Abstract
Th1 immune responses, characterized by production of gamma interferon (IFN-γ), are associated with protective immunity to viruses and intracellular bacteria. Heat-killed Brucella abortus promotes secretion of Th1-inducing cytokines such as interleukin-12 (IL-12) and IFN-γ and has been used as a carrier to induce Th1 responses to vaccines. To explore which bacterial constituents could mediate this response and how it is regulated, murine spleen cells were cultured with B. abortus derived DNA, lipopolysaccharide (LPS), or whole killed organisms. Each constituent induced similar, substantial amounts of IL-10. However, only B. abortus and B. abortus DNA induced high levels of IFN-γ and IL-12. B. abortus and B. abortus DNA-stimulated IL-12 production was maximal by 6 to 18 h, while IL-10 production steadily accumulated over this time period. These kinetics suggested that IL-10 may eventually downmodulate the Th1-like cytokine response to B. abortus and B. abortus DNA, which was confirmed by using neutralizing antibody. In the absence of IL-10, B. abortus LPS induced strong IFN-γ responses, but IL-12 p70 levels were still undetectable from BALB/c spleen cells. LPS induced IL-12 if the spleen cells were primed with IFN-γ and IL-10 was neutralized, indicating that LPS can stimulate IL-12 production under the most favorable conditions. Responses to Escherichia coli LPS and DNA mirrored the responses to B. abortus components, suggesting that immune effects observed with these constituents may be generalizable to many microbial species. In vivo experiments demonstrated the same hierarchy of responses for IL-12 production. These findings support the likelihood that microbial components, if used as carriers or adjuvants, can differ substantially in their ability to effect a Th1 response.
Optimal immunity to viruses and intracellular bacteria is typically mediated by the Th1 subset of CD4+ T lymphocytes. Th1 cells are characterized by the production of IFN-γ, the ability to help CTL responses, and the promotion of complement-fixing antibody isotypes such as immunoglobulin G2a (4, 15, 39, 44). Interleukin-12, chiefly a product of antigen-presenting cells (APC), is usually critical for the development of Th1 responses (28, 36, 54). In contrast, Th2 cells, which produce interleukin-4 (IL-4), IL-5, IL-6, and IL-10, mediate allergic and some antiparasitic responses (15, 17, 18). Ineffective containment of viral infections, such as human immunodeficiency virus, is correlated with Th2-like responses (10, 55). Thus, antiviral vaccine strategies need to focus upon adjuvants which steer the immune response in a Th1 direction. Intense interest is being directed toward the use of bacterial derivatives which promote Th1-like responses. These include monophosphoryl lipid A (modified lipopolysaccharide [LPS]), as well as plasmids and immunostimulatory oligonucleotides, which contain sequences that mimic the stimulatory properties of bacterial DNA (bDNA) (2, 9, 11, 13, 28, 30, 45, 46, 56, 57). Less-well-defined bacterial preparations from intracellular pathogens, such as soluble toxoplasmosis antigens, soluble listerial antigens, and heat-killed Brucella abortus can also be used to stimulate Th1-like responses in mice (16, 26, 47, 49, 51). The purpose of these studies was to directly compare the abilities of different bacterial constituents to stimulate secretion of Th1-inducing cytokines such as IL-12 and gamma interferon (IFN-γ) (27, 34, 36, 54). The requirement for priming with IFN-γ and the sensitivity to IL-10 downregulation was also examined. In this report we demonstrate that B. abortus and bDNA, but not LPS, elicit high amounts of Th1-promoting cytokines from spleen cells in vitro and in vivo, even in the absence of priming with exogenous IFN-γ. Similar levels of endogenous IL-10 production are stimulated by all three constituents, and thus the amount of IL-10 in culture cannot account for the inferior IL-12 and IFN-γ induction by LPS in normal mice. These results indicate that bacterial constituents can differ in their ability to trigger the type of innate immune responses which drive the adaptive response in a Th1 direction. In particular, bDNA, and complex bacterial mixtures such as B. abortus, are more potent IL-12 inducers than is LPS, although they retain the ability to induce IL-10. These considerations are important for the development of Th1-promoting vaccine adjuvants and carriers which are both effective and safe.
MATERIALS AND METHODS
Mice.
IL-10 knockout (KO) mice (on a B10.D2 background) and IFN-γ KO mice (on a BALB/c background) were obtained from the Jackson Laboratory (Bar Harbor, Maine). BALB/c and B10.D2 mice were obtained from Jackson or from the Division of Cancer Treatment, National Cancer Institute (Frederick, Md.). Animals were used according to National Institutes of Health guidelines on animal use and care.
Reagents and antibodies.
LPS and DNA from Escherichia coli and control eukaryotic herring testis DNA were obtained from Sigma (St. Louis, Mo.). Heat-killed B. abortus 1119.3 was kindly provided by Barbara Martin at the U.S. Department of Agriculture (Ames, Iowa). B. abortus LPS was purified by butanol extraction as described previously (24). B. abortus DNA was extracted from B. abortus by using the Qiagen genomic DNA protocol and reagents (Valencia, Calif.). The LPS content of the DNA preparations was determined by using the Limulus amebocyte lysate test (BioWhittaker, Walkersville, Md.), which was performed by Pankaj Amin (Center for Biologics Evaluation and Research, U.S. Food and Drug Administration). The amount of LPS in DNA preparations ranged from 30 to 300 pg/μg of DNA. Recombinant mouse IFN-γ and anti-IL-10 and anti-IL-12 monoclonal antibodies were obtained from Pharmingen (San Diego, Calif.). For neutralization experiments, antibodies were used at 10 μg/ml in culture.
Cell preparation and culture.
Spleens were removed from mice, and single cell suspensions were prepared by gentle teasing through cell strainers (Becton Dickinson, Franklin, N.J.). Erythrocytes were lysed by using ACK lysing buffer (BioWhittaker) and washed three times in phosphate-buffered saline (PBS) before resuspension in RPMI (Life Technologies, Gaithersburg, Md.). RPMI was supplemented with 10% fetal bovine serum (HyClone, Logan, Utah), penicillin-streptomycin, HEPES buffer, 2-mercaptoethanol, nonessential amino acids, and pyruvate. Spleen cells were cultured in 48-well flat-bottom plates (Costar, Cambridge, Mass.) at a concentration of 107 cells/ml. Preliminary experiments determined that this concentration of cells provided optimal IFN-γ production at all time points tested, to LPS, bDNA, and B. abortus. Preliminary dose-response analyses were conducted with bDNA concentrations ranging from 0.5 to 100 μg/ml and LPS concentrations ranging from 3 to 750 μg/ml. Optimal IFN-γ-producing conditions were 25 μg/ml for bDNA and 30 μg/ml for LPS; these concentrations were used for all experiments. Cultures were incubated at 37°C with 5% CO2. Supernatants were harvested and frozen at −20°C for the cytokine assays.
Cytokine ELISA.
Cytokine content in supernatants was determined by enzyme-linked immunosorbent assay (ELISA) by using commercial kits for IFN-γ (Life Technologies), IL-10 and IL-12 p70 (Endogen, Woburn, Mass.), and IL-12 p40 (Biosource, Camarillo, Calif.). Samples were assayed in duplicate, and all experiments were performed at least twice. All data shown are from reproducible experiments. Values are expressed as the means with the standard deviations for duplicate samples. The lower limit of detection for IL-12 p70 was 5 pg/ml.
In vivo experiments.
BALB/c mice were injected intravenously with B. abortus (108 organisms), B. abortus DNA (100 μg), E. coli DNA (100 μg), B. abortus LPS (30 μg), low-dose B. abortus DNA (0.1 μg), or PBS. Injection volumes were 0.1 to 0.2 ml/mouse. The dose of B. abortus was selected because it is nontoxic, suppresses Th2 responses, and induces IL-12 mRNA (49). The 100-μg dose of bDNA has been shown to stimulate IFN-γ secretion in vivo (11). A 30-μg portion of LPS was selected because this is the amount of LPS contained in 108 organisms of the B. abortus preparation. The 0.1-μg dose of bDNA (low dose) used is the amount of bDNA contained in 108 B. abortus organisms. At 3 h after injection, spleens were removed and prepared by gentle teasing through cell strainers. After a washing, cells were cultured in RPMI as detailed above at a concentration of 5 × 106 cells/ml in 48-well plates, without further stimulation. Supernatants were harvested at 18 to 24 h for ELISA.
RESULTS
B. abortus and bDNA are more potent inducers of IFN-γ secretion than is LPS.
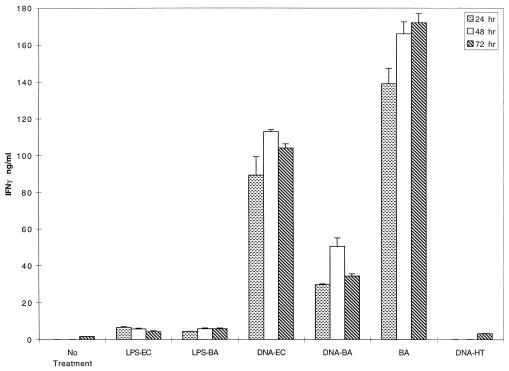
IFN-γ, chiefly a product of T and NK cells, is an important contributor to the development of primary and recall Th1 responses (4–6). In addition, IFN-γ suppresses Th2 development (21, 40, 42, 44). IFN-γ, secreted in an antigen-nonspecific fashion at the onset of an immune response, enhances macrophage activation and antigen uptake and primes macrophages for IL-12 production (4, 20, 25, 33, 55). Therefore, IFN-γ induction is a measure of potential adjuvant activity by bacterial constituents. The ability of bDNA, LPS, and B. abortus to induce IFN-γ was measured in whole spleen cell cultures, which were used in order to provide an environment closest to that seen in vivo. Preliminary dose-response studies indicated that for bDNA and for LPS, 25 to 50 and 30 μg/ml, respectively, were optimal stimulatory doses. B. abortus was added at 108 organisms/ml, a dose which is stimulatory and nontoxic. The amount of bDNA contained in the dose of B. abortus used is 500 ng/ml, which is below the threshold dose of purified bDNA for IFN-γ production. B. abortus and bDNA, but not LPS, promoted high levels of IFN-γ secretion (Fig. 1). Similar results were seen in 10 separate experiments. Typically, IFN-γ production by LPS-treated cells was similar to control levels, although in some experiments it exceeded control culture levels. This interexperimental variation may reflect occasional in vivo priming if mice were exposed to microorganisms in the animal care facility. The source of LPS was not a factor, since neither E. coli nor B. abortus LPS induced high levels of IFN-γ (Fig. 1; see also Fig. 3).
FIG. 1.
IFN-γ induction by B. abortus, bDNA, and LPS. BALB/c spleen cells were cultured with the indicated stimuli; supernatants were harvested at 24, 48, and 72 h, and supernatants were assayed for IFN-γ by ELISA. LPS was derived from either E. coli (LPS-EC) or B. abortus (LPS-BA). Controls include cells stimulated with eukaryotic herring testis DNA (DNA-HT) and untreated cells.
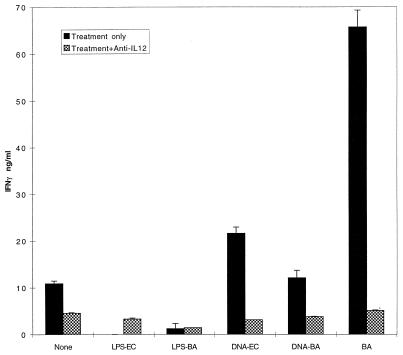
FIG. 3.
IFN-γ induction by B. abortus, LPS, and bDNA is IL-12 dependent. BALB/c spleen cells were cultured for 72 h with or without anti-IL-12 (10 μg/ml), and supernatants were assayed by ELISA. More than 90% of the IFN-γ induction by bDNA and B. abortus was prevented by anti-IL-12. This experiment is representative of four separate experiments in two strains of mice (BALB/c and B10.D2), which all showed significant reduction of IFN-γ secretion by antibodies to IL-12.
Unprimed spleen cells produce IL-12 p70 in response to B. abortus and DNA but not LPS.
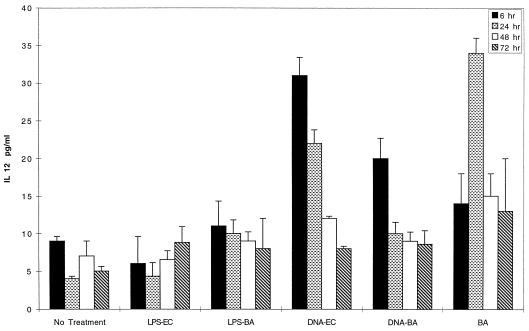
The greater ability of B. abortus and bDNA to induce IFN-γ in vitro, compared to LPS, could be explained by low IL-12 production in response to LPS. Indeed, IL-12 p70 secretion appeared to correlate with subsequent IFN-γ production (Fig. 2). At early time points, both B. abortus and bDNA, but not LPS, noticeably increased IL-12 secretion. IFN-γ is typically required to elicit significant amounts of IL-12 from cultured cells exposed to LPS (20, 37). These results show that robust IL-12 secretion, induced by B. abortus and bDNA, does not require priming with exogenous IFN-γ.
FIG. 2.
IL-12 is induced by B. abortus and bDNA but not LPS. BALB/c spleen cells were cultured with LPS and bDNA (from B. abortus and E. coli) and with B. abortus. Supernatants were removed at the indicated times and assayed for IL-12 p70 levels by ELISA. Cells cultured with LPS, and untreated cells produced similar constitutive amounts of IL-12 p70, at levels near the detection limits of the ELISA (5 pg/ml). In contrast, bDNA and B. abortus both elicited IL-12 production in excess of the control cultures at early time points. The results of one of three similar experiments are shown.
While IL-12 is the most likely cytokine to be responsible for IFN-γ production in this system, other cytokines produced in response to pathogens such as type 1 IFNs and IL-18 (IGIF) can also promote IFN-γ secretion (7, 35, 41, 58). Indeed, human cells secrete IL-18 in response to stimulatory DNA sequences, and B. abortus induces type 1 IFNs (15, 48). To confirm that IFN-γ secretion in this system was dependent upon IL-12, cells were cultured in the presence of anti-IL-12 or isotype control antibody. B. abortus and bDNA-induced IFN-γ production was completely IL-12 dependent (Fig. 3). These data suggest that other IFN-γ-inducing cytokines do not substitute for the IL-12 requirement to stimulate IFN-γ release.
Removal of IL-10 permits LPS induction of IFN-γ and increases B. abortus and bDNA IFN-γ.
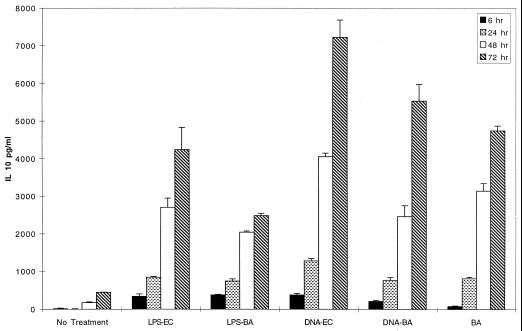
Interleukin-10 is a potent downregulator of Th1 responses (19, 38, 55). Macrophage-secreted IL-10 attenuates IL-12 and IL-1 secretion, thus limiting the strength of inflammatory reactions and therefore potential damage to host tissues (3, 55). LPS, bDNA, and B. abortus can all induce some IL-10 (1, 3, 38, 53). The lack of robust IFN-γ production by spleen cells exposed to LPS could be explained by induction of high IL-10 levels, relative to those induced by B. abortus and bDNA. However, all three bacterially derived preparations induced similar quantities of IL-10 (Fig. 4). The kinetics of IL-10 secretion were inversely correlated with those of IL-12 in that, as IL-10 increased, IL-12 levels diminished to baseline amounts by 72 h in B. abortus and bDNA cultures. The rate of IFN-γ accumulation decreased between 48 and 72 h in most experiments, consistent with downregulation of IL-12, followed by IFN-γ. Thus, the amount of IFN-γ in supernatants was still elevated at 72 h, reflecting production from earlier timepoints.
FIG. 4.
IL-10 production is not enhanced in LPS cultures. BALB/c spleen cells were cultured with the indicated additives for 6 to 72 h. Supernatants were assayed for IL-10 by ELISA. IL-10 production, like IFN-γ, peaked at the latest time point and showed similar kinetics for all constituents.
Although in this system, similar levels of IL-10 were seen after B. abortus, bDNA, and LPS, it was still possible that (potential) LPS-induced IFN-γ secretion is more sensitive to IL-10 downregulation than the pathways used by bDNA and B. abortus. To explore this issue, IFN-γ production was determined after incubation of cells with anti-IL-10 (Fig. 5). In the presence of anti-IL-10, LPS consistently induced small amounts of IFN-γ secretion. Anti-IL-10 also increased production of IFN-γ by cells cultured with bDNA and B. abortus. To confirm these results, cells from IL-10 KO mice were cultured with LPS or bDNA. Cells cultured with E. coli LPS or B. abortus LPS produced large amounts of IFN-γ (72.3 ± 5.6 and 75.2 ± 2.8 ng/ml, respectively), as did cells cultured with E. coli DNA or B. abortus DNA (92.5 ± 1.0 and 73.8 ± 4.3 ng/ml, respectively). Results in IL-10 KO mice differed from those in normal mouse cultures treated with anti-IL-10. The potency of LPS and bDNA for IFN-γ induction was similar in IL-10 KO spleen cultures, but it was dissimilar in normal mice, with bDNA always stimulating more IFN-γ than LPS when spleens were cultured with anti-IL-10. Such results may reflect differences in the reactivity of cell populations in KO mice, which have developed in the complete absence of endogenous IL-10. Overall, however, these results suggest that LPS, while capable of inducing IFN-γ secretion, can only be a potent inducer in the absence of IL-10, whereas the bDNA and pathway of IFN-γ induction can proceed in the presence of IL-10.
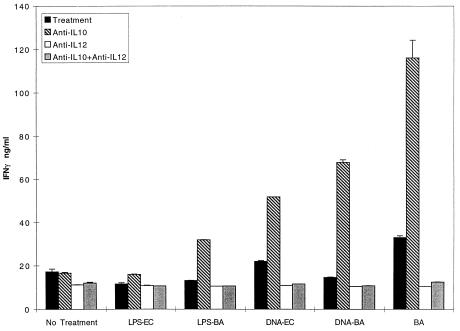
FIG. 5.
IFN-γ produced when endogenous IL-10 is blocked is IL-12 dependent. BALB/c spleen cells were cultured with various stimuli in the presence of anti-IL-10, anti-IL-12, or both antibodies (10 μg/ml). Supernatants were collected at 72 h and analyzed for IFN-γ by ELISA. The results from one of two similar experiments are shown.
Interestingly, even though IFN-γ was increased in LPS–αIL-10-treated cells, no amplification of IL-12 p70 secretion was detected (not shown), suggesting a possible action of another IFN-γ-inducing factor in this setting. To explore this possibility, cells were cultured with LPS–αIL-10 in the presence or absence of αIL-12 (Fig. 5). Coculture with αIL-12 and αIL-10 eliminated the LPS-induced IFN-γ. Thus, small increases of IL-12, which were difficult to detect by ELISA but large enough to cause IFN-γ secretion, were produced when cells were exposed to LPS in the absence of IL-10.
Role of IFN-γ priming for IL-12 production in response to B. abortus bDNA, and LPS.
Murine and human macrophages exposed to IFN-γ have enhanced ability to produce IL-12 when stimulated with LPS (14, 25, 55). The mechanism of this priming effect appears to be an IFN-γ-mediated increase of IL-12 p40 mRNA transcription and stability (25, 33). It has not been determined whether bDNA-induced IL-12 is also susceptible to this priming effect. IFN-γ KO mice were used for these experiments to eliminate the possibility of in vivo priming of cells by IFN-γ due to environmental factors. IFN-γ enhanced IL-12 production by cells cultured with bDNA and B. abortus to a greater extent than those cultured with LPS (Fig. 6). Only B. abortus and bDNA-cultured cells from IFN-γ KO mice produced IL-12 in the presence of anti-IL-10, even without the exogenous addition of IFN-γ. Furthermore, neutralization of IL-10 was synergistic with the addition of IFN-γ for IL-12 production when cells were cultured with B. abortus bDNA, or LPS, but the amount of IL-12 produced was always greatest in B. abortus cultures. As in normal mice, anti-IL-10 addition could not suffice to stimulate measurable increases of IL-12 production from LPS-exposed cells. These results emphasize the limited ability of LPS alone to provide a strong Th1-promoting environment, compared to B. abortus organisms or bDNA.
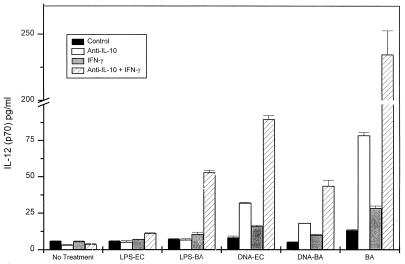
FIG. 6.
B. abortus and bDNA-induced IL-12 are enhanced by IFN-γ priming, especially in the presence of anti-IL-10. LPS only induced detectable IL-12 with the combination of IFN-γ addition and anti-IL-10. Spleen cells from IFN-γ KO mice (BALB/c background) were stimulated with B. abortus, LPS, or bDNA in the presence of IFN-γ (50 ng/ml) and/or anti-IL-10 (10 μg/ml). LPS, bDNA, or B. abortus was added to the culture 10 min after the addition of anti-IL-10 and IFN-γ. IL-12 p70 was measured by ELISA 18 h later. The results of one of three representative experiments are shown. The results in normal BALB/c mice were similar.
B. abortus and B. abortus DNA induce splenic IL-12 in vivo.
Sher et al. reported that IL-12 can be detected in spleen cell cultures after the injection of a soluble parasite extract (47). To determine whether the superior IL-12-inducing ability of B. abortus and B. abortus DNA in vitro could be demonstrated in vivo, BALB/c mice were injected intravenously with B. abortus, bDNA, or LPS. At 3 h after injection, spleens were removed, and cells cultured without further intervention. B. abortus, B. abortus DNA, and E. coli DNA, but not LPS, stimulated IL-12 secretion from in vivo-treated spleen cells (Table 1). B. abortus given in vivo was consistently the most potent IL-12-inducing substance, whereas when exposure was entirely in vitro, bDNA often stimulated as much IL-12 release as had B. abortus. It is possible that bDNA is taken up by other tissues and degraded by DNases before all of it can reach the spleen, which could account for the observed decrease in in vivo potency relative to B. abortus.
TABLE 1.
IL-12 production 3 h after intravenous injection of bacterial constituents in BALB/c micea
| Treatment | IL-12 produced (pg/ml)
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| PBS | 35.7 ± 9.8 | 8.5 ± 0.6 |
| B. abortus | 234 ± 68 | 467 ± 5.4 |
| B. abortus DNA | 104 ± 32.8 | 203 ± 40 |
| B. abortus LPS | 9.7 ± 3.0 | 17.3 ± 2.9 |
| E. coli DNA | ND | 107 ± 27.5 |
| B. abortus DNA (low dose) | 9.9 ± 3.6 | ND |
BALB/c mice were injected with B. abortus (108 organisms), B. abortus LPS (30 μg), B. abortus DNA or E. coli DNA (100 μg), or low dose B. abortus DNA (0.1 μg). Three hours after injection, spleens were removed and cultured individually without further stimulation. Supernatants were harvested at 24 h and assayed by ELISA for IL-12 p40. Spleens removed 8 and 24 h after injection produced less IL-12 than that seen at 3 h, although the relative potency of constituents remained the same (data not shown). The results reflect the means ± the standard error for three mice/treatment. ND, not determined.
DISCUSSION
Vaccine adjuvants which foster Th1 responses are likely to be useful in promoting antiviral immunity. LPS derivatives, bDNA-containing vaccines, and synthesized immunostimulatory DNA sequences are under investigation as enhancers of Th1 and cytotoxic T lymphocyte responses for use against viral and parasitic pathogens. All of these bacterial products stimulate the innate immune system, which in turn influences the type of adaptive T-cell response. To compare the abilities of prototypic bacterial constituents to provide a Th1-favoring environment, we studied the cytokine response to bDNA, LPS, and whole heat-inactivated intracellular bacteria (B. abortus). In addition, the dependence of IL-12 and IFN-γ responses upon IFN-γ and IL-10 was assessed.
Surprisingly, a direct comparison between LPSs from two different bacteria and bDNA showed that bDNA had superior IL-12- and IFN-γ-inducing capacities, in vitro and in vivo. Unlike this work, most studies which show that LPS induces IL-12 have been done in APC-enriched populations or with the exogenous addition of IFN-γ. LPS clearly can induce measurable IL-12 from activated splenic adherent cells (47) and peritoneal or bone marrow-derived macrophages (50, 52). In some studies, LPS induced IL-12 and IFN-γ production from spleens in vivo, but priming by footpad injection of LPS was required (43). Using immunohistochemical methods, a recent study has shown that intravenous injection of LPS elicits detectable IL-12 p40 in splenic dendritic areas, although to a much lesser degree compared to a parasite protein extract (47). Additional support for poor IL-12-inducing activity in the spleen by LPS is provided by the observation that intraperitoneal injection does not prime for the Schwartzman reaction, which is IL-12 dependent (43). Furthermore, most studies have measured IL-12 p40 rather than the bioactive heterodimer p70. In fact, p40 homodimers can antagonize the action of p70 (22, 23). Overall, these reports are consistent with our findings that LPS elicits little IL-12 p70 from spleen cells.
In vitro, priming of spleen cell cultures by IFN-γ increases IL-12 responses to LPS (20, 55). However, other stimuli, such as viable or heat-killed M. tuberculosis, or latex beads, do not require IFN-γ priming for the production of IL-12 by macrophages (31). Our results indicate that bDNA and B. abortus-induced IL-12 does not require exogenous IFN-γ priming, since bDNA and B. abortus stimulation elicited prompt IL-12 and IFN-γ production from normal spleen cells. In IFN-γ KO mice, B. abortus and bDNA alone, still measurably increased IL-12 levels in the absence of IFN-γ, when IL-10 was neutralized. Anti-IL-10 alone did not enhance IL-12 secretion from LPS-treated IFN KO mouse cells, whereas IL-10 neutralization did stimulate IL-12 release from LPS-treated normal mouse cells. The difference between normal and IFN-γ KO mice seen here suggests that constitutive in vivo priming by low levels of environmentally induced IFN-γ occurs in normal mice; this could function to provide a regulated, low level of responsiveness to LPS. In all cases, the combination of anti-IL-10 and exogenous IFN-γ caused the most IL-12 production from IFN-γ KO cells, although B. abortus invariably was the most potent stimulator under these conditions. Taken together, these results support the hypothesis that bDNA- or B. abortus-induced IL-12 is less dependent upon IFN-γ priming than is LPS-induced IL-12.
The increased potency of B. abortus and bDNA relative to LPS could be explained if these bacterial products stimulate different cell populations or subpopulations to produce IL-12. B cells, monocytes, dendritic cells, and polymorphonuclear leukocytes all respond to LPS, and are all capable of secreting IL-12 (32, 47, 54). CpG motifs, contained in bDNA, directly stimulate B cells and macrophages and promote B-cell production of IL-12 (8, 29, 48). If LPS stimulates different cell populations than does bDNA, these populations may be more sensitive to downregulatory cytokines such as IL-10, may be simply less numerous, or may secrete less IL-12/cell. Our experiments in IL-10 KO mice have suggested that bDNA, B. abortus, and LPS-induced IL-12 have similar sensitivities to downmodulation by exogenous IL-10 (not shown). Although IL-10 levels were similar in bDNA and B. abortus cultures, B. abortus typically stimulated higher IL-12 production. A likely explanation is that a greater number of IL-12-secreting cells initially respond to B. abortus than to bDNA. This is supported by recent in vivo immunohistochemical analysis of IL-12 expression in B. abortus- and bDNA-treated mice, showing more numerous IL-12 secreting cells after treatment with B. abortus (49a). Detailed dissection of responding cell populations and the pathways through which they are stimulated should elucidate why bDNA and B. abortus are more effective IL-12 and IFN-γ stimulators than LPS.
B. abortus appeared to be similar to bDNA in its ability to induce IL-12 from spleen cell cultures. Like bDNA, it induced IL-12 more effectively than LPS, and this IL-12 production was downregulated by endogenous IL-10 and upregulated by IFN-γ. However, bDNA contained in B. abortus is not likely to fully explain the potent effects of B. abortus. The amount of bDNA contained in the concentration of B. abortus used is close to 500 ng/ml (12), which is well below our minimal stimulatory bDNA concentration of 2.5 μg/ml. The amount of LPS in the B. abortus preparations was 30 to 35 μg/ml, a level similar to doses of LPS used in these experiments. However, the conclusion that stimulation of IL-12 and IFN-γ secretion by B. abortus is caused by constituents other than bDNA is also not entirely proven by these calculations. B. abortus, a macrophagotropic organism, may preferentially target and activate APCs and thus could deliver its constituents more effectively with other stimulating signals or in a particularly stimulating configuration to responding cells. Indeed, numerous endeavors in this laboratory to remove bDNA from the B. abortus preparations by using DNase digestions coupled with sonication have failed to eliminate PCR-detectable B. abortus DNA sequences, suggesting that bDNA is protected from degradation when contained in heat-killed bacteria. However, efforts are under way to identify other immunostimulating compounds in B. abortus which can promote Th1 responses.
These studies show that bDNA and LPS differ substantially in their ability to create a Th1-promoting environment in vitro and in vivo. We also show for the first time that bDNA-induced IL-12 is enhanced by priming with IFN-γ. Although responses to both constituents and to B. abortus were regulated by IFN-γ and endogenous IL-10, at optimal doses quantitative differences among them are marked. Further understanding of these differences may lead to the design of more effective Th1-promoting adjuvants, which are needed for protective responses to viral and parasitic diseases.
ACKNOWLEDGMENTS
We thank Hana Golding and Ray Donnelly for thoughtful review of this manuscript. Pankaj Amin kindly assayed all bacterial constituents for LPS content. Debra Lowry provided excellent technical assistance.
REFERENCES
- 1.Anitescu M, Chace J H, Tuetken R, Yi A K, Berg D J, Krieg A M, Cowdery J S. Interleukin-10 functions in vitro and in vivo to inhibit bacterial DNA-induced secretion of IL-12. J Interferon Cytokine Res. 1997;12:781–788. doi: 10.1089/jir.1997.17.781. [DOI] [PubMed] [Google Scholar]
- 2.Ballas Z K, Rasmussen W L, Krieg A M. Induction of natural killer activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157:1840–1845. [PubMed] [Google Scholar]
- 3.Berg D J, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, Grunig G, Rennick D. Interleukin-10 is a central regulator of the responses to LPS in murine models of endotoxic shock and the Schwartzman reaction but not endotoxin tolerance. J Clin Investig. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehm U, Klamp T, Groot M, Howard J C. Cellular Responses to Interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 5.Bradley L M, Dalton D K, Croft M. A direct role for IFN-gamma in regulation of Th1 cell development. J Immunol. 1996;157:1350–1358. [PubMed] [Google Scholar]
- 6.Bradley L M, Yoshimoto K, Swain S L. The cytokines IL-4, IFN-gamma, and IL-12 regulate the development of subsets of memory effector helper T cells in vitro. J Immunol. 1995;155:1713–1724. [PubMed] [Google Scholar]
- 7.Brinkmann V, Geiger T, Alkan S, Heusser C H. Interferon alpha increases the frequency of interferon-gamma producing human CD4+T cells. J Exp Med. 1993;178:1655–1663. doi: 10.1084/jem.178.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chace J H, Hooker N A, Mildenstein K L, Krieg A M, Cowdery J S. Bacterial DNA-induced NK cell IFN-gamma production is dependent on macrophage secretion of IL-12. Clin Immunol Immunopathol. 1997;84:185–193. doi: 10.1006/clin.1997.4380. [DOI] [PubMed] [Google Scholar]
- 9.Chu R S, Targoni O S, Krieg A M, Lehmann P V, Harding C V. CpG oligodeoxynucleotides act as adjuvants that switch on T helper (Th1) Immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clerici M, Shearer G M. A Th1 to Th2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 11.Cowdery J S, Chace J H, Yi A-K, Krieg A M. Bacterial DNA induces NK cells to produce IFN-gamma in vivo and increases the toxicity of lipopolysaccharides. J Immunol. 1996;156:4570–4575. [PubMed] [Google Scholar]
- 12.Da Costa M, Guillou J-P, Garin-Bastuji B, Thiebaud M, Dubray G. Specificity of six gene sequences for the detection of the genus Brucellaby DNA amplification. J Appl Bacteriol. 1996;81:267–275. doi: 10.1111/j.1365-2672.1996.tb04328.x. [DOI] [PubMed] [Google Scholar]
- 13.Davis H L, Weeranta R, Waldschmidt T J, Tygrett L, Schorr J, Krieg A M. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 14.DeKruff R H, Gieni R S, Umetsu D T. Antigen-driven but not lipopolysaccharide-driven IL-12 production in macrophages requires triggering of CD40. J Immunol. 1997;158:359–366. [PubMed] [Google Scholar]
- 15.Finkelman F D, Holmes J, Katona I M, Urban J F, Jr, Beckman M P, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 16.Finkelman F D, Katona I M, Mosmann T R, Coffman R L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140:1022–1027. [PubMed] [Google Scholar]
- 17.Finkelman F D, Katona I M, Urban J F, Jr, Holmes J, Ohara J, Tung A S, Sample J v, Paul W E. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335–2341. [PubMed] [Google Scholar]
- 18.Finkelman F D, Shea-Donohue T, Goldhill J, Sullivan C A, Morris S C, Madden K B, Gause W C, Urban J F J. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 19.Fiorentino D F, Zlotnik A, Mosmann T R, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 20.Flesch I E A, Hess J H, Huang S, Aguet M, Rothe J, Bluethmann H, Kaufmann S H E. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon gamma and tumor necrosis factor alpha. J Exp Med. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gajewski T F, Joyce J, Fitch F W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of Th1 and Th2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989;143:15–22. [PubMed] [Google Scholar]
- 22.Gately M K, Carvajal D M, Connaughton S E, Gilessen S, Warrier R R, Kolinsky K D, Wilkinson V L, Dwyer C M, Higgins G F J, Podlaski F J, Faherty D A, Familletti P C, Stern A S, Presky D H. Interleukin-12 antagonist activity of mouse interleukin-12 p40 homodimer in vitro and in vivo. Ann N Y Acad Sci. 1996;795:1–12. doi: 10.1111/j.1749-6632.1996.tb52650.x. [DOI] [PubMed] [Google Scholar]
- 23.Gillessen S, Carvajal D, Ling P, Podlaski F J, Stremlo D L, Familletti P C, Gubler U, Presky D H, Stern A S, Gately M K. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein J, Hoffman T, Frasch C, Lizzio E F, Beining P R, Hochstein D, Lee Y L, Angus R D, Golding B. Lipopolysaccharide (LPS) from Brucella abortus is less toxic that that from Escherichia coli, suggesting the possible use of B. abortus or LPS from B. abortusas a carrier in vaccines. Infect Immun. 1992;60:1385–1389. doi: 10.1128/iai.60.4.1385-1389.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayes M P, Wang J, Norcross M A. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- 26.Hsieh C-S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh C-S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 28.Klinman D M, Yamshchikov G, Ishigatsubo Y. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol. 1997;158:3635–3639. [PubMed] [Google Scholar]
- 29.Klinman D M, Yi A, Beaucage S L, Conover J, Krieg A M. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krieg A M, Yi A K, Schorr J, Davis H L. The role of CpG dinucleotides in DNA vaccines. Trends Microbiol. 1998;6:23–27. doi: 10.1016/S0966-842X(97)01145-1. [DOI] [PubMed] [Google Scholar]
- 31.Ladel C H, Szalay G, Riedel D, Kaufmann S H E. Interleukin-12 secretion by Mycobacterium tuberculosis-infected macrophages. Infect Immun. 1997;65:1936–1938. doi: 10.1128/iai.65.5.1936-1938.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamont A G, Adorini L. IL-12: a key cytokine in immune regulation. Immunol Today. 1996;17:214–217. doi: 10.1016/0167-5699(96)30011-x. [DOI] [PubMed] [Google Scholar]
- 33.Ma X, Chow J M, Gri G, Carra G, Gerosa F, Wolf S F, Dzialo R, Trinchieri G. The interleukin-12 p40 gene promoter is primed by interferon-gamma in monocytic cells. J Exp Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maggi E, Parronchi P, Manetti R, Simonelli C, Piccinni M-P, Rugiu F S, De Carli M, Ricci M, Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992;148:2142–2147. [PubMed] [Google Scholar]
- 35.Manetti R, Annunziato F, Tomasevic L, Gianno V, Parronchi P, Romagnani S, Maggi E. Polyinosinic acid: polycytidylic acid promotes T helper type 1-specific immune responses by stimulating macrophage production of interferon-alpha and interleukin-12. Eur J Immunol. 1995;25:2656–2660. doi: 10.1002/eji.1830250938. [DOI] [PubMed] [Google Scholar]
- 36.Mannetti R, Paronchi P, Giudizi M G, Piccinni M-P, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (IL 12 [IL-12]) induces T helper type I (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maruo S, Oh-hora M, Ahn H, Ono S, Wysocka M, Kaneko Y, Yagita H, Okumura K, Kikutani H, Kishimoto T, Kobayashi M, Hamaoka T, Trinchieri G, Fujiwara H. B cells regulate CD40 ligand-induced IL-12 production in antigen-presenting cells (APC) during T cell/APC interactions. J Immunol. 1997;158:120–126. [PubMed] [Google Scholar]
- 38.Moore K W, O'Garra A, de Waal-Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 39.Mosmann T R, Coffman R L. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 40.Noble A, Staynov D Z, Kemeny D M. Generation of rat Th2-like cells in vitro is interleukin-4-dependent and inhibited by interferon-gamma. Immunology. 1993;79:562. [PMC free article] [PubMed] [Google Scholar]
- 41.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 42.Oriss T B, McCarthy S A, Morel B F, Campana M A K, Morel P A. Crossregulation between T helper cell (Th)1 and Th2. J Immunol. 1997;158:3666–3672. [PubMed] [Google Scholar]
- 43.Ozman L, Pericin M, Hakimi J, Chizzonite R A, Wysocka M, Trinchieri G, Gately M, Garotta G. Interleukin 12, interferon gamma, and tumor necrosis factor alpha are the key cytokines of the generalized Shwartzman reaction. J Exp Med. 1994;180:907–915. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paul W E, Seder R A. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 45.Pisetsky D S. Immune activation by bacterial DNA: a new genetic code. Immunity. 1996;5:303–310. doi: 10.1016/s1074-7613(00)80256-3. [DOI] [PubMed] [Google Scholar]
- 46.Raz E, Tighe H, Sato Y, Corr M, Dudler J A, Roman M, Swain S L, Spiegelberg H L, Carson D A. Preferential induction of Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reis e Sousa C, Hieny S, Scharton-Kerston T, Jankovic D, Charest H, Germain R N, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roman M, Martin-Orozko E, Goodman J S, Nguyen M-D, Sato Y, Ronaghy A, Kornbluth R S, Richman D D, Carson D A, Raz E. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 49.Scott D E, Agranovich I, Inman J, Gober M, Golding B. Inhibition of primary and recall allergen-specific T helper cell type 2-mediated responses by a T helper cell type 1 stimulus. J Immunol. 1997;159:107–116. [PubMed] [Google Scholar]
- 49a.Scott, D. E., and L.-Y. Huang. Unpublished data.
- 50.Skeen M J, Miller M A, Shinnick T M, Ziegler H K. Regulation of murine macrophage IL-12 production. J Immunol. 1996;156:1196–1206. [PubMed] [Google Scholar]
- 51.Street N E, Schumacher J H, Fong T, Bass H, Fiorentino D F, Leverah J A, Mosmann T R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990;144:1629–1639. [PubMed] [Google Scholar]
- 52.Sutterwala F S, Noel G J, Clynes R, Mosser D M. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Svetic A S, Jian Y C, Finkelman F D, Gause W C. Brucella abortus induces a novel cytokine gene expression pattern characterized by elevated IL-10 and IFN-gamma in CD4+T cells. Int Immunol. 1993;5:877–883. doi: 10.1093/intimm/5.8.877. [DOI] [PubMed] [Google Scholar]
- 54.Trinchieri G. IL-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;3:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 55.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 56.Ulrich J T, Myers K R. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharmacol Biotechnol. 1995;6:495–524. [PubMed] [Google Scholar]
- 57.VanCott T C, Kaminski R W, Mascola J R, Kalyanaraman V S, Wassef N M, Alving C R, Ulrich J T, Lowell G H, Birx D L. HIV-1 neutralizing antibodies in the genital and respiratory tracts of mice intranasally immunized with the oligomeric gp160. J Immunol. 1998;160:2000–2012. [PubMed] [Google Scholar]
- 58.Wenner C A, Guler M L, Macatonia S E, O'Garra A, Murphy K M. Roles of IFN-gamma and IFN-alpha in IL-12-induced T helper 1 development. J Immunol. 1996;156:1442–1447. [PubMed] [Google Scholar]