Abstract
Protective immunity to enterotoxigenic Escherichia coli (ETEC) is antibody (Ab) dependent; however, oral immunization with purified ETEC fimbriae fails to elicit protective immunity as a consequence of antigenic alteration by the gastrointestinal (GI) tract. Unless unaltered ETEC fimbriae can reach the inductive lymphoid tissues of the GI tract, immunity to ETEC cannot be induced. To produce immunity, live vectors, such as Salmonella typhimurium, can effectively target passenger antigens to the inductive lymphoid tissues of the GI tract. By convention, oral immunizations with Salmonella vectors induce CD4+ T helper (Th) cell responses by gamma interferon (IFN-γ)-dominated pathways both to the vector and passenger antigen, resulting in serum immunoglobulin G2a (IgG2a) and modest mucosal IgA Ab responses. In the present study, mice orally immunized with a Salmonella vector engineered to stably express ETEC colonization factor antigen I (CFA/I) showed initially elevated serum IgG1 and mucosal IgA anti-CFA/I Ab responses. As expected, mice orally immunized with an E. coli-CFA/I construct elicited poor anti-CFA/I Ab responses. In fact, the addition of cholera toxin during oral E. coli-CFA/I immunization failed to greatly enhance mucosal IgA Ab responses. Seven days after immunization with the Salmonella-CFA/I construct, cytokine-specific ELISPOT showed induction of predominant Th2-type responses in both mucosal and systemic immune compartments supporting the early IgG1 and IgA anti-CFA/I Abs. By 4 weeks, the Th cell response became Th1 cell dominant from the earlier Th2-type responses, as evidenced by increased mucosal and systemic IFN-γ-producing T cells and a concomitant elevation of serum IgG2a Ab responses. This biphasic response offers an alternative strategy for directing Salmonella vector-induced host immunity along a Th2 cell-dependent pathway, allowing for early promotion of mucosal and systemic Abs.
Rational design of vaccines for induction of effective mucosal immunity should carefully consider the types of T helper (Th) cell responses desired. A key aspect to effective induction of mucosal immunity is that the vaccine antigen must reach inductive sites in the mucosal compartment (20, 25). With regard to oral immunization, the Peyer's patches or gut-associated lymphoreticular tissue (GALT) of the small intestine are important sites for stimulation of Th cell subsets for subsequent induction of secretory immunoglobulin A (S-IgA) antibody (Ab) production at local and distal mucosal effector sites.
The recent development of live vector delivery systems, especially those that incorporate vaccine antigens into attenuated Salmonella typhimurium, allows for specific targeting to mucosal inductive sites (4, 5, 28). Previous studies by ourselves (23, 32, 35, 36) and others (21, 26) have shown that Salmonella vectors induced a predominance of Th1 cells against passenger and Salmonella antigens. Salmonella also induces modest S-IgA Ab responses in experimental animals (32, 33) and volunteers (15). Okahashi et al. found that Salmonella-induced S-IgA Ab responses were IL-4 independent and thus differed from S-IgA Ab responses promoted by cholera toxin (CT) (23). There are rare instances when Salmonella vaccines have been shown to induce Th2-type cytokines (33).
Much of the evidence with oral delivery of soluble proteins in conjunction with mucosal adjuvants demonstrates that soluble protein antigens normally induce the development of Th2 responses (16, 37, 38). It remains possible that Salmonella vectors producing soluble vaccine antigens could also elicit Th2-type responses. In this study, we show that mice orally immunized with a Salmonella-CFA/I vaccine induced a biphasic response to the CFA/I fimbriae, characterized early by a Th2-type response, followed by the development of a Th1-type response.
MATERIALS AND METHODS
Oral immunization with S. typhimurium.
BALB/c mice (Frederick Cancer Research Facility, National Cancer Institute, Frederick, Md.) between 6 and 8 weeks old were maintained in horizontal laminar flow cabinets; sterile food and water were provided ad libitum. The S. typhimurium-CFA/I vector vaccine, strain H696, and the E. coli-CFA/I vector, strain H695, were derived as previously described (35). The CFA/I expression plasmid contains a functional asd gene to complement the lethal chromosomal Δasd mutation and stabilize CFA/I expression in the absence of antibiotic selection. By using this expression system, CFA/I fimbriae are expressed on the S. typhimurium or the E. coli vector cell surface as functional fimbriae (11, 35). BALB/c male mice (10/group), pretreated with an oral 50% saturated sodium bicarbonate solution, received a single, oral dose of 5 × 109 of the S. typhimurium-CFA/I construct or the E. coli-CFA/I construct without or with CT (10 μg/dose). The latter group of mice received two oral doses on days 0 and 14. Additional groups of mice were vaccinated in the same manner with isogenic S. typhimurium H647 (lacking the cfaABCE insert) to provide negative control samples.
Ab ELISA.
CFA/I-specific endpoint titers were determined by an enzyme-linked immunosorbent assay (ELISA) (35) by using purified CFA/I fimbrial antigen (12). CT-B-specific endpoint titers were also determined by ELISA (16) by using purified CT-B (Sigma Chemical Co., St. Louis, Mo.). Various dilutions of immune mouse sera or fecal extracts were prepared as previously described (9). The specific reactivities to CFA/I or CT-B were determined by using horseradish peroxidase conjugates of detecting Abs: goat anti-mouse IgG, IgA, IgG1, or IgG2a Abs (Southern Biotechnology Associates, Birmingham, Ala.) and a substrate of 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid)diammonium (Moss, Inc., Pasadena, Calif.). Absorbances were read at 415 nm on a Kinetics Reader model EL312 (Bio-Tek Instruments, Winooski, Vt.). Endpoint titers were expressed as the reciprocal dilution of the last sample dilution giving an absorbance ≥0.1 optical density (OD) units above the OD415 of negative controls after a 1-h incubation.
Lymphoid cell isolation and culture.
Groups of mice were euthanized on days 7 and 28 for collection of blood and external secretions and for derivation of lymphoid tissues. Splenic lymphocytes were isolated by conventional methods (32, 35, 38); Peyer's patch lymphocytes were isolated as previously described (32, 35, 38). Both procedures yielded greater than 95% viability as determined by trypan blue exclusion. Enriched CD4+ T-cell fractions were isolated by a negative selection procedure. Briefly, the total mononuclear cell fraction was subjected to plastic adherence; nonadherent lymphocytes were then subjected to B-cell depletion by panning (24). The resultant T cells were treated with an anti-CD8 Ab (5H10-1; PharMingen, San Diego, Calif.) plus Low-Tox-M baby rabbit complement (Accurate Chemical, Westbury, N.Y.), yielding >95% CD3+ CD4+ T cells. Feeder cells were generated as previously described (34), followed by irradiation at 3,000 rads.
Cytokine detection assays.
Cytokine secretion by stimulated lymphocytes was detected by using the ELISPOT assay (32). Splenic and Peyer's patch CD4+ T cells were cultured at 5 × 106 cells/ml with equal numbers of irradiated feeder cells in medium only, with 10 μg of CFA/I fimbriae per ml, with 10 μg of OVA (Sigma) per ml, or with 10 μg of CT-B (Sigma) per ml for 2 to 3 days at 37°C. Subsequently, the cells were analyzed by cytokine-specific ELISPOT assays (32–34). Reverse transcriptase PCR (RT-PCR) was employed to evaluate the CFA/I-induced IL-4- and IFN-γ-specific mRNA by using cytokine-specific primers (32, 34). The sequences of the oligonucleotides employed were as follows: the IL-4-positive strand, AGA TCA TCG GCA TTT TGA ACG AGG TC, and IL-4-negative strand, CGA GTA ATC CAT TTG CAT GAT GCT C; and the IFN-γ-positive strand, ATA TCT AGA GGA ACT GGC, and IFN-γ-negative strand, TCT AGA CCT TAG GCT AGA TTC TGG. The PCR primers for murine β-actin were purchased (Clontech Laboratories, Inc., Palo Alto, Calif.). The expected sizes (in base pairs) for the cytokine cDNA products after PCR were 345 (IFN-γ), 306 (IL-4), and 349 (β-actin). Restimulated splenic CD4+ T cells, stained with fluorescein isothiocyanate-monoclonal anti-mouse CD4+ antibody (RM4-5; PharMingen), were sorted by flow cytometry and resuspended in Tri-Reagent (Molecular Research Center, Inc., Cincinnati, Ohio) to isolate total RNA (34). To quantify relative amounts of IL-4 and IFN-γ mRNA, the amplified products from the RT-PCR reactions were evaluated by capillary electrophoresis (CE) with a laser-induced fluorescence system (P/ACE 5000; Beckman Instruments, Fullerton, Calif.) (19, 31, 33), and cytokine values were normalized to the corresponding β-actin mRNA (33).
Statistical analysis.
The Student t test was used to evaluate differences between variations in antibody titers. Analysis of variance and the Tukey test were used to compare cytokine production levels.
RESULTS
Oral delivery of Salmonella-CFA/I vaccine induces elevated IgA Ab responses to CFA/I fimbriae, whereas an attenuated E. coli construct is ineffective.
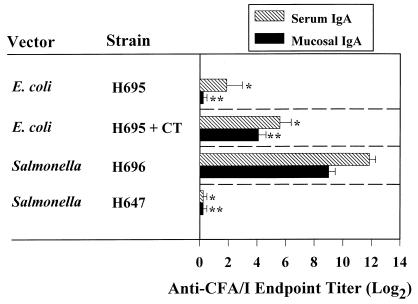
Immunity to ETEC requires stimulation of mucosal inductive sites of the GALT to elicit protective S-IgA Abs (1–3, 8, 17, 18, 22). Previous studies involving human volunteers (7) or experimental animals (6, 27) have shown that oral immunization with intact or encapsulated CFA fimbriae induced poor IgA antibody responses. This has been shown to be in part due to the alteration of fimbrial antigens upon exposure to low pH in the GI tract (29). As such, we attempted to mimic the normal course of fimbrial antigen presentation without producing enterotoxigenic disease by orally immunizing BALB/c mice with an engineered E. coli H695 construct expressing CFA/I fimbriae. A single oral immunization with this construct at 5 × 109 CFU failed to elicit S-IgA and very weak systemic IgA responses (Fig. 1). Likewise, an additional oral immunization with H695 failed to further enhance the IgA anti-CFA/I Ab responses (data not shown). This evidence clearly shows that the E. coli-CFA/I H695 construct was ineffective in stimulating host GI mucosal inductive tissues, suggesting that the CFA/I fimbriae may have been altered by the host GI tract. Alternatively, fimbrial antigens may not have reached the appropriate inductive T and B cells to elicit an immune response. Thus, we questioned whether fimbrial antigens, in the presence of the strong mucosal adjuvant CT, would be able to reach the mucosal inductive tissues for the promotion of antigen-specific S-IgA Ab responses. To test this hypothesis, BALB/c mice received two oral immunizations on days 0 and 14 with E. coli-CFA/I construct in conjunction with CT. Two oral immunizations were required, since no detectable S-IgA anti-CFA/I fimbrial responses could be detected after a single oral immunization (data not shown). Despite the inclusion of CT adjuvant during oral immunization, only weak S-IgA anti-CFA/I fimbria Ab responses, 17.8 ± 1.4 (n = 10; Fig. 1) were obtained, while the S-IgA anti-CT-B response was unabated. Likewise, weak serum IgA responses could be detected (Fig. 1). This experimental evidence shows that CFA/I fimbriae, when presented by this E. coli vector, stimulate poor S-IgA responses, possibly as a result of fimbrial alteration by the GI tract, the fimbrial antigen being unable to reach host mucosal inductive tissues, or a combination of both. Regardless, it is imperative that CFA/I fimbriae can reach the intestinal inductive sites for the development of mucosal IgA Abs.
FIG. 1.
A single oral immunization with the Salmonella-CFA/I construct elicits elevated mucosal and serum IgA Ab responses to CFA/I fimbriae, while an attenuated E. coli elicits poor mucosal and serum IgA Ab responses. BALB/c mice were vaccinated with a single dose of designated live Salmonella vector with (strain H696) or without the cfaABCE insert (strain H647) or with a single oral dose of live E. coli-CFA/I vector (strain H695). A single dose of live E. coli-CFA/I vector failed to elicit a mucosal IgA response. The addition of CT along with the E. coli-CFA/I vector failed to elicit a mucosal IgA response 2 weeks after oral immunization. Consequently, an additional oral dose of E. coli-CFA/I with CT was administered, and an adjuvant effect was observed. Fecal and serum IgA anti-CFA/I Ab titers (log2) were measured by ELISA. Each value represents the mean of 10 mice ± the standard error of the mean. ∗ and ∗∗, where P < 0.001, represent the statistical differences in serum and mucosal IgA anti-CFA/I fimbria levels, respectively, between mice orally immunized with the Salmonella-CFA/I construct and other indicated vaccine strains.
To generate S-IgA anti-CFA/I fimbria responses that would be needed for protection, an attenuated S. typhimurium strain was developed that expressed the CFA/I fimbriae, thereby mimicking the expression of this vaccine antigen by human ETEC (35) as well as being capable of targeting the GI mucosal inductive tissues (4, 5, 28). To test the delivery of CFA/I fimbriae by Salmonella to the host, an S. typhimurium-CFA/I vaccine (strain H696) and a vector control (strain H647) was administered intragastrically to individual groups of BALB/c mice to determine the immunogenicity of the CFA/I fimbriae in strain H696. At 4 weeks after oral immunization with a single dose of 5 × 109 CFU of Salmonella-CFA/I or Salmonella control, fecal and serum IgA anti-CFA/I Ab responses were evaluated (Fig. 1). A fecal IgA titer averaging 512 ± 105 (n = 10) was obtained, while the control S. typhimurium strain lacking CFA/I expression (H647) did not induce measurable S-IgA Abs to CFA/I fimbriae (Fig. 1). IgA anti-CFA/I fimbria Ab-forming cells were detected in the lamina propria of mice vaccinated with H696 construct (35), supporting the notion that the observed fecal IgA Abs was produced locally in the GI tract. Serum IgA anti-CFA/I-specific titers of 3,840 ± 477 were also noted. No specific serum anti-CFA/I Abs were induced by the isogenic strain H647 (Fig. 1). Thus, this evidence shows that a single oral immunization with the S. typhimurium H696 vaccine vector was able to appropriately stimulate GI mucosal inductive tissues without the aid of additional mucosal adjuvants. In addition, to verify that CFA/I expression did not overtly alter the colonization of our recombinant vector strain, CFU were enumerated to determine degree of colonization by both Salmonella vectors in the Peyer's patches and spleen. No statistical differences in the extent of colonization were observed (data not shown). Thus, the expression of CFA/I fimbriae did not appear to quantitatively affect the initial stage of Salmonella attachment and entry.
Induction of Th2 cell responses after oral immunization with Salmonella-CFA/I construct.
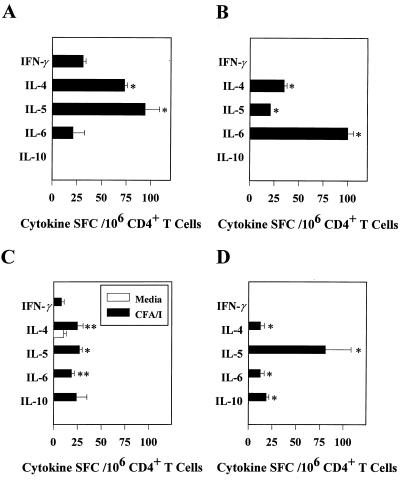
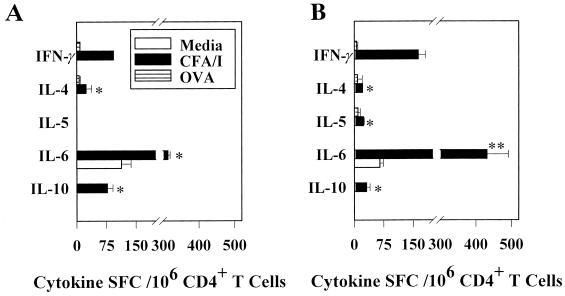
The strength of the S-IgA anti-CFA/I fimbriae responses prompted us to evaluate the CD4+ T-cell cytokine expression patterns. Based on previously reported findings, one would anticipate little or no IL-4-secreting CD4+ T cells and elevated numbers of IFN-γ-secreting CD4+ T cells after oral vaccination with Salmonella (21, 23, 26, 32, 35, 36). However, the kinetics and magnitude of the CFA/I-specific S-IgA Ab response suggested that IL-4 and other Th2-type, IgA-enhancing cytokines (IL-5 and IL-6) might be driving the response. Accordingly, CD4+ T cells from immune BALB/c mice orally immunized with the Salmonella-CFA/I construct were assessed by cytokine-specific ELISPOT assays 1 week after immunization for their ability to produce Th1 and Th2 cytokines. By using freshly isolated CD4+ T cells, the number of splenic IL-4 spot-forming cells (SFC) exceeded the number of IFN-γ SFC by 2.4-fold (Fig. 2A). Moreover, the presence of IL-5 SFC was substantially elevated (94 ± 14.7/106 CD4+ T cells; Fig. 2A). In the Peyer's patches, only Th2-type cytokines and no IFN-γ SFC were detected (Fig. 2B). This result provides evidence for a predominance of Th2 cell-promoting cytokines during the early immune response after oral immunization with the Salmonella-CFA/I construct. However, these cytokine profiles probably represent the summation of Th cell responses to CFA/I fimbrial and Salmonella antigens.
FIG. 2.
Oral immunization with the Salmonella-CFA/I vaccine promotes the induction of Th2 cells in Peyer's patch and splenic CD4+ T-cell populations. Freshly isolated splenic (A) and Peyer's patch (B) CD4+ T cells harvested 1 week after vaccination were examined for cytokine secretion by the cytokine ELISPOT method. Values are expressed as the average number of SFC/106 CD4+ T cells from quadruplicate cultures ± the standard deviation (SD). Values were corrected for spontaneous release by cells obtained for normal, uninfected mice. A representative example from three separate experiments is shown. Splenic and Peyer's patch IL-4 and IL-5 SFC were significantly greater in number when compared to IFN-γ SFC (∗, P < 0.001). No IL-10-producing cells were detected. In addition, Peyer's patch IL-6 SFC were significantly elevated (∗, P < 0.001). Splenic (C) and Peyer's patch (D) CD4+ T cells obtained 1 week after oral immunization of mice with strain H696 were cultured for 2 days with or without CFA/I fimbriae. These cells were harvested and evaluated for Th1 and Th2 cytokine production by the cytokine ELISPOT method. A representative example from three separate experiments is shown. Values are expressed as the average number of SFC/106 CD4+ T cells from quadruplicate cultures ± the SD. Increases in the numbers of IL-4-, IL-5-, and IL-6-producing T cells and minimal IFN-γ-producing T cells were noted compared to unstimulated cells. The numbers of splenic IL-4 and IL-6 (∗∗, P < 0.011), as well as IL-5 SFC (∗, P < 0.001) significantly exceeded splenic IFN-γ SFC; the number of Peyer's patch IL-4, IL-5, IL-6, and IL-10 (∗, P < 0.001) significantly exceeded the numbers of Peyer's patch IFN-γ SFC.
To substantiate that the in vivo T-cell responses were indeed due to CFA/I-specific Th2 cells, immune CD4+ T cells obtained 1 week after immunization of BALB/c mice with the Salmonella-CFA/I construct were cultured for 2 days with or without CFA/I fimbriae and were then evaluated for cytokine production by the ELISPOT assay. Both splenic and Peyer's patch CD4+ T cells exhibited a predilection for Th2 cell cytokine production, as evidenced by the production of IL-4, IL-5, IL-6, and IL-10 (Fig. 2C and D). Minimal or no IFN-γ SFC, especially in the Peyer's patches, were detected following in vitro restimulation with CFA/I fimbriae. Collectively, the data indicate that the expression of CFA/I fimbriae by Salmonella favors the early development of Th2-type responses and that such Th2 cytokines might account for the robust S-IgA Ab responses to CFA/I fimbriae.
Oral immunization with Salmonella-CFA/I construct elicits elevated serum IgG1 Abs.
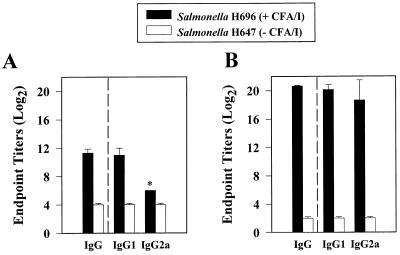
Vaccine delivery by Salmonella vectors typically induces IgG2a-dominated serum Ab responses to passenger antigens, presumably as the result of the Salmonella inducing cytokines characteristic of Th1 cell responses (21, 23, 26, 32). On the basis of the array of cytokines known to support IgG1 and IgA Ab responses, we determined the levels of antigen-specific IgG and IgG subclass responses that were induced by the Salmonella-CFA/I vaccine. As noted above, this construct was particularly effective in generating elevated circulating IgG anti-CFA/I Ab titers (Fig. 3). In contrast to what others have observed (14, 30, 32, 41), elevated serum IgG1 antigen-specific titers were equivalent to IgG2a titers found 4 weeks after oral immunization (Fig. 3B). This serum IgG1 anti-CFA/I titer rose prior to the IgG2a titer shortly after immunization (by 1 week) and exceeded the IgG2a Ab titer by 32-fold (Fig. 3A). Collectively, this evidence corroborates the Th cell subset analysis showing that this construct induces the development of Th2 cells, which in turn, supports IgG1 and IgA anti-CFA/I Ab responses.
FIG. 3.
(A) Antigen-specific serum IgG1 Ab responses increased 1 week after oral immunization with the Salmonella-CFA/I vaccine. (B) While serum IgG2a CFA/I-specific Ab responses are delayed, they become equivalent to serum IgG1 titers by 4 weeks. Serum IgG, IgG1, and IgG2a titers (log2) were determined by using the ELISA procedure. No serum IgG titers against CFA/I fimbriae were elicited in mice orally immunized with the isogenic, H647 construct (which lacks the cfaABCE insert). Values represent the average of duplicate samples from each of 10 mice/group. ∗, P < 0.001.
Consistent with the stimulation of similar Th2-type responses, BALB/c mice orally immunized with the E. coli H695 (Fig. 4A) or in conjunction with CT (Fig. 4B) showed enhanced CFA/I-specific IgG1 titers. Total serum IgG anti-CFA/I titers were enhanced 75-fold upon coimmunization with CT but still remained ∼420-fold less than those obtained with the Salmonella-CFA/I H696 construct. Although the addition of CT during oral immunization with the E. coli-CFA/I construct could enhance serum IgG anti-CFA/I fimbrial responses, as with the mucosal IgA responses, Salmonella was a more effective vaccine vehicle by as shown by its stimulation of both mucosal and systemic antibody responses.
FIG. 4.
(A) Oral immunization with the E. coli-CFA/I H695 construct failed to elicit elevated serum IgG antibodies against CFA/I fimbriae. A group of 10 BALB/c mice received a single oral dose of the H695 construct, and serum IgG, IgG1, and IgG2a antibody titers were measured by an ELISA method. At 4 weeks after immunization, minimal IgG antibody titers against CFA/I fimbriae were induced, and these antibodies were predominantly IgG1. (B) Oral immunization with the E. coli-CFA/I H695 construct in conjunction with CT on days 0 and 14 resulted in enhanced IgG anti-CFA/I titers. A group of 10 BALB/c mice received a single oral immunization with H695 plus CT showed no IgG antibodies against CFA/I fimbriae 2 weeks after a single oral dose. However, subsequent to receiving an additional oral dose of H695 plus CT resulted in induced CFA/I fimbria-specific IgG, IgG1, and IgG2a antibodies, albeit not as elevated as with the Salmonella-CFA/I H696 construct.
Th1 and Th2 cytokines are induced 4 weeks after oral immunization with Salmonella-CFA/I construct.
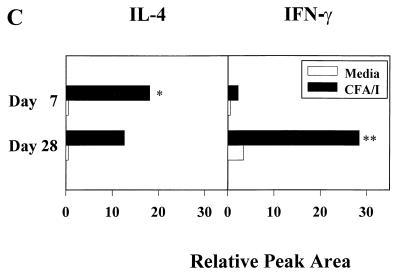
Since the pattern of the serum IgG subclass response markedly changed 4 weeks after oral immunization with the Salmonella-CFA/I construct, as demonstrated by an increase in IgG2a anti-CFA/I fimbria titers, it seemed plausible that Th1 cells may develop later in the vaccinated mice. To determine the cytokines produced by immune CD4+ Th cells that supported the CFA/I-specific IgG2a Ab production, enriched CD4+ T cells were obtained from BALB/c mice 4 weeks after oral immunization with the Salmonella-CFA/I vaccine. These cells were cultured as described above and analyzed by the cytokine ELISPOT assays to quantify the CFA/I-specific cytokine responses (Fig. 5). Immune CD4+ T cells cultured in media alone produced only IL-6 with no detectable IL-4, IL-5, IL-10, or IFN-γ. Increased levels of splenic CFA/I-specific IL-6 (313 ± 6)- and IL-10 (77 ± 14)-producing T cells were also noted compared to unstimulated cells. No IL-5 SFC responses were detected (Fig. 5A); this lack of IL-5 expression was confirmed by ELISA (data not shown). Thus, 4 weeks after vaccination, the levels of Th2-promoting cytokines diminished and the numbers of IFN-γ-producing cells increased. The data suggest that IFN-γ supported the increase in CFA/I-specific IgG2a Abs that developed subsequent to the CFA/I-specific IgG1 response observed 1 week after immunization.
FIG. 5.
Four weeks after oral immunization with the Salmonella-CFA/I vaccine, a predominance of IFN-γ-producing CD4+ Th cells was detected by the cytokine ELISPOT method. Splenic (A) and Peyer's patch (B) CD4+ T cells were cultured for 2 days in medium only, with 10 μg of CFA/I fimbriae, per ml, or with 10 μg of OVA per ml, after which CD4+ T cells were evaluated for Th1 and Th2 cytokine production. Representative data from a total of three experiments are shown. Values are expressed as the average number of SFC/106 CD4+ T cells from quadruplicate cultures ± the SD. Significantly more splenic IFN-γ SFC were detected than IL-4, IL-5, and IL-6 SFC (P < 0.001). Likewise, significantly more Peyer's patch IFN-γ SFC were detected than IL-4, IL-5, and IL-10 SFC ∗, P < 0.001) but less than IL-6 SFC (∗∗, P < 0.002). (C) CE analysis of RT-PCR amplified IL-4 and IFN-γ mRNA isolated from splenic CD4+ T cells harvested from BALB/c mice 1 or 4 weeks after oral immunization with the Salmonella-CFA/I vaccine. Two groups of mice (five/group) were orally immunized with 5 × 109 CFU of Salmonella-CFA/I vaccine; the mice were sacrificed either 1 or 4 weeks postimmunization. Splenic CD4+ T cells were isolated and cultured with irradiated T-cell-depleted splenocytes for 2 days with or without 10 μg of CFA/I antigen per ml. After culture, CD4+ T cells were isolated by flow cytometry and evaluated for IL-4 and IFN-γ mRNA expression by RT-PCR. The results are expressed as the percent increase in cytokine-specific messages relative to the β-actin mRNA level (taken to be 100) according to the peak areas obtained by CE analysis. The results are representative of three separate experiments. ∗, P < 0.001, amplified IL-4 mRNA is significantly greater than IFN-γ amplified mRNA; ∗∗, P = 0.003, amplified IFN-γ mRNA is significantly greater than amplified IL-4 mRNA.
Changes in cytokine patterns for Peyer's patch CD4+ T cells were also observed when compared to the CD4+ T cells from 1-week immunized mice. Although no CFA/I-specific IFN-γ-producing CD4+ T cells were detected 1 week after immunization, by 4 weeks postimmunization, significant numbers of CFA/I-specific IFN-γ-producing CD4+ T cells were evident (Fig. 5B). In fact, the numbers of CFA/I-specific IFN-γ-producing T cells exceeded CFA/I-specific IL-4-producing T cells by seven- to eightfold at this later time point. Minimal IL-5- and IL-10-producing CD4+ T cells were noted; however, an increase in the number of CFA/I-specific IL-6-producing T cells was evident. Taken together, the increased presence of CD4+ Th1 cells in both the spleen and Peyer's patches at 4 weeks postimmunization suggests that the initial Th2 response shifted with time to a Th1 response.
From the cytokine secretion analysis, the levels of CFA/I-stimulated CD4+ T cells clearly demonstrate a time-dependent difference in the numbers of IL-4- and IFN-γ-producing cells between 1 and 4 weeks after oral immunization with the Salmonella-CFA/I vaccine, most notably in the systemic immune compartment. To further substantiate the basis for the systemic production of CFA/I-specific IgG1 versus IgG2a Ab levels, RT-PCR was employed to examine IL-4 and IFN-γ mRNA that were induced upon in vitro restimulation of immune CD4+ T cells. Splenic CD4+ T cells taken from mice 1 or 4 weeks postimmunization with the Salmonella-CFA/I construct were cultured for 2 days in the presence or absence of CFA/I fimbriae and then stained with fluorochrome-conjugated anti-CD4 Ab for the isolation of CD4+ T cells by flow cytometry. Total RNA was extracted from these cells, quantified, and subjected to RT-PCR by using primer pairs specific for IFN-γ, IL-4, and β-actin. PCR-generated products were evaluated on agarose gels and quantified by CE (Fig. 5C). This analysis showed that 1 week after vaccination with the Salmonella-CFA/I construct, increased levels of IL-4 mRNA and minimal IFN-γ mRNA were detected (Fig. 5C), supporting the notion that our Salmonella-CFA/I construct elicited IL-4-secreting CD4+ T cells. This response differs from previously reported T-cell responses to Salmonella-delivered passenger antigens (14, 21, 23, 26, 30, 32, 41). When a similar analysis was performed with CD4+ T cells obtained from mice immunized 4 weeks earlier with the Salmonella-CFA/I construct, the converse was observed. These cells displayed decreased levels of IL-4 and increased levels of IFN-γ mRNA (Fig. 5C). Collectively, this evidence demonstrates that oral administration of Salmonella-CFA/I vaccine elicits transient, early induction of antigen-specific Th2 cells which later develops into more of a Th1-dependent response. This unique switching in Th cell phenotype may represent a mode to overcome Th1-type biases by using Salmonella vaccine delivery and, as a consequence, limit the duration of Th2 cell responses.
Cytokine profiles by CFA/I fimbria-specific CD4+ T cells after oral immunization with E. coli-CFA/I construct.
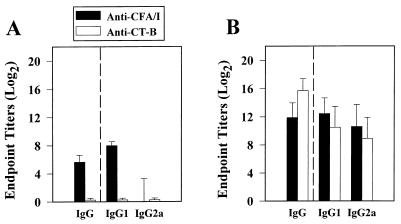
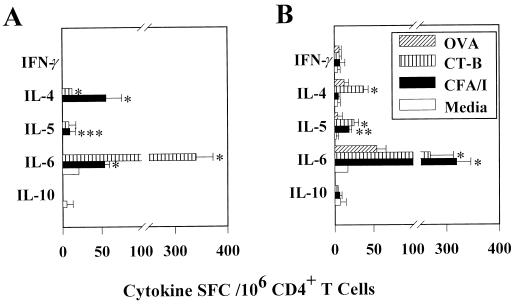
To determine which Th cell cytokines were important for supporting the anti-CFA/I fimbrial antibody responses after oral immunization with the E. coli-CFA/I H695 construct in conjunction with CT, the cytokine ELISPOT assay was employed. Th2-type cytokines were induced after in vitro stimulation with CFA/I fimbriae, as evidenced by increases in splenic and Peyer's patches IL-4-, IL-5-, and IL-6-producing CD4+ T cells compared to unstimulated or OVA-stimulated cells (Fig. 6). No CFA/I fimbria-specific IFN-γ-producing cells were evident. Likewise, the CD4+ anti-CT-B T-cell responses were also dominated by Th2-type cytokines compared to unstimulated or OVA-stimulated cells (Fig. 6). As expected (37, 38), no CT-B-specific induction of IFN-γ was evident (Fig. 6). This evidence further supports the notion for the importance of Th2 cell-dependent immunity against ETEC (1–3, 8, 17, 18, 22). Thus, oral immunization with a CFA/I fimbria-expressing E. coli was not as effective for stimulating antifimbrial B and T cells as the Salmonella-CFA/I construct.
FIG. 6.
At 4 weeks after oral immunization with the E. coli-CFA/I construct, a predominance of CD4+ Th2 cells was detected by the cytokine ELISPOT method. Splenic (A) and Peyer's patch (B) CD4+ T cells were cultured for 2 days in medium only, with CFA/I fimbriae, with CT-B, or with OVA, after which CD4+ T cells were evaluated for Th1 and Th2 cytokine production. Depicted are representative data from a total of three experiments. Values are expressed as the average number of SFC/106 CD4+ T cells from quadruplicate cultures ± the SD. Significantly more CFA/I-specific splenic IL-4, IL-5, and IL-6 SFC were detected than IFN-γ SFC; significantly more CT-B-specific IL-4 and IL-6 SFC were found than IFN-γ SFC. Splenic CD4+ T cells cultured in medium only or with OVA showed minimal cytokine induction. For the Peyer's patches, CFA/I fimbria restimulation induced significantly more IL-5 and IL-6 SFC, while CT-B restimulation significantly induced more IL-4, IL-5, and IL-6 SFC compared to the number of IFN-γ SFC induced. Some IL-6 was induced by OVA, but for the remaining cytokines minimal induction was detected. ∗, P ≤ 0.001; ∗∗, P = 0.006; ∗∗∗, P = 0.018.
DISCUSSION
To underscore the importance of humoral immunity in protection from ETEC, we devised a mucosal vaccine that mimics the invasive properties of this pathogen capable of eliciting strong humoral immunity. A Salmonella-based vaccine was designed because of the effectiveness of this vehicle for delivering vaccines to inductive sites in the GI tract for appropriate stimulation of mucosal B- and T-cell subsets. Consequently, we showed that a single dose of a S. typhimurium-CFA/I vaccine was sufficient to elicit elevated S-IgA and systemic IgG Ab responses to CFA/I fimbriae due to the initial induction of a dominant Th2-type response. The effectiveness of this vaccine in inducing elevated anti-CFA/I fimbria Abs becomes strikingly apparent when compared to the inability of an attenuated E. coli-CFA/I construct to elicit a mucosal response. Moreover, coimmunization with CT, only induced a weak mucosal IgA anti-CFA/I response. This evidence is consistent with past studies, where repeated oral administrations of CFA fimbriae failed to elicit elevated Ab titers (6, 7, 27). Thus, it is apparent that effective targeting of the CFA/I fimbriae to the Peyer's patches is needed to obtain mucosal and enhanced systemic Ab responses.
At mucosal surfaces the seminal event that results in either noninvasive or invasive infection involves attachment to the apical surface of the mucosal epithelium. Protection at mucosal surfaces involves both Th2-type cytokines, which favor the optimal development of S-IgA Ab responses to prevent host attachment and entry, and Th1-promoting cytokines to clear intracellular infections (9, 13). Oral delivery of soluble proteins in the presence of mucosal adjuvants, e.g., a wild-type or mutant CT, favors the development of Th2-promoting cytokines and S-IgA Ab responses (15, 20, 25, 37–40). In contrast, oral delivery of passenger antigens by an attenuated Salmonella vector, which retains the ability to invade and persist intracellularly, results in the development of Th1-promoting cytokines and cell-mediated effector responses (21, 23, 32, 35, 36). The data presented here provide for an alternative mechanism for the development of mucosal immunity involving a biphasic Th cell response. A response of this latter type may allow the host to provide immunity against pathogens that require both Th-type responses such as rotavirus and human immunodeficiency virus. Moreover, the results presented here suggest that mucosal vaccine formulations can be devised that elicit the development of both humoral and cell-mediated arms of mucosal immunity. This type of immunity would provide defense at both pre- and postentry stages of a pathogenic cycle.
The cellular basis for such a biphasic response may be in part due to the summation of two competing pathways: immunity to the extracellular fimbriae and immunity to the intracellular Salmonella. Evidence presented in this study supports the notion that the initial response was heavily influenced by the fimbriae, since a predominance of CD4+ Th2 cells developed. This initial response was supported by the production of IL-4 and concomitant expression of IgG1 and IgA anti-CFA/I Abs. In fact, no CFA/I-specific IFN-γ-producing T cells were noted in the Peyer's patches, and the CD4+ T cells in the immune compartment exhibited a Th2 cell bias. In addition, in the splenic compartment during this early phase, splenic CFA/I-specific IL-4-producing T cells exceeded IFN-γ-producing T cells by a three-to-one ratio. In this regard, the CFA/I fimbriae may be mimicking soluble antigen, resulting in a Th2 cell predominance. However, later (by 4 weeks) in the immune response both mucosal and systemic immune compartments exhibited a predominance of CD4+ Th1 cells, as shown by a fourfold excess of CFA/I-specific IFN-γ-producing T cells compared to that of IL-4-producing T cells. Further, IL-5-secreting CD4+ T cells could not be detected by 4 weeks postimmunization either by IL-5 ELISPOT assay or by RT-PCR of IL-5 mRNA, whereas IL-5 SFC and mRNA were detected 1 week after oral immunization. This phenotypic divergence in the late stage of the Th cell response suggests that the CFA/I fimbriae enter antigen presentation pathways that are similar to those normally accessed by somatic Salmonella antigens. Alternatively, in order to clear the Salmonella vector from its intracellular niche, which is dependent on the development of IFN-γ-producing CD4+ T cells (13, 33), the late-stage immune events become predominated by IFN-γ. Moreover, our data showed elevated CFA/I-specific IgG1 Ab titers, in contrast to previous reports where IgG2a Ab titers dominated the immune response to Salmonella-delivered passenger antigens (14, 30, 35). In fact, the Ab response to CFA/I fimbriae in this study appeared to mimic those generated by adjuvant-boosted oral immunization with soluble subunit antigens, which favor the development of Th2-promoting cytokines and the manifestation of strong S-IgA Ab responses (15, 37, 38).
The observation that a biphasic Th cell response can be generated by using a Salmonella-CFA/I vector opens new avenues for the development of novel mucosal vaccine formulations that exploit this unusual vector construct. Such a shift in Th cell immunity has important implications for human immunity, where Th cell responses are not an all-or-nothing paradigm but rather are mixed Th cell phenotypes (39, 40). Further understanding of the mechanisms that provide the Th2-promoting properties of this vector construct may allow us to design Salmonella vectors that are more effective at generating protective S-IgA Ab responses against other GI pathogens such as Shigella spp., Yersinia spp., and Vibrio cholerae. Furthermore, the development of antigen formulations that mimic the Salmonella-CFA/I vector and thereby induce biphasic mucosal Th cell responses will facilitate the development of a vaccine against human immunodeficiency virus type 1 infection.
ACKNOWLEDGMENTS
We thank Ed C. Boedeker for his review and comments regarding this manuscript and Jamie Bieber for her expert technical assistance.
This work was supported by National Institutes of Health grants AI-41123, AI-18958, DE-44240, DE-09837, AI-35932, AI-35544, DE-08228, AI-41914, AI-42603, and AI-38192, DMID-NIAID contract AI-65299, and S10 RR 11877 and in part by The Montana Agricultural Station.
REFERENCES
- 1.Acres S D, Isaacson R E, Babiuk L A, Kapitany R A. Immunization of calves against enterotoxigenic colibacillosis by vaccinating dams with purified K99 antigen and whole cell bacterins. Infect Immun. 1979;25:121–126. doi: 10.1128/iai.25.1.121-126.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black R E, Brown K H, Becker S, Alim A, Huq I. Longitudinal studies of infectious diseases and physical growth of children in rural Bangladesh: II. Incidence of diarrhea and association with known pathogens. Am J Epidemiol. 1982;115:315–324. doi: 10.1093/oxfordjournals.aje.a113308. [DOI] [PubMed] [Google Scholar]
- 3.Black R E. The epidemiology of cholera and enterotoxigenic Escherichia coli diarrheal disease. In: Holmgren J, Lindberg A, Molby R, editors. Development of Vaccines and Drugs Against Diarrhea. Stockholm, Sweden: Studentlitteratur; 1986. pp. 23–32. [Google Scholar]
- 4.Curtiss R, Kelly S M, Gulig P A, Nakayama K. Selective delivery of antigens by recombinant bacteria. Curr Top Microbiol Immunol. 1989;146:34–49. doi: 10.1007/978-3-642-74529-4_4. [DOI] [PubMed] [Google Scholar]
- 5.Curtiss R, Kelly S M, Hassan J O. Live oral avirulent Salmonellavaccines. Vet Microbiol. 1993;37:397–405. doi: 10.1016/0378-1135(93)90038-9. [DOI] [PubMed] [Google Scholar]
- 6.Edelman R, Russell R G, Losonsky G, Tall B D, Tacket C O, Levine M M, Lewis D H. Immunization of rabbits with enterotoxigenic E. colicolonization factor antigen (CFA/I) encapsulated in biodegradable microspheres of poly (lactide-co-glycolide) Vaccine. 1993;11:155–158. doi: 10.1016/0264-410x(93)90012-m. [DOI] [PubMed] [Google Scholar]
- 7.Evans D G, Graham D Y, Evans D J Jr, Opekun A. Administration of purified colonization factor antigens (CFA/I, CFA/II) of enterotoxigenic Escherichia colito volunteers. Gastroenterology. 1984;87:934–940. [PubMed] [Google Scholar]
- 8.Evans D J, Jr, Evans D G. Colonization factor antigens of human pathogens. Curr Top Microbiol Immunol. 1990;151:129–145. doi: 10.1007/978-3-642-74703-8_7. [DOI] [PubMed] [Google Scholar]
- 9.Ferlin W G, von der Weid T, Cottrez F, Ferrick D A, Coffman R L, Howard M C. The induction of a protective response in Leishmaniamajor-infected BALB/c mice with anti-CD40 mAb. Eur J Immunol. 1998;28:525–531. doi: 10.1002/(SICI)1521-4141(199802)28:02<525::AID-IMMU525>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 10.Fouts T R, Tuskan R G, Chada S, Hone D M, Lewis G K. Construction and immunogenicity of Salmonella typhimuriumvaccine vectors that express HIV-1 gp120. Vaccine. 1995;13:1697–1705. doi: 10.1016/0264-410x(95)00106-b. [DOI] [PubMed] [Google Scholar]
- 11.Girón J A, Xu J-G, González C R, Hone D, Kaper J B, Levine M M. Simultaneous expression of CFA/I and CS3 colonization factor antigens of enterotoxigenic Escherichia coli by ΔaroC, ΔaroD Salmonella typhivaccine strain CVD 908. Vaccine. 1995;13:939–946. doi: 10.1016/0264-410x(95)00003-j. [DOI] [PubMed] [Google Scholar]
- 12.Hall R, Maneval D R, Jr, Collins J H, Theibert J L, Levine M M. Purification and analysis of colonization factor antigen 1, coli surface antigen 1, and coli surface antigen 3 fimbriae from enterotoxigenic Escherichia coli. J Bacteriol. 1989;171:6372–6374. doi: 10.1128/jb.171.11.6372-6374.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hess J, Ladel C, Miko D, Kaufmann S H. Salmonella typhimurium aroA-infection in gene-targeted immunodeficient mice: major role of CD4+TCR-αβ cells and IFN-γ in bacterial clearance independent of intracellular location. J Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 14.Hohmann E L, Oletta C A, Loomis W P, Miller S I. Macrophage-inducible expression of a model antigen in Salmonella typhimuriumenhances immunogenicity. Proc Natl Acad Sci USA. 1995;92:2904–2908. doi: 10.1073/pnas.92.7.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hone D M, Tacket C O, Harris A H, Kay B, Losonsky G, Levine M M. Evaluation in volunteers of a candidate live oral attenuated Salmonella typhivector vaccine. J Clin Investig. 1992;90:412–420. doi: 10.1172/JCI115876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson R J, Fujihashi K, Xu-Amano J, Kiyono H, McGhee J R. Optimizing oral vaccines: induction of systemic and mucosal B cell and antibody responses to tetanus toxoid by use of cholera toxin as an adjuvant. Infect Immun. 1993;61:4272–4279. doi: 10.1128/iai.61.10.4272-4279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine M, Morris J G, Losonsky G, Boedeker E C, Rowe B. Fimbriae (pili) adhesins as vaccine. In: Lark D L, Normark S, Uhlin B-E, Wolf-Watz H, editors. Protein-carbohydrate interactions in biological systems: molecular biology of microbial pathogenicity. London, England: Academic Press; 1986. pp. 143–145. [Google Scholar]
- 18.Levine M M, Girón J A, Noriega F R. Fimbrial vaccines. In: Klemm P, editor. Fimbriae: adhesions, biogenics, genetics, and vaccines. Boca Raton, Fla: CRC Press; 1994. pp. 255–270. [Google Scholar]
- 19.Lu W, Han D-S, Yuan J, Andrieu J-M. Multi-target PCR analysis by capillary electrophoresis and laser-induced fluorescence. Nature. 1994;368:269–271. doi: 10.1038/368269a0. [DOI] [PubMed] [Google Scholar]
- 20.McGhee J R, Kiyono H. New perspectives in vaccine development: mucosal immunity to infections. Infect Agents Dis. 1993;2:55–73. [PubMed] [Google Scholar]
- 21.Mastroeni P, Villareal-Ramos B, Hormaeche C E. Role of T cells, TNF-α and IFN-γ in recall of immunity to oral challenge with virulent Salmonellae in mice vaccinated with live attenuated aro− Salmonellavaccines. Microb Pathog. 1992;13:477–491. doi: 10.1016/0882-4010(92)90014-f. [DOI] [PubMed] [Google Scholar]
- 22.Nagy B. Vaccination of cows with a K99 extract to protect newborn calves against experimental enterotoxic colibacillosis. Infect Immun. 1980;27:21–24. doi: 10.1128/iai.27.1.21-24.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okahashi N, Yamamoto M, VanCott J L, Chatfield S N, Roberts M, Bluethmann H, Hiroi T, Kiyono H, McGhee J R. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect Immun. 1996;64:1516–1525. doi: 10.1128/iai.64.5.1516-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pascual D W, McGhee J R, Kiyono H, Bost K L. Neuroimmune modulation of lymphocyte function: I. Substance P enhances immunoglobulin synthesis in LPS activated murine splenic B cell cultures. Int Immunol. 1991;3:1223–1229. doi: 10.1093/intimm/3.12.1223. [DOI] [PubMed] [Google Scholar]
- 25.Pascual D W, Kiyono H, McGhee J R. Mucosal immunity: molecular and cellular aspects of immune protection to enteric infections. In: Paradise L J, et al., editors. Enteric infections and immunity. New York, N.Y: Plenum Press; 1996. pp. 15–35. [Google Scholar]
- 26.Ramarathinam L, Niesel D W, Klimpel G R. Salmonella typhimuriuminduces IFN-γ production in murine splenocytes: role of natural killer cells and macrophages. J Immunol. 1993;150:3973–3981. [PubMed] [Google Scholar]
- 27.Reid R H, Boedeker E C, McQueen C E, Davis D, Tseng L-Y, Kodak J, Sau K, Wilhelmesen C L, Nellore R, Dalal P, Bhagat H R. Preclinical evaluation of microencapsulated CFA/II oral vaccine against enterotoxigenic E. coli. Vaccine. 1993;11:159–167. doi: 10.1016/0264-410x(93)90013-n. [DOI] [PubMed] [Google Scholar]
- 28.Roberts M, Chatfield S N, Dougan G. Salmonella as carriers of heterologous antigens. In: O'Hagen D T, editor. Novel delivery systems for oral vaccines. Boca Raton, Fla: CRC Press; 1994. pp. 27–58. [Google Scholar]
- 29.Schmidt M, Kelly E P, Tseng L Y, Boedeker E C. Towards an oral E. colipilus vaccine for travelers diarrhea: susceptibility to proteolytic digestion. Gastroenterology. 1985;88:A1575–A1582. [Google Scholar]
- 30.Schodel F, Milich D R, Will H. Hepatitis B virus nucleocapsid/pres-S2 fusion proteins expressed in attenuated Salmonellafor oral vaccination. J Immunol. 1990;145:4317–4321. [PubMed] [Google Scholar]
- 31.Srinivasan K, Girard J E, Williams P, Roby R K, Weedn V W, Morris S, Kline M C, Reeder D J. Electrophoretic separations of polymerase chain reaction-amplified DNA fragments in DNA typing using a capillary electrophoresis-laser induced fluorescence system. J Chromatogr. 1993;652:83–91. [Google Scholar]
- 32.VanCott J L, Staats H F, Pascual D W, Roberts M, Chatfield S N, Yamamoto M, Carter P B, Kiyono H, McGhee J R. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]
- 33.VanCott J L, Chatfield S N, Roberts M, Hone D M, Hohmann E, Pascual D W, Yamamoto M, Yamamoto S, Kiyono H, McGhee J R. Regulation of host immune responses by modification of Salmonellavirulence genes. Nat Med. 1998;4:1247–1252. doi: 10.1038/3227. [DOI] [PubMed] [Google Scholar]
- 34.van Ginkel F W, McGhee J R, Liu C G, Simecka J W, Yamamoto M, Frizzell R A, Sorscher E J, Kiyono H, Pascual D W. Adenoviral gene delivery elicits distinct pulmonary-associated T helper cell responses to the vector and to its transgene. J Immunol. 1997;159:685–693. [PubMed] [Google Scholar]
- 35.Wu S, Pascual D W, VanCott J L, McGhee J R, Maneval D R, Jr, Levine M M, Hone D M. Immune response to Escherichia coli and Salmonella typhimurium vectors that express colonization factor antigen I (CFA/I) of enterotoxigenic E. coli (ETEC) in the absence of the CFA/I positive regulator cfaR. Infect Immun. 1995;63:4933–4938. doi: 10.1128/iai.63.12.4933-4938.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S, Pascual D W, Lewis G K, Hone D M. Induction of mucosal and systemic responses against human immunodeficiency virus type-1 gp120 in mice after oral immunization with a single dose of a Salmonella-HIV vector. AIDS Res Hum Retroviruses. 1997;13:1187–1194. doi: 10.1089/aid.1997.13.1187. [DOI] [PubMed] [Google Scholar]
- 37.Xu-Amano J, Aicher W K, Taguchi T, Kiyono H, McGhee J R. Selective induction of Th2 cells in murine Peyer's patches by oral immunization. Intern Immunol. 1992;4:433–445. doi: 10.1093/intimm/4.4.433. [DOI] [PubMed] [Google Scholar]
- 38.Xu-Amano J, Kiyono H, Jackson R J, Staats H F, Fujihashi K, Burrows P D, Elson C O, Pillai S, McGhee J R. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto M, Briles D E, Yamamoto S, Ohmura M, Kiyono H, McGhee J R. A nontoxic adjuvant for mucosal immunity to pneumococcal surface protein A. J Immunol. 1998;161:4115–4121. [PubMed] [Google Scholar]
- 40.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Fujihashi K, van Ginkel F W, Noda M, Takeda Y, McGhee J R. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang D M, Fairweather N, Button L L, McMaster W R, Kahl L P, Liew F Y. Oral Salmonella typhimurium (AroA−) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990;145:2281–2285. [PubMed] [Google Scholar]