Abstract
Metastasis to the nasal cavity and paranasal sinuses are very rare and only few cases have been reported so far. Metastatic nasal mass with silent primary renal cell carcinoma (RCC) is even rarer. So are giant cell tumors which rarely affects soft tissues whether superficial or deep. These rarely occur in nasal cavity. We would like to discuss 2 cases—one being a 74 year old female with a solitary asymptomatic extensive metastatic lesion in sinonasal area of silent primary renal cell carcinoma and other being a 38 year old female multiple lytic expansile lesions in facial and skull bones who was previously treated for giant cell tumor of long bone-tibia. We aim to bring their occurrence to notice as they are rare, to highlight importance of these tumors in differential diagnosis of sinonasal masses and treatment options for the same.
Keywords: Renal cell carcinoma (RCC), Giant cell tumors (GCT), Sinonasal metastasis
Introduction
Nasal cavity and paranasal sinus cancers are usually primary tumors and metastatic deposits to this region is rarely known. Though rare, renal cell carcinoma is the most common cancer to metastasize to this region (49%). Others include tumors of bronchus, urogenital ridge, breast, and gastrointestinal tract [1, 2]. The other entity that is giant cell tumours which we discuss here more commonly metastasize to lung. Other reported sites to which metastasis occur include lymph nodes (mediastinum, paraaortic), bone, skin, and breast [3]. These giant cell tumors rarely occur in nasal cavity and the exact incidence is not yet recorded in the literature [4].
Renal cell carcinoma (RCC) accounts for approximately 3% of all adult malignancies and 85% of primary renal tumors [5]. It is an aggressive tumor with usual sites of metastasis being lungs (75%), regional lymph nodes (65%), bone (40%), liver (40%), and brain (5%) [5]. Unusual presentation, multiple metastasis, and high vascularity of the tumour make it difficult for the clinician to diagnose and intervene and hence has a poor prognosis.
Giant cell tumors on the other hand constitute about 4–5% of all primary bone tumors [6], are usually benign and occasionally malignant. Most common site of giant cell tumors is the epiphysis of long bones around the knee [7]. Giant cell tumors of head and neck are very rare and constitute about 2% of all giant cell tumors [8]. Giant cell tumor is rare in soft tissues. Though benign, they have been reported to have metastatic potential to distant sites.
Case Report 1
A 74 year old female presented in emergency room with head injury following road traffic accident. Her CT scan of head incidentally detected polypoidal thickening in bilateral frontal sinuses, left ethmoid and left maxillary sinus with suspicious breach in posterior wall of frontal sinus. She had no previous nasal complaints of epistaxis. She had type 2 diabetes well controlled on oral hypoglycaemic agents.
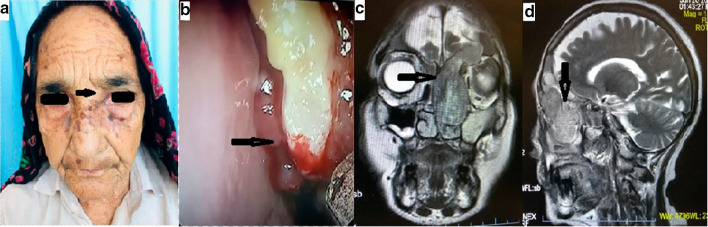
On examination she had hypertension, telecanthus, pulsatile soft tissue swelling in superomedial aspect of left eye with non axial proptosis (Fig. 1a). Diagnostic nasal endoscopy showed pulsatile hypervascular mass in left nasal cavity in region of middle meatus and ethmoids which bled on touch (Fig. 1b). Her routine blood and urine investigations were normal. Differential diagnosis as per CT features were fungal sinusitis, benign tumors including angiogenic lesions and sinonasal primary malignancy were considered.
Fig. 1.
a 74 year female with telecanthus and pulsatile soft tissue swelling in superomedial aspect of left eye.( black arrow) b DNE image showing pulsatile bleeding from mass in left middle meatus (blue arrow) c & d coronal and sagittal views, T2W1 CEMRI showing heterogeneously enhancing mass, in left nasal cavity and maxillary sinus and frontal sinus with anterior cranial fossa involvement
To work up the nasal mass, a contrast enhanced MRI scan of brain, nose and paranasal sinuses (Fig. 1c, d) were performed which showed a heterogeneously enhancing mass, occupying the left nasal cavity and maxillary sinus, bilateral frontal sinus with anterior cranial fossa involvement. Ethmoid sinus component caused bulge in sinus wall encroaching into left orbit with no involvement of intraorbital structures.
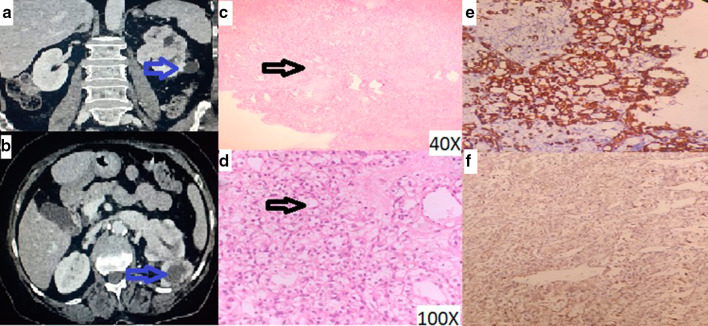
Biopsy of the nasal mass was taken. The mass was friable and bled profusely. However, bleeding got controlled after repeated nasal packing. Histopathology was reported as metastatic renal cell carcinoma-clear cell type (Fig. 2c, d), further immunohistochemistry strongly positive for RCC antigen and vimentin (Fig. 2e, f).
Fig. 2.
a Enhancing left renal mass b Enhancing left renal mass with left renal vein invasion c 40X image showing tumour cells arranged in diffuse sheets d 100X Image showing large tumor cells with clear and vacuolated cytoplasm, round to oval nucleus and prominent nucleolus e Tumor cells positive for Vimentin on IHC f Tumor cells showing positivity for RCC antigen on IHC
We started our further work up. CT urography (Fig. 2a, b) revealed left renal ill defined heterogeneously enhancing neoplastic mass 7 × 3 × 4.5 cm in size with large exophytic component and internal nonenhancing necrotic areas, left renal vein tumor thrombus, and locoregional lymphadenopathy. Metastatic work up including bone scan and CECT thorax showed no other metastasis. Patient was planned for cytoreductive renal surgery and radiotherapy for the sinonasal mass.
Case Report 2
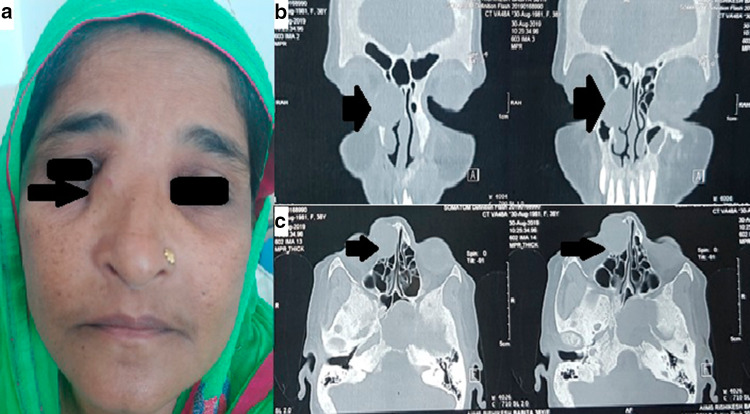
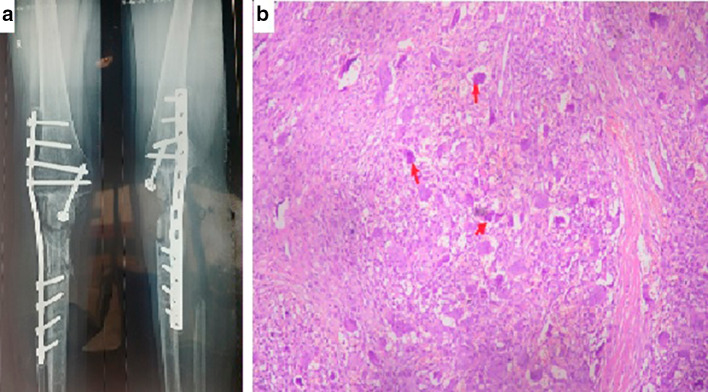
A 38 year old female presented to the ENT OPD with a swelling over the medial canthus of right eye for 6 months (Fig. 3a), with watering from right eye and generalized headache for 3 months. She was a known case of Giant cell tumor of right proximal tibia who underwent wide local excision and limb reconstruction with alcohol inactivated autograft and knee arthrodesis on March 2016 (Fig. 4a). She was on follow up thereafter. Patient did not have any nasal complaints.
Fig. 3.
a clinical image showing 38 year old female with a swelling over the medial side of right eye b NCCT Nose & PNS coronal view showing multiple lytic expansile lesion with areas of cortical breech on facial and skull bones c axial view of same lesion (black arrows marks the lesion)
Fig. 4.
a Giant Cell Tumor—Post-operative X-ray Knee b On HPE numerous osteoclasts like giant cells (red arrow) are seen uniformly distributed throughout the tumor in a background of monomorphic mononuclear cells population. The cells are round to spindled with bland nucleus
Non Contrast Computed Tomography of the paranasal sinuses revealed multiple lytic expansile lesions in facial and skull bones reported as metastatis of giant cell tumour (Fig. 3b, c). Incisional biopsy confirmed the presence of giant cells (Fig. 4b). Medical oncology reference was obtained and therapy with denosumab was advised.
Discussion
The clinical course of RCC is unpredictable, ranging from aggressive nature to spontaneous regression. Metastasis may be found at the time of diagnosis in 25–30% of the patients and upto even 17 years after nephrectomy [9]. Unusual sites of metastases are characteristic of RCC and virtually any organ site can be involved. Metastasis to the head and neck regions account for about 15% of the cases, the paranasal sinuses being highest in the order of frequency [10]. Maxillary sinuses are the more commonly involved (36%), followed by ethmoid (25%), frontal and sphenoid sinuses (17%), and nasal cavity (11%) [11, 12].
RCC mainly affects males between 40 and 60 years [13]. Common presenting symptoms include haematuria (40%), flank pain (40%), and a palpable abdominal mass (25%) [14]. This triad is seen only in 10% of patients. Sinonasal mass as initial presentation has been reported in a few cases [15, 16], while in others it occurred concurrently with renal mass as in our case or occurred years after nephrectomy. Hypervascularity of tumor explains the massive epistaxis which is the most common symptom. Mutation of the VHL gene causes upregulation of hypoxia-induced factor 1a and leads to angiogenesis through VEGF upregulation [17].
In case 1, patient is an elderly female with absence of classical triad of RCC and sinonasal mass which was detected incidentally during a screening CT for head injury. Patient neither had previous episodes of spontaneous epistaxis nor symptomatic nasal obstruction which was surprising after seeing the extent of disease. Clinical suspicion and adequate preoperative haemostatic precautions helped us to control the massive pulsatile bleed which occurred during biopsy and debulking.
In RCC, tumor cells can reach the sinonasal region via two ways: (a) through the great vessels inferior venacava, and subsequently into lungs, heart, and the maxillary artery, (b) through the valveless vertebral venous plexus and intracranial venous plexus.
In the present case, metastasis might have occurred through the second route which by passes the lungs and explains the solitary sinonasal metastatic lesion. In general, work-up for a sinonasal mass should include endoscopic examination, followed by a contrast enhanced CT scan, prior to considering biopsy. This is the imaging test of choice considering its ability to delineate vascularity and skull base involvement. CT angiography and preoperative embolization may be considered which can significantly reduce intraoperative bleeding from external carotid system and internal carotid arteries to some extent. Ultrasound screening for abdominal organs in case of clinical suspicion can detect renal lesions. In CT scan, radiological appearances of metastasis from RCC has similarities to primary malignant lesions of sinonasal cavity. Some indicators of renal origin are enhancement, destruction, and lack of calcification in tumor [18].
Metastatic clear cell RCC on microscopy shows clear cell borders, eosinophilic cytoplasm, round or oval nuclei, and tumor cells arranged in nests with capillaries in between. Immunohistochemical staining for vimentin, EMA, CD10, CA IX, and PAX8 is done for tumor differentiation [19].
Treatment modalities include radiotherapy and immunochemotherapy for metastatic diseases. RCC is considered both radio- and chemo-resistant because of the inability to obtain high doses of radiation in the retroperitoneum, but in the nose and paranasal sinuses effective dose of radiation can be given. Now endoscopic surgery as either complete excision or cytoreduction is gaining more importance as it reduces bleeding in the course of treatment. Two reports describe the successful endoscopic resection of localized disease in the paranasal sinuses with greater 5 year survival [20]. Adjuvant treatment options include anti–vascular endothelial growth factor (VEGF) and mTOR pathway inhibitors, which may improve progression free survival in advanced RCC [21]. Prognosis of metastatic RCC is poor [22]; the survival rate ranges between 15 to 30% at 5 years [23] in case of a single metastasis and upto 7% in patients with multiple metastases [24].
Giant cell tumors commonly present during third and fourth decades in the epiphyseal region of long bones [25]. It can also involve the metaphysis of skeletally immature patient. Distal femur, proximal tibia, distal radius and sacrum are the other commonly involved sites. Other less common sites include proximal fibula, proximal femur, and proximal humerus. Multicentric and synchronous occurrence is rare [26, 27]. GCTs are benign tumors with metastatic potential. Though rare, they may be associated with destruction of local bony architecture. 1 to 9% of cases can show metastasis to distant sites [28].
Pain and soft tissue mass are the common presentation. Pathological fracture may be the presentation in 12% of the cases which indicates more aggressive disease and poor prognosis [27]. On radiography, it has a radiolucent geographical appearance with narrow zone of transition at the margin of the lesion lacking a sclerotic rim. Brown tumor of hyperparathyroidism, aneurysmal bone cyst, telangiectatic osteosarcoma, and malignant fibrous histiocytoma are the differential diagnosis considered [29, 30].
Based on radiographic appearance, GCTs were classified by Enneking and later by Campanacci. They described three stages depending on the aggressiveness and local recurrence: Stage I—latent, Stage II—active and Stage III—aggressive. Radiologically, they are graded as Grade 1, Grade 2 and Grade 3. 20–30% of the cases show local recurrence. Recurrence after three years is less common. Grade 3 has shown to have an increased rate of recurrence [27, 31, 32].
GCT with pulmonary metastasis shows poor prognosis which is seen in 3% of cases [28]. Mean interval noted between the primary tumor and lung metastasis was approximately 18 to 24 months [33]. Giant cell tumors of head and neck are very rare and constitute about 2% of all giant cell tumors [8]. These giant cell tumors rarely occur in nasal cavity and the exact incidence is not yet recorded in the literature [4]. The natural history of metastatic lesions is unpredictable. However, if completely excised, they show good prognosis. Surgery is the mainstay of treatment. Hence, if possible metastatic lesions should be dealt surgically.
Radiotherapy and chemotherapy are of limited value in case of metastasis. At present no effective chemotherapeutic agents are available. Radiotherapy is recommended for unresectable cases like that of spine or sacrum. There have been reports of sarcomatous changes in giant cell tumors following Radiotherapy [34]. In unresectable cases, steroids are also a good option.
There have been reports of topical or systemic use of bisphosphonates as adjuvant therapy: pamidronate or zoledronate. Bisphosphonates targets osteoclast like giant cells and thus results in apoptosis which limits progression of tumor [35, 36]. The giant cells overexpress RANK receptor which plays an important role in osteoclast genesis. The RANK receptor is stimulated by cytokine RANKL. The RANK/RANKL interaction is predominantly responsible for the extensive bone resorption by the tumour. Hence anti-RANKL therapy is the other modality which can be used in giant cell tumors that cannot be surgically excised.[27]. Denosumab, a monoclonal antibody approved by United States Food and Drugs Administration (US FDA) binds to RANKL and has resulted in dramatic responses.[37, 38].
Conclusion
These case reports revealed a silent primary with metastasis to rare distant sites. Histopathology and immunohistochemistry are the mainstay for diagnosis. In both the cases the final diagnosis was surprising and hence sinonasal masses especially those with unusual morphology and hypervascular nature, should undergo a detailed work up. A proper clinical evaluation, radiological correlation and biopsy under precautions are necessary to establish the diagnosis.
Funding
There was no external funding involved in this particular case report.
Compliance with Ethical Standards
Conflict of interest
None.
Informed Consent
Informed consent was obtained from both the participants included in the case report for publishing the details.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Madhu Priya, Email: drpriyamadhu@gmail.com.
Joyson Xavier, Email: Joyson2008karuna@gmail.com.
Saumya John, Email: Saumyamaryjohn@gmail.com.
Sumeet Angral, Email: angralsumeet@gmail.com.
Manu Malhotra, Email: manumalhotralrm@gmail.com.
Abhishek Bhardwaj, Email: abhi04stanley@gmail.com.
Saurabh Varshney, Email: drsaurabh68@gmail.com.
Sneha Venkatesan, Email: Sneha.venkatesan@gmail.com.
References
- 1.Sountoulides P, Metaxa L, Cindolo L. Atypical presentations and rare metastatic sites of renal cell carcinoma: a review of case reports. J Med Case Rep. 2011;5:429. doi: 10.1186/1752-1947-5-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evgeniou E, Menon KR, Jones GL, Whittet H, Williams W. Renal cell carcinoma metastasis to the paranasal sinuses and orbit. BMJ Case Rep. 2012 doi: 10.1136/bcr.01.2012.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities? Clin Orthopaed Rel Res®. 2010;468(3):827–833. doi: 10.1007/s11999-009-0966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuluc M, Zhang X, Inniss S. Giant cell tumor of the nasal cavity: case report. Eur Arch Otorhinolaryngol. 2007;264(2):205. doi: 10.1007/s00405-006-0143-6. [DOI] [PubMed] [Google Scholar]
- 5.Campbell SC, Lane BR. Malignant renal tumors. In: Wein AJ, Kavoussi LR, Novick AC, editors. Campbell-Walsh Urology. 10. Philadelphia, PA: WB Saunders; 2012. pp. 1413–1474. [Google Scholar]
- 6.Som PM (1996) Sinonasal cavities: inflammatory disease, tumors, fractures and postoperative findings. In: Som PM, Curtin HD (eds). Head and neck imaging; vol 1, 3rd edn. Mosby-year Book, Inc. pp 126–318.
- 7.Dubey RB, Tara NP, Desai SM. Giant cell tumor of sinonasal cavity-an uncommon location for a common bone tumor. Indian J Radiol Imag. 2003;13(1):13. [Google Scholar]
- 8.Lanzieri CF. The sinonasal cavity. In: Hagga J, Lanzieri CF, Sartories DJ, Zerhouini DH, editors. Computerised tomography and mangnetic resonance imaging of the whole body. 3. India: Mosby-Year book, Inc.; 1998. pp. 471–492. [Google Scholar]
- 9.Ziari M, Shen S, Amato RJ. Teh BS Metastatic renal cell carcinoma to the nose and ethmoid sinus. Urology. 2006;67(1):199. doi: 10.1016/j.urology.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 10.Dinçbas FO, Atalar B, Oksüz DC, Aker FV, Koca S. Unusual metastasis of renal cell carcinoma to the nasal cavity. J B.U.ON. 2004;9(2):201–204. [PubMed] [Google Scholar]
- 11.Kovaˇci´c M, Krvavica A, Rudi´c M. Renal cell carcinoma metastasis to the sinonasal cavity: case report. Acta Clin Croat. 2015;54(2):223–226. [PubMed] [Google Scholar]
- 12.Bernstein JM, Montgomery WW, Balogh K. Metastatic tumors to the maxilla, nose, and paranasal sinuses. Laryngoscope. 1966;76(4):621–650. doi: 10.1288/00005537-196604000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Lim RY, Bastug DF, Caldwell BL. Metastatic renal cell carcinoma of the nasal septum. W Va Med J. 1989;85(4):143–145. [PubMed] [Google Scholar]
- 14.Skinner DG, Vermillion CD, Pfister RC, Leadbetter WF. Renal cell carcinoma. Am Fam Phys. 1971;4(4):89–94. [PubMed] [Google Scholar]
- 15.Ikeuchi T, Asai N, Hori T, et al. Renal cell carcinoma detected by metastasis to the frontal sinus: a case report. Acta Urol Jpn. 1998;44(2):89–92. [PubMed] [Google Scholar]
- 16.Torres Muros B, Solano Romero JR, Rodr´ıguez Bar´o JG, Bonilla Parrilla R. Maxillary sinus metastasis of renal cell carcinoma. Actas Urol Esp. 2006;30(9):954–957. doi: 10.1016/S0210-4806(06)73565-3. [DOI] [PubMed] [Google Scholar]
- 17.Fyrmpas G, Adeniyi A, Baer S. Occult renal cell carcinoma manifesting with epistaxis in a woman: a case report. J Med Case Rep. 2011;5:79. doi: 10.1186/1752-1947-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Som PM, Norton KI, Shugar JM, et al. Metastatic hypernephroma to the head and neck. Am J Neuroradiol. 1987;8(6):1103–1106. [PMC free article] [PubMed] [Google Scholar]
- 19.Jung SM, Kuo TT. Immunoreactivity of CD10 and inhibin alpha in differentiating hemangioblastoma of central nervous system from metastatic clear cell renal cell carcinoma. Mod Pathol. 2005;18:788–794. doi: 10.1038/modpathol.3800351. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb MD, Roland JT., Jr Paradoxical spread of renal cell carcinoma to the head and neck. Laryngoscope. 1998;108(9):1301–1305. doi: 10.1097/00005537-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt TJ. Targeted therapy for advanced renal cell cancer (RCC): a cochrane systematic review of published randomised trials. BJU Int. 2011;108(10):1556–1563. doi: 10.1111/j.1464-410X.2011.10629.x. [DOI] [PubMed] [Google Scholar]
- 22.Ather MH, Masood N, Siddiqui T. Current management of advanced and metastatic renal cell carcinoma. Urol J. 2010;7(1):1–9. [PubMed] [Google Scholar]
- 23.Torres Muros B, Bonilla Parrilla R, Solano Romero JR, Rodr´ıguez Bar´o JG, Verge Gonz´alez J. Metastasis in maxilar sinus as only manifestation of disseminate renal adenocarcinoma. Anales Otorrinolaringol´ogicos Ibero-Americanos. 2007;34(3):231–236. [PubMed] [Google Scholar]
- 24.Cheng ET, Greene D, Koch RJ. Metastatic renal cell carcinoma to the nose. Otolaryngol Head Neck Surg. 2000;122(3):464. doi: 10.1067/mhn.2000.100500. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd GA. Diagnostic Imaging of the Nose and Paranasal Sinuses. London: Springer; 1988. Giant Cell Lesions of the Nose and Paranasal Sinuses; pp. 89–93. [Google Scholar]
- 26.Hoeffel JC, Galloy MA, Grignon Y, Chastagner P, Floquet J, Mainard L, et al. Giant cell tumor of bone in children and adolescents. Rev Rhum Engl Ed. 1996;63(9):618–623. [PubMed] [Google Scholar]
- 27.Sobti A, Agrawal P, Agarwala S, Agarwal M. Giant cell tumor of bone-an overview. Arch Bone Joint Surg. 2016;4(1):2. [PMC free article] [PubMed] [Google Scholar]
- 28.Siebenrock KA, Unni KK, Rock MG. Giant-cell tumour of bone metastasising to the lungs. A long-term follow-up. J Bone Joint Surg Br. 1998;80(1):43–47. doi: 10.1302/0301-620X.80B1.0800043. [DOI] [PubMed] [Google Scholar]
- 29.Cavanna L, Biasini C, Monfredo M, Maniscalco P, Mori M. Giant cell tumor of bone. Oncologist. 2014;19(11):1207. doi: 10.1634/theoncologist.2014-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakarun CJ, Forrester DM, Gottsegen CJ, Patel DB, White EA, Matcuk GR., Jr Giant cell tumor of bone: review, mimics, and new developments in treatment. Radiographics. 2013;33(1):197–211. doi: 10.1148/rg.331125089. [DOI] [PubMed] [Google Scholar]
- 31.Goldenberg RR, Campbell CJ, Bonfiglio M. Giantcell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg Am. 1970;52(4):619–664. doi: 10.2106/00004623-197052040-00001. [DOI] [PubMed] [Google Scholar]
- 32.Lausten GS, Jensen PK, Schiodt T, Lund B. Local recurrences in giant cell tumour of bone. Long-term follow up of 31 cases. Int Orthop. 1996;20(3):172–176. doi: 10.1007/s002640050057. [DOI] [PubMed] [Google Scholar]
- 33.Chan CM, Adler Z, Reith JD, Gibbs CP., Jr Risk factors for pulmonary metastases from giant cell tumor of bone. J Bone Joint Surg Am. 2015;97(5):420–428. doi: 10.2106/JBJS.N.00678. [DOI] [PubMed] [Google Scholar]
- 34.Boriani S, Sudanese A, Baldini N, Picci P. Sarcomatous degeneration of giant cell tumours. Ital J Orthop Traumatol. 1986;12(2):191–199. [PubMed] [Google Scholar]
- 35.Chang SS, Suratwala SJ, Jung KM, Doppelt JD, Zhang HZ, Blaine TA, et al. Bisphosphonates may reduce recurrence in giant cell tumor by inducing apoptosis. Clin Orthop Relat Res. 2004;426(1):103–109. doi: 10.1097/01.blo.0000141372.54456.80. [DOI] [PubMed] [Google Scholar]
- 36.Cheng YY, Huang L, Lee KM, Xu JK, Zheng MH, Kumta SM. Bisphosphonates induce apoptosis of stromal tumor cells in giant cell tumor of bone. Calcif Tissue Int. 2004;75(1):71–73. doi: 10.1007/s00223-004-0120-2. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Pousa A, Martin Broto J, Garrido T, Vazquez J. Giant cell tumour of bone: new treatments in development. Clin Transl Oncol. 2015;17(6):419–430. doi: 10.1007/s12094-014-1268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matcuk GR, Jr, Patel DB, Schein AJ, White EA, Menendez LR. Giant cell tumor: rapid recurrence after cessation of long-term denosumab therapy. Skeletal Radiol. 2015;44(7):1027–1031. doi: 10.1007/s00256-015-2117-5. [DOI] [PubMed] [Google Scholar]