group of studies there was still a clear reduction of the relapse risk at 12 months by antipsychotic drugs. Therefore, even if only the larger studies were considered, the finding of the superiority of antipsychotic drugs for relapse‐prevention is not threatened. Duval's and Tweedy's trim and fill method did also not suggest a substantial effect from missing small trials (Duval 2000).
4. Subgroup analyses and investigation of heterogeneity
The heterogeneity of many results was statistically significant, which was expected in a review that pooled different drugs and doses, that combined studies that used different relapse definitions, and that were published over a period of 50 years. Nevertheless, in most studies the direction of the effects was the same. Therefore, the heterogeneity reflected only differences in the degree of superiority in relapse prevention. Moreover, most subgroup analyses and meta‐regressions did not reveal any statistically significant differences. This finding is important, because it may be interpreted that the relapse‐preventing effects of antipsychotic drugs can be generalised to many patients.
4.1 People with a first episode of schizophrenia and people in remission
The effects of antipsychotic drugs were similar in first‐episode compared to multiple‐episode participants and if participants were in remission at baseline or not. First‐episode and remitted people with schizophrenia are thought to have a better prognosis, but our results suggest that theybenefit equally from antipsychotic relapse prevention. Approximately 20% of people with a first episode of schizophrenia will not have a second episode within five years (Robinson 1999), but identification of this subgroup in advance is more than problematic.
4.2 People who had been stable for various periods before entering the trials
The relapse‐preventing effects of antipsychotic drugs were independent from the duration that participants had been stable before entering the studies. Even in those participants who had been stable for up to three to six years (Fluphenazine depot 1992; Various drugs 1981b) relapse rates were higher among placebo‐treated than among drug‐treated individuals. This is important for the recommended duration of antipsychotic maintenance treatment in guidelines, because it can be argued that even patients who have taken antipsychotic drugs for such a duration still benefit from them. However, as only two small studies (Fluphenazine depot 1992; Various drugs 1981b) with a total of only 54 participants contributed to this finding, more evidence is clearly needed for solid recommendations.
4.3 Abrupt versus gradual withdrawal of antipsychotic drugs
There is a theory that long‐term treatment with antipsychotic drugs leads to a compensatory up‐regulation of dopamine receptors. If antipsychotic drugs are withdrawn abruptly, dopamine receptors are hypersensitive, leading to rebound psychosis (Moncrieff 2006). This phenomenon has been called 'supersensitivity psychosis'. In contrast to the now outdated report by Viguera 1997, we did not find a difference in relapse reduction between studies in which drugs were abruptly or gradually withdrawn, neither in a dichotomised subgroup analysis applying the same cut‐off as Viguera 1997 (who defined gradual withdrawal by a taper duration of at least three weeks or stopping depot antipsychotic drugs that have a long half‐life), nor in a meta‐regression with duration of taper as a continuous parameter. It should be noted that subgroup analysis and meta‐regression are observational, crude methods and can, therefore, not rule out this theory which needs thorough investigation. It is also possible that supersensitivity psychosis explains a part of the decreasing effect sizes in longer trials (see Figure 7 and below). We would therefore strongly recommend slow tapering of antipsychotic drugs, if withdrawal is needed.
4.4 Single antipsychotic drugs, depot versus oral medication and first‐generation versus second‐generation antipsychotic drugs
There were no differences between the single antipsychotic drugs used apart from depot antipsychotic drugs (in particular depot formulations of haloperidol and fluphenazine) being more effective than oral antipsychotic drugs. Although this result fits to the theory that depot antipsychotic drugs improve the adherence that is crucial for relapse prevention, subgroup analyses are of observational nature. Only head‐to‐head comparisons of oral and depot antipsychotic drugs can decide whether the latter are more effective. A recent update of our systematic review on this question (Leucht 2011) did not find a difference between oral and depot medication (Kishimoto 2014). As a group, so‐called second‐generation antipsychotic drugs did not differ in relapse reduction from first‐generation antipsychotic drugs. This supports previous suggestions that this classification should be abandoned, because there is no single definition that fits to all drugs that are considered to be second‐generation or atypical antipsychotic drugs (Leucht 2009).
4.5 Appropriate versus unclear allocation concealment methods
There was no difference between the effect estimates of studies that used appropriate and unclear allocation concealment methods. It should, however, be noted that the original analyses on this question found larger differences between studies with appropriate and inappropriate allocation concealment than between appropriate and unclear allocation concealment (e.g. Schulz 1995). Studies with inappropriate allocation concealment were excluded a priori from our review.
4.6 Open versus double‐blind studies
Open trials were associated with a stronger difference between drugs and placebo than blinded trials, but as there were only two open RCTs (Various drugs 1993; Various drugs 2011), the impact of this effect was small.
4.7 Meta‐regression on study duration
There was a statistically significant association between longer study duration and smaller relapse reduction by antipsychotic drugs compared with placebo. This result could indicate that antipsychotic drugs lose their efficacy over time. We emphasise that there are also other possible explanations for this counter‐intuitive finding. Participants' severity in shorter and longer trials could be different, and notably the decreasing relapse‐preventing effects could also be an effect of decreasing drug compliance over time. However, studies that last longer than two years and either use depot antipsychotic drugs or thoroughly monitor compliance are needed to investigate the long‐term effects of antipsychotic drugs. Moreover, as antipsychotics prevent relapses, more patients in the drug group stayed in the study as compared to placebo (see also Discussion, section 2.2. "Leaving the study early due to any reasons"). Consequentely, at later time points, there were more at risk for relapse in the drug group than in the placebo group, and the difference in relapses between drug and placebo may get smaller due to this imbalance for people at risk (Davis 1975). Such a phenomenon is indeed expected should antipsychotics not completely prevent but only delay the occurrence of relapses (at least for a proportion of patients).
5. Sensitivity analyses
The results of the primary outcome were not much different when studies that were not clearly described as randomised were excluded, when open studies were excluded, when a fixed‐effect model instead of a random‐effects model was applied, when we used the original authors' assumptions on dropouts instead of our approach, when studies with unclear randomisation or allocation concealment methods were excluded, when only large trials were included, and when studies that did not use operational criteria to diagnose the participants were excluded. These sensitivity analyses underline the robustness of the results.
A final sensitivity analysis in which we analysed only those participants who had not relapsed for various durations after study start again addressed supersensitivity psychosis. It revealed that even in those participants who had not relapsed for nine months, subsequent relapse rates were clearly lower in the drug group than in the placebo group. This finding opposes the theory that many relapses were merely rebound effects after rapid withdrawal (Moncrieff 2006).
Overall completeness and applicability of evidence
The 75 included studies were conducted in various settings (e.g. inpatients and outpatients, different countries, stable superiority antipsychotic drugs in trials from different years), populations (e.g. participants in remission at baseline or not), and methods (e.g. different definitions of relapse). Therefore, we believe that the evidence is quite complete and applicable to routine care. There are, however, several limitations. While almost all studies reported on relapse, there is much less evidence on other outcomes such as hospitalisation, remission, employment status and adverse events, which were often inadequately reported. There were very few studies that lasted longer than one year. Thus, the long‐term effects of maintenance treatment are less clear. Finally, in most studies antipsychotic drugs were withdrawn abruptly. There is a theory that long‐term treatment leads to changes in dopamine receptors ('hypersensitivity psychosis') and re‐emergence of symptoms after abrupt withdrawal (Moncrieff 2006). One study (Olanzapine 1999) was excluded from the original review due to the fact that it provided for a very short duration of follow‐up (three to five days) after abrupt withdrawal (tapering of three to 12 days), and it was therefore difficult to unequivocally distinguish between withdrawal/rebound phenomenon and illness recurrence. In the update we decided to include the study. Sensitivity analyses of the outcomes which the study contributed to were performed, and excluding the study did not change the results. Although our meta‐regression and sensitivity analysis did not detect an effect, future studies should withdraw antipsychotic drugs gradually rather than abruptly and to rule out or confirm this, supersensitivity psychosis should be an important research agenda.
Quality of the evidence
In the review update, judgements on the quality of evidence were consistent with those of the original review. Almost all studies were randomised and double‐blind but for most details were not presented. Therefore it is unclear whether the studies were adequately randomised, whether treatment allocation was really concealed and whether blinding worked. Concerning blinding this may be less important in objective outcomes such as death or weight gain. Concerning allocation concealment we at least found that there was no difference in the primary outcome between studies that used appropriate and unclear methods. Dropout rates were often high, partly because it was specified in many studies' methods that participants had to discontinue once they relapsed. This poses mainly a problem for outcomes other than relapse. While relapse and leaving the studies early was quite consistently reported, the evidence about other outcomes was much more scarce. Without original study protocols being available we cannot judge with absolute certainty whether these were not measured or whether there were cases of selective reporting. The current approach to report only those outcomes that occurred in at least 5% to 10% of the participants should be abandoned, because rare but important side effects might be overlooked.
The most recent studies were often terminated early after pre‐planned interim analyses: this kind of design is clearly useful for practical and ethical reasons, but in the context of meta‐analyses it could be linked to the risk of overestimating treatment effects, especially when the studies stopped early contribute substantial weight in the analysis of some outcomes. However, this potential source of bias was not judged to threaten the quality of the overall evidence, with only a few exceptions (e.g. quality of life data). In individual trials there were also other problems, such as too high or too low doses, early termination of studies, baseline imbalances etc. In summary, the overall quality of the studies according to these criteria is moderate. Nevertheless, due to the consistency of the results in subgroup and sensitivity analyses, the overall superiority of antipsychotic drugs in reducing relapse rates is not challenged.
Potential biases in the review process
Wedecided a priori to pool all antipsychotic drugs in this review. We feel that this is justified for efficacy‐related outcomes, because most antipsychotic drugs do not differ in efficacy and if differences exist between some antipsychotic drugs, these are not large (Leucht 2009; Leucht 2013). The decision to pool all studies irrespective of the antipsychotic drug used is more problematic for adverse effects, because antipsychotic drugs differ to a large extent in this regard. Thus, any differences in side effects compared to placebo cannot be generalised to all antipsychotic compounds. Similarly, we analysed only a selection of common and important adverse effects, but many others exist.
The study search was mainly based on the Cochrane Schizophrenia Group’s register of trials. This is largely made up of searches of published literature. It is possible that there are unpublished studies that we are not aware of and there is a possibility of publication bias, although the funnel plot may also be asymmetrical due to other factors.
As a minor point, the 2017 update‐search did not include all antipsychotic drugs but was restricted to 35 different antipsychotics (including all second‐generation antipsychotics and the most important first‐generation antipsychotics). Therefore, theoretically, studies on specific first‐generation drugs that were not listed, could have been missed. However, we deem it unlikely that many studies (if any at all) with these specific old drugs have been performed after 2008. More sensitive time‐to‐relapse data derived from survival analyses that are considered more appropriate measures were not available for most studies, and, therefore, we had to restrict ourselves to the number of participants relapsed.
We have chosen to use the random‐effects model for our analyses, which does not assume that the populations from which the different trials are derived are the same. This technique does emphasise the results from smaller trials and it is these studies that are likely to be most prone to bias. Nevertheless, the results of a fixed‐effect model in a sensitivity analysis of the primary outcome were similar.
Finally, we highlight that many subgroup and meta‐regression analyses were conducted in this review, many of which were added post‐hoc ‐ after requests from reviewers. This raises the problem of type I errors (i.e. chance findings due to multiple testing).
Agreements and disagreements with other studies or reviews
We are aware of five other reviews that compared maintenance treatment with any antipsychotic drug with placebo (Davis 1975; Baldessarini 1985; Gilbert 1995; Zhao 2016; Kishi 2019). All were consistent with our results because they found that people with schizophrenia who were withdrawn from antipsychotic drugs relapsed significantly more frequently than those who continued them. However, some of these reports did not meet modern criteria of systematic reviews, did not analyse relapse at different points in time and did not address any other outcome. A review by some members of the current review team was restricted to second‐generation antipsychotics (Leucht 2009b, an update of Leucht 2003). Second‐generation antipsychotic drugs clearly reduced relapse rates compared to placebo and the relative risk was similar to that in the current review (RR 0.41, 95% CI 0.28 to 0.59), but the absolute risk difference was smaller (RD 0.20, 95% CI 0.11 to 0.30). The previous review included only seven trials and the inclusion criteria were different (e.g. studies that only followed up acute‐phase responders (a design that corrupts randomisation) were also included and participants were not required to be stable on antipsychotic drugs or to be on antipsychotic drugs at all at study start).
In terms of Cochrane Reviews, Almerie 2007 examined withdrawal of chlorpromazine compared to placebo and also found a significant relapse risk reduction. In the targeted update of this review, which was performed in 2016 (see New Reference) and included 22 RCTs (4334 participants), data on the primary outcome (relapse at one year) were consistent in essence with our results (10 RCTs, RR 0.50, 95% CI 0.38 to 0.66), as well as data on violent/aggressive behaviour. The targeted update also provided data on remission (participants not in remission at one year; 4 RCTs, RR 0.75, 95% CI 0.66 to 0.86); no data on recovery were found, the same as in the present review. As specified before (see Discussion, section 2.9), basing on the targeted update, data maintenance treatment with antipsychotic drugs may make only little difference to quality of life in people with schizophrenia; however, the analysis was based on four studies, and data of one study were entered in the wrong direction of effect; in the present review the superiority of antipsychotic drugs was found to be statistically significant (data based on seven studies), although the certainty of evidence for this outcome was judged as poor due to limitations concerning study design, indirectness and imprecision.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia
For people with schizophrenia it is important to know that antipsychotic drugs are more effective than placebo in preventing relapse. If people stop their antipsychotic drug many will relapse ‐ and quite soon ‐ and more than if they remained on the drug. Taking the drugs is likely to cause a number of adverse effects, such as movement disorders, weight gain and sedation (which differ between compounds). They might tell their doctors that they want to be involved in the choice of the antipsychotic. Stopping the drug could still be the choice of the recipient of care but this review allows more understanding of the risk of this action.
2. For clinicians
Clinicians should know that most studies lasted no longer than one year and that the longest study lasted three years. Thus, nothing is known about the very long‐term effects of antipsychotic drugs compared with placebo. The clear superiority of antipsychotic drugs was consistent for different types of settings (e.g. inpatient and outpatients) and participants (people with a first and multiple episodes, duration of stability before study start), and it was robust to statistical assumptions. Whether antipsychotic drugs save lives by preventing suicides or increase mortality due to their adverse effects could not be clarified by this review. However, this review does make it easier for clinicians to advise the continuation of antipsychotic drugs for many people with schizophrenia. Recognising that this may not be the path chosen by the person with the illness, this review helps inform the clinician of the proportions who are likely to need relapse care in the short and medium term.
3. For managers/policy makers
The data suggest that people maintained on antipsychotic drugs need to be hospitalised less frequently than those stopping medications in favour of placebo. In many countries hospitalisation accounts for a large proportion of the overall costs of schizophrenia. However, less than one third of relapsed participants had such severe relapses that rehospitalisation was necessary. Nevertheless there is such consistency in the findings of this review that it would be understandable that the guidance would adopt a strategy of maintenance of antipsychotic drugs for people with schizophrenia where possible.
Implications for research.
1. General
Outcome reporting remains insufficient in antipsychotic drug trials. Strict adherence to the CONSORT statement (CONsolidated Standards Of Reporting Trials; Moher 2001) would make such studies much more informative. This short‐coming has been highlighted by many reviews of the Cochrane Schizophrenia Group and others, but improvements are still necessary.
2. Specific
Although difficult to conduct due to ethical concerns, it would be interesting to have more studies that last longer than two years. Such studies should not only examine relapse, but also other outcomes such as rehospitalisation, recovery status, outcomes reflecting social participation and death. Participants' compliance should be monitored. Table 2 presents an outline.
1. Design of a future study.
| Methods | Allocation: randomised ‐ clearly described generation of sequence and concealment of allocation Blinding: double ‐ described and tested Duration: 3 years |
| Participants | People with schizophrenia or schizophrenia‐like disorder in remission for at least one month N = 500 Age: any Sex: both History: any (specify duration of illness) |
| Interventions | 1. Any antipsychotic drug (flexible dose within appropriate range) 2. Placebo (after gradual ‐ rather than abrupt ‐ withdrawal of the previous antipsychotic drug) |
| Outcomes | Relapse (primary outcome) Rehospitalisation for psychosis Global state (number of participants improved, in symptomatic and sustained remission) Global state (number of participants in recovery) Leaving the study early (including specific causes) Death (natural and unnatural causes) Violent behaviour Quality of life Satisfaction with care and other measures of subjective well‐being/recovery Side‐effects (well reported) Social functioning, employment and other measures of functioning |
What's new
| Date | Event | Description |
|---|---|---|
| 30 July 2020 | New citation required but conclusions have not changed | New citation |
| 11 September 2019 | New search has been performed | A new update search was done and the references were sent to the team |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 5, 2012
| Date | Event | Description |
|---|---|---|
| 3 July 2018 | Amended | Further update search was done and the references were sent to the team. |
| 10 October 2017 | Amended | Search was done and the references were sent to the team. |
Acknowledgements
We thank the editorial team of the Cochrane Schizophrenia Group for its enormous support. Particularly, we thank Farhad Shokraneh, Information Specialist of the Cochrane Schizophrenia Group, for conducting the update searches. We thank Richard Skodnek, MD, for his work on a preliminary version of this review. We thank Carola Dörries for her help in screening the literature search and extracting data for the updated version of this review. We also thank Astellas, Bristol MyersSquibb, EliLilly, Lundbeck, Pfizer, Johnson & Johnson, SanofiAventis, Eric Chen, George Gardos, Julian Leff, and Erik Denys for sending additional information on their studies. We thank Michael Borenstein and Georgia Salanti for their statistical advice and for conducting statistical analyses.
The Cochrane Schizophrenia Group Editorial Base in Nottingham, UK, produces and maintains standard text for use in the Methods section of their reviews. We have used some of this text as the basis of what appears here and adapted it as required.
Appendices
Appendix 1. Previous searches
1.1 Search in 2008
1.1.1 Electronic searches
1.1.1.1 Cochrane Schizophrenia Group's Study‐Based Register of Trials
We searched the the register (November 2008) with the term: {[cessation* or withdr?w* or discontinu* or halt* or stop* or drop?out* or dropout* or rehospitalis* or relaps* or maintain* or maintenance* or recur* in title, abstract, index terms of REFERENCE] or [withdrawal* in interventions of STUDY]}
This register is compiled by regular systematic searches of major databases including EMBASE, MEDLINE and PsycINFO; handsearches; and conference proceedings (see Group Module). The Cochrane Schizophrenia Group's Specialised Register is maintained on MeerKat 1.5. This version of MeerKat stores references as studies. When an individual reference is selected through a search, all references that have been identified as the same study are also selected.
1.1.2 Searching other resources
1.1.2.1 Reference searching
We inspected the references of all included studies and of previous reviews (Davis 1975; Gilbert 1995) for more trials.
1.1.2.2 Personal contact
We contacted the first author of each included study for missing information and for the existence of further studies.
1.1.2.3 Drug companies
We contacted the manufacturers of antipsychotic drugs and asked them about further relevant studies and for missing information on identified studies.
1.2 Search in 2011
1.2.1 Electronic searches
We searched MEDLINE (2008 to 6th June 2011) and EMBASE (2008 to 6th June 2011) with the term: (cessation* OR withdraw* OR discontinu* OR halt* OR stop* OR drop‐out* OR dropout* OR drop out OR rehospitalis* OR relaps* OR maintain* OR maintenance* OR recur*) AND schizophr* OR schizoaff* Limits: Randomized Controlled Trial. We searched clinicaltrials.gov with the names of 13 second‐generation antipsychotic drugs (amisulpride, aripiprazole, clozapine, iloperidone, lurasidone, olanzapine, quetiapine, risperidone, paliperidone, sertindole, ziprasidone, zotepine).
1.2.1.1 Clinicaltrials.gov (June 08, 2011)
We searched clinicaltrials.gov with the names of 13 second‐generation antipsychotics (amisulpride, aripiprazole, clozapine, iloperidone, lurasidone, olanzapine, quetiapine, risperidone, paliperidone, sertindole, ziprasidone, zotepine)
1.2.1.2 EMBASE (June 06, 2011)
("search"[All Fields] AND Term[All Fields]) AND ((cessation[All Fields] OR cessation/avoidance[All Fields] OR cessation/depletion[All Fields] OR cessation/hypercholesterolemia[All Fields] OR cessation/legislation[All Fields] OR cessation/lifestyle[All Fields] OR cessation/prevention[All Fields] OR cessation/prohibition[All Fields] OR cessation/reduction[All Fields] OR cessation/relapse[All Fields] OR cessation/reperfusion[All Fields] OR cessation/retardation[All Fields] OR cessation/smoking[All Fields] OR cessation/stabilization[All Fields] OR cessation/to[All Fields] OR cessation'[All Fields] OR cessation's[All Fields] OR cessationof[All Fields] OR cessations[All Fields] OR cessations'[All Fields] OR cessationsof[All Fields]) OR (withdraw[All Fields] OR withdraw/limit[All Fields] OR withdraw/pause/advance[All Fields] OR withdraw/retire[All Fields] OR withdraw/withhold[All Fields] OR withdraw'[All Fields] OR withdrawal[All Fields] OR withdrawal/abstinence[All Fields] OR withdrawal/ach[All Fields] OR withdrawal/adaptation[All Fields] OR withdrawal/addition[All Fields] OR withdrawal/advancement[All Fields] OR withdrawal/anhedonia[All Fields] OR withdrawal/anxiety[All Fields] OR withdrawal/apathy/lack[All Fields] OR withdrawal/asocial[All Fields] OR withdrawal/avoidance[All Fields] OR withdrawal/bacteraemia[All Fields] OR withdrawal/challenge[All Fields] OR withdrawal/chronic[All Fields] OR withdrawal/continuation[All Fields] OR withdrawal/conversion[All Fields] OR withdrawal/craving[All Fields] OR withdrawal/decondensation[All Fields] OR withdrawal/delayed[All Fields] OR withdrawal/dependence[All Fields] OR withdrawal/depression[All Fields] OR withdrawal/discontinuation[All Fields] OR withdrawal/disinterest[All Fields] OR withdrawal/dropouts[All Fields] OR withdrawal/failure[All Fields] OR withdrawal/fear[All Fields] OR withdrawal/high[All Fields] OR withdrawal/hospitalisation[All Fields] OR withdrawal/infusion[All Fields] OR withdrawal/inhibition[All Fields] OR withdrawal/intoxication[All Fields] OR withdrawal/isolation[All Fields] OR withdrawal/lethargy[All Fields] OR withdrawal/limitation[All Fields] OR withdrawal/losses[All Fields] OR withdrawal/masking[All Fields] OR withdrawal/minimization[All Fields] OR withdrawal/minipump[All Fields] OR withdrawal/motor[All Fields] OR withdrawal/negative[All Fields] OR withdrawal/no[All Fields] OR withdrawal/numbing[All Fields] OR withdrawal/overcompensation[All Fields] OR withdrawal/periodic[All Fields] OR withdrawal/placebo[All Fields] OR withdrawal/protection[All Fields] OR withdrawal/rebound[All Fields] OR withdrawal/recovery[All Fields] OR withdrawal/refusal[All Fields] OR withdrawal/regulation[All Fields] OR withdrawal/reinfusion[All Fields] OR withdrawal/reinsertion[All Fields] OR withdrawal/reintroduction[All Fields] OR withdrawal/removal[All Fields] OR withdrawal/reperfusion[All Fields] OR withdrawal/replacement[All Fields] OR withdrawal/resistance[All Fields] OR withdrawal/responsiveness[All Fields] OR withdrawal/retardation[All Fields] OR withdrawal/reversal[All Fields] OR withdrawal/rhythm[All Fields] OR withdrawal/ri[All Fields] OR withdrawal/screaming[All Fields] OR withdrawal/social[All Fields] OR withdrawal/stereotypy[All Fields] OR withdrawal/stimulation[All Fields] OR withdrawal/study[All Fields] OR withdrawal/tolerance[All Fields] OR withdrawal/toxic[All Fields] OR withdrawal/transfusion[All Fields] OR withdrawal/uremia[All Fields] OR withdrawal/warnings[All Fields] OR withdrawal/withholding[All Fields] OR withdrawal'[All Fields] OR withdrawal's[All Fields] OR withdrawall[All Fields] OR withdrawallike[All Fields] OR withdrawally[All Fields] OR withdrawalpolicy[All Fields] OR withdrawalpolicyv[All Fields] OR withdrawals[All Fields] OR withdrawals/dropouts[All Fields] OR withdrawan[All Fields] OR withdrawas[All Fields] OR withdrawed[All Fields] OR withdrawel[All Fields] OR withdrawen[All Fields] OR withdrawer[All Fields] OR withdrawers[All Fields] OR withdrawers'[All Fields] OR withdrawial[All Fields] OR withdrawing[All Fields] OR withdrawing/accepting[All Fields] OR withdrawing/donating[All Fields] OR withdrawing/electron[All Fields] OR withdrawing/withholding[All Fields] OR withdrawing'[All Fields] OR withdrawings[All Fields] OR withdrawl[All Fields] OR withdrawls[All Fields] OR withdrawn[All Fields] OR withdrawn/acceptor[All Fields] OR withdrawn/anxious[All Fields] OR withdrawn/black[All Fields] OR withdrawn/depressed[All Fields] OR withdrawn/depression[All Fields] OR withdrawn/depressive[All Fields] OR withdrawn/dysphoric[All Fields] OR withdrawn/inhibited[All Fields] OR withdrawn/ltfu[All Fields] OR withdrawn/not[All Fields] OR withdrawn/psychotic[All Fields] OR withdrawn/timid[All Fields] OR withdrawn/uncommunicative[All Fields] OR withdrawn/uncooperative[All Fields] OR withdrawn/withheld[All Fields] OR withdrawn'[All Fields] OR withdrawness[All Fields] OR withdrawning[All Fields] OR withdrawnn[All Fields] OR withdrawnness[All Fields] OR withdrawol[All Fields] OR withdraws[All Fields]) OR (discontinu[All Fields] OR discontinua[All Fields] OR discontinuacion[All Fields] OR discontinual[All Fields] OR discontinualis[All Fields] OR discontinually[All Fields] OR discontinuance[All Fields] OR discontinuances[All Fields] OR discontinuante[All Fields] OR discontinuanti[All Fields] OR discontinuate[All Fields] OR discontinuated[All Fields] OR discontinuating[All Fields] OR discontinuation[All Fields] OR discontinuation/change[All Fields] OR discontinuation/completion[All Fields] OR discontinuation/continuation[All Fields] OR discontinuation/delay[All Fields] OR discontinuation/dose[All Fields] OR discontinuation/interruption[All Fields] OR discontinuation/interruption/reduction[All Fields] OR discontinuation/reduction[All Fields] OR discontinuation/reintroduction[All Fields] OR discontinuation/retreatment[All Fields] OR discontinuation/safety[All Fields] OR discontinuation/substitution[All Fields] OR discontinuation/switch[All Fields] OR discontinuation/switching[All Fields] OR discontinuation/tapering[All Fields] OR discontinuation/unevaluable[All Fields] OR discontinuation/withdrawal[All Fields] OR discontinuation'[All Fields] OR discontinuations[All Fields] OR discontinuations/bortezomib[All Fields] OR discontinuations/dose[All Fields] OR discontinuations/first[All Fields] OR discontinue[All Fields] OR discontinue/change[All Fields] OR discontinue/reduce[All Fields] OR discontinue/renewal[All Fields] OR discontinue/switch[All Fields] OR discontinued[All Fields] OR discontinued/avoided[All Fields] OR discontinued/switched[All Fields] OR discontinued'[All Fields] OR discontinuence[All Fields] OR discontinueous[All Fields] OR discontinuer[All Fields] OR discontinuer/augmenter/maintainer[All Fields] OR discontinuerlig[All Fields] OR discontinuerlige[All Fields] OR discontinuers[All Fields] OR discontinuers/maintainers/augmenters[All Fields] OR discontinuers'[All Fields] OR discontinues[All Fields] OR discontinuidades[All Fields] OR discontinuing[All Fields] OR discontinuining[All Fields] OR discontinuist[All Fields] OR discontinuist'[All Fields] OR discontinuita[All Fields] OR discontinuite[All Fields] OR discontinuiteit[All Fields] OR discontinuites[All Fields] OR discontinuities[All Fields] OR discontinuities/edges[All Fields] OR discontinuities/line[All Fields] OR discontinuities/splits[All Fields] OR discontinuities'[All Fields] OR discontinuiting[All Fields] OR discontinuity[All Fields] OR discontinuity/fatigue[All Fields] OR discontinuity/occlusion[All Fields] OR discontinuity/otosclerosis[All Fields] OR discontinuity'[All Fields] OR discontinuo[All Fields] OR discontinuos[All Fields] OR discontinuosly[All Fields] OR discontinuosus[All Fields] OR discontinuous[All Fields] OR discontinuous/absent[All Fields] OR discontinuous/conformational[All Fields] OR discontinuous/continuous[All Fields] OR discontinuous/insular[All Fields] OR discontinuous/jumping[All Fields] OR discontinuous/satellite[All Fields] OR discontinuous/sequencing[All Fields] OR discontinuous'[All Fields] OR discontinuously[All Fields] OR discontinuousness[All Fields] OR discontinus[All Fields] OR discontinutation[All Fields] OR discontinute[All Fields] OR discontinuties[All Fields] OR discontinution[All Fields] OR discontinuty[All Fields] OR discontinuum[All Fields]) OR (halt[All Fields] OR halt/moderate[All Fields] OR halt/regress[All Fields] OR halt/reverse[All Fields] OR halt/slow[All Fields] OR halt/standstill[All Fields] OR halt'[All Fields] OR halt's[All Fields] OR halt1[All Fields] OR halt2[All Fields] OR halt3[All Fields] OR halta[All Fields] OR haltafall[All Fields] OR haltagning[All Fields] OR haltai[All Fields] OR haltalin[All Fields] OR haltam[All Fields] OR haltamshire[All Fields] OR haltan[All Fields] OR haltande[All Fields] OR haltas[All Fields] OR haltatari[All Fields] OR haltaufderheide[All Fields] OR haltaufderhyde[All Fields] OR haltbakk[All Fields] OR haltbar[All Fields] OR haltbare[All Fields] OR haltbaren[All Fields] OR haltbarer[All Fields] OR haltbares[All Fields] OR haltbargemachte[All Fields] OR haltbarkeit[All Fields] OR haltbarkeitsberechnung[All Fields] OR haltbarkeitsberechungen[All Fields] OR haltbarkeitsfrist[All Fields] OR haltbarkeitsprufung[All Fields] OR haltbarkeitsspezifische[All Fields] OR haltbarkeitsstudien[All Fields] OR haltbarkeitsverminderung[All Fields] OR haltbarkeitsversuche[All Fields] OR haltbarketi[All Fields] OR haltbarmachung[All Fields] OR haltbestamning[All Fields] OR haltcpbd4dbkgcj3[All Fields] OR halte[All Fields] OR halteapparat[All Fields] OR halteapparates[All Fields] OR haltearbeit[All Fields] OR haltearms[All Fields] OR haltebourg[All Fields] OR haltechnologies[All Fields] OR halted[All Fields] OR haltedon[All Fields] OR halteelement[All Fields] OR halteelemente[All Fields] OR halteelementen[All Fields] OR halteelements[All Fields] OR haltefaden[All Fields] OR haltefeder[All Fields] OR haltefunktion[All Fields] OR haltegerat[All Fields] OR haltegriff[All Fields] OR halteh[All Fields] OR haltehilfe[All Fields] OR halteklemme[All Fields] OR haltekraft[All Fields] OR haltelasten[All Fields] OR halteleistung[All Fields] OR halteleistungsfahigkeit[All Fields] OR haltelement[All Fields] OR halteman[All Fields] OR haltemechanismen[All Fields] OR haltemomentes[All Fields] OR halten[All Fields] OR haltenbanken[All Fields] OR haltende[All Fields] OR haltenden[All Fields] OR haltenhof[All Fields] OR haltenhoff[All Fields] OR haltenhoffstr[All Fields] OR haltenhoffstrasse[All Fields] OR haltenhofstrasse[All Fields] OR halteosen[All Fields] OR haltepersonals[All Fields] OR haltepersonen[All Fields] OR haltequote[All Fields] OR halter[All Fields] OR halter/lead[All Fields] OR halter/wing[All Fields] OR halter's[All Fields] OR halterahmen[All Fields] OR halterapis[All Fields] OR halterata[All Fields] OR haltere[All Fields] OR haltere's[All Fields] OR haltered[All Fields] OR haltereflexe[All Fields] OR halteren[All Fields] OR halterenfrage[All Fields] OR halterenscheibe[All Fields] OR halteres[All Fields] OR halteresassociates[All Fields] OR halterhohung[All Fields] OR halteria[All Fields] OR halteridial[All Fields] OR halteridium[All Fields] OR halteriia[All Fields] OR halteriid[All Fields] OR halteriids[All Fields] OR haltering[All Fields] OR halterj[All Fields] OR halterman[All Fields] OR haltern[All Fields] OR halterna[All Fields] OR halterner[All Fields] OR halternung[All Fields] OR halteromyces[All Fields] OR halterophile[All Fields] OR halterophilus[All Fields] OR halters[All Fields] OR halterung[All Fields] OR halterungstemperatur[All Fields] OR halterwohl[All Fields] OR haltes[All Fields] OR haltestellen[All Fields] OR haltestifte[All Fields] OR haltetechnik[All Fields] OR haltetonus[All Fields] OR haltevorrichtung[All Fields] OR haltevorrichtungen[All Fields] OR haltezange[All Fields] OR haltezeit[All Fields] OR haltfax[All Fields] OR halth[All Fields] OR halthane[All Fields] OR halthcare[All Fields] OR halthon[All Fields] OR halthore[All Fields] OR halthur[All Fields] OR halthy[All Fields] OR halti001[All Fields] OR haltia[All Fields] OR haltiala[All Fields] OR haltialle[All Fields] OR haltiavaara[All Fields] OR haltica[All Fields] OR halticae[All Fields] OR halticella[All Fields] OR haltichella[All Fields] OR halticinae[All Fields] OR halticine[All Fields] OR halticus[All Fields] OR haltigan[All Fields] OR haltige[All Fields] OR haltigem[All Fields] OR haltigen[All Fields] OR haltiger[All Fields] OR haltiges[All Fields] OR haltija[All Fields] OR haltin[All Fields] OR haltiner[All Fields] OR halting[All Fields] OR haltingly[All Fields] OR haltinner[All Fields] OR haltiok[All Fields] OR haltiti[All Fields] OR haltiwanger[All Fields] OR haltli[All Fields] OR haltlib[All Fields] OR haltlichem[All Fields] OR haltlos[All Fields] OR haltm[All Fields] OR haltman[All Fields] OR haltmar[All Fields] OR haltmayer[All Fields] OR haltmayers[All Fields] OR haltmeier[All Fields] OR haltmeyer[All Fields] OR haltmeyr[All Fields] OR haltner[All Fields] OR haltof[All Fields] OR haltohane[All Fields] OR haltom[All Fields] OR halton[All Fields] OR halton's[All Fields] OR haltonhealthcare[All Fields] OR haltore[All Fields] OR haltrabolsi[All Fields] OR haltrecht[All Fields] OR haltrich[All Fields] OR haltrin[All Fields] OR halts[All Fields] OR halts/delays[All Fields] OR halts'[All Fields] OR haltsonen[All Fields] OR halttula[All Fields] OR halttunen[All Fields] OR haltuch[All Fields] OR haltun[All Fields] OR haltundal[All Fields] OR haltunen[All Fields] OR haltung[All Fields] OR haltungen[All Fields] OR haltungs[All Fields] OR haltungsabhangig[All Fields] OR haltungsabhangiger[All Fields] OR haltungsanalyse[All Fields] OR haltungsanalytische[All Fields] OR haltungsanderung[All Fields] OR haltungsanomalien[All Fields] OR haltungsarbeit[All Fields] OR haltungsasymmetrie[All Fields] OR haltungsauffalligkeiten[All Fields] OR haltungsbedigungen[All Fields] OR haltungsbedingte[All Fields] OR haltungsbedingten[All Fields] OR haltungsbedingungen[All Fields] OR haltungsbereich[All Fields] OR haltungsbestimmungen[All Fields] OR haltungsbiofeedback[All Fields] OR haltungsbiologie[All Fields] OR haltungsdauer[All Fields] OR haltungsentwicklung[All Fields] OR haltungsfaktoren[All Fields] OR haltungsfehler[All Fields] OR haltungsfehlern[All Fields] OR haltungsform[All Fields] OR haltungsformen[All Fields] OR haltungsforschung[All Fields] OR haltungsgeschadigten[All Fields] OR haltungsgeschadigter[All Fields] OR haltungshygiene[All Fields] OR haltungsintensitat[All Fields] OR haltungskontrolle[All Fields] OR haltungskorrektur[All Fields] OR haltungslage[All Fields] OR haltungsmangeln[All Fields] OR haltungsmethodik[All Fields] OR haltungsnormalen[All Fields] OR haltungsproblem[All Fields] OR haltungsproblematik[All Fields] OR haltungsprobleme[All Fields] OR haltungsprogrammen[All Fields] OR haltungsreflexe[All Fields] OR haltungsregulation[All Fields] OR haltungsschablone[All Fields] OR haltungsschaden[All Fields] OR haltungsschaeden[All Fields] OR haltungsschwache[All Fields] OR haltungsschwachen[All Fields] OR haltungsschwacher[All Fields] OR haltungssituation[All Fields] OR haltungsspannung[All Fields] OR haltungsstorung[All Fields] OR haltungsstorungen[All Fields] OR haltungssysteme[All Fields] OR haltungssystemen[All Fields] OR haltungssystems[All Fields] OR haltungsszintimyelographie[All Fields] OR haltungstechnik[All Fields] OR haltungstechnische[All Fields] OR haltungstemperaturen[All Fields] OR haltungstherapie[All Fields] OR haltungsturnen[All Fields] OR haltungsturnens[All Fields] OR haltungsumstellung[All Fields] OR haltungsuntersuchung[All Fields] OR haltungsuntersuchungen[All Fields] OR haltungsvariable[All Fields] OR haltungsvarianten[All Fields] OR haltungsverfahren[All Fields] OR haltungsverfall[All Fields] OR haltungsverschleiss[All Fields] OR haltungsvorrichtung[All Fields] OR haltunhan[All Fields] OR haltunkaya[All Fields] OR haltuun[All Fields] OR haltverbesserung[All Fields] OR haltx[All Fields] OR halty[All Fields] OR haltzman[All Fields]) OR (stop[All Fields] OR stop/2[All Fields] OR stop/centering[All Fields] OR stop/centrifugation[All Fields] OR stop/continuancy[All Fields] OR stop/continue[All Fields] OR stop/decrease[All Fields] OR stop/flex[All Fields] OR stop/flow[All Fields] OR stop/free[All Fields] OR stop/fricative[All Fields] OR stop/glide[All Fields] OR stop/go[All Fields] OR stop/hyperperfusion[All Fields] OR stop/increase[All Fields] OR stop/kakapo[All Fields] OR stop/l[All Fields] OR stop/linear[All Fields] OR stop/map6[All Fields] OR stop/nasal[All Fields] OR stop/ndei[All Fields] OR stop/not[All Fields] OR stop/p/in[All Fields] OR stop/pass[All Fields] OR stop/polyadenylation[All Fields] OR stop/q106r[All Fields] OR stop/r[All Fields] OR stop/r262q[All Fields] OR stop/reduce[All Fields] OR stop/repress[All Fields] OR stop/reset[All Fields] OR stop/reverse[All Fields] OR stop/s[All Fields] OR stop/s/sequences[All Fields] OR stop/stall[All Fields] OR stop/start[All Fields] OR stop/stop[All Fields] OR stop/switch[All Fields] OR stop/taper[All Fields] OR stop/tgc972[All Fields] OR stop/trading[All Fields] OR stop/trap[All Fields] OR stop/vowel[All Fields] OR stop/y[All Fields] OR stop'[All Fields] OR stop''[All Fields] OR stop's[All Fields] OR stop1[All Fields] OR stop111c[All Fields] OR stop11503l[All Fields] OR stop126[All Fields] OR stop145[All Fields] OR stop148[All Fields] OR stop152arg[All Fields] OR stop155[All Fields] OR stop160[All Fields] OR stop1p[All Fields] OR stop2[All Fields] OR stop220[All Fields] OR stop221[All Fields] OR stop259taa[All Fields] OR stop28[All Fields] OR stop305[All Fields] OR stop306[All Fields] OR stop330[All Fields] OR stop331[All Fields] OR stop337[All Fields] OR stop351a[All Fields] OR stop373c[All Fields] OR stop373c/e142k[All Fields] OR stop39[All Fields] OR stop398[All Fields] OR stop446[All Fields] OR stop447[All Fields] OR stop45[All Fields] OR stop454[All Fields] OR stop491[All Fields] OR stop523[All Fields] OR stop65[All Fields] OR stop657[All Fields] OR stop660[All Fields] OR stop693[All Fields] OR stop74[All Fields] OR stop78arg[All Fields] OR stop78gly[All Fields] OR stop838[All Fields] OR stop838/nr2a[All Fields] OR stop8546[All Fields] OR stop905[All Fields] OR stopa[All Fields] OR stopac[All Fields] OR stopacciaro[All Fields] OR stopach[All Fields] OR stopadesate[All Fields] OR stopadesatemu[All Fields] OR stopadesatileta[All Fields] OR stopadvies[All Fields] OR stopage[All Fields] OR stopajnik[All Fields] OR stopak[All Fields] OR stopala[All Fields] OR stopali1[All Fields] OR stopalo[All Fields] OR stopalu[All Fields] OR stopalz[All Fields] OR stopami[All Fields] OR stopangin[All Fields] OR stopani[All Fields] OR stopanska[All Fields] OR stopansko[All Fields] OR stopanstva[All Fields] OR stopanstvo[All Fields] OR stopanstvoto[All Fields] OR stopar[All Fields] OR stopard[All Fields] OR stoparic[All Fields] OR stopat[All Fields] OR stopatsams[All Fields] OR stopatschinskaja[All Fields] OR stopatschinskaya[All Fields] OR stopayne[All Fields] OR stopazzoni[All Fields] OR stopband[All Fields] OR stopband/structure[All Fields] OR stopbands[All Fields] OR stopbas[All Fields] OR stopbowitzi[All Fields] OR stopbreastcancer[All Fields] OR stopc[All Fields] OR stopce[All Fields] OR stopchanska[All Fields] OR stopchanskaia[All Fields] OR stopchanskaya[All Fields] OR stopchatuiu[All Fields] OR stopchik[All Fields] OR stopcna[All Fields] OR stopcna1[All Fields] OR stopcna123[All Fields] OR stopcna2[All Fields] OR stopcna3[All Fields] OR stopcna323[All Fields] OR stopcnas[All Fields] OR stopcock[All Fields] OR stopcock'[All Fields] OR stopcocks[All Fields] OR stopcodon[All Fields] OR stopcodons[All Fields] OR stopcoks[All Fields] OR stopcold[All Fields] OR stopcs[All Fields] OR stopcuoglu[All Fields] OR stopczanski[All Fields] OR stopczk[All Fields] OR stopczyk[All Fields] OR stopczyka[All Fields] OR stopczynska[All Fields] OR stopczynski[All Fields] OR stopd[All Fields] OR stopdomesticabuse[All Fields] OR stope[All Fields] OR stopeck[All Fields] OR stoped[All Fields] OR stopedectomy[All Fields] OR stopedeseta[All Fields] OR stopedesetgodisnjica[All Fields] OR stopehylem[All Fields] OR stopek[All Fields] OR stopekova[All Fields] OR stopel[All Fields] OR stopelegeringer[All Fields] OR stopeleire[All Fields] OR stopen[All Fields] OR stopen'[All Fields] OR stopenjska[All Fields] OR stoper[All Fields] OR stopera[All Fields] OR stoperative[All Fields] OR stoperator[All Fields] OR stoperator'[All Fields] OR stopers[All Fields] OR stopes[All Fields] OR stopes's[All Fields] OR stopeskjeen[All Fields] OR stopeteknikk[All Fields] OR stopethyl[All Fields] OR stopethylem[All Fields] OR stopethyloveho[All Fields] OR stopetie[All Fields] OR stopetiniaia[All Fields] OR stopetylove[All Fields] OR stopetylu[All Fields] OR stopf[All Fields] OR stopfbarer[All Fields] OR stopfdruck[All Fields] OR stopfdrucke[All Fields] OR stopfel[All Fields] OR stopfen[All Fields] OR stopfer[All Fields] OR stopferm[All Fields] OR stopfgold[All Fields] OR stopfgoldfullung[All Fields] OR stopfkuchen[All Fields] OR stopflow[All Fields] OR stopflu[All Fields] OR stopfmethode[All Fields] OR stopfnadel[All Fields] OR stopford[All Fields] OR stopfordb[All Fields] OR stopforth[All Fields] OR stopftechniken[All Fields] OR stopgap[All Fields] OR stopgap/solution[All Fields] OR stopgap's[All Fields] OR stopgaps[All Fields] OR stopgo[All Fields] OR stoph[All Fields] OR stophanthidin[All Fields] OR stophantine[All Fields] OR stophanyl[All Fields] OR stophasius[All Fields] OR stophel[All Fields] OR stopher[All Fields] OR stophila[All Fields] OR stophiv[All Fields] OR stophlee[All Fields] OR stophylococcus[All Fields] OR stopi[All Fields] OR stopic[All Fields] OR stopie[All Fields] OR stopie'n[All Fields] OR stopien[All Fields] OR stopiglia[All Fields] OR stopik[All Fields] OR stopikowska[All Fields] OR stopimt[All Fields] OR stopin[All Fields] OR stoping[All Fields] OR stopiniska[All Fields] OR stopinsek[All Fields] OR stopinska[All Fields] OR stopinski[All Fields] OR stopinsky[All Fields] OR stopit[All Fields] OR stopk[All Fields] OR stopk8[All Fields] OR stopka[All Fields] OR stopkan[All Fields] OR stopkan'[All Fields] OR stopkat'e[All Fields] OR stopkatou[All Fields] OR stopkaty[All Fields] OR stopkatym[All Fields] OR stopke[All Fields] OR stopki[All Fields] OR stopkie[All Fields] OR stopkoobraznykh[All Fields] OR stopkou[All Fields] OR stopkova[All Fields] OR stopkovaneho[All Fields] OR stopkowicz[All Fields] OR stopky[All Fields] OR stopler[All Fields] OR stopless[All Fields] OR stoplicht[All Fields] OR stoplicht'[All Fields] OR stoplight[All Fields] OR stoplights[All Fields] OR stoplu[All Fields] OR stopn[All Fields] OR stopni[All Fields] OR stopnia[All Fields] OR stopniach[All Fields] OR stopniau[All Fields] OR stopnicka[All Fields] OR stopnie[All Fields] OR stopniem[All Fields] OR stopning[All Fields] OR stopniowa[All Fields] OR stopniowanego[All Fields] OR stopniowanej[All Fields] OR stopniowanie[All Fields] OR stopniowanych[All Fields] OR stopniowego[All Fields] OR stopniowo[All Fields] OR stopniowy[All Fields] OR stopniowym[All Fields] OR stopnisek[All Fields] OR stopniu[All Fields] OR stopnje[All Fields] OR stopnjo[All Fields] OR stopnogo[All Fields] OR stopnom[All Fields] OR stopochnykh[All Fields] OR stopoi[All Fields] OR stopoiu[All Fields] OR stopol[All Fields] OR stopolianskaia[All Fields] OR stopolyanskij[All Fields] OR stopolyanskiy[All Fields] OR stopom[All Fields] OR stopomer[All Fields] OR stopometriia[All Fields] OR stopopani[All Fields] OR stoporev[All Fields] OR stopornymi[All Fields] OR stoporov[All Fields] OR stopout[All Fields] OR stopov'ych[All Fields] OR stopove[All Fields] OR stopover[All Fields] OR stopover/foraging[All Fields] OR stopovers[All Fields] OR stopovers'[All Fields] OR stopovy[All Fields] OR stopovych[All Fields] OR stopovymi[All Fields] OR stopow[All Fields] OR stopoytch[All Fields] OR stopp[All Fields] OR stopp/start[All Fields] OR stopp'a[All Fields] OR stoppa[All Fields] OR stoppa's[All Fields] OR stoppable[All Fields] OR stoppacciaro[All Fields] OR stoppacher[All Fields] OR stoppaciaro[All Fields] OR stoppad[All Fields] OR stoppade[All Fields] OR stoppage[All Fields] OR stoppage/change[All Fields] OR stoppage/occupational[All Fields] OR stoppages[All Fields] OR stoppain[All Fields] OR stoppalyonnet[All Fields] OR stoppani[All Fields] OR stoppany[All Fields] OR stoppany's[All Fields] OR stoppanys[All Fields] OR stoppar[All Fields] OR stoppard[All Fields] OR stoppard's[All Fields] OR stoppas[All Fields] OR stoppat[All Fields] OR stoppato[All Fields] OR stoppato's[All Fields] OR stoppcodon[All Fields] OR stoppe[All Fields] OR stopped[All Fields] OR stopped/reduced[All Fields] OR stopped/reinitiated[All Fields] OR stopped/released[All Fields] OR stopped/slowed[All Fields] OR stopped/withheld[All Fields] OR stopped'[All Fields] OR stoppee[All Fields] OR stoppeed[All Fields] OR stoppel[All Fields] OR stoppelaar[All Fields] OR stoppelaire[All Fields] OR stoppelbein[All Fields] OR stoppelenburg[All Fields] OR stoppeler[All Fields] OR stoppelhaar[All Fields] OR stoppelhutung[All Fields] OR stoppelkamp[All Fields] OR stoppelli[All Fields] OR stoppelman[All Fields] OR stoppelmann[All Fields] OR stoppels[All Fields] OR stoppen[All Fields] OR stoppenbach[All Fields] OR stoppenbrink[All Fields] OR stoppende[All Fields] OR stoppende'[All Fields] OR stoppenhagen[All Fields] OR stopper[All Fields] OR stopper/glass[All Fields] OR stopper'[All Fields] OR stopperan[All Fields] OR stoppered[All Fields] OR stopperegler[All Fields] OR stopperich[All Fields] OR stoppering[All Fields] OR stoppers[All Fields] OR stoppers/plungers[All Fields] OR stoppers'[All Fields] OR stoppersystem[All Fields] OR stoppes[All Fields] OR stoppet[All Fields] OR stoppia[All Fields] OR stoppicking[All Fields] OR stoppie[All Fields] OR stoppiglia[All Fields] OR stoppin[All Fields] OR stopping[All Fields] OR stopping/continuation[All Fields] OR stopping/continuing[All Fields] OR stopping/crossing[All Fields] OR stopping/decrease[All Fields] OR stopping/decreasing[All Fields] OR stopping/floating[All Fields] OR stopping/keeping[All Fields] OR stopping/limiting[All Fields] OR stopping/reducing[All Fields] OR stopping/release[All Fields] OR stopping/restarting[All Fields] OR stopping/reversing[All Fields] OR stopping/slowing[All Fields] OR stopping/stalling[All Fields] OR stopping/starting[All Fields] OR stopping/switching[All Fields] OR stopping/tapering[All Fields] OR stopping/trying[All Fields] OR stopping'[All Fields] OR stoppings[All Fields] OR stoppini[All Fields] OR stoppinni[All Fields] OR stoppino[All Fields] OR stoppioni[All Fields] OR stoppit[All Fields] OR stoppkotte[All Fields] OR stopple[All Fields] OR stoppler[All Fields] OR stopples[All Fields] OR stoppliquors[All Fields] OR stoppmanns[All Fields] OR stoppo[All Fields] OR stoppok[All Fields] OR stoppoloni[All Fields] OR stopponi[All Fields] OR stoppped[All Fields] OR stopps[All Fields] OR stoppt[All Fields] OR stoppur[All Fields] OR stoppy[All Fields] OR stopr[All Fields] OR stopr3[All Fields] OR stopric[All Fields] OR stoprotsentnykh[All Fields] OR stops[All Fields] OR stops/inconvenience[All Fields] OR stops/market[All Fields] OR stops'[All Fields] OR stops1[All Fields] OR stopsack[All Fields] OR stopschinski[All Fields] OR stopsel[All Fields] OR stopsida[All Fields] OR stopsignal[All Fields] OR stopsinc[All Fields] OR stopsite[All Fields] OR stopsleven[All Fields] OR stopsley[All Fields] OR stopsmokingcenter[All Fields] OR stopstroke[All Fields] OR stopszabaly[All Fields] OR stopt[All Fields] OR stoptb[All Fields] OR stopterapy[All Fields] OR stopti[All Fields] OR stoptik[All Fields] OR stoptrade[All Fields] OR stopu[All Fields] OR stopul[All Fields] OR stopurilor[All Fields] OR stopus[All Fields] OR stopver[All Fields] OR stopwatch[All Fields] OR stopwatch/calculator[All Fields] OR stopwatch'[All Fields] OR stopwatches[All Fields] OR stopwatchesreality[All Fields] OR stopwise[All Fields] OR stopword[All Fields] OR stopwords[All Fields] OR stopwork[All Fields] OR stopy[All Fields] OR stopyouthsuicide[All Fields] OR stopyra[All Fields] OR stopyrowa[All Fields] OR stopzetten[All Fields] OR stopzetting[All Fields] OR stopzyk[All Fields]) OR (drop out[All Fields] OR drop out/new[All Fields] OR drop outline[All Fields] OR drop outmanship[All Fields] OR drop outs[All Fields]) OR (dropout[All Fields] OR dropout/death[All Fields] OR dropout/participation[All Fields] OR dropout/withdrawal[All Fields] OR dropout'[All Fields] OR dropout's[All Fields] OR dropouts[All Fields] OR dropouts/decliners[All Fields] OR dropouts/disciplinary[All Fields] OR dropouts/nonusers[All Fields] OR dropouts/withdrawals[All Fields] OR dropouts'[All Fields]) OR (drop[All Fields] AND out[All Fields]) OR (rehospitalisation[All Fields] OR rehospitalisations[All Fields] OR rehospitalised[All Fields] OR rehospitalisiert[All Fields] OR rehospitalisierung[All Fields] OR rehospitalisierungsfreie[All Fields] OR rehospitalisierungsquote[All Fields] OR rehospitalisierungsrate[All Fields] OR rehospitalisierungsrisiko[All Fields] OR rehospitalisierungszeiten[All Fields]) OR (relaps[All Fields] OR relapsable[All Fields] OR relapsans[All Fields] OR relapsation[All Fields] OR relapsd[All Fields] OR relapse[All Fields] OR relapse/5[All Fields] OR relapse/abstinence[All Fields] OR relapse/breakthrough[All Fields] OR relapse/colectomy[All Fields] OR relapse/continued[All Fields] OR relapse/death[All Fields] OR relapse/defect[All Fields] OR relapse/delivery[All Fields] OR relapse/disease[All Fields] OR relapse/drug[All Fields] OR relapse/exacerbation[All Fields] OR relapse/extrapulmonary[All Fields] OR relapse/failure[All Fields] OR relapse/impending[All Fields] OR relapse/ir[All Fields] OR relapse/loss[All Fields] OR relapse/marked[All Fields] OR relapse/metastasis[All Fields] OR relapse/month[All Fields] OR relapse/no[All Fields] OR relapse/nonrelapse[All Fields] OR relapse/nonresponse[All Fields] OR relapse/outcome[All Fields] OR relapse/patient[All Fields] OR relapse/patient/year[All Fields] OR relapse/persistence[All Fields] OR relapse/persistent[All Fields] OR relapse/person/year[All Fields] OR relapse/pick[All Fields] OR relapse/primary[All Fields] OR relapse/probable[All Fields] OR relapse/progress[All Fields] OR relapse/progression[All Fields] OR relapse/progressive[All Fields] OR relapse/rate[All Fields] OR relapse/reactivation[All Fields] OR relapse/readmission[All Fields] OR relapse/rebound[All Fields] OR relapse/recrudescence[All Fields] OR relapse/recur[All Fields] OR relapse/recurrence[All Fields] OR relapse/reduction[All Fields] OR relapse/refractoriness[All Fields] OR relapse/refractory[All Fields] OR relapse/rehospitalisation[All Fields] OR relapse/rehospitalisation[All Fields] OR relapse/reinfection[All Fields] OR relapse/reinstatement[All Fields] OR relapse/relapses[All Fields] OR relapse/remission[All Fields] OR relapse/remit[All Fields] OR relapse/remitting[All Fields] OR relapse/residual[All Fields] OR relapse/resistance[All Fields] OR relapse/resistant[All Fields] OR relapse/response[All Fields] OR relapse/rising[All Fields] OR relapse/settling[All Fields] OR relapse/shub[All Fields] OR relapse/sustained[All Fields] OR relapse/transformation[All Fields] OR relapse/transformed[All Fields] OR relapse/treatment[All Fields] OR relapse/tumour[All Fields] OR relapse/uncontrolled[All Fields] OR relapse/worsening[All Fields] OR relapse/year[All Fields] OR relapse'[All Fields] OR relapse''[All Fields] OR relapse's[All Fields] OR relapse1[All Fields] OR relapsec[All Fields] OR relapsed[All Fields] OR relapsed/advanced[All Fields] OR relapsed/chemoresistant[All Fields] OR relapsed/metastatic[All Fields] OR relapsed/or[All Fields] OR relapsed/persistent[All Fields] OR relapsed/primary[All Fields] OR relapsed/progressed[All Fields] OR relapsed/progressive[All Fields] OR relapsed/recurrent[All Fields] OR relapsed/refactory[All Fields] OR relapsed/refractor[All Fields] OR relapsed/refractory[All Fields] OR relapsed/relapsed[All Fields] OR relapsed/resistant[All Fields] OR relapsed/secondary[All Fields] OR relapsed'[All Fields] OR relapsefree[All Fields] OR relapseless[All Fields] OR relapselike[All Fields] OR relapsem[All Fields] OR relapsen[All Fields] OR relapser[All Fields] OR relapsers[All Fields] OR relapsers/breakthroughs[All Fields] OR relapsers/incomplete[All Fields] OR relapsers/nonresponders[All Fields] OR relapsers'[All Fields] OR relapses[All Fields] OR relapses/16[All Fields] OR relapses/23[All Fields] OR relapses/28[All Fields] OR relapses/breast[All Fields] OR relapses/chronicity[All Fields] OR relapses/disease[All Fields] OR relapses/exacerbation[All Fields] OR relapses/failures[All Fields] OR relapses/increases[All Fields] OR relapses/metastases[All Fields] OR relapses/metastasis[All Fields] OR relapses/patient[All Fields] OR relapses/patient/month[All Fields] OR relapses/patient/year[All Fields] OR relapses/person/year[All Fields] OR relapses/progressions[All Fields] OR relapses/recurrences[All Fields] OR relapses/recurrent[All Fields] OR relapses/reinfections[All Fields] OR relapses/the[All Fields] OR relapses/woman/year[All Fields] OR relapses/y[All Fields] OR relapses/year[All Fields] OR relapses/yr[All Fields] OR relapses'[All Fields] OR relapset[All Fields] OR relapsf[All Fields] OR relapsi[All Fields] OR relapsin[All Fields] OR relapsing[All Fields] OR relapsing/445[All Fields] OR relapsing/chronic[All Fields] OR relapsing/malignant[All Fields] OR relapsing/non[All Fields] OR relapsing/persistent[All Fields] OR relapsing/persisting[All Fields] OR relapsing/progressing[All Fields] OR relapsing/progressive[All Fields] OR relapsing/refractory[All Fields] OR relapsing/remission[All Fields] OR relapsing/remittent[All Fields] OR relapsing/remitting[All Fields] OR relapsing/residual[All Fields] OR relapsing/resistant[All Fields] OR relapsing/resisting[All Fields] OR relapsing/starting[All Fields] OR relapsing'[All Fields] OR relapsinginflammatory[All Fields] OR relapsingremitting[All Fields] OR relapsingwegener's[All Fields] OR relapsion[All Fields] OR relapsive[All Fields] OR relapsmg[All Fields] OR relapsom[All Fields] OR relapsong[All Fields] OR relapsrate[All Fields] OR relapsu[All Fields] OR relapsujici[All Fields] OR relapsus[All Fields] OR relapsusa[All Fields] OR relapsy[All Fields] OR relapszus[All Fields]) OR (maintain[All Fields] OR maintain/attain[All Fields] OR maintain/avoid[All Fields] OR maintain/elongate[All Fields] OR maintain/expand[All Fields] OR maintain/form[All Fields] OR maintain/improve[All Fields] OR maintain/modulate[All Fields] OR maintain/obtain[All Fields] OR maintain/optimise[All Fields] OR maintain/promote[All Fields] OR maintain/regain[All Fields] OR maintain/regulate[All Fields] OR maintain/remove[All Fields] OR maintain/repair[All Fields] OR maintain/restore[All Fields] OR maintain'[All Fields] OR maintaina[All Fields] OR maintainability[All Fields] OR maintainability'[All Fields] OR maintainable[All Fields] OR maintainace[All Fields] OR maintainance[All Fields] OR maintainat[All Fields] OR maintaince[All Fields] OR maintaind[All Fields] OR maintaine[All Fields] OR maintained[All Fields] OR maintained/altered[All Fields] OR maintained/discontinued[All Fields] OR maintained/enhanced[All Fields] OR maintained/exercised[All Fields] OR maintained/expanded[All Fields] OR maintained/improved[All Fields] OR maintained/increased[All Fields] OR maintained/intermittent[All Fields] OR maintained/modified[All Fields] OR maintained/raised[All Fields] OR maintained/reduced[All Fields] OR maintained'[All Fields] OR maintainedover[All Fields] OR maintainedpto[All Fields] OR maintainement[All Fields] OR maintainenance[All Fields] OR maintainence[All Fields] OR maintainer[All Fields] OR maintainer'[All Fields] OR maintainer's[All Fields] OR maintainers[All Fields] OR maintainers/augmenters[All Fields] OR maintainers'[All Fields] OR maintaines[All Fields] OR maintaing[All Fields] OR maintainig[All Fields] OR maintainindependent[All Fields] OR maintainine[All Fields] OR maintaininf[All Fields] OR maintaining[All Fields] OR maintaining/adjusting[All Fields] OR maintaining/controlling[All Fields] OR maintaining/creating[All Fields] OR maintaining/elongating[All Fields] OR maintaining/expanding[All Fields] OR maintaining/generating[All Fields] OR maintaining/improving[All Fields] OR maintaining/inducing[All Fields] OR maintaining/obtaining/returning[All Fields] OR maintaining/preserving[All Fields] OR maintaining/producing[All Fields] OR maintaining/promoting[All Fields] OR maintaining/publishing[All Fields] OR maintaining/recovering[All Fields] OR maintaining/regular[All Fields] OR maintaining/regulating[All Fields] OR maintaining/restoring[All Fields] OR maintaining/restoring/inducing[All Fields] OR maintaining/spreading[All Fields] OR maintaining/stimulating[All Fields] OR maintaining'[All Fields] OR maintainingthe[All Fields] OR maintainly[All Fields] OR maintainment[All Fields] OR maintainng[All Fields] OR maintainnormal[All Fields] OR maintainremission[All Fields] OR maintains[All Fields] OR maintains/stabilizes[All Fields] OR maintaintained[All Fields] OR maintainted[All Fields] OR maintainually[All Fields]) OR (maintenance[All Fields] OR maintenance/activation[All Fields] OR maintenance/affective[All Fields] OR maintenance/assembly[All Fields] OR maintenance/assembly/differentiation[All Fields] OR maintenance/audit[All Fields] OR maintenance/biogenesis[All Fields] OR maintenance/classification[All Fields] OR maintenance/collision[All Fields] OR maintenance/consolidation[All Fields] OR maintenance/continuation[All Fields] OR maintenance/contraceptive[All Fields] OR maintenance/creation[All Fields] OR maintenance/cue[All Fields] OR maintenance/cyclosporine[All Fields] OR maintenance/d[All Fields] OR maintenance/deployment[All Fields] OR maintenance/depreciation[All Fields] OR maintenance/detergent[All Fields] OR maintenance/detoxification[All Fields] OR maintenance/development[All Fields] OR maintenance/differentiation[All Fields] OR maintenance/discontinuation[All Fields] OR maintenance/disruption[All Fields] OR maintenance/dna[All Fields] OR maintenance/economics[All Fields] OR maintenance/education[All Fields] OR maintenance/efficiency[All Fields] OR maintenance/efforts[All Fields] OR maintenance/efforts/year/patients[All Fields] OR maintenance/elongation[All Fields] OR maintenance/emergence[All Fields] OR maintenance/engineering[All Fields] OR maintenance/environmental[All Fields] OR maintenance/equipment[All Fields] OR maintenance/establishment[All Fields] OR maintenance/ethics[All Fields] OR maintenance/exacerbation[All Fields] OR maintenance/expansion[All Fields] OR maintenance/exportin[All Fields] OR maintenance/exportin1[All Fields] OR maintenance/extended[All Fields] OR maintenance/fat[All Fields] OR maintenance/function[All Fields] OR maintenance/gain[All Fields] OR maintenance/gardening[All Fields] OR maintenance/growth[All Fields] OR maintenance/health[All Fields] OR maintenance/homeostasis[All Fields] OR maintenance/hypertrophy[All Fields] OR maintenance/improvement[All Fields] OR maintenance/induction[All Fields] OR maintenance/inept[All Fields] OR maintenance/inhibition[All Fields] OR maintenance/inspiration[All Fields] OR maintenance/integrity[All Fields] OR maintenance/intensification[All Fields] OR maintenance/interruption[All Fields] OR maintenance/jurisprudence[All Fields] OR maintenance/lifestyle[All Fields] OR maintenance/manpower[All Fields] OR maintenance/mechanical[All Fields] OR maintenance/metabolism[All Fields] OR maintenance/methods[All Fields] OR maintenance/mixed[All Fields] OR maintenance/nutrition[All Fields] OR maintenance/observation[All Fields] OR maintenance/operation[All Fields] OR maintenance/output[All Fields] OR maintenance/periodic[All Fields] OR maintenance/prevention[All Fields] OR maintenance/progression[All Fields] OR maintenance/proliferation[All Fields] OR maintenance/promotion[All Fields] OR maintenance/protection[All Fields] OR maintenance/protective[All Fields] OR maintenance/quality[All Fields] OR maintenance/rebound[All Fields] OR maintenance/reconstitution[All Fields] OR maintenance/reconstruction[All Fields] OR maintenance/recovery[All Fields] OR maintenance/regain[All Fields] OR maintenance/regeneration[All Fields] OR maintenance/regression[All Fields] OR maintenance/regulation[All Fields] OR maintenance/rehearsal[All Fields] OR maintenance/relapse[All Fields] OR maintenance/reliability[All Fields] OR maintenance/remodeling[All Fields] OR maintenance/remodelling[All Fields] OR maintenance/renovation[All Fields] OR maintenance/renovators[All Fields] OR maintenance/repair[All Fields] OR maintenance/replenishment[All Fields] OR maintenance/rescue[All Fields] OR maintenance/resolution[All Fields] OR maintenance/restoration[All Fields] OR maintenance/retrieval[All Fields] OR maintenance/reverberation[All Fields] OR maintenance/segregation[All Fields] OR maintenance/sequential[All Fields] OR maintenance/standards[All Fields] OR maintenance/support[All Fields] OR maintenance/survival[All Fields] OR maintenance/sustainability[All Fields] OR maintenance/system[All Fields] OR maintenance/targeting[All Fields] OR maintenance/transport[All Fields] OR maintenance/treatment[All Fields] OR maintenance/trends[All Fields] OR maintenance/use[All Fields] OR maintenance/utilization[All Fields] OR maintenance/waste[All Fields] OR maintenance/with[All Fields] OR maintenance'[All Fields] OR maintenance's[All Fields] OR maintenance1[All Fields] OR maintenancefed[All Fields] OR maintenancein[All Fields] OR maintenances[All Fields]) OR (recur[All Fields] OR recur/progress[All Fields] OR recur/regrow[All Fields] OR recur'[All Fields] OR recur31a[All Fields] OR recur71a[All Fields] OR recuraresierung[All Fields] OR recurarisation[All Fields] OR recurarisation'[All Fields] OR recurarised[All Fields] OR recurarisierung[All Fields] OR recurarizacao[All Fields] OR recurarizaci'on[All Fields] OR recurarization[All Fields] OR recurarization'[All Fields] OR recurated[All Fields] OR recurculating[All Fields] OR recurdescence[All Fields] OR recurdescences[All Fields] OR recure[All Fields] OR recured[All Fields] OR recureded[All Fields] OR recureence[All Fields] OR recuren[All Fields] OR recurence[All Fields] OR recurences[All Fields] OR recurency[All Fields] OR recurens[All Fields] OR recurensive[All Fields] OR recurent[All Fields] OR recurenta[All Fields] OR recurente[All Fields] OR recurentei[All Fields] OR recurentelor[All Fields] OR recurential[All Fields] OR recurentiala[All Fields] OR recurentis[All Fields] OR recurento[All Fields] OR recurer[All Fields] OR recurerence[All Fields] OR recurerences[All Fields] OR recurerrent[All Fields] OR recures[All Fields] OR recuretage[All Fields] OR recurettage[All Fields] OR recurgitation[All Fields] OR recuriential[All Fields] OR recuriertos[All Fields] OR recuring[All Fields] OR recurit[All Fields] OR recurited[All Fields] OR recuriting[All Fields] OR recuritment[All Fields] OR recurits[All Fields] OR recuronium[All Fields] OR recuronium/hour[All Fields] OR recuroniums[All Fields] OR recuros[All Fields] OR recurr[All Fields] OR recurralo[All Fields] OR recurrance[All Fields] OR recurrances[All Fields] OR recurrancy[All Fields] OR recurrant[All Fields] OR recurrants[All Fields] OR recurre[All Fields] OR recurreat[All Fields] OR recurrebt[All Fields] OR recurrece[All Fields] OR recurrect[All Fields] OR recurred[All Fields] OR recurred/metastasized[All Fields] OR recurred/persisted[All Fields] OR recurred/remnant[All Fields] OR recurred'[All Fields] OR recurren[All Fields] OR recurrenc[All Fields] OR recurrenc/month[All Fields] OR recurrence[All Fields] OR recurrence/100[All Fields] OR recurrence/additional[All Fields] OR recurrence/appearance[All Fields] OR recurrence/cancer[All Fields] OR recurrence/cbc[All Fields] OR recurrence/clearance[All Fields] OR recurrence/complication[All Fields] OR recurrence/death[All Fields] OR recurrence/deterioration[All Fields] OR recurrence/development[All Fields] OR recurrence/diagnosis[All Fields] OR recurrence/disease[All Fields] OR recurrence/distance[All Fields] OR recurrence/distant[All Fields] OR recurrence/economics[All Fields] OR recurrence/enlargement[All Fields] OR recurrence/epidemiology[All Fields] OR recurrence/etiology[All Fields] OR recurrence/excellent[All Fields] OR recurrence/extension[All Fields] OR recurrence/foveal[All Fields] OR recurrence/frank[All Fields] OR recurrence/growth[All Fields] OR recurrence/icd[All Fields] OR recurrence/immunology[All Fields] OR recurrence/incomplete[All Fields] OR recurrence/increased[All Fields] OR recurrence/malignant[All Fields] OR recurrence/marginal[All Fields] OR recurrence/metachronous[All Fields] OR recurrence/metastases[All Fields] OR recurrence/metastasis[All Fields] OR recurrence/metastasis/death[All Fields] OR recurrence/metastatic[All Fields] OR recurrence/methastasis[All Fields] OR recurrence/month[All Fields] OR recurrence/mortality[All Fields] OR recurrence/new[All Fields] OR recurrence/occurrence[All Fields] OR recurrence/pathology[All Fields] OR recurrence/patient[All Fields] OR recurrence/persistence[All Fields] OR recurrence/persistent[All Fields] OR recurrence/pfs[All Fields] OR recurrence/post[All Fields] OR recurrence/prevention[All Fields] OR recurrence/progress[All Fields] OR recurrence/progression[All Fields] OR recurrence/progressions[All Fields] OR recurrence/progressive[All Fields] OR recurrence/prosthesis[All Fields] OR recurrence/radiography[All Fields] OR recurrence/recrudescence[All Fields] OR recurrence/regrowing[All Fields] OR recurrence/regrowth[All Fields] OR recurrence/reinfection[All Fields] OR recurrence/relapse[All Fields] OR recurrence/remission[All Fields] OR recurrence/reoperation[All Fields] OR recurrence/residual[All Fields] OR recurrence/residue[All Fields] OR recurrence/resistance[All Fields] OR recurrence/rest[All Fields] OR recurrence/restaging[All Fields] OR recurrence/second[All Fields] OR recurrence/splenic[All Fields] OR recurrence/spread[All Fields] OR recurrence/spt[All Fields] OR recurrence/stability[All Fields] OR recurrence/stable[All Fields] OR recurrence/stroke[All Fields] OR recurrence/surgery[All Fields] OR recurrence/surgical[All Fields] OR recurrence/survival[All Fields] OR recurrence/therapy[All Fields] OR recurrence/time[All Fields] OR recurrence/transience[All Fields] OR recurrence/treatment[All Fields] OR recurrence/vital[All Fields] OR recurrence/withdrawal[All Fields] OR recurrence/worsening[All Fields] OR recurrence/wrap[All Fields] OR recurrence/year[All Fields] OR recurrence'[All Fields] OR recurrence's[All Fields] OR recurrenced[All Fields] OR recurrencee[All Fields] OR recurrencefree[All Fields] OR recurrencel[All Fields] OR recurrenceless[All Fields] OR recurrenceonline[All Fields] OR recurrencerate[All Fields] OR recurrencerates[All Fields] OR recurrenceree[All Fields] OR recurrences[All Fields] OR recurrences/100[All Fields] OR recurrences/1000[All Fields] OR recurrences/extensions[All Fields] OR recurrences/infections[All Fields] OR recurrences/marginal[All Fields] OR recurrences/metastases[All Fields] OR recurrences/metastatic[All Fields] OR recurrences/month[All Fields] OR recurrences/nonsatisfactory[All Fields] OR recurrences/number[All Fields] OR recurrences/patient[All Fields] OR recurrences/persistent[All Fields] OR recurrences/progressions[All Fields] OR recurrences/reinfections[All Fields] OR recurrences/relapses[All Fields] OR recurrences/residual[All Fields] OR recurrences/tumour[All Fields] OR recurrences/y[All Fields] OR recurrences/year[All Fields] OR recurrences/year/girl[All Fields] OR recurrences/yr[All Fields] OR recurrences'[All Fields] OR recurrencesed[All Fields] OR recurrenceses[All Fields] OR recurrencess[All Fields] OR recurrencewithout[All Fields] OR recurrencia[All Fields] OR recurrencial[All Fields] OR recurrencias[All Fields] OR recurrencies[All Fields] OR recurrenct[All Fields] OR recurrency[All Fields] OR recurrency/reinfection[All Fields] OR recurrencys[All Fields] OR recurrend[All Fields] OR recurrende[All Fields] OR recurrene[All Fields] OR recurreness[All Fields] OR recurrens[All Fields] OR recurrens'[All Fields] OR recurrensa[All Fields] OR recurrensbenulas[All Fields] OR recurrensdurchtrennung[All Fields] OR recurrensidentifizierung[All Fields] OR recurrensinfektion[All Fields] OR recurrenslahmung[All Fields] OR recurrenslahmungen[All Fields] OR recurrensmonitoring[All Fields] OR recurrensparalyse[All Fields] OR recurrensparalysen[All Fields] OR recurrenspares[All Fields] OR recurrensparese[All Fields] OR recurrensparesen[All Fields] OR recurrenspareserate[All Fields] OR recurrensparsen[All Fields] OR recurrensreizung[All Fields] OR recurrensschadigungen[All Fields] OR recurrenstam3[All Fields] OR recurrensund[All Fields] OR recurrensutfall[All Fields] OR recurrensverlamming[All Fields] OR recurrent[All Fields] OR recurrent/advanced[All Fields] OR recurrent/aggressive[All Fields] OR recurrent/chronic[All Fields] OR recurrent/complicated[All Fields] OR recurrent/continued[All Fields] OR recurrent/continuous[All Fields] OR recurrent/cyclic[All Fields] OR recurrent/disseminated[All Fields] OR recurrent/extended[All Fields] OR recurrent/external[All Fields] OR recurrent/feedback[All Fields] OR recurrent/feedforward[All Fields] OR recurrent/founder[All Fields] OR recurrent/habitual[All Fields] OR recurrent/ineffective[All Fields] OR recurrent/infiltrative[All Fields] OR recurrent/inoperable[All Fields] OR recurrent/larger[All Fields] OR recurrent/latent[All Fields] OR recurrent/locally[All Fields] OR recurrent/loculated[All Fields] OR recurrent/metastasized[All Fields] OR recurrent/metastatic[All Fields] OR recurrent/metastic[All Fields] OR recurrent/multifocal[All Fields] OR recurrent/new[All Fields] OR recurrent/nonhealing[All Fields] OR recurrent/nonresponsive[All Fields] OR recurrent/overlapping[All Fields] OR recurrent/persistent[All Fields] OR recurrent/persistent/metastatic[All Fields] OR recurrent/platinum[All Fields] OR recurrent/poor[All Fields] OR recurrent/primary[All Fields] OR recurrent/progressing[All Fields] OR recurrent/progression[All Fields] OR recurrent/progressive[All Fields] OR recurrent/prolonged[All Fields] OR recurrent/protracted[All Fields] OR recurrent/reactivated[All Fields] OR recurrent/recalcitrant[All Fields] OR recurrent/refractory[All Fields] OR recurrent/refractory/poor[All Fields] OR recurrent/regrown[All Fields] OR recurrent/regrowth[All Fields] OR recurrent/relapsed[All Fields] OR recurrent/remaining[All Fields] OR recurrent/residual[All Fields] OR recurrent/resistant[All Fields] OR recurrent/resistant/persistent[All Fields] OR recurrent/retained[All Fields] OR recurrent/second[All Fields] OR recurrent/secondary[All Fields] OR recurrent/severe[All Fields] OR recurrent/stuttering[All Fields] OR recurrent/superior[All Fields] OR recurrent/terminal[All Fields] OR recurrent/unresectable[All Fields] OR recurrent/worsening[All Fields] OR recurrent'[All Fields] OR recurrentbladder[All Fields] OR recurrente[All Fields] OR recurrented[All Fields] OR recurrentes[All Fields] OR recurrential[All Fields] OR recurrentiel[All Fields] OR recurrentielle[All Fields] OR recurrentielles[All Fields] OR recurrentiels[All Fields] OR recurrentis[All Fields] OR recurrentis/b[All Fields] OR recurrentis/species[All Fields] OR recurrently[All Fields] OR recurrentnasal[All Fields] OR recurrents[All Fields] OR recurrenttumors[All Fields] OR recurrentvte[All Fields] OR recurrenty[All Fields] OR recurrenz[All Fields] OR recurrernt[All Fields] OR recurrers[All Fields] OR recurres[All Fields] OR recurretage[All Fields] OR recurretaged[All Fields] OR recurreuce[All Fields] OR recurrey[All Fields] OR recurriculating[All Fields] OR recurrid[All Fields] OR recurring[All Fields] OR recurring/lasting[All Fields] OR recurring/refractory[All Fields] OR recurring/rising[All Fields] OR recurring/terminating[All Fields] OR recurring'[All Fields] OR recurringly[All Fields] OR recurrings[All Fields] OR recurrnet[All Fields] OR recurroids[All Fields] OR recurrrence[All Fields] OR recurrrent[All Fields] OR recurrs[All Fields] OR recurs[All Fields] OR recursa[All Fields] OR recursing[All Fields] OR recursion[All Fields] OR recursions[All Fields] OR recursive[All Fields] OR recursive'[All Fields] OR recursiveclustering[All Fields] OR recursively[All Fields] OR recursiveness[All Fields] OR recursividad[All Fields] OR recursivite[All Fields] OR recursivities[All Fields] OR recursivity[All Fields] OR recurso[All Fields] OR recursor[All Fields] OR recursors[All Fields] OR recursos[All Fields] OR recursosnaturales[All Fields] OR recurt[All Fields] OR recurva[All Fields] OR recurvalis[All Fields] OR recurvartum[All Fields] OR recurvata[All Fields] OR recurvate[All Fields] OR recurvated[All Fields] OR recurvatiane[All Fields] OR recurvatianes[All Fields] OR recurvation[All Fields] OR recurvation/antecurvation[All Fields] OR recurvatios[All Fields] OR recurvatis[All Fields] OR recurvato[All Fields] OR recurvatum[All Fields] OR recurvatum'[All Fields] OR recurvature[All Fields] OR recurvatus[All Fields] OR recurvaum[All Fields] OR recurvazione[All Fields] OR recurve[All Fields] OR recurved[All Fields] OR recurves[All Fields] OR recurvifolia[All Fields] OR recurving[All Fields] OR recurving'[All Fields] OR recurvirostra[All Fields] OR recurvirostrae[All Fields] OR recurvirostridae[All Fields] OR recurvirostrids[All Fields] OR recurvirostrinae[All Fields] OR recurvirostris[All Fields] OR recurvisepala[All Fields] OR recurvispinis[All Fields] OR recurvomyces[All Fields] OR recurvum[All Fields] OR recurvus[All Fields])) AND (schizophr[All Fields] OR schizophr'ene[All Fields] OR schizophr'enes[All Fields] OR schizophr'enie[All Fields] OR schizophr'enies[All Fields] OR schizophr'eniforme[All Fields] OR schizophr'eniformes[All Fields] OR schizophr'enique[All Fields] OR schizophr'eniques[All Fields] OR schizophragma[All Fields] OR schizophrania[All Fields] OR schizophreania[All Fields] OR schizophrehic[All Fields] OR schizophreia[All Fields] OR schizophreie[All Fields] OR schizophreina[All Fields] OR schizophreinia[All Fields] OR schizophreinic[All Fields] OR schizophreken[All Fields] OR schizophrema[All Fields] OR schizophremia[All Fields] OR schizophremic[All Fields] OR schizophremie[All Fields] OR schizophren[All Fields] OR schizophrena[All Fields] OR schizophrenc[All Fields] OR schizophrenci[All Fields] OR schizophrencis[All Fields] OR schizophrencs[All Fields] OR schizophrene[All Fields] OR schizophrene's[All Fields] OR schizophrenek[All Fields] OR schizophrenem[All Fields] OR schizophrenen[All Fields] OR schizophrenengruppe[All Fields] OR schizophrenenproblem[All Fields] OR schizophrener[All Fields] OR schizophrenes[All Fields] OR schizophrenese[All Fields] OR schizophreni[All Fields] OR schizophreni'as[All Fields] OR schizophrenia[All Fields] OR schizophrenia/adhd[All Fields] OR schizophrenia/affective[All Fields] OR schizophrenia/alzheimer's/multiple[All Fields] OR schizophrenia/art[All Fields] OR schizophrenia/autism[All Fields] OR schizophrenia/autistic[All Fields] OR schizophrenia/bacteriological[All Fields] OR schizophrenia/bipolar[All Fields] OR schizophrenia/blood[All Fields] OR schizophrenia/bp[All Fields] OR schizophrenia/cannabis[All Fields] OR schizophrenia/case[All Fields] OR schizophrenia/catatonic[All Fields] OR schizophrenia/cerebrospinal[All Fields] OR schizophrenia/chemistry[All Fields] OR schizophrenia/chronic[All Fields] OR schizophrenia/classification[All Fields] OR schizophrenia/complications[All Fields] OR schizophrenia/control[All Fields] OR schizophrenia/cyclothymia[All Fields] OR schizophrenia/delusion[All Fields] OR schizophrenia/delusional[All Fields] OR schizophrenia/depression[All Fields] OR schizophrenia/diabetes[All Fields] OR schizophrenia/diagnosis[All Fields] OR schizophrenia/differential[All Fields] OR schizophrenia/drugs[All Fields] OR schizophrenia/early[All Fields] OR schizophrenia/economics[All Fields] OR schizophrenia/endocrine[All Fields] OR schizophrenia/enzymology[All Fields] OR schizophrenia/epidemiology[All Fields] OR schizophrenia/ethnology[All Fields] OR schizophrenia/etiology[All Fields] OR schizophrenia/experimental[All Fields] OR schizophrenia/flocculation[All Fields] OR schizophrenia/genetics[All Fields] OR schizophrenia/heredity[All Fields] OR schizophrenia/history[All Fields] OR schizophrenia/hyperglycemic[All Fields] OR schizophrenia/hypothyroidism[All Fields] OR schizophrenia/immunology[All Fields] OR schizophrenia/in[All Fields] OR schizophrenia/insulin[All Fields] OR schizophrenia/jurisprudence[All Fields] OR schizophrenia/learning[All Fields] OR schizophrenia/major[All Fields] OR schizophrenia/manifestations[All Fields] OR schizophrenia/mao[All Fields] OR schizophrenia/mdp[All Fields] OR schizophrenia/metabolism[All Fields] OR schizophrenia/meth[All Fields] OR schizophrenia/microbiology[All Fields] OR schizophrenia/mood[All Fields] OR schizophrenia/mortality[All Fields] OR schizophrenia/no[All Fields] OR schizophrenia/nonsuicide[All Fields] OR schizophrenia/normal[All Fields] OR schizophrenia/nursing[All Fields] OR schizophrenia/nutrition[All Fields] OR schizophrenia/obesity[All Fields] OR schizophrenia/obstetric[All Fields] OR schizophrenia/ocd[All Fields] OR schizophrenia/other[All Fields] OR schizophrenia/others[All Fields] OR schizophrenia/paranoid[All Fields] OR schizophrenia/parasitology[All Fields] OR schizophrenia/pathogenesis[All Fields] OR schizophrenia/pathology[All Fields] OR schizophrenia/pet[All Fields] OR schizophrenia/pharmacological[All Fields] OR schizophrenia/physiology[All Fields] OR schizophrenia/physiopathology[All Fields] OR schizophrenia/prevention[All Fields] OR schizophrenia/prognosis[All Fields] OR schizophrenia/psychology[All Fields] OR schizophrenia/psychoses[All Fields] OR schizophrenia/psychosis[All Fields] OR schizophrenia/psychotherapy[All Fields] OR schizophrenia/psychotic[All Fields] OR schizophrenia/radiography[All Fields] OR schizophrenia/rehabilitation[All Fields] OR schizophrenia/research[All Fields] OR schizophrenia/retinitis[All Fields] OR schizophrenia/schizoaffective[All Fields] OR schizophrenia/schizophrenia[All Fields] OR schizophrenia/schizophrenic[All Fields] OR schizophrenia/schizophreniform[All Fields] OR schizophrenia/schizotypal[All Fields] OR schizophrenia/schizotypy[All Fields] OR schizophrenia/shock[All Fields] OR schizophrenia/sociology[All Fields] OR schizophrenia/spectrum[All Fields] OR schizophrenia/speech[All Fields] OR schizophrenia/spinal[All Fields] OR schizophrenia/ssd[All Fields] OR schizophrenia/statistics[All Fields] OR schizophrenia/substance[All Fields] OR schizophrenia/suicide[All Fields] OR schizophrenia/surgery[All Fields] OR schizophrenia/therapy[All Fields] OR schizophrenia/ultrasonography[All Fields] OR schizophrenia/urine[All Fields] OR schizophrenia/vasospasm[All Fields] OR schizophrenia/virology[All Fields] OR schizophrenia'[All Fields] OR schizophrenia's[All Fields] OR schizophrenia1[All Fields] OR schizophreniaban[All Fields] OR schizophreniac[All Fields] OR schizophreniacs[All Fields] OR schizophreniaes[All Fields] OR schizophreniaforum[All Fields] OR schizophreniagene[All Fields] OR schizophreniai[All Fields] OR schizophreniak[All Fields] OR schizophrenial[All Fields] OR schizophrenialike[All Fields] OR schizophreniaor[All Fields] OR schizophreniara[All Fields] OR schizophreniaresearch[All Fields] OR schizophreniaresearchforum[All Fields] OR schizophreniarol[All Fields] OR schizophrenias[All Fields] OR schizophrenias'[All Fields] OR schizophreniay[All Fields] OR schizophrenic[All Fields] OR schizophrenic/cocaine[All Fields] OR schizophrenic/control[All Fields] OR schizophrenic/nonparanoid[All Fields] OR schizophrenic/normal[All Fields] OR schizophrenic/paranoid[All Fields] OR schizophrenic/psychotic[All Fields] OR schizophrenic/schizoaffective[All Fields] OR schizophrenic/schizophreniform[All Fields] OR schizophrenic/schizotypal[All Fields] OR schizophrenic/vocational[All Fields] OR schizophrenic'[All Fields] OR schizophrenic's[All Fields] OR schizophrenical[All Fields] OR schizophrenically[All Fields] OR schizophrenices[All Fields] OR schizophrenicity[All Fields] OR schizophreniclike[All Fields] OR schizophrenicpatients[All Fields] OR schizophrenics[All Fields] OR schizophrenics/controls[All Fields] OR schizophrenics/s[All Fields] OR schizophrenics'[All Fields] OR schizophrenicss[All Fields] OR schizophrenie[All Fields] OR schizophrenie'[All Fields] OR schizophrenieaehnlichen[All Fields] OR schizophrenieahnliche[All Fields] OR schizophrenieahnlichen[All Fields] OR schizophrenieahnlicher[All Fields] OR schizophrenieartige[All Fields] OR schizophrenieartigen[All Fields] OR schizophrenieartiger[All Fields] OR schizophreniebeggriffs[All Fields] OR schizophreniebegriff[All Fields] OR schizophreniebegriffes[All Fields] OR schizophreniebegriffs[All Fields] OR schizophreniebehandlung[All Fields] OR schizophreniebehandlungen[All Fields] OR schizophreniediagnose[All Fields] OR schizophrenieerkrankten[All Fields] OR schizophrenieerkrankter[All Fields] OR schizophreniefalle[All Fields] OR schizophreniefallen[All Fields] OR schizophrenieform[All Fields] OR schizophrenieforme[All Fields] OR schizophrenieformen[All Fields] OR schizophrenieformer[All Fields] OR schizophrenieforschung[All Fields] OR schizophreniefrage[All Fields] OR schizophreniegenese[All Fields] OR schizophreniekonzepte[All Fields] OR schizophreniekonzepten[All Fields] OR schizophreniekranke[All Fields] OR schizophreniekranken[All Fields] OR schizophreniekranker[All Fields] OR schizophreniekreises[All Fields] OR schizophrenielehre[All Fields] OR schizophrenien[All Fields] OR schizophrenienahe[All Fields] OR schizophreniepatienten[All Fields] OR schizophreniepatientinnen[All Fields] OR schizophrenieproblem[All Fields] OR schizophrenieproblems[All Fields] OR schizophrenierisiko[All Fields] OR schizophrenieritoriality[All Fields] OR schizophrenies[All Fields] OR schizophreniespektrums[All Fields] OR schizophreniespezifitat[All Fields] OR schizophreniestudie[All Fields] OR schizophreniesymptomen[All Fields] OR schizophrenietheorie[All Fields] OR schizophrenietherapie[All Fields] OR schizophrenietypologie[All Fields] OR schizophrenieverlauf[All Fields] OR schizophrenieverlaufe[All Fields] OR schizophrenieverlaufs[All Fields] OR schizophrenieverstandnis[All Fields] OR schizophrenifallen[All Fields] OR schizophreniforems[All Fields] OR schizophreniform[All Fields] OR schizophreniform/paranoid[All Fields] OR schizophreniform/schizoaffective[All Fields] OR schizophreniform'[All Fields] OR schizophreniforme[All Fields] OR schizophreniformen[All Fields] OR schizophreniformes[All Fields] OR schizophreniformic[All Fields] OR schizophreniforms[All Fields] OR schizophrenigenesis[All Fields] OR schizophreniics[All Fields] OR schizophrenikern[All Fields] OR schizophrenine[All Fields] OR schizophreniologists[All Fields] OR schizophreniphorm[All Fields] OR schizophrenique[All Fields] OR schizophreniques[All Fields] OR schizophrenis[All Fields] OR schizophrenisation[All Fields] OR schizophrenism[All Fields] OR schizophreniucs[All Fields] OR schizophrenix[All Fields] OR schizophrenix's[All Fields] OR schizophreniz[All Fields] OR schizophrenization[All Fields] OR schizophrenized[All Fields] OR schizophrenjeforschung[All Fields] OR schizophrenlcs[All Fields] OR schizophrenle[All Fields] OR schizophreno[All Fields] OR schizophrenoform[All Fields] OR schizophrenogenesis[All Fields] OR schizophrenogenic[All Fields] OR schizophrenoid[All Fields] OR schizophrenomimetic[All Fields] OR schizophrenomimetics[All Fields] OR schizophrenosimilar[All Fields] OR schizophrens[All Fields] OR schizophrenuc[All Fields] OR schizophreny[All Fields] OR schizophrerines[All Fields] OR schizophrinic[All Fields] OR schizophrinie[All Fields] OR schizophrnia[All Fields] OR schizophrniekreises[All Fields]) OR (schizoaffecive[All Fields] OR schizoaffectieve[All Fields] OR schizoaffectif[All Fields] OR schizoaffectifs[All Fields] OR schizoaffective[All Fields] OR schizoaffective/bipolar[All Fields] OR schizoaffective/bipolars[All Fields] OR schizoaffective/mania[All Fields] OR schizoaffective/mood[All Fields] OR schizoaffective/schizoaffective[All Fields] OR schizoaffective/unipolars[All Fields] OR schizoaffective'[All Fields] OR schizoaffectives[All Fields] OR schizoaffectives'[All Fields] OR schizoaffectivity[All Fields] OR schizoaffektive[All Fields] OR schizoaffektiven[All Fields] OR schizoaffektiver[All Fields] OR schizoaffettiva[All Fields] OR schizoaffettive[All Fields] OR schizoaffettivo[All Fields]) AND (Randomized Controlled Trial[ptyp] AND ("2008"[PDAT]: "2011"[PDAT]))
1.2.1.3 MEDLINE (June 06, 2011)
(((cessation* or withdraw* or discontinu* or halt* or stop* or drop‐out* or dropout* or drop out or rehospitalis* or relaps* or maintain* or maintenance* or recur*) and schizophr*) or schizoaff*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Appendix 2. R code for meta‐regressions
Restricted‐maximum‐likelihood‐random‐effect meta‐regressions were performed using meta v4.9‐9 (Schwarzer 2007) in R statistical language v3.6.2 (R Core Team 2018).The following code was used:
rm(list=ls()) #R code for the meta‐regressions and Egger test #Last use of code in 06.02.2020 #Libaries used in R version 3.6.2 library(meta) #meta_4.9‐9 library(readxl) #readxl_1.3.1 library(dplyr) #dplyr 0.8.3 #Data import and data cleaning‐‐‐‐ dat <‐ read_excel("data.xlsx", col_names = TRUE, sheet = 1) dat$ndrug<‐as.numeric(dat$ndrug) #participants randomized on antipsychotic dat$npbo<‐as.numeric(dat$npbo) #participants randomized in placebo dat$relapse_7_12_drug<‐as.numeric(dat$relapse_7_12_drug) #participants with relapse at 7‐12 months on antipsychotic dat$relapse_7_12_plb<‐as.numeric(dat$relapse_7_12_plb)#participants with relapse at 7‐12 months on placebo dat$relapse_tot_drug<‐as.numeric(dat$relapse_tot_drug)#participants with relapse at endpoint on anitpsychotic dat$relapse_tot_plb<‐as.numeric(dat$relapse_tot_plb)#participants with relapse at endpoint on anitpsychotic dat$`Mean dose CPZ_R1`<‐as.numeric(dat$`Mean dose CPZ_R1`) #mean dose in chlorpromazine equivalents (mg/day) dat<‐dat %>% rename(CPZ_dose=`Mean dose CPZ_R1`) #rename of the dose variable in order to be used in bubble() dat$duration_stable_weeks<‐as.numeric(dat$duration_stable_weeks) #duration the participants were stable before the start of the study in weeks dat$pbo_taper_R<‐as.numeric(dat$pbo_taper_R) #duration of taper in the placebo group dat$study_duration_metaregression_weeks<‐as.numeric(dat$study_duration_metaregression_weeks) #Study duration in weeks # Primary outcome‐‐‐‐ ## Meta‐analysis==== relapse_7_12 <‐metabin(event.e= relapse_7_12_drug, n.e = ndrug, event.c=relapse_7_12_plb, n.c=npbo, sm="RR", method = "MH", comb.fixed = FALSE, studlab = Author, data = dat[!is.na(dat$relapse_7_12_drug),]) sink('primary_relapse_7_12.txt') relapse_7_12 sink() ## Contour‐enhanced funnel plot png('cefunnel_primary.png', res=300, width=24, height=24, units='cm') funnel(relapse_7_12, level = 0.95, contour = c(0.9, 0.95, 0.99)) dev.off() ## Egger's test==== egger_relapse_7_12<‐metabias(relapse_7_12, method.bias = "linreg") sink('egger_test_primary.txt') print(egger_relapse_7_12) sink() ##Trim‐and‐fill==== trimfill_relapse_7_12<‐trimfill(relapse_7_12) sink('trim_and_fill_primary.txt') print(trimfill_relapse_7_12) sink() ## Meta‐regression analyses==== ### Duration the participants were stable before the start of the study (relapse at 12 months) stability_duration_7_12<‐metareg(relapse_7_12, ~duration_stable_weeks, method.tau = "REML") sink('metareg_duration_stability.txt') print(stability_duration_7_12) sink() png('metareg_duration_stability.png', res=300, width=24, height=24, units='cm') bubble(stability_duration_7_12, xlab='Duration the participants were stable before the start of the study (weeks)', ylab='logRR of relapse at 7‐12 months') dev.off() ### Duration of taper in the placebo group (relapse at 12 months) taper_duration_7_12<‐metareg(relapse_7_12, ~pbo_taper_R, method.tau = "REML") sink('metareg_duration_taper.txt') taper_duration_7_12 sink() png('metareg_duration_taper.png', res=300, width=24, height=24, units='cm') bubble(taper_duration_7_12, xlab='Duration of taper in the placebo group', ylab='logRR of relapse at 7‐12 months') dev.off() ### Mean dose in chlorpromazine equivalents (relapse at 12 months) mean_dose_cpz_7_12<‐metareg(relapse_7_12, ~CPZ_dose, method.tau = "REML") sink('metareg_mean_dose.txt') mean_dose_cpz_7_12 sink() png('metareg_mean_dose.png', res=300, width=24, height=24, units='cm') bubble(mean_dose_cpz_7_12, xlab='Mean dose in chlorpromazine equivalents (mg/day)', ylab='logRR of relapse at 7‐12 months') dev.off() # Relapse independent of timepoint and Meta‐regression for study duration‐‐‐‐ ## Meta‐analysis relapse_total <‐metabin(event.e= relapse_tot_drug, n.e = ndrug, event.c=relapse_tot_plb, n.c=npbo, sm="RR", method = "MH", comb.fixed = FALSE, studlab = Author, data = dat[!is.na(dat$relapse_tot_drug) &!dat$Author=='Goldberg 1981',]) #Goldberg 1981 had 0 events in both antipsychotic and placebo group sink('relapse_endpoint.txt') print(relapse_total) sink() ## Meta‐regression for study duration study_duraiton_endpoint <‐ metareg(relapse_total, ~study_duration_metaregression_weeks, method.tau = "REML") sink('study_duration_endpoint.txt') print(study_duraiton_endpoint) sink() png('metareg_study_duration_endpoint.png', res=300, width=24, height=24, units='cm') bubble(study_duraiton_endpoint, xlab='Study duration (weeks)', ylab='logRR of relapse at endpoint') dev.off()
Data and analyses
Comparison 1. Maintenance treatment with antipsychotic drugs versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Relapse: 1. Within pre‐specified time periods | 71 | 19996 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.33, 0.40] |
| 1.1.1 up to 3 months | 44 | 6362 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.28, 0.40] |
| 1.1.2 4‐6 months | 49 | 7599 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.31, 0.42] |
| 1.1.3 7‐12 months | 30 | 4249 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.32, 0.45] |
| 1.1.4 > 12 months | 10 | 1786 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.33, 0.64] |
| 1.2 Relapse: 2. Independent of duration | 71 | 8666 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.30, 0.40] |
| 1.3 Leaving the study early: 1. Due to any reason (acceptability of treatment) | 56 | 7001 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.49, 0.61] |
| 1.3.1 up to 3 months | 11 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.17, 0.67] |
| 1.3.2 4 to 6 months | 18 | 1792 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.37, 0.65] |
| 1.3.3 7 to 12 months | 24 | 3951 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.48, 0.65] |
| 1.3.4 > 12 months | 5 | 741 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.51, 0.82] |
| 1.4 Leaving the study early: 2. Due to adverse events (overall tolerability) | 53 | 6627 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.85, 1.89] |
| 1.4.1 up to 3 months | 10 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.12, 65.34] |
| 1.4.2 4 to 6 months | 15 | 1852 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.63, 2.28] |
| 1.4.3 7 to 12 months | 23 | 3870 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.69, 1.97] |
| 1.4.4 > 12 months | 5 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 5.70 [1.28, 25.33] |
| 1.5 Leaving the study early: 3. Due to inefficacy | 55 | 6537 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.32, 0.43] |
| 1.5.1 up to 3 months | 11 | 421 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.07, 0.64] |
| 1.5.2 4 to 6 months | 16 | 1661 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.31, 0.54] |
| 1.5.3 7 to 12 months | 24 | 3951 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.31, 0.44] |
| 1.5.4 > 12 months | 4 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.29, 0.64] |
| 1.6 Global state: number of participants improved (at least minimally) | 16 | 1878 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [1.58, 2.85] |
| 1.6.1 up to 3 months | 3 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 4.76 [1.65, 13.68] |
| 1.6.2 4 to 6 months | 8 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [1.69, 3.21] |
| 1.6.3 7 to 12 months | 4 | 388 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.89, 3.13] |
| 1.6.4 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.88, 2.09] |
| 1.7 Global state: number of participants in symptomatic remission | 7 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.20, 2.48] |
| 1.7.1 up to 3 months | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 2.50 [0.63, 10.00] |
| 1.7.2 4 to 6 months | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.79, 3.87] |
| 1.7.3 7 to 12 months | 5 | 807 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [1.11, 2.59] |
| 1.8 Global state: number of participants in sustained remission | 8 | 1807 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.28, 2.19] |
| 1.8.1 7 to 12 months | 6 | 1443 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.49, 2.25] |
| 1.8.2 >12 months | 2 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.13, 1.47] |
| 1.9 Service use: number of participants hospitalised | 21 | 3558 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.32, 0.57] |
| 1.9.1 up to 3 months | 2 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.04, 4.06] |
| 1.9.2 4 to 6 months | 4 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.03, 1.32] |
| 1.9.3 7 to 12 months | 11 | 2119 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.23, 0.56] |
| 1.9.4 > 12 months | 4 | 965 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.44, 0.69] |
| 1.10 Service use: number of participants discharged | 3 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [0.69, 11.06] |
| 1.10.1 4 to 6 months | 3 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [0.69, 11.06] |
| 1.11 Death: due to any reason | 25 | 5181 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.39, 2.11] |
| 1.11.1 up to 3 months | 3 | 415 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.11.2 4 to 6 months | 6 | 1159 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [0.59, 8.98] |
| 1.11.3 7 to 12 months | 15 | 3273 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.11, 1.12] |
| 1.11.4 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 5.18 [0.25, 107.12] |
| 1.12 Death: due to natural causes | 25 | 5226 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.50, 3.60] |
| 1.12.1 up to 3 months | 2 | 379 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.12.2 4 to 6 months | 6 | 1159 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [0.59, 8.98] |
| 1.12.3 7 to 12 months | 16 | 3354 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.11, 2.58] |
| 1.12.4 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 3.11 [0.13, 75.78] |
| 1.13 Death: due to suicide | 19 | 4634 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.12, 2.97] |
| 1.13.1 up to 3 months | 3 | 415 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.13.2 4 to 6 months | 3 | 1033 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.13.3 7 to 12 months | 12 | 2852 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.06, 2.21] |
| 1.13.4 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 3.11 [0.13, 75.78] |
| 1.14 Number with suicide attempts | 12 | 3123 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.19, 1.99] |
| 1.14.1 4 to 6 months | 3 | 776 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 71.51] |
| 1.14.2 7 to 12 months | 9 | 2347 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.13, 1.69] |
| 1.15 Number with suicide ideation | 13 | 3255 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.33, 1.16] |
| 1.15.1 up to 3 months | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 3.88] |
| 1.15.2 4 to 6 months | 1 | 386 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.15.3 7 to 12 months | 10 | 2486 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.24, 1.09] |
| 1.15.4 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.35, 4.74] |
| 1.16 Violent/aggressive behaviour | 12 | 2856 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.24, 0.59] |
| 1.16.1 up to 3 months | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.50] |
| 1.16.2 4 to 6 months | 2 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.20, 1.08] |
| 1.16.3 7 to 12 months | 8 | 2146 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.19, 0.66] |
| 1.16.4 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.28] |
| 1.17 Adverse effects: at least one adverse event | 18 | 4352 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.98, 1.25] |
| 1.17.1 up to 3 months | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.93] |
| 1.17.2 4 to 6 months | 4 | 1079 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.85, 1.12] |
| 1.17.3 7 to 12 months | 12 | 2890 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.99, 1.33] |
| 1.17.4 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.24, 2.45] |
| 1.18 Adverse effects: movement disorders: at least one movement disorder | 29 | 5276 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.25, 1.85] |
| 1.18.1 up to 3 months | 4 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [0.70, 8.33] |
| 1.18.2 4 to 6 months | 8 | 1658 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.06, 1.99] |
| 1.18.3 7 to 12 months | 16 | 3126 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.17, 2.05] |
| 1.18.4 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.58, 2.54] |
| 1.19 Adverse effects: movement disorders: akathisia | 21 | 4214 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.93, 2.38] |
| 1.19.1 up to 3 months | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.68 [0.49, 14.82] |
| 1.19.2 4 to 6 months | 6 | 1191 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [0.50, 9.11] |
| 1.19.3 7 to 12 months | 12 | 2620 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.71, 1.61] |
| 1.19.4 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.42, 7.11] |
| 1.20 Adverse effects: movement disorders: akinesia | 3 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.08, 3.42] |
| 1.20.1 up to 3 months | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.09, 9.92] |
| 1.20.2 7 to 12 months | 2 | 348 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.01, 3.98] |
| 1.21 Adverse effects: movement disorders: dyskinesia | 18 | 3200 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.33, 0.91] |
| 1.21.1 up to 3 months | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.06, 34.91] |
| 1.21.2 4 to 6 months | 3 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.11, 0.84] |
| 1.21.3 7 to 12 months | 13 | 2399 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.37, 1.27] |
| 1.21.4 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.29] |
| 1.22 Adverse effects: movement disorders: dystonia | 13 | 2767 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.99, 2.70] |
| 1.22.1 up to 3 months | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 2.50 [0.13, 49.22] |
| 1.22.2 4 to 6 months | 2 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.94, 3.29] |
| 1.22.3 7 to 12 months | 9 | 2002 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.65, 4.09] |
| 1.22.4 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.28] |
| 1.23 Adverse effects: movement disorders: rigor | 9 | 922 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.70, 2.79] |
| 1.23.1 up to 3 months | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.22, 6.62] |
| 1.23.2 4 to 6 months | 3 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [0.67, 5.85] |
| 1.23.3 7 to 12 months | 4 | 693 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.29, 11.24] |
| 1.24 Adverse effects: movement disorders: tremor | 18 | 3353 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.95, 1.98] |
| 1.24.1 up to 3 months | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.46, 3.16] |
| 1.24.2 4 to 6 months | 3 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.33, 2.61] |
| 1.24.3 7 to 12 months | 12 | 2790 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.04, 2.54] |
| 1.24.4 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.10, 2.79] |
| 1.25 Adverse effects: movement disorders: use of antiparkinson medication | 13 | 2908 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.10, 1.65] |
| 1.25.1 4 to 6 months | 3 | 841 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.90, 2.61] |
| 1.25.2 7 to 12 months | 9 | 1733 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.06, 1.78] |
| 1.25.3 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.64, 1.57] |
| 1.26 Adverse effects: sedation | 18 | 4078 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.24, 1.86] |
| 1.26.1 up to 3 months | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 3.70] |
| 1.26.2 4 to 6 months | 7 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.89, 2.12] |
| 1.26.3 7 to 12 months | 9 | 1844 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.25, 2.53] |
| 1.26.4 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.15, 7.27] |
| 1.27 Adverse effects: weight gain | 19 | 4767 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.21, 2.35] |
| 1.27.1 4 to 6 months | 4 | 1039 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.81, 2.73] |
| 1.27.2 7 to 12 months | 14 | 3394 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [1.17, 2.77] |
| 1.27.3 >12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [1.06, 4.48] |
| 1.28 Participant´s satisfaction with care | 2 | 737 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.10, 1.33] |
| 1.28.1 7 to 12 months | 1 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.02, 1.38] |
| 1.28.2 > 12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.08, 1.38] |
| 1.29 Quality of life (various scales, different timepoints) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.29.1 up to 3 months ‐ Schizophrenia Quality of Life at endpoint (low score=better) | 2 | 379 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐5.80, 1.80] |
| 1.29.2 7 to 12 months ‐ Self‐report Quality of Life Scale change from baseline to endpoint (low score=better) | 2 | 595 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐6.32, ‐1.88] |
| 1.29.3 7 to 12 months ‐ Heinrichs Carpenter Quality of Life Scale change from baseline to endpoint (low score=better) | 1 | 304 | Mean Difference (IV, Random, 95% CI) | ‐11.36 [‐14.67, ‐8.05] |
| 1.29.4 7 to 12 months ‐ European Quality of Life Visual Analog Scale at endpoint (low score=better) | 1 | 277 | Mean Difference (IV, Random, 95% CI) | ‐6.30 [‐23.41, 10.81] |
| 1.29.5 > 12 months ‐ Symptom Questionnaire of Kellner and Sheffield at endpoint (low score=better) | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐14.33, 4.53] |
| 1.30 Quality of life (across all scales and timepoints) | 7 | 1573 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.57, ‐0.07] |
| 1.31 Number of participants in employment | 3 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 1.31.1 7 to 12 months | 2 | 259 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.75, 1.23] |
| 1.31.2 > 12 months | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.97, 2.00] |
| 1.32 Social Functioning (various scales, different timepoints) | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.32.1 up to 3 months ‐ Personal and Social Performance at endpoint (low score=better) | 2 | 379 | Mean Difference (IV, Random, 95% CI) | ‐5.66 [‐11.50, 0.18] |
| 1.32.2 up to 3 months ‐ Global Assessment Scale at endpoint (low score=better) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐3.61 [‐4.66, ‐2.56] |
| 1.32.3 4 to 6 months ‐ Sheehan Disability Schedule change from baseline to endpoint (low score=better) | 1 | 270 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐3.60, ‐0.40] |
| 1.32.4 7 to 12 months ‐ Personal and Social Performance change from baseline to endpoint (low score=better) | 7 | 1823 | Mean Difference (IV, Random, 95% CI) | ‐4.92 [‐5.96, ‐3.89] |
| 1.32.5 7 to 12 months ‐ Global Assessment of Functioning at endpoint (low score=better) | 1 | 275 | Mean Difference (IV, Random, 95% CI) | ‐8.80 [‐13.22, ‐4.38] |
| 1.32.6 7 to 12 months ‐ Specific Levels of Functioning change from baseline to endpoint (low score=better) | 1 | 246 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐4.85, 0.05] |
| 1.32.7 7 to 12 months ‐ Children Global Assessment Scale change from baseline to endpoint (low score=better) | 1 | 146 | Mean Difference (IV, Random, 95% CI) | ‐4.60 [‐9.84, 0.64] |
| 1.32.8 > 12 months ‐ Personal and Social Performance change from baseline to endpoint (low score=better) | 1 | 329 | Mean Difference (IV, Random, 95% CI) | ‐3.60 [‐6.76, ‐0.44] |
| 1.33 Social Functioning (across all scales and timepoints) | 15 | 3588 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.53, ‐0.34] |
Comparison 2. Subgroup analysis (relapse at 12 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Subgroup analysis: participants with a first episode | 29 | 4113 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.33, 0.46] |
| 2.1.1 first episode | 8 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.38, 0.58] |
| 2.1.2 not first episode | 24 | 3585 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.31, 0.46] |
| 2.2 Subgroup analysis: participants in remission at baseline | 29 | 4113 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.32, 0.46] |
| 2.2.1 in remission | 10 | 1050 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.33, 0.60] |
| 2.2.2 not in remission | 19 | 3063 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.30, 0.44] |
| 2.3 Subgroup analysis: various durations of stability before entering the study | 24 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3.1 stable at least 1 month | 6 | 574 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.20, 0.50] |
| 2.3.2 stable at least 3 months | 10 | 2250 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.26, 0.43] |
| 2.3.3 stable at least 6 months | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.69] |
| 2.3.4 stable at least 12 months | 5 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.57] |
| 2.3.5 stable at least 3 to 6 years | 2 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.18, 0.78] |
| 2.4 Subgroup analysis: abrupt withdrawal versus tapering | 29 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4.1 Abrupt withdrawal | 18 | 2348 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.35, 0.53] |
| 2.4.2 Taper | 11 | 1765 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.24, 0.44] |
| 2.5 Subgroup analysis: single antipsychotic drugs | 29 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.5.1 Chlorpromazine | 2 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.36, 0.55] |
| 2.5.2 Fluphenazine depot | 6 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.14, 0.39] |
| 2.5.3 Haloperidol depot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.04, 0.55] |
| 2.5.4 Various, mixed groups of antipsychotic drugs | 10 | 705 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.27, 0.65] |
| 2.5.5 Quetiapine | 1 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.34, 0.69] |
| 2.5.6 Paliperidone | 4 | 1256 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.31, 0.44] |
| 2.5.7 Aripiprazole | 2 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.14, 0.86] |
| 2.5.8 Brexpiprazole | 1 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.23, 0.56] |
| 2.5.9 Ziprasidone | 1 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.39, 0.64] |
| 2.5.10 Cariprazine | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.38, 0.77] |
| 2.6 Subgroup analysis: depot versus oral drugs | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.6.1 depot | 10 | 1705 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.23, 0.39] |
| 2.6.2 oral | 16 | 2187 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.38, 0.55] |
| 2.7 Subgroup analysis: first‐ versus second‐generation antipsychotic drugs | 29 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.7.1 First‐generation antipsychotic drugs | 18 | 1430 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.25, 0.48] |
| 2.7.2 Second‐generation antipsychotic drugs | 11 | 2683 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.32, 0.48] |
| 2.8 Subgroup analysis: appropriate versus unclear allocation concealment | 29 | 4113 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.32, 0.46] |
| 2.8.1 appropriate allocation concealment | 13 | 2708 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.30, 0.45] |
| 2.8.2 unclear allocation concealment | 16 | 1405 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.30, 0.54] |
| 2.9 Subgroup analysis: blinded versus open trials | 29 | 4113 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.32, 0.46] |
| 2.9.1 blinded trials | 27 | 3856 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.33, 0.48] |
| 2.9.2 unblinded trials | 2 | 257 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.17, 0.39] |
Comparison 3. Sensitivity analysis (relapse at 12 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Exclusion of studies that were not explicitly described as randomised | 28 | 4098 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.33, 0.46] |
| 3.2 Exclusion of non‐double‐blind studies | 27 | 3856 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.33, 0.48] |
| 3.3 Fixed‐effects model | 29 | 4113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.35, 0.41] |
| 3.4 Original authors' assumptions on dropouts | 29 | 4113 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.32, 0.46] |
| 3.5 Inclusion of only large studies (> 200 participants) | 10 | 2950 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.31, 0.45] |
| 3.6 Exclusion of studies with clinical diagnosis | 22 | 4054 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.34, 0.48] |
| 3.7 Three months stable | 29 | 4622 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.24, 0.42] |
| 3.8 Six months stable | 20 | 2549 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.20, 0.45] |
| 3.9 Nine months stable | 15 | 1806 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.19, 0.52] |
| 3.10 Exclusion of studies with unclear randomisation method | 11 | 2644 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.29, 0.43] |
| 3.11 Exclusion of studies with unclear allocation concealment method | 13 | 2708 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.30, 0.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aripiprazole 2003.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, no further details. Duration: 26 weeks. Design: parallel. Location: multi‐centre. Setting: in‐ and outpatient, sponsored. | |
| Participants | Diagnosis: chronic schizophrenia (DSM‐IV), at least two years of continuous antipsychotic medication. N = 310. Gender: 174 men, 136 women. Age: mean 42 years. History: duration stable‐ no significant improvement or worsening of symptoms for at least 3 months, but all participants with significant symptoms (PANSS total score of at least 60, but CGI‐severity score no more than moderately ill), duration ill‐ at least 2 years, number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ mean PANSS total score at baseline 81.1, mean CGI severity score at baseline 3.52, approximately 50% were in hospital, 20% were in partially supervised facilities, the rest were outpatients, baseline antipsychotic dose‐ n.i. |
|
| Interventions | 1. Drug: aripiprazole. Fixed dose of 15 mg/day. N = 155. 2. Placebo: duration of taper (days): n.i. (pre‐trial medication was tapered, when appropriate, before stopping treatment). N = 155. Rescue medication: additional antipsychotic drugs were not allowed. |
|
| Outcomes |
Examined Relapse: CGI at least minimally worse, a PANSS score of ‐ 5 (moderately severe) on the subscore items of hostility or uncooperativeness on 2 successive days; or a ‐ 20% increase in PANSS total score. Leaving the study early. Service use ‐ number of participants hospitalised (including non psychiatric reasons). Death. Suicide attempts. Adverse effects. Violent/aggressive behaviour. Unable to use/Not included Global state: CGI (no usable data ). Mental state: PANSS, BPRS (no SD/no predefined outcome of interest). Physiological measures: vital signs (pulse rate, systolic and diastolic blood pressure, no data/no predefined outcome of interest), laboratory (haematology, no data; serum chemistries, no data a apart from creatinine phosphate/no predefined outcome of interest) urine tests, ECG (both no data/no predefined outcome of interest) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Correct randomisation assumed, because recent study from industry.. |
| Allocation concealment (selection bias) | Low risk | Correct allocation concealment assumed, because recent study from industry. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A high number of participants (62.2%) left the study early, mostly because of relapse (61%), which was more frequent in the placebo group. For other outcomes this could be a problem. For the primary outcome survival analysis was used which was not a full ITT (one post‐baseline/dose) but only few participants were excluded. |
| Selective reporting (reporting bias) | High risk | Only those adverse events that occurred in at least 5% of the participants in either group were reported. |
| Other bias | Low risk | No clear other bias. |
Aripiprazole 2017.
| Study characteristics | ||
| Methods | Randomisation: randomised, no further details. Allocation: procedure not described. Blinding: double‐blind, no further details. Duration: terminated early after 37 events (pre‐planned), mean duration of treatment was 164,5 days. Design: parallel. Location: multi‐centre. Setting: outpatients. | |
| Participants | Diagnosis: adolescent patients (13 to 17 years old) with schizophrenia (DSM‐IV‐TR) stabilised on aripiprazole for 7 to 21 weeks before entering the double‐blind phase. N = 146. Gender: 96 men, 50 women. Age: mean 15,4 years (range 13 to 17 years). History: duration stable‐ at least 7 weeks (clinical judgment and rating scale defined), remission at baseline‐ n.i., duration ill‐ 2.1 years, number of previous hospitalisations‐ n.i., age at onset‐ 13.3 years, severity of illness‐ mean PANSS total score 64,6, mean CGI‐S total score 3,1, baseline antipsychotic dose‐n.i. |
|
| Interventions | 1. Drug: aripiprazole. Flexible dose. Allowed dose range: 10 mg/day to 30 mg/day. Mean dose: 19,2 mg/day. N = 98. 2. Placebo: inert placebo. Duration of taper: n.i. N = 48. Rescue medication: not allowed. |
|
| Outcomes |
Examined Relapse: rating scale based and/or need for hospitalisation and/or clinical judgment. Leaving the study early (any reason, adverse events, inefficacy). Global state‐ number of patients in symptomatic remission (Andreasen criteria, LOCF endpoint cross‐sectional criteria) Global state‐ number of patients in sustained remission (Andreasen criteria, maintained for 6 months) Social functioning: Children´s Global Assessment Scale Adverse events Death. Suicidal ideation: Columbia Suicide Severity Rating Scale. Unable to use/Not included Global state ‐ number of participants improved (no usable data). Mental state: PANSS total score and subscores (no predefined outcome of interest). Quality of life: Pediatric Quality of Life Enjoyment and Satisfaction Questionnaire (no usable data). |
|
| Notes | Sponsored by Otzuka Pharmaceutical Development and Commercialization. Adolescent patients subgroup. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised (2:1 ratio), no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The overall attrition rate of 33% could still be acceptable, though higher than 25%, but more participants in the placebo group left the study early, mostly due to relapse. This difference may have biased the results of outcomes other than leaving the study early and relapse, which was assessed using the Kaplan‐Meier survival curve analysis. Data for secondary outcomes were both analysed on an ITT basis (LOCF method) and provided for completers at several time points. |
| Selective reporting (reporting bias) | Low risk | The primary outcome relapse was reported as prespecified; Efficacy and Safety: Quality of Life score not reported completely, but it is not a primary outcome. |
| Other bias | High risk | Terminated early (after 37 relapse events), but it was pre‐planned. |
Aripiprazole depot 2012.
| Study characteristics | ||
| Methods | Randomisation: sponsor‐prepared computer‐generated randomisation code (2:1 ratio), stratified by region and by last stabilisation dose of study drug. Allocation: interactive voice/web response system. Blinding: IM trial medications had different appearance, but were prepared and administered by an unblinded Trial Drug Manager. Duration: terminated early after the first pre‐planned interim analysis (64 events); pre‐planned duration: 52 weeks. Design: parallel. Location: multi‐centre (108 centres). Setting: outpatients. | |
| Participants | Diagnosis: Schizophrenia (DSM‐IV‐TR), diagnosed at least 3 years before. N = 403. Gender: 241 men, 162 women. Age: 40.6 years. History: duration stable‐ at least 12 weeks, duration ill‐ 14.6 years, number of previous hospitalisations‐ n.i., age at onset‐ 26 years, severity of illness‐ mean PANSS total score 54.5, mean CGI‐S total score 2.9, baseline antipsychotic dose‐ 391.6 mg/4 weeks, remission at baseline‐ not in remission (at least moderately ill at CGI‐S). | |
| Interventions | 1. Drug: aripiprazole IM, 1‐month formulation. N = 269 Flexible dose. Mean dose: 396.3 mg/4 weeks. Allowed dose range: either 300 mg or 400 mg/4 weeks. 2. Placebo: duration of taper: depending on the aripiprazole depot half‐life (between 29.9 and 46.5 days). N = 134. Rescue medication: benzodiazepines (maximum 6 mg/day) and anticholinergics (antiparkinson) were permitted, although not within 8 to 12 hours (respectively) of rating scale assessments. |
|
| Outcomes |
Examined Relapse (rating scale defined and/or need for hospitalisation and/or emergent violent behaviour). Leaving the study early (any cause, adverse events, inefficacy). Global state ‐ Number of participants in sustained remission (Andreasen criteria). Service use ‐ Number of participants hospitalised. Participants´satisfaction with care: Patient Satisfaction with Medication Questionnaire. Social functioning: Personal and Social Performance scale. Adverse effects. Death. Suicide ideation and behaviour: CGI‐SS, Columbia Suicide Severity Rating Scale and Columbia Classification Algorythm of Suicide Assessment. Violent/aggressive behaviour. Unable to use/Not included Global state ‐ number of participants improved (no usable data). Mental state: Positive and Negative Symptoms Scale total scores and subscales (no predefined outcome of interest). Neurocognitive function: Trail Making Test (A), Tower of London, Letter‐Number Span (no predefined outcome of interest). Compliance to treatment: Drug Attitude Inventory Score, Medication Adherence Questionnaire (no predefined outcome of interest). Carer´s satisfaction with care: Investigator´s Assessment Questionnaire (no usable data). |
|
| Notes | Sponsored by Otsuka. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sponsor‐prepared computer‐generated randomisation code (2:1 ratio), stratified by region and by last stabilisation dose of study drug |
| Allocation concealment (selection bias) | Low risk | Interactive voice/web response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | IM trial medications had different appearance (aripiprazole: milky white suspension; placebo: clear solution); they were prepared and administered by an unblinded Trial Drug Manager. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | IM trial medications had different appearance (aripiprazole: milky white suspension; placebo: clear solution); they were prepared and administered by an unblinded Trial Drug Manager. Two participants were unblinded at the site level (one incident with depot dose log, one incidental access to the drug storage cabinet given by the monitor), both withdrawn from the study. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | IM trial medications had different appearance (aripiprazole: milky white suspension; placebo: clear solution); they were prepared and administered by an unblinded Trial Drug Manager. Two participants were unblinded at the site level (one incident with depot dose log, one incidental access to the drug storage cabinet given by the monitor), both withdrawn from the study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The total attrition rate (35%) was higher than 25% but could still be acceptable. However more participants in the placebo group dropped out, mostly due to relapse (reasons are unbalanced between groups). The primary outcome (relapse) was assessed using the Kaplan‐Meier survival curve analysis. Data for secondary outcomes were analysed on an ITT basis (LOCF). |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | Study terminated early after an interim analysis (64 relapse events), but it was pre‐planned. |
Asenapine 2011.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, identical capsules in taste. Duration: 6 months. Design: parallel. Location: multi‐centre. Setting: unclear. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). N = 386. Gender: 221 men, 165 women. Age: mean 38.9 years. History: duration stable‐ 30 weeks, duration ill‐ mean 12.7 years, number of previous hospitalisations‐ n.i., age at onset‐ mean 26.7 years, severity of illness‐ n.i., baseline antipsychotic dose‐ all on asenapine 10 mg/day or 20 mg/day. |
|
| Interventions | 1. Drug: asenapine. Fixed dose (same dose as at end of stabilisation phase): mean 17.6 mg/day. N = 194. 2. Placebo: duration of taper: 0 days. N = 192. Rescue medication: benzodiazepines, anticholinergics, antidepressants. |
|
| Outcomes |
Examined Relapse: CGI‐severity >=4, moderately ill for one week was accompanied by: PANSS total score increase >=20% (a 10 point increase if PANSS was lower than 50), a PANSS item score >=5 on hostility of uncooperativeness or a PANSS item score >=5 and two items of unusual thought content, conceptual disorganisation or hallucinatory behaviour. Relapse was also judged to appear if in the investigator's opinion schizophrenia, risk of violence to self or others, or suicide risk increased so >=1 of the following was required: an additional >=2mg/day lorazepam, compared with the highest open‐label dose for 1 week, addition of antipsychotic, addition or dosage increase of an antidepressant or mood‐stabiliser, increased psychiatric care, arrest or imprisonment, electroconvulsive therapy, or other relevant measures. Suicidal ideation and behaviour. Adverse effects: at least one adverse event, at least one movement disorder, akathisia, sedation, weight gain. Unable to use/Not included Mental state: PANSS (no predefined outcome of interest). Global state: CGI (no usable data ). Leaving the study early (data are unclear). Electrocardiogram (no predefined outcome of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules in taste. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules in taste. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules in taste. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate, but exact number of dropouts could not be calculated. Dropouts were not clearly enough reported. Survival curve analysis was used for the primary outcome relapse. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear evidence for other bias. |
Brexpiprazole 2017.
| Study characteristics | ||
| Methods | Randomisation: sponsor‐prepared computer‐generated randomisation code (1:1 ratio), assignment of blocks of randomisation numbers to trial centres and individual numbers to patients. Allocation: interactive voice/web response system. Blinding: double‐blind, identical capsules. Duration: terminated early after the first pre‐planned interim analysis (64 events); pre‐planned duration: 52 weeks. Design: parallel. Location: multi‐centre (49 sites across 7 countries). Setting: inpatients and outpatients | |
| Participants | Diagnosis: Schizophrenia (DSM‐IV‐TR), diagnosed at least 3 years before; stabilised on study drug after resolution of an acute exacerbation. N = 202. Gender: 123 men, 79 women. Age: 40.3 years. History: duration stable‐ at least 12 weeks, duration ill‐ 13 years, number of previous hospitalisations‐ n.i., age at onset‐ 27.2 years, severity of illness‐ mean PANSS total score 57.3, mean CGI‐S total score 3.1, baseline antipsychotic dose‐ n.i., remission at baseline‐ n.i. (probably not in remission). | |
| Interventions | 1. Drug: Brexpiprazole. N = 97 Fixed dose. Mean dose: 3.6 mg/day. Allowed dose range: 1 mg/day to 4 mg/day. 2. Placebo: duration of taper: no. N = 105. Rescue medication: n.i. |
|
| Outcomes |
Examined Relapse (rating scale defined and/or need for hospitalisation and/or emergent violent/suicidal behaviour). Leaving the study early (any cause, adverse events, inefficacy). Social functioning: Personal and Social Performance scale. Adverse effects Suicidal ideation and behavior: Columbia Suicide Severity Scale Unable to use/Not included Global state ‐ number of participants improved: CGI‐I defined (no usable data). Global state ‐ number of participants in remission: CGI‐S defined (no usable data). Mental state: Positive and Negative Symptoms Scale total score and subscales (no predefined outcome of interest) Affective symptoms: PANSS excited component score, PANSS Marder Anxiety/Depression score (no predefined outcome of interest). Neurocognitive function: Cogstate computerized cognitive test battery (no predefined outcome of interest) Social functioning: Global Assessment of functioning (already used data regarding Personal and Social Performance scale). |
|
| Notes | Sponsored by Otsuka. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sponsor‐prepared computer‐generated randomisation code, assignment of blocks of randomisation numbers to trial centres and individual numbers to patients. |
| Allocation concealment (selection bias) | Low risk | Interactive voice/web response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall attrition rate of 46% was high, and more participants in the placebo group left the study early, mostly due to relapse. This difference may have biased the results of outcomes other than leaving the study early and relapse, which was assessed using the Kaplan‐Meier survival curve analysis. Data for secondary outcomes were analysed on an ITT basis. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | Terminated early after an interim analysis, but it was pre‐planned. |
Cariprazine 2016.
| Study characteristics | ||
| Methods | Randomisation: randomised (1:1 ratio), no further details. Allocation: interactive web response system. Blinding: double‐blind, identical appearing capsules. Duration: open‐ended, duration varied from 26 to 72 weeks (mean exposure duration: 232 days in the double‐blind phase). Design: parallel. Location: multi‐centre. Setting: outpatients. | |
| Participants | Diagnosis: Schizophrenia (DSM‐IV‐TR), stabilized on treatment for at least 12 weeks before double‐blind phase. N = 200. Gender: 132 men, 68 women. Age: 38.5 years. History: duration stable‐ at least 12 weeks (duration of treatment), duration ill‐ 11.2 years, mean duration of hospitalisation‐ n.i., number of previous hospitalisations‐ mean 4.6., age at onset‐ n.i, severity of illness‐ mean PANSS total score 50.9, mean CGI‐S total score 2.7, baseline antipsychotic dose‐n.i. (for the cariprazine arm: 7.1 mg/day), remission at baseline‐ 85% of the participants met symptomatic remission criteria (Andreasen) at double‐blind baseline. | |
| Interventions | 1. Drug: cariprazine. N = 101 Fixed dose. Mean dose: 7.07 mg/day. Allowed dose range: 3 mg/day to 9 mg/day. 2. Placebo: duration of taper: n.i. N = 99. Rescue medication: anticholinergics (antiparkinson), beta‐blocker (akatihsia), lorazepam or oxazepam (anxiety/agitation). |
|
| Outcomes |
Examined Relapse (clinical judgement and/or need of hospitalisation). Leaving the study early (any reason, inefficacy, adverse events) Global state ‐ number of participants in sustained remission (Andreasen criteria) Social functioning: Personal and Social Performance Scale Adverse events Death Suicide attempts Suicidal ideation: Columbia‐Suicide Severity Rating Scale Unable to use/Not included Mental state: PANSS total score and subscores (no predefined outcome of interest), negative symptoms assessed with NSA‐16 (no predefined outcome of interest). Global state ‐ number of improved participants: CGI‐S and CGI‐I scores (no usable data). |
|
| Notes | Sponsored by Forest, Actavis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Low risk | Investigators and patients were blinded to the double‐blind treatment assignment through an interactive web‐response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical appearing capsules. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical appearing capsules. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical appearing capsules. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall attrition rate was high (68%); it was slightly different between the study arms, and reasons were different (more participants in the placebo group left the study early due to relapse). This difference may have biased the results of outcomes other than leaving the study early and relapse, which was assessed using the Kaplan‐Meier survival curve analysis. No full ITT analysis for other outcomes (only patients that had at least one evaluation were included). |
| Selective reporting (reporting bias) | Low risk | No clear evidence of selective reporting. |
| Other bias | High risk | Terminated early, but it was pre‐planned. |
Chlorpromazine 1959.
| Study characteristics | ||
| Methods | Randomisation: matched and then randomised by a research assistant. Allocation: by a research assistant who carefully guarded the identity of patients and the assigned treatment regimen. Furthermore, medication was assigned by the director of professional services who kept the names for use in case a patient had to be withdrawn from the study. Blinding: double‐blind, identical capsules. Duration: 26 weeks. Design: parallel. Location: single‐centre. Setting: inpatient. | |
| Participants | Diagnosis: chronic schizophrenia (clinical diagnosis), less than 50 years, on chlorpromazine for at least six months, had reached a stable improved state. N = 80. Gender: n.i.. Age: all <50 years. History: duration stable‐ n.i., duration ill‐ n.i., number of previous hospitalisations‐ n.i., but median duration of current hospitalisation eight years, age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: chlorpromazine. Fixed dose of 200 mg/day. N = 40. 2. Drug: reserpine*. Fixed dose of 2 mg/day. N = 40. 3. Placebo: duration of taper 0 days. N = 40. Rescue medication: not indicated, probably not allowed. |
|
| Outcomes |
Examined Leaving the study early. Suicide attempts. Unable to use/Not included Mental state: Lorr Multidimensional Scale for Rating Psychiatric Patients (no SD/no predefined outcome of interest). Behaviour: Psychiatric Behaviour Rating Scales (no SD / no predefined outcome of interest). |
|
| Notes | *this group was not used in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Matched and then randomised by a research assistant. |
| Allocation concealment (selection bias) | Low risk | By a research assistant who carefully guarded the identity of patients and the assigned treatment regimen. Furthermore, medication was assigned by the director of professional services who kept the names for use in case a patient had to be withdrawn from the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 11% dropped out, most of them due to relapse (88%) in the placebo group. As relapse, dropout and suicide were the only outcomes, this did not produce a risk of bias. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear evidence for other bias. |
Chlorpromazine 1962.
| Study characteristics | ||
| Methods | Randomisation: participants were ranked for morbidity, then matched, then randomised. Allocation: procedure not described. Blinding: double‐blind, identical capsules, each participant was provided medication in individual container. Staff guessed on which medication the participants were but could not guess adequately. Duration: 26 weeks. Design: parallel. Location: single‐centre. Setting: in hospital. | |
| Participants | Diagnosis: chronic, long term hospitalised male psychotics (clinical diagnosis), 86 schizophrenia, 6 chronic brain syndrome, 2 personality disorders, 2 n.i.. N = 96. Gender: 96 men. Age: 43.6 years. History: duration stable‐ treated with chlorpromazine for at least 2 months, not ready for discharge, not assaultive, duration ill‐ n.i. but duration of current hospitalisation 12.3 years, number of previous hospitalisations NI‐, age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ 224 mg chlorpromazine per day. |
|
| Interventions | 1. Drug: chlorpromazine ‐ Flexible dose. Allowed dose range: n.i.. Mean dose: n.i.. N = 48. 2. Placebo: duration of taper: 0 days. N = 48. Rescue medication: occasional use of sedatives, antipsychotics were not allowed. |
|
| Outcomes |
Examined Relapse: condition worsened to such a point that ordinarily a complete change in treatment would be considered. Leaving early due to inefficacy. Unable to use/Not included Behaviour: Lyon’s Behaviour Scale (no SD / no prespecified outcome of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were ranked for morbidity, then matched, then randomised. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical capsules, each participant was provided medication in individual container. Staff guessed on which medication the participants were but could not guess adequately. |
| Blinding (performance bias and detection bias) Subjective outcomes | Low risk | Double‐blind, identical capsules, each participant was provided medication in individual container. Staff guessed on which medication the participants were but could not guess adequately. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules, each participant was provided medication in individual container. Staff guessed on which medication the participants were but could not guess adequately. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It could be that there were participants leaving the study early but this was not clearly reported. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | Blinding was broken once a participant relapsed. |
Chlorpromazine 1968.
| Study characteristics | ||
| Methods | Randomisation: "randomly assigned”, no further details. Allocation: procedure not described. Blinding: double‐blind, liquid form. no further details. Duration: 24 weeks. Design: parallel. Location: multi‐centre. Setting: inpatient. | |
| Participants | Diagnosis: schizophrenia (clinical diagnosis), continuously hospitalised for at least two years. N = 420. Gender: n.i.. Age: mean 41.6 years. History: duration stable‐ patients were observed on their normal hospital medication for eight weeks, duration ill‐ mean 17.4 years, mean age at first hospitalisation 24.2 years, mean duration of current hospitalisation‐ mean 13.1 years, number of previous hospitalisations‐ n.i., age at onset‐ mean 24.2 years, severity of illness‐ on the average markedly ill, participants were required to show positive or negative symptoms, baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: chlorpromazine. Fixed dose of 300 mg/day. N = 208. *2. Drug: chlorpromazine. Fixed dose of 2000 mg/day (titrated within 45 days, dose reduction to 1500 mg/day was possible). N = 208. 3. Placebo: duration of taper: 0 days. N = 212. *4. Routine treatment (any antipsychotic medication, any dose). N = 210. Rescue medication: n.i., but probably not allowed. |
|
| Outcomes |
Examined Relapse: a patient was considered relapsed if he regressed and had to be returned to known medication before the end of the 24‐week period. Leaving the study early. Global state: number of participants improved. Death. Adverse effects: based on clinical interview. Unable to use/Not included Mental state: Inpatient Multidimensional Psychiatric Scale, Brief Psychiatric Rating Scale (both only P values/no predefined outcome of interest). Global state: number of participants in remission (no usable data, only reported for a subgroup of patients evaluated by the same rater during the trial). Behaviour: Nurses’ Observation Scale for Inpatient Evaluation (no predefined outcome of interest). Readiness for discharge: Discharge‐Readiness Inventory (no predefined outcome of interest). Ophthalmologic examination (no predefined outcome of interest). |
|
| Notes | Quote: *We only analysed the low dose group, because the high dose was excessively high (2000mg chlorpromazine per day) and because the conventional treatment group was not double‐blind. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, liquid formulation. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, liquid formulation. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, liquid formulation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall 26% dropped out (of which 87% due to relapse). 15% of the participants in the drug group compared to 38% of the participants in the placebo group left the study early. This difference in attrition is a problem for the analysis of other outcomes than relapse. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear other bias. |
Chlorpromazine 1973.
| Study characteristics | ||
| Methods | Randomisation: randomly assigned, no further details. Allocation: procedure not described. Blinding: double‐blind, identical capsules, no further details. Duration: 2 to 3 years (data available up to 2 years). Design: parallel. Location: three centres. Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia (DSM‐II, undifferentiated type 46.3%, paranoid 39%, acute differentiated 8%, schizoid affective 2.7%, other 3.8%), currently hospitalised for less than 2 years. N = 374. Gender: 159 men, 215 women. Age: mean 34.4 years. History: duration stable‐ 2 months transition phase, those who relapsed during this time were replaced, duration ill‐ n.i., number of previous hospitalisations‐ mean 2.6, age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ mean 265mg chlorpromazine per day. |
|
| Interventions | Previous medication was gradually shifted to chlorpromazine for two months.
1. Drug: chlorpromazine ‐ Flexible dose. Allowed dose range: 100 mg/day. Mean dose: ~ 260 mg/day. N = 192. 2. Placebo: duration of taper: 0 days. N = 182. Rescue medication: not indicated, but probably not allowed. |
|
| Outcomes |
Examined Relapse: clinical deterioration of such magnitude that hospitalisation appeared imminent. Service use: number of participants hospitalised. Unable to use/Not included Leaving the study early (numbers not specified for each group separately). Mental state: Brief Psychiatric Rating Scale, Inpatient Multidimensional Psychiatric Scale, Springfield Symptom Index, Hopkin’s Symptom Distress Check List (all no SDs and data only given for subgroups/no predefined outcome of interest). Social behaviour and adjustment: Katz Adjustment Scale, Major Role Adjustment Inventory (no usable data). Number of participants employed (no usable data). |
|
| Notes | Half of the participants randomly received major role therapy in addition to chlorpromazine or placebo. For the purpose of this review the four resulting groups were pooled as described above. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Relatively few participants left the study early due to reasons other than relapse which was the only outcome (n = 31). Although it is unclear in which group they occurred the small percentage does not represent an important risk of bias. |
| Selective reporting (reporting bias) | Low risk | No clear evidence for selective reporting. |
| Other bias | Low risk | No clear other bias. |
Chlorpromazine 1975.
| Study characteristics | ||
| Methods | Randomisation: random number table. Allocation: all personnel except for the treating psychiatrist remained unaware of the code until the end of the study. Blinding: double‐blind (patients, scientists, nurses, only the treating psychiatrist knew the treatment). Duration: 12 days. Design: parallel. Location: single‐centre. Setting: inpatient. | |
| Participants | Diagnosis: chronic schizophrenia (clinical diagnosis). N = 14. Gender: 14 women. Age: n.i.. History: duration stable‐ n.i., duration ill‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: chlorpromazine ‐ Fixed dose. Allowed dose range n.i.. Mean dose n.i.. N = 7. 2. Placebo: duration of taper: 0 days. N = 7. Rescue medication: benztropine. |
|
| Outcomes |
Examined Relapse: worsening of psychotic symptoms. Leaving the study early. Unable to use/Not included Behaviour: NOSIE (no data / no prespecified outcome of interest). Extrapyramidal symptoms: clinical and electrophysiological evaluation (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | All personnel except for the treating psychiatrist remained unaware of the code until the end of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind (patients, scientists, nurses, only the treating psychiatrist knew the treatment). |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind (patients, scientists, nurses, only the treating psychiatrist knew the treatment). |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind (patients, scientists, nurses, only the treating psychiatrist knew the treatment). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant in the placebo group left the study prematurely which is an acceptable rate. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear other bias. |
Chlorpromazine 1976.
| Study characteristics | ||
| Methods | Randomisation: randomised, no further details. Allocation: pharmacists held the key. Blinding: double‐blind, identical capsules. Duration: 42 weeks. Design: parallel. Location: single‐centre. Setting: hospital. | |
| Participants | Diagnosis: schizophrenia (clinical diagnosis), continuously in hospital for at least 6 years (mean 28 years). N = 32. Gender: 32 men. Age: mean 58 years. History: duration stable‐8 weeks, duration ill NI‐ mean duration of hospitalisation 28 years, number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ mean Wing Behaviour Scale Withdrawal Score 2.14, baseline antipsychotic dose‐216 mg/day CPZ equivalent | |
| Interventions | 1. Drug: Chlorpromazine ‐ mean dose: 216 mg/day. N = 15. Allowed dose range: the participants were kept on their initial dose. 2. Placebo: duration of taper 0 days. N = 17. Rescue medication: benzodiazepines, anticholinergics. |
|
| Outcomes |
Examined Relapse (need of antipsychotic medication). Leaving the study early. Unable to use/Not included Behaviour: Ward Behaviour Rating Scale of Wing (no SD / no prespecified outcome of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Low risk | Pharmacists held the key. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No obvious other bias. |
Fluphenazine 1979.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐dummy technique, procyclidine was added to fluphenazine to avoid unmasking by extrapyramidal side effects. Duration: one year. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia (hospital diagnosis, there was an additional evaluation based on research criteria (Kraepelinian), but the results of all participants are presented here), in remission (no positive symptoms, but other symptoms could be present). Patients who were uncooperative in the stabilisation phase were not included in the study. N = 73. Gender: 50 men, 23 women. Age: mean 23.3 years. History: duration stable‐ at least four weeks stable on fluphenazine before randomisation, duration ill‐ n.i., number of previous hospitalisations‐ mean 1.72 previous episodes, age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: fluphenazine decanoate combined with procyclidine flexible dose of 0.5 mL to 2.0 mL biweekly. Mean dose: n.i.. N = 23. 2. Drug: oral fluphenazine combined with procyclidine. Flexible dose of 5 mg/day to 20 mg/day. Mean dose: n.i.. N = 28. 3. Placebo: duration of taper: 0 days. N = 22. Rescue medication: not clearly indicated, but probably not allowed. Prophylactic antiparkinson medication. |
|
| Outcomes |
Examined Relapse: substantial deterioration with a potential of marked social impairment. Leaving the study early. Adverse effects: dropout due to specific adverse events. Death. Unable to use/Not included Global state (CGI ‐ no SD, data for relapsed subgroup only). Mental state (Brief Psychiatric Rating Scale ‐ no SD, data for relapsed subgroup only). Employment status (no usable data, unclearly reported). Social adjustment: Katz Adjustment Scale ‐ no SD, only data for relapsed subgroup and a matched but not randomised subsample. Akinesia: Periodic Evaluation Record (no SD, data for relapsed subgroup only). Suicide attempts (unclearly reported, probably not for the global sample). |
|
| Notes | * 11 out of 73 patients were then diagnosed as non schizophrenic by the research staff. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy technique, procyclidine was added to fluphenazine to avoid unmasking by extrapyramidal side effects. |
| Blinding (performance bias and detection bias) Subjective outcomes | Low risk | Double‐dummy technique, procyclidine was added to fluphenazine to avoid unmasking by extrapyramidal side effects. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐dummy technique, procyclidine was added to fluphenazine to avoid unmasking by extrapyramidal side effects. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 67% of the participants discontinued the study due to relapse (41%) or other reasons. More participants in the drug group discontinued due to adverse events, while more participants in the placebo group discontinued due to relapse. This differential attrition can cause bias. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No evidence for other bias. |
Fluphenazine 1980.
| Study characteristics | ||
| Methods | Randomisation: random 2:1, no further details. Allocation: procedure not described. Blinding: double‐blind, no further details. Duration: 15 weeks. Design: parallel. Location: four hospitals. Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia (DSM‐II). N = 67. Gender: 34 men, 33 women. Age: mean 31.7 years. History: duration stable‐ continuously and successfully treated for one year, duration ill‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ oral fluphenazine mean 24.4 mg/day, depot fluphenazine 30.9 mg/3 weeks. |
|
| Interventions | 1. Drug: oral fluphenazine (n = 6) or depot fluphenazine (n = 11). Fixed/flexible dose: unclear. Allowed dose range: unclear. Mean dose: unclear. N = 17. 2. Placebo: duration of taper: 0 days. N = 50. Rescue medication: n.i., but antipsychotics were probably not allowed. |
|
| Outcomes |
Examined Relapse: rehospitalisation or deterioration in clinical condition which could not be managed within protocol limits (e.g. increased psychological support or adjustment of dosage). Adverse effects: tardive dyskinesia (AIMS). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random 2:1, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were participants who left the study early. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear evidence for other bias. |
Fluphenazine 1982.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, all participants received both pills and injections (active or placebo) to maintain double‐blind conditions. Duration: 1 year. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: first episode schizophrenia (clinical diagnosis), no evidence of drug abuse or important medical illnesses. When diagnoses were reassessed by Research Diagnostic Criteria, 19 had schizophrenia, 3 had unspecific schizophrenic psychoses, 4 had other psychiatric disorders, one mania with schizotypal features and one depression with schizotypal features. N = 28. Gender: 14 men, 14 women. Age: mean 21.9 years. History: duration stable‐ stable remission of at least 4 weeks, mean 16.9 weeks, duration ill‐ mean 17.6 weeks, number of previous hospitalisations‐ 0, age at onset‐ mean 21.5 years, severity of illness‐ n.i., baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: oral fluphenazine ‐ Flexible dose. Allowed dose range: 5‐20 mg/day. Mean dose: n.i.. N = n.i.. 2. Drug: depot fluphenazine ‐ Flexible dose. Allowed dose range: 12.5 mg to 50 mg biweekly. Mean dose: n.i.. N = n.i.. 2. Placebo: duration of taper: 0 days. N = 17. Rescue medication: not indicated. |
|
| Outcomes |
Examined Relapse: a substantial clinical deterioration with a potential for marked social impairment. Patients were considered dropouts only if they showed no signs of clinical deterioration at the time they left the study. Leaving the study early. Unable to use/Not included Social aspects of premorbid personality: Premorbid Asocial Adjustment Scale (data on placebo group only/no predefined outcome of interest). |
|
| Notes | The design was changed during the study in that only non‐compliant patients were randomised to depot fluphenazine or depot placebo, and the randomisation was changed to 2‐1‐1 (placebo, oral fluphenazine, depot fluphenazine). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, all participants received both pills and injections (active or placebo) to maintain double‐blind conditions. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, all participants received both pills and injections (active or placebo) to maintain double‐blind conditions. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, all participants received both pills and injections (active or placebo) to maintain double‐blind conditions. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20 out of 28 participants left the study early, 10 for other reasons than relapse, which was the only outcome apart from leaving the study early. This may present a bias. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Unclear risk | The design was changed during the study in that only non‐compliant patients were randomised to depot fluphenazine or depot placebo, and the randomisation was changed to 2‐1‐1 (placebo, oral fluphenazine, depot fluphenazine). It is unclear whether this biased the results. |
Fluphenazine depot 1968.
| Study characteristics | ||
| Methods | Randomisation: randomly assigned, no further details. Allocation: procedure not described. Blinding: double‐blind, placebo treated participants received injections of sesame oil in a similar amount. Duration: 12 weeks. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: chronic schizophrenia (clinical diagnosis), 12 paranoid, 3 hebephrenic, 2 catatonic, 1 simple, 6 chronic undifferentiated, on antipsychotic medication for a mean duration of 2 years. N = 24. Gender: 4 men, 20 women. Age: mean 36 years. History: duration stable‐ minimum six weeks stable on oral fluphenazine, duration ill‐ mean 12.4 years, number of previous hospitalisations‐ n.i., age at onset‐ mean 23.6 years, severity of illness‐ n.i., baseline antipsychotic dose‐ mean 28.5 mg fluphenazine decanoate biweekly. |
|
| Interventions | 1. Drug: fluphenazine decanoate ‐ Flexible doses. Allowed dose range: 12.5 to 75/mg biweekly. Mean dose: n.i.. N = 13 2. Placebo: sesame oil injections. Duration of taper: 0 days. N = 11. Rescue medication: antiparkinson medication, additional fluphenazine decanoate ‐ but this was considered to be a relapse. |
|
| Outcomes |
Examined Relapse: clinical deterioration requiring additional antipsychotic drug treatment. Leaving study early. Service use: number of participants hospitalised. Adverse effects (at least one movement disorder). Unable to use / Not included: Global state: 7‐point scale of severity (no usable data for the two study arms ). Mental state: scale published by the authors (no SD/no predefined outcome of interest). Adverse effects: scale published by the authors (no numbers). Physiological measures: ECG, EEG, laboratory (all no data/no predefined outcomes of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo treated participants received injections of sesame oil in a similar amount. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, placebo treated participants received injections of sesame oil in a similar amount. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, placebo treated participants received injections of sesame oil in a similar amount. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant left the study early. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | In case of deterioration the participants received additional antipsychotic drugs. This is a problem for the analysis of side effects. |
Fluphenazine depot 1973.
| Study characteristics | ||
| Methods | Randomisation: randomly allocated by research assistant. Allocation: a part from the research assistant no one knew who was on drug or placebo until the data were analysed. Blinding: double‐blind, sesame oil injections, unmarked ampoules. Blinding was tested at the end of the trial and it worked. Duration: 9 months. Design: parallel. Location: two centres. Setting: outpatient. | |
| Participants | Diagnosis: chronic schizophrenia (Present State Examination), chronicity defined by at least 2 admissions or 1 admission lasting longer than 6 months, 71 schizophrenic psychosis with delusions or auditory hallucinations, six non affective delusional psychoses, three catatonic schizophrenia. N = 81. Gender: 52 men, 29 women. Age: mean 43.4 years. History: duration stable‐ at least 8 weeks, duration ill‐ n.i., number of previous hospitalisations‐ 24 had ≤3 and 57 had ≥4), age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ 86% fluphenazine depot 25 mg/month, no additional antipsychotic medication. |
|
| Interventions | 1. Drug ‐ Fixed/flexible dose: allowed dose range: 25 mg/month ‐ no upper limit. Mean dose: 26.4 mg/month. N = 41. 2. Placebo: duration of taper: n.i.. N = 40. Rescue medication: antidepressants, antiparkinson medication |
|
| Outcomes |
Examined Relapse: deterioration of condition to a degree that participant had to be taken out of the trial to ensure that active medication was prescribed, prescription of oral phenothiazines. Leaving the study early. Service use: number of participants hospitalised. Number of participants employed. Death. Violent/aggressive behaviour. Adverse effects: use of antiparkinson medication. Unable to use/Not included Mental state: Present State Examination (no data/no predefined outcome of interest). Social functioning: Social Performance Schedule, Events Schedule of Bron and Birley (no usable data) Suicidal ideation (no usable data, only reported as referred by the patients´ informants to the study rater). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated by research assistant. |
| Allocation concealment (selection bias) | Low risk | Apart from the research assistant no one knew who was on drug or placebo until the data were analysed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, sesame oil injections, unmarked ampoules. Blinding was tested at the end of the trial and it worked. |
| Blinding (performance bias and detection bias) Subjective outcomes | Low risk | Double‐blind, sesame oil injections, unmarked ampoules. Blinding was tested at the end of the trial and it worked. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, sesame oil injections, unmarked ampoules. Blinding was tested at the end of the trial and it worked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall, 43% of the participants left the study early (no complete ITT for some outcomes). |
| Selective reporting (reporting bias) | Low risk | No evidence for selected reporting. |
| Other bias | Low risk | No evidence of other bias |
Fluphenazine depot 1979a.
| Study characteristics | ||
| Methods | Randomisation: no details (just reported as a "randomised study”). Allocation: procedure not described. Blinding: "double‐blind” ("patients and authors were not aware of the allocated treatment”). Duration: 9 months. Design: randomised, parallel (enriched design: patients, who responded to fluphenazine long‐acting treatment (25 mg or 50 mg/month) for at least six to 12 months before study entry, were randomised to continue that treatment or to placebo). Ten out of 20 patients had been previously recruited in a study comparing fluphenazine with trifluorazine. Location: no clear details. Setting: outpatients. | |
| Participants | Diagnosis: chronic schizophrenia with an acute episode within 6 to 12 months before study entry (no details about diagnostic criteria). N = 20. Gender: all men. Age: 19 to 32 years. History: duration stable at least six months, duration ill‐ some were first episode patients, some were patients with recurrence, number of previous hospitalisations‐ no data, age at onset‐ no data, severity of illness‐ fluphenazine group had a mean BPRS baseline score of 24.56 (SD 3.56); placebo group had a mean BPRS baseline score of 21.71, baseline antipsychotic dose (25 mg or 50 mg/month). |
|
| Interventions | 1. Drug: fluphenazine depot. Fixed dose: 25 mg or 50 mg/month (long‐acting formulation). Mean dose: n.i.. N = 10 randomised (but data available only for 9 patients who completed the study). 2. Placebo: duration of taper (days): n.i.. N = 10 randomised (but data available only for 7 patients who completed the study). Rescue medication: antiparkinson medication at study entry (and then progressively tapered off, without a prespecified schedule). |
|
| Outcomes |
Examined Relapse: defined as worsening of clinical status needing an adjunctive new antipsychotic treatment. Leaving the study early. Global state: number of participants improved. Unable to use/Not included Mental state: BPRS (no prespecified outcome of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details (just reported as a "randomised study”). |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind ("patients and authors were not aware of the allocated treatment"). |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind ("patients and authors were not aware of the allocated treatment”). |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind ("patients and authors were not aware of the allocated treatment”). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 25% of the participants dropped out, all due to relapse. This may still be acceptable. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear other bias. |
Fluphenazine depot 1979b.
| Study characteristics | ||
| Methods | Randomisation: matched then each pair randomised, no further details. Allocation: procedure not described. Blinding: double‐blind, no further details. Duration: 6 months. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: probable or definite schizophrenia, any subtype (Research Diagnostic Criteria), in remission for at least 4 weeks or at stable clinical plateau despite vigorous chemotherapy. N = 16. Gender: 14 men, 2 women. Age: 26.7 years. History: duration stable‐ mean 22.9 months in remission (minimum 6 months), duration ill‐ mean 6.1 years, number of previous hospitalisations‐ n.i., but a mean of 2.4 previous episodes, age at onset‐ mean 20.6 years, severity of illness‐ n.i., baseline antipsychotic dose‐ 3.8 mg fluphenazine biweekly, remission at baseline: yes.. |
|
| Interventions | 1. Drug: fluphenazine decanoate ‐ Flexible dose. Allowed dose range: 1.25 mg to 5.0mg biweekly. Mean dose: n.i.. N = 8. 2. Placebo: duration of taper: 0 days, but previously treated with depot medication. N = 8. Rescue medication: minor tranquillisers, additional antipsychotic drugs were not allowed. |
|
| Outcomes |
Examined Relapse: increase in or re‐emergence of significant symptoms suggesting imminent psychotic relapse. Leaving the study early. Unable to use/Not included Adverse effects (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Matched, then each pair randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants in the drug group (1 relapse, 1 unclear) left the study early, and 7/8 participants in the placebo group dropped out due to relapse. As relapse and dropout were the only outcomes, this did not lead to bias. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear evidence for other bias. |
Fluphenazine depot 1981.
| Study characteristics | ||
| Methods | Randomisation: randomly assigned. Allocation: procedure not described. Blinding: double‐blind, placebo injection. Duration: 6 weeks. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: chronic schizophrenic outpatients (DSM‐III). N = 31. Gender: n.i.. Age: 37 years. History: duration stable‐ 2 years on fluphenazine decanoate 3 weekly, duration ill‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ 24 years, severity of illness‐ mean GAS (Global Assessment Scale Endicott 1976 by Spitzer & Endicott 1976), baseline antipsychotic dose‐ 39.3 mg/3 weekly fluphenazine decanoate. |
|
| Interventions | 1. Drug: fluphenazine decanoate‐ Fixed doses. Allowed dose range: n.i. ‐ same dose as before. Mean dose: n.i.. N = 14. 2. Placebo: duration of taper: 0 days, but all on depot. N = 17. Rescue medication: n.i.. | |
| Outcomes |
Examined Relapse: clinical judgement. Leaving the study early. Service use: number of participants hospitalised. Social functioning: Global Assessment Scale (GAS). Unable to use/Not included Community adjustment: Weissman Social Adjustment scale (no usable data) Depression: SADS (no mean, no SD/no prespecified outcome of interest). Adverse effects: tardive diskinesia (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo injection. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, placebo injection. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, placebo injection. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 out of 30 participants (10%) left the study early which is an acceptable rate, irrespective of the statistical analysis (completer analysis). |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No evidence for other bias. |
Fluphenazine depot 1982.
| Study characteristics | ||
| Methods | Randomisation: participants were matched for age, sex, duration of illness, and severity of symptoms in the preceding episode and then assigned based on a randomised schedule. Allocation: procedure not described. Blinding: double‐blind, evaluating psychiatrist and participants were unaware of the contents of their injections. It seems that the treating psychiatrist was aware of the treatment. Duration: 12 months. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia (ICD‐9 and Present State Examination), with two or more episodes and several first rank symptoms in previous episode, free of psychopathology for at least 12 months, on fluphenazine decanoate for at least 2 years. N = 70. Gender: n.i.. Age: n.i.. History: duration stable‐ at least 12 months free of psychopathology, duration ill‐ n.i., number of previous hospitalisations‐ n.i., but at least two previous episodes, age at onset‐ n.i., severity of illness‐ BPRS < 10 in all participants, baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: fluphenazine decanoate. Fixed dose of 50 mg IM four/eight weekly. N = 35. 2. Placebo: vitamin B complex IM. Duration of taper: 0 days. N = 35. Rescue medication: nitrazepam for sleep and benzhexol for extrapyramidal side‐effects; additional antipsychotic drugs were not allowed. |
|
| Outcomes |
Examined Relapse (re‐emergence of definite schizophrenic psychopathology necessitating hospital admission or other major treatment change). Leaving the study early. Death. Adverse effects: tardive dyskinesia (Aquired Involuntary Movements Scale). Unable to use/Not included Mental state: BPRS (no mean, no SD/no predefined outcome of interest). Adverse effects: extrapyramidal symptoms ‐ use of antiparkinson medication (combined with nitrazepam), use of additional nitrazepam for sleep (combined with use of antiparkinson medication). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were matched for age, sex, duration of illness, and severity of symptoms in the preceding episode and then assigned based on a randomised schedule. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Double‐blind, evaluating psychiatrist and participants were unaware of the contents of their injections. It seems that treating psychiatrist was aware of the treatment. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, evaluating psychiatrist and participants were unaware of the contents of their injections. It seems that treating psychiatrist was aware of the treatment. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, evaluating psychiatrist and participants were unaware of the contents of their injections. It seems that treating psychiatrist was aware of the treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout rate drug 40% versus placebo 66%, most due to relapse. This poses a risk for bias for other outcomes. Completer analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear evidence for other sources of bias. |
Fluphenazine depot 1992.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, placebo was sesame oil of identical volume and identical in physical appearance. Duration: 12 months. Design: parallel. Location: single‐centre. Setting: inpatient, sponsored. | |
| Participants | Diagnosis: chronic schizophrenia (Research Diagnostic Criteria), stable for at least 5 years (absence of clinical deterioration and/or an increase of neuroleptic medication, retrospectively and in addition prospectively for at least 12 months), all on fluphenazine decanoate. N = 24. Gender: n.i.. Age: mean 57.3 years. History: duration stable‐ retrospectively at least 5 years, prospectively for 12 months, mean 7 years, duration ill‐ mean 33.1 years, number of previous hospitalisations‐ n.i., but mean duration of hospitalisation 24.9 years (unclear whether current or life‐time total), age at onset‐ mean 24.3 years, severity of illness‐ mean BPRS total score 24.9, baseline antipsychotic dose‐ mean 41.9 mg fluphenazine/4 weeks, remission at baseline: yes (study defined). |
|
| Interventions | 1. Drug: fluphenazine decanoate.Fixed dose: mean 50.4 mg/4 weeks. N = 12. 2. Placebo: duration of taper: 0 days, but all participants were on depot medication before the study. N = 12. Rescue medication: n.i., but probably not allowed. |
|
| Outcomes |
Examined Relapse: at least 25% increase of BPRS total score and judgement of by nurse according to Psychotic Inpatient Profile. Unable to us /Not included Mental state: BPRS total, Psychotic Inpatient Profile (for both scales means for subgroups only / no predefined outcome of interest). Physiological measures: prolactin levels (no SD’s/no predefined outcome of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo was sesame oil of identical volume and identical in physical appearance, |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, placebo was sesame oil of identical volume and identical in physical appearance. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, placebo was sesame oil of identical volume and identical in physical appearance. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is no statement on participants leaving the study early. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | There was a baseline imbalance in terms of gender and in terms of baseline fluphenazine dose. |
Haloperidol 1973.
| Study characteristics | ||
| Methods | Randomisation: unclear, randomisation assumed due to double‐blinding. Allocation: procedure not described. Blinding: double‐blind, all participants received both (placebo) tablets and (placebo) liquid, no further details. Duration: 90 days. Design: parallel. Location: single‐centre. Setting: inpatient. | |
| Participants | Diagnosis: chronic schizophrenia (clinical diagnosis), all had previously responded to haloperidol and were adequately maintained on it. N = 49. Gender: 24 men, 20 women. Age: mean 42.5 years. History: duration stable‐ all stabilised for 30 days on haloperidol concentrate, duration ill‐ n.i., but mean duration of hospitalisation 13.7 years, number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ mean BPRS 46.6 (16 items scale, rating system unclear), mean Clinical Global Impression of severity 4.9, baseline antipsychotic dose‐ mean 9.3 mg haloperidol/day. |
|
| Interventions | 1. Drug: haloperidol tablets.* Flexible dose. Allowed dose range: n.i.. Mean dose: mean 8.8mg/day. N = 17. 2. Drug: haloperidol liquid.* Flexible dose. Allowed dose range: n.i.. Mean dose: 10.4 mg/day. N = 16. 3. Placebo: duration of taper: 0 days. N = 16. Rescue medication: antiparkinson medication was allowed. |
|
| Outcomes |
Examined Relapse: deterioration of global state. Leaving the study early. Global state ‐ number of participants improved. Adverse effects (movement disorders). Suicide ideation. Unable to use/Not included Mental state: Brief Psychiatric Rating Scale (no SD/no predefined outcome of interest). Global state: Clinical Global Impression of Severity (no SD/no predefined outcome of interest). Behaviour: Nurses Observation Scale for Inpatient Evalutation (NOSIE) (no SD/no predefined outcome of interest). Adverse effects: laboratory (insufficient data/no predefined outcome of interest), vital signs (insufficient data/no predefined outcome of interest). |
|
| Notes | *Groups 1 and 2 were pooled for the purpose of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear, randomisation assumed due to double‐blinding. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, all participants received both (placebo) tablets and (placebo) liquid, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, all participants received both (placebo) tablets and (placebo) liquid, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, all participants received both (placebo) tablets and (placebo) liquid, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Acceptable dropout rate (10%), which should not affect other outcomes (completer analysis). |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear other bias. |
Haloperidol 1991.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, "participants and investigators were blind to treatment”, no further details. Duration: 6 months. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia (DSM‐III), not dangerous to themselves, no hospitalisation in the last year. N = 23. Gender: 23 men. Age: > 50 years, mean 60.1 years. History: duration stable‐ at least 1 month, last hospitalisation an average of 12.8 years ago, duration ill‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ mean BPRS psychosis subscale 6.2, baseline antipsychotic dose‐ 325 chlorpromazine equivalents (according to Davis’s equivalents). |
|
| Interventions | Before randomisation all participants were put on haloperidol for one month or until they were considered stable. 1. Drug: haloperidol. Fixed dose (dose before randomisation was maintained). Mean dose: n.i.. N = 11. 2. Placebo: duration of taper: 14 days. N = 12. Rescue medication: n.i., but probably not allowed. |
|
| Outcomes |
Examined Relapse: significant clinical design defined by either reoccurrence of symptoms or worsening of existing symptoms or prodromals signs such as sleep problems or anxiety. Leaving the study early. Service use: number of participants hospitalised. Death. Unable to use/Not included Mental state: BPRS (no data for each group/no predefined outcome of interest). Quality of life: Heinrich Scale (no data for each group). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, "participants and investigators were blind to treatment", no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, "participants and investigators were blind to treatment”, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, "participants and investigators were blind to treatment”, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 47% of the participants left the study early, most of them due to a relapse (55%). This attrition can be a source of bias for other outcomes than relapse. |
| Selective reporting (reporting bias) | High risk | Data on quality of life were not reported. |
| Other bias | Low risk | No clear evidence for other bias. |
Haloperidol depot 1982.
| Study characteristics | ||
| Methods | Randomisation: randomly assigned according to pre‐established randomisation code. Allocation: randomisation code was unknown to the evaluating investigators. Blinding: double‐blind, administered by a particular nurse. Duration: 16 weeks. Design: parallel. Location: single‐centre. Setting: inpatient. | |
| Participants | Diagnosis: chronic schizophrenia (Feighner’s criteria), treated with antipsychotic drugs for at least 2 years and currently under control. N = 32. Gender: 9 men, 23 women. Age: mean 46.5 years. History: duration stable‐ n.i., but treated with antipsychotic drugs for at least 2 years and currently under control, duration ill‐ mean 24.4 years, number of previous hospitalisations‐ n.i., but mean duration of current hospitalisation 9.6 years, age at onset‐ mean 22.1 years, severity of illness‐ n.i., baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: haloperidol decanoate. Flexible dose. Allowed dose range: starting dose 1.5 mL (= 150 mg) four‐weekly, maximum 3 mL (= 300 mg) four‐weekly. Median dose 1.5 mL four‐weekly. N = 16. 2. Placebo: duration of taper: 0 days. N = 16. Rescue medication: antiparkinson medication, oral haloperidol, but this was considered to be a relapse. |
|
| Outcomes |
Examined Relapse: addition of oral haloperidol. Leaving the study early. Global state: number of participants improved. Unable to use/Not included Mental state: Brief Psychiatric Rating Scale (no SD/no predefined outcome of interest). Behaviour: NOSIE (no SD/no predefined outcome of interest). Adverse effects (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned according to pre‐established randomisation code. |
| Allocation concealment (selection bias) | Low risk | Randomisation code was unknown to the evaluating investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, administered by a particular nurse. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, administered by a particular nurse. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, administered by a particular nurse. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 out of 16 participants in the placebo group compared to 0 out of 16 in the haloperidol group were withdrawn from the trial due to inefficacy of treatment. As the only outcomes were relapse, number of participants improved and leaving the study early this should not have been a problem. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No other bias. |
Haloperidol depot 1991.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, placebo injections, no further details. Duration: 48 weeks. Design: parallel. Location: single‐centre. Setting: in‐ and outpatients. | |
| Participants | Diagnosis: schizophrenia (Research Diagnostic Criteria), requiring neuroleptic maintenance treatment to prevent relapse. N = 43. Gender: n.i.. Age: mean 51.7 (range 25 to 65) years. History: duration stable‐ remained in the study after 15 weeks of haloperidol decanoate, duration ill‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ 60 mg haloperidol decanoate per month (~3.5 mg/day haloperidol). |
|
| Interventions | 1. Drug: haloperidol decanoate 60 mg/4 weeks. Fixed dose. N = 20. 2. Placebo: duration of taper: 0 days, but all on depot medication before study. N = 23. Rescue medication: anticholinergics and sedation. |
|
| Outcomes |
Examined Relapse: clinical judgement. Leaving the study early. Unable to use/Not included Mental state: Comprehensive Psychopathological Rating Scale (no mean, no SD/no prespecified outcome of interest). Adverse effects: extrapyramidal side‐effects, tardive dyskinesia (no mean, no SD/continuous side‐effect results were not among the prespecified outcomes). Physiological measures: laboratory (prolactin and haloperidol levels, no mean/SD/no prespecified outcomes of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo injections, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, placebo injections, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, placebo injections, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A considerable number of participants (42%) left the study early. The number was clearly higher in the placebo group and the reasons differed. Data were analysed on an ITT basis. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | No clear other bias. |
Iloperidone 2016.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated random sequence (1:1 ratio). Allocation: centralised, interactive voice response system. Blinding: double‐blind, identical appearing capsules. Duration: terminated early after 68 relapse events, patients were followed up for up to 26 weeks. Design: parallel. Location: multi‐centre. Setting: outpatients. | |
| Participants | Diagnosis: Schizophrenia (DSM‐IV), not hospitalised ar the time of screening, then stabilised on iloperidone for at least 12 weeks before the relapse‐prevention phase. N = 303. Gender: 178 men, 125 women. Age: 38.3 years. History: duration stable‐ clinically stable for at least 12 weeks, no change in treatment for at least 4 weeks, duration ill‐ 12.4 years, mean duration of hospitalisation‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ 25.9 years, severity of illness‐ mean PANSS total score 55,4, mean CGI‐S total score 3,3, baseline antipsychotic dose‐ n.i., remission at baseline‐ n.i.. | |
| Interventions | 1. Drug: Iloperidone. N = 153 Flexible dose. Mean dose: 15 mg/day. Allowed dose range: 8 mg/day to 24 mg/day. 2. Placebo: duration of taper: n.i. N = 150. Rescue medication: anticholinergics (antiparkinson medication), lorazepam (agitation, severe restlessness, insomnia), zolpidem (insomnia). |
|
| Outcomes |
Examined Relapse (rating scale based and/or clinical judgement and/or need for hospitalisation or increase in the level of psychiatric care). Leaving the study early ‐ due to adverse events. Social functioning: Sheehan Disability Scale. Adverse effects Death Unable to use/Not included Leaving the study early ‐ due to any cause/inefficacy (no usable data, no crude numbers for relapse are available, subjects withdrawn due to early termination counted as dropouts). Global state‐ Number of participants improved (no usable data) Global state ‐ Number of participants in remission (no usable data, CGI‐I not reported). Mental state: PANSS change in total score, BPRS change in total score (no predefined outcomes of interest) |
|
| Notes | Sponsored by Vanda Pharma. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Centralised, interactive voice response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical appearing capsules |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical appearing capsules |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical appearing capsules |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall attrition rate (57%) was high, and more participants in the placebo group left the study early due to relapse. This difference may have biased the results of outcomes other than leaving the study early and relapse, which was assessed using the Kaplan‐Meier survival curve analysis. Data for secondary outcomes were analysed on an ITT basis (not full ITT, because only participants who received at least one dose of study medication were included) . |
| Selective reporting (reporting bias) | Unclear risk | Some secondary efficacy outcomes are reported incompletely so that they cannot be entered in the meta‐analysis (e.g. CGI‐I scores). |
| Other bias | High risk | Terminated early, but it was pre‐planned. |
Lurasidone 2016.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated randomisation scheme (1:1 ratio). Allocation: interactive voice/web response system. Blinding: double‐blind, identically‐matched placebo. Duration: 28 weeks. Design: parallel. Location: multi‐centre. Setting: n.i. | |
| Participants | Diagnosis: Schizophrenia (DSM‐IV‐TR), stabilised on study drug (after experiencing an acute exacerbation) for at least 12 weeks before randomisation. 87% paranoid type, 8% undifferentiated type, 4.9% disorganised type. N = 285. Gender: 178 men, 107 women. Age: 42,7 years. History: duration stable‐ at least 12 weeks, duration ill‐ 17,1 years, number of previous hospitalisations‐ 74% of the participants had at least 1 prior hospitalisation, 50% had four or more prior hospitalisations for schizophrenia, number of psychotic exacerbations in the previous 2 years‐ 1.8, age at onset‐ 23.7 years, severity of illness‐ mean PANSS total score 54.4, mean CGI‐S total score 2.72, baseline antipsychotic dose‐, remission at baseline‐ n.i.. | |
| Interventions | 1. Drug: Lurasidone. N = 144 Flexible dose. Mean dose: 78.9 mg/day. Allowed dose range: 40 mg/day to 80 mg/day. 2. Placebo: duration of taper: no taper. N = 141. Rescue medication: anticholinergics (antiparkinson), benzodiazepines (insomnia, anxiety/agitation ‐ with restrictions). Limited use of psychotropic medications (including antipsychotics other than lurasidone) immediately prior to study discontinuation was permitted. |
|
| Outcomes |
Examined Relapse (rating scale based and/or clinical judgement and/or need for hospitalisation or increase in the level of psychiatric care). Leaving the study early (any cause,adverse events, inefficacy). Service use ‐ Number of participants hospitalised. Participants´satisfaction with care: Health Economics Exit Questionnaire. Quality of life: EuroQol 5 Dimensions, Visual Analog Scale (EQ‐VAS). Social functioning: Specific Levels of Functioning, modified. Adverse effects. Death. Suicidal ideation and behaviour: Columbia Suicide Severity Rating Scale. Unable to use/Not included Global state: CGI‐S change scores (no usable data). Mental state: Positive and Negative Syndrome Scale total score and subscores (no predefined outcomes of interest). Depressive symptoms: Montgomery Asberg Depression Rating Scale (no predefined outcome of interest). Compliance to treatment: Brief Adherence Rating Scale (no predefined outcome of interest). Service use: Health Services Utilization Questionnaire (no usable data). Quality of life: Short Form‐12v2 Health Survey (SF‐12) (no usable data, available only for the Physical Component score, subscore of the scale. Chose the EuroQol data over these data) Smoking attitude (no predefined outcome of interest). |
|
| Notes | Sponsored by Sunovion, Takeda. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence generation, |
| Allocation concealment (selection bias) | Low risk | Interactive Voice/Web Response System. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identically‐matched placebo. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identically‐matched placebo. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identically‐matched placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The overall attrition rate (54%) was high, substantially similar between the two groups. Only slightly more participants in the placebo group left the study early due to inefficacy/relapse. The primary efficacy outcome (relapse) was assessed using the Kaplan‐Meier survival curve analysis. Data for secondary outcomes were all analysed on an ITT basis (MMRM). |
| Selective reporting (reporting bias) | Unclear risk | Many secondary efficacy outcomes (but not the primary outcome) are reported incompletely so that they could not be entered in a meta‐analysis. |
| Other bias | Unclear risk | Following the end of the subject’s participation in the study, the PI or an authorized delegate had to report SAEs. |
Olanzapine 1999.
| Study characteristics | ||
| Methods | Randomisation: randomly assigned (1:1), no further details. Allocation: procedure not described. Blinding: double‐blind, no further details. Duration: 3 to 5 days. Design: parallel (optional crossover treatment for relapsed patients). Location: multi‐centre. Setting: inpatients and outpatients (numbers not available). | |
| Participants | Diagnosis: schizophrenia (DSM‐IV), received clozapine for a minimum of 4 weeks before entering the study, had to undergo an elective discontinuation of clozapine (49% due to patient inconvenience, 37% due to adverse events, 13% due to partial response). N = 106. Gender: 75 men, 31 women. Age: mean 38.8 years History: duration stable‐ received clozapine for at least 4 weeks before study entrance, duration ill‐ n.i., mean duration of antipsychotic treatment‐ range from 4 weeks to >1 year (4 weeks to 6 months: N = 39, 6months‐1year: N = 18, >1year: N = 59), number of previous hospitalizations‐ n.i., age at onset‐ n.i., severity of illness‐ mean PANSS total score 64.5 points, baseline antipsychotic dose‐ clozapine 324 mg/day (gradual tapering from baseline dose to 300 mg/day in 2 to 12 days). |
|
| Interventions | 1. Drug: olanzapine. Fixed dose, 10 mg/day. N = 53. 2. Placebo: inert placebo. duration of taper 2 to 12 days. N = 53. Rescue medication: only benzodiazepines (for agitation) and anticholinergic (for evident EPS). Patients who relapsed could be removed from the double‐blind treatment and enter and optional open‐label cross‐over treatment (clozapine+olanzapine). |
|
| Outcomes |
Examined Relapse: worsening on at least one of the following COSTART events: schizophrenic reaction, hallucinations, delusions, thinking abnormal. Leaving the study early (any reason, inefficacy) Unable to use/Not included Global state: CGI‐S mean change (no usable data). Mental state: PANSS (no predefined outcome of interest). Depressive symptoms: Montgomery Asberg Depression Rating Scale (no predefined outcome of interest). Performance tests: MiniMental State Examination (no predefined outcome of interest). Adverse events: no usable data. Suicidality: no usable data. |
|
| Notes | Not explicitly stated, probably sponsored (EliLilly) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned (1:1), no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The overall attrition rate of 10% was acceptable, but more participants in the placebo group than in the olanzapine group left the study early due to inefficacy. This may be a source of bias for outcomes other than relapse and leaving the study early, but data on such other outcomes are not available. Analyses were all done on an ITT basis. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Unclear risk | Very short follow‐up (3 to 5 days, difficult to discriminate between withdrawal symptoms and illness recurrence); 13% of the randomised patients had partial response to clozapine. |
Olanzapine 2003.
| Study characteristics | ||
| Methods | Randomisation: randomised, 2:1 ratio, by an interactive voice response system. Allocation: interactive voice response system. Blinding: double‐blind, no further details. Duration: one year, but the study was terminated early. Maximum length was 30 weeks. Design: parallel. Location: multi‐centre. Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia (n = 266) or schizoaffective disorder (n = 60, DSM‐IV). BPRS total score < 36, positive symptoms at most mild, Global Assessment of Functioning at least 40, currently on maintenance antipsychotic medication. N = 326. Gender: 173 men, 153 women. Age: mean 35.9 years. History: duration stable‐ 8 weeks, duration ill‐ mean 11.1 years, number of previous hospitalisations‐ n.i., age at onset‐ mean 24.7 years, severity of illness‐ mean PANSS total score at baseline 43, baseline antipsychotic dose‐ mean 13.4 mg olanzapine/day. |
|
| Interventions | Participants were first converted to olanzapine and then stabilised for 8 weeks before randomisation. 1. Drug: olanzapine ‐ Fixed dose of either 10, 15 mg/day or 20 mg/day. Mean dose 13.4 mg/day. N = 224. 2. Placebo: duration of taper: 0 days. N = 102. Rescue medication: a one‐time increase of the same medication (olanzapine or placebo) was allowed. Furthermore, antiparkinson medication and benzodiazepines were allowed. |
|
| Outcomes |
Examined Relapse: any BPRS positive item > 4, absolute increase of a positive item or of the positive subscore, hospitalisation due to positive symptoms, suicide or suicide attempt. Leaving the study early. Adverse effects. Death, Suicide attempts. Violent/aggressive behaviour Quality of life: Heinrich Carpenter Quality of Life Scale (QLS). Service use ‐ number of participants hospitalised. Unable to use/Not included Mental state: PANSS (no prespecified outcome of interest). Adverse effects: adverse effects with an incidence < 10% (no data), laboratory, EPS‐scales (in part no data/no prespecified outcome of interest), EPS‐scales (no SD/continuous side‐effect results were not among the prespecified outcomes). Physiological measures: vital signs (no prespecified outcome of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, 2:1 ratio, by an interactive voice response system. |
| Allocation concealment (selection bias) | Low risk | Interactive voice response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall attrition of 26% was acceptable, but many more participants in the placebo group than in the olanzapine group left the study early. Kaplan‐Meier survival analysis was used for the analysis of relapse, ANOVA based on LOCF was used for continuous outcomes. |
| Selective reporting (reporting bias) | High risk | Only those adverse events with a frequency of at least 10% were reported. Use of antiparkinson medication has not been reported. |
| Other bias | High risk | The study was terminated early when there was a sufficient difference, but this was preplanned. |
Paliperidone 2007.
| Study characteristics | ||
| Methods | Randomisation: randomised, computerised randomisation and stratification scheme. Allocation: interactive voice‐response system. Blinding: double‐blind, no further details. Duration: variable. Design: parallel. Location: multi‐centre. Setting: outpatient, sponsored. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV), 80% paranoid subtype, 14% undifferentiated subtype, initially with acute exacerbation, then 8 weeks run in and 6 weeks stabilisation phase. N = 207. Gender: 121 men, 86 women. Age: 38.3 years. History: duration stable‐ at least 8 weeks, duration ill‐ mean 12.1 years, number of previous hospitalisations‐ median 3, age at onset‐ 26.2 years, severity of illness‐ mean PANSS total score 52.2, mean CGI severity 2.6, baseline antipsychotic dose‐ 10.8 mg/day paliperidone. |
|
| Interventions | 1. Drug: paliperidone‐ Flexible doses. Allowed dose range: 3 mg/day to 15 mg/day Mean dose: 10.8 mg/day. N = 105. 2. Placebo: duration of taper: 0 days. N = 102. Rescue medication: benzodiazepines, antiparkinson medication, propanolol, antidepressants when the dose was stable for at least 3 months before the study. |
|
| Outcomes |
Examined Relapse: (a) psychiatric hospitalisation (involuntary or voluntary admission); b) increase in PANSS total score by 25% for 2 consecutive days for patients who scored more than 40 at randomisation or a 10‐point increase for patients who scored 40 or below at randomisation; c) increase in the Clinical Global Impression‐Severity (CGI‐S) score to at least 4, for patients who scored 3 or below at randomisation, or to at least 5, for patients whose CGI‐S scores were 4 at randomisation, for 2 consecutive days; d) deliberate self‐injury or aggressive behavior, or suicidal or homicidal ideation and aggressive behavior that was clinically significant; e) increase in prespecified individual PANSS item scores to at least 5, for patients whose scores were 3 or below at randomisation, or to at least 6, for patients whose scores were 4 at randomisation, for 2 consecutive days). Leaving the study early. Global state ‐ number of participants improved. Global state ‐ number of participants in remission (CGI‐based). Service use: number of participants hospitalised. Quality of life: Schizophrenia Quality‐of‐Life Scale (SQLS) Social functioning: Personal and Social Performance scale. Adverse effects. Violent/aggressive behaviour. Death; Suicide attempts. Unable to use/Not included Mental state: PANSS (no predefined outcome of interest). Physiological measures: laboratory (except for metabolic problems no data), vital signs, ECG, prolactin (all no data/no predefined outcomes of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, computerised randomisation and stratification scheme. |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 28 out of 207 participants left the study prematurely for another reason than relapse. Therefore, missing outcomes may not pose a problem for the primary outcome which was assessed with the Kaplan‐Meier method. Nevertheless, high discontinuations due to relapse (75/207) which were much more frequent in the placebo group than in the drug group pose a major problem for secondary outcomes. No full ITT (participants had to receive at least one dose post‐baseline) but only two participants were excluded on this basis. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | Study was terminated after an interim analysis showed a clear advantage of paliperidone. |
Paliperidone 2014.
| Study characteristics | ||
| Methods | Randomisation: computer‐based randomisation (1:1 ratio). Allocation: interactive voice/web response system (online IWRS/IVRS) Blinding: double‐blind, matching placebo. Duration: terminated early after an interim analysis. Mean duration of exposure in the double‐blind phase: 10 weeks; the longest patient remained in the study for 54 weeks. Design: parallel. Location: multi‐centre Setting: outpatients. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV‐TR), stabilised on paliperidone before double‐blind phase. N = 136. Gender: 55 men, 80 women. Age: 31,7 years. History: duration stable‐ at least 6 weeks, duration ill‐ at least 1 year, mean duration of hospitalisation‐ n.i., number of previous hospitalisations‐ 48% were previously hospitalised, age at onset‐ n.i., severity of illness‐ mean PANSS total score 52,4, mean CGI‐S total score 2,9, baseline antipsychotic dose‐ n.i., remission at baseline‐ 76,5% were in remission at baseline (CGI‐S based). | |
| Interventions | .1. Drug: Paliperidone ER N = 65 Fixed dose. Mean dose: 9.5 mg/day. Allowed dose range: 3 mg/day to 12 mg/day. 2. Placebo: duration of taper: n.i. N = 71. Rescue medication: benzodiazepines (anxiety, agitation), antiparkinson medication (anticholinergics). |
|
| Outcomes |
Examined Relapse (rating scale based and/or clinical judgement and/or need for hospitalisation or increase in the level of psychiatric care) Leaving the study early (any reason, adverse events, inefficacy). Global state ‐ number of participants in remission (CGI‐S defined). Service use ‐ number of patients hospitalised Social functioning: Personal and Social Performance scale. Adverse effects Death Suicidal ideation and behavior: Columbia Suicide Severity Rating Scale. Violent/aggressive behaviour Unable to use/Not included Global state ‐ number of participants improved (no usable data). Mental state: PANSS total score and subscores (no predefined outcomes of interest) Depressive symptoms: PANSS Marder Anxiety/Depression (no predefined outcome of interest). Subjective sleep measures: Sleep Visual Analog scale (no predefined outcome of interest) |
|
| Notes | Sponsored by Janssen Research & Development, LLC. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation. |
| Allocation concealment (selection bias) | Low risk | Interactive web/voice response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, matching placebo. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, matching placebo. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, matching placebo. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall attrition rate of 67% was high, and more participants in the placebo group left the study early, mostly due to relapse. This difference may have biased the results of outcomes other than leaving the study early and relapse, which was assessed using the Kaplan‐Meier survival curve analysis. Data for secondary outcomes were analysed on an ITT basis. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | Terminated early after an interim analysis, but it was pre‐planned. |
Paliperidone depot1M 2010.
| Study characteristics | ||
| Methods | Randomisation: patients were randomised in a 1 to 1 ratio (via a sponsor prepared, computer generated randomisation scheme, assigned by an interactive voice system). Allocation: interactive voice system. Blinding: double‐blind, no further details. Duration: variable (the trial was terminated early after an interim analysis). Design: parallel. Location: multi‐centre. Setting: n.i.. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV‐TR). N = 410. Gender: 220 men, 88 women. Age: mean 39 years. History: duration stable‐ 12 weeks prospectively stable on fixed dose paliperidone, duration ill‐ mean 12 years, number of previous hospitalisations‐ median 2.6, age at onset‐ mean 27.3 years, severity of illness‐ PANSS total mean 53 points, baseline antipsychotic dose‐ n.i., remission at baseline: 36% of the patients met remission criteria. |
|
| Interventions | 1. Drug: paliperidone palmitate depot ‐ Fixed dose: originally 25 mg, 50 mg or 100 mg/4 weeks; this dose was maintained. Mean dose: n.i.. N = 206. 2. Placebo: duration of taper: 0 days. N = 204. Rescue medication: n.i.. |
|
| Outcomes |
Examined Relapse: psychiatric rehospitalisation, deliberate self‐injury or violent behaviour, suicidal or homicidal ideation, certain predefined PANSS score. Leaving the study early. Rehospitalisation. Global state ‐ number of participants in sustained remission (Andreasen criteria). Quality of life: Schizophrenia Quality of Life Scale (SQLS) Social functioning: Personal and Social Performance scale (PSP). Suicidal ideation and attempts. Violent/aggressive behaviour. Death. Adverse effects. Unable to use/Not included Mental state: PANSS (no predefined outcome of interest). Prolactin levels (no predefined outcome of interest). |
|
| Notes | The study was stopped early after a significant interim analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised in a 1 to 1 ratio (via a sponsor prepared, computer‐generated randomisation scheme, assigned by an interactive voice system). |
| Allocation concealment (selection bias) | Low risk | Interactive voice system. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall high dropout rate (45%). Clearly more participants in the placebo group (95) than in the drug group (31) left the study early due to relapse. This imbalance may have biased the results of other outcomes such as adverse events. Kaplan‐Meier survival curve analysis was used for the primary outcome relapse. |
| Selective reporting (reporting bias) | Low risk | Those adverse events that occurred in at least 2% of the participants and severe adverse events were presented. We feel that is acceptable. |
| Other bias | High risk | Study was stopped early after an interim analysis. |
Paliperidone depot1M 2015.
| Study characteristics | ||
| Methods | Randomisation: sponsor‐prepared computer‐generated randomisation scheme (stratified by absence or presence of mood stabilisers or antidepressants and study centre). Allocation: central, interactive voice/web response system. Blinding: double‐blind, matching placebo injections, administered by a person distinct from other study personnel. Duration: 65 weeks. Design: parallel. Location: multi‐centre. Setting: inpatients and outpatients (only outpatients during double‐blind phase). | |
| Participants | Diagnosis: schizoaffective disorder (Structured Clinical Interview for DSM‐IV Axis I Disorders). N = 334. Gender: 169 men, 165 women. Age: 38.6 years. History: duration stable‐ 12 weeks (scale defined, duration of the stabilisation phase, with paliperidone palmitate as monotherapy or adjunctive therapy), duration ill‐ 12.2 years, mean duration of hospitalisation‐ n.i, number of previous hospitalisations‐ mean 3.9, age at onset‐ 30,9 years, severity of illness‐ mean PANSS total score 51.5, mean CGI‐S total score 2.4, baseline antipsychotic dose‐ n.i, remission at baseline‐ yes (97% according to the CGI‐S‐SCA rating system) | |
| Interventions | 1. Drug: Paliperidone palmitate depot (1‐month formulation). N = 164 Fixed dose. Mean dose: 114.3 mg eq/month. Allowed dose range: 50 mg to 150 mg eq/month. 2. Placebo: duration of taper: depending on the long elimination half‐life of paliperidone depot. N = 170 Rescue medication: anticholinergics (antiparkinson medication); benzodiazepines not allowed within 8 hours prior to efficacy assessments, further antipsychotics or initiation of antidepressants or mood stabilizers were not allowed. |
|
| Outcomes |
Examined Relapse (rating scale based and/or clinical judgement and/or need for hospitalisation or increase in the level of psychiatric care) Leaving the study early (any reason, inefficacy, adverse events). Global state ‐ number of participants improved (CGI‐S‐SCA defined, as reported by the authors) Global state ‐ number of participants in sustained remission (at least 'mildly ill' at CGI‐S‐SCA evaluation). Patient´s satisfaction with care: Medication Satisfaction Questionnaire. Service use ‐ number of participants hospitalised: Resource Utilization Questionnaire Social functioning: Personal and Social Performance Scale. Number of participants employed Adverse effects (clinical judgement and rating scale based) Suicidal ideation: Columbia‐ Suicide Severity Rating Scale. Violent/aggressive behaviour Death Unable to use/Not included Mental state:PANSS total score and subscores (no predefined outcomes of interest). Affective symptoms: Hamilton Depression Rating Scale, Young Mania Rating Scale (no predefined outcomes of interest). |
|
| Notes | Sponsored by Janssen Research & Development, LLC. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sponsor‐prepared computer‐generated stratified randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Central, interactive voice/web response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, matching placebo injections, administered by a person distinct from other study personnel |
| Blinding (performance bias and detection bias) Subjective outcomes | Low risk | Double‐blind, matching placebo injections, administered by a person distinct from other study personnel |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, matching placebo injections, administered by a person distinct from other study personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The total attrition rate was high (51%), and differed substantially among study arms (drug arm: 39% versus placebo arm: 61%); almost double participants in the placebo group left the study early due to relapse. This may be a source of bias for outcomes other than dropouts and relapse, which was assessed using the Kaplan Mayer survival´s curve analysis. Data for secondary outcomes were analysed on an ITT basis (LOCF method). |
| Selective reporting (reporting bias) | Low risk | No clear evidence for selective reporting. |
| Other bias | Low risk | No clear evidence for bias. |
Paliperidone depot3M 2015.
| Study characteristics | ||
| Methods | Randomisation: sponsor‐prepared computer‐generated randomisation scheme, balanced using permuted blocks across the treatment groups and stratified by study centre to ensure balance of treatment allocation within a centre. Allocation: interactive voice/web response system. Blinding: double‐blind, identical appearing capsules, administered by a person distinct from other study personnel. Duration: open‐ended after 66 weeks (longest patient), variable duration of the double‐blind phase (median: 158 days). Design: parallel. Location: multi‐centre (64 centres in 8 countries). Setting: outpatients. | |
| Participants | Diagnosis: Schizophrenia (DSM‐IV‐TR), symptomatically stable when enrolled, then stabilised on paliperidone LAI for at least 12 weeks before randomisation. N = 305. Gender: 228 men, 77 women. Age: 37.8 years. History: duration stable‐ at least 12 weeks (duration of the open‐label maintenance phase), duration ill‐ 10.8 years, mean duration of hospitalisation‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ 26.9 years, severity of illness‐ mean PANSS total score 54.5, mean CGI‐S total score 2.7, baseline antipsychotic dose‐ paliperidone palmitate 3 months formulation 210 mg eq/3 months, remission at baseline‐ 53% of the participants were remitters at baseline. | |
| Interventions | 1. Drug: Paliperidone palmitate depot (3‐month formulation). N = 160 Fixed dose. Mean dose: 402 mg eq/3 months. Allowed dose range: 175 mg to 525 mg eq/3 months. 2. Placebo: duration of taper: depending on the elimination half‐life of paliperidone depot (between 84 and 139 days) N = 145. Rescue medication: anticholinergics (antiparkinson), beta‐blocker (akathisia), lorazepam (anxiety/agitation), zolpidem (sleep disturbances). |
|
| Outcomes |
Examined Relapse (rating scale based and/or clinical judgement and/or need for hospitalisation or increase in the level of psychiatric care) Leaving the study early (any reason, inefficacy, adverse events). Social functioning: Personal and Social Performance Scale. Adverse effects (clinical judgement and rating scale based). Violent/aggressive behaviour. Death. Suicide ideation: Columbia Suicide Severity Rating scale based. Unable to use/Not included Global state ‐ number of participants improved (no usable data). Global state ‐ number of participants in remission (no usable data, only available for a subgroup of patients in remission at baseline, reported as change in remitter status, PANSS defined). Mental state: PANSS total score and subscores (no predefined outcomes of interest). Depressive symptoms: PANSS Marder factors (no predefined outcome of interest). |
|
| Notes | Sponsored by Janssen Research & Development, LLC. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sponsor‐prepared computer‐generated randomisation scheme, balanced using permuted blocks across the treatment groups and stratified by study centre to ensure balance of treatment allocation within a centre. |
| Allocation concealment (selection bias) | Low risk | Interactive voice/web response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical appearing capsules, administered by a person distinct from other study personnel. |
| Blinding (performance bias and detection bias) Subjective outcomes | Low risk | Double‐blind, identical appearing capsules, administered by a person distinct from other study personnel. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical appearing capsules, administered by a person distinct from other study personnel. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall attrition rate of 30% could still be acceptable, though higher than 25%, but three times as many participants in the placebo group left the study early due to relapse. This difference may have biased the results of outcomes other than leaving the study early and relapse, which was assessed using the Kaplan‐Meier survival curve analysis. Data for secondary outcomes were analysed on an ITT basis. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. All the outcomes have been reported in the protocol‐specified way. |
| Other bias | High risk | Study terminated early after an interim analysis, but this was pre‐planned. |
Penfluridol 1970.
| Study characteristics | ||
| Methods | Randomisation: matched pairs were formed and then randomised, no further details. Allocation: procedure not described. Blinding: double‐blind, indistinguishable placebo. Duration: 10 weeks. Design: parallel. Location: single‐centre. Setting: hospital, sponsored. | |
| Participants | Diagnosis: chronic psychotic hospitalised patients mainly with schizophrenic and paranoid behaviour patterns, suspected of relapsing after withdrawal of medication (clinical diagnosis). N = 26. Gender: 26 men. Age: n.i. History: duration stable‐ 8 months pre‐treatment with penfluridol to find optimum dose, duration ill‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ n.i., but hospitalised, baseline antipsychotic dose‐ 23.4 mg/day. | |
| Interventions | 1. Drug: penfluridol once weekly ‐ Fixed dose, mean dose: n.i., range 10 mg to 40 mg/weekly. N = 13. 2. Placebo: duration of taper: 0 days. N = 13. Rescue medication: sedative neuroleptics allowed for 2 weeks, dexbenzitide. |
|
| Outcomes |
Examined Relapse: need of medication as decided by two psychiatrists. Leaving the study early Unable to use/Not included Global state ‐ number of participants improved (no usable data). Mental state: Psychiatric Evaluation Scale (no predefined outcome of interest). Adverse effects: movement disorders (Factor Construct Outcome Scale, no data for randomised phase/continuous side‐effect results were not among the prespecified outcomes of interest), neurologic effects (graphometric and tapping test, no data for randomised phase/no prespecified outcomes of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Matched pairs were formed and then randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, indistinguishable placebo. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, indistinguishable placebo. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, indistinguishable placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Apart from those participants who relapsed, no participant left the study early and relapse was the only outcome. |
| Selective reporting (reporting bias) | High risk | Adverse events were not reported for the double‐blind phase. |
| Other bias | Low risk | No obvious other bias. |
Penfluridol 1974a.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, no further details. Duration: 12 weeks. Design: parallel. Location: single‐centre. Setting: inpatient. | |
| Participants | Diagnosis: severely ill, chronically hospitalised people with schizophrenia (clinical diagnosis). N = 50. Gender: 25 men, 25 women. Age: medium 41.5 years. History: duration stable‐ 12 weeks stabilisation phase., but how long the participants were stable is unclear, duration ill‐ n.i., number of previous hospitalisations‐ n.i., but median duration of current hospitalisation 15.5 years, age at onset‐ n.i., severity of illness‐ all severely ill (Clinical Global Impression Score = 6), baseline antipsychotic dose‐ 100 mg to 160 mg/week penfluridol. |
|
| Interventions | 1. Drug: penfluridol once weekly. Fixed dose. Allowed dose range: 40 mg to 160 mg/week. Mean dose: n.i.. N = 25. 2. Placebo: duration of taper: 0 days. N = 25. Rescue medication: antiparkinson medication. |
|
| Outcomes |
Examined Relapse: worsening of global state. Leaving the study early. Global state: number of participants improved (CGI based). Adverse effects: extrapyramidal side effects. Unable to use/Not included Mental state: BPRS (no mean, no SD/no prespecified outcome of interest). Behaviour: NOSIE (no mean, no SD/no prespecified outcome of interest). Physiological measures: laboratory, ECG, photosensitivity tests, ophthalmologic examinations, vital signs (no clear data/no prespecified outcomes of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It is not entirely clear, whether there were dropouts in addition to 18 participants (7 drug, 11 placebo, 36%) who left the study early due to relapse. However, the 36% dropout rate can be a problem for other outcomes. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No evidence for other bias. |
Penfluridol 1974b.
| Study characteristics | ||
| Methods | Randomisation: divided into two comparable groups by an unbiased statistician. Allocation: procedure not explained. Blinding: double‐blind, identical capsules. Duration: 6 months. Design: parallel. Location: single‐centre. Setting: inpatient, sponsored. | |
| Participants | Diagnosis: chronic psychotic inpatients (clinical diagnosis), 13 schizophrenia, 1 dementia, 1 paranoia. N = 15. Gender: 6 men, 9 women. Age: median 54 years. History: duration stable‐ n.i., duration ill‐ mean 17.7 years, number of previous hospitalisations‐ n.i., but mean duration of hospitalisation 11.3 years, age at onset‐ mean 36.3 years, severity of illness‐ n.i., baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: penfluridol. Fixed/flexible dose: unclear, but different doses according to pretrial medication. Allowed dose range: unclear, but all participants received 40 mg/week. Mean dose: 40 mg/week. N = 7. 2. Placebo: duration of taper: 0 days. N = 8. Rescue medication: Dexetimide was given prophylactically to prevent extrapyramidal side effects. |
|
| Outcomes |
Examined Relapse: need of additional antipsychotic medication. Leaving the study early. Unable to use/Not included Mental state: Zwanikken Scale (no mean, no SD / no predefined outcome of interest). Behaviour: Zwanikken Scale (no mean, no SD / no predefined outcome of interest). Adverse effects: interview (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Divided into two comparable groups by an unbiased statistician. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not explained. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant left the study early. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear other bias. |
Penfluridol 1974c.
| Study characteristics | ||
| Methods | Randomisation: randomised, no further details. Allocation: procedure not described. Blinding: double‐blind, identical capsules. Duration: 6 months Design: parallel. Location: single‐centre. Setting: inpatient. | |
| Participants | Diagnosis: chronic schizophrenia (DSM‐II), catatonic type (n = 2), residual type (n = 15), hebephrenic type (n = 1), simple type (n = 2), paranoid type (n = 1). N = 21. Gender: 21 women. Age: mean 58 years. History: duration stable‐ successfully maintained on penfluridol for at least 6 months, duration ill‐ mean 28.5 years, median duration of current hospitalisation 21 years, number of previous hospitalisations‐ n.i., age at onset‐ 29.5 years, severity of illness‐ n.i., baseline antipsychotic dose‐ mean 43 mg/week penfluridol. |
|
| Interventions | 1. Drug: penfluridol. Fixed dose, mean 43 mg/week. N = 10. 2. Placebo: duration of taper: 0 days. N = 11. Rescue medication: antiparkinson medication, haloperidol, but this was considered to be a sign of relapse. |
|
| Outcomes |
Examined Relapse: use of additional haloperidol. Leaving the study early. Unable to use/Not included Mental state: Zwanikken scale (no data/no predefined outcome of interest). Adverse effects: Zwanikken scale (no data / continuous side‐effect results were not among the predefined outcomes of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant left the study prematurely. |
| Selective reporting (reporting bias) | High risk | Data on side effects and the mental state were not reported. |
| Other bias | Low risk | No evidence for other bias. |
Penfluridol 1975.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, identical capsules. Duration: 12 weeks. Design: parallel. Location: single‐centre. Setting: inpatient. | |
| Participants | Diagnosis: chronic schizophrenia (clinical diagnosis). N = 35. Gender: 19 men, 16 women. Age: mean 43.9 years. History: duration stable‐ maintained on neuroleptic for at least 3 months, prospective 12 week stabilisation phase during which participants were switched to penfluridol, duration ill‐ n.i., number of previous hospitalisations‐ mean 1.34, age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ mean 64.1 mg/week penfluridol. |
|
| Interventions | 1. Drug: penfluridol ‐ Flexible dose. Allowed dose range: 20 mg to 120 mg/week. Mean dose:n.i.. N = 18. 2. Placebo: duration of taper: 0 days. N = 17. Rescue medication: antiparkinson medication, it seems that haloperidol was not allowed in the double‐blind phase. |
|
| Outcomes |
Examined Relapse: psychiatric decompensation that could not be controlled by dose increase. Leaving the study early. Adverse effects (at least one movement disorder). Unable to use/Not included Global state: Clinical Global Impression Scale (no usable data for remission). Mental state: BPRS (no numbers/no predefined outcomes of interest). Behaviour: NNOSIE (no numbers/no predefined outcomes of interest). Physiological measures: vital signs (weight, pulse, blood pressure, respiratory frequency, temperature ‐ no numbers/no predefined outcomes of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 of 35 participants left the study early (34%), 11 of them were in the placebo group. As all participants in the placebo group discontinued due to relapse, the primary outcome is not affected. But the results of all other outcomes are biased by this effect. |
| Selective reporting (reporting bias) | Low risk | Results on rating scales have not been reported, but these were not outcomes of interest in our review. |
| Other bias | Low risk | No clear other bias |
Penfluridol 1987.
| Study characteristics | ||
| Methods | Randomisation: n.i., but double‐blind study. Allocation: procedure not described. Blinding: double‐blind, identical capsules. Duration: 12 weeks. Design: parallel. Location: single‐centre. Setting: unclear. | |
| Participants | Diagnosis: chronic schizophrenia (DSM‐III), all on maintenance medication for control of continuous symptoms, all stable for at least 6 months. N = 30. Gender: 16 men, 12 women. Age: mean 36.0 years. History: duration stable‐ at least 6 months, duration ill‐ mean 11.1 years, number of previous hospitalisations‐ n.i., age at onset‐ 24.9 years, severity of illness‐ n.i., baseline antipsychotic dose‐ mean 297.5 mg/day chlorpromazine equivalent. |
|
| Interventions | 1. Drug: penfluridol. Fixed dose of 55 mg/week. N = 15. 2. Placebo: duration of taper: 0 days. N = 15. Rescue medication: antiparkinson medication and haloperidol, but this was considered to be a relapse. |
|
| Outcomes |
Examined Relapse (need of additional haloperidol medication). Leaving the study early. Unable to use/Not included Mental state (Scale for the Assessment of Positive Symptoms and Negative Symptoms ‐ no data /no predefined outcome of interest). Adverse effects: extrapyramidal side‐effects (Simpson Angus Scale (SAS) ‐ no data / continuous side‐effect results were not among the prespecified outcomes). Physiological measures: mean body weight, pulse rate, blood pressure, laboratory (all no data/no prespecified outcomes of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N.i., but double‐blind study. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double, identical capsules. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double, identical capsules. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double, identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only study completers were used in the final analysis, but as there were only two dropouts (one in each group) this was not necessarily a problem. |
| Selective reporting (reporting bias) | Low risk | Rating scale results were not reported, but these were not of interest for the review. |
| Other bias | Low risk | No clear evidence for other bias. |
Perphenazine 1963.
| Study characteristics | ||
| Methods | Randomisation: arbitrarily allocated. Allocation: procedure not described. Blinding: double‐blind (only the pharmacist knew which bottles were active. Participants were asked whether they were aware of the medication, but only one realised a change in taste. Nurses were also asked, but did not guess the right medication better than by chance alone. Duration: 10 weeks. Design: parallel. Location: two centres. Setting: inpatient. | |
| Participants | Diagnosis: chronic schizophrenia (clinical diagnosis by at least two psychiatrists), all with paranoid condition, two additionally catatonic tendencies and one hebephrenic features, six were leucotomised. N = 26. Gender: 26 men. Age: mean 50.7 years. History: duration stable‐ n.i., but all had been receiving maintenance doses of perphenazine for a mean of 16 months, duration ill‐ n.i., but mean duration of current hospitalisation 16.5 years, number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ two 12 mg three times, one 20 mg three times per day, all other 8 mg three times per day and most less. |
|
| Interventions | 1. Drug: perphenazine liquid. Fixed dose (same dose as before the start of the study) two 12 mg three times, one 20 mg three times, all other 8mg three times and most less. Mean dose: see above. N = 13. 2. Placebo. duration of taper: 0 days. N = 13. 3. No medication*. duration of taper: 0 days. N = 13. Rescue medication: not allowed apart from antiparkinson medication. |
|
| Outcomes |
Examined Relapse: "major relapse" = replaced on active medication. Violent/aggressive behaviour. Unable to use/Not included Mental state: self‐developed psychiatric rating scale ‐ unpublished scale (no predefined outcome of interest). Behaviour: Fergus Falls Behaviour Rating Scale (no predefined outcome of interest). |
|
| Notes | *This group was not used for the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Arbitrarily allocated. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind ‐ only the pharmacist knew which bottles were active. Participants were asked whether they were aware of the medication, but only one realised a change in taste. Nurses were also asked, but did not guess the correct medication better than by chance alone. |
| Blinding (performance bias and detection bias) Subjective outcomes | Low risk | Double‐blind ‐ only the pharmacist knew which bottles were active. Participants were asked whether they were aware of the medication, but only one realised a change in taste. Nurses were also asked, but did not guess the correct medication better than by chance alone. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind ‐ only the pharmacist knew which bottles were active. Participants were asked whether they were aware of the medication, but only one realised a change in taste. Nurses were also asked, but did not guess the correct medication better than by chance alone. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts were not reported. It is not clear, whether there really no dropouts. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear evidence for other bias. |
Pimozide 1971.
| Study characteristics | ||
| Methods | Randomisation: randomised, no further details. Allocation: procedure not described. Blinding: double‐blind, no further details. Duration: 12 weeks. Design: parallel. Location: single‐centre. Setting: inpatients. | |
| Participants | Diagnosis: chronic schizophrenia (clinical diagnosis), receiving maintenance treatment. N = 20. Gender: only male participants. Age: 42.6 years. History: duration stable‐n.i., duration ill‐ n.i., mean duration of hospitalisation‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ mean BPRS total score 34.6, mean CGI‐S total score 3.73, baseline antipsychotic dose‐ n.i., remission at baseline‐ 40% were in remission at baseline (CGI‐S defined). | |
| Interventions | 1. Drug: Pimozide. N = 10 Flexible dose. Mean dose: 40 mg/day. 2. Placebo: duration of taper: abrupt withdrawal. N = 10. Rescue medication: n.i. |
|
| Outcomes |
Examined Leaving the study early. Global state ‐ number of participants improved (CGI‐I defined). Global state ‐ number of participants in remission (CGI‐S defined). Adverse events. Unable to use/Not included Mental state: BPRS (no predefined outcome of interest) Behavior: NOSIE (no predefined outcome of interest). |
|
| Notes | Sponsored by McNeil Laboratories. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only study completers were used in the final analysis, but as there was only one dropout (in the drug arm, before receiving the first dose of medication) this was not necessarily a problem. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | The pimozide doses (40 mg/day) were very high for current standards. |
Pimozide 1973.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, identical capsules. Duration: 26 weeks. Design: parallel. Location: single‐centre. Setting: in hospital. | |
| Participants | Diagnosis: residual schizophrenia (DSM‐II), chronic, currently treated with antipsychotic drugs. N = 40. Gender: 40 women. Age: mean 58.5 years. History: duration stable‐ all participants were switched to two months treatment with pimozide and only those who were treated effectively (=markedly improved) were randomised, duration ill‐ mean 30.5 years, duration of current hospitalisation mean 24.5 years (range 1‐43), number of previous hospitalisations‐ n.i., age at onset‐ mean 28 years, severity of illness‐ n.i., baseline antipsychotic dose‐ pimozide mean 7.72mg/day. |
|
| Interventions | 1. Drug: pimozide. Flexible dose. Allowed dose range: n.i.. Mean dose: n.i.. N = 20. 2. Placebo: duration of taper: 0 days. N = 20. Rescue medication: not allowed, only dose increase of pimozide or placebo‐pimozide was possible. Additional use of haloperidol meant relapse. |
|
| Outcomes |
Examined Relapse: need of additional haloperidol) Leaving the study early. Adverse effects: number of participants with at least one movement disorder, rigor and tremor. Death. Violent/aggressive behaviour. Unable to use/Not included Mental state: Overall Factor Construct Scale (no mean, no SD/no prespecified outcome of interest) Behaviour: ‘Psychiatric Evaluation Scale’ (no mean, no SD/no prespecified outcome of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 (5%) of the participants left the study early which is an acceptable rate. Both participants were included in the endpoint analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear evidence for other bias. |
Quetiapine 2007.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, identical capsules. Duration: 52 weeks, however terminated early after a mean duration of 120 days. Design: parallel. Location: multi‐centre. Setting: probably mainly outpatients. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV), duration ill at least 2 years, Positive and Negative Syndrome Scale total score < 60 before randomised phase, Clinical Global Impression Severity Scale not more than moderately ill. N = 197. Gender: 103 men, 69 women. Age: mean 35 years. History: duration stable‐ at least 20 weeks, retrospectively at least one month (no change of overall severity and medication), prospectively 16 weeks stabilisation phase during which all participants were switched to quetiapine, duration ill‐ mean 8.7 years, number of previous hospitalisations‐ n.i., but mean number of episodes 4.3, age at onset‐ mean 26.5 years, severity of illness‐ mean Clinical Global Impression of severity 2.7, mean PANSS total score 48.2, baseline antipsychotic dose‐ quetiapine 646 mg/day, remission at baseline ‐ yes (92% of the randomised patients met criteria for symptomatic remission). |
|
| Interventions | 1. Drug: quetiapine XR. Flexible dose 400 mg to 800 mg/day. Mean dose: 669 mg/day. N = 94. 2. Placebo: duration of taper: 4 days.N = 103. Rescue medication: anticholinergic medication, sleep medication, lorazepam, no additional antipsychotic drugs. |
|
| Outcomes |
Examined Relapse: increase of PANSS by at least 30 percent from baseline, Clinical Global Impression Scale much or very much worse, need for additional antipsychotic medication. Leaving the study early. Global state: number of participants in symptomatic remission (Andreasen criteria). Global state: number of participants in sustained remission (Andreasen criteria, maintained for at least 6 months). Adverse events: open interviews. Death. Extrapyramidal side‐effects: use of antiparkinson medication, Barnes Akathisia Scale, SAS, Aquired Involuntary Movements Scale. Unable to use/Not included Global state: number of participants improved (no usable data). Service use ‐ number of participants hospitalised (unclearly reported). Mental state: PANSS/no predefined outcome of interest. Laboratory: haematology, chemistry, glucose, Hba1c, insulin, lipids, urine analysis, thyroid function), ECG, vital signs, mean weight gain (all no predefined outcomes of interest). Compliance (pill count/no predefined outcome of interest). |
|
| Notes | No participant terminated the preplanned study duration of one year. The authors reported that data after 6 months are not reliable because only a few patients were left. Therefore, relapse data after 6 months were not extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Correct randomisation assumed, because recent study from industry. |
| Allocation concealment (selection bias) | Low risk | Correct allocation concealment assumed, because recent study from industry. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall drop‐out rate was 41%, most of them due to relapse (76%), which occurred much more frequently in the placebo group. This difference in attrition may have biased the results of other outcomes than relapse. Kaplan‐Meier survival curve analysis was used for the primary outcome relapse. |
| Selective reporting (reporting bias) | High risk | Only adverse events with a frequency of at least 5% were reported. |
| Other bias | High risk | The study was terminated early after an interim analysis showed a clear superiority of quetiapine; there were certain baseline discrepancies in terms of mean age, duration ill and number of previous episodes. |
Quetiapine 2009a.
| Study characteristics | ||
| Methods | Randomisation: randomised (1:1:1 ratio) to bifeprunox, quetiapine or placebo, no further detail. Allocation: procedure not described. Blinding: double‐blind, encapsulated tablets. Duration: 12 weeks. Design: parallel. Location: multi‐centre. Setting: inpatients and outpatients. | |
| Participants | Diagnosis: Schizophrenia (DSM‐IV‐TR), in the maintenance phase (no acute exacerbation and medication unchanged for at least 4 weeks). N = 144. Gender: 78 men, 66 women. Age: 39 years. History: duration stable‐ at least 4 weeks, duration ill‐ n.i., mean duration of hospitalisation‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ mean PANSS total score 80.2, mean CGI‐S total score 4,2, baseline antipsychotic dose‐ n.i., remission at baseline‐ not in remission (still experiencing clinically significant symptoms, not sufficiently controlled with medication, at least moderately ill at CGI‐S at baseline. | |
| Interventions | 1. Drug: Quetiapine. N = 76 Fixed dose. Mean dose: 600 mg/day. 2. Placebo: duration of taper: 21 days, N = 68. Rescue medication: n.i. |
|
| Outcomes |
Examined Quality of life: Schizophrenia Quality of Life (S‐QoL) scale. Social functioning: Personal and Social Performance scale. Death Unable to use/Not included Relapse (no usable data) Leaving the study early (no usable data, available only for the whole study duration) Global state ‐ number of participants in remission/improved: CGI‐S, CGI‐I (no usable data) Mental state: PANSS total score and subscores (no predefined outcomes of interest) Depressive symptoms: Calgary Depression Scale for Schizophrenia (no predefined outcome of interest) Service use ‐ number of participants hospitalised (no usable data) Social functioning: Global Assessment of Functioning (PSP data used) Adverse effects (no usable data) |
|
| Notes | Sponsored by Lundbeck. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only stated randomised, no further detail. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, encapsulated tablets. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, encapsulated tablets. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, encapsulated tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Global and per‐arm attrition rate are not reported for the randomised placebo‐controlled period, but only for the total duration of the study. Efficacy and safety were assessed on a ITT base. |
| Selective reporting (reporting bias) | Unclear risk | Relapse was not a pre‐specified outcome. several efficacy outcomes cited as assessed but not reported throughout the text. |
| Other bias | High risk | Terminated early for negative efficacy results after a pooled interim analysis. |
Quetiapine 2009b.
| Study characteristics | ||
| Methods | Randomisation: randomised (1:1:1 ratio) to bifeprunox, quetiapine or placebo, no further detail. Allocation: procedure not described. Blinding: double‐blind, encapsulated tablets. Duration: 12 weeks. Design: parallel. Location: multi‐centre. Setting: inpatients and outpatients. | |
| Participants | Diagnosis: Schizophrenia (DSM‐IV‐TR), in the maintenance phase (no acute exacerbation and medication unchanged for at least 4 weeks). N = 235. Gender: 127 men, 108 women. Age: 38 years. History: duration stable‐ at least 4 weeks, duration ill‐ n.i., mean duration of hospitalisation‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ mean PANSS total score 79,5, mean CGI‐S total score 4,2, baseline antipsychotic dose‐ n.i., remission at baseline‐ not in remission (still experiencing clinically significant symptoms, not sufficiently controlled with medication, at least moderately ill at CGI‐S at baseline. | |
| Interventions | 1. Drug: Quetiapine. N = 116. Fixed dose. Mean dose: 600 mg/day. 2. Placebo: Duration of taper: 21 days, N = 119. Rescue medication: n.i. |
|
| Outcomes |
Examined Quality of life: Schizophrenia Quality of Life (S‐QoL) scale. Social functioning: Personal and Social Performance scale. Death Unable to use/Not included Relapse (no usable data) Leaving the study early (no usable data, available only for the whole study duration) Global state ‐ number of participants in remission/improved: CGI‐S, CGI‐I (no usable data, available data on "at least much improved") Mental state: PANSS total score and subscores (no predefined outcomes of interest) Depressive symptoms: Calgary Depression Scale for Schizophrenia (no predefined outcome of interest) Service use ‐ number of participants hospitalised (no usable data) Social functioning: Global Assessment of Functioning (PSP data used) Adverse effects (no usable data) Suicide attempts (no usable data). |
|
| Notes | Sponsored by Lundbeck. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only stated randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, encapsulated tablets. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, encapsulated tablets. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, encapsulated tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Global and per‐arm attrition rate are not reported for the randomised placebo‐controlled period, but only for the total duration of the study. Efficacy and safety were assessed on an ITT base. |
| Selective reporting (reporting bias) | Unclear risk | Relapse was not a pre‐specified outcome. several efficacy outcomes cited as assessed but not reported throughout the text. |
| Other bias | High risk | Terminated early for negative outcome data after a pooled interim analysis. 8 patients in the quetiapine arm and 11 patients in the placebo arm either continued taking or started concomitant new antipsychotic treatment during the study. |
Quetiapine 2010.
| Study characteristics | ||
| Methods | Randomisation: sequence by computer, fixed block size of four without stratification. Allocation: AstraZeneca prepared individually numbered sets of study drugs, packed them according to the randomisation sequence and then shipped them to the study team in numbered but apparently identical sets. Blinding: identical capsules, "investigators, patients and all research staff were blind to the study drugs and the block size". Duration: 1 year. Design: parallel. Location: single‐centre (all in Early Assessment Service for Young People with Psychosis (EASY) in Hong Kong). Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia and related psychoses (DSM‐IV), all first episode, all well remitted, all had remained well on maintenance medication for 1 year. N = 178. Gender: 80 men, 98 women. Age: 24.2 years. History: duration stable‐ 1 year, duration ill‐ 2.3 years, number of previous hospitalisations‐ 0 (first episode), age at onset‐ 21.9 years, severity of illness‐ mean PANSS 36, baseline antipsychotic dose‐ 153 mg/day chlorpromazine equivalents. |
|
| Interventions | 1. Drug: quetiapine. Fixed dose of 400 mg/day. N = 89. 2. Placebo: duration of taper (days): 35. N = 89. Rescue medication: antipsychotics not allowed. |
|
| Outcomes |
Examined Relapse: (i) an increase in at least one of the following PANSS psychotic symptom items to a threshold score (delusion, hallucinatory behaviour, conceptual disorganisation, unusual thought content, suspiciousness; (ii) Clinical Global Impression Severity of Illness 3 or above and (iii) CGI change 5 or above). Leaving the study early. Rehospitalisation. Suicide attempts. Adverse effects: akathisia, tardive dyskinesia, tremor, sedation, weight gain. Open employment status. |
|
| Notes | Supported by investigator initiated trial award from AstraZeneca. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence by computer, fixed block size of four without stratification. |
| Allocation concealment (selection bias) | Low risk | AstraZeneca prepared individually numbered sets of study drugs, packed them according to the randomisation sequence and then shipped them to the study team in numbered but apparently identical capsules. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Identical capsules, "investigators, patients and all research staff were blind to the study drugs and the block size". |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Identical capsules, "investigators, patients and all research staff were blind to the study drugs and the block size". |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Identical capsules, "investigators, patients and all research staff were blind to the study drugs and the block size". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 72% of the participants left the study early. As most participants dropped out after relapse this outcome was not affected, but it is a source of bias for other outcomes. Survival analysis for the primary outcome relapse. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear other bias. |
Trifluoperazine 1969.
| Study characteristics | ||
| Methods | Randomisation: randomised, no further details. Allocation: procedure not described. Blinding: double‐blind, identical capsules. Whether blinding was successful was not assessed, although in one group high doses associated with a lot of side effects were administered. Duration: 24 weeks. Design: parallel. Location: multi‐centre. Setting: inpatient. | |
| Participants | Diagnosis: chronic schizophrenia (clinical diagnosis), hospitalised for at least 2 years.
N = 341. Gender: n.i.. Age: mean 41.8 years. History: duration stable‐ not clearly indicated, all were observed on their normal hospital medication for 4 weeks, quote "we may assume that the patients were well stabilised”, duration ill‐ n.i., number of previous hospitalisations‐ n.i., but mean length of current hospitalisation 15 years, age at onset‐ n.i. , severity of illness‐ n.i., baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: high‐dose trifluoperazine. Fixed dose of 80 mg/day (reached within 35 days). N = 117. 2. Drug: low‐dose trifluoperazine. Fixed dose of 15 mg/day. N = 113. 3. Placebo: duration of taper: 0 days. N = 111. Rescue medication: not indicated, but probably not allowed. |
|
| Outcomes |
Examined Relapse: worsening of global state. Leaving the study early. Global state: number of participants improved. Service use; number of participants discharged. Adverse effects: clinical interview based on 40 items checklist. Unable to use/Not included Mental state: Inpatient Multidimensional Psychiatric Scale, BPRS (both only P values/no predefined outcome of interest). Behaviour: NOSIE (only P values/no predefined outcome of interest). Ophthalmologic examination (no predefined outcome of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules. Whether blinding was successful was not assessed, although in one group high doses associated with a lot of side effects were administered. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind identical capsules. Whether blinding was successful was not assessed, although in one group high doses associated with a lot of side effects were administered. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules. Whether blinding was successful was not assessed, although in one group high doses associated with a lot of side effects were administered. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall attrition was considerable (33%) and clearly more participants discontinued the study early in the placebo group (53%) than in the two drug groups (23%), mainly due to inefficacy, which can be a problem for other outcomes than relapse. Not all participants were included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | The high‐dose group used too high doses (80 mg/day) for current standards, even the low‐dose would nowadays be considered to be quite high (15 mg/day). |
Trifluoperazine 1972.
| Study characteristics | ||
| Methods | Randomisation: randomised, no further details. Allocation: capsules dispensed by the hospital pharmacist who was the only person who knew what the capsules were and to whom they were given. Blinding: double‐blind, placebo capsules, no further details. Duration: range 13‐22 weeks, mean 16 weeks. Design: parallel. Location: 2 centres. Setting: inpatient. | |
| Participants | Diagnosis: chronic schizophrenia (clinical diagnosis), >70% of them with extrapyramidal side effects after long treatment with phenothiazines. N = 63. Gender: 32 men, 31 women. Age: mean 57 years. History: duration stable‐ n.i., duration ill‐ n.i., but currently hospitalised for at least 4 years and treated with phenothiazines for a mean duration of 9.4 years, number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ mean 17 mg/day trifluoperazine (86% of the participants). |
|
| Interventions | 1. Drug: trifluoperazine ‐ Fixed dose (maintaining the initial dose, necessity of dose increase was considered to be a relapse). Mean dose: 17 mg/day. N = 31. 2. Placebo: duration of taper: 0 days. N = 32. Rescue medication: n.i.. |
|
| Outcomes |
Examined Relapse: deterioration of participant’s condition to such a degree that additional antipsychotic medication was necessary. Leaving the study early. Death. Unable to use/Not included Adverse effects: movement disorders (no randomised data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Low risk | Capsules dispensed by the hospital pharmacist who was the only person who knew what the capsules were and to whom they were given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo capsules, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, placebo capsules, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, placebo capsules, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16% left the study early, all but one due to relapse. This appears acceptable. relapse and death were the only outcomes. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | Participants with a relapse were probably removed from the study and the blind broken. Study was probably terminated early. |
Various drugs 1960.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, unidentifiable capsules. Duration: 6 months. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: chronic psychotic patients (mainly schizophrenia, clinical diagnosis). N = 144. Gender: n.i.. Age: n.i.. History: duration stable‐ "observed on the same drugs for 4.5 months”, duration ill‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: continuation of antipsychotic taken before the study ‐ Fixed/flexible dose: unclear. Allowed dose range: unclear. Mean dose: n.i.. N = 46. 2. Placebo: duration of taper: "4 weeks to five months, usually 2 months”. N = 98. Rescue medication: n.i.. |
|
| Outcomes |
Examined Relapse: clinical diagnosis. Unable to use/Not included Social adjustment: (not reported for the randomised participants). Rehospitalisation (unclear numbers). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, unidentifiable capsules. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, unidentifiable capsules. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, unidentifiable capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Whether participants left the study early is unclear. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | In case of relapse the blind was broken. |
Various drugs 1961.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: only the hospital pharmacist had the code on what medication the patient was on. Blinding: double‐blind, identical capsules, each participant had his own container. Duration: 16 weeks. Design: parallel. Location: single‐centre. Setting: inpatient. | |
| Participants | Diagnosis: schizophrenia (clinical diagnosis), all withdrawn of subject to periodic disturbances, all needed supervision or management. N = 80. Gender: 80 men. Age: younger than 55 years, mean 40.6 years. History: duration stable‐ n.i. ("participants had attained and maintained some degree of improvement”), duration ill‐ n.i., but mean duration of current hospitalisation 10 years, number of previous hospitalisations‐ mean 1.6, age at onset‐ n.i., severity of illness‐ n.i., but "most required closed ward care”, median baseline antipsychotic dose‐ chlorpromazine 475 mg/day (N = 30), mepazine 200 mg/day (N = 35), trifluoperazine 30 mg/day (N = 6), prochlorpromazine (N = 2, dose not indicated), combinations of drugs (N = 7, doses not indicated) |
|
| Interventions | 1. Drug: chlorpromazine; flexible dose; allowed dose range 200 mg/day to 1000 mg/day; mean dose: 894 mg/day (here mean maximum dose); N = 20 2. Drug: trifluoperazine; flexible dose; allowed dose range 10 to 50 mg/day; mean dose: 29 mg/day (here mean maximum dose); N=20 3. Drug: thioridazine; flexible dose; allowed dose range 200 mg/day to 1000 mg/day; mean dose: 958 mg/day (here mean maximum dose); N = 20 4. Placebo: duration of taper: 0 days; N = 20 Rescue medication: phenobarbital and bentropine methansulfonate, no additional antipsychotic drugs |
|
| Outcomes |
Examined Relapse: worsening of global state Leaving the study early. Global state ‐ number of participants improved (clinical judgement, categories comparable to CGI). Adverse effects: clinical interview, number of participants receiving antiparkinson medication Unable to use/Not included Behaviour: Manifest Behaviour Scale (no SD/no predefined outcome of interest) Personality traits: Minnesota Multiphasic Personality Inventory (MMPI) (no SD/no predefined outcome of interest) |
|
| Notes | The results of all drug groups were pooled. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details |
| Allocation concealment (selection bias) | Low risk | Only the hospital pharmacist had the code on what medication the patient was on |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules, each participant had his own container. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules, each participant had his own container. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules, each participant had his own container. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 out of 80 participants left the study early and the reasons were well described. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | No evidence for other bias. |
Various drugs 1962a.
| Study characteristics | ||
| Methods | Randomisation: randomly selected and then assigned. Allocation: procedure not described. Blinding: identical pink capsules. Nurses, raters and patients were blind to the procedure. Treating physician was led to believe that half of the patients were on placebo, the other half on drug. Duration: 30 days. Design: parallel. Location: single‐centre. Setting: inpatients. | |
| Participants | Diagnosis: chronically mentally ill, 67% to 83% schizophrenia (clinical diagnosis), in hospital and apparently treated with antipsychotic drugs for the last 18 months. N = 60. Gender: n.i.. Age: mean 51 years. History: duration stable‐ at least 60 days plus 30 days prospectively, duration ill‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ n.i. |
|
| Interventions | 1. Drug: chlorpromazine or thioridazine. Fixed/flexible dose: n.i.. Allowed dose range: n.i.. Mean dose: n.i.. N = 30. 2. Placebo: duration of taper: 0 days. N = 30. Rescue medication: not indicated. |
|
| Outcomes |
Examined Relapse: attrition because of behavioural upset. Unable to use/Not included Behaviour: various scales (no data reported/no predefined outcome of interest). |
|
| Notes | There were several study phases (alternation between drug and placebo). Only the first phase was of interest for the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly selected and then assigned. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Identical pink capsules. Nurses, raters and patients were blind to the procedure. Treating physician was led to believe that half of the patients were on placebo, the other half on drug. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Identical pink capsules. Nurses, raters and patients were blind to the procedure. Treating physician was led to believe that half of the patients were on placebo, the other half on drug. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Identical pink capsules. Nurses, raters and patients were blind to the procedure. Treating physician was led to believe that half of the patients were on placebo, the other half on drug. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear how many participants left the study during the first month. |
| Selective reporting (reporting bias) | High risk | Data on behaviour scales were not reported, including aggressive behaviour which was an outcome in our review. |
| Other bias | Low risk | No evidence for other bias. |
Various drugs 1962b.
| Study characteristics | ||
| Methods | Randomisation: randomised, no further details. Allocation: psychiatrist without contact to the participants held the key and filled the medication containers. Blinding: double‐blind, exact placebo replicas. Duration: ~ 43 weeks. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia without positive symptoms (clinical diagnosis). N = 43. Gender: 16 men, 27 women. Age: typically 40 to 50 years. History: duration stable‐ out of hospital for at least a year (typically 2 to 4 years), duration ill‐ n.i., number of previous hospitalisations‐ typically 2‐3, age at onset n.i., severity of illness n.i., but no positive symptoms at baseline, baseline antipsychotic dose‐ maximum 300 mg chlorpromazine per day. |
|
| Interventions | 1. Drug: various phenothiazines, mainly chlorpromazine. Fixed/flexible dose: flexible. Allowed dose range: not limited, but complete discontinuation was not allowed. Mean dose: 150 mg/day to 200 mg/day chlorpromazine. N = 24. 2. Placebo: duration of taper: 0 days. N = 19. Rescue medication: not allowed. |
|
| Outcomes |
Examined Relapse: clinical judgement. Service use: number of participants rehospitalised. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Low risk | Psychiatrist without contact to the participants held the key and filled the medication containers. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, exact placebo replicas. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, exact placebo replicas. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, exact placebo replicas. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear ‐ whether participants discontinued the study prematurely was not reported. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | Some placebo participants continued to take medication, study terminated early. |
Various drugs 1964a.
| Study characteristics | ||
| Methods | Randomisation: randomised, no further details. Allocation: procedure not described. Blinding: double‐blind, identical tablets. However, placebo dose reduction group received medication only every other day. Therefore, blinding was not fully maintained. Duration: 16 weeks. Design: parallel. Location: multi‐centre. Setting: in hospital. | |
| Participants | Diagnosis: schizophrenia (clinical diagnosis), one third paranoid subtype, without central nervous system disease, without lobotomy. N = 259. Gender: all men. Age: mean 40 years. History: duration stable‐ stable doses for at least 3 months before the study, duration ill‐ n.i., but currently hospitalised for a mean of 10 years, number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ chlorpromazine mean 400 mg/day, thioridazine mean 350 mg/day. |
|
| Interventions | 1. Drug: chlorpromazine or thioridazine.* Fixed dose, continuation of the dose given in the stabilisation phase. Mean dose: chlorpromazine mean 400 mg/day, thioridazine mean 350 mg/day. N = 88. 2. Placebo: duration of taper: 1 ‐ 8 days. N = 171. Rescue medication: not indicated. |
|
| Outcomes |
Examined Relapse: definitive worsening of the condition and medication again necessary, usually joint decision of treatment team. Unable to use/Not included Mental state: Inpatient Multidimensional Psychiatric Scale (IMPS) (no prespecified outcome of interest). Behaviour: Psychotic Reaction Profile Scale (no prespecified outcome of interest). |
|
| Notes | * There was another group which received half the original dose. It was not considered in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Double‐blind, identical tablets. However, placebo dose reduction group received medication only every other day. Therefore, blinding was not fully maintained. |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Double,‐blind identical tablets. However, placebo dose reduction group received medication only every other day. Therefore, blinding was not fully maintained. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical tablets. However, placebo dose reduction group received medication only every other day. Therefore, blinding was not fully maintained. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were dropouts or whether the authors analysed only study completers. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Unclear risk | 22 participants who had relapsed in the first 8 weeks were entered in the study again. As the number is small, it is unclear whether they affected the results. |
Various drugs 1964b.
| Study characteristics | ||
| Methods | Randomisation: randomly assigned. Allocation: procedure not described. Blinding: double‐blind ‐ (apart from previous antipsychotic group) ‐ three different colours which were again changed. Double‐blind condition maintained for patients, ward nurses and psychiatrists. Duration: 7 months. Design: parallel. Location: single‐centre. Setting: inpatient. | |
| Participants | Diagnosis: chronic psychotic patients, treatment resistive in closed wards. No seizures, no antidepressants, no candidates for discharge. N = 88. Gender: 38 men, 40 women. Age: 47 years. History: duration stable‐ 1 year on medication, duration ill‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ mean 28.1 years, severity of illness‐ mean 11.6 on modified Psychotic Reaction Profile (PRP), baseline antipsychotic dose‐ 39.3mg/ 3 weekly fluphenazine decanoate. |
|
| Interventions | 1. Drug: trifluoperazine (10 mg/day to 90 mg/day), chlorprothixene (50 mg/day to 450 mg/day), same medication (various drugs). Flexible doses. Allowed dose range: n.i.. Mean dose: n.i.. N = 54. 2. Placebo: duration of taper: 0 days. N = 34. Rescue medication: antiparkinson, barbiturate sedation. | |
| Outcomes |
Examined Relapse: clinical judgement. Leaving the study early. Adverse effects. Unable to use/Not included Ward behaviour: unpublished rating scale (no predefined outcome of interest). Urinary excretion (no predefined outcome of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, different colours. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, different colours. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, different colours. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts 10 out of 88 is acceptable (11%), although only completers were analysed. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No evidence for other bias. |
Various drugs 1966a.
| Study characteristics | ||
| Methods | Randomisation: matched in three groups according to age and hospitalisation, then randomised using a table of random numbers. Allocation: procedure not described. Blinding: double‐blind, no further details. Duration: 22 weeks (experimental phase). Design: parallel. Location: single‐centre. Setting: inpatient. | |
| Participants | Diagnosis: schizophrenia (clinical diagnosis), undifferentiated type (N = 10), hebephrenic (N = 6), catatonic (5), paranoid (5), acute undifferentiated (N = 1). N = 27. Gender: 27 women. Age: mean 42.4 years. History: duration stable‐ on continuous phenothiazine medication at sufficient dose for at least 6 months, then stabilised another 2 months on the ward, total 8 months, duration ill NI‐ duration of current hospitalisation mean 11.42 years, number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ chlorpromazine mean 610 mg/day (N = 17), thioridazine mean 480 mg/day (N = 5), trifluoperazine mean 25 mg/day (N = 3), perphenazine 24 mg/day (N = 1), prochlorperazine 60 mg/day (N = 1). |
|
| Interventions | 1. Drug: remained on previous antipsychotic medication (chlorpromazine, thioridazine, trifluoperazine, perphenazine, prochlorperazine). Fixed/flexible dose: not clear, but probably fixed. Allowed dose range: n.i.. Mean dose: n.i., because it is unclear which patients were allocated to which group. N = 9. 2. Placebo: duration of taper: 7 days. N = 9**. Rescue medication: tranquilliser (= benzodiazepine). |
|
| Outcomes |
Examined Relapse: worsening by three points on the factor scores of the Inpatient Multidimensional Psychiatric Scale (IMPS) or withdrawn due to being worse. Leaving the study early. Global state: improvement by three points on the factor scores of the IMPS or withdrawn due to being ready for discharge. Service use: number of participants discharged. Unable to use/Not included Mental state: IMPS (no data/no prespecified outcome of interest). Behaviour: Psychotic Reaction Profile (no data / no prespecified outcome of interest). |
|
| Notes | ** a second placebo group that was referred to a specialised ward was not used in our calculations (N = 9). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Matched in three groups according to age and hospitalisation, then randomised using a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a considerable number of participants leaving the study early (28%). The approach how missing data were handled is not specified. |
| Selective reporting (reporting bias) | Low risk | Only two factors of the IMPS were presented, but this was no outcome of interest. |
| Other bias | Low risk | No clear other bias. |
Various drugs 1966b.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, "the staff, patients and investigators were not aware of which patients were to receive placebo instead of their medication”. Duration: 6 weeks. Design: parallel. Location: single‐centre. Setting: inpatient. | |
| Participants | Diagnosis: schizophrenia (clinical diagnosis), paranoid schizophrenia (N = 19), undifferentiated schizophrenia (N = 8), catatonic schizophrenia (N = 8), hebephrenic schizophrenia (N = 4), acute schizophrenic reaction (N = 1). N = 40. Gender: 20 men, 20 women. Age: n.i.. History: duration stable‐ not indicated, but mean 4.6 months on current medication, duration ill‐ mean 12.18 years, number of previous hospitalisations‐ n.i. but mean duration of current hospitalisation 18 months, age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: chlorpromazine (N = 6) or thioridazine (N = 14). Flexible dose. Allowed dose range: 100 mg/day to 600 mg/day. Mean dose: n.i.. N = 20. 2. Placebo: duration of taper (days): 0 days. N = 20. Rescue medication: n.i.. |
|
| Outcomes |
Examined Relapse: symptoms similar to those which had characterized the patient’s illness prior to successful treatment by phenothiazines. Unable to use/Not included Withdrawal symptoms (no numbers for each group separately/no predefined outcome of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, "the staff, patients and investigators were not aware of which patients were to receive placebo instead of their medication”. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, "the staff, patients and investigators were not aware of which patients were to receive placebo instead of their medication”. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, "the staff, patients and investigators were not aware of which patients were to receive placebo instead of their medication”. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was not reported whether participants left the study early, but it is well possible that there were not any, because it was a relatively short inpatient study. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear evidence for other bias. |
Various drugs 1968.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: the hospital pharmacist was responsible for supplying placebo and active drugs to the ward, no one concerned with the care of patients knew which patients were started on placebo. Blinding: double‐blind, identical tablets, but in most cases nurses made correct forecasts on who was on drug and who was on placebo. Blind was broken when a participant relapsed. Duration: 6 months. Design: parallel. Location: single‐centre. Setting: inpatient. | |
| Participants | Diagnosis: chronic schizophrenia (clinical diagnosis by two psychiatrists). N = 40. Gender: 40 men. Age: 25 to 55 years. History: duration stable‐ maintenance doses of tranquiliser had been administered for at least 18 months, in six participants who had to change treatment no change in symptoms was noted during 6 weeks, duration ill‐ n.i., number of previous hospitalisations‐ n.i., but duration of current hospitalisation > 2 years, age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ all but six participants were on chlorpromazine or trifluoperazine, dose n.i.. |
|
| Interventions | 1. Drug: chlorpromazine or trifluoperazine. Fixed/flexible dose: n.i.. Allowed dose range: n.i.. Mean dose: n.i.. N = 20. 2. Placebo: duration of taper: 0 days. N = 20. Rescue medication: n.i.. |
|
| Outcomes |
Examined Relapse: worsening of global state. Global state: number of participants improved. Adverse effects. Unable to use/Not included Mental state: Wing Scale (no SD/no predefined outcome of interest). Behaviour: Wing Scale (no SD/no predefined outcome of interest). Leaving the study early (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Low risk | The hospital pharmacist was responsible for supplying placebo and active drugs to the ward, no one concerned with the care of patients knew which patients were started on placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Double‐blind, identical tablets, but in most cases nurses made correct forecasts on who was on drug and who was on placebo. |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Double‐blind, identical tablets, but in most cases nurses made correct forecasts on who was on drug and who was on placebo. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical tablets, but in most cases nurses made correct forecasts on who was on drug and who was on placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were dropouts. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | Blinding was broken when a participant relapsed. |
Various drugs 1971.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: trial medication was held by the unit secretary and dispensed to Julian Leff who gave it to the treating consultant. Only the unit secretary knew which pills were active drug and which were placebo. Blinding: double‐blind, no further details. But side‐effects were not troublesome in any patient and therefore doctors concerned probably received no clues about whether a patient was on active drug or not. Duration: one year. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia (Present State Examination (PSE)), recently recovered from an acute episode, 32 florid schizophrenia, 3 delusional psychosis. N = 35. Gender: n.i.. Age: 16 to 55 years. History: duration stable‐ n.i., but stabilised at the pre‐admission level during a 6 to 12 weeks outpatient period and recently recovered from an acute episode, duration ill‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: trifluoperazine or chlorpromazine (depending on the previous medication so that so far as the patient was concerned there was no apparent change in medication). Flexible dose. Allowed dose range: trifluoperazine 5 mg/day to 25 mg/day, chlorpromazine 100 mg/day to 500 mg/day. Mean dose: chlorpromazine 157.1 mg/day, trifluoperazine 12.3 mg/day. N = 20. 2. Placebo: duration of taper: not indicated, probably 0 days. N = 15. Rescue medication: antiparkinson medication, antidepressants, no antipsychotics (doctors received a letter asking them not to prescribe other medication). |
|
| Outcomes |
Examined Relapse: physician was sufficiently concerned about the patient’s status to want to be certain that he was on active drug. Leaving the study early. Service use: number of participants hospitalised. Adverse effects. Death. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Low risk | Trial medication was held by the unit secretary and dispensed to Julian Leff who gave it to the treating consultant. Only the unit secretary knew which pills were active drug and which were placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, no further details. But side‐effects were not troublesome in any patient and therefore doctors concerned probably received no clues about whether a patient was on active drug or not. |
| Blinding (performance bias and detection bias) Subjective outcomes | Low risk | Double‐blind, no further details. But side‐effects were not troublesome in any patient and therefore doctors concerned probably received no clues about whether a patient was on active drug or not. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. But side‐effects were not troublesome in any patient and therefore doctors concerned probably received no clues about whether a patient was on active drug or not. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout rate was 60%, almost all due to relapse which occurred much more frequently in the placebo group. This poses a problem for other outcomes than relapse. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear other bias. |
Various drugs 1974.
| Study characteristics | ||
| Methods | Randomisation: randomised, no further details. Allocation: procedure not described. Blinding: double‐blind, identical capsules. Duration: 16 weeks. Design: parallel. Location: single‐centre. Setting: inpatient. | |
| Participants | Diagnosis: chronic schizophrenia (clinical diagnosis) with positive or negative symptoms, responsive to treatment with antipsychotic drugs, all so ill that they required continuous treatment with antipsychotic medication for at least 3 months. N = 61. Gender: 37 men, 24 women. Age: mean 45.7 years. History: duration stable‐ all participants had received a neuroleptic for at least 4 weeks, then stabilized on a fixed dose for 2 weeks, the last 2 weeks of which they were stabilized on a fixed dose, duration ill‐ at least 2 years, number of previous hospitalisations‐ n.i., age at onset‐ n.i. , severity of illness‐ n.i., baseline antipsychotic dose‐ chlorpromazine maximum dose 500 mg/day, thioridazine 500 mg/day, fluphenazine 30 mg/day, trifluoperazine 30 mg/day, other equipotent antipsychotics or combinations not exceeding the maximum doses. |
|
| Interventions | 1. Drug: pimozide ‐ Flexible dose. Allowed dose range: 2 mg/day to 12 mg/day. Mean dose: 6.3 mg/day. N = 21. 2. Drug: trifluoperazine. Flexible dose. Allowed dose range: 5 mg/day to 30 mg/day. Mean dose: 17.5 mg/day. N = 20. 3. Placebo: duration of taper: 21 days. N = 20. Rescue medication: chloralhydrate, antiparkinson medication. |
|
| Outcomes |
Examined Relapse: at least minimally worse on CGI. Leaving the study early. Global state: number of participants improved (CGI defined). Unable to use/Not included Mental state: BPRS (no predefined outcome of interest). Global state: number of participants in remission (no usable data). Social activity: Family Rating Form (no SD/no predefined outcome of interest). Social adjustment: Harbor View House Residents Rating Report (no SD/no predefined outcome of interest). Adverse effects: open interview (no data). Physiological measures: vital signs, laboratory (both no data/no predefined outcome of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall number of participants leaving the study early (41%) was considerable, with a higher dropout rate in the placebo group. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear evidence for other bias. |
Various drugs 1975.
| Study characteristics | ||
| Methods | Randomisation: random, in blocks of eight, stratified for age, duration ill and time since last admission. Allocation: procedure not described. Blinding: double‐blind, identical capsules, each participant had an individual stock bottle. Duration: 24 weeks. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: chronic schizophrenia (clinical diagnosis), 22 undifferentiated, 7 paranoid, 1 schizoaffective, no severe other psychiatric or somatic illnesses, no severely ill participants. N = 40. Gender: 40 women. Age: mean 42.8 years (range 24 to 60). History: duration stable‐ maintained on medication in an outpatient status for at least 3 months, "relatively stable state of health”, duration ill‐ mean 11.6 years, number of previous hospitalisations‐ mean 6.1, age at onset ‐ NI, severity of illness‐ mean CGI severity score 2.94, baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: pimozide.* Flexible dose. Allowed dose range: 2 mg/day to 20 mg/day. Mean dose: 5.3 mg/day. N = 15. 2. Drug: thioridazine.* Flexible dose. Allowed dose range: 75 mg/day to 750 mg/day. Mean dose: 189 mg/day. N = 15. 3. Placebo: duration of taper: 0 days. N = 10. Rescue medication: antiparkinson medication, bedside sedation. |
|
| Outcomes |
Examined Relapse (worsening of global state). Leaving the study early. Global state: number of participants improved (CGI based). Global state ‐ number of participants in remission (CGI based) Adverse effects: binary outcomes ‐ open interview. Unable to use/Not included Mental state: BPRS (no SD/no prespecified outcome of interest). Functioning: Katz Lyerly Scale of Social Adjustment, Patient Rating Form, Family Rating Form (no data available ) Physiological measures: biological parameters (temperature, mean weight, pulse, blood pressure, all no data/all no prespecified outcomes of interest), laboratory (blood count, urine analysis, liver enzymes, blood sugar, protein bound iodine, all no prespecified outcomes of interest). |
|
| Notes | * The results of pimozide and thioridazine were combined in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random, in blocks of eight, stratified for age, duration ill and time since last admission. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules, each participant had an individual stock bottle. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules, each participant had an individual stock bottle. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules, each participant had an individual stock bottle. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall 36% left the study early. The specific reasons why the participants dropped out were not indicated by group. |
| Selective reporting (reporting bias) | Low risk | No clear source for selective reporting. |
| Other bias | Low risk | No clear other sources of bias. |
Various drugs 1981a.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, thiamine chloride used as placebo, participants and nurses were told that a new medication was given, but nurses soon new that this was a placebo. Duration: 16 weeks. Design: parallel. Location: single‐centre. Setting: in hospital. | |
| Participants | Diagnosis: schizophrenia (clinical diagnosis). N = 45. Gender: 45 men. Age: 20 to 40 years. History: duration stable‐ n.i., but clinically tranquilised and making a satisfactory adjustment on phenothiazine medication, duration ill‐ n.i., but mean length of current hospitalisation 45 months (range 3 to 129), number of previous hospitalisations‐ all more than one, age at onset‐ n.i., severity of illness‐ n.i., but all in open hospital ward, baseline antipsychotic dose‐ prochlorpromazine 15 mg/day to 150mg/day, perphenazine 12 mg/day to 24 mg/day, chlorpromazine 50 mg/day to 800 mg/day, promazine 200 mg/day to 400 mg/day, trifluoperazine 6 mg/day. |
|
| Interventions | 1. Drug: prochlorpromazine, perphenazine, chlorpromazine, promazine or trifluoperazine. Fixed doses continued with the same drug and dose taken before the study. Mean dose: n.i. N = 30. 2. Placebo: duration of taper: 0 days. N = 15*. Rescue medication: not allowed. |
|
| Outcomes |
Examined Relapse (need of medication or deterioration of state or transfer to closed ward) Leaving the study early. Global state ‐ number of participants improved Service use ‐ number of participants hospitalised/discharged. Unable to use/Not included Behaviour: Patient Adjustment Report (no prespecified outcome of interest). Mental state: Taylor Manifest Anxiety Scale (no prespecified outcome of interest). |
|
| Notes | * Another 15 participants were treated only for 8 weeks with placebo and then switched back to their initial antipsychotic drug. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Double‐blind, thiamine chloride used as placebo, participants and nurses were told that a new medication was given, but nurses soon knew that this was a placebo. |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Double‐blind, thiamine chloride used as placebo, participants and nurses were told that a new medication was given, but nurses soon knew that this was a placebo. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, thiamine chloride used as placebo, participants and nurses were told that a new medication was given, but nurses soon knew that this was a placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only completers were included in the statistical analysis, but because the drop‐out rate was only 13% we did not consider this a source of bias. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear other risk of bias. |
Various drugs 1981b.
| Study characteristics | ||
| Methods | Randomisation: randomly in group of 15 each, no further details. Allocation: procedure not indicated. Blinding: double‐blind, no further details. Duration: 18 months. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia (mainly Schneiderian first‐rank symptoms), last relapse 30 to 60 months ago, fully remitted since and maintained on antipsychotic drugs. N = 30. Gender: 12 men, 18 women. Age: 39.9 years. History: duration stable‐ mean 44 months, duration ill‐ mean 10.2 years, number of previous hospitalisations‐ mean 1.6, age at onset‐ mean 29.7 years, severity of illness‐ n.i., baseline antipsychotic dose‐ 151 mg/day chlorpromazine equivalents. |
|
| Interventions | 1. Drug: switched to various antipsychotic drugs with similar profile as the previous one. Fixed/flexible dose: probably flexible. Allowed dose range: n.i.. Mean dose: n.i.. N = 15. 2. Placebo: benzodiazepine (‘active placebo’). Duration of taper 0 days. N = 15. Rescue medication: n.i.. |
|
| Outcomes |
Examined Relapse: recurrence of symptoms definitely of schizophrenic type, or symptoms not diagnostic of schizophrenia (e.g. sleep problems) which could not be controlled with other measures than antipsychotic drugs or ECT. Leaving the study early. Quality of life: subjective distress (Symptom Questionnaire of Kellner and Sheffield, SQKS). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly in group of 15 each, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 participants left the study early (40%), among those 10 from the placebo group and 8 for relapse. Outcomes other than relapse and leaving early are clearly prone to bias due to this difference in leaving the study early. |
| Selective reporting (reporting bias) | Low risk | Use of benzodiazepines was not indicated, but this was not an outcome of interest in our review. |
| Other bias | Low risk | No evidence for other bias. |
Various drugs 1981c.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, placebo sesame oil. Duration: 26 weeks. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia (according to Bleuler’s concept, with at least three primary symptoms ‐ e.g. autism, disturbance of affects, association and volition ‐ and at least two secondary symptoms ‐ hallucinations, persecution‐), duration ill at least 2 years. N = 41. Gender: 15 men, 23 women. Age: mean 43.1 years. History: duration stable‐ outpatient and continuous antipsychotic treatment for at least one year, on flupenthixol depot or fluphenazine depot for at least three months, prospective stabilisation phase of 6 months, duration ill‐ mean 13.3 years, number of previous hospitalisations‐ n.i., age at onset‐ mean 29.8 years, severity of illness‐ mean Comprehensive Psychopathological Rating Scale schizophrenia score 2.3, baseline antipsychotic dose‐ mean 21.42 mg fluphenazine/3 weeks or 27.5 mg flupenthixol/three weeks. |
|
| Interventions | 1. Drug: fluphenazine depot (most around 12.5 mg to 25 mg/3 weeks, mean 21.42 mg/3 weeks) or flupenthixol depot (most around 20 mg to 40 mg/3 weeks, mean 27.5/3 weeks) ‐ Fixed dose. N = 24. 2. Placebo: duration of taper: 0 days. N = 17. Rescue medication: chloral hydrate, antiparkinson medication, additional antipsychotic drugs were not allowed. |
|
| Outcomes |
Examined Relapse: psychotic behaviour or increase in six subscales of the Comprehensive Psychopathological Rating Scale. Leaving the study early. Service use: number of participants hospitalised. Adverse effects. Unable to use/Not included Mental state: Comprehensive Psychopathological Rating Scale (no SD/no predefined outcome of interest). Behaviour: NOSIE (no SD/no predefined outcome of interest). Physiological measures: various laboratory tests (no data/no predefined outcome of interest). Life events (Life Event Scale/no predefined outcome of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo sesame oil. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, placebo sesame oil. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, placebo sesame oil. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall, 3 (7%) out of 41 participants left the study early. Although only completers were analysed, due to the low rate this is not a problem. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No obvious risk for other bias. |
Various drugs 1982.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, drug appearance was made identical with respect to taste, colour and volume by adding a kind of "stomatics”. Duration: three years. Design: cross‐over Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia (clinical diagnosis) in remission. N = 30. Gender: 21 men, 9 women. Age: mean 33.2 years. History: duration stable‐ "in remission”, but details were not reported, duration ill‐ mean 7.3 years, number of previous hospitalisations‐ mean 2.4, age at onset‐ 25.9 years, severity of illness‐ "in remission”, baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: chlorpromazine. Fixed dose of 75 mg/day. N = 10. 2. Drug: haloperidol. Fixed dose of 3 mg/day. N = 10. 3. Placebo: duration of taper (days): 0 days. N = 10. Rescue medication: only nitrazepam for sleep and biperiden for extrapyramidal side‐effects, no additional antipsychotic drugs. |
|
| Outcomes |
Examined Relapse: clinical judgement. Leaving the study early (due to adverse events). Global state ‐ number of participants in sustained remission (study defined). Unable to use/Not included Number of symptom‐free days (no SD’s/no predefined outcome of interest). |
|
| Notes | There were also a diazepam and an imipramine group which were not of interest for the current review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, drug appearance was made identical with respect to taste, colour and volume by adding a kind of "stomatics”. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, drug appearance was made identical with respect to taste, colour and volume by adding a kind of "stomatics”. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, drug appearance was made identical with respect to taste, colour and volume by adding a kind of "stomatics”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant left the study early due to other reasons than relapse in the first phase of the study, the only outcome apart from leaving the study early. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | The doses used were very low for Western standards. The study was initially planned as a cross‐over trial, but due to high dropout rates after the first phase only the first treatment phase was analysed. Nevertheless, this did not interfere with the aims of our review. |
Various drugs 1984a.
| Study characteristics | ||
| Methods | Randomisation: randomised, 3:1 ratio. Allocation: procedure not described. Blinding: double‐blind, ‘matching placebos’ and sesame oil for fluphenazine decanoate treated participants. Duration: 10 weeks. Design: parallel. Location: two centres. Setting: outpatient. | |
| Participants | Diagnosis: chronic psychotic outpatients (DSM‐III), schizophrenia (N = 26), mental retardation with psychosis (N = 9), organic brain syndrome (N = 1). N = 36. Gender: 17 men, 19 women. Age: mean 45.8 years. History: duration stable‐ n.i., but all receiving maintenance neuroleptic therapy, all for at least 5 years, duration ill‐ n.i., but mean duration of neuroleptic treatment 13.4 years, number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ mean 365 mg/day chlorpromazine equivalents. |
|
| Interventions | 1. Drug: various antipsychotic drugs. Fixed dose: keeping the dose of the antipsychotic the participant was on at the beginning of the study. Mean dose: 365 mg/day chlorpromazine equivalents. N = 9. 2. Placebo: duration of taper: 28 days. N = 27. Rescue medication: n.i.. |
|
| Outcomes |
Examined Relapse: major clinical deterioration. Leaving the study early. Death. Unable to use/Not included Global state: Clinical Global Impression (CGI) (no data for each group separately/no prespecified outcome of interest). Mental state:BPRS, Profile of Mood Symptoms (PRS) (no data for each group separately/no prespecified outcome of interest). Adverse effects: extrapyramidal side‐effects (Abnormal Involuntary Movement Scale (AIMS), Dyskinesia Rating Scale, no data for each group separately/continuous side‐effect results were not among the prespecified outcomes), other adverse effects (Treatment Emergent Symptoms Scale, no data for each group separately/continuous side‐effect results were not among the prespecified outcomes). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, 3:1 ratio (information obtained from author). |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, ‘matching placebos’ and sesame oil for fluphenazine decanoate treated participants. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, ‘matching placebos’ and sesame oil for fluphenazine decanoate treated participants. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, ‘matching placebos’ and sesame oil for fluphenazine decanoate treated participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The differential dropout rate (placebo group 8/27, 0/9 maintenance group, all due to relapse) can have biased other outcomes than relapse and leaving the study early. But data on such other outcomes were not available anyway. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No evidence for other bias. |
Various drugs 1984b.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double‐blind, drug appearance was made identical with respect to powder, colour, taste and volume by adding a gastric acid. Duration: one year. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia (DSM‐III), in remission or residual state. N = 87. Gender: 53 men, 34 women. Age: mean 41 years. History: duration stable‐ n.i., but in remission, duration ill‐ mean 8.2 years, number of previous hospitalisations‐ mean 3.4, age at onset‐ mean 32.8 years, severity of illness‐ in remission or residual symptoms, baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: haloperidol combined with biperidine and nitrazepam. Fixed dose: 1 mg, 3 mg or 6 mg/day.* N = 37. 2. Drug: propericiazine combined with biperidine and nitrazepam. Fixed dose: 10, 30 mg/day or 60 mg/day.* N = 37. 3. Placebo combined with biperidine and nitrazepam. Duration of taper: 0 days. N = 13. Rescue medication: not indicated, probably no additional antipsychotic medication allowed. |
|
| Outcomes |
Examined Relapse: clinical judgement. Leaving the study early. Global state ‐ number of participants in sustained remission (study defined). Unable to use/Not included Prolactin levels (no predefined outcome of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, drug appearance was made identical with respect to powder, colour, taste and volume by adding a gastric acid. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, drug appearance was made identical with respect to powder, colour, taste and volume by adding a gastric acid. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, drug appearance was made identical with respect to powder, colour, taste and volume by adding a gastric acid. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | While in the placebo group and in the haloperidol group the rates of participants leaving early due to other reasons were low, 9 out of 12 participants in the propericiazine group discontinued due to overdose. It is questionable whether relapse rates could be accurately measured, because most participants did not reach the endpoint. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No evidence for other bias. |
Various drugs 1986a.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: allocation lists prepared by pharmacy for five antipsychotic drugs mentioned below, concealment is unclear. Blinding: double‐blind, no further details. Duration: 104 weeks. Design: parallel. Location: multi‐centre. Setting: outpatient. | |
| Participants | Diagnosis: first episode of schizophrenia (Present State Examination). N = 120. Gender: 74 men, 46 women. Age: mean 26.3 years (range 16 to 59 years). History: duration stable‐ 30 days after discharge all on active medication, duration ill‐ 2.8 months (between illness onset and admission to hospital), number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ most participants were ‘well’ at the beginning of the study (91 well, 13 psychotic features, 10 defect state, 6 unspecific symptoms), baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: flupenthixol IM, chlorpromazine, haloperidol, pimozide, trifluoperazine Flexible dose. Allowed dose range: no upper limit, but lower limit was flupenthixol IM 40 mg/month, chlorpromazine 200 mg/day, haloperidol 3 mg/day, pimozide 4 mg/day, trifluoperazine 5 mg/day. Mean dose: flupenthixol 84 mg/month (N = 31), chlorpromazine 366 mg/day (N = 3), haloperidol 11.8 mg/day (N = 3), pimozide 7.8 mg/day (N = 5), trifluoperazine 11.5 mg/day (N = 12). N=54. 2. Placebo: duration of taper (days): 30 days on drug, then received half dose for 30 days before they were put on placebo. N = 66. Rescue medication: antiparkinson medication, antidepressants, anxiolytics. |
|
| Outcomes |
Examined Relapse: rehospitalisation or rehospitalisation thought necessary although not possible or need of medication. Leaving the study early. Unable to use/Not included Hallucinations, delusions (no data/no predefined outcomes of interest). Global state: clinical judgment of patients global state at endpoint (not usable data/no predefined outcome of interest) Death, Suicide attempts (no usable data, only reported for the total sample). Disturbed behaviour/non‐cooperation (no usable data, only reported for the total sample). Use of antiparkinson medication (unclearly reported, probably referred to the baseline intake). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Allocation lists prepared by pharmacy for five antipsychotic drugs mentioned below, concealment is unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No clear bias. overall rate of leaving early of 11% is acceptable. Survival curve analysis was used for the primary outcome relapse. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | Blind was broken when a participant relapsed. |
Various drugs 1986b.
| Study characteristics | ||
| Methods | Randomisation: random, no further details. Allocation: procedure not explained. Blinding: double‐blind, placebo matching in kind and dose the previous medication. Duration: 10 weeks. Design: parallel. Location: three hospitals. Setting: probably inpatients. | |
| Participants | Diagnosis: schizophrenia (Schedule for Affective Disorder and Schizophrenia, and Research Diagnostic Criteria), all had previously responded to antipsychotic drugs. N = 100. Gender: 73 men, 27 women. Age: mean 32.6 years. History: duration stable‐ prospectively participants had remained for 10 weeks on the same medication before the study, duration ill‐ mean 9.7 years, number of previous hospitalisations‐ n.i., average cumulative hospitalisation 6.5 years, age at onset‐ mean 22.9 years, severity of illness‐ n.i., baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: various antipsychotic drugs. Fixed/flexible dose: probably flexible. Allowed dose range: n.i.. Mean dose: n.i.. N = 36. 2. Placebo: duration of taper 0 days. N = 64. Rescue medication: n.i.. |
|
| Outcomes |
Examined Relapse: first signs of symptoms according to ward staff and project nurse, full deterioration was not waited for. Unable to use/Not included Performance tests: Rohrschach test, Wechsler Adult Intelligence Scale (all no clear mean’s, n´s, no SD’s / no predefined outcomes of interest). Mental state: BPRS (no clear mean, no number of participants, no SD/no predefined outcome of interest). Thought disorder: Thought Disorder Index, PRS (all no clear mean’s, no SDs/no predefined outcomes of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not explained. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double, placebo matching in kind and dose the previous medication. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, placebo matching in kind and dose the previous medication. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, placebo matching in kind and dose the previous medication. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear, because these have not been indicated. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Unclear risk | Unclear, baseline data have not been presented for both groups separately. |
Various drugs 1989.
| Study characteristics | ||
| Methods | Randomisation: assumed, because study was double‐blind and because the first study phase was randomised (no further details). Allocation: procedure not described. Blinding: double‐blind, no further details. Duration: 12 months. Design: parallel. Location: single‐centre. Setting: outpatient. | |
| Participants | Diagnosis: first episode schizophrenia (Present State Examination, Feighner criteria and Research Diagnostic Criteria). N = 15. Gender: n.i. Age: n.i. History: duration stable‐ 1 year, duration ill‐ n.i., number of previous hospitalisations‐ n.i., age at onset‐ n.i., severity of illness‐ n.i., baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: pimozide once weekly or IM flupenthixol. Flexible doses. Allowed dose range: n.i.. Mean dose: n.i.. N = 8. 2. Placebo: duration of taper: 0 days N = 7. Rescue medication: antiparkinson medication. | |
| Outcomes |
Examined Relapse: re‐admission. Rehospitalisation. Unable to use/Not included Leaving the study early (no data). Global state ‐ number of participants in remission (no data for withdrawal study). Social adjustment (no data for withdrawal study). Cognition (no data for withdrawal study / no predefined outcome of interest). Adverse effects: parkinsonism, tardive dyskinesia (no data for withdrawal study). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation assumed. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were missing data. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Unclear risk | Not entirely clear. |
Various drugs 1993.
| Study characteristics | ||
| Methods | Randomisation: centrally randomised by a specialised unit using an "adaptive randomisation method”. Allocation: procedure not described. Blinding: open, only key rating scales were additionally rated by a second blind assessor. Duration: 2 years. Design: parallel. Location: multi‐centre. Setting: outpatient. | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (ICD‐9 and Research Diagnostic Criteria). N = 237. Gender: 124 women, 113 men. Age: mean 34.6 years. History: duration stable‐ at least 3 months in addition titrated to minimally effective dose which was maintained for at least 4 weeks, duration ill‐ mean 7.3 years, number of previous hospitalisations‐ n.i., age at onset‐ mean 27.3 years, severity of illness‐ mean CGI 3.8; mean BPRS total score 28.5, baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: various antipsychotic drugs. Flexible dose, minimum 100 mg/day chlorpromazine equivalent. Allowed dose range: 100 mg ‐ unlimited chlorpromazine equivalents/day. Mean dose: 201 mg/day. N = 122. 2. No treatment (= crisis management, medication was only given in case of a full relapse). Duration of taper: 50% every two weeks, thus after 6 weeks only 12.5% of initial dose left, thus 42 days. Note that participants were not withdrawn after they had received crisis intervention. N = 115. Rescue medication: in the no treatment group additional antipsychotic medication could only be given in case of relapse. |
|
| Outcomes |
Examined Relapse: BPRS total score ‐ >10 increase, GAS < 20 reduction, deterioration Clinical Global Impression Scale CGI >7. Leaving the study early. Service use: number of participants hospitalised. Unable to use/Not included Global state: CGI (no usable data ). Mental state: BPRS, AMDP system, Paranoid Depression Scale (all no means, no SDs / no predefined outcome of interest). Functioning: Strauss and Carpenter scale, another scale validated by the study group (no usable data) Subjective well‐being (own scale ‐ no mean, no SD/no predefined outcome of interest). Adverse effects: extrapyramidal side‐effects (AIMS ‐ no SD, SAS, Dosage Record and Treatment Emergent Symptoms Scale ‐ all no means, no SDs/continuous side‐effect results were not among the predefined outcomes of interest). Concept of illness (concept of illness scale ‐ no mean, no SD). Compliance: doctors’ assessment (no predefined outcome of interest). Physiological measures: routine laboratory, ECG, EEG (no data/no predefined outcome of interest). |
|
| Notes | There was a third group using intermittent treatment which was not of interest for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally randomised by a specialised unit using an "adaptive randomisation method”. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open, only key rating scales were additionally rated by a second blind assessor. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Open, only key rating scales were additionally rated by a second blind assessor. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Open, only key rating scales were additionally rated by a second blind assessor. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High two‐year discontinuation rate of 43.7%. Analysis was ITT based on Kaplan‐Meier survival curve analysis, completer analyses were presented in addition if different. A risk of bias can not be excluded given the high discontinuation rate. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear evidence for other bias. |
Various drugs 2011.
| Study characteristics | ||
| Methods | Randomisation: an independent rater created randomisation lists stratified for gender with randomly permuted blocks of 4 allocation groups. Allocation: procedure not described. Blinding: open. Duration: 24 months. Design: parallel. Location: multi‐centre. Setting: outpatient. |
|
| Participants | Diagnosis: first episode schizophrenia (DSM‐IV). N = 20. Gender: 17 men, 3 women. Age: mean 29.8 years. History: duration stable‐ 1 year, duration ill‐ 2.6 years, number of previous hospitalisations‐ 0, age at onset‐ 27.3 years, severity of illness‐ PANSS total score 49, baseline antipsychotic dose‐ 3 mg/day haloperidol equivalents (olanzapine, risperidone, quetiapine, zuclopenthixol). |
|
| Interventions | 1. Drug: olanzapine, risperidone, quetiapine, zuclopenthixol. Flexible doses. Mean dose: n.i. N = 9. 2. No treatment: duration of taper: 6 to 12 weeks. N = 11. Rescue medication: not indicated. |
|
| Outcomes |
Examined Relapse: scale defined or need of hospitalisation for any psychiatric indication. Leaving the study early. Rehospitalisation. |
|
| Notes | Sponsor: The Netherlands Organisation for Health Research and Development and EliLilly. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent rater created randomisation lists stratified for gender with randomly permuted blocks of 4 allocation groups. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study. |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open study. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Open study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 out of 20 participants left the study early (25%). Probably an acceptable rate, there was no big difference between drug and placebo group. Kaplan‐Meier survival curves were used for the primary outcome relapse. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | High risk | Premature termination after interim analysis. |
Ziprasidone 2002.
| Study characteristics | ||
| Methods | Randomisation: random, computer‐generated randomisation code. Allocation: drug treatment cards numbered for each subject entering the double‐blind phase; investigator and pharmacist allocated numbers to subjects in strict sequence of entry into the study. Blinding: double‐blind, identical capsules. Duration: 12 months. Design: parallel. Location: multi‐centre (26 European centres). Setting: inpatient. | |
| Participants | Diagnosis: chronic, stable schizophrenia (DSM‐III‐R), less than markedly ill on Clinical Global Impression Scale. 56% of the participants had predominantly negative symptoms at baseline. N = 278 (originally 294 were randomised, but 16 were then excluded from all the analyses due to protocol deviations in one centre). Gender: 203 men, 75 women. Age: mean 49.7 years. History: duration stable‐ n.i., duration ill‐ mean 21.8 years, number of previous hospitalisations‐ mean 10.1, duration of current hospitalisation‐ 68 months, age at onset‐ mean 27.9 years, severity of illness‐ mean PANSS 85.8, mean CGI severity 4.02, baseline antipsychotic dose n.i.. |
|
| Interventions | 1. Drug: ziprasidone ‐ Fixed doses of 40 mg/day, 80 mg/day or 160 mg/day.** N = 207 (originally 219 randomised). 2. Placebo: duration of taper < 3 days. N = 71 (originally 75 randomised). Rescue medication: anticholinergics, lorazepam, temazepam, no additional antipsychotic medication. |
|
| Outcomes |
Examined Relapse: (CGI of much worse or more, PANSS items hostility or uncooperativeness > 6, or in need for additional treatment for exacerbation of symptoms). Leaving the study early. Social functioning: Global Assessment of Functioning scale. Adverse events Violent/aggressive behaviour. Death. Suicidal ideation. Unable to use/Not included Mental state: PANSS total score and subscores (no predefined outcome of interest). Global state: Clinical Global Impression Severity Scale (no usable data for remission). Service use ‐ number of participants hospitalised (no usable data, unclearly reported). Subjective well‐being: own scale (no usable data). Concept of illness: Concept of illness scale (no predefined outcome of interest). Adverse effects: extrapyramidal symptoms (SAS, BAS, AIMS ‐ all no SD/continuous side‐effect results were not among the prespecified outcomes of interest). Physiological measures: ECG, vital signs, weight, ophthalmological assessment, lab tests (all no SD, no data/not prespecified outcomes of interest). |
|
| Notes | ** The results of the three dose groups were pooled. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, computer‐generated randomised code. |
| Allocation concealment (selection bias) | Low risk | Drug treatment cards numbered for each participant entering the double‐blind phase; investigator and pharmacist allocated numbers to participants in strict sequence of entry into the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, identical capsules. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 64% of the participants left the study early, most due to relapse. The rate was higher in the placebo group (86%) than in the medication group (~57%). This was probably not a problem for the primary outcome relapse, but for secondary outcomes for which the LOCF method was used. Appropriate survival curve analysis was used for the primary outcome relapse. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | No obvious other bias. |
Zotepine 2000.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated randomisation list. Allocation: allocation to treatment was on a double‐blind basis, codes were not broken until the time of analysis. Blinding: double‐blind, no further details. Duration: 26 weeks. Design: parallel. Location: multi‐centre, multi‐national. Setting: inpatient (N = 33) and outpatient (N = 86), sponsored. | |
| Participants | Diagnosis: chronic schizophrenia (DSM‐III‐R), at least mildly ill according to CGI, had a history of recurrence in last 18 months, currently maintained on antipsychotic medication. N = 121. Gender: 82 men, 37 women (intent‐to‐treat dataset). Age: 42.3 years. History: duration stable‐ n.i., duration ill‐ mean 13.6 years, number of previous hospitalisations‐ n.i., age at onset‐ mean 28.7 years, severity of illness‐ mean BPRS 49.1, mean CGI 4.2, baseline antipsychotic dose‐ n.i.. |
|
| Interventions | 1. Drug: zotepine. Fixed dose of 300 mg/day which could be reduced once to 150 mg/day. Mean dose: n.i.. N = 63. 2. Placebo:duration of taper: 0 days. N = 58. Rescue medication: antipsychotic drugs not allowed, but benzodiazepines. |
|
| Outcomes |
Examined Relapse: (i) a moderate clinical deterioration from baseline (an increase in CGI severity score of at least 2 points plus an increase of 2 points in at least two positive symptom items on the BPRS persisting for two assessments over 3 days, but not requiring hospitalisation; (ii) deterioration requiring hospitalisation accompanied, on one assessment, by an increase in CGI severity score of at least 2 points plus an increase of 2 points in at least two positive symptom items on the BPRS; and (iii) severe clinical deterioration (an increase in CGI severity score to ‘severely ill’ for 24 hours, or, if in hospital, requiring special observation for suicidal or aggressive behaviour). Leaving the study early. Global state: number of participants improved (CGI based). Global state: number of participants in remission (CGI based). Adverse effects: binary outcomes ‐ open interview. Suicide ideation Unable to use/Not included Mental state: BPRS, SANS (no prespecified outcomes of interest). Adverse effects: extrapyramidal side‐effects (SAS, AIMS, no SD/continuous side‐effect results were not among the prespecified outcomes). Physiological measures: laboratory, vital signs, ECG (all no data/no prespecified outcomes of interest). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Allocation to treatment was on a double‐blind basis, codes were not broken until the time of analysis. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind, no further details. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Double‐blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall rate of participants leaving the study early was very high (76%) and many more participants in the placebo group than in the drug group dropped out due to relapse. Kaplan‐Meier survival analysis was used for primary outcome relapse. No full ITT analysis, only those participants with at least one post‐baseline assessment were included, but only two participants were excluded on this basis. |
| Selective reporting (reporting bias) | High risk | Only those adverse events that were reported on at least four occasions and serious adverse events were reported. |
| Other bias | Low risk | No clear other bias. |
General abbreviations
CNS: central nervous system CPZ: chlorpromazine DSM: Diagnostic and Statistical Manual of Mental Disorders ECG: electrocardiography ECT: electroconvulsive therapy EASY: Early Assessment Service for Young People with Psychosis EEG: electroencephalography EPS: extrapyramidal symptoms HbA1c: glycated haemoglobin ICD: International Statistical Classification of Diseases and Related Health Problems IM: intramuscular injection ITT: intention to treat LAI: long‐acting injectable LOCF: last observation carried forward n.i.: not indicated SD: standard deviation Rating scales
AIMS: Abnormal Involuntary Movement Scale AMDP: Arbeitsgemeinschaft für Methodik und Dokumentation in der Psychiatrie BAS: Barnes Akathisia Scale BPRS: Brief Psychiatric Rating Scale CGI: Clinical Global Impression ‐S: severity, ‐I: improvement GAS: Global Assessment Scale IMPS: Inpatient Multidimensional Psychiatric Rating Scale MMPI: Minnesota Multiphasic Personality Inventory NOSIE: Nurses Observation Scale for Inpatient Evaluation PANSS: Positive And Negative Syndrome Scale PRP: Psychotic Reaction Profile PRS: Psychiatric Rating Scale PSE: Present State Examination RDC: Research Diagnostic Criteria SADS: Schedule for Affective Disorders SANS: Scale for the Assessment of Negative Symptoms SAS: Simpson‐Angus Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 1997 | Allocation: controlled clinical trial, not randomised. |
| Bai 2003 | Allocation: randomised. Participants: not stabilised on antipsychotic drugs. |
| Bechdolf 2016 | Allocation: randomised. Participants: including only those at clinical high risk for psychosis (CHR). |
| Bo 2017 | Allocation: randomised. Participants: clinically stable for at least 4 weeks, treated with risperidone monotherapy at an optimal dose. Intervention: risperidone (baseline dose), risperidone (gradual dose reduction by 50%), no placebo arm. |
| Bourin 2008 | Allocation: randomised. Participants: not stabilised on antipsychotic drugs. |
| Branchey 1981 | Allocation: not randomised, matched groups. |
| Breier 1987 | Allocation: not randomised. |
| Brown 2018 | Allocation: randomised. Participants: mild‐to‐moderate schizophrenia, unclear clinical stability. Intervention: BI 409306 (inhibitor of phosphodiesterase 9A), not currently approved for schizophrenia, versus placebo. |
| Cather 2018 | Allocation: cluster randomised. Participants: first episode of non‐affective psychosis, Intervention: community care compared to NAVIGATE program, which included individual resilience therapy, family education, supported employment and personalised medication management. The specific effect of maintenance therapy with antipsychotic treatment cannot be assumed from this design. |
| Cheng 2019 | Allocation: randomised. Participants: first episode schizophrenia, stabilised on antipsychotic medication. Intervention: risperidone versus olanzapine versus aripiprazole; participants failing the initially‐assigned antipsychotic were switched to one of the other two. No real placebo or discontinuation arm. |
| Chopra 2019 | Allocation: randomised,no hint for real maintenance study design. Participants: first episode schizophrenia; inclusion criteria allow acute patients. |
| Chouinard 1980 | Allocation: not randomised. |
| Chouinard 1993 | Allocation: randomised. Participants: not clinically stable. |
| Claghorn 1974 | Allocation: randomised. Participants: schizophrenia. Intervention: thiothixene alone versus thiothixene plus group therapy versus chlorpromazine alone versus chlorpromazine plus group therapy. |
| Clark 1967 | Allocation: randomised. Participants: not stabilised on antipsychotic drugs (discontinued medication for at least 6 months before study entry). |
| Collins 1967 | Allocation: not randomised. |
| Condray 1995 | Allocation: not randomised. |
| Curson 1985 | Allocation: not randomised. |
| Degkwitz 1970 | Allocation: not randomised. |
| Diamond 1960 | Allocation: not randomised. |
| Double 1993 | Allocation: randomised. Participants: schizophrenia. Intervention: all participants were on neuroleptics and antiparkinson medication at baseline. They were then randomised to neuroleptics plus continuation of antiparkinson medication versus neuroleptics alone. |
| Durgam 2016 | Allocation: randomised. Participants: acute exacerbation of schizophrenia. |
| Engelhardt 1967 | Allocation: randomised. Participants: outpatients with chronic schizophrenia not truly stabilised on antipsychotic drugs. |
| Fleischhacker 2014 | Allocation: randomised. Participants: schizophrenia, receiving maintenance treatment, stabilised on study drug for at least 8 weeks. Intervention: aripiprazole LAI (400 mg/4 weeks), aripiprazole oral (10 mg/day to 30 mg/day), aripiprazole LAI suboptimal dose (50 mg/4 weeks), no real placebo arm. |
| Francey 2018 | Allocation: randomised. Participants: first episode of psychosis, not stable on antipsychotic medication. |
| Freedman 1982 | Allocation: randomised. Participants: discontinued antipsychotic medication for some weeks, then kept in the study only if showing signs of psychotic exacerbation. |
| Gallant 1964 | Allocation: randomised. Participants: unclear baseline clinical status and unclear whether they were stabilised on antipsychotic medication. Intervention: I. butaperazine, trifluoperazine, inert placebo; II. trifluperidol, trifluoperazine, phenobarbital. Outcome: no predefined outcome of interest. |
| Gitlin 1988 | Allocation: randomised (no further details). Participants: schizophrenia or schizoaffective disorder, stabilised on the same depot medication for 1 year. Intervention: fluphenazine decanoate, placebo (cross‐over design). Outcome: no usable data for relevant outcomes (data up to the point of first cross‐over are not available). |
| Gitlin 2001 | Allocation: randomised. Participants: schizophrenia or schizoaffective disorder, clinically stable and with stabilised maintenance antipsychotic therapy. Intervention: fluphenazine decanoate, placebo; cross‐over design. Outcome: no usable data (data up to the point of first cross‐over are not available). |
| Gleeson 2004 | Allocation: randomised. Participants: first‐episode psychosis. Intervention: treatment as usual (including antipsychotics) versus multimodal relapse prevention therapy (including antipsychotics and cognitive behavioral therapy/family intervention). |
| Goldberg 1967 | Allocation: not randomised. |
| Good 1958 | Allocation: randomised. Participants: schizophrenia. Interventions: chlorpromazine versus placebo. Outcomes: no usable outcomes. |
| Greenberg 1966 | Allocation: randomised. Participants: patients with chronic schizophrenia. Intervention: abrupt versus gradual withdrawal of chlorpromazine, but chlorpromazine was withdrawn from both groups. Thus not appropriate control group. |
| Hine 1958 | Allocation: not randomised. |
| Hirsch 1989 | Allocation: randomised. Participants: schizophrenia, clinically stable for at least 6 months, no florid psychotic symptoms. Intervention: fluphenazine decanoate, placebo. Outcome: no usable data for relevant outcomes. |
| Hirsch 1996 | Allocation: randomised (no further details). Participants: schizophrenia (DSM‐III‐R), clinically stable and receiving maintenance treatment. Intervention: fluphenazine depot, placebo. Outcome: no usable data for relevant outcomes (not presented for the randomised subset). |
| Hunt 1967 | Allocation: not randomised. |
| Ionescu 1983 | Allocation: not randomised. |
| Janecek 1963 | Allocation: randomised. Participants: 50% not diagnosed as with schizophrenia. |
| Johnstone 1988 | Allocation: not randomised. |
| Keefe 2018 | Allocation: randomised. Participants: schizophrenia with relevant negative symptoms, unclear clinical stability. Intervention: MIN‐101 (roluperidone), not currently approved, versus placebo. |
| Kellam 1971 | Allocation: not randomised. |
| Lauriello 2005 | Allocation: randomised. Participants: participants were acutely ill, not stable. |
| Lecrubier 1997 | Allocation: randomised. Participants: not stable, not all on antipsychotics before the study. |
| Liu 2018 | Allocation: randomised. Participants: schizophrenia‐related psychotic disorders, under remitted states. Intervention: maintenance therapy with antipsychotics versus guided dose reduction; no real discontinuation or placebo arm. |
| Loo 1997 | Allocation: randomised. Participants: participants were not stable, most not on antipsychotics before the study. |
| Mahal 1975 | Allocation: randomised. Participants: schizophrenia, on maintenance phenotiazine medication Intervention: pimozide, placebo; cross‐over design. Outcome: no usable data for relevant outcomes. |
| Marder 1994 | Allocation: randomised. Participants: not clinically stable. |
| Mathur 1981 | Allocation: randomised. Participants: chronic schizophrenia, stabilised on antipsychotic treatment for at least 6 months before study entry. Intervention: chlorpromazine, placebo; cross‐over design. Outcome: no usable data for relevant outcomes (data up to the point of first cross‐over are not available). |
| Meehan 2019 | Allocation: randomised. Participants: schizophrenia, not adequately stable. |
| Mefferd 1958 | Allocation: randomised. Participants: men with schizophrenia. Intervention: chlorpromazine versus placebo. Outcome: no usable outcome. |
| Mosher 1975 | Allocation: not randomised. |
| Müller 1982 | Allocation: some of the participants were matched, not randomised. |
| NCT03559426 | Allocation: randomised. Participants: schizophrenia spectrum disorders, currently on antipsychotic medication. Intervention: maintenance treatment with antipsychotics versus dose reduction programme; no real discontinuation or placebo arm. |
| Nishikawa 1989 | Allocation: randomised. Participants: outpatients diagnosed with schizophrenia, in remission at baseline. Intervention: timiperone, sulpiride, placebo. The placebo arm was only retrospective, derived from data from previous studies. |
| Oosthuizen 2003 | Allocation: randomised. Participants: first episode of psychosis, not clinically stable. |
| Pasamanick 1967 | Allocation: randomised. Participants: 152 state hospital patients with schizophrenia (severely impaired at time of enrollment), 29 ambulatory schizophrenia patients (not acutely ill but recruited only if severe enough to warrant hospitalisation). |
| Paul 1972 | Allocation: not randomised. |
| Peet 1981 | Allocation: randomised. Participants: schizophrenia. Intervention: chlorpromazine versus chlorpromazine plus propranolol. |
| Pickar 1986 | Allocation: not randomised. |
| Pickar 2003 | Allocation: not randomised. |
| Pigache 1993 | Allocation: randomised. Participants: chronic schizophrenia. Intervention: chlorpromazine, placebo, orphenadrine. Outcome: no relevant outcome, only auditory attention task. |
| Ran 2002 | Allocation: randomised. Participants: chronic schizophrenia, unclear clinical status, 30% uncovered Intervention: antipsychotic therapy + family psychoeducational intervention, antipsychotic treatment alone, control group (in which quote: "medication was not encouraged nor discouraged"). |
| Rassidakis 1970 | Allocation: not randomised. |
| Ravaris 1965 | Allocation: randomised. Participants: chronic schizophrenia. Intervention: fluphenazine elixir plus placebo injection versus fluphenazine enanthate injection plus oral placebo. |
| Ruiz 1975 | Allocation: randomised. Participants: chronic schizophrenia, same antipsychotic treatment for at least one month before study entry. Intervention: pimozide, placebo. Outcome: no usable data for relevant outcomes (data for the double‐blind phase are not avaiable). |
| Ruiz Veguilla 2013 | Allocation: randomised. Participants: diagnosed with non‐affective psychosis (first episode), receiving antipsychotic treatment for 12 months since clinical stabilisation, at the same dose for at least 4 months. Intervention: continual antipsychotic treatment, treatment discontinuation. Outcome: study not performed (stopped after recruitment of 16 patients), no data available. |
| Schlossberg 1978 | Allocation: randomised. Participants: not stable. |
| Singer 1971 | Allocation: randomised. Participants: unclear if clinically stable, probabily not taking antipsychotics before study entry. Intervention: thiopropazate, placebo; cross‐over design. Outcome: no usable data for relevant outcomes (data up to the point of first cross‐over are not available). |
| Singh 1990 | Allocation: not randomised. |
| Smelson 2006 | Allocation: not randomised. |
| Soni 1990 | Allocation: randomised. Participants: schizophrenia, not stabilised on antipsychotic drugs, because all had been withdrawn from antipsychotic drugs for 8 to 20 months before study start. |
| Stuerup 2017 | Allocation: randomised. Participants: participants with newly diagnosed schizophrenia spectrum disorder, from the outpatient early intervention program (OPUS), meeting remission criteria for at least 3 months before study entry. Intervention: maintenance treatment with antipsychotics versus tapering/discontinuation; the control arm does not necessarily imply complete discontinuation of antipsychotic medication in all cases. |
| Sumitomo 2008 | Allocation: randomised. Participants: schizophrenia, not described as clinically stable in inclusion criteria. |
| Vaddadi 1986 | Allocation: randomised. Participants: schizophrenia. Intervention: depot antipsychotics (fluphenazine depot, flupenthixol depot or clopenthixol depot) plus oral dihomo gammalinolenic acid (DHLA) versus oral DHLA plus placebo injections versus DHLA placebo capsules and placebo injections. What is lacking is a depot antipsychotic only group. |
| Van Kammen 1982 | Allocation: not randomised. |
| Van Praag 1973 | Allocation: randomised. Participants: psychotic participants. Intervention: fluphenazine enanthate versus fluphenazine decanoate. |
| Vanover 2018 | Allocation: randomised. Participants: acute exacerbation of schizophrenia. |
| Weller 2018 | Allocation: randomised. Participants: young people with a first episode of affective/non‐affective psychosis (unclear proportion), meeting remission criteria for at least 3 months. Intervention: maintenance treatment with antipsychotic drugs versus dose reduction strategy; no real discontinuation or placebo arm. |
| Wiedemann 2001 | Allocation: randomised. Participants: schizophrenia. Intervention: continuation of current antipsychotic versus gradual withdrawal. However, antipsychotic was given again when early warning signs appeared, i.e. intermittent treatment, a design that was excluded a prior by our protocol. |
| Wright 1964 | Allocation: not randomised. |
| Wunderink 2006 | Allocation: randomised. Participants: schizophrenia and related psychotic disorder. Intervention: continuation of current antipsychotic versus gradual withdrawal. However, antipsychotic was given again when early warning signs appeared, i.e. intermittent treatment, a design that was excluded by the protocol. Approximately 50% of participants were never withdrawn. |
| Zeller 1956 | Allocation: all participants were in hospital. 95 were allocated to placebo (not randomly). Then 81 participants were quote: "selected at random to match” the intervention group. We feel that this is not an appropriate method of randomisation. |
| Zou 2018 | Allocation: randomised. Participants: people with schizophrenia, experiencing an acute episode. |
| Zwanikken 1973 | Allocation: randomised. Participants: more than 50% had mental retardation, not schizophrenia. |
DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, Third Edition‐Revised LAI: LAI: long‐acting injectable
Characteristics of studies awaiting classification [ordered by study ID]
Ascher‐Svanum 2011.
| Methods | Allocation: post‐hoc analysis of a 1‐year randomised open‐label trial. |
| Participants | Diagnosis: schizophrenia. |
| Interventions | 1. Switching of antipsychotic medication 2. Discontinuation of antipsychotic therapy |
| Outcomes | Change scores on standard efficacy and tolerability measures (no usable data reported). |
| Notes | The conference abstract did not report any usable data for outcomes of interest in this review. We tried to contact the trials Authors to ask for further data but did not receive any reply. |
Decot 2011.
| Methods | Allocation: the study is described as double‐blind; no details about random sequence generation and allocation concealment procedure. Cross‐over design. |
| Participants | Diagnosis: schizophrenia. No details about the baseline clinical status. |
| Interventions | 1. Standard antipsychotics 2. Placebo |
| Outcomes | Efficacy, analysis based on the COMT Val108/158Met polymorphism (no usable data reported). |
| Notes | The conference abstract did not report any usable data for outcomes of interest in this review. We tried to contact the trial Authors to ask for further data but did not receive any reply. |
Eisenberg 2016.
| Methods | Allocation: the study is described as blinded; no details about random sequence generation and allocation concealment. |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder. No details about the baseline clinical status. |
| Interventions | 1. Atypical antipsychotic monotherapy 2. Placebo |
| Outcomes | Cognitive function, positron emission tomography scanning (no predefined outcomes of interest). |
| Notes | The conference abstract did not report any usable data for outcomes of interest in this review. We tried to contact the trial authors to ask for further data but did not receive an informative reply. |
EUCTR2005‐005499‐34.
| Methods | Allocation: randomised. Location: multi‐centre. |
| Participants | Diagnosis: stable schizophrenia. |
| Interventions | 1, Bifeprunox (not marketed drug) 2. Quetiapine 3. Placebo |
| Outcomes | Standard efficacy, safety and tolerability measures. |
| Notes | Prematurely ended, no data available. We were not able to find further information. |
Zhang 2006.
| Methods | Allocation: randomised. Setting: inpatients. |
| Participants | Diagnosis: "deteriorated" schizophrenia, on antipsychotic treatment before study entry (no details). |
| Interventions | 1. Standard antipsychotic treatment 2. Discontinuation of antipsychotic treatment |
| Outcomes | Efficacy on negative symptoms (no predefined outcome of interest). |
| Notes | The paper did not report any usable data for outcomes of interest in this review. We tried to contact the trial authors to ask for further data but did not receive an informative reply. |
Characteristics of ongoing studies [ordered by study ID]
NCT03503318.
| Study name | A multicenter, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy, safety, and tolerability of risperidone extended‐release injectable suspension (TV‐46000) for subcutaneous use as maintenance treatment in adult and adolescent patients with schizophrenia. |
| Methods | Randomised controlled trial. |
| Participants | Individuals diagnosed with schizophrenia, clinically stable and eligible for risperidone treatment. |
| Interventions | Risperidone ER injectable suspension, subcutaneous injections (two different dose regimens) versus placebo. |
| Outcomes | Efficacy, safety and tolerability outcomes (time to impending relapse, number maintaining stability, number acheiving remission, number with adverse events). |
| Starting date | April 2018 |
| Contact information | USMedInfo@tevapharm.com |
| Notes |
NCT03593213.
| Study name | Clinical trial evaluating the efficacy, safety and tolerability of cariprazine in a dose‐reduction paradigm in the prevention of relapse in patients with schizophrenia. |
| Methods | Randomised controlled trial. |
| Participants | Schizophrenia, in maintenance/relapse prevention phase. |
| Interventions | Cariprazine (two different dose regimens) versus placebo. |
| Outcomes | Time to impending relapse. |
| Starting date | July 2018 |
| Contact information | IR‐CTRegistration@Allergan.com |
| Notes |
NCT03893825.
| Study name | A study to test if TV‐46000 is safe for maintenance treatment of schizophrenia |
| Methods | Randomised controlled trial. |
| Participants | Schizophrenia, in maintenance/relapse prevention phase. |
| Interventions | TV‐46000 (risperidone extended release injectable suspension) versus matching placebo. |
| Outcomes | Number of adverse events, dropouts due to adverse events. |
| Starting date | April 2019 |
| Contact information | USMedInfo@tevapharm.com |
| Notes |
Differences between protocol and review
In the original review, instead of Stata 2002 we used Comprehensive Meta‐analysis Version 2 (Borenstein 2006) for the meta‐regression; in the review update the meta‐regression analyses were performed using meta v4.9‐2 (Schwarzer 2007) in R statistical language v3.5 (R Core Team 2018), number needed to treat for an additional beneficial outcome (NNTB) and number needed to treat for an additional harmful outcome (NNTH) were calculated as the inverse of the risk difference rather than using Visual Rx. Various subgroup and meta‐regression analyses were added and the method section on the investigation of heterogeneity changed to reflect this. Post‐hoc analyses were clearly marked as such using an asterisk*. We only contacted the manufacturers of so‐called second‐generation antipsychotic drugs for further trials (Sanofi‐Aventis, Astellas, Bristol‐Myers Squibb, Novartis, Eli Lilly, AstraZeneca, Janssen‐Cilag, Lundbeck and Pfizer; asenapine, iloperidone and lurasidone were not available at the time of our first search and therefore not contacted). Our attempts to contact the manufacturers of old "first‐generation antipsychotic drugs" had not been successful and most of these trials had been published more than 15 years ago (the official time trial documents must be stored in many countries).
Methodological differences between original review and update
Four additional secondary outcomes were addressed (symptomatic remission, sustained remission, recovery, social functioning); certainty of the evidence on social functioning (instead of satisfaction with care) was also investigated using the GRADE approach. For continuous outcomes, change data were preferred over endpoint data. In order to adapt to the progressive development in systematic review methods, the assessment of the risk of bias due to blinding of participants and personnel was added as a further element (in the original review, only blinding of subjective and objective outcomes assessment was addressed).
Contributions of authors
Anna Ceraso: designing the review update, study selection, data extraction, statistical analysis and writing of the report. Jessie Lin: designing the review update, study selection, data extraction, statistical analysis and writing of the report. Johannes Schneider‐Thoma: designing the review update, study selection, data extraction, statistical analysis and writing of the report. Spyridon Siafis: statistical analysis and writing of the report. Magdolna Tardy: designing the original review, study selection, data extraction, statistical analysis and writing of the report. Katja Komossa: designing the original review, study selection, data extraction and writing of the report. Stephan Heres: designing the original review, data extraction and writing of the report. Werner Kissling: designing the original review, data extraction and writing of the report. John M. Davis: designing the original review, data extraction, statistical analysis and writing of the report. Stefan Leucht: designing the original review and the update, study selection, data extraction, statistical analysis and writing of the report.
All the authors of the original review have agreed to a co‐publication of this review in the Lancet (Leucht 2012a).
Sources of support
Internal sources
Freistaat Bayern, Germany
Psychiatric Institute, University of Chicago at Illinois, USA
University of Brescia, Italy
External sources
Bundesministerium für Bildung und Forschung Grant number 01KG0816 88166528, Germany
University of Hong Kong/China Medical Board (CMB) Grant, Hong Kong
Declarations of interest
Anna Ceraso: none to declare. Jessie Lin: none to declare. Johannes Schneider‐Thoma: none to declare. Spyridon Siafis: none to declare. Magdolna Tardy: none to declare. Katja Komossa: none to declare. Stephan Heres: received speaker honoraria from Janssen‐Cilag, Eli Lilly, Sanofi‐Aventis, Otsuka, Lundbeck and Johnson & Johnson; accepted travel or hospitality payment from Janssen‐Cilag, Sanofi‐Aventis, Johnson & Johnson, Pfizer, Bristol‐Myers‐Squibb, AstraZeneca, Lundbeck, Novartis and Eli Lilly; participated in clinical trials sponsored or supported by Eli Lilly, Janssen Cilag, Johnson & Johnson, Bristol‐Myers‐Squibb, AstraZeneca, Lundbeck, Novartis, Servier, Pierre Fabre, Pfizer, Organon, Roche and Merck; and received honoraria for participation in advisory‐boards or activities as a consultant from Lundbeck, Otsuka, Eli Lilly, Roche, Teva, Janssen and Johnson & Johnson. Werner Kissling: has received honoraria for board memberships, consulting and lectures from Janssen and Eli Lilly; honoraria for development of educational materials from Janssen; grant support from Janssen and AstraZeneca; and travel/accommodation expenses from AstraZeneca, Eli Lilly and Janssen. John M Davis: none to declare. Stefan Leucht: In the last three years Stefan Leucht has received honoraria for lectures or consulting from LB Pharma, Otsuka, Lundbeck, Boehringer Ingelheim, LTS Lohmann, Janssen, Johnson&Johnson, TEVA, MSD, Sandoz, SanofiAventis, Angelini, Recordati, Sunovion, Geodon Richter.
These authors contributed equally to this work.
These authors contributed equally to this work.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aripiprazole 2003 {published data only}6164
- Baker RA, Meyer JM, Kaplita S, Forbes A, Grossman F, Pikalov A, Marcus RN. Long-term effects of aripiprazole on the lipid profiles of patients with schizophrenia in a 26-week placebo-controlled trial. Schizophrenia Bulletin 2007;33(2):S419. [CSZG: 15132] [Google Scholar]
- Carson W, McQuade R, Saha A, Torbeyns A, Stock E. Aripiprazole versus placebo for relapse prevention in patients with chronic schizophrenia. European Neuropsychopharmacology 2002;12(Suppl 3):S288. [CSZG: 9067] [Google Scholar]
- Carson W, Pigott T, Saha A, Ali M, McQuade RD, Torbeyns AF, et al. Aripiprazole vs placebo in the treatment of stable, chronic schizophrenia. In: Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17-22; San Francisco, California, USA. 2003. [CSZG: 9986]
- Carson W, Weiden P, Waldeck R, Tafesse E, Cislo P, L'Italien G, Iwamoto T. Aripiprazole is not associated with increased diabetes risk: a long-term model. Schizophrenia Research 2004;67(Suppl 1):181. [CSZG: 13175] [Google Scholar]
- Carson WH, Ingenito GG, McQuade RD, Stock EG, Iwamoto T. Schizophrenia: safety/tolerability of aripiprazole. In: Proceedings of the 12th World Congress of Psychiatry; 2002 Aug 24-29; Yokohama, Japan. 2002. [CSZG: 19426]
- Carson WH, Pigott TA, Saha AR, Ali MW, McQuade RD, Torbeyns AF, et al. Aripiprazole vs placebo in the treatment of chronic schizophrenia. International Journal of Neuropsychopharmacology 2002;5(Suppl 1):S187. [CSZG: 8326] [Google Scholar]
- Casey D, Saha A, Marcus R, Carson WH, McQuade RD, Torbeyns AF, et al. Aripiprazole versus placebo for relapse prevention in patients with chronic schizophrenia. Schizophrenia Research 2003;60:276. [CSZG: 9689] [Google Scholar]
- Casey D, Stock E, Jody D, Kaplita S, Saha A, Liston H, et al. Long-term treatment with aripiprazole: effects on plasma lipis levels and glycaemic control. Int J Neuropsychopharmacol 2004 Jun;7(Suppl 2):S417. [CSZG: 13177] [Google Scholar]
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 1. http://www.fda.gov/cder/foi/nda/2002/21-436_Abilify.htm 2002:1-50. [CSZG: 9980]
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 2. http://www.fda.gov/cder/foi/nda/2002/21-436_Abilify.htm 2002:50-110. [CSZG: 9981]
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 3. http://www.fda.gov/cder/foi/nda/2002/21-436_Abilify.htm 2002:111-75. [CSZG: 9982]
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. http://www.fda.gov/cder/foi/nda/2002/21-436_Abilify.htm 2002:176-232. [CSZG: 9983]
- Marder SR, Kaplita S, Saha AR, Carson WH Jr, Torbeyns A, Stock EG. Glycemic control and plasma lipids in long-term aripiprazole treatment. In: Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17-22; San Francisco, California, USA. 2003. [CSZG: 9799]
- McQuade R, Kostic D, Marcus R, Carson W, Torbeyns A, Kerselaers W, Yocca F. Aripiprazole versus olanzapine in a 52-week, open-label extension study. Schizophrenia Bulletin 2005;31:S496-7. [CSZG: 11624] [Google Scholar]
- Mortimer AM. Aripiprazole is effective for relapse prevention in people with chronic stable schizophrenia. Evidence-Based Mental Health 2004;7(2):41. [CSZG: 13962] [DOI] [PubMed] [Google Scholar]
- Pigott TA, Carson WH, Saha AR, Torbeyns AF, Stock EG, Ingenito GG. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. Journal of Clinical Psychiatry 2003;64:1048-56. [CSZG: 9896] [DOI] [PubMed] [Google Scholar]
- Pigott TA, Saha AR, Ali MW, McQuade RD, Torbeyns AF, William HC, et al. Aripiprazole versus placebo in the treatment of chronic schizophrenia. In: Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18-23; Philadelphia, Pennsylvania, USA. 2002. [CSZG: 9018]
- Stock E, Marder SR, Jody D, Kaplita S, Saha A, Carson W, et al. Plasma lipids levels and glycemic control in long-term treatment with aripiprazole. European Neuropsychopharmacology 2003;13(4):S327. [CSZG: 9854] [Google Scholar]
- Torbeyns A, Marder SR, Carson W, Jody D, Kaplita S, Saba A, et al. Glycemic control and plasma lipids in long-term treatment with aripiprazole. Schizophrenia Research 2004;67(1):192-3. [CSZG: 11339] [Google Scholar]
Aripiprazole 2017 {published data only}16575
- Correll C, Kohegyi E, Zhao C, Baker RA, McQuade R, Salzman P, et al. Oral aripiprazole is an effective maintenance treatment in adolescents with schizophrenia. Neuropsychopharmacology 2014:S349-S350. [CSZG: 29288]
- Correll CU, Kohegyi E, Zhao C, Baker RA, McQuade R, Salzman PM, et al. Oral aripiprazole as maintenance treatment in adolescent schizophrenia: results from a 52-week, randomized, placebo-controlled withdrawal study. Journal of the American Academy of Child and Adolescent Psychiatry 2017;56(9):784-92. [CSZG: 36321] [DOI] [PubMed] [Google Scholar]
- NCT01149655. A long-term multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy, safety, and tolerability of aripiprazole (opc 14597) as maintenance treatment in adolescent patients with schizophrenia. http://ClinicalTrials.gov/show/NCT01149655 2010. [CSZG: 21085]
Aripiprazole depot 2012 {published data only}12553
- Baker R, Fleischhacker W, Sanchez R, Perry P, Jin N, Johnson B, et al. Long-term safety and tolerability of once-monthly aripiprazole intramuscular depot for maintenance treatment in schizophrenia. International Journal of Neuropsychopharmacology 2012;15:111. [CSZG: 28035] [Google Scholar]
- Baker RA, Carson WH, Perry PP, Sanchez R, Zhao J, McQuade RD, et al. Effects of aripiprazole once-monthly vs placebo on domains of personal and social performance in younger and older patients with schizophrenia. CNS Spectrums 2013;18(6):376-7. [CSZG: 28526] [Google Scholar]
- Baker RA, Eramo A, Carson WH, Perry P, Sanchez R, Zhao J, et al. Effects of aripiprazole once-monthly vs. placebo on domains of personal and social performance in younger and older patients with schizophrenia. Schizophrenia Bulletin 2013;39(Suppl 1):S321-S322. [CSZG: 28131] [Google Scholar]
- Baker RA, Eramo A, Nylander AG, Tsai LF, Peters-Strickland TS, Kostic D, et al. The effect of previous dose or oral aripiprazole (10 or 30 mg/day) on the efficacy and tolerability of aripiprazole once-monthly: Results from post-hoc analyses from two double-blind, randomized, controlled trials. Schizophrenia Research 2014;153:S293. [CSZG: 29386] [Google Scholar]
- Carson WH, Perry PP, Sanchez R, Jin N, Forbes RA, McQuade RD, et al. Effects of ARI-IM-depot on secondary efficacy outcomes in maintenance treatment of schizophrenia. International Journal of Neuropsychopharmacology 2012;15:112-3. [CSZG: 28030] [Google Scholar]
- Eramo A, Fleischhacker WW, Sanchez R, Perry P, Jin N, Johnson B, et al. A placebo-controlled study of efficacy and safety of aripiprazole oncemonthly for long-term maintenance treatment in schizophrenia. European Psychiatry 2013;28(1):1. [CSZG: 28187] [Google Scholar]
- Eramo A, Fleischhacker WW, Sanchez R, Tsai LF, Peters-Strickland TS, Baker RA, et al. All-cause discontinuation and safety of aripiprazole once-monthly for the treatment of schizophrenia: A pooled analysis of two double-blind, randomized, controlled trials. Schizophrenia Research 2014;153:S174. [CSZG: 29437] [Google Scholar]
- Fleischhacker WW, Baker RA, Eramo A, Sanchez R, Tsai LF, Peters-Strickland T, et al. Effects of aripiprazole once-monthly on domains of personal and social performance: results from 2 multicenter, randomized, double-blind studies. Schizophrenia Research 2014;159(2-3):415-20. [CSZG: 32163] [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Perry P, Sanchez R, Jin N, Peters-Strickland T, Johnson B, et al. Functional outcomes with aripiprazole once-monthly in two double-blind, placebo- and active-controlled studies (aspire us 246 and aspire eu 247) for the treatment of schizophrenia. Value in Health 2013;16(7):A550. [CSZG: 28480] [Google Scholar]
- Fleischhacker WW, Sanchez R, Johnson B, Jin N, Forbes RA, McQuade R, et al. Long-term safety and tolerability of aripiprazole once-monthly in maintenance treatment of patients with schizophrenia. International Clinical Psychopharmacology 2013;28:171-6. [CSZG: 28391] [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Sanchez R, Lan-Feng T, Peters-Strickland T, Baker RA, Eramo A, et al. Safety and Effectiveness of Aripiprazole Oncemonthly for the Treatment of Schizophrenia: A Pooled Analysis of Two Double-blind, Randomized, Controlled Trials. Neuropsychopharmacology 2013;38:S394-S395. [CSZG: 28459] [Google Scholar]
- Kane JM, Sanchez R, Baker RA, Eramo A, Peters-Strickland T, Perry PP, et al. Patient-centered outcomes with aripiprazole once-monthly for maintenance treatment in patients with schizophrenia: Results from two multicenter, randomized, double-blind studies. Clinical Schizophrenia and Related Psychoses 2015;9(2):79-87. [CSZG: 32183] [DOI] [PubMed] [Google Scholar]
- Kane JM, Sanchez R, Perry PP, Jin N, Johnson B, Forbes RA, et al. Efficacy of aripiprazole-IM-depot for long-term maintenance treatment of schizophrenia. International Journal of Neuropsychopharmacology 2012;15:S119. [CSZG: 28033] [Google Scholar]
- Kane JM, Sanchez R, Perry PP, Jin N, Johnson BR, Forbes RA, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: A 52-week, multicenter, randomized, double-blind, placebo-controlled study. Journal of Clinical Psychiatry 2012;73(5):617-24. [CSZG: 24369] [DOI] [PubMed] [Google Scholar]
- Loze J, Carson WH, Perry PP, Sanchez R, Jin N, Forbes RA, et al. Psychosocial and overall effectiveness of aripiprazole intramuscular depot vs. placebo for maintenance treatment in schizophrenia. European Neuropsychopharmacology 2012;22:S328. [CSZG: 24886] [Google Scholar]
- NCT00705783. Aripiprazole (OPC-14597) as maintenance treatment in patients with schizophrenia. https://www.ClinicalTrials.gov/ct/show/ 2008. [CSZG: 16546]
- Sanchez R, Johnson B, Jin N, Forbes RA, Carson WH, McQuade RD, et al. Patient-reported outcomes (PROs) with aripiprazole intramuscular depot (ARI-IMD) for maintenance treatment in schizophrenia. International Journal of Neuropsychopharmacology 2012;15:133. [CSZG: 28040] [Google Scholar]
- Sanchez R, Perry PP, Jin N, Johnson, RA Forbes, RD McQuade, WH, et al. A placebo-controlled study of efficacy/ safety of aripiprazole intramuscular depot for long-term maintenance treatment of schizophrenia. European Neuropsychopharmacology 2012;(Suppl 2):S327. [CSZG: 24893] [Google Scholar]
Asenapine 2011 {published data only}10424
- Kane JM, Mackle M, Snow-Adami L, Zhao J, Szegedi A, Panagides J. A randomised placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. Journal of Clinical Psychiatry 2011;72(3):349-55. [CSZG: 22873] [DOI] [PubMed] [Google Scholar]
- Kane JM, Mackle M, Snow-Adami L, Zhao J, Szegedi A, Panagides J. Double-blind, placebo-controlled trial of asenapine in prevention of relapse after long-term treatment of schizophrenia. International Journal of Neuropsychopharmacology 2010;13:223. [CSZG: 21634] [Google Scholar]
- Mackle M, Snow-Adami L, Zhao J, Szegedi A, Panagides J. Double-blind, placebo-controlled trial of asenapine in prevention of relapse after long-term treatment of schizophrenia. European Neuropsychopharmacology 2009;19(Suppl 3):S543. [CSZG: 19377] [Google Scholar]
- NCT00150176. A randomized, placebo-controlled, double-blind trial of asenapine in the prevention of relapse after long-term treatment of schizophrenia. https://www.ClinicalTrials.gov/ct/show/ 2005. [CSZG: 14708]
- The NHSC . Asenapine (Saphris) for schizophrenia. Report 2010. [CSZG: 21910]
Brexpiprazole 2017 {published data only}26950
- Baker R, Hobart M, Forbes A, Ouyang J, Weiller E. Effect of brexpiprazole on long-term functioning in adults with schizophrenia. Australian and New Zealand Journal of Psychiatry 2017;51(Suppl 1):148. [CSZG: 35955] [Google Scholar]
- Correll CU, Shi L, Weiss C, Hobart M, Eramo A, Duffy RA, et al. Successful switching of patients with acute schizophrenia from another antipsychotic to brexpiprazole: comparison of clinicians' choice of cross-titration schedules in a post-hoc analysis of a randomized double-blind maintenance treatment study. CNS Spectrums 2019;24(5):507-17. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Hobart M, Ouyang J, Forbes A, Pfister S, McQuade R, et al. Brexpiprazole (OPC-34712) efficacy and safety as maintenance therapy in adults with schizophrenia: Randomised, double-blind, placebo-controlled study. European Neuropsychopharmacology 2015;25:S527. [CSZG: 33322] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischhacker WW, Hobart M, Ouyang J, Forbes A, Pfister S, McQuade RD, et al. Efficacy and sfety of Brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: a randomized, double-blind, placebo-controlled study. International Journal of Neuropsychopharmacology 2017;20(1):11-21. [CSZG: 35475] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01668797. Efficacy, Safety, and tolerability of Brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia. https://ClinicalTrials.gov/show/NCT01668797 2012. [CSZG: 35608]
- Therrien F, Weiss C, Jin N, Baker RA, MacKenzie E, Meehan SR. Effect of brexpiprazole on patient functioning in patients with schizophrenia: results from a long-term, randomised, double-blind, placebo-controlled, maintenance study. Neuropsychopharmacology 2017;43((Suppl.1)):S244-S245. [CSZG: 37935] [Google Scholar]
- Weiller E, Hobart M, Pfster S, Forbes A, Ouyang J, Weiss C. Effect of brexpiprazole on long-term remission in adults with schizophrenia: Results from a randomized, double-blind, placebo-controlled, maintenance study. Schizophrenia Bulletin 2017;43:S202-3. [CSZG: 36050] [Google Scholar]
- Weiss C, Forbes A, Hobart M, Pfster S, Ouyang J, Weiller E. Short-term and long-term efficacy of brexpiprazole in adults with schizophrenia: Effect across marder factors. Schizophrenia Bulletin 2017;43:S155. [CSZG: 36051] [Google Scholar]
- Weiss C, Ouyang J, Eramo A, Duffy RA, Weiller E, Baker RA. Switching patients with acute schizophrenia to brexpiprazole: post-hoc analysis of a double-blind randomized maintenance treatment study. Neuropsychopharmacology 2015;40:S227-8. [CSZG: 33450] [Google Scholar]
- Weiss C, Weiller E, Hobart M, Ouyang J. Effect of brexpiprazole on weight and metabolic parameters: analysis of a maintenance trial in schizophrenia. European Neuropsychopharmacology 2016;26:S556. [CSZG: 35746] [Google Scholar]
Cariprazine 2016 {published data only}18421
- Correll CU, Potkin SG, Durgam S, Chang CT, Szatmari B, Laszlovszky I, et al. Sustained remission with cariprazine treatment: post-hoc analysis of a randomised, double-blind, schizophrenia relapse prevention trial. Europan Neuropsychopharmacology 2017;27((Suppl. 4)):S935-S936. [CSZG: 37757] [Google Scholar]
- Correll CU, Potkin SG, Zhong Y, Harsányi J, Szatmári B, Earley W. Long-term remission with cariprazine treatment in patients with schizophrenia: a post-hoc analysis of a randomized, double-blind, placebo.controlled relapse prevention trial. Journal of Clinical Psychiatry 2019;80(2):18m12495. [DOI] [PubMed] [Google Scholar]
- Durgam S, Earley W, Li R, Li D, Lu K, Laszlovszky I, et al. Corrigendum to "Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: A randomized, double-blind, placebo-controlled trial" [Schizophr. Res. 176 (2016) 264-71]. Schizophrenia Research 2017;InPress:InPress. [CSZG: 35204] [DOI] [PubMed] [Google Scholar]
- Durgam S, Earley W, Li R, Li D, Lu K, Laszlovszky I, et al. Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: A double-blind, placebo-controlled trial. European Neuropsychopharmacology 2015;25:S512-3. [CSZG: 33317] [Google Scholar]
- Durgam S, Earley W, Li R, Li D, Lu K, Laszlovszky I, et al. Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: A randomized, double-blind, placebo-controlled trial. Schizophrenia Research 2016;176(2-3):264-71. [CSZG: 35204] [DOI] [PubMed] [Google Scholar]
- Durgam S, Earley W, Li R, Li D, Lu K, Laszlovszky I, et al. Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: additional analysis from a randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology 2015;40:S380-1. [CSZG: 33316] [Google Scholar]
- Earley W, Guo H, Luchini R. Modified cariprazine relapse prevention clinical trial results. Schizophrenia Research 2018;199:452-3. [DOI] [PubMed] [Google Scholar]
- Kunovac J, Kane J, Durgam S, Chang CT, Nemeth G, Laszlovszky I, et al. Characteristics associated with relapse in patients with schizophrenia: Post hoc analysis of a randomized, double-blind, placebo-controlled, cariprazine relapse prevention trial. Schizophrenia Bulletin 2017;43:S166-7. [CSZG: 36002] [Google Scholar]
- NCT01412060. Cariprazine relative to placebo in the prevention of relapse of symptoms in patients with schizophrenia. http://ClinicalTrials.gov/show/NCT01412060 2011. [CSZG: 23106]
- Potkin S, Correll C, Chang CT, Szatmari B, Laszlovszky I, Earley W. Long-term remission with cariprazine treatment in patients with schizophrenia: a post hoc analysis of a randomized, double-blind, placebo-controlled, relapse prevention trial. Schizophrenia Bulletin 2017;43:S118. [CSZG: 36026] [DOI] [PubMed] [Google Scholar]
Chlorpromazine 1959 {published data only}2474
- Shawver JR, Gorham DR, Leskin LW, Good WW, Kabnick DE. Comparison of chlorpromazine and reserpine in maintenance drug therapy. Diseases of the Nervous System 1959;20:452-7. [CSZG: 2560] [PubMed] [Google Scholar]
Chlorpromazine 1962 {published data only}759
- Freeman LS, Alson E. Prolonged withdrawal of chlorpromazine in chronic patients. Diseases of the Nervous System 1962;23:522-5. [CSZG: 809] [PubMed] [Google Scholar]
Chlorpromazine 1968 {published data only}3766
- Crane GE. Tardive dyskinesia in schizophrenic patients treated with psychotropic drugs. Agressologie 1968;9(2):209-16. [CSZG: 3170] [PubMed] [Google Scholar]
- De Long SL. Incidence and significance of chlorpromazine-induced eye changes. Diseases of the Nervous System 1968;29:19-22. [CSZG: 18229] [PubMed] [Google Scholar]
- Gardos G, Cole JO, LaBrie RA. A 12-year follow-up study of chronic schizophrenics. Psychopharmacology 1982;33:983-4. [CSZG: 853] [DOI] [PubMed] [Google Scholar]
- Prien RF, Cole JO, Belkin NF. Relapse in chronic schizophrenics following abrupt withdrawal of tranquillizing medication. British Journal of Psychiatry 1968;115:679-86. [CSZG: 3951] [DOI] [PubMed] [Google Scholar]
- Prien RF, Cole JO. High dose chlorpromazine therapy in chronic schizophrenia. Report of National Institute of Mental Health - psychopharmacology research branch collaborative study group. Archives of General Psychiatry 1968;18:482-95. [CSZG: 3950] [PubMed] [Google Scholar]
- Prien RF, DeLong SL, Cole JO, Levine J. Ocular changes occurring with prolonged high dose chlorpromazine therapy. Results from a collaborative study. Archives of General Psychiatry 1970;23:464-8. [CSZG: 3952] [DOI] [PubMed] [Google Scholar]
- Prien RF, Levine J, Cole JO. Indications for high dose chlorpromazine therapy in chronic schizophrenia. Diseases of the Nervous System 1970;31:739-45. [CSZG: 2345] [PubMed] [Google Scholar]
- Prien RF, Levine J, Switalski RW. Discontinuation of chemotherapy for chronic schizophrenics. Hospital and Community Psychiatry 1971;22(1):4-7. [CSZG: 2346] [DOI] [PubMed] [Google Scholar]
Chlorpromazine 1973 {published data only}1800
- Goldberg SC, Schooler NR, Hogarty GE, Roper M. Prediction of relapse in schizophrenic outpatients treated by drug and sociotherapy. Archives of General Psychiatry 1977;34:171-84. [CSZG: 1873] [DOI] [PubMed] [Google Scholar]
- Hogarty G, Goldberg SC. Drug and social therapy in schizophrenic outpatients. Unknown Source 1994:18. [CSZG: 5049]
- Hogarty GE, Goldberg S, Schooler N. Proceedings: drug and sociotherapy in the aftercare of schizophrenic patients. Psychopharmacology Bulletin 1974;10(4):47. [CSZG: 4745] [PubMed] [Google Scholar]
- Hogarty GE, Goldberg SC, Schooler NR, Ulrich RF. Drug and sociotherapy in the aftercare of schizophrenic patients. II. Two-year relapse rates. Archives of General Psychiatry 1974;31:603-8. [CSZG: 1876] [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Goldberg SC, Schooler NR. Drug and sociotherapy in the aftercare of schizophrenic patients. III. Adjustment of nonrelapsed patients. Archives of General Psychiatry 1974;31:609-18. [CSZG: 1875] [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Goldberg SC. Drug and sociotherapy in the aftercare of schizophrenic patients. One-year relapse rates. Archives of General Psychiatry 1973;28:54-64. [CSZG: 1874] [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Munetz MR. Pharmacogenic depression among outpatient schizophrenic patients: a failure to substantiate. Journal of Clinical Psychopharmacology 1984;4:17-24. [CSZG: 1877] [PubMed] [Google Scholar]
- Hogarty GE, Schooler NR, Ulrich R, Mussare F, Ferro P, Herron E. Fluphenazine and social therapy in the aftercare of schizophrenic patients. Relapse analyses of a two-year controlled study of fluphenazine decanoate and fluphenazine hydrochloride. Archives of General Psychiatry 1979;36:1283-94. [CSZG: 1277] [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Ulrich RF. Temporal effects of drug and placebo in delaying relapse in schizophrenic outpatients. Archives of General Psychiatry 1977;34:297-301. [CSZG: 1878] [DOI] [PubMed] [Google Scholar]
- Hogarty GE. NIHM-PRB collaborative outpatient schizophrenic study. Psychopharmacology Bulletin 1974;10(2):54-5. [CSZG: 1276] [Google Scholar]
Chlorpromazine 1975 {published data only}1827
- Elie R, Gagnon MA, Gauthier R, Jequier JC. Effects of neuroleptic withdrawal on the drug-induced extrapyramidal syndrome of chronic schizophrenia [Effets d'un sevrage neuroleptique sur le syndrome extrapyramidal medicamenteux de la schizophrenie chronique]. Union Medicale du Canada 1975;104:909-14. [CSZG: 1911] [PubMed] [Google Scholar]
Chlorpromazine 1976 {published data only}208
- Andrews P, Hall JN, Snaith RP. A controlled trial of phenothiazine withdrawal in chronic schizophrenic patients. British Journal of Psychiatry 1976;128:451-5. [CSZG: 226] [DOI] [PubMed] [Google Scholar]
Fluphenazine 1979 {published data only}2340
- Rifkin A, Quitkin F, Kane J, Klein DF, Ross D. The effect of fluphenazine upon social and vocational functioning in remitted schizophrenics. Biological Psychiatry 1979;14:499-508. [CSZG: 2428] [PubMed] [Google Scholar]
- Rifkin A, Quitkin F, Kane J, Klein DF. Fluphenazine decanoate, oral fluphenazine, and placebo in the treatment of remitted schizophrenics. II. Rating scale data. Psychopharmacology Bulletin 1977;13:40-50. [CSZG: 2424] [PubMed] [Google Scholar]
- Rifkin A, Quitkin F, Klein DF. Fluphenazine decanoate, oral fluphenazine, and placebo in treatment of remitted schizophrenics. II. Rating scale data. Archives of General Psychiatry 1977;34:15-9. [CSZG: 3959] [DOI] [PubMed] [Google Scholar]
- Rifkin A, Quitkin F, Rabiner CJ, Klein DF. Comparison of fluphenazine decanoate, oral fluphenazine, and placebo in remitted outpatient schizophrenics. Psychopharmacology Bulletin 1976;12:24-6. [CSZG: 2426] [PubMed] [Google Scholar]
- Rifkin A, Quitkin F, Rabiner CJ, Klein DF. Fluphenazine decanoate, fluphenazine hydrochloride given orally, and placebo in remitted schizophrenics. I. Relapse rates after one year. Archives of General Psychiatry 1977;34:43-7. [CSZG: 3960] [DOI] [PubMed] [Google Scholar]
Fluphenazine 1980 {published data only}3772
- Gelenberg AJ, Doller JC, Schooler NR, Mieske M, Severe J, Mandel MR. Acute extrapyramidal reactions with fluphenazine hydrochloride and fluphenazine decanoate. American Journal of Psychiatry 1979;136:217-9. [CSZG: 3961] [DOI] [PubMed] [Google Scholar]
- Levine J, Schooler NR, Severe J, Escobar J, Gelenberg A, Mandel M, et al. Discontinuation of oral and depot fluphenazine in schizophrenic patients after one year of continuous medication: a controlled study. Advances in Biochemical Psychopharmacology 1980;24:483-93. [CSZG: 1683] [PubMed] [Google Scholar]
- Mandel MR, Severe JB, Schooler NR, Gelenberg AJ, Mieske M. Development and prediction of postpsychotic depression in neuroleptic-treated schizophrenics. Archives of General Psychiatry 1982;39:197-203. [CSZG: 3962] [DOI] [PubMed] [Google Scholar]
- Schooler NR, Levine J, NIMH-PRB Collaborative Fluphenazine Study Group. The initiation of long-term pharmacotherapy in schizophrenia: dosage and side effect comparisons between oral and depot fluphenazine. Pharmacopsychiatry 1976;9:159-69. [CSZG: 3964] [PubMed] [Google Scholar]
- Schooler NR, Levine J, Severe JB, Brauzer B, DiMascio A, Klerman GL, et al. Prevention of relapse in schizophrenia. An evaluation of fluphenazine decanoate. Archives of General Psychiatry 1980;37:16-24. [CSZG: 3965] [DOI] [PubMed] [Google Scholar]
- Schooler NR, Levine J, Severe JB. Depot fluphenazine in the prevention of relapse in schizophrenia: evaluation of a treatment regimen. Psychopharmacology Bulletin 1979;15:44-7. [CSZG: 2521] [PubMed] [Google Scholar]
- Schooler NR, Levine J. Dosage and side effect comparisons between oral and depot fluphenazine. Psychopharmacology Bulletin 1977;13:29-31. [PubMed] [Google Scholar]
Fluphenazine 1982 {published data only}1251
- Kane JM, Rifkin A, Quitkin F, Nayak D, Ramos-Lorenzi J. Fluphenazine vs. placebo in patients with remitted, acute first-episode schizophrenia. Archives of General Psychiatry 1982;39:70-3. [CSZG: 1304] [DOI] [PubMed] [Google Scholar]
- Kane JM, Rifkin A, Woerner M, Reardon G. Low-dose neuroleptics in outpatient schizophrenics. Psychopharmacology Bulletin 1982;18:20-1. [CSZG: 1602] [Google Scholar]
Fluphenazine depot 1968 {published data only}1259
- Keskiner A, Holden JMC, Itil TM. Maintenance treatment of schizophrenic outpatients with a depot phenothiazine. Psychosomatics 1968;9:166-71. [CSZG: 1312] [DOI] [PubMed] [Google Scholar]
- Keskiner A, Simeon J, Fink M, Itil TM. Long-acting phenothiazine (fluphenazine decanoate) treatment of psychosis. Archives of General Psychiatry 1968;18:477-81. [DOI] [PubMed] [Google Scholar]
- King DJ, Devaney N, Cooper SJ, Blomqvist M, Mitchel MJ. Pharmacokinetics and antipsychotic effect of remoxipride in chronic schizophrenic patients. Journal of Psychopharmacology 1990;4:83-9. [CSZG: 1323] [DOI] [PubMed] [Google Scholar]
- Kinross-Wright J, Charalampous KD. A controlled study of a very long-acting phenothiazine preparation. International Journal of Neuropsychiatry 1965;1:66-70. [CSZG: 1335] [Google Scholar]
Fluphenazine depot 1973 {published data only}3723
- Hankoff LD, Engelhardt DM, Freedman N, Mann D, Maroolis R. Denial of illness in schizophrenic outpatients: effects of psychopharmacological treatment. Archives of General Psychiatry 1960;3:657-66. [CSZG: 959] [DOI] [PubMed] [Google Scholar]
- Hirsch SR, Gaind R, Rohde PD, Stevens BC, Wing JK. Outpatient maintenance of chronic schizophrenic patients with long-acting fluphenazine: double-blind placebo trial. Report to the Medical Research Council Committee on Clinical Trials in Psychiatry. British Medical Journal 1973;1:633-7. [CSZG: 3799] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fluphenazine depot 1979a {published data only}1444
- Dotti A, Bersani G, Rubino IA, Eliseo C. Double-blind trial of fluphenazine decanoate against placebo in ambulant maintenance treatment of chronic schizophrenics [Studio in doppio cieco della flufenazina decanoato versus placebo nella terapia ambulatoriale di mantenimento di pazienti schizofrenici cronici]. Rivista di Psichiatria 1979;14:374-83. [CSZG: 1514] [Google Scholar]
Fluphenazine depot 1979b {published data only}1233
- Kane JM, Rifkin A, Quitkin F, Nayak D, Saraf K, Ramos Lorenzi JR, et al. Low dose fluphenazine decanoate in maintenance treatment of schizophrenia. Psychiatry Research 1979;1:341-8. [CSZG: 1286] [DOI] [PubMed] [Google Scholar]
- Kane JM. Low dose medication strategies in the maintenance treatment of schizophrenia. Schizophrenia Bulletin 1983;9(4):528-32. [DOI] [PubMed] [Google Scholar]
Fluphenazine depot 1981 {published data only}4324
- Goldberg SC, Shenoy RS, Sadler A, Hamer R, Ross B. The effects of a drug holiday on relapse and tardive dyskinesia in chronic schizophrenics. Psychopharmacology Bulletin 1981;17:116-7. [CSZG: 5007] [PubMed] [Google Scholar]
- Shenoy RS, Sadler AG, Goldberg SC, Hamer RM, Ross B. Effects of a six-week drug holiday on symptom status, relapse, and tardive dyskinesia in chronic schizophrenics. Journal of Clinical Psychopharmacology 1981;1:141-5. [CSZG: 2577] [DOI] [PubMed] [Google Scholar]
Fluphenazine depot 1982 {published data only}2098
- Odejide OA, Aderounmu AF. Double-blind placebo substitution: withdrawal of fluphenazine decanoate in schizophrenic patients. Journal of Clinical Psychiatry 1982;43:195-6. [CSZG: 2184] [PubMed] [Google Scholar]
Fluphenazine depot 1992 {published data only}2407
- Sampath G, Shah A, Krska J, Soni SD. Neuroleptic discontinuation in the very stable schizophrenic patient - relapse rates and serum neuroleptic levels. Human Psychopharmacology 1992;7:255-64. [CSZG: 2493] [Google Scholar]
- Soni SD, Mallik A, Schiff AA. Sulpiride in negative schizophrenia: a placebo-controlled double-blind assessment. Human Psychopharmacology 1990;5:233-8. [CSZG: 2697] [Google Scholar]
Haloperidol 1973 {published data only}2232
- Ota KY, Kurland AA. A double-blind comparison of haloperidol oral concentrate, haloperidol solutabs and placebo in the treatment of chronic schizophrenia. Journal of Clinical Pharmacology 1973;13:99-110. [CSZG: 2318] [DOI] [PubMed] [Google Scholar]
Haloperidol 1991 {published data only}2385
- Ruskin PE, Nyman G. Discontinuation of neuroleptic medication in older, outpatient schizophrenics. A placebo-controlled, double-blind trial. Journal of Nervous and Mental Disease 1991;179:212-4. [CSZG: 2471] [DOI] [PubMed] [Google Scholar]
Haloperidol depot 1982 {published data only}3162
- Zissis NP, Psaras M, Lyketsos G. Haloperidol decanoate, a new long-acting antipsychotic, in chronic schizophrenics: double-blind comparison with placebo. Current Therapeutic Research 1982;31:650-5. [CSZG: 3256] [Google Scholar]
Haloperidol depot 1991 {published data only}9291
- Eklund K, Forsman A. Minimal effective dose and relapse - double-blind trial: haloperidol decanoate vs. placebo. Clinical Neuropharmacology 1991;14:S7-S15. [CSZG: 747] [PubMed] [Google Scholar]
- Eklund K. Low dose of haloperidol decanoate is effective against relapses in schizophrenia patients. A double-blind placebo controlled study. In: Proceedings of the 17th Collegium Internationale Neuro-Psychopharmacologicum Congress; 1990 Sep 10-14; Kyoto, Japan. 1990:287. [CSZG: 12924]
Iloperidone 2016 {published data only}17965
- CTRI/2010/091/001409. A clinical trial to study prevention of relapse in patients with schizophrenia reveiving either flexible dose iloperidone or placebo in long-term use.. http://ctri.nic.in/Clinicaltrials/login.php 2010.
- NCT01291511. Efficacy in prevention of relapse of schizophrenia in subjects taking either placebo or iloperidone. http://ClinicalTrials.gov/show/NCT01291511 2011. [CSZG: 22671]
- Weiden PJ, Manning R, Wolfgang CD, Ryan JM, Mancione L, Han G, et al. A randomized trial of iloperidone for prevention of relapse in schizophrenia: the REPRIEVE study. CNS drugs 2016;30(8):735-47. [CSZG: 35243] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lurasidone 2016 {published data only}18736
- NCT01435928. PEARL Schizophrenia Maintenance. http://ClinicalTrials.gov/show/NCT01435928 2011. [CSZG: 23439]
- Tandon R, Cucchiaro J, Phillips D, Hernandez D, Mao Y, Pikalov A, et al. A double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophrenia. Journal of Psychopharmacology 2016;30(1):69-77. [CSZG: 33434] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon R, Loebel A, Philips D, Pikalov A, Hernandez D Mao Y, et al. Lurasidone for maintenance of efficacy in patients with schizophrenia. European Neuropsychopharmacology 2014;24(Suppl 2):S559-560. [CSZG: 27840] [Google Scholar]
- Tandon R, Loebel A, Philips D, Pikalov A, Hernandez D, Mao Y, Cucchiaro J. A double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophrenia. Schizophrenia Research 2014;153(1):S372. [CSZG: 29681] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon R, Loebel A, Philips D, Pikalov A, Hernandez D, Mao Y, et al. A double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophrenia. Int J Neuropsychopharmacol 2014;17:S153-154. [CSZG: 29954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olanzapine 1999 {published data only}4622
- Dellva MA, Tollefson GD, Mattler CA, Kinon BJ, Grundy SL. A controlled, double-blind investigation of the clozapine discontinuation syndrome with conversion to either olanzapine or placebo. Schizophrenia Research 1999;36:277-8. [CSZG: 5651] [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Dellva MA, Mattler CA, Kane JM, Wirshing DA, Kinon BJ, et al. Controlled, double-blind investigation of the clozapine discontinuation symptoms with conversion to either olanzapine or placebo. Journal of Clinical Psychopharmacology 1999;19:435-43. [CSZG: 5305] [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Dellva MA, Mattler CA, Kinon BJ, Grundy SL. A controlled, double-blind investigation of the clozapine discontinuation syndrome with conversion to either olanzapine or placebo. In: Proceedings of the 11th European College of Neuropsychopharmacology Congress; 1998 Oct 31 - Nov 4; Paris, France. 1998. [CSZG: 4859]
Olanzapine 2003 {published data only}14624
- Beasley C Jr, Hamilton S, Dossenbach M. Relapse prevention with olanzapine. European Neuropsychopharmacology 2000;10(Suppl 3):S304. [CSZG: 4620] [Google Scholar]
- Beasley CM, Hamilton SH, Dossenbach M. Relapse prevention with olanzapine. Schizophrenia Research 2000;41(1):196-7. [CSZG: 4874] [Google Scholar]
- Beasley CM, Sutton VK, Hamilton SH, Walker DJ, Dossenbach M, Taylor CC, et al. A double-blind, randomised, placebo-controlled trial of olanzapine in the prevention of psychotic relapse. Journal of Clinical Psychopharmacology 2003;23:582-94. [CSZG: 10010] [DOI] [PubMed] [Google Scholar]
- Beasley CM, Sutton VK, Taylor CC, Sethuraman G, Dossenbach M, Naber D, Pickar D. Does maintenance treatment with atypical antipsychotics worsen quality of life among stable patients with schizophrenia? Neuropsychopharmacology 2005;30(S1):S189. [CSZG: 13107] [Google Scholar]
- Beasley CM, Sutton VK, Taylor CC, Sethuraman G, Dossenbach M, Naber D. Is quality of life among minimally symptomatic patients with schizophrenia better following withdrawal or continuation of antipsychotic treatment? Journal of Clinical Psychopharmacology 2006;26:40-4. [CSZG: 14454] [DOI] [PubMed] [Google Scholar]
- Beasley CM, Walker DJ, Sutton VK, Alaka K, Naber D, Namjoshi M. The effectiveness of olanzapine versus placebo in maintaining the quality of life in stabilized patients with schizophrenia. European Neuropsychopharmacology 2003;13:S342. [CSZG: 9849] [Google Scholar]
- EliLilly Company. Olanzapine relapse prevention versus placebo in the treatment of schizophrenia. http://www.Clinicalstudyresults.org/. 2005. [CSZG: 18733]
Paliperidone 2007 {published data only}15435
- Eerdekens. Evaluate the efficacy in the prevention of recurrence of the symptoms of schizophrenia. Sponsor: Johnson & Johnson Pharmaceutical Research & Development, LL.C 2010. [CTG: ]
- Kramer M, Kushner S, Sherr J, Vijapurkar U, Lim P, Eerdekens M. Delaying symptom recurrence in patients with schizophrenia with paliperidone extended-release tablets: an international, randomized, double-blind, placebo-controlled study. Schizophrenia Bulletin 2007;33(2):438. [CSZG: 15185] [DOI] [PubMed] [Google Scholar]
- Kramer M, Kushner S, Vijapurkar U, Lim P, Eerdekens M. Delaying symptom recurrence in patients with schizophrenia treated with paliperidone extended-release tablets. European Neuropsychopharmacology 2006;16(Suppl 4):S386. [CSZG: 13313] [Google Scholar]
- Kramer M, Kushner S, Vijapurkar U, Lim P, Eerdekens M. Delaying symptom recurrence in patients with schizophrenia with paliperidone extended-release tablets: an international, randomized, double-blind, placebo-controlled study. International Journal of Neuropsychopharmacology 2006;9(Suppl 1):S274. [CSZG: 19545] [Google Scholar]
- Kramer M, Simpson G, Maciulis V, Kushner S, Liu Y, Lim P, et al. One-year open-label safety and efficacy study of paliperidone extended-release. CNS Spectrums 2010;15(8):506-14. [CSZG: 20885] [DOI] [PubMed] [Google Scholar]
- Kramer M, Simpson G, Maciulis V, Kushner S, Vijapurkar U, Lim P, et al. Paliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomised, double-blind, placebo-controlled study. Journal of Clinical Psychopharmacology 2007;27:6-14. [CSZG: 13907] [DOI] [PubMed] [Google Scholar]
- Markowitz M, Fu DJ, Levitan B, Gopal S, Turkoz I, Alphs L. Long-acting injectable paliperidone palmitate versus oral paliperidone extended release. Annals of General Psychiatry 2013;12:22. [CSZG: 28633] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Gopal S, Baker S, Narayan VA. Trajectories and changes in individual items of positive and negative syndrome scale among schizophrenia patients prior to impending relapse. NPJ Schizophrenia 2018;4(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paliperidone 2014 {published data only}22628
- Rui Q, Wang Y, Liang S, Liu Y, Wu Y, Wu Q, et al. Relapse prevention study of paliperidone extended-release tablets in Chinese patients with schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2014;53:45-53. [CSZG: 29320] [DOI] [PubMed] [Google Scholar]
- Zhang H, Li H, Liu Y, Wu C, Wu Q, Nuamah I. Safety and efficacy of paliperidone extended-release in Chinese patients with schizophrenia. Neuropsychiatric Disease and Treatment 2016;12:69-77. [CSZG: 33455] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paliperidone depot1M 2010 {published data only}11886
- Alphs L, Bossie C, Kern-Sliwa J, Ma Y-W, Haskins JT. Paliperidone palmitate: clinical response in subjects with schizophrenia with recent vs. longer-term duration of illness. In: Proceedings of the 162nd Annual Meeting of the American Psychiatric Association. San Francisco, CA., 2009 May 16-21. [CSZG: 19067]
- Emsley R, Nuamah I, Gopal S, Hough D, Fleischhacker WW. Relapse after antipsychotic discontinuation in schizophrenia as a withdrawal phenomenon vs illness recurrence: a post hoc analysis of a randomized placebo-controlled study. Journal of Clinical Psychiatry 2018 Jun;79(4):17m11874. [CSZG: 38184] [DOI] [PubMed] [Google Scholar]
- Emsley R, Nuamah I, Hough D, Gopal S. Treatment response after relapse in a placebo-controlled maintenance trial in schizophrenia. Schizophrenia Research 2012;138(1):29-34. [CSZG: 24214] [DOI] [PubMed] [Google Scholar]
- Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M, Hough D. A 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophrenia. Journal of Psychopharmacology 2011;25(5):685-97. [CSZG: 22903] [DOI] [PubMed] [Google Scholar]
- Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M. Long-term efficacy, safety and tolerability of paliperidone palmitate in patients with schizophrenia. In: Proceedings of the 162nd Annual Meeting of the American Psychiatric Association. San Francisco, CA, 2009 May 16-21. [CSZG: 19076]
- Hough D, Gopal S, Vijapukar U, Lim P, Morozova M, Eerdekens M. Paliperidone palmitate in prevention of symptom recurrence in patients with schizophrenia: a randomised, double-blind, placebo-controlled study. In: Proceedings of the 161st Annual Meeting of the American Psychiatric Association; 2008 May 3-8, Washington DC, USA. 2008. [CSZG: 16486]
- Hough D, Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M. Paliperidone palmitate, an injectable antipsychotic, in prevention of symptom recurrence in patients with schizophrenia: A randomized, double-blind, placebo-controlled study. In: Proceedings of the 63rd Annual Convention of the Society of Biological Psychiatry. Washington, DC, USA: Elsevier Science Inc, 2008:S285-6. [CSZG: 20281]
- Hough D, Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M. Paliperidone palmitate, an injectable antipsychotic, in prevention of symptom recurrence in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Biological Psychiatry 2008;63(7 Suppl):285S-6S. [CSZG: 19530] [Google Scholar]
- Hough D, Gopal S, Vjapukar U, Lim P, Morozowa M, Eerdekens M. Paliperidone palmitate maintenance treatment in delaying the time to relapse in patients with schizophrenia: a randomised, double-blind, placebo-controlled study. Schizophrenia Research 2010;116:107-17. [CSZG: 19704] [DOI] [PubMed] [Google Scholar]
- Hulihan J, Bossie CA, Fu DJ, Kern Sliwa J, Ma Y-W, Alphs LD. Remission with continued paliperidone palmitate treatment in stable subjects with schizophrenia. Schizophrenia Research 2012;136:S252. [CSZG: 29467] [Google Scholar]
- Kozma CM, Slaton T, Dirani R, Fastenau J, Gopal S, Hough D. Changes in schizophrenia-related hospitalization and ER use among patients receiving paliperidone palmitate. Current Medical Resarch and Opinion 2011;27(8):1603-11. [DOI] [PubMed] [Google Scholar]
- Sliwa JK, Bossie CA, Fu DJ, Turkoz I, Alphs L. Long-term tolerability of once-monthly injectable paliperidone palmitate in subjects with recently diagnosed schizophrenia. Neuropsychiatric Disease and Treatment 2012;8:375-85. [CSZG: 28007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwa JK, Fu DJ, Bossie CA, et al. Body mass index and metabolic parameters in patients with schizophrenia during long-term treatment with paliperidone palmitate. BMC Psychiatry 2014;14:S52. [CSZG: 29148] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Gopal S, Baker S, Narayan VA. Trajectories and changes in individual items of positive and negative syndrome scale among schizophrenia patients prior to impending relapse. NPJ Schizophrenia 2018;4(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paliperidone depot1M 2015 {published data only}16601
- Bossie C, Turkoz I, Alphs L, Fu DJ. Monotherapy with once monthly paliperidone palmitate for psychotic, depressive, and manic symptoms in schizoaffective disorder. Schizophrenia Bulletin 2015;41:S303. [CSZG: 29400] [Google Scholar]
- Bossie CA, Turkoz I, Alphs L, Mahalchick L, Fu DJ. Paliperidone palmitate once-monthly treatment in recent onset and chronic illness patients with schizoaffective disorder. Journal of Nervous and Mental Ddisease 2017;205(4):324-8. [CSZG: 35417] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu DJ, Alphs L, Lindenmayer JP, Schooler N, Simonson RB, Turkoz I, et al. Paliperidone palmitate long-acting injectable delays psychotic and mood symptom relapse in schizoaffective disorder. European Neuropsychopharmacology 2014;24:S534-5. [CSZG: 27785] [Google Scholar]
- Fu DJ, Bossie C, Turkoz I, Mahalchick L, Alphs L. Paliperidone palmitate treatment response in early and chronic illness schizoaffective disorder patients. Schizophrenia Bulletin 2015;41:S312. [CSZG: 29446] [Google Scholar]
- Fu DJ, Turkoz I, Simonson RB, Walling D, Schooler N, Lindenmayer JP, et al. Effect of paliperidone palmitate once-monthly in improving and maintaining functioning in subjects with schizoaffective disorder using the domains of the personal and social performance scale. Neuropsychopharmacology 2014;39:S365. [CSZG: 29278] [Google Scholar]
- Fu DJ, Turkoz I, Simonson RB, Walling D, Schooler N, Lindenmayer JP, et al. Paliperidone palmitate delays relapse in patients with schizoaffective disorder. Biological Psychiatry 2014;1:263S. [CSZG: 28974] [Google Scholar]
- Fu DJ, Turkoz I, Simonson RB, Walling D, Schooler N, Lindenmayer JP, et al. Paliperidone palmitate long-acting injectable in acute exacerbation of schizoaffective disorder. Schizophrenia Bulletin 2013;39:S331. [CSZG: 28167] [Google Scholar]
- Fu DJ, Turkoz I, Simonson RB, Walling D, Schooler N, Lindenmayer JP, et al. Paliperidone palmitate once-monthly injectable treatment for acute exacerbations of schizoaffective disorder. Journal of Clinical Psychopharmacology 2016;36(4):372-6. [CSZG: 35803] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu DJ, Turkoz I, Simonson RB, Walling DP, Schooler NR, Lindenmayer JP, et al. Paliperidone palmitate once-monthly reduces risk of relapse of psychotic, depressive, and manic symptoms and maintains functioning in a double-blind, randomized study of schizoaffective disorder. Journal of Clinical Psychiatry 2015;76(3):253-62. [CSZG: 29752] [DOI] [PubMed] [Google Scholar]
- Fu DJ, Turkoz I, Walling D, Lindenmayer JP, Schooler NR, Alphs L. Paliperidone palmitate once-monthly maintains improvement in functioning domains of the Personal and Social Performance scale compared with placebo in subjects with schizoaffective disorder. Schizophrenia Research 2018;192:185-93. [CSZG: 35804] [DOI] [PubMed] [Google Scholar]
- Joshi K, Lin J, Lingohr-Smith M, Fu D. Medical cost reductions for paliperidone palmitate versus placebo in randomized double-blind trial of patients with schizoaffective disorder. Journal of Managed Care and Specialty Pharmacy 2015;21:536-7. [DOI] [PubMed] [Google Scholar]
- Joshi K, Lin J, Lingohr-Smith M, Fu DJ. Estimated medical cost reductions for paliperidone palmitate vs placebo in a randomized, double-blind relapse-prevention trial of patients with schizoaffective disorder. Journal of Medical Economics 2015;18:629-36. [CSZG: 33677] [DOI] [PubMed] [Google Scholar]
- NCT01193153. A study to evaluate the efficacy of paliperidone palmitate in the prevention of relapse of the symptoms of schizoaffective disorder. http://ClinicalTrials.gov/show/NCT01193153 2010. [CSZG: 21094]
- Turkoz I, Fu DJ, Walling D, Schooler NR, Lindenmayer JP, Simonson RB, et al. Effect of paliperidone palmitate once-monthly in improving and maintaining functioning in subjects with schizoaffective disorder using the domains of the personal and social performance scale. Schizophrenia Bulletin 2015;41:S193-4. [CSZG: 29687] [Google Scholar]
Paliperidone depot3M 2015 {published data only}18982
- Bell LKS, Turkoz I, Kim E. Paliperidone palmitate once-every-3-months in adults with early illness schizophrenia. Early Intervention in Psychiatry 2019;13(3):667-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwaerts J, Liu Y, Gopal S, Nuamah I, Xu H, Savitz A, et al. Efficacy and safety of paliperidone palmitate 3 month formulation: a randomized, double-blind, placebo-controlled study. Schizophrenia Bulletin 2015;41:S302. [CSZG: 29393] [Google Scholar]
- Berwaerts J, Liu Y, Gopal S, Nuamah I, Xu H, Savitz A, et al. Efficacy and safety of paliperidone palmitate 3-month formulation in schizophrenia: a randomized, double-blind, placebo-controlled study. European Psychiatry 2015;30:272. [CSZG: 32149] [Google Scholar]
- Berwaerts J, Liu Y, Gopal S, Nuamah I, Xu H, Savitz A, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry 2015;72(8):830-9. [CSZG: 33027] [DOI] [PubMed] [Google Scholar]
- Chirila C, Zheng Q, Sawyerr G, Nuamah I. Healthcare resource use of paliperidone palmitate 3-month injection vs placebo. In: 168th Annual Meeting of American Psychiatric Association; Toronto, Canada. May 16-20, 2015. [CSZG: 33035]
- Gopal S, Furey M, Hibar D, Kolb H, Saad Z, Savitz A, et al. Potential biomarkers of impending schizophrenia relapse. Biological Psychiatry 2017;(10 Suppl):S126-S127. [CSZG: 36107] [Google Scholar]
- Gopal S, Xu H, McQuarrie K, Savitz A, Nuamah I, Woodruff K, et al. Caregiver burden in schizophrenia following paliperidone palmitate long acting injectables treatment: pooled analysis of two double-blind randomized phase three studies. NPJ Schizophrenia 2017;3(1):23. [CSZG: 36325] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M, Nuamah I, Savitz A, Gopal S. Efficacy and safety of paliperidone palmitate 3-month formulation vs placebo for relapse prevention of Schizophrenia: Treatment effect using a number needed to treat analysis. Biological Psychiatry 2017;81(10 Suppl 1):S350-1. [CSZG: 36437] [Google Scholar]
- NCT01529515. A study of paliperidone palmitate 3 month formulation for the treatment of patients with schizophrenia. http://ClinicalTrials.gov/show/NCT01529515 2012. [CSZG: 23705]
- Wang D, Gopal S, Baker S, Narayan VA. Trajectories and changes in individual items of positive and negative syndrome scale among schizophrenia patients prior to impending relapse. NPJ Schizophrenia 2018;4(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penfluridol 1970 {published data only}279
- Baro F, Brugmans J, Dom R, Van Lommel R. Maintenance therapy of chronic psychotic patients with a weekly oral dose of R 16341. A controlled double-blind study. Journal of Clinical Pharmacology 1970;10:330-41. [CSZG: 297] [DOI] [PubMed] [Google Scholar]
Penfluridol 1974a {published data only}799
- Gallant DM, Mielke DH, Spirtes MA, Swanson WC, Bost R. Penfluridol: an efficacious long-acting oral antipsychotic compound. American Journal of Psychiatry 1974;131:699-702. [CSZG: 849] [DOI] [PubMed] [Google Scholar]
Penfluridol 1974b {published data only}2354
- Roelofs GA. Penfluridol (R 16341) as a maintenance therapy in chronic psychotic patients: a double-blind clinical evaluation. Acta Psychiatrica Scandinavica 1974;50:219-24. [CSZG: 2440] [DOI] [PubMed] [Google Scholar]
Penfluridol 1974c {published data only}2833
- Vandecasteele AJ, Vereecken JL. A double-blind clinical evaluation of penfluridol (R 16 341) as a maintenance therapy in schizophrenia. Acta Psychiatrica Scandinavica 1974;50:346-53. [CSZG: 2919] [DOI] [PubMed] [Google Scholar]
Penfluridol 1975 {published data only}1515
- Kurland AA, Ota KY, Slotnick VB. Penfluridol: a long-acting oral neuroleptic. A controlled study. Journal of Clinical Pharmacology 1975;15:611-21. [CSZG: 1586] [DOI] [PubMed] [Google Scholar]
- Kurland AA, Ota KY. Penfluridol: once-a-week oral maintenance neuroleptic in the management of chronic schizophrenics. Psychopharmacology Bulletin 1975;11:12-4. [CSZG: 1585] [PubMed] [Google Scholar]
Penfluridol 1987 {published data only}4221
- Channabasavanna SM, Michael A. Penfluridol maintenance therapy in schizophrenia: a controlled study. Indian Journal of Psychiatry 1987;29(4):333-6. [CSZG: 1094] [PMC free article] [PubMed] [Google Scholar]
Perphenazine 1963 {published data only}
- Whittaker CB, Hoy RM. Withdrawal of perphenazine in chronic schizophrenia. British Journal of Psychiatry 1963;109:422-7. [DOI] [PubMed] [Google Scholar]
Pimozide 1971 {published data only}1548
- Huber W, Serafetinides EA, Colmore JP, Clark M. Pimozide in chronic schizophrenic patients. Journal of Clinical Pharmacology 1971;11(4):304-9. [CSZG: 1618] [PubMed] [Google Scholar]
Pimozide 1973 {published data only}1356
- Denijs EL, Vereeken JL. Pimozide (OrapR, R 6238) in residual schizophrenia. A clinical evaluation with long-term double-blind follow-up. Psychiatria, Neurologia, Neurochirurgia 1973;76:47-59. [CSZG: 1409] [PubMed] [Google Scholar]
Quetiapine 2007 {published data only}10847
- D1441C00131. A 1-year, international, multicenter, randomized, double-blind, parallel-group, placebo-controlled phase IV study to evaluate prevention of relapse in patients in stable condition with chronic schizophrenia receiving either quetiapine fumarate IR (SEROQUEL) or placebo. https://astrazenecagrouptrials.pharmacm.com/ST/Submission/View?id=385 2003.
- D1444C00004. A 1-year, international, multicenter, randomized, double-blind, parallelgroup, placebo-controlled phase iii study to evaluate prevention of relapse in patients in stable condition with chronic schizophrenia receiving either sustained-release quetiapine fumarate (SEROQUEL) or placebo. https://astrazenecagrouptrials.pharmacm.com/ST/Submission/View?id=1418 2006.
- NCT00228462. A 1-year, multicenter, randomized, double-blind, parallel group, placebo-controlled phase 3 study to evaluate prevention of relapse in patients in stable chronic schizophrenia receiving either sustained-release quetiapine fumarate (SEROQUEL) or placebo (abbreviated). https://www.ClinicalTrials.gov/ct/show/ 2005. [CSZG: 14874]
- Peuskens J, Trivedi J, Malyarov S, Brecher M, Svensson O, Miller F, et al. Prevention of schizophrenia relapse with extended release quetiapine fumarate dosed once daily: a randomised, placebo-controlled trial in clinically stable patients. Psychiatry 2007;4(11):34-50. [CSZG: 16025] [PMC free article] [PubMed] [Google Scholar]
- Peuskens J, Trivedi JK, Brecher M, Miller F, Study I . Long-term symptomatic remission of schizophrenia with once-daily extended release quetiapine fumarate: Post-hoc analysis of data from a randomized withdrawal, placebo-controlled study. International Clinical Psychopharmacology 2010;25(3):183-7. [CSZG: 20670] [DOI] [PubMed] [Google Scholar]
- Peuskens J, Trivedi JK, Brecher M, Svensson O, Miller F, Meulien D. Long-term symptomatic remission of schizophrenia with once-daily extended release quetiapine fumarate. In: Proceedings of the 161st Annual Meeting of the American Psychiatric Association; 2008 May 3-8; Washington DC, USA. 2008. [CSZG: 16500]
- Peuskens J, Trivedi JK, Brecher M, Svensson O, Miller F, Meulien D. Long-term symptomatic remission of schizophrenia with once-daily extended release quetiapine fumarate. Schizophrenia Research 2008;98:14-5. [CSZG: 16344] [Google Scholar]
- Peuskens J, Trivedi JK, Malyarov S, Brecher M, Svensson O, Miller F, et al. A randomized, placebo-controlled, relapse-prevention study with once-daily quetiapine sustained release in patients with schizophrenia. Proceedings of the 11th International Congress on Schizophrenia Research; 2007 Mar 28-Apr 1; Colorado Springs, Colorado, USA 2007. [CSZG: 15271]
- Peuskens JC, Trivedi JK, Malyarov S, Brecher M, Svensson O, Miller F, et al. A randomized, placebo-controlled, relapse-prevention study with once daily quetiapine sustained release in patients with schizophrenia. Schizophrenia Bulletin 2007;33(2):453. [CSZG: 15225] [Google Scholar]
Quetiapine 2009a {published and unpublished data}12006
- NCT00658645. A multinational, randomised, double-blind, fixed-dose, bifeprunox study combining a 12-week placebo-controlled, quetiapine-referenced phase with a 12-month quetiapine-controlled phase in patients with schizophrenia. https://www.clinicaltrials.gov/ct2/show/NCT00658645?term=NCT00658645&rank=1. [CSZG: 16241]
Quetiapine 2009b {published and unpublished data}12579
- NCT00704509. A multinational, randomised, double-blind, fixed-dose, bifeprunox study combining a 12-week placebo-controlled, quetiapine-referenced phase with a 12-month quetiapine-controlled phase in patients with schizophrenia. https://www.clinicaltrials.gov/ct2/show/NCT00704509?term=NCT00704509&rank=1. [CSZG: 16566]
Quetiapine 2010 {published data only}10486
- Chen E, Hui C, Chang WC, Chan S, Lee E, Honer W. The effect of early medication discontinuation on long-term clinical outcome in first episode psychosis. Schizophrenia Bulletin 2018;44(Suppl 1):S97. [Google Scholar]
- Chen E, Hui C, Lam M, Law C, Chiu C, Chung D, et al. Assessing the efficacy of relapse prevention in single episode psychosis patients with stable maintenance treatment for at least 1 year: a double-blind randomized placebo-controlled. Early Intervention in Psychiatry 2008;2(Suppl 1):A5. [CSZG: 19113] [Google Scholar]
- Chen EY, Hui CL, Lam M, Law CW, Chiu CP, Chung DW, et al. A double-blind randomised placebo-controlled study of relapse prevention in remitted first-episode psychosis patients following one year of maintenance therapy. Schizophrenia Research 2008;98:11-2. [CSZG: 16325] [Google Scholar]
- Chen EY, Hui CL, Lam M, Law CW, Chiu CP, Chung DW, et al. A double-blind randomized placebo-controlled relapse prevention study in remitted first-episode psychosis patients following one year of maintenance treatment. Early Intervention in Psychiatry 2010;4(Suppl 1):13. [CSZG: 23475] [Google Scholar]
- Chen EY, Hui CL, Lam MM, Chiu CP, Law CW, Chung DW, et al. Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial. BMJ 2010;341:c4024. [CSZG: 20857] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Hui LM, Lam M, Law CW, Chiu PY, Chung WS, et al. A double-blind randomized placebo-controlled relapse prevention study in remitted first-episode psychosis patients following one year of maintanence therapy. European Psychiatry 2008;23:S107-S8. [CSZG: 20468] [Google Scholar]
- Hui C L, Wong GH, Tang JY, Chang WC, Chan SK, Lee EH, et al. Predicting 1-year risk for relapse in patients who have discontinued or continued quetiapine after remission from first-episode psychosis. Schizophrenia Research 2013;150(1):297-302. [CSZG: 28601] [DOI] [PubMed] [Google Scholar]
- Hui C, Chen E, Lam M, Law C, Chiu C, Chung D, et al. Can we predict relapse in single episode psychosis patients with stable maintenance treatment for at least one year? Early Intervention in Psychiatry 2008;2(Suppl 1):A124. [CSZG: 19123] [Google Scholar]
- Hui CL, Chen EY, Lam M, Law CW, Chiu CP, Chung DW, et al. Predictors for relapse in a double-blind randomized placebo-controlled discontinuation study of remitted first-episode psychosis patients. Schizophrenia Research 2008;98:13. [CSZG: 16333] [Google Scholar]
- Hui CL, Honer W, Lee E, Chan S, Chang WC, Chen E. The 10-year clinical outcome of early medication discontinuation in remitted first episode psychosis. Early Intervention in Psychiatry 2018;12(1):46. [Google Scholar]
- Hui CL, Honer WG, Lee EH, Chang WC, Chan SK, Chen ES, et al. Long-term effects of discontinuation from antipsychotic maintenance following first-episode schizophrenia and related disorders: a 10 year follow-up of a randomised, double-blind trial. Lancet Psychiatry 2018 May;5(5):432-42. [CSZG: 38003] [DOI] [PubMed] [Google Scholar]
- Hui CL, Honer WG, Lee EH, Chang WC, Chan SK, Chen ES, et al. Predicting first-episode psychosis patients who will never relapse over 10 years. Psychological Medicine 2019;49(13):2206-14. [DOI] [PubMed] [Google Scholar]
- Hui CL, Honer WG, Lee EH, Chang WC, Chan SK, Chen EY. Factors associated with successful medication discontinuation after a randomized clinical trial of relapse prevention in first-episode psychosis: a 10-year follow-up. JAMA Psychiatry 2019;76(2):217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui CL, Li YK, Li AW, Lee EH, Chang WC, Chan SK, et al. Visual working memory deterioration preceding relapse in psychosis. Psychological Medicine 2016;46:2435-44. [CSZG: 33663] [DOI] [PubMed] [Google Scholar]
- Hui LM, Chen YH, Lam M, Law CW, Chiu PY, Chung WS, et al. A double-blind randomized placebo-controlled study of relapse predictors in remitted first-episode psychosis patients. European Psychiatry 2008;23:S120-S1. [CSZG: 20469] [Google Scholar]
- NCT00334035. Duration of maintenance anti-psychotic therapy after first –episode schizophrenia: a double-blind randomized placebo-control relapse prevention study. https://www.ClinicalTrials.gov/ct/show/ 2006. [CSZG: 14607]
Trifluoperazine 1969 {published data only}2258
- Prien RF, Levine J, Cole JO. High dose trifluoperazine therapy in chronic schizophrenia. American Journal of Psychiatry 1969;126:305-13. [CSZG: 2344] [DOI] [PubMed] [Google Scholar]
- Prien RF, Levine J, Switalski RW. Discontinuation of chemotherapy for chronic schizophrenics. Hospital and Community Psychiatry 1971;22(1):4-7. [CSZG: 2346] [DOI] [PubMed] [Google Scholar]
Trifluoperazine 1972 {published data only}951
- Hershon HI, Kennedy PF, McGuire RJ. Persistence of extra-pyramidal disorders and psychiatric relapse after withdrawal of long-term phenothiazine therapy. British Journal of Psychiatry 1972;120(554):41-50. [CSZG: 1001] [DOI] [PubMed] [Google Scholar]
- Stevens BC. Role of fluphenazine decanoate in lessening the burden of chronic schizophrenics on the community. Psychological Medicine 1973;3:141-58. [CSZG: 2739] [DOI] [PubMed] [Google Scholar]
Various drugs 1960 {published data only}877
- Gross M, Hitchman IL, Reeves WP, Lawrence J, Newell PC. Discontinuation of treatment with ataractic drugs. A preliminary report. American Journal of Psychiatry 1960;116:931-2. [CSZG: 927] [DOI] [PubMed] [Google Scholar]
Various drugs 1961 {published data only}6740
- Schiele BC, Vestre ND, Stein KE. A comparison of thioridazine, trifluoperazine, chlorpromazine, and placebo: a double-blind controlled study on the treatment of chronic, hospitalized, schizophrenic patients. Journal of Clinical and Experimental Psychopathology and Quarterly Review of Psychiatry and Neurology 1961;22:151-62. [CSZG: 9157] [PubMed] [Google Scholar]
Various drugs 1962a {published data only}31690
- Olson GW, Peterson DB. Intermittent chemotherapy for chronic psychiatric inpatients. Journal of Nervous and Mental Disease 1962;134:1459. [CSZG: 38115] [DOI] [PubMed] [Google Scholar]
- Olson GW, Peterson DB. Sudden removal of tranquilizing drugs from chronic psychiatric patients. Journal of Mental and Nervous Disease 1960;131:252-5. [DOI] [PubMed] [Google Scholar]
Various drugs 1962b {published data only}2769
- Troshinsky C, Aaronson HG, Stone RK. Maintenance phenothiazines in aftercare of schizophrenic patients. Pennsylvania Psychiatric Bulletin 1962;2:11-5. [CSZG: 2855] [Google Scholar]
Various drugs 1964a {published data only}3067
- Caffey EM, Diamond LS, Frank TV, Grasberger JC, Herman L, Klett CJ, et al. Discontinuation or reduction of chemotherapy in chronic schizophrenics. Journal of Chronic Diseases 1964;17:347-58. [CSZG: 3153] [DOI] [PubMed] [Google Scholar]
- Caffey EM, Forrest IS, Frank TV, Klett CJ. Phenothiazine excretion in chronic schizophrenics. American Journal of Psychiatry 1963;120:578-82. [CSZG: 420] [DOI] [PubMed] [Google Scholar]
Various drugs 1964b {published data only}4149
- Marjerrison G, Irvine D, Stewart CN, Williams R, Matheu H, Demay M. Withdrawal of long-term phenothiazines from chronically hospitalized psychiatric patients. Canadian Psychiatric Association Journal 1964;9(4):290-8. [CSZG: 4833] [DOI] [PubMed] [Google Scholar]
Various drugs 1966a {published data only}3195
- Garfield SL, Gershon S, Sletten I, Neubauer H, Ferrel E. Withdrawal of ataractic medication in schizophrenic patients. Diseases of the Nervous System 1966;27:321-5. [CSZG: 3289] [PubMed] [Google Scholar]
Various drugs 1966b {published data only}3495
- Melnyk WT, Worthington AG, Laverty SG. Abrupt withdrawal of chlorpromazine and thioridazine from schizophrenic in-patients. Canadian Psychiatric Association Journal 1966;11:410-3. [CSZG: 3590] [Google Scholar]
Various drugs 1968 {published data only}2006
- Morton MR. A study of the withdrawal of chlorpromazine or trifluoperazine in chronic schizophrenia. American Journal of Psychiatry 1968;124:1585-8. [CSZG: 2092] [DOI] [PubMed] [Google Scholar]
Various drugs 1971 {published data only}3723
- Hirsch SR, Gaind R, Rohde PD, Stevens BC, Wing JK. Outpatient maintenance of chronic schizophrenic patients with long-acting fluphenazine: double-blind placebo trial. Report to the Medical Research Council Committee on Clinical Trials in Psychiatry. British Medical Journal 1973;1(854):633-7. [CSZG: 3799] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch SR, Leff JP, Wing JK. Outpatient maintenance of chronic schizophrenics with long-acting. British Medical Journal 1973;2(5868):715-6. [CSZG: 3839] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff JP, Hirsch SR, Gaind R, Rohde PD, Stevens BC. Life events and maintenance therapy in schizophrenic relapse. British Journal of Psychiatry 1973;123:659-60. [DOI] [PubMed] [Google Scholar]
- Leff JP, Wing JK. Trial of maintenance therapy in schizophrenia. British Medical Journal 1971;3:599-604. [CSZG: 3840] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff JP. Influence of selection of patients on results of clinical trials. British Medical Journal 1973;4(5885):156-8. [CSZG: 7717] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B. The social value of fluphenazine decanoate. Acta Psychiatrica Belgica 1976;76(5):792-804. [CSZG: 2745] [PubMed] [Google Scholar]
- Stevens BC. Role of fluphenazine decanoate in lessening the burden of chronic schizophrenics on the community. Psychological Medicine 1973;3(2):141-58. [CSZG: 2739] [DOI] [PubMed] [Google Scholar]
Various drugs 1974 {published data only}3237
- Gross HS. A double-blind comparison of once-a-day pimozide, trifluoperazine, and placebo in the maintenance care of chronic schizophrenic outpatients. Current Therapeutic Research 1974;16:696-705. [CSZG: 3331] [PubMed] [Google Scholar]
Various drugs 1975 {published data only}3868
- Clark ML, Huber WK, Hill D, Wood F, Costiloe JP. Pimozide in chronic schizophrenic outpatients. Diseases of the Nervous System 1975;36(3):137-41. [CSZG: 1235] [PubMed] [Google Scholar]
- Clark. Pimozide vs thioridazine vs placebo. Bulletin 1973;9:59-63. [CSZG: 4106] [Google Scholar]
Various drugs 1981a {published data only}6229
- Blackburn HL, Allen JL. Behavioral effects of interrupting and resuming tranquilizing medication among schizophrenics. Journal of Nervous and Mental Disease 1981;133:303-8. [CSZG: 8369] [DOI] [PubMed] [Google Scholar]
Various drugs 1981b {published data only}442
- Cheung HK. Schizophrenics fully remitted on neuroleptics for 3-5 years - to stop or continue drugs? British Journal of Psychiatry 1981;138:490-4. [CSZG: 460] [DOI] [PubMed] [Google Scholar]
Various drugs 1981c {published data only}3827
- Wistedt B, Jorgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology 1982;78:301-4. [CSZG: 27894] [DOI] [PubMed] [Google Scholar]
- Wistedt B, Palmstierna T. Depressive symptoms in chronic schizophrenic patients after withdrawal of long-acting neuroleptics. Journal of Clinical Psychiatry 1983;44:369-71. [CSZG: 3068] [PubMed] [Google Scholar]
- Wistedt B, Ranta J. Comparative double-blind study of flupenthixol decanoate and fluphenazine decanoate in the treatment of patients relapsing in a schizophrenic symptomatology. Acta Psychiatrica Scandinavica 1983;67:378-88. [CSZG: 3069] [DOI] [PubMed] [Google Scholar]
- Wistedt B, Wiles D, Jorgensen A. A depot neuroleptic withdrawal study neurological effects. Psychopharmacology 1983;80:101-5. [CSZG: 3070] [DOI] [PubMed] [Google Scholar]
- Wistedt B. A depot neuroleptic withdrawal study. A controlled study of the clinical effects of the withdrawal of depot fluphenazine decanoate and depot flupenthixol decanoate in chronic schizophrenic patients. Acta Psychiatrica Scandinavica 1981;64:65-84. [CSZG: 27892] [DOI] [PubMed] [Google Scholar]
- Wistedt B. Neuroleptics and depression. Archives of General Psychiatry 1982;39:745. [CSZG: 4059] [DOI] [PubMed] [Google Scholar]
Various drugs 1982 {published data only}2081
- Nishikawa T, Tsuda A, Tanaka M, Koga I, Uchida Y. Prophylactic effect of neuroleptics in symptom-free schizophrenia. Psychopharmacology 1982;77:301-4. [CSZG: 2167] [DOI] [PubMed] [Google Scholar]
Various drugs 1984a {published data only}
- Gardos G, Cole JO, Rapkin RM, LaBrie RA, Baquelod E, Moore P, et al. Anticholinergic challenge and neuroleptic withdrawal. Changes in dyskinesia and symptom measures. Archives of General Psychiatry 1984;41:1030-5. [DOI] [PubMed] [Google Scholar]
Various drugs 1984b {published data only}2082
- Nishikawa T, Tsuda A, Tanaka M, Hoaki Y, Koga I, Uchida Y. Prophylactic effect of neuroleptics in symptom-free schizophrenics: a comparative dose response study of haloperidol and propericiazine. Psychopharmacology 1984;82:153-6. [CSZG: 2168] [DOI] [PubMed] [Google Scholar]
Various drugs 1986a {published data only}599
- Crow TJ, MacMillan JF, Johnson AL, Johnstone EC. A randomised controlled trial of prophylactic neuroleptic treatment. British Journal of Psychiatry 1986;148:120-7. [CSZG: 648] [DOI] [PubMed] [Google Scholar]
Various drugs 1986b {published data only}2626
- Spohn HE, Coyne L, Larson J, Mittleman F, Spray J, Hayes K. Episodic and residual thought pathology in chronic schizophrenics: effect of neuroleptics. Schizophrenia Bulletin 1986;12:394-407. [CSZG: 2712] [DOI] [PubMed] [Google Scholar]
Various drugs 1989 {published data only}3775
- McCreadie RG, Wiles D, Grant S, Crockett GT, Mahmood Z, Livingston MG, et al. The Scottish first episode schizophrenia study. VII two-year follow-up. Acta Psychiatrica Scandinavica 1989;80:597-602. [CSZG: 3968] [DOI] [PubMed] [Google Scholar]
- Scottish Schizophrenia Research Group. The Scottish First Episode Schizophrenia Study II. Treatment. British Journal of Psychiatry 1987;150:334-8. [CSZG: 3969] [DOI] [PubMed] [Google Scholar]
- Scottish Schizophrenia Research Group. The Scottish First Episode Schizophrenia Study V. One-year follow-up. British Journal of Psychiatry 1988;152:470-476. [CSZG: 3970] [DOI] [PubMed] [Google Scholar]
- Scottish Schizophrenia Research Group. The Scottish First Episode Schizophrenia Study. III. Cognitive performance. British Journal of Psychiatry 1987;150:338-340. [CSZG: 16867] [DOI] [PubMed] [Google Scholar]
- Scottish Schizophrenia Research Group. The Scottish first episode schizophrenia study. VIII. Five-year follow-up. British Journal of Psychiatry 1992;161:496-500. [CSZG: 2542] [PubMed] [Google Scholar]
Various drugs 1993 {published data only}2205
- Gaebel W, Frick U, Kopcke W, Linden M, Mueller P, Mueller Spahn F, et al. Early neuroleptic intervention in schizophrenia: are prodromal symptoms valid predictors of relapse? British Journal of Psychiatry Supplementum 1993;163:8-12. [CSZG: 831] [PubMed] [Google Scholar]
- Gaebel W, Janner M, Frommann N, Pietzcker A, Kopcke W, Linden M, et al. First vs multiple episode schizophrenia: two-year outcome of intermittent and maintenance medication strategies. Schizophrenia Research 2002;53(1-2):145-59. [CSZG: 8197] [DOI] [PubMed] [Google Scholar]
- Gaebel W, Moeller HJ. Treatment strategies in first episode schizophrenia. In: Proceedings of the 12th World Congress of Psychiatry; 2002 Aug 24-29; Yokohama, Japan. 2002. [CSZG: 8597]
- Pietzcker A, Gaebel W, Koepcke W, Linden M, Mueller P, Mueller-Spahn F, et al. Intermittent versus maintenance neuroleptic long-term treatment in schizophrenia - 2-year results of a German multicenter study. Journal of Psychiatric Research 1993;27:321-39. [CSZG: 3944] [Google Scholar]
- Pietzcker A, Gaebel W, Kopcke W, Linden M, Muller P, Muller-Spahn, et al. A German multicenter study on the neuroleptic long-term therapy of schizophrenic patients. Preliminary report. Pharmacopsychiatry 1986;19:161-6. [CSZG: 3943] [Google Scholar]
Various drugs 2011 {published data only}10307
- Boonstra G, Burger H, Grobbe DE, Kahn RS. Antipsychotic prophylaxis is needed after remission from a first psychotic episode in schizophrenia patients: results from an aborted randomised trial. International Journal of Psychiatry in Clinical Practice 2011;15(2):128-34. [CSZG: 22940] [DOI] [PubMed] [Google Scholar]
- Boonstra G. Schizophrenia termination of pharmacotherapy-STOP-trial. Current Controlled Trials 2005. [CSZG: 14487] [ISRCTN: 6332944]
Ziprasidone 2002 {published data only}227
- Arato M, O'Connor R, Bradbury JE, Meltzer H. Long-term ziprasidone in schizophrenia. In: Proceedings of the 151st Annual Meeting of the American Psychiatric Association; 1998 May 30 - Jun 4; Toronto, Ontario, Canada. 1998. [CSZG: 3189]
- Arato M, O'Connor R, Bradbury JE, Meltzer H. The efficacy of ziprasidone in the long-term treatment of negative symptoms and prevention of exacerbation of schizophrenia. In: Proceedings of the 21st Collegium Internationale Neuro-Psychopharmacologicum Congress; 1998 Jul 12-16; Glasgow, UK. Amsterdam, Netherlands: Cambridge University Press, 1998. [CSZG: 3188]
- Arato M, O'Connor R, Bradbury JE, Meltzer H. Ziprasidone in the long-term treatment of negative symptoms and prevention of exacerbation of schizophrenia. In: Proceedings of the 9th Congress of the Association of European Psychiatrists; 1998 Sep 20-24; Copenhagen, Denmark. 1998. [CSZG: 3190]
- Arato M, O'Connor R, Bradbury JE, Meltzer H. Ziprasidone in the long-term treatment of negative symptoms and prevention of exacerbation of schizophrenia. Schizophrenia Research 1999;36(1-3):270. [CSZG: 5626] [Google Scholar]
- Arato M, O'Connor R, Meltzer H, Bradbury J. Ziprasidone: efficacy in the prevention of relapse and in the long-term treatment of negative symptoms of chronic schizophrenia. In: Proceedings of the 10th European College of Neuropsychopharmacology Congress; 1997 Sep 13-17; Vienna, Austria. 1997. [CSZG: 245]
- Arato M, O'Connor R, Meltzer HY. A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the Ziprasidone Extended Use in Schizophrenia (ZEUS) study. International Clinical Psychopharmacology 2002;17:207-15. [CSZG: 18486] [DOI] [PubMed] [Google Scholar]
- Bernardo M, Aranza JR, Rubio-Terres C, Rejas J. Cost-effectiveness analysis of schizophrenia relapse prevention. Clinical Drug Investigation. New Zealand 2006;26(8):447-57. [CSZG: 13898] [DOI] [PubMed] [Google Scholar]
- Bernardo M, Aranza JR, Rubio-Terres C, Rejas J. Cost-effectiveness analysis of the prevention of relapse in schizophrenia in the ZEUS longitudinal study Ziprasidone Extended Use in Schizophrenia (ZEUS). Acta Espanolas de Psiquiatria 2007;35(4):259-62. [CSZG: 15396] [PubMed] [Google Scholar]
- Meltzer HY, O´Connor R. Long-term efficacy of ziprasidone in schizophrenia. Schizophrenia research 2001;(1-2):S. 239. [CSZG: 6050] [Google Scholar]
- Meltzer HY, O´Connor R. Path analysis of the ZEUS study provides evidence of a direct effect of ziprasidone on primary negative symptoms in chronic, stable schizophrenia. Conference Poster 1997. [CSZG: 2033]
- Meltzer HY, O'Connor R. Long-term efficacy of ziprasidone in schizophrenia: results of two controlled trials. In: Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000 Dec 10-14; San Juan, Puerto Rico. 2000. [CSZG: 7609]
- O'Connor R, Schooler NR. Penultimate observation carried forward (POCF): a new approach to analysis of long-term symptom change in chronic relapsing conditions [letter]. Schizophrenia Research 2003;60:319-20. [CSZG: 9365] [DOI] [PubMed] [Google Scholar]
- Pfizer 2000. Multicentre double-blind study of ziprasidone versus placebo in relapse prevention for hospitalised patients with chronic or subchronic schizophrenia. Study report Unpublished.
- Schooler N. A basis for optimism: the implications of long term treatment. In: Proceedings of the 9th Congress of the Association of European Psychiatrists; 1998 Sep 20-24; Copenhagen, Denmark. 1998. [CSZG: 5864]
Zotepine 2000 {published data only}544
- Cooper SJ, Butler A, Tweed J, Raniwalla J, Welch C. Zotepine in the prevention of relapse. Biological Psychiatry 1997;42:41s. [CSZG: 17007] [Google Scholar]
- Cooper SJ, Butler A, Tweed J, Raniwalla J, Welch C. Zotepine in the prevention of relapse. In: Proceedings of the 6th World Congress of Biological Psychiatry; 1997 June 22-27; Nice, France. 1997. [CSZG: 569]
- Cooper SJ, Butler A, Tweed J, Welch C, Raniwalla J. Zotepine in the prevention of recurrence: a randomised, double-blind, placebo-controlled study for chronic schizophrenia. Psychopharmacology 2000;150:237-43. [CSZG: 5732] [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Butler A, Tweed JA, Welch CP, Wighton AJ, Appleby P, et al. Zotepine is effective in preventing recurrence in patients with chronic schizophrenia. In: Proceedings of the 11th European College of Neuropsychopharmacology Congress; 1998 Oct 31 - Nov 4; Paris, France. 1998. [CSZG: 5784]
- Cooper SJ, Butler A, Tweed JA, Welch CP, Wighton AJ, Appleby P, et al. Zotepine is effective in preventing recurrence in patients with chronic schizophrenia. Schizophrenia Research 2000;41:207-8. [CSZG: 4925] [Google Scholar]
References to studies excluded from this review
Allen 1997 {published data only}
- Allen DN, Gilbertson MW, Barry E, Kammen DP, Gurklis JA. Haloperidol improves memory in schizophrenia. In: Proceedings of the 150th Annual Meeting of the American Psychiatric Association; 1997 May 17-22; San Diego, California, USA. 1997.
Bai 2003 {published data only}
- Bai YM, Yu SC, Chen JY, Lin CY, Chou P, Lin CC. Risperidone for pre-existing severe tardive dyskinesia: a 48-week prospective follow-up study. International Clinical Psychopharmacology 2005;20:79-85. [DOI] [PubMed] [Google Scholar]
- Bai YM, Yu SC, Lin CC. Risperidone for severe tardive dyskinesia: a 12 week randomised, double-blind, placebo-controlled study. Journal of Clinical Psychiatry 2003;64:1342-8. [PubMed] [Google Scholar]
Bechdolf 2016 {published data only}
- Bechdolf A, Mueller H, Gaebel W, Hasan A, Maier W, Juckel G, et al. PREVENT: a randomized controlled trial comparing cognitive behavioural therapy, clinical management and aripiprazole and clinical management and placebo for the prevention of first episode psychosis. Early Intervention in Psychiatry 2016;10(Suppl 1):40. [Google Scholar]
Bo 2017 {published data only}
- Bo Q, Li F, Li X, Wang Z, Dong F, He F, et al. Symptomatic remission in schizophrenia: results from a risperidone maintenance treatment study. Psychiatry Research 2017;258:289-94. [DOI] [PubMed] [Google Scholar]
Bourin 2008 {published data only}
- Bourin M, Debelle M, Heisterberg J, Josiassen MK, Ostergaard JB, Sands E. Efficacy of bifeprunox in patients in the post-acute, maintenance phase of schizophrenia: findings from a 6-month study. Schizophrenia Research 2008;98:158. [Google Scholar]
Branchey 1981 {published data only}
- Branchey MH, Branchey LB, Richardson MA. Effects of gradual decrease and discontinuation of neuroleptics on clinical condition and tardive dyskinesia. Psychopharmacology Bulletin 1981;17:118-20. [PubMed] [Google Scholar]
- Branchey MH, Branchey LB, Richardson MA. Effects of neuroleptic adjustment on clinical condition and tardive dyskinesia in schizophrenic patients. American Journal of Psychiatry 1981;138:608-12. [DOI] [PubMed] [Google Scholar]
Breier 1987 {published data only}
- Breier A, Wolkowitz OM, Doran AR, Roy A, Boronow J, Hommer DW, et al. Neuroleptic responsivity of negative and positive symptoms in schizophrenia. American Journal of Psychiatry 1987;144:1549-55. [DOI] [PubMed] [Google Scholar]
Brown 2018 {published data only}
- Brown D, Daniels K, Pichereau S, Sand M. A phase IC study evaluating the safety, tolerability, pharmacokinetics and cognitive outcomes of BI409306 in patients with mild to moderate schizophrenia. Neurology and Therapy 2018;7(1):129-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand M, Nakagome K, Cordes J, Brenner R, Gründer G, Keefe R, et al. Effects of BI409306 on positive and negative syndrome scale in schizophrenia: a randomized double-blind placebo-controlled phase II trial. Biological Psychiatry 2018;83(9):S207-8. [Google Scholar]
Cather 2018 {published data only}
- Cather C, Brunette MF, Mueser KT, Babbin SF, Rosenheck R, Correll CU, et al. Impact of comprehensive treatment for first episode psychosis and substance use outcomes: a randomized controlled trial. Psychiatry Research 2018;268:303-11. [DOI] [PubMed] [Google Scholar]
- Fulford D, Piskulic D, Addington J, Kane JM, Schooler N, Mueser KT. Prospective relationships between motivation and functioning in recovery after a first episode of schizophrenia. Schizophrenia Bulletin 2018;44(2):369-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT, Meyer-Kalos PS, Glynn SM, Lynde DW, Robinson DG, Gingerich S, et al. Implementation and fidelity assessment of the NAVIGATE treatment program for first episode psychosis in a multi-site study. Schizophrenia Research 2019;204:271-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler N, Mueser K, Estroff S, Correll C, Robinson D, Marcy P, et al. Elements of recovery in first episode psychosis: developing measurable criteria. Early Interventions in Psychiatry 2016;10(Suppl 1):149. [Google Scholar]
Cheng 2019 {published data only}
- Cheng Z, Yuan Y, Han X, Yang L, Zeng X, Yang F, et al. Rates and predictors of one year antipsychotic treatment discontinuation in first episode schizophrenia: results from ab open-label randomized "real-world" clinical trial. Psychiatry Research 2019;273:631-40. [DOI] [PubMed] [Google Scholar]
Chopra 2019 {published data only}
- Chopra S, Fornito A, Francey S, O’Donoghue B, Nelson B, Graham J, et al. Gray matter changes in medicated and unmedicated first episode psychosis: a randomized placebo-controlled trial. Schizophrenia Bulletin 2019;45:S172-3. [Google Scholar]
Chouinard 1980 {published data only}
- Chouinard G, Annable L, Jones BD, Collu R. Lack of tolerance to long-term neuroleptic treatment in dopamine tuberoinfundibular system. Acta Psychiatrica Scandinavica 1980;64:353-62. [DOI] [PubMed] [Google Scholar]
Chouinard 1993 {published data only}
- Andris J. Clinical trials van risperidone [Clinical trails van risperidone]. Unknown Source 1997:14.
- Chouinard G, Albright PS. Economic and health state utility determinations for schizophrenic patients treated with risperidone or haloperidol. Journal of Clinical Psychopharmacology 1997;17(4):298-307. [DOI] [PubMed] [Google Scholar]
- Chouinard G, Arnott W. The effect of risperidone on extrapyramidal symptoms in chronic schizophrenic patients. Biological Psychiatry 1992;31:158. [Google Scholar]
- Chouinard G, Jones B, Remington G, Bloom D, Addington D, MacEwan GW, et al. A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. Journal of Clinical Psychopharmacology 1993;13(1):25-40. [PubMed] [Google Scholar]
- Chouinard G. Effects of risperidone in tardive dyskinesia: an analysis of the Canadian multicenter risperidone study. Journal of Clinical Psychopharmacology 1995;15(Suppl 1):S36-44. [DOI] [PubMed]
- Czobor P, Volavka J, Meibach RC. Effect of risperidone on hostility in schizophrenia. Journal of Clinical Psychopharmacology 1995;15(4):243-9. [DOI] [PubMed] [Google Scholar]
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. Journal of Clinical Psychological Medicine 1997;58(12):538-46. [DOI] [PubMed] [Google Scholar]
- McEvoy JP. Efficacy of risperidone on positive features of schizophrenia. Journal of Clinical Psychiatry 1994;55(5 Suppl):18-21. [PubMed] [Google Scholar]
- Moller HJ, Muller H, Borison RL, Schooler NR, Chouinard G. A path-analytical approach to differentiate between direct and indirect drug effects on negative symptoms in schizophrenic patients. A re- evaluation of the North American risperidone study. European Archives of Psychiatry and Clinical Neuroscience 1995;245(1):45-9. [DOI] [PubMed] [Google Scholar]
- Musser WS, Kirisci L. Critique of the Canadian multicenter placebo-controlled study of risperidone and haloperidol. Journal of Clinical Psychopharmacology 1995;15(3):226-8. [DOI] [PubMed] [Google Scholar]
- NCT00249132. Risperidone versus haloperidol versus placebo in the treatment of chronic schizophrenia. https://www.ClinicalTrials.gov/ct/show/ 2005.
- Simpson GM, Lindenmayer JP. Extrapyramidal symptoms in patients treated with risperidone. Journal of Clinical Psychopharmacology 1997;17(3):194-201. [DOI] [PubMed] [Google Scholar]
Claghorn 1974 {published data only}
- Claghorn JL, Johnstone EE, Cook TH, Itschner L. Group therapy and maintenance treatment of schizophrenics. Archives of General Psychiatry 1974;31:361-5. [DOI] [PubMed] [Google Scholar]
Clark 1967 {published data only}
- Clark ML, Ray TS, Paredes A, Ragland RE, Costiloe JP, Smith CW, et al. Chlorpromazine in women with chronic schizophrenia: the effect on cholesterol levels and cholesterol-behavior relationships. Psychosomatic Medicine 1967;29(6):634-42. [DOI] [PubMed] [Google Scholar]
Collins 1967 {published data only}
- Collins AD, Dundas J. A double-blind trial of amitriptyline-perphenazine, perphenazine and placebo in chronic withdrawn inert schizophrenics. British Journal of Psychiatry 1967;113:1425-9. [DOI] [PubMed] [Google Scholar]
Condray 1995 {published data only}
- Condray R, Van Kammen DP, Steinhauer SR, Kasparek A, Yao JK. Language comprehension in schizophrenia: trait or state indicator? Biological Psychiatry 1995;38:287-96. [DOI] [PubMed] [Google Scholar]
Curson 1985 {published data only}
- Curson DA, Barnes TR, Bamber RW, Platt SD, Hirsch SR, Duffy JC. Long term depot maintenance of chronic schizophrenic out patients: the seven year follow up of the Medical Research Council fluphenazine/placebo trial. II. The incidence of compliance problems, side effects, neurotic symptoms and depression. British Journal of Psychiatry 1985;146:469-74. [DOI] [PubMed] [Google Scholar]
- Curson DA, Barnes TR, Bamber RW, Platt SD, Hirsch SR, Duffy JC. Long-term depot maintenance of chronic schizophrenic out-patients: the seven year follow-up of the Medical Research Council fluphenazine- placebo trial I. Course of illness, stability of diagnosis, and the role of a special maintenance clinic. British Journal of Psychiatry 1985;146:464-9. [DOI] [PubMed] [Google Scholar]
- Curson DA, Barnes TR, Bamber RW, Platt SD, Hirsch SR, Duffy JC. Long-term depot maintenance of chronic schizophrenic out-patients: the seven year follow-up of the Medical Research Council fluphenazine/placebo trial. III. Relapse postponement or relapse prevention? The implications for long-term outcome. British Journal of Psychiatry 1985;146:474-80. [DOI] [PubMed] [Google Scholar]
Degkwitz 1970 {published data only}
- Degkwitz R, Bauer MP, Gruber M, Hampel G, Luxenburger O, Richartz M, et al. Time relationship between the appearance of persisting extrapyramidal hyperkineses and psychotic recurrences following sudden interruption of prolonged neuroleptic therapy of chronic schizophrenic patients. Arzneimittelforschung 1970;20(7):890-3. [PubMed] [Google Scholar]
Diamond 1960 {published data only}
- Diamond LS, Marks JB. Discontinuance of tranquilizers among chronic schizophrenic patients receiving maintenance dosage. Journal of Nervous and Mental Disease 1960;131:247-51. [DOI] [PubMed] [Google Scholar]
Double 1993 {published data only}
- Double DB, Warren GC, Evans M, Rowlands RP. Efficacy of maintenance use of anticholinergic agents. Acta Psychiatrica Scandinavica 1993;88:381-4. [DOI] [PubMed] [Google Scholar]
- Elie R, Morin L, Tetreault L. Effects of ethopropazine and trihexyphenidyl on several parameters of the neuroleptic syndrome [Effets de l'ethopropazine et du trihexyphenidyle sur quelques parametres du syndrome neuroleptique]. Encephale 1972;61:32-52. [PubMed] [Google Scholar]
Durgam 2016 {published data only}
- Barabassy A, Szatmàri B, Laszlovszky I, Harsanyi J, Acsai K, Sebe B, et al. Efficacy of cariprazine in the treatment of negative symptoms of schizophrenia: post hoc analysis versus aripiprazole. European Neuropsychopharmacology 2019;29(Suppl 1):S417-8. [Google Scholar]
- Cutler AJ, Durgam S, Lu K, Laszlovszky I, Szalai E, Edwards J, Earley W. Efficacy of cariprazine in negative, cognitive, social function symptoms in schizophrenia: post hoc analysis of a randomized controlled trial. European Neuropsychopharmacology 2016;26(2):S551-2. [Google Scholar]
- Durgam S, Earley W, Lu K, Németh G, Laszlovszky I, Szatmari B, Patel M, Nasrallah H. Cariprazine for negative symptoms of schizophrenia: a pooled post-hoc analysis of 2 randomized double-blind placebo and active controlled trials. In: 29th European College of Neuropsychopharmacology Congress. Vienna, Austria, 2016, Sep 17-20.
- Marder S, Fleischhacker WW, Earley W, Kaifeng L, Zhong Y, Németh G. Efficacy of cariprazine across symptom domains in patients with acute exacerbation of schizophrenia: pooled analysis from 3 phase II/III studies. European Neuropsychopharmacology 2019;29(1):127-36. [DOI] [PubMed] [Google Scholar]
Engelhardt 1967 {published data only}
- Engelhardt DM, Rosen B, Freedman N, Margolis R. Phenothiazines in prevention of psychiatric hospitalisation. Archives of General Psychiatry 1967;16:98-101. [DOI] [PubMed] [Google Scholar]
Fleischhacker 2014 {published data only}
- Fleischhacker WW, Sanchez R, Perry PP, Jin N, Peters-Strickland T, Johnson BR, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. British Journal of Psychiatriy 2014;205(2):135-44. [DOI] [PubMed] [Google Scholar]
Francey 2018 {published data only}
- Francey S, Nelson B, Jessica G, Lara B, Yuen HP, O'Donoghue B, et al. Antipsychotic medication in first episode psychosis: an RCT to assess the risk benefit ratio. Early Intervention in Psychiatry 2018;12(Suppl 1):70. [Google Scholar]
- Francey S. A randomized placebo-controlled trial of intensive psychosocial treatment plus or minus antipsychotic medication for first episode psychosis with low risk of self harm of aggression. The STAGES study: staged treatment and acceptability guidelines in early psychosis. Early Interventions in Psychiatry 2016;10(Suppl 1):9. [DOI] [PubMed] [Google Scholar]
- O'Donoghue B, Francey SM, Nelson B, Ratheesh A, Allott K, Grahan J, et al. Staged treatment and acceptability guidelines in early psychosis study (STAGES): a randomized placebo-controlled trial of intensive psychosocial treatment plusof minus antipsychotic medication for first episode with low risk of self harm or aggression: study protocol and baseline characteristics of patients. Early Interventions in Psychiatry 2019;13(4):953-60. [DOI] [PubMed] [Google Scholar]
Freedman 1982 {published data only}
- Freedman R, Kirch D, Bell J, Adler LE, Pecevich M, Pachtman E, et al. Clonidine treatment of schizophrenia: double blind comparison to placebo and neuroleptic drugs. Acta Psychiatrica Scandinavica 1982;65:35-45. [DOI] [PubMed] [Google Scholar]
Gallant 1964 {published data only}
- Gallant DM, Edwards CG, Bishop MP, Galbraith GC. Withdrawal symptoms after abrupt cessation of antipsychotic compounds: clinical confirmation in chronic schizophrenics. American Journal of Psychiatry 1964;121:491-3. [DOI] [PubMed] [Google Scholar]
Gitlin 1988 {published data only}
- Gitlin MJ, Midha KK, Fogelson D, Nuechterlein KH. Persistence of fluphenazine in plasma after decanoate withdrawal. Journal of Clinical Psychopharmacology 1988;8:53-6. [PubMed] [Google Scholar]
Gitlin 2001 {published data only}
- Gitlin M, Nuechterlein K, Subotnik KL, Ventura J, Mintz J, Fogelson DL, et al. Clinical outcome following neuroleptic discontinuation in patients with remitted recent-onset schizophrenia. American Journal of Psychiatry 2001;158:1835-42. [DOI] [PubMed] [Google Scholar]
Gleeson 2004 {published data only}
- Gleeson J, Wade D, Mcgorry P, Albiston D, Castle D, Gilbert M, et al. Episode ii: prevention of relapse following early psychosis. Schizophrenia Research 2004;70:61-2. [Google Scholar]
Goldberg 1967 {published data only}
- Goldberg SC, Schooler NR, Mattsson N. Paranoid and withdrawal symptoms in schizophrenia: differential symptom reduction over time. Journal of Nervous and Mental Disease 1967;145:158-62. [DOI] [PubMed] [Google Scholar]
- Mefferd RB Jr, Labrosse EH, Gawienowski AM, Williams RJ. Influence of chlorpromazine on certain biochemical variables of chronic male schizophrenics. Journal of Nervous and Mental Disease 1958;127:167-79. [DOI] [PubMed] [Google Scholar]
Good 1958 {published data only}
- Good WW, Sterling M, Holtzman WH. Termination of chlorpromazine with schizophrenic patients. American Journal of Psychiatry 1958;115:443-8. [DOI] [PubMed] [Google Scholar]
- Mefferd RB, Labrosse EH, Gawienowski AM, Williams RJ. Influence of chlorpromazine on certain biochemical variables of chronic male schizophrenics. Journal of Nervous and Mental Disease 1958;127:167-79. [DOI] [PubMed] [Google Scholar]
Greenberg 1966 {published data only}
- Greenberg LM, Roth S. Differential effects of abrupt versus gradual withdrawal of chlorpromazine in hospitalized chronic schizophrenic patients. American Journal of Psychiatry 1966;123:221-6. [DOI] [PubMed] [Google Scholar]
Hine 1958 {published data only}
- Hine FR. Chlorpromazine in schizophrenic withdrawal and in the withdrawn schizophrenic. Journal of Nervous and Mental Disease 1958;127:220-7. [DOI] [PubMed] [Google Scholar]
Hirsch 1989 {published data only}
- Hirsch SR, Jolley AG, Barnes TR, Liddle PF, Curson DA, Patel A, et al. Dysphoric and depressive symptoms in chronic schizophrenia.. Schizophrenia Research 1989;2(3):259-64. [DOI] [PubMed] [Google Scholar]
- Hirsch SR, Jolley AG. The dysphoric syndrome in schizophrenia and its implications for relapse [Das dysphorische Syndrom in der Schizophrenie und seine Implikationen fuer den Rueckfall]. In: Boeker W//Brenner HD//Waldvogel DM, editors(s). Schizophrenie Als Systemische Stoerung. Die Bedeutung Intermediaerer Prozesse Fuer Theorie Und Therapie. Bern, Switzerland: Verlag Hans Huber, 1989:94-103. [Google Scholar]
- Hirsch SR, Jolley AG. The dysphoric syndrome in schizophrenia and its implications for relapse. British Journal of Psychiatry. Supplements 1989;155(Suppl 5):46-50. [PubMed] [Google Scholar]
Hirsch 1996 {published data only}
- Hirsch SR, Bowen JT, Emami J, Cramer P, Jolley A, Haw C, et al. A one year prospective study of the effect of life events and medication in the aetiology of schizophrenic relapse. British Journal of Psychiatry 1996;168:49-56. [DOI] [PubMed] [Google Scholar]
- Hirsch SR, Bowen JT, Emami J. The effect of life events and medication in the aetiology of schizophrenic relapse. Schizophrenia Research 1993;9:266. [Google Scholar]
Hunt 1967 {published data only}
- Hunt PV. A comparison of the effects of oxypertine and trifluoperazine in withdrawn schizophrenics. British Journal of Psychiatry 1967;113:1419-24. [DOI] [PubMed] [Google Scholar]
Ionescu 1983 {published data only}
- Ionescu R, Tiberiu C, Miklos R, Angelescu C, Persiceanu AM. Penfluridol in the maintenance therapy of schizophrenia. Neurologie et Psychiatrie 1983;21:33-41. [PubMed] [Google Scholar]
Janecek 1963 {published data only}
- Janecek J, Schiele BC, Bellville T, Anderson R. The effects of withdrawal of trifluoperazine on patients maintained on the combination of tranylcypromine and trifluoperazine: a double-blind study. Current Therapeutic Research, Clinical and Experimental 1963;85:608-15. [PubMed] [Google Scholar]
Johnstone 1988 {published data only}
- Johnstone EC, Crow TJ, Frith CD, Owens DG. The Northwick Park "functional" psychosis study: diagnosis and treatment response. Lancet 1988;2:119-25. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Owens DGC, Frith CD. The Northwick Park 'functional' psychosis study. Phase 2 - maintenance treatment. Journal of Psychopharmacology 1991;5:388-95. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Owens DGC. Does early treatment have an effect on outcome? In: Proceedings of the 10th European College of Neuropsychopharmacology Congress; 1997 Sep 13-17; Vienna, Austria. 1997.
Keefe 2018 {published data only}
- Keefe RS, Harvey PD, Khan A, Saoud JB, Staner C, Davidson M, et al. Cognitive effects of MIN-101 in patients with schizophrenia and negative symptoms: results from a randomized controlled trial. Journal of Clinical Psychiatry 2018;79(3):17m11753. [DOI] [PubMed] [Google Scholar]
Kellam 1971 {published data only}
- Kellam AM, Jones KS. A double-blind controlled trial of thiothixene and perphenazine in chronic schizophrenics shown to require maintenance therapy. Acta Psychiatrica Scandinavica 1971;47:174-85. [DOI] [PubMed] [Google Scholar]
Lauriello 2005 {published data only}
- Lauriello J, McEvoy JP, Rodriguez S, Bossie CA, Lasser RA. Long-acting risperidone vs. placebo in the treatment of hospital inpatients with schizophrenia. Schizophrenia Research 2005;72:249-58. [DOI] [PubMed] [Google Scholar]
Lecrubier 1997 {published data only}
- Lecrubier Y. Amisulpride in deficit schizophrenia. In: Proceedings of the 6th World Congress Biological Psychiatry, 1997 Jun 22-27; Nice, France. 1997.
Liu 2018 {published data only}
- Liu CC. A proposed alternative between discontinuation and maintenance of antipsychotics: a guided dose reduction trial for patients with remitted psychosis. Schizophrenia Bulletin 2018;44(Suppl 1):S414. [Google Scholar]
Loo 1997 {published data only}
- Loo H, Poirier-Littre MF, Theron M, Rein W, Fleurot O. Amisulpride versus placebo in the medium-term treatment of the negative symptoms of schizophrenia. British Journal of Psychiatry 1997;170:18-22. [DOI] [PubMed] [Google Scholar]
Mahal 1975 {published data only}
- Mahal AS, Janakiramaiah N. A double blind placebo controlled trial of pimozide (R6238) on 49 hospitalized chronic schizophrenics. Indian Journal of Psychiatry 1975;17(1):45-55. [Google Scholar]
Marder 1994 {published data only}
- Andris J. Clinical trials van risperidone [Clinical trails van risperidone]. Unknown Source 1997:14.
- Czobor P, Volavka J, Meibach RC. Effect of risperidone on hostility in schizophrenia. Journal of Clinical Psychopharmacology 1995;15(4):243-9. [DOI] [PubMed] [Google Scholar]
- Czobor P, Volavka J. Quantitative electroencephalogram examination of effects of risperidone in schizophrenic patients. Journal of Clinical Psychopharmacology 1993;13(5):332-42. [PubMed] [Google Scholar]
- Lindenmayer JP, =The RSG. Incidence of EPS with risperidone compared with haloperidol and placebo in patients with chronic schizophrenia. In: Proceedings of the 146th Annual Meeting of the American Psychiatric Association; 1993 May 22-27; San Francisco, California, USA. 1993.
- Marder S. Risperidone versus haloperidol versus placebo in the treatment of chronic schizophrenia. Clinical Research Report (RIS-INT-3) 1991.
- Marder SR, Chouinard G, Davis JM. The clinical actions of risperidone. In: Proceedings of the 10th Congress of the European College of Neuropsychopahrmacology; 1997 Sep 13-17, Vienna, Austria. 1997.
- Marder SR, Chouinard G, Davis JM. The clinical actions of risperidone. In: Proceedings of the 35th Annual Meeting of the American College of Neuropsychopharmacology; 1996 Dec 9-13; San Juan, Puerto Rico. 1996.
- Marder SR, Chouinard G, Davis JM. The clinical actions of risperidone. In: Proceedings of the 6th World Congress of Biological Psychiatry; 1997 Jun 22-27; Nice, France. 1997.
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. Journal of Clinical Psychological Medicine 1997;58(12):538-46. [DOI] [PubMed] [Google Scholar]
- Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. American Journal of Psychiatry 1994;151(6):825-35. [DOI] [PubMed] [Google Scholar]
- McEvoy JP. Efficacy of risperidone on positive features of schizophrenia. Journal of Clinical Psychiatry 1994;55(5 Suppl):18-21. [PubMed] [Google Scholar]
- Moller HJ, Muller H, Borison RL, Schooler NR, Chouinard G. A path-analytical approach to differentiate between direct and indirect drug effects on negative symptoms in schizophrenic patients. A re- evaluation of the North American risperidone study. European Archives of Psychiatry and Clinical Neuroscience 1995;245(1):45-9. [DOI] [PubMed] [Google Scholar]
- NCT00249132. Risperidone versus haloperidol versus placebo in the treatment of chronic schizophrenia. https://www.ClinicalTrials.gov/ct/show/ 2005.
- Simpson GM, Lindenmayer JP. Extrapyramidal symptoms in patients treated with risperidone. Journal of Clinical Psychopharmacology 1997;17(3):194-201. [DOI] [PubMed] [Google Scholar]
Mathur 1981 {published data only}
- Mathur S, Hall JN. Phenothiazine withdrawal in schizophrenics in a hostel. British Journal of Psychiatry 1981;138:271-2. [DOI] [PubMed] [Google Scholar]
Meehan 2019 {published data only}
- Meehan SR, Zhang P, Hobart M, Hefting N, Baker RA, Weiss C. Short term efficacy of brexpiprazole in patients with schizophrenia with clinically relevant levels of negative symptoms. European Neuropsychopharmacology 2019;29(Suppl 1):S424. [Google Scholar]
Mefferd 1958 {published data only}
- Mefferd RB, Labrosse EH, Gawienowski AM, Williams RJ. Influence of chlorpromazine on certain biochemical variables of chronic male schizophrenics. Journal of Nervous and Mental Disease 1958;127(2):167-79. [DOI] [PubMed] [Google Scholar]
Mosher 1975 {published data only}
- Bola JR, Mosher LR. Treatment of acute psychosis without neuroleptics: two-year outcomes from the Soteria project. Journal of Nervous and Mental Disease 2003;191:219-29. [DOI] [PubMed] [Google Scholar]
- Matthews SM, Roper MT, Mosher LR, Menn AZ. A non neuroleptic treatment for schizophrenia: analysis of the two year postdischarge risk of relapse. Schizophrenia Bulletin 1979;5:322-33. [DOI] [PubMed] [Google Scholar]
- Mosher LR, Menn A, Matthew SM. Soteria: evaluation of a home-based treatment for schizophrenia. American Journal of Orthopsychiatry 1975;45:455-67. [DOI] [PubMed] [Google Scholar]
- Mosher LR, Menn AZ. Community residential treatment for schizophrenia: two year follow up. Hospital and Community Psychiatry 1978;29:715-23. [DOI] [PubMed] [Google Scholar]
Müller 1982 {published data only}
- Müller P, Hartmann W, Jung F, Kind J, Lohrengel S, Steuber H. To prevent relapse of schizophrenic psychoses [Zur Rezidivprophylaxe schizophrener Psychosen]. Ferdinand Enke Verlag Stuttgart 1982;14:1-144. [Google Scholar]
NCT03559426 {published data only}
- NCT03559426. Research into antipsychotic discontinuation and reduction (RADAR): a randomized controlled trial. https://clinicaltrials.gov/.
Nishikawa 1989 {published data only}
- Nishikawa T, Tanaka M, Tsuda A, Koga I, Uchida Y. Prophylactic effects of neuroleptics in symptom-free schizophrenics: a comparative dose-response study of timiperone and sulpiride. Biological Psychiatry 1989;25(7):861-6. [DOI] [PubMed] [Google Scholar]
- Nishikawa T. Personal Communication. Personal Communication 2009.
Oosthuizen 2003 {published data only}
- Oosthuizen PP. Treatment of first episode schizophrenia with low-dose haloperidol: a study in the Western Cape province of South Africa. Dissertation presented for the Degree of Doctor of Philosophy at the University of Stellenbosch 2003 March.
Pasamanick 1967 {published data only}
- Davis AE, Dinitz S, Pasamanick B. The prevention of hospitalization in schizophrenia: five years after an experimental program. American Journal of Orthopsychiatry 1972;42(3):375-88. [DOI] [PubMed] [Google Scholar]
- Pasamanick B, Scarpitti FR, Dinitz S. Schizophrenic in theCcommunity: an Experimental Study in the Prevention of Hospitalisation. New York: Meredith Publishing Company, 1967. [Google Scholar]
- Pasamanick B, Scarpitti FR, Lefton M, Dinitz F, Wernert JJ, McPheeters H. Home vs hospital care for schizophrenics. JAMA 1964;187(3):177-81. [DOI] [PubMed] [Google Scholar]
Paul 1972 {published data only}
- Paul GL, Tobias LL, Holly BL. Maintenance psychotropic drugs in the presence of active treatment programs. A "triple-blind" withdrawal study with long-term mental patients. Archives of General Psychiatry 1972;27:106-15. [DOI] [PubMed] [Google Scholar]
Peet 1981 {published data only}
- Peet M, Middlemiss DN, Yates RA. Propranolol in schizophrenia II. Clinical and biochemical aspects of combining propranolol with chlorpromazine. British Journal of Psychiatry 1981;138:112-7. [DOI] [PubMed] [Google Scholar]
Pickar 1986 {published data only}
- Pickar D, Labarca R, Doran AR, Wolkowitz OM, Roy A, Breier A, et al. Longitudinal measurement of plasma homovanillic acid levels in schizophrenic patients. Correlation with psychosis and response to neuroleptic treatment. Archives of General Psychiatry 1986;43:669-76. [DOI] [PubMed] [Google Scholar]
Pickar 2003 {published data only}
- Pickar D, Bartko JJ. Effect size of symptom status in withdrawal of typical antipsychotics and subsequent clozapine treatment in patients with treatment-resistant schizophrenia. American Journal of Psychiatry 2003;160:1133-8. [DOI] [PubMed] [Google Scholar]
Pigache 1993 {published data only}
- Pigache RM, Norris HN. Measurement of drug action in schizophrenia. Clinical Science 1973;44:28P. [DOI] [PubMed] [Google Scholar]
- Pigache RM, Norris HN. Selective attention as an index of the anti-psychotic action of chlorpromazine in schizophrenia. British Psychological Society Bulletin 1973;20:160. [Google Scholar]
- Pigache RM. Effects of placebo, orphenadrine, and rising doses of chlorpromazine, on PAT performance in chronic schizophrenia. A two year longitudinal study. Schizophrenia Research 1993;10:51-9. [DOI] [PubMed] [Google Scholar]
- Pigache RM. The clinical relevance of an auditory attention task (PAT) in a longitudinal study of chronic schizophrenia with placebo substitution for chlorpromazine. Schizophrenia Research 1993;10:39-50. [DOI] [PubMed] [Google Scholar]
Ran 2002 {published data only}
- Ran MS, Xiang MZ, Chan CL, Leff J, Simpson P, Huang MS, et al. Effectiveness of psychoeducational intervention for rural Chinese families experiencing schizophrenia. Social Psychiatry and Psychiatric Epidemiology 2003;38:69-75. [DOI] [PubMed] [Google Scholar]
- Ran MS, Xiang MZ, Huang MS. A control study of psychoeducational family intervention for relatives of schizophrenics in a Chinese rural community. Chinese Journal of Psychiatry 2001;34(2):98-101. [Google Scholar]
- Ran MS. Community mental health in China - A randomized controlled trial of psychoeducational family intervention for carers of persons with schizophrenia in a rural area in Chengdu. http://hdl.handle.net/10722/35402 submitted at the University of Hong Kong in March 2002.
Rassidakis 1970 {published data only}
- Rassidakis NC, Kondakis X, Papanastassiou A, Michalakeas A. Withdrawal of antipsychotic drugs from chronic psychiatric patients. Bulletin of the Menninger Clinic 1970;34(4):216-22. [PubMed] [Google Scholar]
Ravaris 1965 {published data only}
- Ravaris CL, Weaver LA, Brooks GW. A controlled study of fluphenazine enanthate in chronic schizophrenic patients. Diseases of the Nervous System 1965;25:33-9. [PubMed] [Google Scholar]
- Ravaris CL, Weaver LA, Brooks GW. Further studies with fluphenazine enanthate: II. Relapse rate in patients deprived of medication. American Journal of Psychiatry 1967;124:248-9. [DOI] [PubMed] [Google Scholar]
Ruiz 1975 {published data only}
- Ruiz Ruiz M, Miro Quintana L, Sentis Vilalta J. A clinical study with pimozide in chronic schizophrenics [Estudio clinico con pimocide en las esquizophrenias de evolucion cronica]. Revista de Psiquiatria Y Psicologia Medica de Europa Y America Latinas 1975;12(4):247-56. [Google Scholar]
Ruiz Veguilla 2013 {published data only}
- NCT01765829. Clinical trial to evaluate the efficacy of treatment vs discontinuation in a first episode of non-affective psychosis (NONSTOP). https://www.clinicaltrials.gov/ct2/show/NCT01765829?term=NCT01765829&rank=1 2013.
Schlossberg 1978 {published data only}
- Schlossberg A, Shadmi M. A comparative controlled study of two long-acting phenothiazines: pipotiazine palmitate and fluphenazine decanoate. Current Therapeutic Research 1978;23(5):642-54. [Google Scholar]
Singer 1971 {published data only}
- Singer K, Cheng MN. Thiopropazate hydrochloride in persistent dyskinesia. British Medical Journal 1971;4:22-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 1990 {published data only}
- Singh H, Hunt JI, Vitiello B, Simpson GM. Neuroleptic withdrawal in patients meeting criteria for supersensitivity psychosis. Journal of Clinical Psychiatry 1990;51:319-21. [PubMed] [Google Scholar]
Smelson 2006 {published data only}
- Smelson DA, Tunis SL, Nyhuis AW, Faries DE, Kinon BJ, Ascher-Svanum H. Antipsychotic treatment discontinuation among individuals with schizophrenia and co-occurring substance use. Journal of Clinical Psychopharmacology 2006;26:666-7. [DOI] [PubMed] [Google Scholar]
Soni 1990 {published data only}
- Soni SD, Mallik A, Schiff AA. Sulpiride in negative schizophrenia: a placebo-controlled double-blind assessment. Human Psychopharmacology 1990;5:233-8. [Google Scholar]
Stuerup 2017 {published data only}
- Dolmer S, Nielsen M, Birk M, Mors O, Sturup AE, Albert N, et al. Tailor tapered discontinuation versus maintenance therapy of antipsychotic medication in patients with newly diagnosed schizophrenia spectrum disorders in remission of psychotic symptoms. Schizophrenia Bulletin 2018;44(Suppl 1):S134-S135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EudraCT 2016-000565-23. TAILOR - a randomized clinical trial: Tapered discontinuation versus maintenance therapy of antipsychotic medication in patients with newly diagnosed schizophrenia or schizophreniform psychosis in remission of psychotic symptoms. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2016-000565-23 2017. [DOI] [PMC free article] [PubMed]
- Stuerup AE, Jensen H, Dolmer S, Albert N, Birk M, Hjorthoj C, et al. Discontinuation versus maintenance therapy with antipsychotic medication in schizophrenia. TAILOR: a randomized controlled trial. Early Interventions in Psychiatry 2018;12:141. [Google Scholar]
- Stuerup AE, Jensen HD, Dolmer S, Birk M, Albert N, Nielsen M, et al. TAILOR - tapered discontinuation versus maintenance therapy of antipsychotic medication in patients with newly diagnosed schizophrenia or persistent delusional disorder in remission of psychotic symptoms: study protocol for a randomized clinical trial. Trials 2017;18(1):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sumitomo 2008 {published data only}
- Sumitomo Dainippon Pharma. Randomized, placebo-controlled, double-blind, parallel-group, confirmatory study of SM-13496 (lurasidone HCl) in patients with schizophrenia: a phase III study. Summary of study results. Sumitomo Dainippon Pharma Reports 2008.
Vaddadi 1986 {published data only}
- Vaddadi KS, Gilleard CJ, Mindham RH, Butler R. A controlled trial of prostaglandin E1 precursor in chronic neuroleptic resistant schizophrenic patients. Psychopharmacology 1986;88:362-7. [DOI] [PubMed] [Google Scholar]
Van Kammen 1982 {published data only}
- Van Kammen DP, Bunney WE Jr, Docherty JP, Marder SR, Ebert MH, Rosenblatt JE, et al. D-Amphetamine-induced heterogeneous changes in psychotic behavior in schizophrenia. American Journal of Psychiatry 1982;139:991-7. [DOI] [PubMed] [Google Scholar]
- Van Kammen DP, Bunney WE, Docherty JP, Jimerson DC, Post RM, Siris P, et al. Amphetamine-induced catecholamine activation in schizophrenia and depression: behavioral and physiological effects. Advances in Biochemical Psychopharmacology 1977;16:655-9. [PubMed] [Google Scholar]
- Van Kammen DP, Docherty JP, Marder SR, Schulz SC, Bunney WE Jr. Lack of behavioral supersensitivity to d-amphetamine after pimozide withdrawal. A trial with schizophrenic patients. Archives of General Psychiatry 1980;37:287-90. [DOI] [PubMed] [Google Scholar]
- Van Kammen DP, Docherty JP, Marder SR, Siris SG, Bunney WE Jr. D amphetamine raises serum prolactin in man: evaluations after chronic placebo, lithium and pimozide treatment. Life Sciences 1978;23:1487-92. [DOI] [PubMed] [Google Scholar]
Vanover 2018 {published data only}
- Vanover K, Dmitrienko A, Glass S, Kozauer S, Saillard J, Weingart M, et al. Lumateperone (ITI-007) for the treatment of schizophrenia: placebo-controlled clinical trials and an open-label safety switching study. Schizophrenia Bulletin 2018;44(Suppl 1):S341. [Google Scholar]
Van Praag 1973 {published data only}
- Van Praak HM, Dols LC. Fluphenazine enanthate and fluphenazine decanoate: a comparison of their duration of action and motor side effects. American Journal of Psychiatry 1973;130:801-4. [DOI] [PubMed] [Google Scholar]
Weller 2018 {published data only}
- Weller A, Killackey E, Gleeson J, Allott K, Alvarez-Jimenez M, Bendall S, et al. Less is more? An Australian RCT comparing dose reduction of anti-psychotic medication to maintenance treatment. Early Interventions in Psychiatry 2018;12(Suppl 1):207. [Google Scholar]
- Weller A, et al. Can antipsychotic dose reduction lead to better functional recovery in first episode psychosis? A randomized controlled trial of antipsychotic dose reduction: the REDUCE trial: study protocol. Early Intervention in Psychiatry 2019;13(6):1345-1356. [DOI] [PubMed] [Google Scholar]
Wiedemann 2001 {published data only}
- Wiedemann G, Hahlweg K, Muller U, Feinstein E, Hank G, Dose M. Effectiveness of targeted intervention and maintenance pharmacotherapy in conjunction with family intervention in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience 2001;251:72-84. [DOI] [PubMed] [Google Scholar]
Wright 1964 {published data only}
- Wright RL, Lynes PG. Value of continuous drug administration for chronic long-term mental hospital patients. Canadian Psychiatric Association Journal 1964;60:352-7. [DOI] [PubMed] [Google Scholar]
Wunderink 2006 {published data only}
- Faber G, Smid HG, Van Gool AR, Wiersma D, Van Den Bosch RJ. The effect of guided discontinuation of antipsychotics on neurocognition in first onset psychosis. European Psychiatry 2012;27:275-80. [DOI] [PubMed] [Google Scholar]
- Wunderink A, Nienhuis, Sytema S, Wiersma D. Guided discontinuation versus maintenance treatment in remitted first episode psychosis: relapse rates and functional outcome. Schizophrenia Research 2006;86:S51. [DOI] [PubMed] [Google Scholar]
Zeller 1956 {published data only}
- Zeller WW, Graffagnino PN, Cullen CF, Rietman HJ. Use of chlorpromazine and reserpine in the treatment of emotional disorders. Journal of the American Medical Association 1956;160:179-84. [DOI] [PubMed] [Google Scholar]
Zou 2018 {published data only}
- Zou X, Zhu Y, Jackson JW, Bellavia A, Fitxmaurice GM, Centorrino F, et al. The role of PANSS symptoms and adverse events in explaining the effects of paliperidone on social functioning: a causal mediation analysis approach. NPJ Schizophrenia 2018;4(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwanikken 1973 {published data only}
- Zwanikken 1973. Penfluridol (R 16341). A long-acting oral neuroleptic, as maintenance therapy for schizophrenic and mentally retarded patients. Psychiatria, Neurologia, Neurochirurgia 1973;76:83-92. [PubMed] [Google Scholar]
References to studies awaiting assessment
Ascher‐Svanum 2011 {published data only}
- Ascher-Svanum H, Nyhuis A, Faries D, Novick D, Kinon B. Differences between schizophrenia patients who switch vs discontinue antipsychotic therapy. Schizophrenia Bulletin 2011 March;37(Suppl 1, Abstracts for the 13th International Congress on Schizophrenia Research (ICOSR)):96. [Google Scholar]
Decot 2011 {published data only}
- Decot H, Zhang F, Weinberger DR, Apud J. Effect of placebo and reintroduction of antipsychotics in patients with schizophrenia based on COMT Val108/158Met polymorphism. In: International Congress on Schizophrenia Research 84, downloaded from: schizophreniabulletin.oxfordjournals.org,. 2011.
Eisenberg 2016 {published data only}
- Eisenberg D, Yankowitz L, Kohn P, Hegarty C, Ianni A, Rubinstein D, Gregory M, Dickinson D, Apud J, Berman K. Neuroleptic-induced striatal blood flow changes and neurocognitive functioning in schizophrenia. Neuropsychopharmacology 2016;41:S223-4. [Google Scholar]
EUCTR2005‐005499‐34 {published and unpublished data}
- EUCTR2005-005499-34. A double-blind, randomised, placebo-controlled, quetiapine-referenced, multicentre study of the long-term bifeprunox efficacy, safety and tolerability in patients with stable schizophrenia. www.clinicaltrialsregister.eu.
Zhang 2006 {published data only}
- Zhang Q, He FR, Tian YL, Zhao S, Sun ZH. Double blind study of the contact between antipsychotic and the negative symptoms of the deteriorated schizophrenia. Medical Journal of Chinese People Health 2006;3:182-3. [Google Scholar]
References to ongoing studies
NCT03503318 {published data only}
- NCT03503318. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy, safety, and tolerability of risperidone extended-release injectable suspension (TV-46000) for subcutaneous use as maintenance treatment in adult and adolescent patients with schizophrenia. https://clinicaltrials.gov.
NCT03593213 {published data only}
- NCT03593213. Clinical trial evaluating the efficacy, safety and tolerability of cariprazine in a dose-reduction paradigm in the prevention of relapse in patients with schizophrenia. https://clinicaltrials.gov.
NCT03893825 {published data only}
- NCT03893825. A study to test if TV-46000 is safe for maintenance treatment of schizophrenia. https://clinicaltrials.gov.
Additional references
Almerie 2007
- Almerie MQ, Alkhateeb H, Essali A, Matar HE, Rezk E. Cessation of medication for people with schizophrenia already stable on chlorpromazine. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD006329. [DOI: 10.1002/14651858.CD006329] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
American Psychiatric Association 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd rev edition. Washington DC: American Psychiatric Association, 1987. [Google Scholar]
Andreasen 2005
- Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. American Journal of Psychiatry 2005;162(3):441-9. [DOI] [PubMed] [Google Scholar]
Auquier 2003
- Auquier P, Simeoni MC, Sapin C, Reine G, Aghababian V, Cramer J, et al. Development and validation of a patient-based health-related quality of life questionnaire in schizophrenia: the S-QoL. Schizophrenia Research 2003;63(1-2):137-49. [DOI] [PubMed] [Google Scholar]
Baldessarini 1985
- Baldessarini RJ. Chemotherapy in Psychiatry: Principles and Practice. Cambridge, MA: Harvard University Press, 1985. [Google Scholar]
Barnes 1989
- Barnes TR. A rating scale for drug-induced akathisia. British Journal of Psychiatry 1989;154:672-6. [DOI] [PubMed] [Google Scholar]
Berger 2003
- Berger M. Psychische Erkrankungen. Klinik und Therapie. 2nd edition. München: Urban & Fischer, 2003. [Google Scholar]
Bland 1997
- Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405-11. [PubMed] [Google Scholar]
Borenstein 2006 [Computer program]
- Comprehensive Meta-analysis Version 2. Borenstein M, Hedges LV, Higgins JP, Rothstein H. Biostat, 2006.
Boyer 2010
- Boyer L, Simeoni MC, Loundou A, D'Amato T, Reine G, Lancon C, et al. The development of the S-QoL 18: a shortened quality of life questionnaire for patients with schizophrenia. Schizophrenia Research 2010;121:241-50. [DOI] [PubMed] [Google Scholar]
Burns 2007
- Burns T, Patrick D. Social functioning as an outcome measure in schizophrenia studies. Acta Psychiatrica Scandinavica 2007;116:403-18. [DOI] [PubMed] [Google Scholar]
Carpenter 1994
- Carpenter WT, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681-90. [DOI] [PubMed] [Google Scholar]
Davis 1974
- Davis JM. Dose equivalence of the antipsychotic drugs. Journal of Psychiatric Research 1974;11:65-9. [DOI] [PubMed] [Google Scholar]
Davis 1975
- Davis JM. Overview: maintenance therapy in psychiatry: I. Schizophrenia. American Journal of Psychiatry 1975;132(12):1237-45. [DOI] [PubMed] [Google Scholar]
Davis 1989
- Davis JM, Barter JT, Kane JM. Antipsychotic drugs. In: Kaplan HI, Sadock BJ, editors(s). Comprehensive Textbook of Psychiatry. 5th edition. Baltimore: Williams & Wilkins, 1989:1591-626. [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta-analyses of binary data. In: Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25-28; Cape Town, South Africa. 2000.
Der‐Simonian 1986
- Der-Simonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
Divine 1992
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623-9. [DOI] [PubMed] [Google Scholar]
Duggan 2005
- Duggan L, Fenton M, Rathbone J, Dardennes R, El-Dosoky A, Indran S. Olanzapine for schizophrenia. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD001359. [DOI: 10.1002/14651858.CD001359.pub2] [DOI] [PubMed] [Google Scholar]
Duval 2000
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-63. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey-Smith G, Schneider M, Minder CS. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;13:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31:140-9. [DOI] [PubMed] [Google Scholar]
Endicott 1976
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry 1976;33(6):766-71. [DOI] [PubMed] [Google Scholar]
EuroQol 1990
- The EuroQol Group. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy 1990;16(3):199-208. [DOI] [PubMed] [Google Scholar]
Gardner 2010
- Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. American Journal of Psychiatry 2010;167(6):686-93. [DOI] [PubMed] [Google Scholar]
Gilbert 1995
- Gilbert P, Harris MJ, McAdams LA, Bowers MB, Charney DS, Glazer WM. Neuroleptic withdrawal in schizophrenic patients. A review of the literature. Archives of General Psychiatry 1995;52:173-88. [DOI] [PubMed] [Google Scholar]
Gopal 2010
- Gopal S, Gassman-Mayer C, Palumbo J, Samtani MN, Shiwach R, Alphs L. Practical guidance or dosing and switching paliperidone palmitate treatment in patients with schizophrenia. Current Medical Research and Opinion 2010;26(2):377-87. [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876-83. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. ECDEU Assessment Manual for Psychopharmacology-revised 1976. Washington, DC: DHEW. National Institute of Mental Health, 1976. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Kalali 1999
- Kalali A. Patient satisfaction with, and acceptability of, atypical antipsychotics. Current Medical Research and Opinion 1999;15:135-7. [DOI] [PubMed] [Google Scholar]
Kane 1988
- Kane JM, Honigfeld G, Singer J, Meltzer H, and the Clozaril Collaborative study group. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Archives of General Psychiatry 1988;45:789-96. [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive And Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi-Health Systems, 1986. [Google Scholar]
Kellner 1973
- Kellner R, Sheffield BF. A self-rating scale of distress. Psychological Medicine 1973;3:88-100. [DOI] [PubMed] [Google Scholar]
Kishi 2019
- Kishi T, Ikuta T, Matsui Y, Inada K, Matsuda Y, Mishima K, et al. Effect of discontinuation vs maintenance of antipsychotic medication on relapse rates in patients with remitted/stable first-episode psychosis: a meta-analysis. Psychological Medicine 2019;49(5):772-9. [DOI] [PubMed] [Google Scholar]
Kishimoto 2014
- Kishimoto T, Robenzadeh A, Leucht C, Leucht S, Watanabe K, Mimura M, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophrenia Bulletin 2014;40(1):192-213. [DOI] [PMC free article] [PubMed]
Klein 1969
- Klein DF, Davis JM. Diagnosis and Drug Treatment of Psychiatric Disorders. Baltimore: Williams & Wilkins, 1969. [Google Scholar]
Leon 2006
- Leon AC, Mallinckrodt CH, Chuang-Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomised controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59:1001-5. [DOI] [PubMed] [Google Scholar]
Leucht 2003
- Leucht S, Barnes TR, Kissling W, Engel RR, Correll C, Kane JM. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomised, controlled trials. American Journal of Psychiatry 2003;160:1209-22. [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale scores. British Journal of Psychiatry 2005;187:366-71. [DOI] [PubMed] [Google Scholar]
Leucht 2005b
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophrenia Research 2005;79(2-3):231-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leucht 2009
- Leucht S, Corves C, Arbter D, Engel R, Li C, Davis JM. A meta-analysis comparing second-generation and first-generation antipsychotics for schizophrenia. Lancet 2009;373:31-41. [DOI] [PubMed] [Google Scholar]
Leucht 2009a
- Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, et al. A meta-analysis of head to head comparisons of second generation antipsychotics in the treatment of schizophrenia. American Journal of Psychiatry 2009;166:152-63. [DOI] [PubMed] [Google Scholar]
Leucht 2009b
- Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Molecular Psychiatry 2009;14:429-47. [DOI] [PubMed] [Google Scholar]
Leucht 2011
- Leucht C, Heres S, Kane JM, Kissling W, Davis JM, Leucht S. Oral versus depot antipsychotic drugs for schizophrenia - a critical systematic review and meta-analysis of randomised long-term trials. Schizophrenia Research 2011;127:83-92. [DOI] [PubMed] [Google Scholar]
Leucht 2013
- Leucht S, Cipriani A, Spineli L, Mavridis M, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013;382(9896):951962. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249-52. [DOI] [PubMed] [Google Scholar]
Marvaha 2004
- Marwaha S, Johnson S. Schizophrenia and employment? A review. Social Psychiatry and Psychiatric Epidemiology 2004;39:337-49. [DOI] [PubMed] [Google Scholar]
McGrath 2008
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality.. Epidemiologic Reviews 2008;30:67-76. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. JAMA 2001;285:1987-1. [DOI] [PubMed] [Google Scholar]
Moncrieff 2006
- Moncrieff J. Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse. Acta Psychiatrica Scandinavica 2006;114(1):3-13. [DOI] [PubMed] [Google Scholar]
Morosini 2000
- Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatrica Scandinavica 2000;101:323-9. [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799-12. [Google Scholar]
Papanikolaou 2004
- Papanikolaou PN, Churchill R, Wahlbeck K, Ioannidis JP. Safety reporting in randomised trials of mental health interventions. American Journal of Psychiatry 2004;161:1692-7. [DOI] [PubMed] [Google Scholar]
Peters 2008
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61:991-6. [DOI] [PubMed] [Google Scholar]
Pope 2010
- Pope A, Adams C, Paton C, Weaver T, Barnes TR. Assessment of adverse effects in clinical studies of antipsychotic medication: survey of methods used. British Journal of Psychiatry 2010;197:67-72. [DOI] [PubMed] [Google Scholar]
R Core Team 2018 [Computer program]
- Foundation for Statistical Computing R: A language and environment for statistical computing. R Core Team, Version 3.6.2. Vienna: Foundation for Statistical Computing, 2018.
Ray 2009
- Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. New England Journal of Medicine 2009;360:225-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 1999
- Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Archives of General Psychiatry 1999;56:241-7. [DOI] [PubMed] [Google Scholar]
Schneider 1983
- Schneider LC, Struening EL. SLOF: a behavioural rating scale for assessing the mentally ill. Social Work Research & Abstracts 1983 Fall;19:9-21. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. [DOI] [PubMed] [Google Scholar]
Schwarzer 2007
- Schwarzer G. Meta: an R package for meta-analyses. R News 2007;7(3):40-5. [Google Scholar]
Shaffer 1983
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A Children's Global Assessment Scale (CGAS). JAMA Psychiatry 1983;40:1228-31. [DOI] [PubMed] [Google Scholar]
Sheenan 1983
- Sheenan DV. The Sheenan Disability Scale. In: The Anxiety Disease and How to Overcome it. New York: Charles Scribner and Sons, 1983:151. [Google Scholar]
Shokraneh 2017
- Shokraneh F, Adams CE. Study-based registers of randomized controlled trials: Starting a systematic review with data extraction or meta-analysis. BioImpacts 2017;4:209-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 1970
- Simpson EN, Angus JW. A rating scale for extrapyramidal side-effects. Acta Psychiatrica Scandinavica Supplementum 1970;212:11-9. [DOI] [PubMed] [Google Scholar]
Srisurapanont 2004
- Srisurapanont M, Maneeton B, Maneeton N. Quetiapine for schizophrenia. Cochrane Database of Systematic Reviews 2004, Issue 2. Art. No: CD000967. [DOI: 10.1002/14651858.CD000967.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stata 2002 [Computer program]
- Intercooled Stata 7.0 for Windows. Stata Corporation. Stata Corporation, 2002.
Tiihonen 2009
- Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet 2009;374:620-7. [DOI] [PubMed] [Google Scholar]
Tsuang 1978
- Tsuang MT. Suicide in schizophrenics, manics, depressives, and surgical controls: a comparison with general population suicide mortality. Archives of General Psychiatry 1978;35:153-5. [DOI] [PubMed] [Google Scholar]
Vernon 2010
- Vernon MK, Revicki DA, Awad G, Dirani R, Panish J, Canuso CM, et al. Psychometric evaluation of the Medication Satisfaction Questionnaire (MSQ) to assess satisfaction with antipsychotic medication among schizophrenia patients. Schizophrenia Research 2010;118(1-3):271-8. [DOI] [PubMed] [Google Scholar]
Viguera 1997
- Viguera AC, Baldessarini RJ, Hegarty JD, Kammen DP, Tohen M. Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Archives of General Psychiatry 1997;54:49-55. [DOI] [PubMed] [Google Scholar]
Vita 2018
- Vita A, Barlati S. Recovery from schizophrenia: is it possible? Curr Opin Psychiatry 2018;31(00):1-10. [DOI] [PubMed] [Google Scholar]
Wahlbeck 1999
- Wahlbeck K, Cheine M, Essali MA, Rezk E. Clozapine vs typical neuroleptic medication for schizophrenia. Cochrane Database of Systematic Reviews 1999, Issue 2. Art. No: CD000059. [DOI: 10.1002/14651858.CD000059] [DOI] [PubMed] [Google Scholar]
Walsh 2002
- Walsh E, Buchanan A, Fahy T. Violence and schizophrenia: examining the evidence. British Journal of Psychiatry 2002;180:490-5. [DOI] [PubMed] [Google Scholar]
Weinmann 2009
- Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophrenia Research 2009;113(1):1-11. [DOI] [PubMed] [Google Scholar]
Wilkinson 2000
- Wilkinson G, Hesdon B, Wild D, Cookson R, Farina C, Sharma V, et al. Self-report quality of life measure for people with schizophrenia: the SQLS. British Journal of Psychiatry 2000;177:42-6. [DOI] [PubMed] [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254-7. [Google Scholar]
Zhao 2016
- Zhao YJ, Lin L, Teng M, Khoo AL, Soh LB, Furukawa TA, et al. Long-term antipsychotic treatment in schizophrenia: systematic review and network meta-analysis of randomised controlled trials. BJPsych Open 2016;2(1):59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bergman 2016
- Bergman H, Davies S, Dawson S, Kakourou A, Spineli L. Targeted update of Maintenance treatment with antipsychotic drugs for schizophrenia. The Cochrane Collaboration, Targeted Updates Project 2016. [https://community.cochrane.org/news/targeted-updates-project-case-study-b]
Komossa 2009
- Komossa K, Depping AM, Heres S, Kissling W, Davis JM, Leucht S. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD008016. [DOI: 10.1002/14651858.CD008016] [DOI] [PubMed] [Google Scholar]
Leucht 2012a
Leucht 2012b
- Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Davis JM. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD008016. [DOI: 10.1002/14651858.CD008016.pub2] [DOI] [PubMed] [Google Scholar]


