Abstract
The stimulation of gamma interferon (IFN-γ) has been shown to be essential in resolving infections by intracellular pathogens. As such, several different cytokines including, interleukin-12 (IL-12) and IL-18, can induce IFN-γ. To resolve Salmonella infections, the stimulation of IL-12 and IFN-γ are important for mediating its clearance. In this present study, the relevance of IL-18 in protection against oral challenge with Salmonella typhimurium was investigated to determine the role of this IFN-γ-promoting cytokine. Rabbit anti-murine IL-18 antisera was generated and administered prior to the oral challenge of BALB/c and IL-12p40-deficient knockout (IL-12KO) mice with a wild-type S. typhimurium strain. The median survival time was reduced by 2 days for the anti-IL-18-treated BALB/c mice, while no significant reduction in survival rate for the anti-IL-18-treated IL-12KO mice was observed compared to vehicle-treated mice. To investigate the contribution of IL-18 to resolving Salmonella infections, an attenuated aro-negative mutant (H647) was orally administered to BALB/c mice. This Salmonella infection induced both IL-12 and IFN-γ in both the Peyer's patches and the spleens. In vehicle-treated mice, Peyer's patch IL-12 peaked by 24 h, while IL-18 levels peaked at 3 days, suggesting sequential support by these cytokines for IFN-γ. Anti-IL-18 treatment exerted its greatest effect upon the mucosal compartment, limiting early IFN-γ production. However, anti-IL-18 treatment had little effect upon splenic IFN-γ levels until late in the response. Infection of IL-12KO mice with H647 strain induced IFN-γ, but it was not supported by IL-18, although IL-18 levels were reduced by this treatment. These results suggest that IL-18 does contribute to the clearance of S. typhimurium and that endogenously induced IL-18 could not substitute for IL-12.
Protection against intracellular pathogens depends upon the stimulation of a T helper 1 (Th1)-type immunity (15, 34). Interleukin-12 (IL-12) has been shown to be essential in the clearance of intracellular pathogens, e.g., Salmonella typhimurium (4–6, 17, 24, 30). IL-12 is a 70-kDa heterodimeric cytokine consisting of two subunits, p35 and p40, produced by phagocytic cells in response to bacteria, bacterial products, and intracellular parasites (40). IL-12 induces IFN-γ cytokine production from natural killer (NK) and T cells and drives Th1 cell development (39, 40). A recently identified cytokine, gamma interferon (IFN-γ)-inducing factor (IGIF) or IL-18 has been shown to share many properties with IL-12 (29, 32, 42).
IL-18 was first isolated and cloned from livers of mice infected with Propionibacterium acnes and challenged with lipopolysaccharide (31). IL-18 is produced as a precursor protein of 192 amino acids which is cleaved into a mature protein of 152 amino acids or approximately 18 kDa by the IL-1β-converting enzyme (10, 12). IL-18 promotes the production of IFN-γ and granulocyte-macrophage colony-stimulating factor and inhibits IL-10 production (38). IL-18 also enhances NK cell activity and proliferation of T cells (28, 29, 37) and upregulates Fas ligand expression (36, 41).
IL-18 has recently been shown to be important in the clearance of several intracellular pathogens, including Yersinia enterocolitica, a gram-negative, rod-shaped bacterium (3), and the fungal pathogen Cryptococcus neoformans (16, 46). Although IL-18 and IL-12 share many properties, including the induction of IFN-γ, it appears that IL-18 and IL-12 are independently regulated and are functionally different with respect to receptor binding and signal transduction pathways (28). The interaction of IL-18 and IL-12 in the clearance of intracellular pathogens is not yet fully understood.
While IFN-γ is essential for the clearance of Salmonella (4–6, 17, 24, 30) and supposably attenuated Salmonella vectors can behave lethally in mice deficient of IFN-γ (14, 43), it is unclear whether IL-12 is solely responsible for IFN-γ stimulation or whether other IFN-γ-promoting cytokines contribute to the clearance of Salmonella. In this present study, we investigated the role of IL-18 in protection against S. typhimurium.
MATERIALS AND METHODS
Reagents.
Cells were cultured in RPMI 1640 medium with l-glutamine (BioWhittaker, Walkersville, Md.) supplemented with 10 mM HEPES buffer, 1 mM sodium pyruvate solution, 1× nonessential amino acids (Cellgro; Mediatech, Inc., Herndon, Va.), 100 IU of penicillin per ml, and 100 μg of streptomycin per ml (Cellgro; Mediatech). Fetal bovine serum was supplied by HyClone Laboratories, Inc. (Logan, Utah). Antibodies and cytokines included murine IL-12 (R&D Systems, Inc., Minneapolis, Minn.), murine IL-12p40 (PharMingen, San Diego, Calif.), murine IL-18 (PreproTech, Inc., Rocky Hill, N.J.), murine IFN-γ (Genzyme Corp., Cambridge, Mass.), hamster and rat anti-mouse IL-12p35 cocktail (PharMingen), rat anti-mouse IL-12p40 (clone C15.6; PharMingen), biotinylated rat anti-mouse IL-12p40 (clone C17.8; PharMingen), anti-mouse IFN-γ (clone R4-6A2; PharMingen), biotinylated rat anti-mouse IFN-γ (clone XMG1.2; PharMingen), horseradish peroxidase (HRP)-goat anti-rabbit immunoglobulin G (IgG; Southern Biotechnology Associates, Birmingham, Ala.), alkaline phosphatase (AP)-goat anti-biotin (Vector Laboratories, Inc., Burlingame, Calif.), and HRP-goat anti-biotin (Vector Laboratories).
Bacterial strains.
Wild-type S. typhimurium H71 was kindly provided by David M. Hone (Institute of Human Virology, Medical Biotechnology Center, University of Maryland at Baltimore). Attenuated S. typhimurium ΔaroA Δasd mutant strain H647 was previously described (2, 9, 44). Bacteria strains were streaked on Luria broth (LB) agar plates and incubated overnight at 37°C. A single colony was used to inoculate LB cultures. Bacterial colony forming units were quantified by reading the absorbance at an optical density at 600 nm on a Spectronic 20 (Bausch & Lomb) spectrometer and interpolating from a standard curve for the respective bacterial strain. Bacterial counts were verified by plating serial dilutions of bacterial suspensions on LB agar plates.
Molecular cloning of murine IL-18.
Murine IL-18 mRNA was isolated from activated RAW 264.7 cells, a murine macrophage cell line (ATCC TIB-71; American Type Cell Culture, Manassas, Va.). The RAW 264.7 cells were infected with the attenuated S. typhimurium H647 for 24 h at 37°C, and mRNA was phenol-chloroform extracted with Tri-Reagent (Molecular Research Center, Inc., Cincinnati, Ohio). The entire coding region for IL-18 protein was amplified by reverse transcriptase PCR and cloned into the BamHI-HindIII site of the pmal-c2 plasmid (New England BioLabs, Beverly, Mass.). The IL-18 cDNA sequence (31) was confirmed by DNA sequencing. The IL-18 gene was inserted downstream from the maltose-binding protein (MBP), and recombinant murine IL-18 was expressed as a maltose-binding fusion protein. Expression of recombinant MBP–IL-18 was induced by the addition of 0.6 mM IPTG to a mid-log-phase bacterial culture. Cells were harvested 4 h later, and the recombinant MBP-IL-18 fusion protein was purified on an amylose affinity column according to the manufacturer's protocol. In addition, a thioredoxin IL-18 fusion protein was also generated by cloning into the pET32a vector (Novagen, Inc., Milwaukee, Wis.) at the EcoRI and HindIII sites. This fusion protein was expressed and isolated according to manufacturer's protocol.
Rabbit anti-mouse IL-18 antiserum.
The MBP–IL-18 fusion protein was conjugated to keyhole limpet hemocyanin (KLH; Calbiochem, La Jolla, Calif.) with 10 mM glutaraldehyde (Sigma) as previously described (33). New Zealand White rabbits were immunized with the KLH–MBP–IL-18 conjugate (200 μg of IL-18 fusion protein) emulsified in complete Freund adjuvant (Sigma), followed with booster immunizations (100 μg of IL-18 fusion protein) with incomplete Freund adjuvant (Sigma) for the production of polyclonal anti-mouse IL-18 antibodies. To test the specificity of the rabbit anti-IL-18 antisera, microtiter wells (35) were coated overnight with 1.0 μg of recombinant mouse IL-18 thioredoxin (Trx)–IL-18 fusion protein, recombinant mouse IFN-γ, recombinant mouse IL-12p70, or Trx (Sigma) per ml. After the blocking step, various dilutions of immune rabbit anti-IL-18 antisera were added, followed by incubation for 3 h at 37°C. After a wash step, a 1:1,000 dilution of HRP-goat anti-rabbit IgG (heavy-chain-specific) antibody was added for 90 min at 37°C. After a washing step, 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid)diammonium (ABTS; Moss, Inc., Pasadena, Calif.) was added, and the absorbance was measured at 415 nm with a Kinetics Reader model EL312 (Bio-Tek Instruments, Winooski, Vt.).
Mice.
BALB/c mice were obtained from The National Cancer Institute (Frederick, Md.). BALB/c mice containing a disruption in the gene encoding the p40 subunit of IL-12 (22, 23, 27) were obtained from Jackson Laboratories (Bar Harbor, Maine). Breeding pairs of the IL-12p40 knockout (IL-12KO) mice, stock mice, and infected experimental mice were maintained under microisolator conditions. Sterile food and water were provided ad libitum.
Salmonella infections.
Groups of 5- to 8-week-old BALB/c mice and IL-12KO mice were given intraperitoneal (i.p.) injections of either vehicle (phosphate-buffered saline [PBS]) or rabbit anti-mouse IL-18 antiserum prior to and after oral challenge. On the day of infection, mice were pretreated with 50% saturated sodium bicarbonate solution (2) and then orally infected with 5 × 107 CFU/0.2 ml of wild-type S. typhimurium H71 strain. Mice were observed twice daily, and the extent of survival was recorded.
To investigate the development of IFN-γ after oral exposure to Salmonella, BALB/c mice received an oral gavage with a 50% saturated sodium bicarbonate solution and were then orally challenged with 5 × 109 CFU of the attenuated Salmonella sp. strain H647 (2, 44). On days −1, 2, 4, and 6 postinfection (p.i.), mice received i.p. injections of PBS (or normal rabbit serum) or rabbit anti-mouse IL-18 antisera that had been previously dialyzed against PBS. On days 0.5, 1, 3, and 7 p.i., spleens and Peyer's patches were harvested. Tissues from individual mice (three animals/group) were Dounce homogenized in 1 ml of PBS. Cellular debris was removed by centrifugation, and the supernatants were stored at −20°C until analysis for cytokine levels. This experiment was repeated three times.
To evaluate the development of IFN-γ in the absence of IL-12, IL-12KO mice were orally gavaged with S. typhimurium H647. On days 0.5, 1, 3, and 7 days p.i., spleens and Peyer's patches were harvested as described above and stored for cytokine analysis.
Cytokine-specific ELISAs.
Levels of IFN-γ were measured by an antigen-capture enzyme-linked immunosorbent assay (ELISA) as previously described (35). This ELISA procedure was adapted to measure IL-12p70 and IL-12p40 with microtiter plates coated at 16 μg of anti-IL-12p35 antibody and 6 μg of anti-IL-12p40 (clone C15.6) antibody per ml, respectively. IL-12p70 and IL-12p40 were detected by using the biotinylated rat anti-mouse IL-12p40 (clone C17.8) antibody and AP-goat anti-biotin antibody. To measure IL-18 levels, a rat monoclonal anti-IL-18 antibody was used to coat microtiter wells at 2.0 μg/ml in sterile PBS overnight at room temperature. After blocking with PBS plus 1% bovine serum albumin for 1 h at 37°C, tissue homogenates in PBS and recombinant IL-18 (PreproTech, Inc.) were added to the wells and incubated overnight at 4°C. After a washing, a biotinylated goat anti-mouse IL-18 antibody (200 ng/ml) was added to the wells for 2 h at 37°C. After a washing, a 1:1,000 dilution of HRP-goat anti-biotin antibody was added to the wells and incubated for 1 h at room temperature. After a washing, ABTS substrate was added, and the absorbance was measured at 415 nm on a Kinetics Reader model EL312.
Statistical analysis.
The Kaplan-Meier method (GraphPad Prism; GraphPad Software, Inc., San Diego, Calif.) was applied to obtain the survival fractions after infection with a lethal dose of wild-type S. typhimurium. By using the Mantel-Haenszel log rank test, the P values for the statistical differences between vehicle and anti-IL-18 antibody treatments were discerned at the 95% confidence interval. All other data were analyzed by Student t test, and significant values were recorded at a P value of <0.05.
RESULTS
Anti-IL-18 sera diminishes survival against wild-type S. typhimurium infection.
To assess the relevance of IL-18 in protection against oral challenge with S. typhimurium, a recombinant IL-18 was generated as an MBP fusion which allowed for the production of an IL-18-specific, neutralizing antiserum. This antiserum recognized commercially available recombinant murine IL-18 but not recombinant murine IL-12 or IFN-γ. The antiserum was also able to inhibit IL-18-induced IFN-γ by anti-CD3-stimulated T cells (data not shown). To block endogenous IL-18 production, the rabbit anti-mouse IL-18 serum was administered to BALB/c mice 1 day prior to and on the day of oral challenge with wild-type S. typhimurium. Such anti-IL-18 treatment did result in a significant reduction (P < 0.005) in the survival rate compared to the normal serum or to PBS-treated mice (Fig. 1). The median survival rate for the anti-IL-18-treated group (n = 19) was 6 days compared to the vehicle-treated group (n = 21), and the median survival rate was 8 days. Thus, the anti-IL-18 treatment accelerated death by approximately 2 days, implicating the importance of IL-18 in resolving infection with wild-type S. typhimurium. The extent of colonization in the two subject groups was also examined. No statistical difference in the extent of colonization in the Peyer's patches and spleens was noted at 3 days p.i. with the wild-type S. typhimurium.
FIG. 1.
Anti-IL-18 treatment diminishes survival rates after oral challenge of BALB/c, but not IL-12p40 KO, mice with wild-type S. typhimurium. Five- to eight-week-old age-matched mice were given vehicle (PBS or normal rabbit serum) or rabbit anti-mouse IL-18 sera by the i.p. route 1 day prior to and on the day of oral challenge with 5 × 107 wild-type S. typhimurium H71. Deaths were recorded daily. The anti-IL-18-treated BALB/c mice (n = 19) showed a median survival rate of 6 days, while vehicle-treated BALB/c mice (n = 21) showed a median survival of 8 days. This difference in survival rates was statistically significant by the Mantel-Haenszel log-rank test (P < 0.005). No statistical differences in survival rates were evident between vehicle-treated (n = 10) and anti-IL-18-treated (n = 10) IL-12KO mice (P = 0.48). Symbols: ●, BALB/c; ○, anti-IL-18-treated BALB/c; ⧫, IL-12KO; and ◊, anti-IL-18-treated IL-12KO.
Since IL-12 has been shown to be important for resolving Salmonella infections, we sought to determine whether endogenous IL-18 could substitute for IL-12 in orally challenged IL-12KO mice. There was no significant difference in the survival rates between anti-IL-18- and vehicle-treated IL-12KO mice (Fig. 1). Both vehicle (n = 10)- and anti-IL-18 (n = 10)-treated groups exhibited a median survival rate of 6 days. Collectively, the data suggest that IL-12 and IL-18 contribute to protective immunity against wild-type S. typhimurium infections.
Role of IL-18 subsequent infection with an avirulent Salmonella vector.
Diminished survival was evident with the anti-IL-18-treated BALB/c mice after oral challenge with wild-type S. typhimurium, suggesting that IL-18 contributes to the development of protective immunity against Salmonella. To consider how IFN-γ is induced by IL-18, we selected to use an avirulent, aro-negative Salmonella vaccine vector for infection in order to monitor the early events in the mucosal and systemic compartments. Such experimentation would permit the study of how IFN-γ is induced ultimately for protection, eliminating the concerns attributed to Salmonella virulence.
BALB/c mice were orally infected with the attenuated Salmonella sp. strain H647 vaccine vector. The tissue weights of Peyer's patches and spleens were recorded for each individual mouse, and the tissue homogenates were analyzed for IL-12, IL-12p40, and IFN-γ cytokine levels. Administration of anti-mouse IL-18 antisera prior to and during oral challenge with attenuated Salmonella sp. strain H647 resulted in a lesser change in Peyer's patch weight at 12 h after infection compared to vehicle-treated mice (Fig. 2A) but, by 24 h p.i., was dramatically enhanced (Fig. 2A). Upon analysis for cytokine levels in the Peyer's patch homogenates, we observed induction of IFN-γ as early as 12 h, with maximal expression by 7 days p.i. Anti-IL-18 treatment showed enhanced IFN-γ production by 12 h, and this was significantly diminished (P < 0.001) up to 3 days after infection (Fig. 2B). Between 3 and 7 days p.i., significant increases (P < 0.021) in IFN-γ levels between the two groups were evident (Fig. 2B). These IFN-γ levels were supported by concomitant increases in IL-12 (Fig. 2C). As evident at 7 days p.i., significantly higher levels of IL-12 were observed in the anti-IL-18 treatment group compared to the vehicle-treated group (Fig. 2C). Low levels of IL-12p40 were observed in the Peyer's patches throughout the course of infection, with minor but significant differences (P < 0.001) in the IL-12p40 levels between the two groups (Fig. 2D).
FIG. 2.
In vivo treatment with anti-IL-18 sera alters Peyer's patch IFN-γ, IL-12, and IL-12p40 levels after oral infection with an avirulent, aro-negative S. typhimurium strain. Five- to eight-week-old age-matched mice were given up to four doses of either PBS or normal rabbit serum (n = 9) or rabbit anti-murine IL-18 serum (n = 9) i.p. on days −1, 2, 4, and 6 p.i. with 5 × 109 CFU of attenuated S. typhimurium H647. Peyer's patches were harvested, weighed, and homogenized in PBS on the indicated days. Values represent the mean ± the standard error of the mean (SEM) per group per time period. (A) Peyer's patch weights expressed as a percent increase from noninfected BALB/c age-matched mice. Peyer's patches from H647-infected mice were collected on the indicated days p.i. and were assessed for IFN-γ (B), IL-12p70 (C), and IL-12p40 (D) levels by using cytokine-specific ELISA methods. The two groups were compared against each other within each time period by one-way analysis of variance. ∗, P < 0.001; ∗∗, P = 0.015; ∗∗∗, P = 0.021.
A different immune response was observed in the systemic immune compartment with the administration of anti-IL-18 antisera prior to and during oral challenge with Salmonella sp. strain H647. There was a delay in the change in the splenic weights, with the anti-IL-18 treatment inducing greater increases in weight by day 3 p.i. and a significant inflammation of splenic tissue by day 7 p.i. (P ≤ 0.001; Fig. 3A). Unlike the Peyer's patches, no significant differences in IFN-γ levels in spleens were observed between vehicle-treated and anti-IL-18-treated groups until 7 days p.i., at which point significantly less (P ≤ 0.001) IFN-γ was observed with the anti-IL-18 group (Fig. 3B). Anti-IL-18 treatment of orally challenged BALB/c mice with attenuated Salmonella sp. strain H647 strain also resulted in significant decreases in IL-12 levels at each day tested (P ≤ 0.001 and P = 0.011; Fig. 3C). Although an initial rise in IL-12p40 levels was observed at 12 h p.i., the IL-12p40 levels were reduced on days 3 and 7 p.i. for the anti-IL-18-treated group compared to the vehicle-treated group (P ≤ 0.001; Fig. 3D). Thus, these data suggest that the mucosal and systemic immune compartments behave differently as a result of the anti-IL-18 treatment during oral challenge with an attenuated Salmonella strain.
FIG. 3.
In vivo treatment with anti-IL-18 serum alters IFN-γ, IL-12, and IL-12p40 levels after oral infection with attenuated, aro-negative S. typhimurium H647. Five- to eight-week-old age-matched mice were given up to four doses of either PBS or normal rabbit serum (n = 9) or rabbit anti-murine IL-18 serum (n = 9) i.p. on days −1, 2, 4, and 6 p.i. with 5 × 109 CFU of attenuated S. typhimurium H647. Spleens were harvested, weighed, and homogenized in PBS. Values represent the mean ± the SEM per group per time period. (A) Splenic weights expressed as the percent increase from noninfected BALB/c age-matched mice. Spleens from H647-infected mice were collected on the indicated days p.i. and were assessed for IFN-γ (B), IL-12p70 (C), and IL-12p40 (D) levels by using cytokine-specific ELISA methods. The two groups were compared against each other within each time period by one-way analysis of variance. ∗, P ≤ 0.001; ∗∗, P = 0.011.
Anti-IL-18 treatment lowers in vivo levels of IL-18 in BALB/c mice.
To assess whether IL-18 is induced after oral infection with H647 strain, Peyer's patch and splenic homogenates were assessed for the presence of IL-18. Except for the 12-h time point, the anti-IL-18 treatment significantly reduced IL-18 levels in the Peyer's patches (Fig. 4A). Peak induction of IL-18 was evident 3 days after oral infection with the attenuated Salmonella sp. strain H647, which corresponded to decreases in IL-12 levels in vehicle-treated mice (Fig. 2C). This reduction of IL-18 also coincided with the observed reduction in Peyer's patch IFN-γ levels (Fig. 2B), suggesting that the mucosally induced IFN-γ was supported in part by IL-18.
FIG. 4.
Anti-IL-18 treatment reduces IL-18 levels in both Peyer's patches (A) and spleens (B). BALB/c mice received up to four doses of either PBS or normal rabbit serum (n = 9) or rabbit anti-murine IL-18 serum (n = 9) i.p. on days −1, 2, 4, and 6 p.i. with 5 × 109 CFU of attenuated, aro-negative S. typhimurium H647. Spleens were harvested, weighed, and homogenized in PBS on the indicated days. Values represent the mean ± the SEM per group per time period. IL-18 was measured in samples from individual mice by using an IL-18-specific ELISA. Antibodies used for detection were different from the rabbit anti-IL-18 antibody used in the treatment groups. Values represent the mean ± the SEM. ∗, P < 0.001; ∗∗, P < 0.007; ∗∗∗, P = 0.045.
The anti-IL-18 treatment exerted no effect upon splenic tissue IL-18 levels until 3 days p.i., and reduced IL-18 levels were observed for 7 days p.i. (P = 0.003 and P < 0.001, respectively; Fig. 4B). At 3 and 7 days p.i., there were concurrent reductions in IL-12 (Fig. 3C). As with the Peyer's patches, peak induction of splenic IL-18 occurred at 3 days p.i. (Fig. 4B).
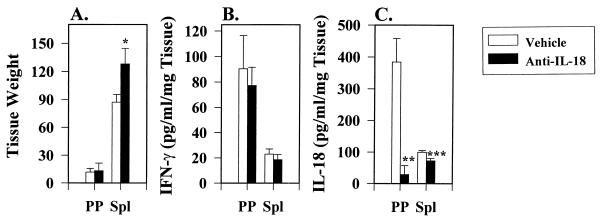
Role of IL-18 subsequent infection of IL-12KO mice with an attenuated Salmonella.
In the absence of IL-12, we sought to determine whether IL-18 could be induced and whether it could contribute to IFN-γ production. Thus, H647-infected IL-12KO mice were examined at 3 days p.i., at which point IL-18 appeared to be induced to its greatest extent. Anti-IL-18 treatment of IL-12KO mice resulted in no significant change in Peyer's patch weight when compared to vehicle-treated mice (Fig. 5A). In contrast, anti-IL-18-treated IL-12KO mice did show significant increases in splenic weights by 3 days p.i. with H647 strain (P < 0.001; Fig. 5A). Although these mice were deficient in bioactive IL-12, IFN-γ was still in induced (Fig. 5B); however, anti-IL-18 treatment exerted no significant effect upon this IFN-γ generation (Fig. 5B) in contrast to the effect observed with the Peyer's patches from normal BALB/c mice (Fig. 2B). To determine whether IL-18 could be induced in the absence of bioactive IL-12, both the Peyer's patches and the spleen were able to generate IL-18 (Fig. 5C). The Peyer's patch tissue IL-18 levels were similar to that observed in normal BALB/c mice but were approximately one-third less than that observed for splenic tissue IL-18 levels (Fig. 4). Anti-IL-18 treatment did result in significant reduction of Peyer's patch and splenic tissue IL-18 (P = 0.011 and P = 0.02, respectively; Fig. 5C). Thus, endogenously induced IL-18 did not compensate for the absence of IL-12 in IFN-γ production.
FIG. 5.
Anti-IL-18 treatment does not alter Peyer's patch and splenic IFN-γ levels, but it does reduce IL-18 levels in IL-12KO mice. Five- to eight-week-old age-matched IL-12KO mice were given vehicle (n = 6) or rabbit anti-mouse IL-18 antisera (n = 6) i.p. on days −1 and 2 p.i. with S. typhimurium H647. Treated IL-12KO mice were orally gavaged with attenuated, aro-negative S. typhimurium H647 on day 0. At 3 days after oral infection, Peyer's patches (PP) and spleens (Sp1) were harvested, weighed, and homogenized. (A) Tissue weights represent the mean ± the standard deviation per group at 3 days p.i. Only spleens showed a significant increase in size. (B) No statistical difference in IFN-γ cytokine levels between Peyer's patches and spleens were observed. (C) However, IL-18 levels were greatly reduced by the anti-IL-18 treatment. ∗, P < 0.001; ∗∗, P = 0.011; ∗∗∗, P = 0.02.
DISCUSSION
The cytokine IFN-γ produced by T and NK cells plays an important role in the inhibition of bacterial growth and in stimulating early host defenses against virulent S. typhimurium (15). IL-12, an important inducer of IFN-γ, is essential in the clearance of intracellular pathogens, including S. typhimurium (17). A recently identified cytokine, IGIF or IL-18, has been shown to have properties similar to those of IL-12 and together with IL-12 in synergistically induced IFN-γ (28). In the present study, the role of IL-18 in eliciting a protective immune response against oral challenge with S. typhimurium was investigated.
IL-18 did contribute to protection against oral challenge with wild-type S. typhimurium, as evidenced by these studies inhibiting IL-18 action. Comparisons of the extent of survival showed that the anti-IL-18 treatment reduced the median survival rate by 2 days compared to vehicle-treated mice. Our results are in agreement with a recent study in which mice treated in vivo with recombinant IL-18 were protected upon systemic challenge with S. typhimurium (25). As with Salmonella, it appears that IL-18 contributes to protective immunity against a number of intracellular pathogens but to varying degrees (1, 3, 7, 8, 16, 41, 45). Kawakami et al. (16) showed that in vivo treatment with IL-18 could augment survival after pulmonary challenge with C. neoformans. Similarly, IL-18 participated in the susceptibility to infection with Y. enterocolitica when infected mice were treated with an anti-IL-18 antibody but failed to augment colonization upon treatment with recombinant IL-18 (3). Similarly, we were unable to notice any significant change in colonization caused by the anti-IL-18 treatment of mice infected with wild-type S. typhimurium at 3 days p.i. Likewise, only a minor change in Salmonella colonization was observed when mice were treated in vivo with recombinant IL-18 (25). Collectively, these studies suggest that IL-18 contributes to resistance, perhaps by enhancing IL-12 or other IFN-γ-promoting cytokines. As such, the role for IL-18 may be secondary to IL-12, serving in a cofactor capacity, since IL-18 is dependent upon secreted IL-12 for activation. IL-12 regulates IL-18 by directing the expression of the IL-18 receptor (1, 45). Bohn et al. (3) observed that IL-18-induced IFN-γ stimulation is IL-12 dependent, with minimal IL-18 activity observed in the absence of functional IL-12. In contrast, IL-12 does not rely upon IL-18, since IL-12-induced IFN-γ was only slightly reduced in the presence of anti-IL-18 antibodies (3). While these studies implicate IL-18 as a cofactor, a recent study showed that indeed IL-18 could contribute to resistance, as evidenced by reversing death due to herpes simplex virus type 1 infection (8). Since IL-18 upregulates Fas ligand expression (7, 41), this may enhance cytotoxic-T-cell activity or augment IFNs to resolve HSV-1 infections. It is important to note that immunity to such viruses may rely more on specific rather than on innate immunity.
To assess how IL-18 contributes to innate immunity after infection with an attenuated Salmonella vector, cytokine analyses of isolated mucosal and systemic lymphoid tissues were performed. Such information should enhance our understanding regarding how IFN-γ is induced by subsequent infection with such vaccine vectors. To this end, we investigated the presence of IFN-γ, IL-12, and IL-18 at specific time points during the first week of infection with the attenuated S. typhimurium strain H647. Anti-IL-18 treatment enhanced the early production of IFN-γ evident by 12 h, production then declined by 24 h for at least 3 days and, by 7 days p.i., slightly enhanced IFN-γ levels were detected. A portion of the early IFN-γ does appear to be supported by IL-12, since anti-IL-18 treatment did induce greater levels of IL-12. Likewise, IL-18 levels were also increased by 12 h by the anti-IL-18 treatment, but it is unclear how much of this cytokine was being released. Clearly, the levels of IL-12 in the Peyer's patches were important during the early phase of infection, with maximal levels evident at 24 h p.i. Subsequently, Peyer's patch IL-12 levels begin to decline for 7 days p.i., with a concomitant rise in IL-18. IL-18 reached maximal levels by 3 days p.i. Thus, the Peyer's patch IFN-γ may be more dependent on IL-18 later in the infection or, alternatively, IL-18 may have an additive effect on the IL-12 dependence.
For the later stage of the mucosal infection, Peyer's patch IL-12 levels dramatically increased in the anti-IL-18-treated BALB/c mice, and this may be due to a compensation for the limiting IL-18. This compensation mechanism was affected by the absence of IL-12. In IL-12KO mice, the anti-IL-18 treatment greatly reduced Peyer's patch levels of IL-18, although significant levels of IFN-γ were still induced. In the IL-12KO spleen, IL-18 levels were slightly but significantly reduced by the anti-IL-18 treatment, but IFN-γ levels were not affected. Thus, in the absence of IL-12, the reduction in splenic IL-18 did not correspond to a change in IFN-γ levels, suggesting other compensating mechanisms for supporting IFN-γ generation in the systemic compartment.
By using an IL-12p70-specific ELISA, the IL-12 detected in the mucosal and systemic compartments is the IL-12p70 heterodimer. IL-12p40, implicated as an IL-12 antagonist (11, 13, 21, 26), and previous studies that relied solely on the detection of p40 (19, 20) may have inadvertently overestimated the amounts of p70 heterodimer. This is particularly important in Salmonella infections, where substantial amounts of IL-12 p40 homodimer are induced during the early phase of an S. dublin infection. In fact, the IL-12p40 was expressed to concentrations exceeding the IL-12p70 by more than a 100-fold (5). As shown in our study, the IL-12p40 levels in the Peyer's patches reflected the changes of IL-12 concentrations. In the systemic compartment, this also was true, except at 12 h, when more IL-12p40 was detected for the anti-IL-18-treated mice. This slight increase in IL-12p40 over IL-12p70 levels as a consequence of anti-IL18 treatment could be due to the early events associated with the regulation of IL-12p40, since endogenous IL-12p40 could be detected in normal BALB/c spleens. Moreover, IL-12p40 is more sensitive to upregulation than IL-12p70, as has been previously shown (5).
The regulation of IL-18 and IL-12 is not fully understood. IL-18 may induce IL-12 indirectly by inducing low amounts of IFN-γ, which in turn activates macrophages to produce IL-12, and IL-12, together with IL-18, synergistically induces higher levels of IFN-γ (3). IL-18, unlike IL-12, is incapable of inducing naive CD4+ T cells to develop into Th1 cells; however, IL-18 is a costimulatory factor that enhances IFN-γ production by stimulated Th1 clones (18, 28).
IL-18 and IL-12 are both important for protection against S. typhimurium. It is not known why these two similar cytokines contribute to immunity against intracellular pathogens, but our evidence suggests that they exhibit an overlapping effect which may be time dependent. Further studies are needed to understand how IL-18 and IL-12 interact and regulate each other and how they direct cellular immunity.
ACKNOWLEDGMENTS
We thank Peter Hillemeyer and Karyn Carlson for their expert technical assistance.
This work was in part supported by NIH-NIAID grant AI-41123 and NRI-CRGP/USDA grant 9602195.
REFERENCES
- 1.Ahn H-J, Maruo S, Tomura M, Mu J, Hamaoka T, Nakanishi K, Clark S, Kurimoto M, Okamura H, Fujiwara H. A mechanism underlying synergy between IL-12 and IFN-γ-inducing factor in enhanced production of IFN-γ. J Immunol. 1997;159:2125–2131. [PubMed] [Google Scholar]
- 2.Asćon M A, Hone D M, Walters N, Pascual D W. Oral immunization with a Salmonella vaccine vector expressing recombinant enterotoxigenic Escherichia coliK99 fimbriae elicits elevated antibody titers for protective immunity. Infect Immun. 1998;66:5470–5476. doi: 10.1128/iai.66.11.5470-5476.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohn E, Sing A, Zumbihl R, Bielfeldt C, Okamura H, Kurimoto M, Heesemann J, Autenrieth I B. IL-18 (IFN-γ-inducing factor) regulates early cytokine production in, and promotes resolution of bacterial infection in mice. J Immunol. 1998;160:299–307. [PubMed] [Google Scholar]
- 4.Bost K L, Clements J D. In vivo induction of interleukin-12 mRNA expression after oral immunization with Salmonella dublin or the B subunit of Escherichia coliheat labile enterotoxin. Infect Immun. 1995;63:1076–1083. doi: 10.1128/iai.63.3.1076-1083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bost K L, Clements J D. Intracellular Salmonella dublininduces substantial secretion of the 40-kilodalton subunit of interleukin-12 (IL-12) but minimal secretion of IL-12 as a 70-kilodalton protein in murine macrophages. Infect Immun. 1997;65:3186–3192. doi: 10.1128/iai.65.8.3186-3192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong C, Bost K L, Clements J D. Differential production of interleukin-12 mRNA by murine macrophages in response to viable or killed Salmonellaspp. Infect Immun. 1996;64:1154–1160. doi: 10.1128/iai.64.4.1154-1160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dao T, Ohashi K, Kayano T, Kurimoto M, Okamura H. Interferon-gamma-inducing factor, a novel cytokine, enhances Fas ligand-mediated cytotoxicity of murine T helper 1 cells. Cell Immunol. 1996;173:230–235. doi: 10.1006/cimm.1996.0272. [DOI] [PubMed] [Google Scholar]
- 8.Fujioka N, Akazawa R, Ohashi K, Fujii M, Ikeda M, Kurimoto M. Interleukin-18 protects mice against acute herpes simplex virus type 1 infection. J Virol. 1999;73:2401–2409. doi: 10.1128/jvi.73.3.2401-2409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galan J E, Nakayama K, Curtiss R., III Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonellavaccine. Gene. 1990;94:29–35. doi: 10.1016/0378-1119(90)90464-3. [DOI] [PubMed] [Google Scholar]
- 10.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 11.Gillessen S, Carvajal D, Ling P, Podlaski F J, Stremlo D L, Familletti P C, Gubler U, Presky D H, Stern A S, Gately M K. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 12.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell R A, Sato V, Harding M W, Livingston D J, Su M S-S. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 13.Heinzel F P, Hujer A M, Ahmed F N, Rerko R M. In vivo production and function of IL-12 p40 homodimers. J Immunol. 1997;158:4381–4388. [PubMed] [Google Scholar]
- 14.Hess J, Ladel C, Miko D, Kaufmann S H. Salmonella typhimurium aroA-infection in gene-targeted immunodeficient mice: major role of CD4+TCR-αβ cells and IFN-γ in bacterial clearance independent of intracellular location. J Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 15.Jones B D, Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami K, Qureshi M H, Zhang T, Okamura H, Kurimoto M, Saito A. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformansby inducing IFN-γ production. J Immunol. 1997;159:5528–5534. [PubMed] [Google Scholar]
- 17.Kincy-Cain T, Clements J D, Bost K L. Endogenous and exogenous interleukin-12 augment the protective immune response in mice orally challenged with Salmonella dublin. Infect Immun. 1996;64:1437–1440. doi: 10.1128/iai.64.4.1437-1440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. IFN-γ-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 19.Ladel C H, Blum C, Kaufmann S H. Control of natural killer cell-mediated innate resistance against the intracellular pathogen Listeria monocytogenesby gamma/delta T lymphocytes. Infect Immun. 1996;64:1744–1749. doi: 10.1128/iai.64.5.1744-1749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladel C H, Szalay G, Riedel D, Kaufmann S H. Interleukin-12 secretion by Mycobacterium tuberculosis-infected macrophages. Infect Immun. 1997;65:1936–1938. doi: 10.1128/iai.65.5.1936-1938.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling P, Gately M K, Gubler U, Stern A S, Lin P, Hollfelder K, Su C, Pan Y C, Hakimi J. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995;154:116–1127. [PubMed] [Google Scholar]
- 22.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C-Y, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. IL-12-deficient mice are defective in IFN-γ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 23.Magram J, Sfarra J, Connaughton S, Faherty D, Warrier R, Carvajal D, Wu C-Y, Stewart C, Sarmiento U, Gately M K. IL-12-deficient mice are defective but not devoid of type 1 cytokine responses. Ann N Y Acad Sci. 1996;795:60–70. doi: 10.1111/j.1749-6632.1996.tb52655.x. [DOI] [PubMed] [Google Scholar]
- 24.Mastroeni P, Villareal-Ramos B, Hormaeche C E. Role of T cells, TNF-α and IFN-γ in recall of immunity to oral challenge with virulent salmonellae in mice vaccinated with live attenuated aro−Salmonellavaccines. Microb Pathog. 1992;13:477–491. doi: 10.1016/0882-4010(92)90014-f. [DOI] [PubMed] [Google Scholar]
- 25.Mastroeni P, Clare S, Khan S, Harrison J A, Hormaeche C E, Okamura H, Kurimoto M, Dougan G. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect Immun. 1999;67:478–83. doi: 10.1128/iai.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattner F, Fischer S, Guckes S, Jin S, Kaulen H, Schmitt E, Rude E, Germann T. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur J Immunol. 1993;23:2202–2208. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- 27.Mattner F, Magram J, Ferrante J, Launois P, Padova K D, Behin R, Gately M K, Louis J A, Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania majorand mount a polarized Th2 cell response. Eur J Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 28.Micallef M J, Ohtsuki T, Kohno K, Tahabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kutimoto M. Interferon-γ-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-γ production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K, Okamura H, Nagata K, Komatsu T, Tamura T. Purification of a factor which provides a costimulatory signal for gamma interferon production. Infect Immun. 1993;61:64–70. doi: 10.1128/iai.61.1.64-70.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nauciel C, Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimuriuminfection. Infect Immun. 1992;60:450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamura H, Nagata K, Komatsu T, Tanimoto T, Nukata Y, Tanabe F, Akita K, Torigoe K, Okura T, Fukuda S, Kurimoto M. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect Immun. 1995;63:3966–3972. doi: 10.1128/iai.63.10.3966-3972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 33.Pascual D W, Blalock J E, Bost K L. Anti-peptide antibodies which recognize a lymphocyte substance P receptor. J Immunol. 1989;143:3697–3702. [PubMed] [Google Scholar]
- 34.Pascual D W, Kiyono H, McGhee J R. Mucosal immunity: molecular and cellular aspects of immune protection to enteric infections. In: Paradise L J, et al., editors. Enteric infections and immunity. New York, N.Y: Plenum Press; 1996. pp. 15–35. [Google Scholar]
- 35.Pascual D W, Walters N, Hillemeyer P. Repeated intratracheal instillations of non-replicating adenovirus 2 vector attenuate CTL responses and IFN-γ production. J Immunol. 1998;160:4465–4472. [PubMed] [Google Scholar]
- 36.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 37.Tomura M, Zhou X-Y, Maruo S, Ahn H-J, Hamaoka T, Okamura H, Nakanishi K, Tanimoto T, Kurimoto M, Fujiwara H. A critical role for IL-18 in the proliferation and activation of NK1.1+ CD3−cells. J Immunol. 1998;160:4738–4746. [PubMed] [Google Scholar]
- 38.Tone M, Thompson S A J, Tone Y, Fairchild P J, Waldman H. Regulation of IL-18 (IFN-γ-inducing factor) gene expression. J Immunol. 1997;159:6156–6163. [PubMed] [Google Scholar]
- 39.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 40.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 41.Tsutsui H, Matsui K, Kawada N, Hyodo Y, Hayashi N, Okamura H, Higashino K, Nakanishi K. IL-18 accounts for both TNF-α- and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J Immunol. 1997;159:3961–3967. [PubMed] [Google Scholar]
- 42.Ushio S, Namba M, Okura T, Hattori K, Nukada Y, Akita K, Tanabe F, Konishi K, Micallef M, Fujii M, Torigoe K, Tanimoto T, Fukada S, Ikeda M, Okamura H, Kurimoto M. Cloning of the cDNA for human IFN-γ-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–4279. [PubMed] [Google Scholar]
- 43.VanCott J L, Chatfield S N, Roberts M, Hone D M, Hohmann E, Pascual D W, Yamamoto M, Yamamoto S, Kiyono H, McGhee J R. Regulation of host immune responses by modification of Salmonellavirulence genes. Nat Med. 1998;4:1247–1252. doi: 10.1038/3227. [DOI] [PubMed] [Google Scholar]
- 44.Wu S, Pascual D W, VanCott J L, McGhee J R, Maneval D R, Jr, Levine M M, Hone D M. Immune responses to novel Escherichia coli and Salmonella typhimurium vectors that express colonization factor antigen I (CFA/I) of enterotoxigenic E. coli in the absence of the CFA/I positive regulator cfaR. Infect Immun. 1995;63:4933–4938. doi: 10.1128/iai.63.12.4933-4938.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S-I, Okamura H, Akira S, Nakanishi K. IL-12 up-regulates IL-18 receptor expression on T cells, and B cells: synergism with IL-18 for IFN-γ production. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- 46.Zhang T, Kawakami K, Qureshi M H, Okamura H, Kurimoto M, Saito A. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformansthrough production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–3599. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]