Abstract
Introduction
The efficacy of immunotherapy for treatment of patients with oligometastatic non-small cell lung cancer (NSCLC) at different metastatic sites remains controversial. We investigated the effect of different metastatic sites on immunotherapy for oligometastatic NSCLC following local treatment (LT).
Methods
We retrospectively analyzed patients with oligometastatic NSCLC from the latest 2018 registry on the SEER Stat software (8.3.9. Version) and a Chinese single-center cohort. The effects of immunotherapy on OS (overall survival) and CSS (cancer specific survival) were estimated for patients with different metastatic sites.
Results
A total of 483 patients in the SEER-18 database and 344 patients in the single-center cohort were included. Immunotherapy was significantly correlated with improved OS (SEER: Hazard ratio 0.754, 95% CI 0.609–0.932; P=0.044; China: Hazard ratio 0.697, 95% CI 0.542–0.896; P=0.005) and CSS (SEER: Hazard ratio 0.743, 95% CI 0.596–0.928; P=0.009; China: Hazard ratio 0.725, 95% CI 0.556–0.945; P=0.018). Subgroup analysis showed that OS was improved after immunotherapy in the BRM (SEER: Hazard ratio 0.565, 95% CI 0.385–0.829; P=0.004; China: Hazard ratio 0.536, 95% CI 0.312–0.920; P=0.024) and MOM (SEER: Hazard ratio 0.524, 95% CI 0.290–0.947; P=0.032; China: Hazard ratio 0.469, 95% CI 0.235–0.937; P=0.032) subgroups, but not in the BOM (SEER: P=0.334; China: P=0.441), LIM (SEER: P=0.301; China: P=0.357), or OTM (SEER: P=0.868; China: P=0.489) subgroups.
Conclusions
This study showed that immunotherapy conferred survival benefits on patients with oligometastatic NSCLC. Our subgroup analysis suggested that patients with oligometastatic NSCLC in the brain or multiple organs may particularly benefit from aggressive front-line therapies.
Keywords: non-small cell lung cancer, immunotherapy, oligometastases, overall survival, metastatic sites
Introduction
Non-small cell lung cancer accounts for 85% of lung cancer and is the leading cause of cancer-related mortality (1). Non-small cell lung cancer is the primary cause of cancer-related death in the world mainly because patients with lung cancer often develop advanced metastases (2). Approximately 25–50% of patients with NSCLC exhibit an oligometastatic status (3). The concept of “oligometastasis” builds on two models of cancer progression: the Halsted model of continuous progression and a systemic model that hypothesizes that disease is a manifestation of extensive systemic clinical involvement (4). Patients with oligometastatic NSCLC may achieve long-term disease control and may even be cured. In retrospective studies, survival in patients with oligometastatic NSCLC had better overall survival (OS) than patients with a large number of metastases (3, 5). Therefore, it is important to investigate selection of treatment for these patients with limited metastatic NSCLC.
A number of previous studies (6–8) on oligometastatic NSCLC have shown that use of local treatment (LT) at all metastatic sites resulted in longer OS and progression-free survival (PFS) than those following palliative treatment. Immunotherapy has become the standard treatment for patients with metastatic NSCLC (9). The KEYNOTE-189 (10) and KEYNOTE-407 (11) studies have shown that pembrolizumab in combination with specific chemotherapy improved OS and PFS in patients with untreated metastatic NSCLC, regardless of PD-L1 expression. Therefore, use of LT combined with immunotherapy for treatment of oligometastatic NSCLC is an area of active investigation.
Joshua M et al (12) reported in a phase II clinical study that pembrolizumab improved PFS in patients with oligometastatic NSCLC after LT. Although this study showed that pembrolizumab could improve the prognosis of patients with oligometastatic NSCLC, this study did not consider the effect of factors such as the location and the number of organs affected by oligometastases on prognosis, and there are no phase III clinical prospective trials focused on oligometastatic NSCLC. Several previous studies have confirmed that the prognosis of patients with oligometastatic NSCLC was closely related to the site and number of metastatic organs (3, 13, 14). Ashworth AB et al (14) reported that prognosis in patients with oligometastatic NSCLC was associated with brain metastasis but did not address the relationship between prognosis and other metastatic sites.
No study has elucidated the effects of immunotherapy in patients with oligometastatic NSCLC at different metastatic sites. Therefore, our goal was to investigate the effect of different metastatic sites on immunotherapy for oligometastatic NSCLC following LT.
Methods
Patient cohort
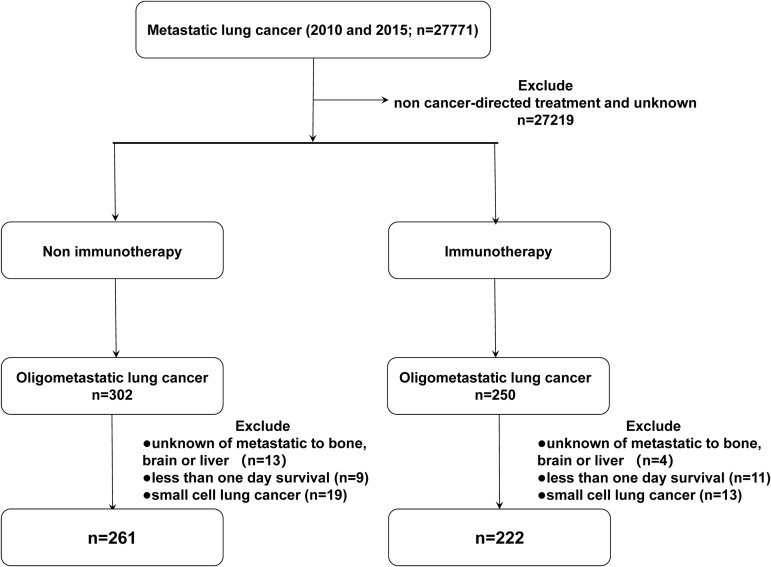
Patient data were collected from the incidence-SEER 18 registries from the National Cancer Institute SEER Stat software (Version 8.3.9), with additional treatment fields added. According to the third edition of the International Classification of Diseases for Oncology (ICD-O-3) and the AJCC 7th TNM staging of NSCLC, we screened patients who were first primarily diagnosed with malignant tumors of the lung and bronchus with M1b stage in 2010 and 2015. We selected patients with oligometastatic NSCLC based on the time of approval of immunotherapy as the primary treatment (2015), with patients diagnosed in 2010 as a comparison. The inclusion criteria were as follows: 1) patients with metastatic non-small cell lung cancer treated with cancer-directed treatment; 2) no younger than 20 years old. The exclusion criteria were: 1) more than one primary tumor; 2) incomplete data on metastases of the bone, liver, and brain at diagnosis; 3) incomplete treatment and follow-up information; 4) 0 days of survival. The following variables were selected: ID number, age, sex, year of diagnosis, race (white, black, or other), marital status, site recode ICD-0-3, affected side, tissue grade, histology, T stage (AJCC 7th), N stage (AJCC 7th), metastases of the bone, liver, and brain at diagnosis, radiation (radiation or other), chemotherapy (yes or no), surgery information, survival months, COD to site rec KM, vital status record. Patients were divided into five subgroups: BOM (bone metastasis), BRM (brain metastasis), LIM (liver metastasis), MOM (multiple organ metastasis), and OTM (other metastasis). The detailed inclusion and exclusion criteria are summarized in Figure 1 .
Figure 1.
Flow diagram of the patient selection process from the SEER Database.
An independent cohort of patients with oligometastatic NSCLC from 2016 to 2019 from Shandong Cancer Hospital in China was used for external validation. These patients met the same inclusion and exclusion criteria as those from the SEER Database.
Statistical analysis
The Pearson chi-squared test was used to compare demographics, pathology, and site of metastasis in patients with or without immunotherapy. We chose 36 months as the cutoff value. Overall survival and CSS were analyzed using the Kaplan–Meier (KM) method via the log-rank test. Univariate and multivariate analyses was performed using the Cox proportional hazards risk model. Variables that were significantly associated with survival in the univariate analysis were included in the multivariate Cox analysis. All tests were two-sided tests, and P <0.05 was considered statistically significant. SPSS 26 (IBM, Armonk, USA) and R 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for analysis.
Results
Patient characteristics
A total of 483 patients in the SEER cohort and 344 patients in the single-center cohort who met the eligibility criteria were included in this study. The baseline characteristics of each cohort are listed in Tables 1 and 2 . In the SEER cohort, at a median follow-up of 24.8 months (range, 1-107 months), 370 patients died, among whom 339 died of lung cancer. And in the single-center cohort, 245 deaths, including 219 cases dying from NSCLC with a median follow-up of 20.3 months (range, 3-53 months).
Table 1.
Baseline characteristics of all patients in the SEER and the single-center cohorts.
| Characteristics | SEER CohortN=483(%) | Single-Center CohortN=344(%) | P |
|---|---|---|---|
| Age | |||
| <65 | 262 (54.2%) | 120 (34.9%) | <0.001 |
| ‗65 | 221 (45.8%) | 224 (65.1%) | |
| Sex | |||
| Male | 275 (56.9%) | 198 (57.6%) | 0.859 |
| Female | 208 (43.1%) | 146 (42.4%) | |
| Origin | |||
| Left | 205 (42.4%) | 136 (39.5%) | 0.402 |
| Right | 278 (57.6%) | 208 (60.5%) | |
| Histology | |||
| Squamous cell carcinoma | 146 (30.2%) | 119 (34.6%) | 0.185 |
| Others | 337 (69.8%) | 225 (65.4%) | |
| T stage | |||
| T1 | 119 (24.6%) | 44 (12.8%) | <0.001 |
| T2 | 85 (17.6%) | 113 (32.8%) | |
| T3 | 168 (34.8%) | 143 (41.6%) | |
| T4 | 108 (22.4%) | 38 (11.0%) | |
| Unknown | 3 (0.6%) | 6 (1.7%) | |
| N stage | |||
| N0 | 200 (41.4%) | 161 (46.8%) | 0.201 |
| N1 | 64 (13.3%) | 41 (11.9%) | |
| N2 | 147 (30.4%) | 91 (26.5%) | |
| N3 | 56 (11.6%) | 46 (13.4%) | |
| Unknown | 16 (3.3%) | 5 (1.5%) | |
| Radiation status | |||
| No | 217 (44.9%) | 159 (46.2%) | 0.713 |
| Yes | 266 (55.1%) | 185 (53.8%) | |
| Immunotherapy | |||
| No | 261 (54.0%) | 163 (47.4%) | 0.059 |
| Yes | 222 (46.0%) | 181 (52.6%) | |
| Bone metastasis | |||
| No | 323 (66.9%) | 218 (63.4%) | 0.297 |
| Yes | 160 (33.1%) | 126 (36.6%) | |
| Brain metastasis | |||
| No | 283 (58.6%) | 216 (62.8%) | 0.224 |
| Yes | 200 (41.4%) | 128 (37.2%) | |
| Liver metastasis | |||
| No | 400 (82.8%) | 268 (77.9%) | 0.094 |
| Yes | 83 (17.2%) | 76 (22.1%) | |
| Metastasis site | |||
| BOM | 103 (21.3%) | 79 (23.0%) | 0.274 |
| BRM | 160 (33.1%) | 82 (23.8%) | |
| LIM | 45 (9.3%) | 30 (8.7%) | |
| MOM | 59 (12.2%) | 67 (19.5%) | |
| OTM | 116 (24.0%) | 86 (25.0%) | |
BOM, bone metastases only; BRM, brain metastases only; LIM, liver metastases only; MOM, multiple organ metastases; OTM, other metastases.
*P value is significant
Table 2.
Characteristics of all patients according to receipt of immunotherapy in each cohort.
| Variable | SEER Cohort | Single-Center Cohort | ||||
|---|---|---|---|---|---|---|
| No immunotherapyN=261 (%) | ImmunotherapyN=222 (%) | P | No immunotherapyN=163 (%) | ImmunotherapyN=181 (%) | P | |
| Age | ||||||
| <65 | 146 (55.9%) | 116 (52.3%) | 0.418 | 56 (34.4%) | 64 (35.4%) | 0.845 |
| ‗65 | 115 (44.1%) | 106 (47.7%) | 107 (65.6%) | 117 (64.6%) | ||
| Sex | ||||||
| Male | 152 (58.2%) | 123 (55.4%) | 0.531 | 98 (60.1%) | 100 (55.2%) | 0.361 |
| Female | 109 (41.8%) | 99 (44.6%) | 65 (39.9%) | 81 (44.8%) | ||
| Origin | ||||||
| Left | 111 (42.5%) | 94 (42.3%) | 0.967 | 68 (41.7%) | 68 (37.6%) | 0.432 |
| Right | 150 (57.5%) | 128 (57.7%) | 95 (58.3%) | 113 (62.4%) | ||
| Histology | ||||||
| Squamous cell carcinoma | 89 (34.1%) | 57 (25.7%) | 0.056 | 60 (36.8%) | 59 (32.6%) | 0.412 |
| Others | 172 (65.9%) | 165 (74.3%) | 103 (63.2%) | 122 (67.4%) | ||
| T stage | ||||||
| T1 | 69 (26.4%) | 50 (22.5%) | 0.086 | 28 (17.2%) | 16 (8.8%) | 0.110 |
| T2 | 45 (17.2%) | 40 (18.0%) | 52 (31.9%) | 61 (33.7%) | ||
| T3 | 95 (36.4%) | 73 (32.9%) | 65 (39.9%) | 78 (43.1%) | ||
| T4 | 49 (18.8%) | 59 (26.6%) | 14 (8.6%) | 24 (13.3%) | ||
| Unknown | 3 (1.1%) | 0 (0.0%) | 4 (2.5%) | 2 (1.1%) | ||
| N stage | ||||||
| N0 | 114 (43.7%) | 86 (38.7%) | 0.483 | 82 (50.3%) | 79 (43.6%) | 0.666 |
| N1 | 34 (13.0%) | 30 (13.5%) | 18 (11.0%) | 23 (12.7%) | ||
| N2 | 81 (31.0%) | 66 (29.7%) | 43 (26.4%) | 48 (26.5%) | ||
| N3 | 25 (9.6%) | 31 (14.0%) | 18 (11.0%) | 28 (15.5%) | ||
| Unknown | 7 (2.7%) | 9 (4.1%) | 2 (1.2%) | 3 (1.7%) | ||
| Radiation status | ||||||
| No | 117 (44.8%) | 100 (45.0%) | 0.962 | 69 (42.3%) | 90 (49.7%) | 0.206 |
| Yes | 144 (55.2%) | 122 (55.0%) | 94 (57.7%) | 91 (50.3%) | ||
| Bone metastasis | ||||||
| No | 182 (69.7%) | 141 (63.5%) | 0.148 | 108 (66.3%) | 110 (60.8%) | 0.292 |
| Yes | 79 (30.3%) | 81 (36.5%) | 55 (33.7%) | 71 (39.2%) | ||
| Brain metastasis | ||||||
| No | 153 (58.6%) | 130 (58.6%) | 0.989 | 109 (66.9%) | 107 (59.1%) | 0.169 |
| Yes | 108 (41.4%) | 92 (41.4%) | 54 (33.1%) | 74 (40.9%) | ||
| Liver metastasis | ||||||
| No | 223 (85.4%) | 185 (83.3%) | 0.524 | 34 (20.9%) | 42 (23.2%) | 0.601 |
| Yes | 38 (14.6%) | 37 (16.7%) | 129 (79.1%) | 139 (76.8%) | ||
| Metastasis site | ||||||
| BOM | 55 (21.1%) | 55 (21.6%) | 0.225 | 40 (24.5%) | 39 (21.5%) | 0.563 |
| BRM | 92 (35.2%) | 68 (30.6%) | 41 (25.2%) | 41 (22.7%) | ||
| LIM | 27 (10.3%) | 18 (8.1%) | 13 (8.0%) | 17 (9.4%) | ||
| MOM | 24 (9.6%) | 35 (15.8%) | 26 (16.0%) | 41 (22.7%) | ||
| OTM | 63 (23.8%) | 53 (23.9%) | 43 (26.4%) | 43 (23.8%) | ||
BOM, bone metastases only; BRM, brain metastases only; LIM, liver metastases only; MOM, multiple organ metastases; OTM, other metastases.
*p value is significant
In the SEER cohort, 54.2% were aged <65. The majority of patients were men (56.9%). The distribution of the metastasis sites was 21.3%, 33.1%, 9.3%, 12.2%, and 24.0% for BOM, BRM, LIM, MOM, and OTM, respectively ( Table 1 ). Moreover, this cohort enrolled 222 (46.0%) patients who received immunotherapy, 275 (57.0%) male patients, and 285 (59.0%) patients with adenocarcinoma histology. The different metastasis site subgroups were evenly distributed between the no immunotherapy and immunotherapy groups ( Table 2 ). None of the factors, including age, sex, origin, histology, T stage, N stage, radiation status, chemotherapy, bone metastasis, brain metastasis, and liver metastasis differed significantly between the two groups ( Table 2 ).
In the validation cohort, the metastasis sites included BOM (23.0%), BRM (23.8%), LIM (8.7%), MOM (19.5%), and OTM (25.0%) ( Table 2 ). In this cohort, no factors, including metastasis-site subgroups, age, sex, origin, histology, T stage, or N stage correlated with immunotherapy.
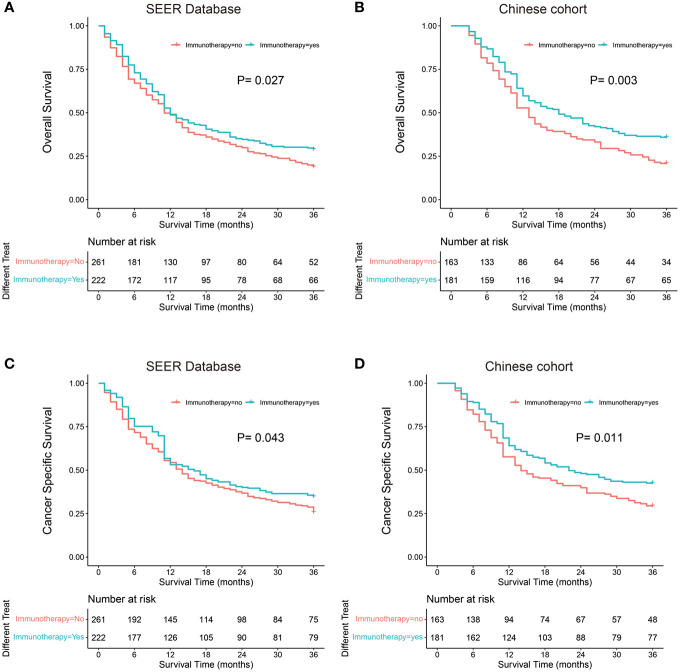
Immunotherapy and survival outcomes
Kaplan–Meier probability plots showed improved OS and CSS after immunotherapy in both cohorts ( Figure 2 ). We performed univariate and multivariate cox proportional hazards analyses to determine whether any clinical or pathological features were associated with OS and CSS ( Table 3 ). In the univariate analysis, we found that immunotherapy was associated with better OS (SEER: Hazard ratio 0.797, 95% CI 0.649–0.980; P=0.027; China: Hazard ratio 0.693, 95% CI 0.539–0.890; P=0.004) and CSS (SEER: Hazard ratio 0.796, 95% CI 0.642–0.987; P=0.043; China: Hazard ratio 0.715, 95% CI 0.548–0.932; P=0.013). Moreover, we showed that sex, site, T stage, N stage, chemotherapy, and liver metastasis were significantly associated with clinical outcome in the univariate analysis. These factors were adjusted to evaluate the effect of immunotherapy. In the multivariate analysis, we found that immunotherapy was significantly correlated with improved OS (SEER: Hazard ratio 0.754, 95% CI 0.609–0.932; P=0.044; China: Hazard ratio 0.697, 95% CI 0.542–0.896; P=0.005) and CSS (SEER: Hazard ratio 0.743, 95% CI 0.596–0.928; P=0.009; China: Hazard ratio 0.725, 95% CI 0.556–0.945; P=0.018) ( Table 3 ). Male patients had significantly worse OS (SEER: Hazard ratio 1.311, 95% CI 1.057–1.626; P=0.014; China: Hazard ratio 1.599, 95% CI 1.225–2.088; P=0.001) and CSS (SEER: Hazard ratio 1.282, 95% CI 1.020–1.610; P=0.033; China: Hazard ratio 1.502, 95% CI 1.143–1.976; P=0.004) than female patients in the multivariate analyses. Patients with lesions located in the main bronchus had a worse prognosis than those with lesions located at other sites. Patients with higher T and N stage showed better prognosis. Liver metastasis was associated with poor prognosis for the SEER cohort but not for the single-center cohort.
Figure 2.
Kaplan-Meier probability plots of Overall Survival and Cancer Specific Survival between the Immunotherapy group and the No immunotherapy group: SEER Database (A, C) and Single-Center cohort (B, D).
Table 3.
Univariate and multivariate analyses of OS and CSS for different characteristics of oligometastatic NSCLC in each cohort.
| SEER Cohort | Single-Center Cohort | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Univariate analysis (OS) | Multivariate analysis (OS) | Univariate analysis (OS) | Multivariate analysis (OS) | |||||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||||||||
| Sex | |||||||||||||||
| Female | 1.353 (1.098-1.666) | 0.004* | 1.311 (1.057-1.626) | 0.014* | 1.603 (1.237-2.077) | <0.001* | 1.599 (1.225-2.088) | 0.001* | |||||||
| Male | |||||||||||||||
| T stage | 0.002* | 0.013* | 0.016* | 0.126 | |||||||||||
| T1 | Reference | Reference | Reference | Reference | |||||||||||
| T2 | 0.878 (0.632-1.219) | 0.437 | 0.850 (0.602-1.202) | 0.359 | 0.968 (0.640-1.465) | 0.878 | 0.911 (0.599-1.385) | 0.838 | |||||||
| T3 | 1.120 (0.854-1.469) | 0.414 | 1.234 (0.933-1.632) | 0.140 | 1.041 (0.697-1.555) | 0.844 | 0.989 (0.656-1.490) | 0.827 | |||||||
| T4 | 1.608 (1.199-2.158) | 0.002 | 1.475 (1.080-2.015) | 0.015 | 1.775 (1.113-2.830) | 0.016 | 1.501 (0.929-2.425) | 0.076 | |||||||
| Unknown | 1.693(0.535-5.361) | 0.371 | 0.801(0.247-2.597) | 0.711 | 2.006(0.706-5.698) | 0.191 | 1.377(0.473-4.011) | 0.558 | |||||||
| N stage | 0.001* | <0.001* | * | 0.015* | 0.014* | ||||||||||
| N0 | Reference | Reference | Reference | Reference | |||||||||||
| N1 | 1.347(0.977-1.857) | 0.069 | 1.692(1.211-2.363) | 0.002 | 1.222(0.815-1.832) | 0.331 | 1.333(0.887-2.004) | 0.166 | |||||||
| N2 | 1.389(1.084-1.779) | 0.009 | 1.570(1.207-2.043) | 0.001 | 0.979(0.705-1.360) | 0.900 | 1.032(0.742-1.435) | 0.853 | |||||||
| N3 | 1.944(1.412-2.676) | <0.001 | 2.062(1.471-2.890) | <0.001 | 1.714(1.221-2.406) | 0.002 | 1.780(1.266-2.501) | 0.001 | |||||||
| Unknown | 1.255(0.711-2.216) | 0.434 | 1.572(0.874-2.830) | 0.131 | 1.834(0.748-4.501) | 0.185 | 1.902(0.775-4.668) | 0.161 | |||||||
| Immunotherapy | |||||||||||||||
| No | 0.797 (0.649-0.980) | 0.031* | 0.754 (0.609-0.932) | 0.044* | 0.693 (0.539-0.890) | 0.004* | 0.697 (0.542-0.896) | 0.005* | |||||||
| Yes | |||||||||||||||
| Univariate analysis (CSS) | Multivariate analysis (CSS) | Univariate analysis (CSS) | Multivariate analysis (CSS) | ||||||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||||||||
| Sex | |||||||||||||||
| Female | 1.363 (1.094-1.697) | 0.006* | 1.282 (1.020-1.610) | 0.033* | 1.519 (1.156-1.998) | 0.003* | 1.502 (1.143-1.976) | 0.004* | |||||||
| Male | |||||||||||||||
| T stage | 0.005* | 0.030* | 0.024* | 0.170* | |||||||||||
| T1 | Reference | Reference | Reference | Reference | |||||||||||
| T2 | 0.923(0.655-1.301) | 0.647 | 0.884(0.618-1.264) | 0.500 | 0.888(0.570-1.385) | 0.601 | 0.825(0.526-1.294) | 0.403 | |||||||
| T3 | 1.154(0.867-1.537) | 0.326 | 1.228(0.917-1.645) | 0.168 | 1.080(0.705-1.654) | 0.723 | 0.933(0.644-1.531) | 0.974 | |||||||
| T4 | 1.638(1.201-2.235) | 0.002 | 1.414(1.019-1.962) | 0.038 | 1.747(1.068-2.857) | 0.026 | 1.438(0.867-2.386) | 0.160 | |||||||
| Unknown | 0.636(0.088-4.581) | 0.654 | 0.281(0.038-2.054) | 0.221 | 1.405(0.427-4.626) | 0.576 | 0.992(0.295-3.341) | 0.990 | |||||||
| N stage | 0.001* | <0.001* | 0.015* | 0.014* | |||||||||||
| N0 | Reference | Reference | Reference | ||||||||||||
| N1 | 1.466(1.049-2.049) | 0.025 | 1.825(1.289-2.583) | 0.001 | 1.397(0.921-2.119) | 0.116 | 1.496(0.984-2.274) | 0.060 | |||||||
| N2 | 1.539(1.188-1.995) | 0.001 | 1.747(1.329-2.297) | <0.001 | 1.046(0.740-1.477) | 0.800 | 1.097(0.775-1.552) | 0.601 | |||||||
| N3 | 1.905(1.347-2.693) | <0.001 | 1.981(1.376-2.851) | <0.001 | 1.600(1.111-2.304) | 0.012 | 1.637(1.136-2.359) | 0.008 | |||||||
| Unknown | 1.342(0.741-2.429) | 0.332 | 1.649(0.894-3.043) | 0.110 | 2.254(0.916-5.550) | 0.077 | 2.329(0.945-5.738) | 0.066 | |||||||
| Immunotherapy | |||||||||||||||
| No | 0.796 (0.642-0.987) | 0.043* | 0.743 (0.596-0.928) | 0.009* | 0.715 (0.548-0.932) | 0.013* | 0.725 (0.556-0.945) | 0.018* | |||||||
| Yes | |||||||||||||||
*p value is significant.
Subgroup analysis and validation
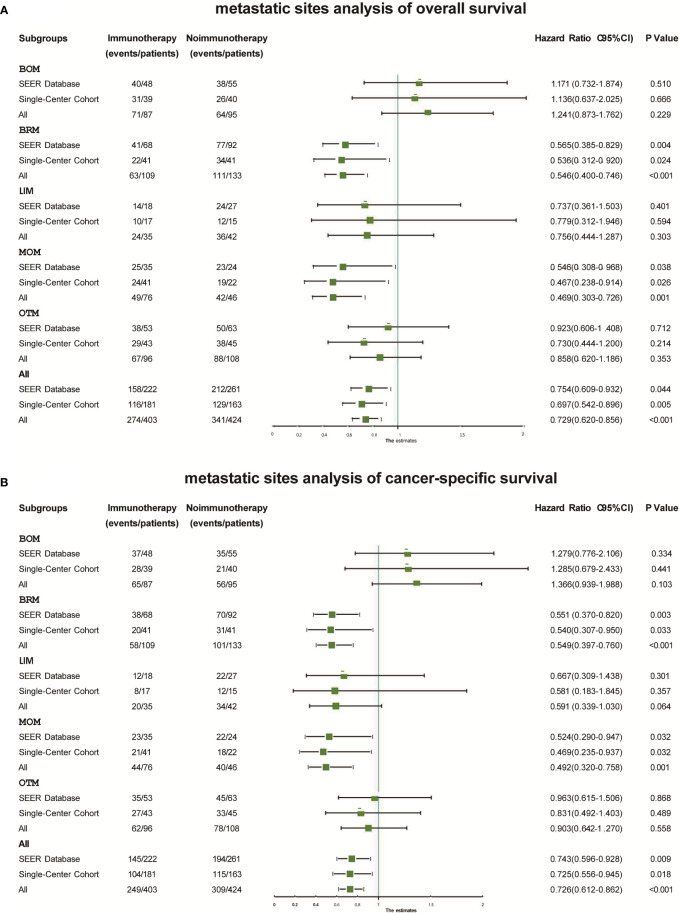
In the subgroup analysis, Kaplan–Meier probability plots displayed differences in OS and CSS improvement after immunotherapy in both cohorts and among the five metastatic subgroups in both cohorts ( Figure S1 ). As shown in Figure 3 , immunotherapy had similar effects on the different metastatic subgroups. No improvement in OS was found in the BOM (SEER: Hazard ratio 1.171, 95% CI 0.732–1.874; P=0.510; China: Hazard ratio 1.136, 95% CI 0.637–2.025; P=0.666), LIM (SEER: Hazard ratio 0.737, 95% CI 0.361–1.503; P=0.401; China: Hazard ratio 0.779, 95% CI 0.312–1.946; P=0.594), or OTM (SEER: Hazard ratio 0.923, 95% CI 0.606–1.408; P=0.712; China: Hazard ratio 0.730, 95% CI 0.444–1.200; P=0.214) subgroups. However, immunotherapy significantly increased OS in the BRM (SEER: Hazard ratio 0.565, 95% CI 0.385–0.829; P=0.004; China: Hazard ratio 0.536, 95% CI 0.312–0.920; P=0.024) and MOM (SEER: Hazard ratio 0.546, 95% CI 0.308–0.968; P=0.038; China: Hazard ratio 0.467, 95% CI 0.238–0.914; P=0.026) subgroups. Immunotherapy significantly improved CSS in the BRM (SEER: Hazard ratio 0.551, 95% CI 0.370–0.820; P=0.003; China: Hazard ratio 0.540, 95% CI 0.307–0.950; P=0.033) and MOM (SEER: Hazard ratio 0.524, 95% CI 0.290–0.947; P=0.032; China: Hazard ratio 0.469, 95% CI 0.235–0.937; P=0.032) subgroups, but not in the BOM (SEER: P=0.334; China: P=0.441), LIM (SEER: P=0.301; China: P=0.357), or OTM (SEER: P=0.868; China: P=0.489) subgroups ( Figure 3 ).
Figure 3.
Forest plots displaying the relationship between immunotherapy and overall survival (A) and cancer-specific survival (B) within different subgroups in each cohort. BOM, bone metastases only; BRM, brain metastases only; CSS, cancer-specific survival; LIM, liver metastases only; MOM, multiple organ metastases; OS, overall survival; OTM, other metastases.
We also merged the data from the two cohorts. Adjuvant immunotherapy improved OS (Hazard ratio 0.729, 95% CI 0.620–0.856; P<0.001) and CSS (Hazard ratio 0.726, 95%CI 0.612–0.862; P<0.001) significantly in the merged cohort ( Figure 3 ). We found different benefits from immunotherapy within each metastatic subgroup. This benefit was greatest in the BRM (OS: Hazard ratio 0.565, 95% CI 0.385–0.829; P=0.004; CSS: Hazard ratio 0.536, 95% CI 0.312–0.920; P=0.024) and MOM (OS: Hazard ratio 0.565, 95% CI 0.385–0.829; P=0.004; CSS: Hazard ratio 0.536, 95% CI 0.312–0.920; P=0.024) subgroups. In the BOM, LIM, and OTM subgroups, immunotherapy did not result in significant differences in OS or CSS.
Discussion
We analyzed the prognostic significance of immunotherapy at different sites in patients with oligometastatic NSCLC from the SEER and the single-center cohorts. To our knowledge, this was the largest sample size, and the first analysis to assess the role of immunotherapy in treating oligometastatic NSCLC according to different metastatic sites. Immunotherapy is US Food and Drug Administration approved for use alone or in combination with chemotherapy for patients with NSCLC, and has been evaluated after locally ablative therapy for oligometastatic disease (12). However, phase III clinical studies are still lacking in the field of immunotherapy treatment of oligometastatic NSCLC. Whether immunotherapy can confer a survival benefit in patients with oligometastatic NSCLC remains controversial, particular in those with different metastatic sites. Our study showed that immunotherapy improved OS and CSS in patients with oligometastatic NSCLC. These findings agreed with previous findings of improved survival following treatment of metastatic NSCLC with PD-1 inhibitors (15, 16). Recent clinical trials focused on use of immunotherapy to treat various solid tumors have shown a clear association between immunotherapy and increased OS and improved prognosis (17, 18). We also observed reduced OS in patients in the LIM and MOM subgroups. This was consistent with the findings of previous studies showing that patients with NSCLC that had metastasized to multiple organs or the liver had worse OS relative to those with other metastases (19–22). These results showed the value of immunotherapy for treatment of oligometastatic NSCLC patients with liver and multiple organ metastases.
We found that being male was significantly associated with poorer prognosis, which agreed with the results of a study by Radkiewicz C et al, who reported that men with NSCLC had consistently poorer lung cancer-specific survival across stages (23). Moreover, our results showed that patient prognosis was related to tumor location, and patients with tumors located in the main bronchus had worse outcomes than those who had tumors in other locations, which agreed with the results of a study by Li C et al (24). A recent study showed that chemotherapy combined with immunotherapy had superior efficacy for improving anticancer activity (25). Radiotherapy is not an independent prognostic factor for improvement of OS and CSS, due to the remarkable effects of recently developed targeted therapies.
The definition of oligometastatic NSCLC differed across many previous studies, with the maximum number of metastases ranging from 3 to 6 (26). Recently, a number of studies have shown that LT improved the prognosis of patients with oligometastatic NSCLC (27, 28). However, several studies have also shown that immunotherapy was effective for treatment of patients with oligometastatic NSCLC, especially for those at high risk of distant metastases (29, 30). In the most recent NCCN guidelines, immunotherapy is a recognized front-line treatment approach for metastatic NSCLC (31). Therefore, we studied the efficacy of immunotherapy for treatment of oligometastatic NSCLC based on different metastatic sites.
Previous studies focused on metastatic NSCLC have demonstrated that different sites of metastasis can predict clinical outcomes (20, 21, 32).Patients with NSCLC with bone metastasis have the best prognosis (32), which we further confirmed in the present study. We found that patients in the LOM and MOM subgroups had the worst OS and CSS compared to those in other subgroups, which was consistent with the results of a study by Yang J et al, which identified that the mortality risk was highest with MOM and liver metastases (20). We also found that immunotherapy improved OS and CSS in the BRM and MOM subgroups. A recent study found that local control and overall survival were both improved in patients with NSCLC who received concurrent immune checkpoint inhibitor treatment with radiosurgery (33), which was consistent with our results. Another study (34) that brain irradiation induces the strongest immune activation effects and patient outcome compared with those achieved through irradiation of other organs, which may be consistent with our results. However, patients in the LOM subgroup did not benefit from immunotherapy, which may have been associated with poorer response of liver metastasis to immunotherapy. A study reported that liver metastases diminish immunotherapy efficacy systemically in patients and preclinical models, resulting in a systemic immune desert through siphoning of activated CD8+ T cells from the systemic circulation (35). Our results showed that patients in the MOM subgroup benefited most from immunotherapy, which was consistent with the results of a study by Ma SC et al (36), who reported that increased number of metastatic sites (≥2) we associated with pronounced OS benefits from atezolizumab versus docetaxel. This may indicate that these patients represent a subgroup that may benefit from aggressive treatment.
Our study had several limitations. First, the SEER database does not provide a detailed description of the specific immunotherapy used. Second, limited information is provided by the SEER database, and there may be some degree of error in the coding of individual patients. Transfer of patients in a particular group (bone, brain, liver) were restricted to this organ, although patients may have had metastases at other sites not explicitly documented in the SEER database (the most common unrecorded site may be the adrenal). Finally, this study was a retrospective study, and the efficacy of immunotherapy in oligometastatic NSCLS with different metastatic sites should be evaluated in prospective clinical trials.
Conclusion
Our study showed that immunotherapy improved OS and CSS in patients in the SEER and the single-center cohorts. Furthermore, we found that immunotherapy improved the prognosis of patients in the BRM and MOM subgroups, but not those in the BOM, OTM, or LIM subgroups. The MOM subgroup experienced the greatest benefit from immunotherapy in both cohorts. Therefore, metastatic sites may affect the efficacy of immunotherapy. These subgroups may benefit from aggressive treatment, and our results may have implications for clinical guidance. However, our findings need to be validated in future large-sample prospective clinical trials.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
J-CM, J-XZ, FW, DC and JY contributed to the study. DC and JY designed the project and approved the final manuscript. J-XZ and FW are responsible for modifying and editing this article. J-CM collected the clinical patients information and drafted the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (82172676), Science Foundation of Shandong (ZR2021YQ52, ZR2020LZL016), Foundation of Bethune Charitable Foundation (2021434953), and the Young Elite Scientist Sponsorship Program by CAST (YESS20210137).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1039157/full#supplementary-material
Kaplan-Meier probability plots of Overall Survival and Cancer Specific Survival between the Immunotherapy group and the No immunotherapy group within different metastatic site subgroups: BOM group (SEER: a and b; Single-Center: k and l), BRM group (SEER: c and d; Single-Center: m and n), LIM group (SEER: e and f; Single-Center: o and p), MOM group (SEER: g and h; Single-Center: q and r), and OTM group (SEER: i and j; Single-Center: s and t). Abbreviations: MOM, multiple organ metastases; OTM, other metastases.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2. Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet (2013) 382:709–19. doi: 10.1016/S0140-6736(13)61502-0 [DOI] [PubMed] [Google Scholar]
- 3. Parikh RB, Cronin AM, Kozono DE, Oxnard GR, Mak RH, Jackman DM, et al. Definitive primary therapy in patients presenting with oligometastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys (2014) 89:880–7. doi: 10.1016/j.ijrobp.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 4. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol (1995) 13:8–10. doi: 10.1200/JCO.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- 5. Torok JA, Gu L, Tandberg DJ, Wang X, Harpole DH, Jr., Kelsey CR, et al. Patterns of distant metastases after surgical management of non-small-cell lung cancer. Clin Lung Cancer (2017) 18:e57–70. doi: 10.1016/j.cllc.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 6. Gomez DR, Tang C, Zhang J, Blumenschein GR, Jr., Hernandez M, Lee JJ, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-Small-Cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol (2019) 37:1558–65. doi: 10.1200/JCO.19.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gomez DR, Blumenschein GR, Jr., Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol (2016) 17:1672–82. doi: 10.1016/S1470-2045(16)30532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet (2019) 393:2051–8. doi: 10.1016/S0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]
- 9. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 10. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-Small-Cell lung cancer. N Engl J Med (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 11. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-Small-Cell lung cancer. N Engl J Med (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 12. Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, et al. Pembrolizumab after completion of locally ablative therapy for oligometastatic non-small cell lung cancer: A phase 2 trial. JAMA Oncol (2019) 5:1283–90. doi: 10.1001/jamaoncol.2019.1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hong JC, Ayala-Peacock DN, Lee J, Blackstock AW, Okunieff P, Sung MW, et al. Classification for long-term survival in oligometastatic patients treated with ablative radiotherapy: A multi-institutional pooled analysis. PLoS One (2018) 13:e0195149. doi: 10.1371/journal.pone.0195149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ashworth AB, Senan S, Palma DA, Riquet M, Ahn YC, Ricardi U, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer (2014) 15:346–55. doi: 10.1016/j.cllc.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 15. Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol (2016) 17:976–83. doi: 10.1016/S1470-2045(16)30053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldberg SB, Schalper KA, Gettinger SN, Mahajan A, Herbst RS, Chiang AC, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol (2020) 21:655–63. doi: 10.1016/S1470-2045(20)30111-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (2018) 359:1350–5. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: A systematic review and meta-analysis. JAMA Oncol (2020) 6:375–84. doi: 10.1001/jamaoncol.2019.5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hendriks LE, Derks JL, Postmus PE, Damhuis RA, Houben RM, Troost EG, et al. Single organ metastatic disease and local disease status, prognostic factors for overall survival in stage IV non-small cell lung cancer: Results from a population-based study. Eur J Cancer (2015) 51:2534–44. doi: 10.1016/j.ejca.2015.08.008 [DOI] [PubMed] [Google Scholar]
- 20. Yang J, Zhang Y, Sun X, Gusdon AM, Song N, Chen L, et al. The prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: a SEER-based study. J Cancer Res Clin Oncol (2018) 144:1835–42. doi: 10.1007/s00432-018-2702-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu Z, Yang Q, Chen X, Zheng L, Zhang L, Yu Y, et al. Clinical associations and prognostic value of site-specific metastases in non-small cell lung cancer: A population-based study. Oncol Lett (2019) 17:5590–600. doi: 10.3892/ol.2019.10225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Campos-Balea B, De Castro Carpeno J, Massuti B, Vicente-Baz D, Perez Parente D, Ruiz-Gracia P, et al. Prognostic factors for survival in patients with metastatic lung adenocarcinoma: An analysis of the SEER database. Thorac Cancer (2020) 11:3357–64. doi: 10.1111/1759-7714.13681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radkiewicz C, Dickman PW, Johansson ALV, Wagenius G, Edgren G, Lambe M. Sex and survival in non-small cell lung cancer: A nationwide cohort study. PLoS One (2019) 14:e0219206. doi: 10.1371/journal.pone.0219206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li C, Liu J, Lin J, Li Z, Shang X, Wang H. Poor survival of non-small-cell lung cancer patients with main bronchus tumor: a large population-based study. Future Oncol (2019) 15:2819–27. doi: 10.2217/fon-2019-0098 [DOI] [PubMed] [Google Scholar]
- 25. Lazzari C, Karachaliou N, Bulotta A, Viganó M, Mirabile A, Brioschi E, et al. Combination of immunotherapy with chemotherapy and radiotherapy in lung cancer: is this the beginning of the end for cancer? Ther Adv Med Oncol (2018) 10:1758835918762094. doi: 10.1177/1758835918762094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dingemans AC, Hendriks LEL, Berghmans T, Levy A, Hasan B, Faivre-Finn C, et al. Definition of synchronous oligometastatic non-small cell lung cancer-a consensus report. J Thorac Oncol (2019) 14:2109–19. doi: 10.1016/j.jtho.2019.07.025 [DOI] [PubMed] [Google Scholar]
- 27. Bansal P, Rusthoven C, Boumber Y, Gan GN. The role of local ablative therapy in oligometastatic non-small-cell lung cancer: hype or hope. Future Oncol (2016) 12:2713–27. doi: 10.2217/fon-2016-0219 [DOI] [PubMed] [Google Scholar]
- 28. Tibdewal A, Agarwal JP, Srinivasan S, Mummudi N, Noronha V, Prabhash K, et al. Standard maintenance therapy versus local consolidative radiation therapy and standard maintenance therapy in 1-5 sites of oligometastatic non-small cell lung cancer: a study protocol of phase III randomised controlled trial. BMJ Open (2021) 11:e043628. doi: 10.1136/bmjopen-2020-043628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schanne DH, Heitmann J, Guckenberger M, Andratschke NHJ. Evolution of treatment strategies for oligometastatic NSCLC patients - a systematic review of the literature. Cancer Treat Rev (2019) 80:101892. doi: 10.1016/j.ctrv.2019.101892 [DOI] [PubMed] [Google Scholar]
- 30. Gauvin C, Krishnan V, Kaci I, Tran-Thanh D, Bedard K, Albadine R, et al. Survival impact of aggressive treatment and PD-L1 expression in oligometastatic NSCLC. Curr Oncol (2021) 28:593–605. doi: 10.3390/curroncol28010059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines insights: Non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw (2021) 19:254–66. doi: 10.6004/jnccn.2021.0013 [DOI] [PubMed] [Google Scholar]
- 32. Gu Y, Zhang J, Zhou Z, Liu D, Zhu H, Wen J, et al. Metastasis patterns and prognosis of octogenarians with NSCLC: A population-based study. Aging Dis (2020) 11:82–92. doi: 10.14336/AD.2019.0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abdulhaleem M, Johnston H, D'agostino R, Jr., Lanier C, Lecompte M, Cramer CK, et al. Local control outcomes for combination of stereotactic radiosurgery and immunotherapy for non-small cell lung cancer brain metastases. J Neurooncol (2022) 157 (1):101–7. doi: 10.1007/s11060-022-03951-7 [DOI] [PubMed] [Google Scholar]
- 34. Wu M, Liu J, Wu S, Liu J, Wu H, Yu J, et al. Systemic immune activation and responses of irradiation to different metastatic sites combined with immunotherapy in advanced non-small cell lung cancer. Front Immunol (2021) 12:803247. doi: 10.3389/fimmu.2021.803247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med (2021) 27:152–64. doi: 10.1038/s41591-020-1131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma SC, Tang XR, Long LL, Bai X, Zhou JG, Duan ZJ, et al. Integrative evaluation of primary and metastatic lesion spectrum to guide anti-PD-L1 therapy of non-small cell lung cancer: results from two randomized studies. Oncoimmunology (2021) 10:1909296. doi: 10.1080/2162402X.2021.1909296 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier probability plots of Overall Survival and Cancer Specific Survival between the Immunotherapy group and the No immunotherapy group within different metastatic site subgroups: BOM group (SEER: a and b; Single-Center: k and l), BRM group (SEER: c and d; Single-Center: m and n), LIM group (SEER: e and f; Single-Center: o and p), MOM group (SEER: g and h; Single-Center: q and r), and OTM group (SEER: i and j; Single-Center: s and t). Abbreviations: MOM, multiple organ metastases; OTM, other metastases.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.