Abstract
During the past decade, several inflammation-based periferic prognostic systems have been reported in the field of chronic rhinosinusitis with nasal polyps (CRSwNP). Recently, C-reactive protein (CRP) and albümin ratio (CAR) showed its impact on a large variety of diseases conditions that cause chronic inflammation. We aimed to compare the inflammatory markers in patients with recurrent and non-recurrent nasal polyps and if a significant inflammatory profile is associated with multiple recurrences. The study concerned 144 patients who underwent FESS for CRSwNP from 2012 to 2017 and had a postoperative follow-up longer than 12 months and 120 healthy individuals. We evaluated the impact of the CAR, neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio, eosinophil-to-lymphocyte ratio (ELR) between patients with and without polyp recurrences and control groups. There was a statistically significant difference in CRP, CAR and ELR values between multiple recurrence group and no-recurrence group (p = 0.02; 0.004; 0.019 respectively), mean eosinophil and CRP values, CAR, NLR and ELR was significantly higher in NP patients than control group (p < 0.001). The receiver operating curve analysis showed CAR and ELR as a potential marker of recurrence of NP (AUC = 0.713 and 0.613, respectively p < 0.001). The cutoff values for were 1.03 for CAR and 0.22 for ELR. The mean CRP, CAR and ELR were significantly higher in patients with CRSwNP whose disease recurred after surgery. CAR may be a potential marker to predict the recurrence before endoscopic sinus surgery as well as ELR in CRSwNP disease.
Keywords: Sinonasal polyposis, C-reactive protein-to-albumin ratio, Neutrophil-to-lymphocyte ratio, Eosinophil-to-lymphocyte-ratio, Trombosite-to-lymphocyte-ratio, Recurrence, FESS
Introduction
Chronic rhinosinusitis (CRS) is categorized into two main clinical subsection. CRS without nasal polyps (CRSsNP) and CRS with nasal polyps (CRSwNP) [1]. The etiology of NP is controversial, some theories have speculated that polyps occur as an outcome of conditions that cause chronic inflammation in the nasal cavity and paranasal sinuses characterized by variable cellular infiltration and stromal edema [2]. Functional endoscopic sinus surgery (FESS) is an effective treatment option for patients with CRSwNP resistant to medical therapy. However, inspite of repeated FESS combined with aggressive medical therapy as oral or topical corticosteroids and antibiotics, some patients have a tendency to be uncontrollable and have a high recurrence rate of NP [3]. This disturbing character of the disease, the symptoms including nasal obstruction, nasal discharge, and/or loss of smell, the recurrent polypoid lesions, and the absence of satisfactory treatment methods all make CRSwNP a serious life-quality, clinical, and economic problem [4]. The predominant inflammatory patterns are thought to influence the clinical manifestations and responses to current medical and surgical interventions. Patients with multiple recurrences of NP was found higher baseline eosinophil counts compared to single exacerbations of NP before [5, 6]. During the past decade, several inflammation-based prognostic systems have been reported in the field of inflammatory based diseases [7] Neutrophil to lymphocyte ratio (NLR), C-reactive protein (CRP) and albümin ratio (CAR) platelet to lymphocyte ratio (PLR) and eosinophil-to-lymphocyte ratio (ELR) are the most populars.
In this study we aimed to compare the inflammatory markers in patients with recurrent and non recurrent CRSwNP and if a significant inflammatory profile is associated with long term recurrence.
Materials and Methods
A retrospective review was performed using the clinical records of the patients who underwent primary or revision FESS after being unresponsive to medical management for NP and the control group from healthy individuals. All the procedures had been performed by the same surgical team at Bagcilar Training and Research Hospital, between January 2012 and December 2017. The study protocol and the written informed consent were approved by the Committee of Ethics in Human Research of Bagcilar Training and Research Hospital (2019.12.1.03.88). The diagnosis of CRSwNP was made according to the International Consensus Statement on Allergy and Rhinology: Rhinosinusitis [8].
Inclusion and Exclusion Criterias
The inclusion criteria of this study were as follow: (1) Clinically diagnosed as CRSsNP or CRSwNP and operated by our department (2) having pre-treatment blood sampling for complete blood count (CBC), CRP and albumin measurement, and (3) Regular Follow up.
Patients who had any inflammatory, systemic or local autoimmune disease affecting the upper airway (e.g., systemic lupus erythematosus, Sjogren’s syndrome, systemic sclerosis, small vessel vasculitis)and/or malnutritional condition, secondary malignancy and any trauma and medication history that might affect the level of blood parameters, who had sinonasal malignancies, autoimmune diseases, impaired mucociliary function (e.g., cystic fibrosis, Kartagener syndrome), hematologic disorders or were immunocompromised as indicated on patient history, and those without baseline eosinophil and basophil values were excluded from the study. Reported antibiotic and oral/nasal steroids utilization in the 4 weeks before blood samples, antrochoanal polyps, cystic fibrosis, inverted papilloma, unilateral diseases were also excluded.
Surgery Type
All patients were started on a course of a prednisone (20 mg) tablets once daily for 1 week, then 10 mg tablets once daily for 1 week) starting 1-week prior to surgery All patients were assessed with a comprehensive head and neck examination including flexible direct laryngoscopy and imaging (computerised tomography (CT) scans). Grading was determined using modification of the method described before [9] 0—no visible polyp seen; 1—polypoid disease confined within the middle meatus 2—multiple polyps occupying the middle meatus; 3—polyps extending beyond the middle meatus, but not completely obstructing the nasal cavity; and 4—polyps completely obstructing the nasal cavity. The operation included bilateral endoscopic polyps removal, while surgical treatment of nasal septum, anterior and posterior ethmoid, frontal, maxillary or sphenoid sinuses were nasalised depending on the specific necessity of the patient. All patients received the same postoperative treatment, including nasal saline irrigation, ointment, and intranasal corticosteroids as standard treatment. No systemic steroids were prescribed during the follow-up period for the subjects included in the study.
Clinical Data Extraction
We reviewed baseline characteristic of participants, including age, gender, disease stage, smoking status, Postoperative clinical controls for this study were performed after 1 month, after 6 months and after 1 year. Postoperative assessment during the controls was performed with endoscopic evaluation. Recurrence of polyps was defined if in the period from 6 month after surgery; by nasal endoscopy or CT scan was discovered the presence of polypoid mucosa together with bothersome symptoms that persisted for at least 1 month and if patient needed maximal medical treatment or revision surgery. Blood samples were tested prior to initial treatment. We noted the blood parameters including CBC, CRP and albumin. First, we compared the CAR, NLR, EOS, values of the groups. Then, we investigated the most sensitive and specific indicator parameter associated with the activity of aphthous lesions.
Statistical Analysis
Independent samples t test comparing age between the multiple recurrences or single exacerbation group against no recurrences group. Fisher’s exact test comparing categorical characteristics between the multiple recurrences or single exacerbation group against no recurrences group if expected cell count less than 5. Chi square test comparing categorical characteristics between the multiple recurrences or single exacerbation group against no recurrences group. To detect the most significantly associated parameter with the activity of RAS, The receive operating characteristic (ROC) curve analysis was carried out to assess the cut-off of CAR, NLR, EOS, The optimal cutoff values were identified as the values (sensitivity + specificity − 1). We used SPSS 15.00 software for Windows (SPSS Inc., Chicago, IL, USA). All tests were two-sided and a p value < 0.05 was considered statistically significant. Additionally, for post hoc comparison tests, we used Bonferroni correction of three groups (triple combination) and a p value less than 0.017 (0.05/3) was considered statistically significant.
Results
Of the 284 patients identified with CRSwNP, 140 patients were excluded due to exclusion criterias and 144 were included to the study Demographic and clinical characteristics of the study and control groups were described in Table 1. Mean follow-up duration was 16.3 ± 2.1 months after FESS. According to the control group, patients with NP were significantly have allergic diseases [3.3% and 25%, respectively (p < 0.05)]. Moreover patients who experienced multiple polyp recurrence had more alergic component especially aspirin intolerance and asthma rather than non-recurrent group. As described in Table 1, 16.6% of the study group and 20% of the control group were smokers. Smoking status was not statistically significant (p = 0.567) No statistical differences were found in the NP scores of patients in the CRSwNP subgroups.
Table 1.
Baseline characteristics of CRSsNP and control group
| No recurrence | Single recurrence | Multiple recurrence | NP total | Control group | |
|---|---|---|---|---|---|
| Total N | 66 (%) [SD] | 38 (%) [SD] | 40 (%) [SD] | 144 (%) [SD] | 120 (%) [SD] |
| Age [SD] | 46.3 [± 12.3] | 47.15 [± 11.7] | 43.95 [± 12.2] | 46.5 [± 11.4] | 47 [± 11.1] |
| Gender | |||||
| Male | 36 (54.5%) | 20 (52.6%) | 16 (40%) | 72 (50%) | 64 (53.3%) |
| Female | 30 (45.5%) | 18 (47.4%) | 24 (60%) | 72 (50%) | 56 (47.7%) |
| Smoking | |||||
| Yes | 10 (15.1%) | 6 (15.7%) | 8 (20%) | 24 (16.6%) | 24 (20%) |
| No | 56 (64.8%) | 32 (84,3%) | 32 (80%) | 120 (83.3%) | 96 (80%) |
| Asthma-allergy | |||||
| Yes | 10 (15.1%) | 14 (36.8%) | 12 (30%) | 36 (25%) | 4 (3.3%) |
| No | 56 (84.9%) | 52 (63.1%) | 28 (70%) | 108 (75%) | 116 (96.6%) |
| NP score | 2.90 ± [± 0.91] | 2.63 [± 1.16] | 3.05 [± 0.60] | 2.87 [± 0.91] | – |
NP nasal polyp, SD standard deviation
The measured laboratory values of the groups are compared in Table 2. Mean laboratory count with the standard deviation of CRP were significantly higher in the multiple recurrences than the no recurrences group (p = 0.02). Eosinophil count was higher in both single and multiple recurrence groups than the group without recurrence however it was not statistically significant. Platelet, lymphocyte and neutrophil count did not show any significant difference between patients and controls (p = 0.72, p = 0.065 and p = 0.071, respectively).
Table 2.
Comparison of mean [SD] laboratory data and predictive ratios (CAR–NLR–PLR–ELR) between no-recurrence, single recurrence, multiple recurrence, overall CRSsNP and control groups
| No recurrence | Single recurrence | Multiple recurrence | NP total | Control group | Multiple versus none p | Multiple versus single p | NP total versus control | |
|---|---|---|---|---|---|---|---|---|
| Neutrophils | 5.54 ± 0.93 | 6.03 ± 1.12 | 5.85 ± 0.82 | 5.76 ± 0.96 | 4.30 ± 1.16 | 0.225 | 0.353 | 0.02 |
| Lymphocytes | 2.26 ± 0.48 | 2.26 ± 0.42 | 2.21 ± 0.38 | 2.25 ± 0.43 | 2.38 ± 0.45 | 0.993 | 0.632 | 0.045 |
| Eosinophils | 0.53 ± 0.67 | 0.6 ± 0.65 | 0.78 ± 0.75 | 0.67 ± 0.70 | 0.22 ± 0.24 | 0.593 | 0.093 | < 0.001** |
| Thrombocytes | 279 ± 21.05 | 268.2 ± 32.4 | 282.3 ± 11.2 | 277.2 ± 23.01 | 291.6 ± 18.38 | 0.890 | 0.160 | 0.72 |
| CRP | 3.96 ± 1.30 | 6.67 ± 3.66 | 7.12 ± 5.13 | 5.55 ± 3.66 | 2.47 ± 0.43 | 0.02* | 0.978 | < 0.001** |
| Albumin | 4.23 ± 0.47 | 4.23 ± 0.33 | 3.98 ± 0.55 | 4.16 ± 0.47 | 4.36 ± 0.28 | 0.72 | 0.186 | 0.58 |
| CAR | 0.92 ± 0.24 | 1.59 ± 0.92 | 1.74 ± 1.25 | 1.32 ± 0.89 | 0.56 ± 0.09 | 0.004* | 0.955 | < 0.001** |
| NLR | 2.50 ± 0.45 | 2.71 ± 0.59 | 2,68 ± 0.41 | 2.60 ± 0.49 | 1.80 ± 0.42 | 0.183 | 0.789 | < 0.001** |
| PLR | 127.7 ± 24 | 125.2 ± 25.2 | 132.2 ± 22.4 | 128.2 ± 23.73 | 126.8 ± 24.8 | 0.826 | 0.136 | 0.747 |
| ELR | 0.25 ± 0.33 | 0.27 ± 0.35 | 0.34 ± 0.38 | 0.31 ± 0.33 | 0.09 ± 0.11 | 0.019* | 0.249 | < 0.001** |
*p < 0.05; **p < 0.001
There was a statistically significant difference in CRP, CAR and ELR values between multiple recurrence group and no-recurrence group (p = 0.02, 0.004, 0.019 respectively). There was a highly significant difference between total NP patients and control group according to Eosinophil, CRP, CAR, NLR and ELR (p < 0.001). In the Kruskal–Wallis test, the median values of PLR did not significantly differ among study and control groups (p = 0.747).
Table 3 shows the analytically calculated cutoffs to predict multiple recurrences after FESS when comparing preoperative serum CAR, NLR, PLR, ELR levels between the total of recurrences group and the no recurrences group. The receiver operating characteristic (ROC) approach was used to find the analytically best-fitting NLR cut-off for prognostic purposes (CRSwNP recurrence). Patients with ELR higher than 0.22 and CAR higher than 1.03 had ORs of 4.26 (sensitivity: 61.5%, specificity: 72.7%) and 3.57 (sensitivity: 66.6%, specificity: 66.6%), respectively, of experiencing recurrences after FESS. These results are significantly higher predictive value than NLR and PLR (p < 0.001).
Table 3.
Comparison of the predictive values of CAR, ELR, NLR, PLR for recurrence of nasal polyp
| Calculated cut-off (ROC) | Area under curve (AUC) | Odds ratio (OD) | 95% CI | p | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|---|
| CAR | 1.03 | 0.713 (0.586–0.840) | 3.57 | 1.34–9.47 | < 0.001 | 66.6 | 66.6 |
| ELR | 0.22 | 0.653 (0.524–0.782) | 4.26 | 1.56–11.61 | < 0.001 | 61.5 | 72.7 |
| NLR | 2.49 | 0.596 (0.463–0.729) | 1.72 | 0.67–4.40 | 0.693 | 58.9 | 57.5 |
| PLR | 121 | 0.483 (0.341–0.625) | 1.52 | 0.79–5.63 | 0.782 | 51.7 | 45.4 |
CAR C-reactive protein (CRP) and albümin ratio, ELR eosinophil-to-lymphocyte ratio, NLR neutrophil to lymphocyte ratio, PLR platelet to lymphocyte ratio, CI confidence interval, ROC receiver operating characteristic
*p < 0.05; **p < 0.001
The graph of the ROC analysis of the parameters included CAR, ELR, NLR and PLR for determining the predictive importance are shown at Fig. 1. ROC analysis revealed that, CAR had a greater area under curve (0.713) compared both to ELR (0.653), NLR (0.596) and PLR (0.485) (p < 0.001). Thus, CAR and ELR were significantly the most associated parameter with the recurrence of NP (p < 0.001 and p < 0.001, respectively). The value of PLR and NLR was not significant for predicting recurrence possibility. (p = 0.782 and p = 0.693, respectively).
Fig. 1.
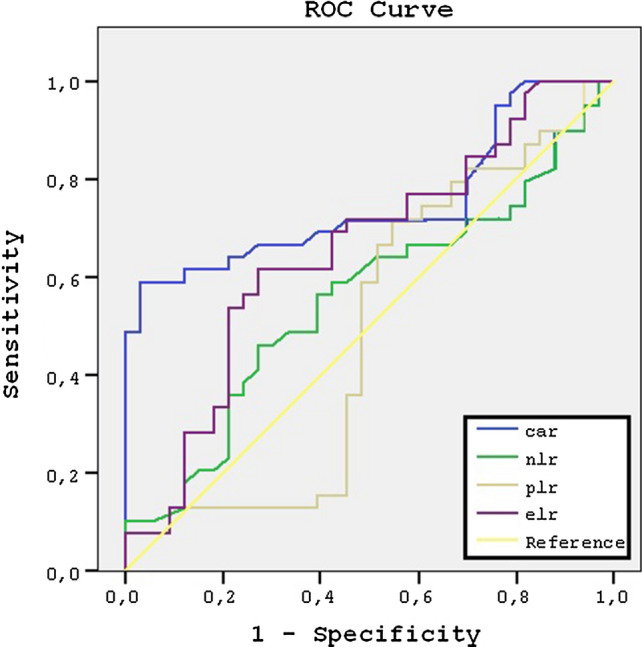
The graph of the ROC analysis. ROC receiver operating characteristic, CAR C-reactive protein to albumin ratio, NLR neutrophil to lymphocyte ratio, ELR eosinophil-to-lymphocyte ratio, PLR platelet to lymphocyte ratio
Discussion
Our study focused on various blood parameters that might be associated with the recurrence of the CRSwNP. The pathophysiology of CRSwNP remains poorly understood, resulting in disease which neither medical nor surgical approaches might control the progression or recurrence. However, recently 0, the high efficacy is achieved with surgery, followed by pharmacotherapy [10]. NP are found in 36% of patients with salicylate intolerance, 7% of those with asthma an 0.1% in childhood. The recurrence rate of CRSwNP is 35–40% even in the most effective treatment [11]. Several factors, including allergy, asthma, acetylsalicylic acid (ASA)intolerance, number of eosinophils and basophils in the nasal tissue and blood, and IgE levels, have been observed related to CRSwNP recurrence [12, 13] In our study, we observed a significant collelation between asthma and possibility of recurrence. Additionally patients with CRSwNP have a highly rate of allergic disease comparing healthy individuals in accordance with the literature.
Hypertrophic epithelia cells generate various inflammatory cytokines including tumor necrosis factor (TNF-a), IL-8, granulocyte–macrophage colony stimulating factor (GM-CSF), IL-6 and IL-1b, and vascular endothelial growth factor (VEGF). These cytokines lead to eosinophilia by increasing peripheral circulation of eosinophils [14]. In the sinus mucosa, activated eosinophils release proinflammatory mediators that lead to inflammation of the mucosa, tissue injury, and edema. The importance of eosinophilic inflammation in CRS is well known moreover the understanding that eosinophilic CRS observed in CRSwNP is especially difficult to manage and control. [15]. Soler et al. found that sinus tissue eosinophilia correlates with objective disease severity as measured by nasal endoscopy, computed tomography (CT), and smell test scores [16]. It was reported that patients who experienced plural recurrences of NP had significantly higher baseline eosinophil counts and percentages of total white blood cells. Increased serum eosinophils and basophils was found to correlate with polyp growth and disease recurrence and have a significant impact on the quality of life [5, 17] According to our study, in patients who were operated multiple times than those with any recurrences, eosinophil counts were found significantly higher, suggesting truly refractory disease has an inflammatory nature coming through by eosinophilic cascade.
According to previous studies, the entire situation of the body is reflected by thrombocytosis, peripheral lymphopenia and neutrophilia [18, 19]. NLR, a novel potential marker for identifying inflammation in various diseases, is a valuable, easily attainable and unexpensive parameter unlike the costly inflammatory markers such as IL-6, IL-1b. Cayir et al. reported that higher NLR were associated with poor prognosis in patients with Bell’s palsy and recurrent aftous stomatitis [7]. Brescia et al. investigated the role of the NLR for predicting recurrence rate of CRSwNP and the mean NLR were found to be significantly higher [20]. Inconsistent with the prior literature, we found the median NLR doesn’t have a predictive value in determining recurrence possibility in CRSwNP However our results showed that patients with NP had a higher rate of NLR compared to the control group.
Acute-phase proteins are systemic precursors of inflammatory response which are used in the diagnosis and prognosis of the process of inflammation. In the literature, cardiologists and rheumatologists are also interested in acute-phase proteins, in the context of the etiopathogenesis and prognosis in diseases connected with chronic inflammatory processes. CRP is a one of them synthesized by hepatocytes that is regulated by proinflammatory cytokines, particularly IL-6 [21]. In a broad participation study, it have shown that CRP, previously considered only in its inflammatory aspect, has become a strong and independent prognostic factor in the risk assessment of cardiovascular disease [22, 23]. Partyka et al. reported that serum ferritin and hs-CRP concentrations might be helpful in early detection of inflammatory state in patients with nasal polyps and for the effectiveness of therapy [24]. The CRP concentration has been found to be useful in evaluating both the severity of inflammation and treatment method in cases of respiratory tract inflammation and myocardial infarction [13, 25]. Although the association between NLR, ELR and CRSwNP was investigated yet to the best of our knowledge, thus far, no studies have addressed the association between CAR and CRSwNP. In our study on this basis the CAR is likely to be a more specific marker of CRSwNP recurrence than the ELR, PLR and NLR.
The eosinophil-to-lymphocyte ratio (ELR) has recently been proposed as possible marker of ongoing systemic reactions and inflammation in patients with CRSwNP by Yenigün et al. In their study preoperative NLR and ELR were found significantly higher in CRSwNP patients with recurrent CRSwNP than unrecurrent group [26]. We demonstrated higher ELR in patients with recurrent NP when compared with the non-recurrent NP consistent with the literature. In addition, our cohort also had very significantly elevated baseline preoperative eosinophil counts compared with the healthy individuals.
Conclusion
In our study, we investigated the role of circulating inflammatory markers before the treatment and verified their connection with the recurrence of CRSwNP. Among our sample of patients with CRSwNP, the mean CRP, CAR and ELR were significantly higher in those whose disease recurred after surgery. None of the prognostic parameters were found to predict the number of recurrence. However our preliminary experience suggests that the power of the CAR and ELR to identify cases more likely to recur is valuable for the purposes of clinical practice.
Acknowledgements
The authors received no grants or other scientific support related to the preparation of this manuscript.
Authors’ Contribution
A.B.C. H.D.T.: Study design and concept. All authors: Data acquisition, analysis, or interpretation. B.C.G.: drafting of the manuscript. M.F.O., S.O.: Critical revision of the manuscript for important intellectual content. A.B.C., H.D.T.: Statistical analysis. All authors: administrative, technical, or material support; and A.B.C: Study supervision.
Funding
The authors declare that they have no funding
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies were in accordance with the ethical standards of Committee in Bagcilar Training and Research Hospital with number and date 2019.12.1.03.03/13.12.2019.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abdurrahman Bugra Cengiz, Email: drcengiz@gmail.com.
Bekir Can Gumuslu, Email: bekircan-gumuslu@hotmail.com.
Hasan Deniz Tansuker, Email: hasandeniztansuker@gmail.com.
Sahin Ogreden, Email: drsahinogreden@gmail.com.
Mehmet Faruk Oktay, Email: farukoktay@hotmail.com.
References
- 1.Thomas M, Yawn BP, Price D, et al. EPOS primary care guidelines: European position paper on the primary care diagnosis and management of rhinosinusitis and nasal polyps 2007—a summary. Prim Care Respir J. 2008;17(2):79–89. doi: 10.3132/pcrj.2008.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman ND, Fahy C, Woolford TJ. Nasal polyps: still more questions than answers. J Laryngol Otol. 2003;117(1):1–9. doi: 10.1258/002221503321046577. [DOI] [PubMed] [Google Scholar]
- 3.Lou H, Meng Y, Piao Y, Zhang N, Bachert C, Wang C, Zhang L. Cellular phenotyping of chronic rhinosinusitis with nasal polyps. Rhinology. 2016;54(2):150–159. doi: 10.4193/Rhin15.271. [DOI] [PubMed] [Google Scholar]
- 4.Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, Bousquet PJ, Brozek G, Bruno A, Dahlen SE, Forsberg B, Gunnbjornsdottir M, Kasper L, Kramer U, Kowalski ML, Lange B, Lundback B, Salagean E, Todo-Bom A, Tomassen P, Toskala E, van Drunen CM, Bousquet J, Zuberbier T, Jarvis D, Burney P. Chronic rhinosinusitis in Europe—an underestimated disease. A GA(2)LEN study. Allergy. 2011;66(9):1216–1223. doi: 10.1111/j.1398-9995.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 5.Guo M, Alasousi F, Okpaleke C, Habib AR, Javer A. Prognosis of chronic rhinosinusitis with nasal polyps using preoperative eosinophil/basophil levels and treatment compliance. Am J Rhinol Allergy. 2018;32(5):440–446. doi: 10.1177/1945892418793523. [DOI] [PubMed] [Google Scholar]
- 6.Drake VE, Rafaels N, Kim J. Peripheral blood eosinophilia correlates with hyperplastic nasal polyp growth. Int Forum Allergy Rhinol. 2016;6(9):926–934. doi: 10.1002/alr.21793. [DOI] [PubMed] [Google Scholar]
- 7.Cayir S, Hizli O, Kayabasi S. Is C-reactive protein to albumin ratio an indicator of poor prognosis in Bell’s palsy? Eur Arch Otorhinolaryngol. 2020;277(1):115–119. doi: 10.1007/s00405-019-05691-3. [DOI] [PubMed] [Google Scholar]
- 8.Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, Batra PS, Bernal-Sprekelsen M, Bhattacharyya N, Chandra RK, Chiu A, Citardi MJ, Cohen NA, DelGaudio J, Desrosiers M, Dhong HJ, Douglas R, Ferguson B, Fokkens WJ, Georgalas C, Goldberg A, Gosepath J, Hamilos DL, Han JK, Harvey R, Hellings P, Hopkins C, Jankowski R, Javer AR, Kern R, Kountakis S, Kowalski ML, Lane A, Lanza DC, Lebowitz R, Lee HM, Lin SY, Lund V, Luong A, Mann W, Marple BF, McMains KC, Metson R, Naclerio R, Nayak JV, Otori N, Palmer JN, Parikh SR, Passali D, Peters A, Piccirillo J, Poetker DM, Psaltis AJ, Ramadan HH, Ramakrishnan VR, Riechelmann H, Roh HJ, Rudmik L, Sacks R, Schlosser RJ, Senior BA, Sindwani R, Stankiewicz JA, Stewart M, Tan BK, Toskala E, Voegels R, de Wang Y, Weitzel EK, Wise S, Woodworth BA, Wormald PJ, Wright ED, Zhou B, Kennedy DW. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–S209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 9.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, Adinoff AD, Bachert C, Borish L, Chinchilli VM, Danzig MR, Ferguson BJ, Fokkens WJ, Jenkins SG, Lund VJ, Mafee MF, Naclerio RM, Pawankar R, Ponikau JU, Schubert MS, Slavin RG, Stewart MG, Togias A, Wald ER, Winther B. Rhinosinusitis: developing guidance for clinical trials. J Allergy Clin İmmunol. 2006;118(5 Suppl):S17–S61. doi: 10.1016/j.jaci.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Dijkstra MD, Ebbens FA, Poublon RML, Fokkens WJ. Fluticasone propionate aqueous nasal spray does not influence the recurrence rate of chronic rhinosinusitis and nasal polyps 1 year after functional endoscopic sinus surgery. Clin Exp Allergy. 2004;34(9):1395–1400. doi: 10.1111/j.1365-2222.2004.02044.x. [DOI] [PubMed] [Google Scholar]
- 11.Settipane GA. Epidemiology of nasal polyps. Allergy Asthma Proc. 1996;17(5):231–236. doi: 10.2500/108854196778662246. [DOI] [PubMed] [Google Scholar]
- 12.Gelardi M, Fiorella R, Fiorella ML, Russo C, Soleti P, Ciprandi G. Nasal-sinus polyposis: clinical-cytological grading and prognostic index of relapse. J Biol Regul Homeost Agents. 2009;23(3):181–188. [PubMed] [Google Scholar]
- 13.Brescia G, Marioni G, Franchella S, et al. A prospective investigation of predictive parameters for post-surgical recurrences in sinonasal polyposis. Eur Arch Otorhinolaryngol. 2016;273(3):655–660. doi: 10.1007/s00405-015-3598-5. [DOI] [PubMed] [Google Scholar]
- 14.Wittekindt C, Hess A, Bloch W, Sultanie S, Michel O. Immunohistochemical expression of VEGF and VEGF receptors in nasal polyps as compared to normal turbinate mucosa. Eur Arch Otorhinolaryngol. 2002;259(6):294–298. doi: 10.1007/s00405-002-0467-9. [DOI] [PubMed] [Google Scholar]
- 15.Poznanovic SA, Kingdom TT. Total IgE levels and peripheral eosinophilia: correlation with mucosal disease based on computed tomographic imaging of the paranasal sinus. Arch Otolaryngol Head Neck Surg. 2007;133(7):701–704. doi: 10.1001/archotol.133.7.701. [DOI] [PubMed] [Google Scholar]
- 16.Soler ZM, Sauer DA, Mace J, Smith TL. Relationship between clinical measures and histopathologic findings in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2009;141(4):454–461. doi: 10.1016/j.otohns.2009.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kountakis SE, Arango P, Bradley D, Wade ZK, Borish L. Molecular and cellular staging for the severity of chronic rhinosinusitis. Laryngoscope. 2004;114(11):1895–1905. doi: 10.1097/01.mlg.0000147917.43615.c0. [DOI] [PubMed] [Google Scholar]
- 18.Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6(4):283–287. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 19.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol (London, England) 2010;6(1):149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 20.Brescia G, Pedruzzi B, Barion U, Cinetto F, Giacomelli L, Martini A, Marioni G. Are neutrophil-, eosinophil-, and basophil-to-lymphocyte ratios useful markers for pinpointing patients at higher risk of recurrent sinonasal polyps? Am J Otolaryngol. 2016;37:339–345. doi: 10.1016/j.amjoto.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Roxburgh CS, McMillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer. 2014;110(6):1409–1412. doi: 10.1038/bjc.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 23.Fritz P, Saal JG, Wicherek C, König A, Laschner W, Rautenstrauch H. Quantitative photometrical assessment of iron deposits in synovial membranes in different joint diseases. Rheumatol Int. 1996;15(5):211–216. doi: 10.1007/BF00290523. [DOI] [PubMed] [Google Scholar]
- 24.Partyka R, Pałac J, Paluch Z, Szyguła-Jurkiewicz B, Namysłowski G, Misiołek M, Jałowiecki P, Kokocińska D. Evaluation of usefulness of hs-CRP and ferritin assays in patients with nasal polyps. Dis Markers. 2014;2014:794060. doi: 10.1155/2014/794060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/nejm200003233421202. [DOI] [PubMed] [Google Scholar]
- 26.Yenigün A. Assessment of patients with nasal polyposis by the neutrophil-to-lymphocyte ratio and eosinophil-to-lymphocyte ratio. Kulak Burun Bogaz Ihtis Derg. 2015;25(4):193–199. doi: 10.5606/kbbihtisas.2015.10734. [DOI] [PubMed] [Google Scholar]


